
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 18 June 2021
Sec. Reproduction
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.694083
This article is part of the Research Topic Endocrinology and COVID-19: A Cross-Disciplinary Topic View all 18 articles
 Lukas Lanser1
Lukas Lanser1 Francesco Robert Burkert1
Francesco Robert Burkert1 Lis Thommes1
Lis Thommes1 Alexander Egger2
Alexander Egger2 Gregor Hoermann2,3
Gregor Hoermann2,3 Susanne Kaser4
Susanne Kaser4 Germar Michael Pinggera5
Germar Michael Pinggera5 Markus Anliker2
Markus Anliker2 Andrea Griesmacher2
Andrea Griesmacher2 Günter Weiss1
Günter Weiss1 Rosa Bellmann-Weiler1*
Rosa Bellmann-Weiler1*Background: Male sex is related to increased COVID-19 severity and fatality although confirmed infections are similarly distributed between men and women. The aim of this retrospective analysis was to investigate the impact of sex hormones on disease progression and immune activation in men with COVID-19.
Patients and Methods: We studied for effects of sex hormones on disease severity and immune activation in 377 patients (230 men, 147 women) with PCR-confirmed SARS-CoV-2 infections hospitalized at the Innsbruck University Hospital between February and December 2020.
Results: Men had more severe COVID-19 with concomitant higher immune system activation upon hospital admission when compared to women. Men with a severe course of infection had lower serum total testosterone (tT) levels whereas luteinizing hormone (LH) and estradiol (E2) levels were within the normal range. tT deficiency was associated with elevated CRP (rs = - 0.567, p < 0.001), IL-6 levels (rs = - 0.563, p < 0.001), lower cholesterol levels (rs = 0.407, p < 0.001) and an increased morbidity and mortality. Men with tT levels < 100 ng/dL had a more than eighteen-fold higher in-hospital mortality risk (OR 18.243 [95%CI 2.301 – 144.639], p = 0.006) compared to men with tT levels > 230 ng/dL. Moreover, while morbidity and mortality showed a positive correlation with E2 levels at admission, we detected a negative correlation with the tT/E2 ratio upon hospital admission.
Conclusion: Hospitalized men with COVID-19 present with rather low testosterone levels linked to more advanced immune activation, severe clinical manifestations translating into an increased risk for ICU admission or death. The underlying mechanisms remain elusive but may include infection driven hypogonadism as well as inflammation mediated cholesterol reduction causing gonadotropin suppression and impaired androgen formation. Finally, in elderly late onset hypogonadism might also contribute to lower testosterone levels.
Coronavirus disease 2019 (COVID-19) is still influencing the daily life of people all over the world. Most patients infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have developed mild symptoms, while up to 20% need hospitalization due to severe disease course characterized by shortness of breath and hypoxia (1). Several comorbidities including hypertension, obesity, diabetes, cardiovascular disease, and chronic pulmonary disease as well as age were shown to be related to more severe COVID-19 courses (2–5). Additionally, male sex is related to more severe COVID-19 manifestation, even though there are no sex differences in the absolute number of confirmed COVID-19 cases (6). Currently, up to 60% of hospitalized patients and even up to 82% of patients treated in the intensive care unit (ICU) are men (3, 4, 6). Accordingly, the case fatality rate (CFR) is 1.7 times higher in men compared to women (6–8). Inflammatory biomarkers were shown to be associated with COVID-19 morbidity and mortality in men and women: interleukin 6 (IL-6) as main inducer of C-reactive protein (CRP) in the liver (9), neopterin reflecting macrophage activation and thus T-helper cell type I (Th1) immune response (10) as well as other acute phase proteins including procalcitonin (PCT) and ferritin (11).
Several reviews have elucidated potential mechanisms underlying these sex differences in COVID-19 patients and will be only shortly summarized hereafter (6, 8, 12). Biological sex affects immune responses to invading pathogens through hormonal regulation, gene expression and environmental factors (8). Generally, women have a stronger innate and adaptive immune response than men resulting in faster pathogen clearance (13). Many genes located on the sex chromosomes regulate innate immune function by encoding for pattern recognition receptors, cytokine receptors or transcriptional factors (14). On the other hand, androgen response elements and estrogen (E2) response elements are found in promoters of several innate immunity genes thereby affecting their expression (15, 16). These mechanisms are suggested to contribute to faster containment and clearance of SARS-CoV-2 in female patients (8). Moreover, sex-associated differences in expression and activity of the virus entry receptor angiotensin-converting 2 (ACE2) and its co-receptor transmembrane protease serine subtype 2 (TMPRSS2) are suggested to contribute to a higher mortality in men (17, 18). The ACE2 gene is located on the X chromosome and estrogens were shown to upregulate its expression (19), while TMPRSS2 was shown to be regulated by androgen receptor signalling (20–22). Preclinical studies of ACE2 tissue expression have shown different results depending on the tissue type (23): ACE2 expression seems to be higher in the lungs, heart or kidney of male (24–26), while pancreatic ACE2 expression seems to be higher in female (27). However, the relationship of tissue ACE2 expression and circulating ACE2 activity is still not well understood and data from the literature is partial contradictory (23): in human, the ACE2 activity was shown to be higher in healthy men and men with heart failure compared to matched women (28, 29) while other studies showed no sex-related differences in serum ACE2 activity (30, 31). Interestingly, ACE2 activity does not differ between young and old men but is significantly higher in older compared to younger women (31).
However, whether these mechanisms actually affect disease severity and clinical outcome of COVID-19 patients or explain the sex differences remains unknown. Therefore, we analyzed different sex hormones and their interactions with inflammatory markers to assess the impact on disease severity and outcome in men: the androgen testosterone is the primary male sex hormone playing a key role in the male reproductivity and produced by testicular Leydig cells upon stimulation by luteinizing hormone (LH) released by the pituitary gland and regulated by the gonadotropin-releasing hormone (GnRH) released by the hypothalamus (32). Estradiol (E2) is the primary female sex hormone but also involved in male reproduction and synthesized from androgens by aromatase (33). Sex hormones are transported in the human body unbound in the serum or bound to the sex hormone binding protein (SHBG) (32).
We analyzed 377 patients with polymerase chain reaction (PCR)-proven COVID-19 hospitalized at our department at the Medical University of Innsbruck, Austria, between February and December 2020. Information on past medical history, concomitant medication, clinical characteristics and laboratory parameters were obtained from the local clinical electronic data management. The severity of the disease was categorized according to the score by the WHO Working Group on the Clinical Characterization and Management of COVID-19 infection (34). Events during hospital stay including death, ICU admission and need for mechanical ventilation were recorded for outcome analysis.
The study was conformed to the principles outlined in the Declaration of Helsinki and was approved by the ethics committee of the Innsbruck Medical University and patients had given informed consent (ID of ethical vote: 1167/2020).
All analyses were conducted at the ISO 15189 accredited Central Institute of Clinical and Chemical Laboratory Diagnostics (Medical University of Innsbruck, Austria) according to the manufacturers’ procedures. Blood samples for detection of sex hormones were taken guideline conform till 11 a.m. within the first three days after hospital admission. A fully automated analyzer (Cobas 8000) by Roche Diagnostics GmbH (Mannheim, Germany) comprising an indirect potentiometric unit (ISE module), a chemistry unit (module 702), and an immunological unit (module e602) was used to determine the following parameters: creatinine (CREP2, enzymatic), aspartate transaminase (AST; ASTPM), alanine aminotransferase (ALT; ALTPM), alkaline phosphatase (ALP; ALP2), interleukin 6 (IL-6; Elecsys IL-6), cholesterol (CHOL2), high-density lipoprotein (HDL; HDLC4), low-density lipoprotein (LDL; LDLC3), triglycerides (TRIGL), estradiol (E2; Elecsys Estradiol III), procalcitonin (PCT; Elecsys BRAHMS PCT), C-reactive protein (CRP; CRP4), and ferritin (FERR4). Glycated hemoglobin (HbA1c) was measured by liquid chromatography on a TOSOH G8 instrument (Tosoh Corporation, Shiba, Minato-ku, Japan). Fibrinogen was determined on a Siemens analyzer (BCS-XP) using reagents from Siemens (Multifibren U). Androstenedione, dehydroepiandrosterone (as sulfate), luteinizing hormone (LH), follicle-stimulating hormone (FSH), and sexual hormone-binding globulin (SHBG) were quantified using reagents from Siemens on an IMMULITE ® 2000 XP analyzer. Neopterin was measured using the ELISA from IBL International (Hamburg, Germany) on a Dynex DS2 automated ELISA system (Dynex Technologies, Chantilly, USA) and total testosterone (tT) was determined using high-pressure liquid chromatography hyphenated with tandem mass spectrometry via an in-house developed method. Free testosterone (fT) was measured via a CLIA assay obtained from IDS iSYS (Immunodiagnostic Systems GmbH, Frankfurt am Main, Germany) on an IDS-iSYS Multi-Discipline Automated System. All hematological parameters (thrombocytes, leucocytes, lymphocytes, hemoglobin, and hematocrit) were measured on a Sysmex automated hematology analyzer (XN series).
We calculated the luteinizing hormone to total testosterone (LH/tT) ratio to specify the hypothalamic-pituitary-gonadal axis (35) and the total testosterone to estradiol (tT/E2) ratio to reflect aromatase activity (36). Reference ranges for total testosterone (tT) levels were based on the guideline of the investigation, treatment and monitoring of functional hypogonadism in males by the European Academy of Andrology (EAA): serum total testosterone (tT) levels were classified to be reduced when tT levels ≤ 230 ng/dL, borderline when tT levels between 231 – 350 ng/dL and normal when tT levels > 350 ng/dL (37).
Parameters are reported as n (%) or medians (25th, 75th percentile) since the data were not normally distributed (Shapiro-Wilk test). To test for differences between men and women we used the Mann-Whitney-U test or Pearson chi-square tests. Kruskal-Wallis test was performed to test for significant differences between more than two groups. Analysis of the effect of risk factors on the probability of death or ICU admission during the in-hospital stay was performed with logistic regression analysis (not normally distributed parameters were logarithmized with the natural logarithm). All tests were two-tailed and p-values < 0.05 were regarded as statistically significant. Statistical analysis was performed using SPSS Statistics Version 27 (IBM Corporation, Armonk, NY, USA).
Within this retrospective analysis we investigated clinical, hormonal and inflammatory parameters in 230 men (61.0%) and 147 women (39.0%) with PCR-confirmed COVID-19 disease and a median age of 67 years in men and 70 years in women (p = 0.540). Upon initial hospital admission men presented with significantly lower serum cholesterol (121 vs. 141 mg/dL, p < 0.001), LDL (72 vs. 83 mg/dL, p = 0.001) and HDL levels (31 vs. 40 mg/dL, p < 0.001) as well as significantly higher immune activation markers, namely C-reactive protein (CRP; 6.01 mg/dL vs. 3.70 mg/dL, p < 0.001), interleukin 6 (IL-6; 44.5 vs. 24.0 ng/L, p < 0.001), procalcitonin (PCT; 0.13 vs. 0.07 ng/mL, p < 0.001), neopterin (51.2 vs. 41.3 nmol/L, p = 0.002), fibrinogen (511 vs. 439 G/L, p = 0.002) and ferritin levels (662 vs. 265 μg/L, p < 0.001) as well as higher leukocytes counts (5.80 vs. 5.00 G/L, p = 0.003) compared to women. The prevalence of cardiovascular disease (53.2 % vs. 49.0 %, p = 0.007) and diabetes mellitus (35.7 % vs. 25.9 %, p = 0.046) was also significantly higher in men than women. Finally, men were at higher risk to die during hospital stay (16.2 % vs. 6.8 %, p = 0.008) with significantly longer hospitalizations compared to women (11 vs. 8 days, p = 0.002). We will focus on sex hormones in SARS-CoV-2 infected men in the following analysis. Baseline characteristics for men upon hospital admission are depicted in Table 1.
Sex hormones were available from 267 patients (155 men, 112 women) in the first three days after hospital admission. Most hospitalized men with available sex hormones upon initial hospital admission (n = 155) presented with reduced total testosterone (tT) levels ≤ 230 ng/dL (n = 107, 69.0 %), while 22 men (14.2 %) presented with borderline tT levels between 231 – 350 ng/dL and 24 men (15.5 %) with normal tT levels > 350 ng/dL. When differentiating men according to their age, men over the age of 60 had a significantly higher prevalence of reduced tT levels (81.5% vs. 52.5 %) and a lower prevalence of borderline tT levels (9.8 % vs. 21.3 %) when compared to men under the age of 60 (p < 0.001). Irrespective of the median age of 67 years, only 15 men out of 155 (9.7 %) were on pharmacological therapy for their benign prostate enlargement with a 5-alpha-reductase inhibitor, generally accepted to not influence the tT concentrations; indeed, these men showed median tT levels of 191 ng/dL (65 – 419 ng/dL) and median free testosterone (fT) levels of 3.94 ng/L (2.05 – 5.65 ng/L) as compared to men without 5-alpha reductase inhibitor therapy with tT levels of 148 ng/dL (70 – 262 ng/dL, p = 0.494) and fT levels of 4.19 ng/L (2.49 – 5.89 ng/L, p = 0.510). Interestingly, although tT levels were rather low in the majority of hospitalized men, levels of the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) as well as the steroid hormone estradiol (E2) were within the upper normal range (Table 1). However, when differentiating men again according to their age, men over the age of 60 presented with slightly elevated LH levels (7.6 U/L [4.8 – 12.0]) which were also significantly higher compared to men under the age of 60 (4.8 [3.5 – 6.1], p < 0.001). Conversely, tT levels were significantly lower in men over the age of 60 compared to men under the age of 60 (130 ng/dL [50 – 198] vs. 219 ng/dL [120 – 359], p < 0.001),
Cardiovascular disease, arterial hypertension, diabetes mellitus and hypercholesterinemia were frequently encountered in men within our study. Interestingly, serum low-density lipoprotein (LDL) and high-density lipoprotein (HDL) were quite low even in men without vs. with lipid-lowering therapy (LDL: 81 mg/dL vs. 57 mg/dL; HDL: 32 mg/dL vs. 30 mg/dL). Low serum cholesterol, LDL and HDL levels were associated with older age, higher immune activation, WHO-score and temperature and lower SpO2 with concomitant higher O2 requirements (Table 2). Moreover, low tT levels as well as a lower tT/E2 and higher LH/tT ratio were associated with lower cholesterol, LDL and HDL levels (Table 3).
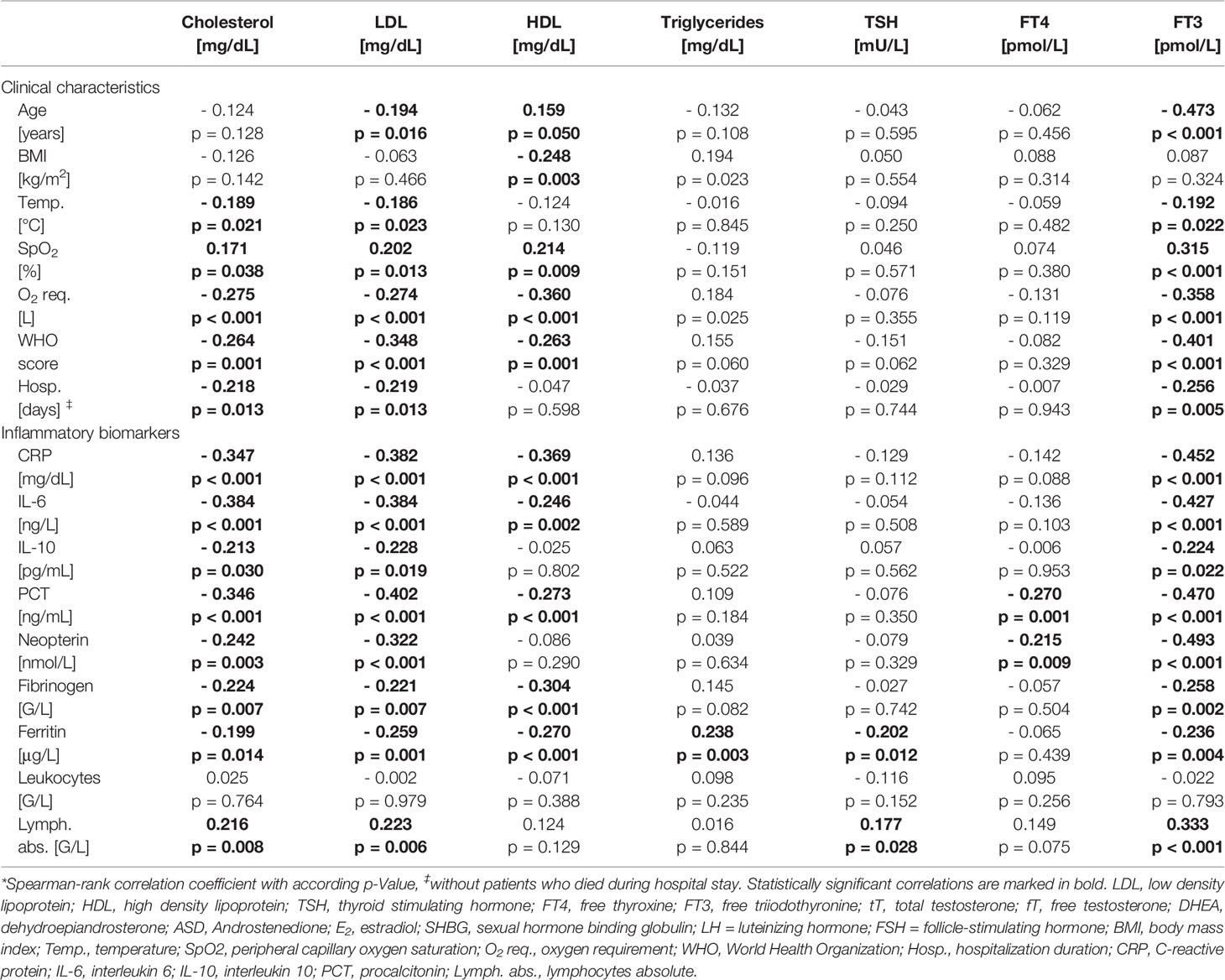
Table 2 Correlations of lipoproteins and thyroid hormones with clinical characteristics and laboratory parameters in men.*
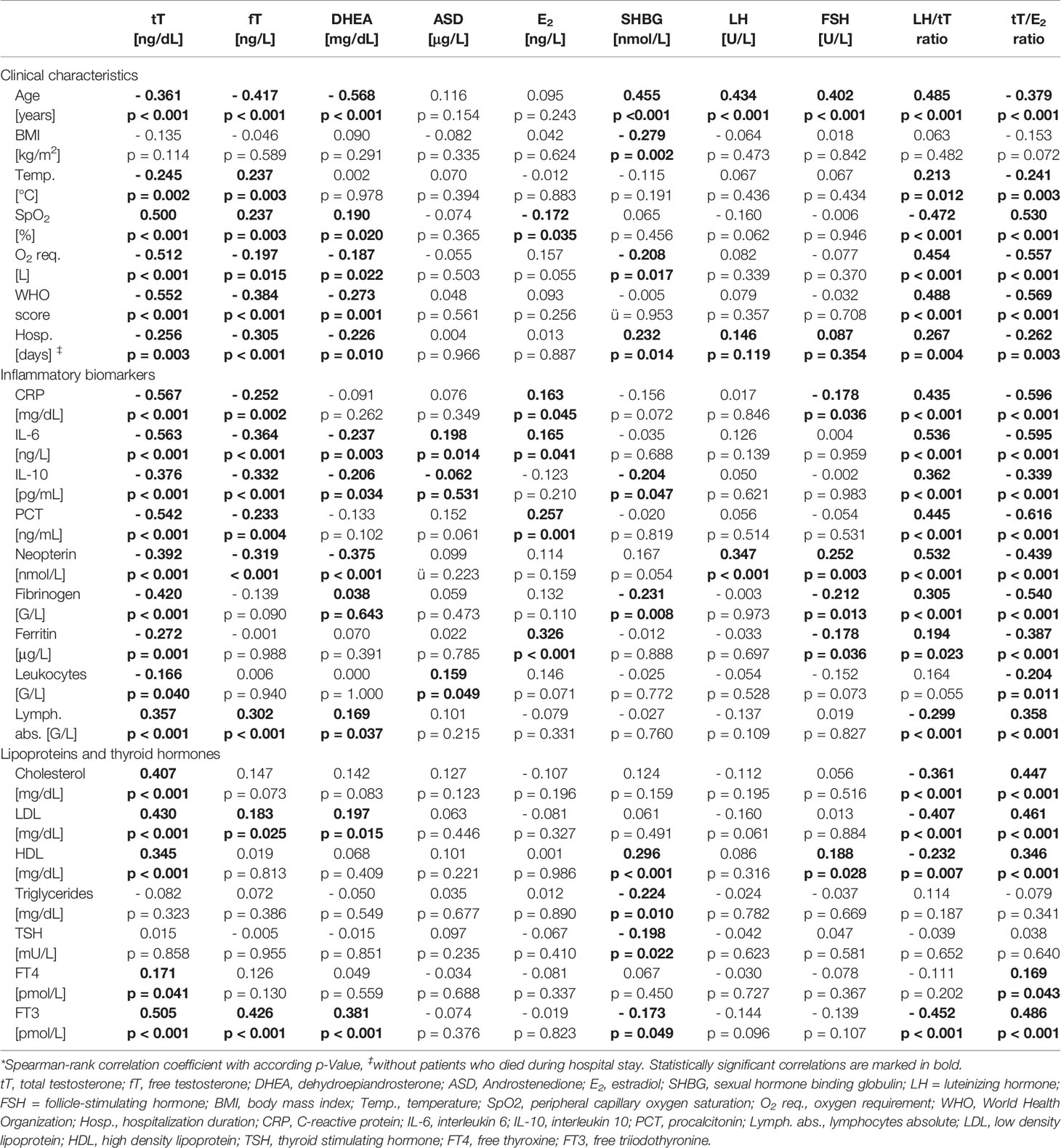
Table 3 Correlations of sex hormones with clinical characteristics and laboratory parameters in men.*
In men (n = 155) lower tT and fT levels upon hospital admission were correlated with older age, higher WHO score and temperature, as well as lower SpO2 with concomitant higher O2 requirement and longer hospital stay (Table 3). Also, lower dehydroepiandrosterone (DHEA) levels were significantly correlated with an older age, higher WHO score and lower SpO2, again with higher O2 requirement and longer hospital stay. SHBG was positively correlated with age and hospitalization duration and negatively with BMI and O2 requirement. Androstenedione (ASD), E2, LH and FSH were not related to disease severity. Finally, a higher LH/tT and a lower tT/E2 ratio were associated with an older age, higher WHO score and temperature as well as lower SpO2 with accordingly higher O2 requirements and longer hospital stay (Table 3).
When analyzing relations of sex hormones with markers of immune activation, tT and fT levels negatively correlated with CRP, IL-6, IL-10, neopterin, PCT, fibrinogen and ferritin levels as well as with leukocyte counts and positively correlated with absolute lymphocyte numbers. Also, other sex hormones correlated widely with different biomarkers of immune activation depicted in Table 3. Interestingly, a higher LH/tT and lower tT/E2 ratio was associated with higher CRP, IL-6, IL-10, neopterin, PCT, fibrinogen and ferritin levels as well as lower absolute lymphocyte counts. (Table 3)
Univariate logistic regression analysis showed that lower tT, fT and DHEA levels, but higher ASD and E2 levels, as well as a lower tT/E2 and higher LH/tT ratio, were associated with an increased risk to die during hospital stay (Table 4). Men with tT levels < 100 ng/dL (n = 52) had a more than eighteen-fold higher risk to die during hospital stay when compared to men with tT levels > 230 ng/dL (n = 46; OR 18.243 [95%CI 2.301 – 144.639], p = 0.006, Figure 1). Actually, 19 out of 20 men who died had reduced tT levels ≤ 230 ng/dL; yet one man who died had a tT level of 244 ng/dL.
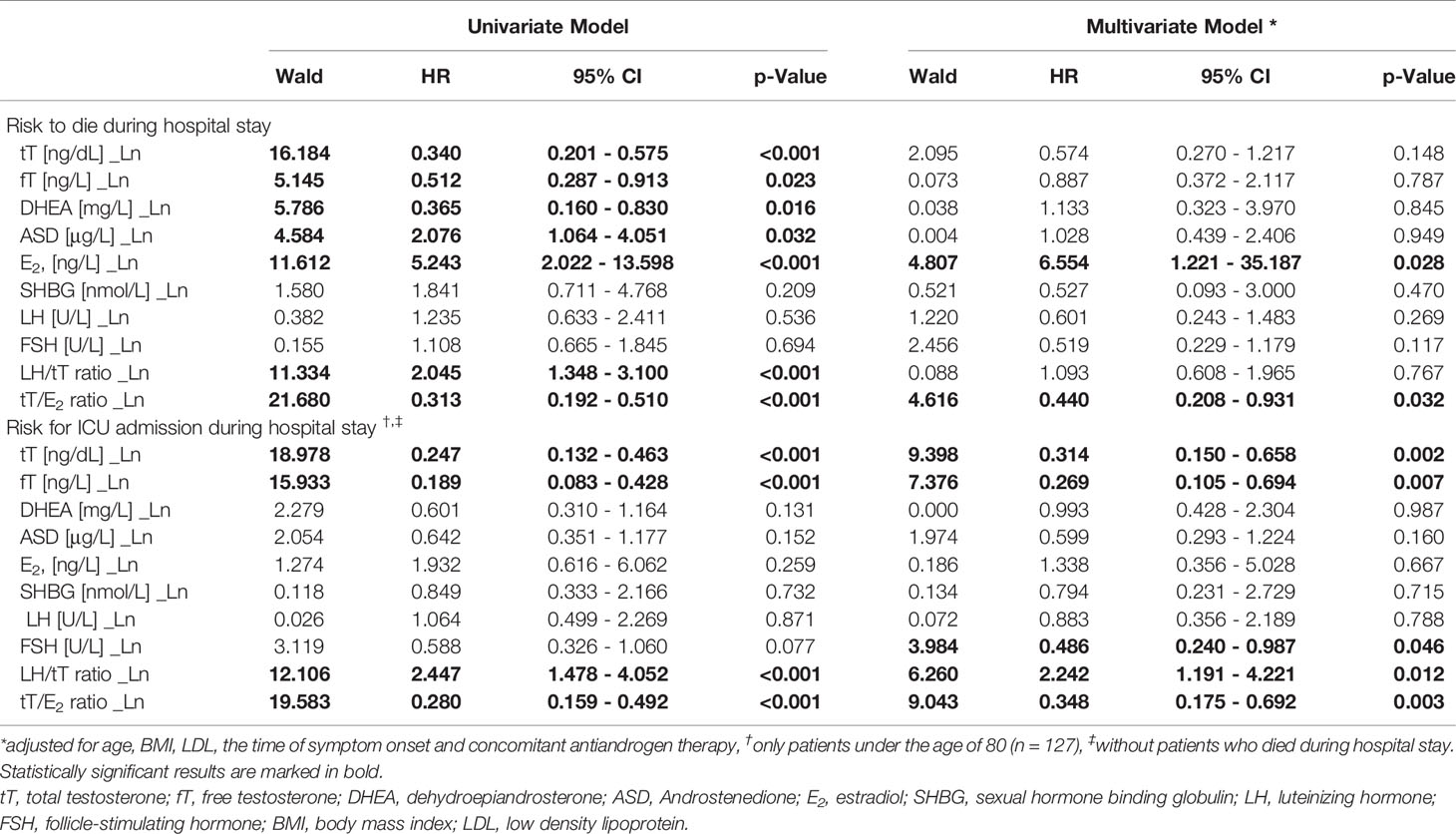
Table 4 Logistic regression analysis of sex hormones and the risk do die or admission to ICU during hospital stay in men.
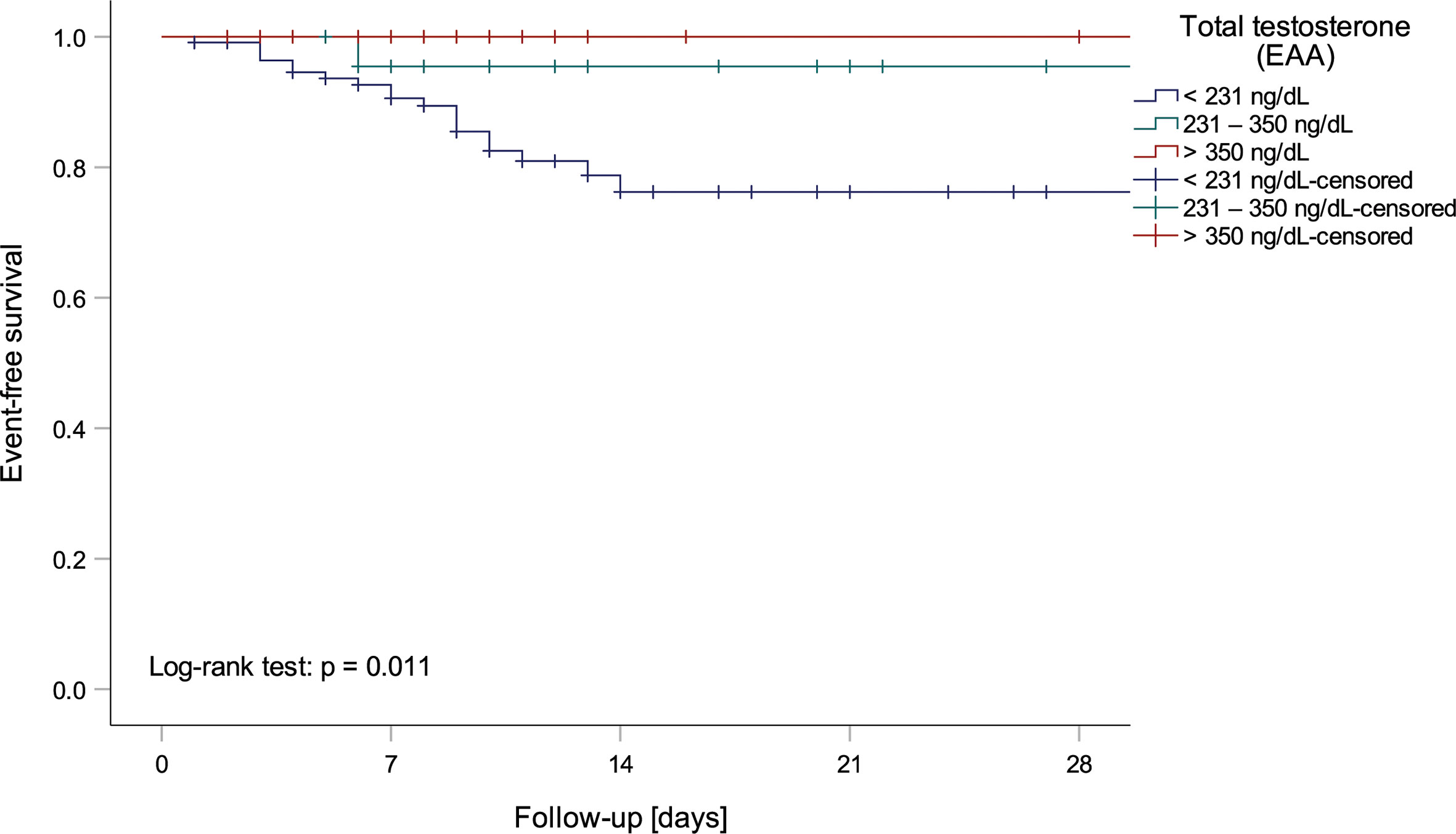
Figure 1 Kaplan-Meier curve depicting mortality of men within testosterone normality ranges by the European Academy of Andrology (EAA) (37).
Moreover, men with a tT/E2 ratio < 3.30 (n = 52) had a more than 22-fold higher mortality risk when compared to men with a tT/E2 ratio > 7.30 (n = 51; OR 22.222 [95%CI 2.818 – 175.265], p = 0.003), while those with a LH/tT ratio > 6.95 (n = 46) had a more than 15-fold higher mortality risk when compared to men with a LH/tT ratio < 2.85 (n = 45; OR 15.529 [95%CI 1.924 – 125.367], p = 0.010). However, in multivariate logistic regression analysis adjusted for age, BMI, LDL, and the time of symptom onset only E2 and the tT/E2 ratio were significantly predicting mortality (Table 4).
Since men over the age of 80 were less probably transferred to the ICU because of their age, we only included men below the age of 80 (n = 127) in the following analyses. Increased risk for ICU admission during hospital stay was found in men with lower baseline tT or fT levels as well as in those with a lower tT/E2 ratio and higher LH/tT ratio. These findings were independent of age, BMI, LDL and the time of symptom onset in multivariate Cox regression analysis (Table 4). All 26 men who subsequently needed be transferred to the ICU had median tT baseline levels of only 80 ng/dL (range: 41 – 122 ng/dL), thus were hypogonadal per definition. Further subdivision in tertiles showed that men with tT levels < 100 ng/dL (n = 37) had a 12-fold higher risk for ICU admission compared to men with tT levels ≥ 100 ng/dL (n = 88; OR 12.214 [95%CI 4.467 – 33.398], p < 0.001). Additionally, men with a tT/E2 ratio < 3.30 (n = 37) had a more than 39-fold higher risk for ICU admission compared to men with a tT/E2 > 7.30 (n = 47; OR 39.100 [95%CI 4.865 – 314.225], p < 0.001), while men with a LH/tT ratio > 6.95 (n = 32) had a 24-fold higher risk for ICU admission compared to men with a LH/tT ratio < 2.85 (n = 41; OR 24.000 [95%CI 2.911 – 197.845], p = 0.003).
In the present study we found that low serum total and free testosterone levels upon hospital admission are associated with disease severity indicated by lower oxygen saturation, higher WHO score and increased risk for ICU admission or death during the hospital stay in men with SARS-CoV-2 infection. These data are in line with recent published small cohort studies suggesting that low testosterone levels predict clinical adverse outcome (38, 39). Our results strongly suggest that SARS-CoV-2 infected men with the need for hospitalization present with distinct low testosterone levels that are further not compensated by hypothalamic-hypophyseal feedbacks especially in younger men, since LH levels were within the normal range. Testosterone itself was shown to be associated with a reduced cytokine response following cellular immune activation in men but not in women (40). This might be due to already rather low testosterone levels in women who are therefore not further strongly affected by inflammation (41). In addition, androgen receptor expression in male immune cells is more distinctive than in female ones (18, 42). Thus testosterone is suggested to be partly responsible for the lower prevalence of autoimmune disease but also for the higher incidence of cancer in men compared to women (43). Interestingly, it was also shown that androgens can reduce lung pathology in influenza-infected mice (44) suggesting that testosterone prevents inappropriate overwhelming immune activation following infection (45). In the case of low testosterone levels, such suppressive mechanism may be reduced, which might result in an imbalance between immunosuppressive and pro-inflammatory regulating mechanism in men (46). It’s generally assumed that this can lead to enhanced pro-inflammatory cytokine response following a SARS-CoV-2 infection, also known as a cytokine storm. Therefore, a reduced innate immune response toward viral infections in men compared to women results in a higher and longer persisting viral load (47, 48) with a subsequently more pronounced cellular immune response (49). Such situation is mainly present in men with low serum levels of the immunosuppressive acting testosterone. Accordingly, serum testosterone levels were negatively correlated with all investigated inflammatory biomarkers including IL-6, IL-10, neopterin (Th1 immune response), PCT, CRP, fibrinogen and ferritin which all were associated with a poorer clinical course and higher risk for ICU admission or death in SARS-CoV-2 infected patients (10, 48, 50, 51). Vice versa, it was shown in previous studies, that the testosterone production in Leydig cells is also downregulated by immune activation itself (52). The findings of our study, that rather low than high testosterone levels predict mortality in men, contrast with recent findings showing that androgen stimulates the expression of the ACE2 coreceptor TMPRSS2 (53), which further provides access of SARS-CoV-2 to cells (54), suggesting that higher testosterone levels with consequently higher receptor density provides more docking sites for SARS-CoV-2. However, high mortality in COVID-19 is suggested to be caused by hyperinflammation and not actually by higher viral loads (55). Thus, results of our study suggest that low levels of testosterone might be even more immunosuppressive than high testosterone levels with consequently higher SARS-CoV-2 receptor expression.
However, other risk factors for severe COVID-19 disease also interact with hormonal status. Particularly, lower serum testosterone levels are associated with older age, higher BMI and lower cholesterol levels. Several studies have shown that viral and bacterial infections cause decreased cholesterol levels (56) and that the alterations in lipid levels correlate with the severity of the underlying infection (57, 58). Since cholesterol is the precursor of testosterone, its deficiency also causes testosterone deficiency (59). Actually, a highly significant positive correlation of testosterone with lipoproteins was also found in our cohort. Interestingly, statin therapy, which was frequently encountered in our patients, was found to cause lower total testosterone levels in men with diabetes mellitus (60). However, testosterone levels did not significantly differ between men with or without lipid lowering therapy and also the predictive value of testosterone was independent of concomitant use of lipid lowering drugs.
Testosterone levels are typically decreased in older men (61) due to several factors, including a decline in ability of Leydig cells to produce adequate testosterone in response to LH stimulation (62), which might also partly contribute to the higher COVID-19 related morbidity and mortality within this vulnerable group. Actually, the prevalence of hypogonadism in geriatric hospitalized men over the age of 65 was found to be 53.3 % (63). This is supported by the finding that higher LH/tT ratio was also related to morbidity and mortality. LH levels were within the normal range suggesting the presence of normogonadotropic hypogonadism (64). The absence of compensatory LH secretion in case of low testosterone levels (65) most probably might be caused by altered GnRH and consecutive LH suppression by cytokines as found in patients with immune activation (66, 67). Actually, men were hospitalized in median one week after symptom onset providing a long period for suppression of gonadotropins. The finding that older men over the age of 60 had again lower tT levels compared to young men with concurrent slightly elevated (but still low) LH levels suggests that in older men also the occurrence of late onset hypogonadism might contribute to this rather low tT levels. This is further supported by the positive correlation of the LH/tT ratio with age. Unfortunately, a differentiation of whether these men had age-related Leydig cell dysfunction before COVID-19 or whether low testosterone levels are primarily due to inflammation-related Leydig cell dysfunction or impaired testicular steroid-biosynthesis, cannot be provided by our results.
Testosterone expression can also be suppressed by cortisol which is produced in the adrenal cortex upon stimulation by the adrenocorticotropic hormone (ACTH) secreted by the pituitary gland upon stress-related corticotropin-releasing hormone (CRH) stimulation (68, 69). Activation of the hypothalamic-pituitary-adrenal axis (HPA) in patients with COVID-19 reflected by elevated total serum cortisol levels was shown to be associated with an increased mortality (70) and might also contribute to low testosterone levels in these patients. However, testosterone was also shown to suppress CRH-stimulated cortisol production in men (71) which is why loss of this suppressive mechanism might promote stress-induced HPA activation. Unfortunately, we do not have detected serum hormonal levels of the hypothalamic-pituitary-adrenal axis. Interestingly, hydroxysteroid dehydrogenases, which catalyse steroid biosynthesis (e.g. DHEA to androstenedione, androstenedione to testosterone), is also suggested to be suppressed by immune activation (40).
On the other hand, obesity, linked to disease severity and outcome in SARS-CoV-2 infected patients, is considered to be stringently linked to testosterone deficiency (72). Testosterone can be aromatized to estradiol by the aromatase which is found in abundance in the visceral fatty tissue but also in male gonads (typically in Leydig cells (73), placenta, brain, muscle, bone and vascular tissues (74), and stimulated by cytokines such as IL-6 (75) or Tumor necrosis factor alpha (TNF-α) (76). This is supported by the highly significant negative correlation of inflammatory markers with the tT/E2 ratio. Interestingly, estradiol levels were within the normal range thus representing a disturbed relation of testosterone to estradiol primarily caused by quite low testosterone levels rather than increased aromatase activity. Finally, the median BMI was normal. This would also explain the contrary findings in airway epithelial cells in which estradiol was demonstrated to downregulate ACE2 expression (77). Also, recent study results suggest that estrogen receptor signaling is actually protective in mice infected with SARS-CoV-2 (78) by suppressing ACE2 expression as well as pro-inflammatory pathways (79). Th1 immune activation might have stronger impacts on ACE2 expression than estradiol receptor signaling (80). Moreover, lower levels of cardiovascular- and lung-protective ACE2 might also aggravate existing co-morbidities (81). However, higher estradiol level (but within normal range) as well as a lower tT/E2 ratio were associated with mortality and morbidity suggesting that this might primarily reflect inflammation-induced aromatase activity, which only slightly increase estradiol levels in men with distinctive testosterone deficiency.
Finally, low DHEA levels upon hospital admission were (similarly to testosterone) also related to higher morbidity and outcome. In vivo experiments showed that DHEA increases macrophage function and promotes a shift of Th1/Th2 balance toward Th1 immunity (82) thus enhancing immunity against viral infection (83). At the same time, DHEA suppresses the expression of various pro-inflammatory cytokines thus preventing overwhelming immune activation (83), suggesting that its deficiency enables overwhelming inflammation with reduced immunity.
This was a retrospective explorative analysis of continuous COVID-19 patients hospitalized at our department. Additionally, sex hormones were not available of all men initially included in the study, which might be an unmeasured bias although baseline characteristics of all men and only those with available sex hormones were almost the same (Table 1). This represents a risk for possible type I and II errors. We further have no information about the hormonal status of the patients before their COVID-19 infection which does not allow any conclusion whether these rather low testosterone levels represent hypogonadal hypogonadism or decreased following SARS-CoV-2 related immune activation.
Our results indicate that men hospitalized due to COVID-19 present with lower testosterone levels showing advanced immune activation and having high risk for an adverse clinical course and a poor prognosis. The origin of testosterone deficiency in these patients might be primarily caused by altered cholesterol biosynthesis in the case of SARS-CoV-2 infection as well as being due to inflammation-induced gonadotrophin suppression. The impact of enzymatic aromatase activation on hypogonadism pathogenesis might be negligible. The latter is supported by the finding that high estradiol levels (but within the normal range) were associated with more severe SARS-CoV-2 infections. Whether these men already had hypogonadotropic hypogonadism before COVID-19 cannot be clarified by our study. Effects of testosterone supplementation on outcome in men with severe SARS-CoV-2 infections should be evaluated in further studies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by ethics committee of the Innsbruck Medical University. The patients/participants provided their written informed consent to participate in this study.
Conceptualization and methodology, AG and RB-W. Software and formal analysis, LL. Investigation and data curation, AE, FB, GH, LL, LT and RB-W. Resources, AG, AE, FB, GH, GW, MA and RB-W. Writing – original draft preparation, LL. Writing – review and editing, AG, AE, FB, GH, GW, LT, MA, RB-W, G-MP and SK. Supervision, GW. All authors contributed to the article and approved the submitted version.
Author GH was employed by company MLL Munich Leukemia Laboratory.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK Patients in Hospital With Covid-19 Using the ISARIC Who Clinical Characterisation Protocol: Prospective Observational Cohort Study. BMJ (Clinical Res ed) (2020) 369:m1985. doi: 10.1136/bmj.m1985
2. Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, et al. Hospitalization Rates and Characteristics of Patients Hospitalized With Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morbidity mortality weekly Rep (2020) 69(15):458–64. doi: 10.15585/mmwr.mm6915e3
3. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With Covid-19 in the New York City Area. Jama (2020) 323(20):2052–9. doi: 10.1001/jama.2020.6775
4. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-Cov-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA (2020) 323(16):1574–81. doi: 10.1001/jama.2020.5394
5. Sonnweber T, Sahanic S, Pizzini A, Luger A, Schwabl C, Sonnweber B, et al. Cardiopulmonary Recovery After COVID-19 – An Observational Prospective Multi-Center Trial. Eur Respir J (2020) 57:2003481. doi: 10.1183/13993003.03481-2020
6. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of Sex and Gender on COVID-19 Outcomes in Europe. Biol Sex Differ (2020) 11(1):29. doi: 10.1186/s13293-020-00304-9
7. Penna C, Mercurio V, Tocchetti CG, Pagliaro P. Sex-Related Differences in COVID-19 Lethality. Br J Pharmacol (2020) 177(19):4375–85. doi: 10.1111/bph.15207
8. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering How Biological Sex Impacts Immune Responses and COVID-19 Outcomes. Nat Rev Immunol (2020) 20(7):442–7. doi: 10.1038/s41577-020-0348-8
9. Chen LYC, Hoiland RL, Stukas S, Wellington CL, Sekhon MS. Confronting the Controversy: Interleukin-6 and the COVID-19 Cytokine Storm Syndrome. Eur Respir J (2020) 56:2003006. doi: 10.1183/13993003.03006-2020
10. Bellmann-Weiler R, Lanser L, Burkert F, Seiwald S, Fritsche G, Wildner S, et al. Neopterin Predicts Disease Severity in Hospitalized Patients With COVID-19. Open Forum Infect Dis (2020) 8(1). doi: 10.1093/ofid/ofaa521
11. Laguna-Goya R, Utrero-Rico A, Talayero P, Lasa-Lazaro M, Ramirez-Fernandez A, Naranjo L, et al. Il-6-based Mortality Risk Model for Hospitalized Patients With COVID-19. J Allergy Clin Immunol (2020) 146(4):799–807. doi: 10.1016/j.jaci.2020.07.009
12. Giagulli VA, Guastamacchia E, Magrone T, Jirillo E, Lisco G, De Pergola G, et al. Worse Progression of COVID-19 in Men: Is Testosterone a Key Factor? Andrology (2021) 9(1):53–64. doi: 10.1111/andr.12836
13. Klein SL, Flanagan KL. Sex Differences in Immune Responses. Nat Rev Immunol (2016) 16(10):626–38. doi: 10.1038/nri.2016.90
14. Libert C, Dejager L, Pinheiro I. The X Chromosome in Immune Functions: When a Chromosome Makes the Difference. Nat Rev Immunol (2010) 10(8):594–604. doi: 10.1038/nri2815
15. Hannah MF, Bajic VB, Klein SL. Sex Differences in the Recognition of and Innate Antiviral Responses to Seoul Virus in Norway Rats. Brain Behav Immun (2008) 22(4):503–16. doi: 10.1016/j.bbi.2007.10.005
16. Kadel S, Kovats S. Sex Hormones Regulate Innate Immune Cells and Promote Sex Differences in Respiratory Virus Infection. Front Immunol (2018) 9:1653(1653). doi: 10.3389/fimmu.2018.01653
17. Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 Expression and Sex Disparity in COVID-19. Cell Death Discovery (2020) 6(1):37. doi: 10.1038/s41420-020-0276-1
18. Younis JS, Skorecki K, Abassi Z. The Double Edge Sword of Testosterone’s Role in the COVID-19 Pandemic. Front Endocrinol (Lausanne) (2021) 12:607179(81). doi: 10.3389/fendo.2021.607179
19. Bukowska A, Spiller L, Wolke C, Lendeckel U, Weinert S, Hoffmann J, et al. Protective Regulation of the ACE2/ACE Gene Expression by Estrogen in Human Atrial Tissue From Elderly Men. Exp Biol Med (2017) 242(14):1412–23. doi: 10.1177/1535370217718808
20. Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID-19: Serendipity or Opportunity for Intervention? Cancer Discovery (2020) 10(6):779–82. doi: 10.1158/2159-8290.Cd-20-0451
21. Lin B, Ferguson C, White JT, Wang S, Vessella R, True LD, et al. Prostate-Localized and Androgen-Regulated Expression of the Membrane-Bound Serine Protease TMPRSS2. Cancer Res (1999) 59(17):4180–4.
22. Lucas JM, Heinlein C, Kim T, Hernandez SA, Malik MS, True LD, et al. The Androgen-Regulated Protease TMPRSS2 Activates a Proteolytic Cascade Involving Components of the Tumor Microenvironment and Promotes Prostate Cancer Metastasis. Cancer Discovery (2014) 4(11):1310–25. doi: 10.1158/2159-8290.Cd-13-1010
23. Salah HM, Mehta JL. Hypothesis: Sex-Related Differences in ACE2 Activity May Contribute to Higher Mortality in Men Versus Women With Covid-19. J Cardiovasc Pharmacol Ther (2021) 26(2):114–8. doi: 10.1177/1074248420967792
24. Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-Cell RNA Expression Profiling of ACE2, the Receptor of SARS-CoV-2. American J Respi Critical Care Med (2020) 202(5):756-9. doi: 10.1164/rccm.202001-0179LE
25. Liu J, Ji H, Zheng W, Wu X, Zhu JJ, Arnold AP, et al. Sex Differences in Renal Angiotensin Converting Enzyme 2 (ACE2) Activity are 17β-Oestradiol-Dependent and Sex Chromosome-Independent. Biol Sex Differ (2010) 1(1):6. doi: 10.1186/2042-6410-1-6
26. Dalpiaz PL, Lamas AZ, Caliman IF, Ribeiro RF Jr., Abreu GR, Moyses MR, et al. Sex Hormones Promote Opposite Effects on ACE and ACE2 Activity, Hypertrophy and Cardiac Contractility in Spontaneously Hypertensive Rats. PloS One (2015) 10(5):e0127515. doi: 10.1371/journal.pone.0127515
27. Pedersen KB, Chodavarapu H, Porretta C, Robinson LK, Lazartigues E. Dynamics of ADAM17-Mediated Shedding of ACE2 Applied to Pancreatic Islets of Male Db/Db Mice. Endocrinology (2015) 156(12):4411–25. doi: 10.1210/en.2015-1556
28. Úri K, Fagyas M, Kertész A, Borbély A, Jenei C, Bene O, et al. Circulating ACE2 Activity Correlates With Cardiovascular Disease Development. J Renin-Angiotensin-Aldosterone System (2016) 17(4):1470320316668435. doi: 10.1177/1470320316668435
29. Sama IE, Ravera A, Santema BT, van Goor H, ter Maaten JM, Cleland JGF, et al. Circulating Plasma Concentrations of Angiotensin-Converting Enzyme 2 in Men and Women With Heart Failure and Effects of Renin–Angiotensin–Aldosterone Inhibitors. Eur Heart J (2020) 41(19):1810–7. doi: 10.1093/eurheartj/ehaa373
30. Bernardi S, Toffoli B, Tonon F, Francica M, Campagnolo E, Ferretti T, et al. Sex Differences in Proatherogenic Cytokine Levels. Int J Mol Sci (2020) 21(11):3861. doi: 10.3390/ijms21113861
31. Fernández-Atucha A, Izagirre A, Fraile-Bermúdez AB, Kortajarena M, Larrinaga G, Martinez-Lage P, et al. Sex Differences in the Aging Pattern of Renin–Angiotensin System Serum Peptidases. Biol Sex Differ (2017) 8(1):5. doi: 10.1186/s13293-017-0128-8
32. Mooradian AD, Morley JE, Korenman SG. Biological Actions of Androgens. Endocr Rev (1987) 8(1):1–28. doi: 10.1210/edrv-8-1-1
33. Nelson LR, Bulun SE. Estrogen Production and Action. J Am Acad Dermatol (2001) 45(3):S116–S24. doi: 10.1067/mjd.2001.117432
34. Marshall JC, Murthy S, Diaz J, Adhikari NK, Angus DC, Arabi YM, et al. A Minimal Common Outcome Measure Set for COVID-19 Clinical Research. Lancet Infect Dis (2020) 20(8):e192–e7. doi: 10.1016/S1473-3099(20)30483-7
35. Wiechno PJ, Kowalska M, Kucharz J, Sadowska M, Michalski W, Poniatowska G, et al. Dynamics of Hormonal Disorders Following Unilateral Orchiectomy for a Testicular Tumor. Med Oncol (2017) 34(5):84. doi: 10.1007/s12032-017-0943-0
36. Figtree GA, Ngo DTM, Bubb KJ. Testosterone to Estradiol Ratio and Plaque Inflammation: Mechanistic Insights and Biomarker Potential? Cardiovasc Res (2018) 115(2):255–7. doi: 10.1093/cvr/cvy260
37. Corona G, Goulis DG, Huhtaniemi I, Zitzmann M, Toppari J, Forti G, et al. European Academy of Andrology (EAA) Guidelines on Investigation, Treatment and Monitoring of Functional Hypogonadism in Males. Andrology (2020) 8(5):970–87. doi: 10.1111/andr.12770
38. Çayan S, Uğuz M, Saylam B, Akbay E. Effect of Serum Total Testosterone and its Relationship With Other Laboratory Parameters on the Prognosis of Coronavirus Disease 2019 (COVID-19) in SARS-CoV-2 Infected Male Patients: A Cohort Study. Aging Male (2020) 23:1–11. doi: 10.1080/13685538.2020.1807930
39. Rastrelli G, Di Stasi V, Inglese F, Beccaria M, Garuti M, Di Costanzo D, et al. Low Testosterone Levels Predict Clinical Adverse Outcomes in SARS-CoV-2 Pneumonia Patients. Andrology (2020) 9(1):88–98. doi: 10.1111/andr.12821
40. Tsugita M, Iwasaki Y, Nishiyama M, Taguchi T, Shinahara M, Taniguchi Y, et al. Differential Regulation of 11beta-Hydroxysteroid Dehydrogenase Type-1 and -2 Gene Transcription by Proinflammatory Cytokines in Vascular Smooth Muscle Cells. Life Sci (2008) 83(11-12):426–32. doi: 10.1016/j.lfs.2008.07.005
41. Roved J, Westerdahl H, Hasselquist D. Sex Differences in Immune Responses: Hormonal Effects, Antagonistic Selection, and Evolutionary Consequences. Hormones Behav (2017) 88:95–105. doi: 10.1016/j.yhbeh.2016.11.017
42. McCrohon JA, Death AK, Nakhla S, Jessup W, Handelsman DJ, Stanley KK, et al. Androgen Receptor Expression is Greater in Macrophages From Male Than From Female Donors. A Sex Difference With Implications for Atherogenesis. Circulation (2000) 101(3):224–6. doi: 10.1161/01.cir.101.3.224
43. Trigunaite A, Dimo J, Jørgensen TN. Suppressive Effects of Androgens on the Immune System. Cell Immunol (2015) 294(2):87–94. doi: 10.1016/j.cellimm.2015.02.004
44. Tuku B, Stanelle-Bertram S, Sellau J, Beck S, Bai T, Kouassi NM, et al. Testosterone Protects Against Severe Influenza by Reducing the Pro-Inflammatory Cytokine Response in the Murine Lung. Front Immunol (2020) 11:697(697). doi: 10.3389/fimmu.2020.00697
45. Tavares LP, Teixeira MM, Garcia CC. The Inflammatory Response Triggered by Influenza Virus: A Two Edged Sword. Inflammation Res (2017) 66(4):283–302. doi: 10.1007/s00011-016-0996-0
46. Schroeder M, Tuku B, Jarczak D, Nierhaus A, Bai T, Jacobsen H, et al. The Majority of Male Patients With COVID-19 Present Low Testosterone Levels on Admission to Intensive Care in Hamburg, Germany: A Retrospective Cohort Study. medRxiv (2020). doi: 10.1101/2020.05.07.20073817
47. Jaillon S, Berthenet K, Garlanda C. Sexual Dimorphism in Innate Immunity. Clin Rev Allergy Immunol (2019) 56(3):308–21. doi: 10.1007/s12016-017-8648-x
48. Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, et al. Viral Load Dynamics and Disease Severity in Patients Infected With SARS-CoV-2 in Zhejiang Province, China, January-March 2020: Retrospective Cohort Study. BMJ (Clinical Res ed) (2020) 369:m1443. doi: 10.1136/bmj.m1443
49. Pozzilli P, Lenzi A. Commentary: Testosterone, a Key Hormone in the Context of COVID-19 Pandemic. Metabolism (2020) 108:154252–. doi: 10.1016/j.metabol.2020.154252
50. Manson JJ, Crooks C, Naja M, Ledlie A, Goulden B, Liddle T, et al. Covid-19-associated Hyperinflammation and Escalation of Patient Care: A Retrospective Longitudinal Cohort Study. Lancet Rheumatol (2020) 2(10):e594–602. doi: 10.1016/S2665-9913(20)30275-7
51. Bellmann-Weiler R, Lanser L, Barket R, Rangger L, Schapfl A, Schaber M, et al. Prevalence and Predictive Value of Anemia and Dysregulated Iron Homeostasis in Patients With COVID-19 Infection. J Clin Med (2020) 9(8):2429. doi: 10.3390/jcm9082429
52. Leisegang K, Henkel R. The In Vitro Modulation of Steroidogenesis by Inflammatory Cytokines and Insulin in TM3 Leydig Cells. Reprod Biol Endocrinol (2018) 16(1):26. doi: 10.1186/s12958-018-0341-2
53. Mikkonen L, Pihlajamaa P, Sahu B, Zhang FP, Jänne OA. Androgen Receptor and Androgen-Dependent Gene Expression in Lung. Mol Cell Endocrinol (2010) 317(1-2):14–24. doi: 10.1016/j.mce.2009.12.022
54. Samuel RM, Majd H, Richter MN, Ghazizadeh Z, Zekavat SM, Navickas A, et al. Androgen Signaling Regulates SARS-CoV-2 Receptor Levels and Is Associated With Severe Covid-19 Symptoms in Men. Cell Stem Cell (2020) 27(6):876–89.e12. doi: 10.1016/j.stem.2020.11.009
55. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. Covid-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet (2020) 395(10229):1033–4. doi: 10.1016/S0140-6736(20)30628-0
56. Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, et al. Effects of Infection and Inflammation on Lipid and Lipoprotein Metabolism: Mechanisms and Consequences to the Host. J Lipid Res (2004) 45(7):1169–96. doi: 10.1194/jlr.R300019-JLR200
57. Pizzini A, Kurz K, Orth-Hoeller D, Fille M, Rabensteiner J, Lunger F, et al. The Impact of Bacteremia on Lipoprotein Concentrations and Patient’s Outcome: A Retrospective Analysis. Eur J Clin Microbiol Infect Dis (2019) 38(7):1279–86. doi: 10.1007/s10096-019-03543-w
58. Deniz O, Gumus S, Yaman H, Ciftci F, Ors F, Cakir E, et al. Serum Total Cholesterol, HDL-C and LDL-C Concentrations Significantly Correlate With the Radiological Extent of Disease and the Degree of Smear Positivity in Patients With Pulmonary Tuberculosis. Clin Biochem (2007) 40(3-4):162–6. doi: 10.1016/j.clinbiochem.2006.10.015
59. Ieko T, Sasaki H, Maeda N, Fujiki J, Iwano H, Yokota H. Analysis of Corticosterone and Testosterone Synthesis in Rat Salivary Gland Homogenates. Front Endocrinol (Lausanne) (2019) 10:479(479). doi: 10.3389/fendo.2019.00479
60. Stanworth RD, Kapoor D, Channer KS, Jones TH. Statin Therapy is Associated With Lower Total But Not Bioavailable or Free Testosterone in Men With Type 2 Diabetes. Diabetes Care (2009) 32(4):541–6. doi: 10.2337/dc08-1183
61. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal Effects of Aging on Serum Total and Free Testosterone Levels in Healthy Men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab (2001) 86(2):724–31. doi: 10.1210/jcem.86.2.7219
62. Chen H, Ge R-S, Zirkin BR. Leydig Cells: From Stem Cells to Aging. Mol Cell Endocrinol (2009) 306(1-2):9–16. doi: 10.1016/j.mce.2009.01.023
63. Iglesias P, Prado F, Macías MC, Guerrero MT, Muñoz A, Ridruejo E, et al. Hypogonadism in Aged Hospitalized Male Patients: Prevalence and Clinical Outcome. J Endocrinol Invest (2014) 37(2):135–41. doi: 10.1007/s40618-013-0009-x
64. Huhtaniemi I, Forti G. Male Late-Onset Hypogonadism: Pathogenesis, Diagnosis and Treatment. Nat Rev Urol (2011) 8(6):335–44. doi: 10.1038/nrurol.2011.47
65. Finkelstein JS, Whitcomb RW, O’Dea LS, Longcope C, Schoenfeld DA, Crowley WF Jr. Sex Steroid Control of Gonadotropin Secretion in the Human Male. I. Effects of Testosterone Administration in Normal and Gonadotropin-Releasing Hormone-Deficient Men. J Clin Endocrinol Metab (1991) 73(3):609–20. doi: 10.1210/jcem-73-3-609
66. Wu S, Wolfe A. Signaling of Cytokines is Important in Regulation of GnRH Neurons. Mol Neurobiol (2012) 45(1):119–25. doi: 10.1007/s12035-011-8224-y
67. Daniel JA, Elsasser TH, Martínez A, Steele B, Whitlock BK, Sartin JL. Interleukin-1beta and Tumor Necrosis Factor-Alpha Mediation of Endotoxin Action on Growth Hormone. Am J Physiol Endocrinol Metab (2005) 289(4):E650–7. doi: 10.1152/ajpendo.00489.2004
68. Smith SM, Vale WW. The Role of the Hypothalamic-Pituitary-Adrenal Axis in Neuroendocrine Responses to Stress. Dialogues Clin Neurosci (2006) 8(4):383–95. doi: 10.31887/DCNS.2006.8.4/ssmith
69. Cumming DC, Quigley ME, Yen SS. Acute Suppression of Circulating Testosterone Levels by Cortisol in Men. J Clin Endocrinol Metab (1983) 57(3):671–3. doi: 10.1210/jcem-57-3-671
70. Tan T, Khoo B, Mills EG, Phylactou M, Patel B, Eng PC, et al. Association Between High Serum Total Cortisol Concentrations and Mortality From COVID-19. Lancet Diabetes Endocrinol (2020) 8(8):659–60. doi: 10.1016/s2213-8587(20)30216-3
71. Rubinow DR, Roca CA, Schmidt PJ, Danaceau MA, Putnam K, Cizza G, et al. Testosterone Suppression of CRH-stimulated Cortisol in Men. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol (2005) 30(10):1906–12. doi: 10.1038/sj.npp.1300742
72. Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals With Obesity and COVID-19: A Global Perspective on the Epidemiology and Biological Relationships. Obes Rev (2020) 21(11):e13128. doi: 10.1111/obr.13128
73. Payne AH, Kelch RP, Musich SS, Halpern ME. Intratesticular Site of Aromatization in the Human. J Clin Endocrinol Metab (1976) 42(6):1081–7. doi: 10.1210/jcem-42-6-1081
74. de Ronde W, de Jong FH. Aromatase Inhibitors in Men: Effects and Therapeutic Options. Reprod Biol Endocrinol (2011) 9(1):93. doi: 10.1186/1477-7827-9-93
75. Larralde C, Morales J, Terrazas I, Govezensky T, Romano MC. Sex Hormone Changes Induced by the Parasite Lead to Feminization of the Male Host in Murine Taenia Crassiceps Cysticercosis. J Steroid Biochem Mol Biol (1995) 52(6):575–80. doi: 10.1016/0960-0760(95)00062-5
76. Zhao Y, Nichols JE, Valdez R, Mendelson CR, Simpson ER. Tumor Necrosis Factor-Alpha Stimulates Aromatase Gene Expression in Human Adipose Stromal Cells Through Use of an Activating Protein-1 Binding Site Upstream of Promoter 1.4. Mol Endocrinol (1996) 10(11):1350–7. doi: 10.1210/mend.10.11.8923461
77. Stelzig KE, Canepa-Escaro F, Schiliro M, Berdnikovs S, Prakash YS, Chiarella SE. Estrogen Regulates the Expression of SARS-CoV-2 Receptor ACE2 in Differentiated Airway Epithelial Cells. Am J Physiol-Lung Cell Mol Physiol (2020) 318(6):L1280–L1. doi: 10.1152/ajplung.00153.2020
78. Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection. J Immunol (2017) 198:1601896. doi: 10.4049/jimmunol.1601896
79. Straub RH. The Complex Role of Estrogens in Inflammation. Endocr Rev (2007) 28(5):521–74. doi: 10.1210/er.2007-0001
80. Sajuthi SP, DeFord P, Li Y, Jackson ND, Montgomery MT, Everman JL, et al. Type 2 and Interferon Inflammation Regulate SARS-CoV-2 Entry Factor Expression in the Airway Epithelium. Nat Commun (2020) 11(1):5139. doi: 10.1038/s41467-020-18781-2
81. Samavati L, Uhal BD. Ace2, Much More Than Just a Receptor for SARS-COV-2. Front Cell Infect Microbiol (2020) 10:317(317):5139. doi: 10.3389/fcimb.2020.00317
82. Cao J, Yu L, Zhao J, Ma H. Effect of Dehydroepiandrosterone on the Immune Function of Mice In Vivo and In Vitro. Mol Immunol (2019) 112:283–90. doi: 10.1016/j.molimm.2019.06.004
Keywords: testosterone, estradiol, inflammation, COVID-19, SARS-CoV-2, disease severity, outcome
Citation: Lanser L, Burkert FR, Thommes L, Egger A, Hoermann G, Kaser S, Pinggera GM, Anliker M, Griesmacher A, Weiss G and Bellmann-Weiler R (2021) Testosterone Deficiency Is a Risk Factor for Severe COVID-19. Front. Endocrinol. 12:694083. doi: 10.3389/fendo.2021.694083
Received: 12 April 2021; Accepted: 01 June 2021;
Published: 18 June 2021.
Edited by:
Alexandra Kautzky-Willer, Medical University of Vienna, AustriaReviewed by:
Hao Chen, Nantong University, ChinaCopyright © 2021 Lanser, Burkert, Thommes, Egger, Hoermann, Kaser, Pinggera, Anliker, Griesmacher, Weiss and Bellmann-Weiler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rosa Bellmann-Weiler, cm9zYS5iZWxsbWFubi13ZWlsZXJAaS1tZWQuYWMuYXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.