- 1Department of Obstetrics and Gynaecology, The University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong
- 2Discipline of Obstetrics & Gynaecology, School of Women’s & Children’s Health, University of New South Wales, Sydney, NSW, Australia
- 3Biotherapeutics Division, National Institute for Biological Standards and Control, Potters Bar, United Kingdom
Serum anti-Mullerian hormone (AMH) is a widely used marker of functional ovarian reserve in the assessment and treatment of infertility. It is used to determine dosing of gonadotropins used for superovulation prior to in vitro fertilization, as well as to determine the degree of damage to ovarian reserve by cytotoxic treatments such as chemotherapy. AMH is also now used to predict proximity to menopause and potentially provides a sensitive and specific test for polycystic ovarian syndrome. Twenty one different AMH immunoassay platforms/methods are now commercially available. Of those compared, the random-access platforms are the most reliable. However, to date there has not been an agreed common international AMH reference preparation to standardize calibration between the various immunoassays. Recently, a purified human AMH preparation (code 16/190) has been investigated by the World Health Organization as a potential international reference preparation. However, this was only partially successful as commutability between it and serum samples was observed only in some but not all immunoassay methods. Development of a second generation reference preparation with wider commutability is proposed.
Introduction
Anti-Mullerian hormone (AMH), also known as Mullerian-inhibiting substance (MIS), was first recognized in the 1940s as the factor which determines regression of the Mullerian duct in the male fetus although it was only formally characterized and cloned in the 1980s (1, 2). Its production in the adult female was first reported in 1990 (3). With the development of serum AMH immunoassays it has become apparent that AMH is a clinically useful marker of functional ovarian reserve (4, 5) and thus of clinical value in the treatment of infertility, where measuring the follicle reserve is important.
Serum AMH is now used as a diagnostic test in infertile women undergoing controlled ovarian stimulation as part of an in vitro fertilization (IVF) program (6) and in the assessment of polycystic ovarian syndrome (PCOS), risk of ovarian hyperstimulation syndrome, prediction of menopause and monitoring the impact of cytotoxic chemotherapy and radiotherapy on ovarian function. Currently there are commercial kits available from more than 14 manufacturers (7, 8). However, the absence of an agreed international AMH reference preparation has resulted in confusion in defining clinical reference ranges between different kits. The aim of this report is to describe the development of AMH immunoassays and AMH reference preparations, and discuss the recently described WHO AMH Reference Reagent for immunoassay standardization.
AMH Gene and Molecular Structure
AMH is a member of the transforming growth factor-beta (TGF- ß) superfamily. It is a 140 kDa homodimeric glycoprotein consisting of two identical glycoprotein subunits linked by disulphide bonds. In humans, AMH is encoded by the AMH gene, which is located on chromosome 19 p13.3. The AMH gene is 275 bp in length and consists of five exons. The GC-rich 3’ end of the fifth exon codes for the bioactive part of the AMH molecule (8, 9).
The AMH gene encodes a pre-protein of 560 amino acid residues (pre-proAMH) which is cleaved to produce the precursor (proAMH) (AMH25-560) that has no binding to the AMH receptor. It undergoes proteolytic cleavage by subtilisin/kexin proprotein convertases to the bioactive form, AMHN,C. AMHN,C is a complex consisting of the N-terminal fragment (AMHN) and the C-terminal fragment (AMHC) associated non-covalently. The AMHN fragment is a 110 kDa homo-dimer formed by two 57kDa subunits, whereas the AMHC fragment is a 25 kDa homo-dimer formed by two 12.5 kDa subunits. Only AMHN,C and AMHC are bioactive on AMH receptors (Supplementary Figure 1). ProAMH and AMHN,C are the circulating forms detectable in the blood in varying ratios, whereas the free AMHC and AMHN are not detectable in the blood circulation in physiological conditions. Current commercially available immunoassays detect both proAMH and AMHN,C, and the reported values are a composite of both. The physiological role of proAMH in the circulation is currently not clear (10–15).
Correlation of Serum AMH with Ovarian Reserve
Although the primordial follicle count is conceptually the definitive parameter representing the ovarian reserve in a woman, it can only be measured directly by histological examination of the whole ovary after oophorectomy. Hence, surrogate markers which correlate with the primordial follicle count have been explored for clinical use, and both the AMH measured in serum and the antral follicle count (AFC) measured sonographically have been demonstrated to serve this purpose.
One study of 42 women undergoing oophorectomy for benign gynaecological conditions revealed a significant correlation (p<0.0001) between serum AMH and the ovarian primordial follicle count determined histologically, both unadjusted (r=0.72) as well as after adjustment for chronological age (r=0.48); the correlation coefficients were similar between AFC and primordial follicle count (unadjusted r=0.78 and adjusted r=0.53) (16). Most of the available studies showed a high correlation between serum AMH level and AFC (17–19).
Intra- and Inter-Cycle Variations of Serum AMH
Most studies have demonstrated small fluctuations in serum AMH across the normal menstrual cycle with a decline in the late follicular phase. This pattern has been explained by a decrease in AMH secretion from the lead follicle as it achieves dominance prior to ovulation. However, the magnitude of these intra-cycle fluctuations is small and is not generally considered clinically relevant (20–24). One study revealed that the intra-cycle fluctuations remained within the same quintile in 72% of women and crossed two quintiles in only 1% of women (22). Two prospective studies (22, 25) explored the inter-cycle variability and suggested that between-cycle reproducibility of serum AMH is higher than that of serum FSH or AFC, and that only 11% of the variability resulted from intra-individual fluctuations between cycles (intra-class coefficient 0.89).
There are a small number of situations in which the intracycle fluctuation in AMH should be taken into account when assessing ovarian reserve. In particular, late reproductive aged women have a reduced number of follicular waves through the cycle, and hence AFC may be low and serum AMH can show marked changes (26) paralleling the pattern of the follicular waves. Similarly, following chemotherapy, the antral follicle reserve may be severely reduced and serum AMH profile may vary across the cycle or treatment period. In cases where the ovarian reserve is low, the more sensitive AMH immunoassays (sensitivity ~0.1ng/ml) are needed. Depending on its application, standardising the serum collection time in the cycle would appear to be a wise prerequisite in some situations. Sample collection alongside FSH and LH in the early follicular phase of the cycle allows for standardisation of timing in such situations. If the woman does not have a natural cycle then a random sample would be acceptable.
Clinical Applications
A number of clinical situations have been identified in which serum AMH can be a useful diagnostic marker.
a) Ovarian Reserve Testing
Prediction of ovarian response to superovulation is the most common application of serum AMH (6, 27). Two individual patient data meta-analyses (28, 29) have shown that both serum AMH and AFC had good performance in predicting poor ovarian response as well as excessive response. Ovarian stimulation regimes are now individualised to provide the optimum number of oocytes while avoiding risk of severe OHSS (30). In the IVF context, low oocyte yield in “poor responder” patients inevitably results in a smaller pool of cryopreserved embryos, thereby reducing the cumulative livebirth rate (LBR) from one IVF cycle, whereas larger numbers of eggs/embryos offers a higher cumulative LBR.
b) PCOS
It is now well recognised that serum AMH is elevated in women with PCOS (5, 31, 32). In a recent study, AMH exhibited high specificity:sensitivity based on the receiver-operating characteristic (ROC) curve in predicting PCOS compared with age-matched controls (32).
c) Prediction of Menopause
Several studies (33–35) have explored the use of single or multiple AMH samples over time as a means to predict menopause. Studies by Finkelstein (35) assessed the probability of AMH predicting menopause in women in the late reproductive age (~47y) and showed that in combination with age and body mass index, AMH measurement predicted the occurrence of menopause within 12 to 36 months (area under the ROC curve = 0.88-0.99). These conclusions were derived from an ultrasensitive AMH enzyme-linked immunosorbent assay (ELISA) with a lower detection limit of <2pg/ml. Assessment in women over a longer lead time (14 years) showed an improved prediction of menopause when including knowledge of the AMH decline rate (34).
d) Monitoring the Return of Fertility in Those Women With Cancer Treated With Chemotherapy
Recovery of fertility in women following chemotherapy is a poorly defined area with evidence of shortened reproductive lifespan and infertility (3). Using AMH to monitor this process has revealed a complex pattern of recovery which is dependent both on the type of chemotherapy used and the woman’s age at treatment (36–38). In the study of Su et al. (38) dried blood analyses were undertaken using validated ELISA methodology with a sensitivity of 30pg/ml. The AMH level post treatment was 140pg/ml requiring sensitive ELISAs. Only 7% of samples were undetectable in this study.
e) Serving as a Tumour Marker for Some Cancers
AMH can serve as a tumour marker for the detection or follow-up for recurrence of granulosa cell tumors (39–41).
Evolution of AMH Assay Methods
Measurement of AMH in serum from adult women using ELISA was first reported in 1990 (3, 42). In the early 2000’s, when clinical studies utilizing AMH measurement were initiated, two commercial AMH ELISAs were available, manufactured by Diagnostic Systems Laboratories, Inc. (DSL, Webster, Texas, USA) and Immunotech (Marseille, France). DSL and Immunotech were subsequently acquired by Beckman Coulter, Inc. with the development of a second generation ELISA under the name “AMH Gen II ELISA”. This ELISA utilized the antibodies from the DSL kit and the AMH reference preparations from the Immunotech kit (43). Following its introduction in 2010, the AMH Gen II ELISA became the most widely used assay for AMH. However, its reliability was questioned due poor assay reproducibility, particularly following sample dilution and sample storage under different conditions (5, 44). The poor reproducibility was subsequently attributed to assay interference due to binding of serum complement protein C1q to the capture antibody. A pre-mixing protocol was then recommended by the manufacturer to overcome this problem. It was postulated that pre-mixing the test sample with the highly anionic buffer inactivated complement, hence reducing the interference. However, serum AMH values generated by the pre-mixing protocol are significantly higher compared with the conventional protocol (45).
More recently, additional AMH immunoassay kits have become available, including the ultrasensitive AMH/MIS ELISA kit (Ansh Laboratories, Texas, USA), the automated Access AMH kit (Beckman-Coulter Diagnostics, USA) and Elecsys® AMH Immunoassay (Roche Diagnostics International Ltd, Indiana, USA). The latter two are automated immunoassays that utilize chemiluminescence for detection and are not susceptible to interference by serum complement (46). Table 1 lists the analytical characteristics of the common commercial AMH assays that are currently available. Additional new AMH immunoassays are presented in the recent article by Ferguson et al. (8).
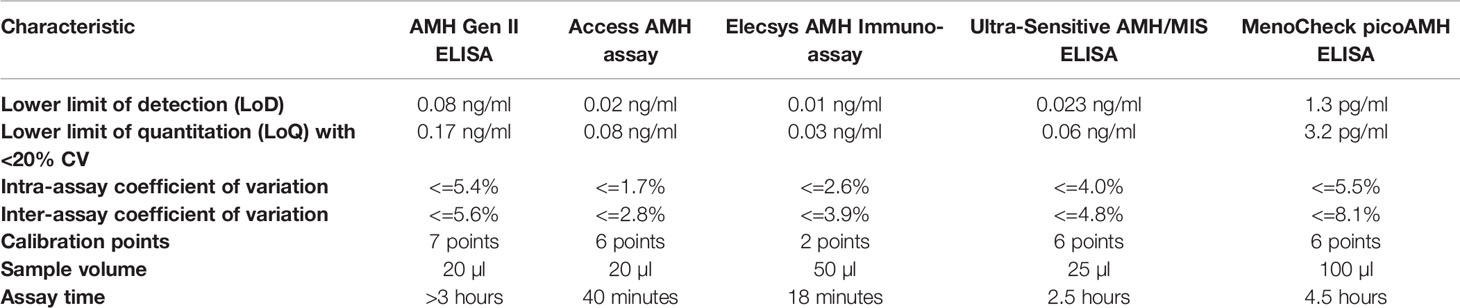
Table 1 Analytical characteristics of the common commercial AMH assay methods according to information from the manufacturers.
Comparison Between the Various AMH Assay Methods
In the earlier years, a comparison between the Immunotech and DSL ELISAs showed widely different regression relationships, with lower, comparable or higher serum AMH values by Immnotech compared with DSL ELISA being reported in different studies (47–50).
Studies comparing the AMH Gen II ELISA, DSL and Immunotech AMH ELISAs (30, 50, 51) showed good correlations of AMH Gen II ELISA with both the DSL and Immunotech kits although higher numerical values were shown by AMH Gen II ELISA compared to the latter. A higher numerical value was generated by the AnshLabs assay compared with the Gen II assay (43, 50–52).
AMH values obtained with the Gen II kit were well correlated with those generated by the Access and Elecsys® automated immunoassay methods, with correlation coefficients being >0.9 (p<0.001) in all pairwise comparisons (52). Passing and Bablok regression revealed that the values generated by the Access AMH assay were comparable to those generated by the Gen II assay, whereas those generated by the Elecsys AMH Immunoassay were systematically lower (Supplementary Figure 2). The bias between the Beckman-Coulter platforms (Gen II assay and Access AMH Assay) and the Elecsys AMH Immunoassay was uniform across the whole range of values studied. The finding concurred with previous reports, although the Elecsys AMH Immunoassay was claimed to be standardized against the Gen II assay (53–57). This differential calibration should be kept in mind when results generated by the different assay methods are interpreted or compared in clinical practice or research settings.
Assay Stability Upon Different Sample Storage Conditions
In the Access AMH assay and Elecsys AMH Immunoassay, serum samples frozen at -20°C and -80°C gave significantly lower AMH values (p<0.05) compared with freshly collected samples (52), with significantly lower values for those stored at -20°C compared with -80°C (p<0.05). The magnitude of the difference between immunoassays is small (<0.2 ng/ml) and may not be clinically important. The basis for this loss of immunoactivity with frozen storage remains to be explored.
Development of an International AMH Reference Reagent for Immunoassay
There has long been an unmet need for an internationally available reference material for AMH. This would allow calibration of diagnostic AMH immunoassays against a standard, which would then allow values obtained from different assay systems to be compared. An international collaborative study (7, 8) was thus undertaken by the World Health Organization (WHO) to produce a reference material purified from media from a stable human ovarian cancer cell line and available in ampoules (coded 16/190), and to derive an immunopotency evaluation for this human AMH preparation. The AMH preparation consisted of the full length 140kDa form with a modification of the internal cleavage site to ensure maximum cleavage between the pro-hormone and mature forms of the molecule (58) which represent the major forms of AMH in human serum (14, 15, 32). The WHO study involved the distribution the WHO reference preparation to participant laboratories, along with 21 human serum samples of varying origin. Each participant laboratory was requested to include these samples in their own immunoassay system using the AMH reference preparation of their own kit. Study participants used 21 different assay methods, 19 of which were different methods/platforms (either manual or automated) combinations. Since there is currently no recognized AMH preparation to act as an international reference, the results for serum values and the WHO reference reagent varied markedly between assays. The immunopotencies of the WHO reference reagent for the 21 laboratories ranged from ~350 to ~1200 ng/vial. In order to develop a consensus potency for the WHO Reference reagent, results of those 16 methods which were statistically comparable were combined to yield a robust geometric mean of 489 ng/ampoule. In addition, the bias of individual serum AMH samples for all kit results from the consensus means were also determined. In parallel, the bias attributed to the WHO AMH reference reagent in individual assays from the consensus mean was determined and compared with the corresponding bias observed with the serum samples.
Interestingly, in many of the assays the bias for the WHO AMH reference reagent within assay was statistically dissimilar to that observed with serum samples. Thus, while the use of the WHO AMH reference reagent as an International reference preparation should reduce the variability between some assays, it is apparent that the WHO AMH reference reagent is not being recognized in a similar manner to serum samples in all methods. The reason for the dissimilar responses between serum and WHO AMH reference reagent in these assays is not apparent (8). Clearly, the selection of kit reference preparation used in these methods is important, but other explanations regarding the different methodologies can be considered. From a global perspective one should not be too surprised by these results. AMH is a large complex glycoprotein which is found in the circulation in both precursor and processed forms (14, 15, 32). Little is known of the various heterogeneous AMH forms found in serum and it is unclear to what extent these forms are comparable with the purified recombinant preparation used as the reference reagent. Recently, AMH isoforms have been identified in human follicular fluid and granulosa cell extracts which do not match recognized consensus forms, suggesting that additional, as yet unknown, processing occurs (59). Additionally, the choice of antibodies used in the respective assays is also critical. Immunoassays of this sort are comparative assays where the adage ‘like vs. like’ strictly applies. For a serum assay, the most appropriate reference preparation should be serum-based, reflecting the samples under investigation, and yet the matrix used in the 16/190 preparation was bovine casein-based instead of human serum-based. The question of using a serum pool as a reference preparation was discussed by Ferguson and colleagues (8) but was not progressed due to problems of availability, standardization and continuity of a pooled serum standard supply. In contrast, the WHO AMH reference reagent satisfies many of the requirements expected of an international reference preparation and is the first widely available, stable, lyophilized preparation of AMH that can be used for harmonization of the current clinically relevant immunoassays. Its introduction should lead to greater consistency between the different kit assays. However, although the WHO reference reagent is likely to be commutable in a proportion of AMH assays, commutability with clinical samples has been demonstrated only in some but not all assays. As such, the reference reagent may not effectively harmonize the results for clinical samples in all assays, and because of this, has not been established as a WHO International Standard. Instead, its status as a WHO reference reagent represents its intended use as a common material with which manufacturers can investigate assay performance characteristics. This is critical as a first step in the continuum toward eventual AMH assay harmonization and will likely pave the way for a second generation of reference material(s) with which a more universal demonstration of commutability with clinical samples will be possible.
Discussion
AMH immunoassays are now widely available for assessing ovarian reserve and have application in a number of reproductive conditions where the size of the ovarian reserve is clinically important. Immunoassays are now available both in automated and manual formats with the automated platforms showing superior assay characteristics. In addition, new sensitive immunoassays are now available for situations where AMH serum concentrations are low, as seen in young women following chemotherapy and in women approaching menopause.
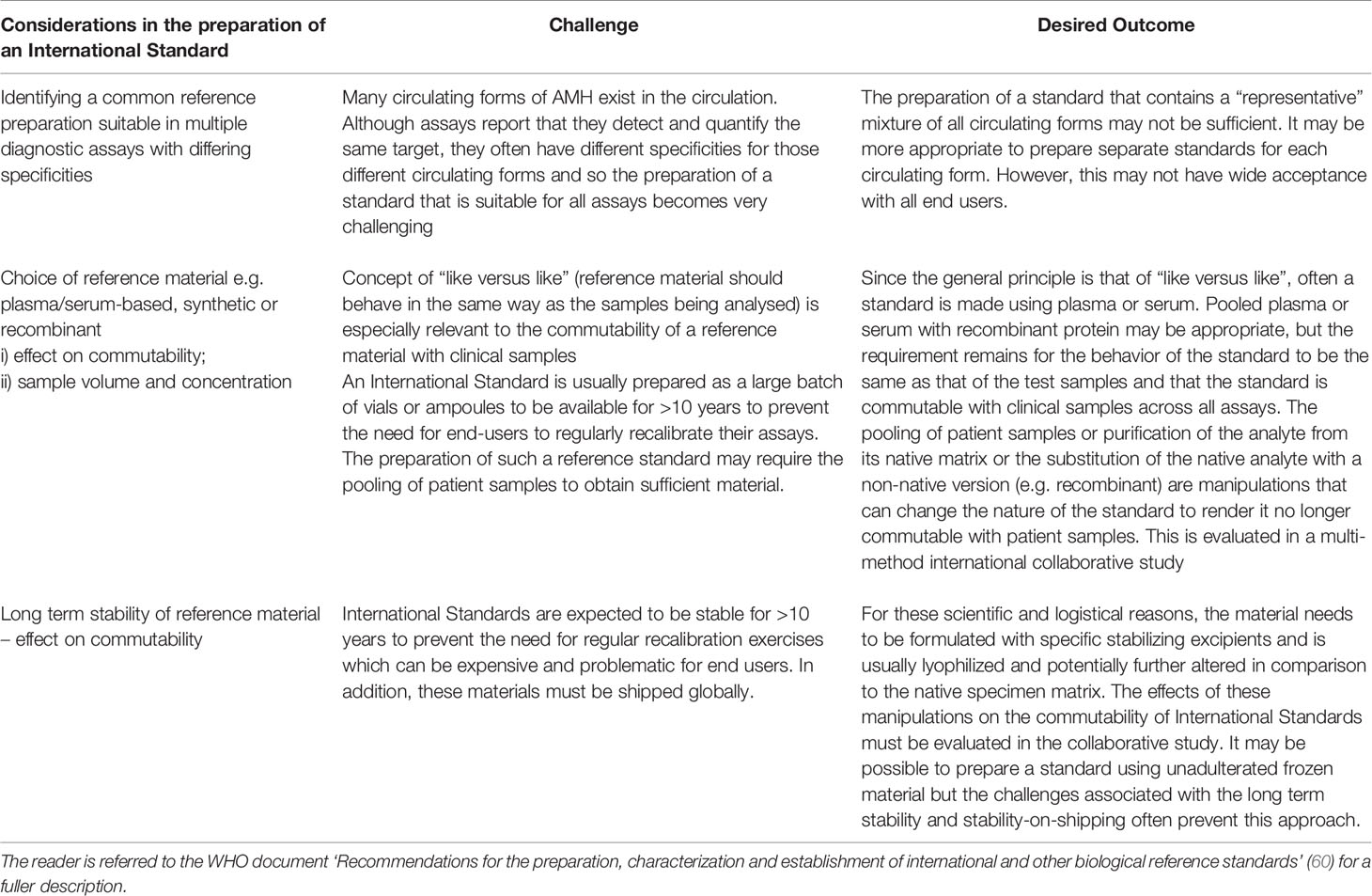
At recent count there are AMH kits provided by more than 14 manufacturers, most with their own AMH reference preparations which to date have not been calibrated against a common (international) reference preparation. The WHO AMH Study (8) was an attempt to establish such an appropriate reference preparation to aid in the harmonization of assay results. One of the primary goals in the harmonization of immunoassays is that, for a given analyte, the same numeric result should be obtained for a clinical sample irrespective of the assay method used to derive that result. This facilitates the derivation and effective use of clinical practice guidelines and supports evidence based medicine. A lack of harmonization can lead to different methods providing divergent results for the same clinical sample and clinicians and other healthcare professionals, who are often unaware of these differences, may wrongly classify a patient’s health status. Central to improving agreement between the results of different assay methods is the traceability of calibration to reference preparations and there is now acceptance that these reference preparations should be commutable, i.e. they should behave in the same way as the native analyte itself. The mathematical definition of commutability in effect states that for two samples (e.g. test and reference), the ratio of the results from the samples will be the same for each assay method. Based on the lack of commutability of the WHO AMH reference reagent with serum samples in some assay methods, this preparation cannot be considered as a suitable universal immunoassay reference preparation, although it will play an intermediate role while a second generation preparation is identified. Identification of that preparation will require knowledge of serum forms of AMH so that a suitable compatible reference preparation can be identified. The current challenges of creating an international reference preparation are summarized in Table 2.
Author Contributions
HL and WL conceived the idea of this article. HL and DR conducted literature search and wrote the manuscript, with critical input from CB and WL. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.691432/full#supplementary-material
Supplementary Figure 1 | Multiple forms of AMH generated by post-translation cleavage. The amino acid sequence is numbered from the N-terminus of the pre-proAMH in this figure. The proAMH molecule itself contains 230 amino acid residues.
Supplementary Figure 2 | Correlation between the Beckman-Coulter Gen II assay (Gen II), Access AMH assay (Access), and Elecsys AMH Immunoassay (Roche) for determination of AMH (n=94). The upper panels represent the Passing and Bablok regression plots whereas the lower panels represent the Bland-Altman plots. (Reproduced with permission from Li et al., 2016) (50).
References
1. Picard JY, Benarous R, Guerrier D, Josso N, Kahn A. Cloning and Expression of cDNA for Anti-Mvillerian Hormone. Proc Natl Acad Sci USA (1986) 83:5464–8. doi: 10.1073/pnas.83.15.5464
2. Cate RL, Mattaliano RJ, Hession C, Tizard R, Farber NM, Cheung A, et al. Isolation of the Bovine and Human Genes for Mullerian Inhibiting Substance and Expression of the Human Gene in Animal Cells. Cell (1986) 45(5):685–98. doi: 10.1016/0092-8674(86)90783-x
3. Hudson PL, Dougas I, Donahoe PK, Cate RL, Epstein J, Pepinsky RB, et al. An Immunoassay to Detect Human Mullerian Inhibiting Substance in Males and Females During Normal Development. J Clin Endocrinol Metab (1990) 70:16–22. doi: 10.1210/jcem-70-1-16
4. Moolhuijsen LME, Visser JV. Anti-Müllerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J Clin Endocrinol Metab (2020) 105:3361–73. doi: 10.1210/clinem/dgaa513
5. Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The Physiology and Clinical Utility of Anti-Mullerian Hormone in Women. Hum Reprod Update (2014) 20:370–85. doi: 10.1093/humupd/dmt062
6. Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early Follicular Serum Mullerian-Inhibiting Substance Levels Are Associated With Ovarian Response During Assisted Reproductive Technology Cycles. Fertil Steril (2002) 77:468–71. doi: 10.1016/S0015-0282(01)03201-0
7. Ferguson JM, Pépin D, Duru C, Matejtschuk P, Donahoe PK, Burns CJ. Towards International Standardization of Immunoassays for Müllerian Inhibiting Substance/anti-Müllerian Hormone. Reprod BioMed Online (2018) 37:631–40. doi: 10.1016/j.rbmo.2018.08.012
8. Ferguson J, Hockley J, Rigsby P, Burns C. Establishment of a WHO Reference Reagent for Anti-Mullerian Hormone. Reprod Biol Endocrinol (2020) 18:86. doi: 10.1186/s12958-020-00641-9
9. Cohen-Haguenauer O, Picard JY, Mattéi MG, Serero S, Nguyen VC, de Tand MF, et al. Mapping of the Gene for Anti-Müllerian Hormone to the Short Arm of Human Chromosome 19. Cytogenet Cell Genet (1987) 44:2–6. doi: 10.1159/000132332
10. Rey R, Lukas-Croisier C, Lasala C, Bedecarrás P. Amh/Mis: What We Know Already About the Gene, the Protein and its Regulation. Mol Cell Endocrinol (2003) 211:21–31. doi: 10.1016/j.mce.2003.09.007
11. Pankhurst MW, McLennan IS. Human Blood Contains Both the Uncleaved Precursor of Anti-Mullerian Hormone (proAMH) and a Complex of the NH2- and COOH-terminal Peptides. Am J Physiol Endocrinol Metab (2013) 305:E1241–1247. doi: 10.1152/ajpendo.00395.2013
12. Pankhurst MW, Chong YH, McLennan IS. Enzyme-Linked Immunosorbant Assay Measurements of Antimullerian Hormone (AMH) in Human Blood Are a Composite of the Uncleaved and Bioactive Cleaved Forms of AMH. Fertil Steril (2014) 101:846–50. doi: 10.1016/j.fertnstert.2013.12.009
13. McLennan IS, Pankhurst MW. Anti-Mullerian Hormone Is a Gonadal Cytokine With Two Circulating Forms and Cryptic Actions. J Endocrinol (2015) 226:R45–57. doi: 10.1530/JOE-15-0206
14. Pankhurst MW, McLennan IS. A Specific Immunoassay for proAMH, the Uncleaved Proprotein Precursor of anti-Müllerian Hormone. Mol Cell Endocrinol (2016) 419:165–71. doi: 10.1016/j.mce.2015.10.013
15. Peigné M, Pigny P, Pankhurst MW, Drumez E, Loyens A, Dewailly D, et al. The Proportion of Cleaved Anti-Müllerian Hormone is Higher in Serum But Not Follicular Fluid of Obese Women Independently of Polycystic Ovary Syndrome. Reprod BioMed Online (2020) 41:1112–21. doi: 10.1016/j.rbmo.2020.07.020
16. Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of Ovarian Reserve Tests With Histologically Determined Primordial Follicle Number. Fertil Steril (2011) 95(1):170–5. doi: 10.1016/j.fertnstert.2010.04.006
17. Nardo LG, Christodoulou D, Gould D, Roberts SA, Fitzgerald CT, Laing I. Anti-Müllerian Hormone Levels and Antral Follicle Count in Women Enrolled in In Vitro Fertilization Cycles: Relationship to Lifestyle Factors, Chronological Age and Reproductive History. Gynecol Endocrinol (2007) 23:486–93. doi: 10.1080/09513590701532815
18. Anderson RA, Anckaert E, Bosch E, Dewailly D, Dunlop CE, Fehr D, et al. Prospective Study Into the Value of the Automated Elecsys Antimüllerian Hormone Assay for the Assessment of the Ovarian Growing Follicle Pool. Fertil Steril (2015) 103:1074–80. doi: 10.1016/j.fertnstert.2015.01.004
19. Li HWR, Ko JKY, Lee VCY, Yung SSF, Lau EYL, Yeung WSB, et al. Comparison of Antral Follicle Count and Serum Anti Müllerian Hormone Level for Determination of Gonadotropin Dosing in In-Vitro Fertilization: Randomized Trial. Ultrasound Obstet Gynecol (2020) 55:303–9. doi: 10.1002/uog.20402
20. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum Anti-Mullerian Hormone Throughout the Human Menstrual Cycle. Hum Reprod (2006) 21:3103–7. doi: 10.1093/humrep/del291
21. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Müllerian Hormone Levels in the Spontaneous Menstrual Cycle Do Not Show Substantial Fluctuation. J Clin Endocrinol Metab (2006) 91:4057–63. doi: 10.1210/jc.2006-0331
22. van Disseldorp J, Lambalk CB, Kwee J, Looman CW, Eijkemans MJ, Fauser BC, et al. Comparison of Inter- and Intra-Cycle Variability of Anti-Mullerian Hormone and Antral Follicle Counts. Hum Reprod (2010) 25:221–7. doi: 10.1093/humrep/dep366
23. Sowers M, McConnell D, Gast K, Zheng H, Nan B, McCarthy JD, et al. Anti-Müllerian Hormone and Inhibin B Variability During Normal Menstrual Cycles. Fertil Steril (2010) 94:1482–6. doi: 10.1016/j.fertnstert.2009.07.1674
24. Gracia CR, Shin SS, Prewitt M, Chamberlin JS, Lofaro LR, Jones KL, et al. Multi-Center Clinical Evaluation of the Access Amh Assay to Determine AMH Levels in Reproductive Age Women During Normal Menstrual Cycles. J Assist Reprod Genet (2018) 35:777–83. doi: 10.1007/s10815-018-1141-5
25. Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High Reproducibility of Serum Anti-Mullerian Hormone Measurements Suggests a Multi-Staged Follicular Secretion and Strengthens its Role in the Assessment of Ovarian Follicular Status. Hum Reprod (2005) 20:923–7. doi: 10.1093/humrep/deh688
26. Robertson DM, Kumar A, Kalra B, Shah S, Pruysers E, Vanden Brink H, et al. Detection of Serum Antimüllerian Hormone in Women Approaching Menopause Using Sensitive Antimüllerian Hormone Enzyme-Linked Immunosorbent Assays. Menopause (2014) 21:1277–86. doi: 10.1097/GME.0000000000000244
27. Tal R, Seifer DB, Tal R, Grainger E, Wantman E, Tal O. Amh Highly Correlates With Assisted Reproduction Cumulative Live Birth Rate in Women With Diminished Ovarian Reserve Independent of Age: An Analysis of 34,540 Cycles From the SART Database for 2014-2016. J Clin Endocrinol Metab (Forthcoming 2021). doi: 10.1210/clinem/dgab168
28. Broer SL, van Disseldorp J, Broeze KA, Dolleman M, Opmeer BC, Bossuyt P, et al. Added Value of Ovarian Reserve Testing on Patient Characteristics in the Prediction of Ovarian Response and Ongoing Pregnancy: An Individual Patient Data Approach. Hum Reprod Update (2013) 19(1):26–36. doi: 10.1093/humupd/dms041
29. Broer SL, Dólleman M, van Disseldorp J, Broeze KA, Opmeer BC, Bossuyt PM, et al. Prediction of an Excessive Response in In Vitro Fertilization From Patient Characteristics and Ovarian Reserve Tests and Comparison in Subgroups: An Individual Patient Data Meta-Analysis. Fertil Steril (2013) 100(2):420–9.e7. doi: 10.1016/j.fertnstert.2013.04.024
30. Andersen AN, Nelson SM, Fauser BCJ, García-Velasco JA, Klein BM, Arce JC, et al. Individualized Versus Conventional Ovarian Stimulation for In Vitro Fertilization: A Multicenter, Randomized, Controlled, Assessor-Blinded, Phase 3 Noninferiority Trial. Fertil Steril (2017) 107:387–96. doi: 10.1016/j.fertnstert.2016.10.033
31. Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM. Can Anti-Mullerian Hormone Predict the Diagnosis of Polycystic Ovary Syndrome? A Systematic Review and Meta-Analysis of Extracted Data. J Clin Endocrinol Metab (2013) 98(8):3332–40. doi: 10.1210/jc.2013-1393
32. Wissing ML, Mikkelsen AL, Kumar A, Kalra B, Pors SE, Flachs EM, et al. Associations of Different Molecular Forms of Antimüllerian Hormone and Biomarkers of Polycystic Ovary Syndrome and Normal Women. Fertil Steril (2019) 112:149–155.e1. doi: 10.1016/j.fertnstert.2019.03.002
33. de Kat AC, van der Schouw YT, Eijkemans MJC, Broer SL, Verschuren WMM, Broekmans FJM. Can Menopause Prediction be Improved With Multiple Amh Measurements? Results From the Prospective Doetinchem Cohort Study. J Clin Endocrinol Metab (2019) 104:5024–31. doi: 10.1210/jc.2018-02607
34. Ramezani Tehrani F, Bidhendi Yarandi R, Solaymani-Dodaran M, Tohidi M, Firouzi F, Azizi F. Improving Prediction of Age At Menopause Using Multiple Anti-Müllerian Hormone Measurements: The Tehran Lipid-Glucose Study. J Clin Endocrinol Metab (2020) 105:dgaa083. doi: 10.1210/clinem/dgaa083
35. Finkelstein JS, Lee H, Karlamangla A, Neer RM, Sluss PM, Burnett-Bowie SM, et al. Antimullerian Hormone and Impending Menopause in Late Reproductive Age: The Study of Women’s Health Across the Nation. J Clin Endocrinol Metab (2020) 105:e1862–71. doi: 10.1210/clinem/dgz283
36. Dezellus A, Barriere P, Campone M, Lemanski C, Vanlemmens L, Mignot L, et al. Prospective Evaluation of Serum Anti-Müllerian Hormone Dynamics in 250 Women of Reproductive Age Treated With Chemotherapy for Breast Cancer. Eur J Cancer (2017) 79:72–80. doi: 10.1016/j.ejca.2017.03.035
37. Trapp E, Steidl J, Rack B, Kupka MS, Andergassen U, Jückstock J, et al. Anti-Müllerian Hormone (AMH) Levels in Premenopausal Breast Cancer Patients Treated With Taxane-Based Adjuvant Chemotherapy - A Translational Research Project of the SUCCESS A Study. Breast (2017) 35:130–5. doi: 10.1016/j.breast.2017.07.007
38. Su HI, Kwan B, Whitcomb BW, Shliakhsitsava K, Dietz AC, Stark SS, et al. Modeling Variation in the Reproductive Lifespan of Female Adolescent and Young Adult Cancer Survivors Using Amh. J Clin Endocrinol Metab (2020) 105:2740–51. doi: 10.1210/clinem/dgaa172
39. Rey RA, Lhommé C, Marcillac I, Lahlou N, Duvillard P, Josso N, et al. Antimullerian Hormone as a Serum Marker of Granulosa Cell Tumors of the Ovary: Comparative Study With Serum Alpha-Inhibin and Estradiol. Am J Obstet Gynecol (1996) 174(3):958–65. doi: 10.1016/s0002-9378(96)70333-2
40. Chong YH, Campbell AJ, Farrand S, McLennan IS. Anti-Mullerian Hormone Level in Older Women: Detection of Granulosa Cell Tumor Recurrence. Int J Gynecol Cancer (2012) 22(9):1497–9. doi: 10.1097/IGC.0b013e318270ac69
41. Chen S, Yang B, Fan J. Diagnostic Value of Anti-Mullerian Hormone in Ovarian Granulosa Cell Tumor: A Meta-Analysis. Eur J Obstet Gynecol Reprod Biol (2020) 253:266–72. doi: 10.1016/j.ejogrb.2020.08.011
42. Josso N, Legeai L, Forest MG, Chaussain JL, Brauner R. An Enzyme Linked Immunoassay for Anti-Müllerian Hormone: A New Tool for the Evaluation of Testicular Function in Infants and Children. J Clin Endocrinol Metab (1990) 70:23–7. doi: 10.1210/jcem-70-1-23
43. Kumar A, Kalra B, Patel A, McDavid L, Roudebush WE. Development of a Second Generation Anti-Mullerian Hormone (AMH) ELISA. J Immunol Methods (2010) 362(1-2):51–9. doi: 10.1016/j.jim.2010.08.011
44. Rustamov O, Smith A, Roberts SA, Yates AP, Fitzgerald C, Krishnan M, et al. Anti-Mullerian Hormone: Poor Assay Reproducibility in a Large Cohort of Subjects Suggests Sample Instability. Hum Reprod (2012) 27:3085–91. doi: 10.1093/humrep/des260
45. Han X, McShane M, Sahertian R, White C, Ledger W. Pre-Mixing Serum Samples With Assay Buffer is a Prerequisite for Reproducible Anti-Mullerian Hormone Measurement Using the Beckman Coulter Gen II Assay. Hum Reprod (2014) 29:1042–8. doi: 10.1093/humrep/deu050
46. Turner K, Larson B, Willrich MA, Bornhourst J. Assessment of Complement Interference in Anti-Müllerian Hormone (AMH) Immunoassays. Am J Clin Pathol (2018) 150(Suppl 1):S158–9. doi: 10.1515/cclm-2019-0496
47. Freour T, Mirallie S, Bach-Ngohou K, Denis M, Barriere P, Masson D. Measurement of Serum Anti-Mullerian Hormone by Beckman Coulter ELISA and DSL Elisa: Comparison and Relevance in Assisted Reproduction Technology (ART). Clin Chim Acta (2007) 375:162–4. doi: 10.1016/j.cca.2006.06.013
48. Bersinger NA, Wunder D, Birkhäuser MH, Guibourdenche J. Measurement of Anti-Mullerian Hormone by Beckman Coulter ELISA and DSL ELISA in Assisted Reproduction: Differences Between Serum and Follicular Fluid. Clin Chim Acta (2007) 384:174–5. doi: 10.1016/j.cca.2007.05.011
49. Lee JR, Kim SH, Jee BC, Suh CS, Kim KC, Moon SY. Antimullerian Hormone as a Predictor of Controlled Ovarian Hyperstimulation Outcome: Comparison of Two Commercial Immunoassay Kits. Fertil Steril (2011) 95(8):2602–4. doi: 10.1016/j.fertnstert.2011.01.126
50. Li HWR, Ng EHY, Wong BPC, Anderson RA, Ho PC, Yeung WSB. Correlation Between Three Assay Systems for Anti-Müllerian Hormone (AMH) Determination. J Assist Reprod Genet (2012) 29:1443–6. doi: 10.1007/s10815-012-9880-1
51. Wallace AM, Faye SA, Fleming R, Nelson SM. A Multicentre Evaluation of the New Beckman Coulter Anti-Mullerian Hormone (AMH) Immunoassay (AMH Gen II). Ann Clin Biochem (2011) 48:370–3. doi: 10.1258/acb.2011.010172
52. Li HWR, Wong BPC, Ip WK, Yeung WSB, Ho PC, Ng EHY. Comparative Evaluation of Three New Commercial Immunoassays for Anti-Müllerian Hormone Measurement. Hum Reprod (2016) 31:2796–802. doi: 10.1093/humrep/dew248
53. Gassner D, Jung R. First Fully Automated Immunoassay for Anti-MüLlerian Hormone. Clin Chem Lab Med (2014) 52:1143–52. doi: 10.1515/cclm-2014-0022
54. Nelson SM, Pastuszek E, Kloss G, Malinowska I, Liss J, Lukaszuk A, et al. Two New Automated, Compared With Two Enzyme-Linked Immunosorbent, Antimullerian Hormone Assays. Fertil Steril (2015) 104:1016–21. doi: 10.1016/j.fertnstert.2015.06.024
55. van Helden J, Weiskirchen R. Performance of the Two New Fully Automated Anti-Mullerian Hormone Immunoassays Compared With the Clinical Standard Assay. Hum Reprod (2015) 30:1918–26. doi: 10.1093/humrep/dev127
56. Hyldgaard J, Bor P, Ingerslev HJ, Tørring N. Comparison of Two Different Methods for Measuring Anti-Mullerian Hormone in a Clinical Series. Reprod Biol Endocrinol (2015) 13:107. doi: 10.1186/s12958-015-0101-5
57. Anckaert E, Öktem M, Thies A, Cohen-Bacrie M, Daan NM, Schiettecatte J, et al. Multicenter Analytical Performance Evaluation of a Fully Automated Anti-Müllerian Hormone Assay and Reference Interval Determination. Clin Biochem (2016) 49(3):260–7. doi: 10.1016/j.clinbiochem.2019.11.007
58. Pépin D, Hoang M, Nicolaou F, Hendren K, Benedict LA, Al-Moujahed A, et al. An Albumin Leader Sequence Coupled With a Cleavage Site Modification Enhances the Yield of Recombinant C-Terminal Mullerian Inhibiting Substance. Technology (2013) 1:63–71. doi: 10.1142/S2339547813500076
59. Mamsen LS, Bøtkjær JA, Kristensen SG, Pors SE, Jeppesen JV, Kumar A, et al. High Variability of Molecular Isoforms of AMH in Follicular Fluid and Granulosa Cells From Human Small Antral Follicles. Front Endocrinol (Lausanne) (2021) 12:617523. doi: 10.3389/fendo.2021.617523
Keywords: anti-Müllerian hormone, enzyme-linked immunosorbent assay (ELISA), automated chemiluminescence immunoassay, reference preparation, international standard
Citation: Li HWR, Robertson DM, Burns C and Ledger WL (2021) Challenges in Measuring AMH in the Clinical Setting. Front. Endocrinol. 12:691432. doi: 10.3389/fendo.2021.691432
Received: 06 April 2021; Accepted: 11 May 2021;
Published: 24 May 2021.
Edited by:
Antonio La Marca, University of Modena and Reggio Emilia, ItalyReviewed by:
Rodolfo A Rey, Hospital de Niños Ricardo Gutiérrez, ArgentinaDavid Seifer, Yale University, United States
Copyright © 2021 Li, Robertson, Burns and Ledger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hang Wun Raymond Li, cmF5bW9uZGxpQGhrdS5oaw==
 Hang Wun Raymond Li
Hang Wun Raymond Li David Mark Robertson
David Mark Robertson Chris Burns
Chris Burns William Leigh Ledger
William Leigh Ledger