
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 01 September 2021
Sec. Clinical Diabetes
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.690343
 Yotsapon Thewjitcharoen1*
Yotsapon Thewjitcharoen1* Ekgaluck Wanothayaroj1
Ekgaluck Wanothayaroj1 Sirinate Krittiyawong1
Sirinate Krittiyawong1 Soontaree Nakasatien1
Soontaree Nakasatien1 Tsz Fung Tsoi2
Tsz Fung Tsoi2 Cadmon K. P. Lim2
Cadmon K. P. Lim2 Juliana C. N. Chan2,3
Juliana C. N. Chan2,3 Thep Himathongkam1
Thep Himathongkam1Glucokinase-Maturity-Onset Diabetes of the Young (GCK-MODY) is characterized by asymptomatic, non-progressive and fasting hyperglycemia, albeit not without phenotypic variability. We used next generation sequencing (NGS) to screen for 34 MODY genes in a non-obese person with familial young-onset diabetes followed by screening in 24 family members within three generations with varying presentations of young-onset diabetes and sensorineural hearing loss. The index patient was found to carry a paternally-inherited heterozygous missense variant (c.716 A>G) of GCK in exon 7 with amino acid change (Q239R). This variant was associated with phenotypic heterogeneity ranging from normal glucose tolerance to diabetes with complications amongst the siblings which might be modified by obesity and chronic hepatitis B infection. Two paternally-inherited variants of SLC29A3 encoding a nucleoside transporter protein and Apo-A1 genes also co-segregated with glucose and lipid traits. Co-occurrence of diabetes and deafness in maternal aunts led to discovery of WFS1 (Wolfram syndrome type 1) as a cause of non-syndromic deafness in multiple members of the maternal pedigree. Our findings highlight the complex causes of familial young-onset diabetes and the need of a multidisciplinary approach to interpret the clinical relevance of discoveries made by NGS in this era of genomic medicine.
Earlier studies, conducted mainly in Caucasians, suggested that Glucokinase-Maturity-Onset Diabetes of the Young (GCK-MODY), is a mild condition with stable fasting hyperglycemia not requiring medical treatment (1). However, there are sporadic reports on severe forms of GCK-MODY due to co-existence of autoimmune diabetes or obesity-driven insulin resistance (2, 3). Diagnosis of MODY is an exemplary example of precision medicine where delayed diagnosis and intervention could lead to serious complications while family screening can enable early diagnosis and treatment. Besides, patients with different MODY subtypes might respond differently to oral drugs or insulin, making precise diagnosis important in guiding treatment selection (4).
Both clinical acumen and modern technology are needed in our pursuit of precision medicine. The use of next generation sequencing (NGS) has reduced the cost and time of genetic discovery with new forms of monogenic diabetes being reported. Moreover, co- occurrence of various subtypes of MODY and mitochondrial diabetes within the same family had been reported (5, 6). Herein, we report the phenotypic and genetic heterogeneity of a large multigenerational Thai family with GCK-MODY.
This study was approved by the Ethics Committee of Theptarin Hospital. All participants gave written informed consent.
A total of 25 family members within three generations of young-onset diabetes with or without sensorineural hearing loss was studied. Genomic DNA was extracted from peripheral blood for sequencing. Clinical information and biochemical characteristics were documented and 75-gram oral glucose tolerance test (OGTT) was performed to verify glycemic status. Assessment of insulin resistance by homeostasis model assessment (HOMA-IR) was evaluated (7). Whole Exome Sequencing (WES) based on NGS using Human Core Exome Kit and Human RefSeq Panel from Twist Bioscience was used to screen for all coding regions including intron-exon junctions of 34 MODY genes (Supplementary Material Table 1) and a mitochondrial mutation for maternally-inherited deafness and diabetes (MIDD, mt A3243G) (GemVCare, Shatin, Hong Kong). Sanger sequencing covering all coding regions and intron-exon junctions of candidate gene was used to confirm the mutations in other family members.
Sequence data were aligned to the reference genome GRCh38 to identify variants. We evaluated possible functional significance of the variants using computational predictive programs PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2) and SIFT (http://sift.jcvi.org/). Sequence variations were described using nomenclature of the Human Gene Mutation Database (HGMD). Based on the American College of Medical Genetics and Genomics (ACMG) standards and guidelines, these sequence variants were interpreted as pathogenic, likely pathogenic, variant of uncertain significance (VUS), likely benign, and benign (8).
The proband [female, II-1, aged 41, body mass index (BMI) 23.9 kg/m2] was detected to have high fasting plasma glucose (FPG) of 120 mg/dL at the age of 34. She was diagnosed to have type 2 diabetes (T2D) when aged 37 with a FPG of 150 mg/dL and glycated hemoglobin (A1C) of 7.0%. Since then, she was put on metformin 1000 mg daily with a A1C range of 5.9-7.1%. Prior to the date of 75-gram OGTT, she had discontinued metformin for nearly 1 year. Her mother (I-2, aged 66) had T2D since the age of 38 with A1C of 7.5-8.7% and complicated by retinopathy and kidney disease. Her two maternal aunties (I-3, I-4) had diabetes and deafness, the latter affecting some of their family members (Figure 1).
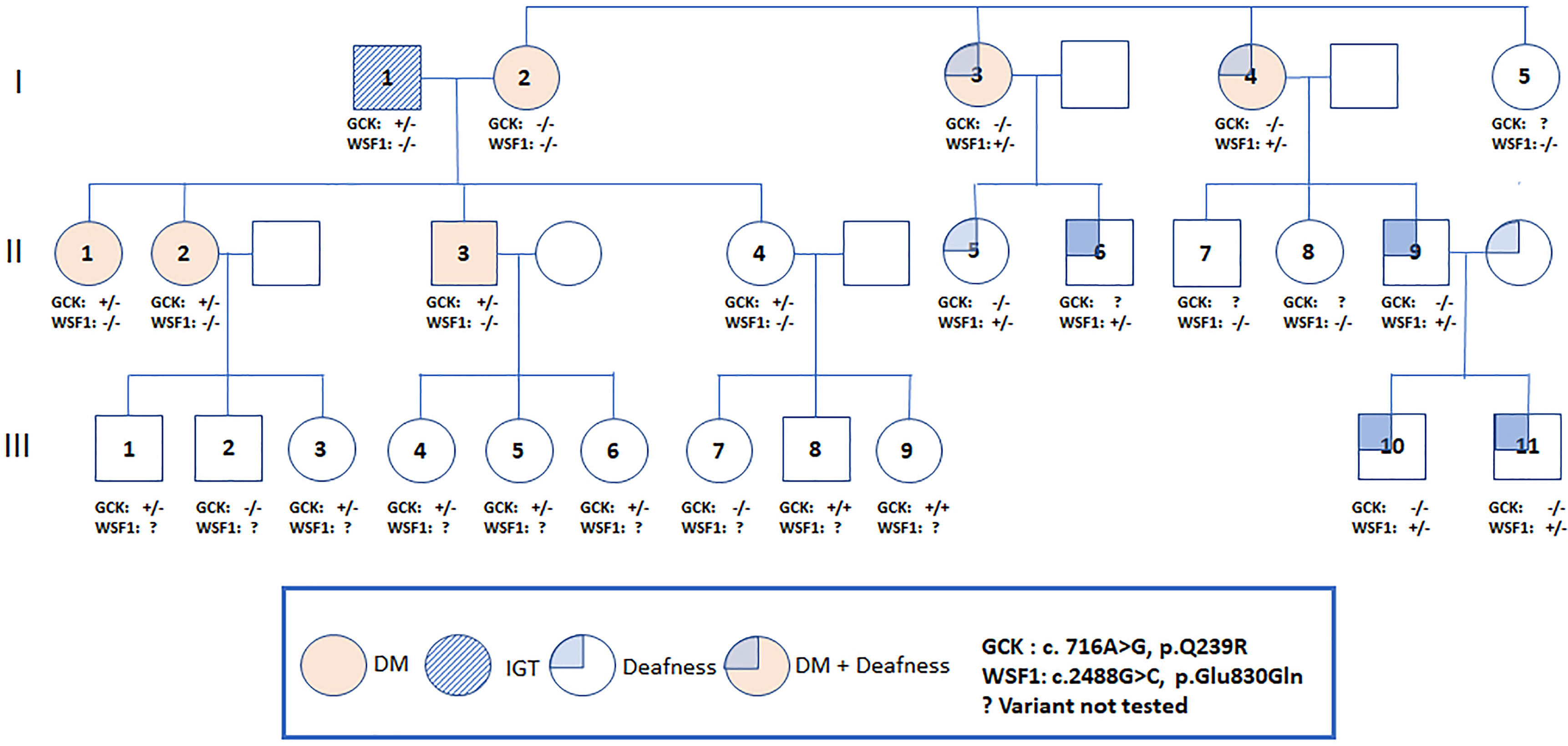
Figure 1 Pedigree of a Thai family with the Q239R mutation in the GCK gene. An arrow indicates the proband. Filled symbols indicate diabetes and non-filled symbols indicate normal or unknown glucose tolerance. Carrier status is indicated in those who underwent genotyping (+/+ = homozygous, +/- =heterozygous, -/- = non-carrier).
The proband’s younger sister (II-2, aged 40) had insulin-treated gestational diabetes when aged 31 and was diagnosed to have T2D at the age of 38 during screening (FPG 170 mg/dL, A1C 7.7%). She had hypertension, obesity (BMI 28.6 kg/m2) and proteinuria and received multiple drugs with A1C of 7.5-8.3%. Autoantibody for glutamic acid decarboxylase was negative in II-1 and II-2.
We conducted a research study to investigate possible genetic causes of diabetes in this family. We used NGS to screen for all coding regions including intron-exon junctions of 34 MODY genes (Supplementary Material Table 1) and mt A3243G in I-1, I-2, II-1, II-2, II-3 and II-4 and Sanger sequencing to confirm mutations associated with diabetes and deafness in other family members.
The proband and II-2 were heterozygous for a previously reported missense variant (c.716 A>G) of GCK gene with amino acid change (Q239R) (9). The allele frequency was 0.00054 (The Genome Aggregation Database, gnomAD, East Asians) (Supplementary Material Table 2) and located within exon 7, a hotspot for mutations. This variant was classified as VUS by the ACMG guidelines due to its high frequency in Asian population (Supplementary Material Table 2). Both patients were also heterozygous for a missense variant (c.640 G>A) of SLC29A3 in exon 5 with amino acid change (V214M). In-silico analysis predicted damaging effect, classified as VUS by the ACMG guidelines. The allele frequency was less than 0.00962 (gnomAD, East Asians) and not found in 50 Thai healthy subjects.
We offered 75-gram OGTT to the father (I-1) in his 70s and 2 siblings (II-3, II-4) in their 40s with no known history of diabetes. The father (I-1) had impaired glucose tolerance (IGT), the younger brother (II-3) had diabetes and the youngest sister (II-4) had normal glucose tolerance. The results of OGTT and HOMA-IR were summarized in Table 1. The father and all children were heterozygous for GCK Q239R and SLC29A3 V214M variants. The mother (I-2) carried neither of these two variants (Figure 1). Due to the heterogeneous phenotypes amongst the carriers of these two VUS, we screened for surface antigen of hepatitis B (HBsAg) which had been reported to increase the risk of T2D by 3-fold in Asian carriers versus non-carriers (10). The mother I-2 (BMI 23.8 kg/m2) and sibling II-3 (BMI, 31.0 kg/m2) and II-4 (BMI, 26.0 kg/m2) were HBsAg carriers. Sibling II-3 with both obesity and positive HBsAg status had diabetes. In the third generation, amongst the 9 children (age 2 to 12), 5 were heterozygous and 2 were homozygous carriers for GCK Q239R. None of them had history of diabetes although formal 75g OGTT was not performed.
During screening for cardiometabolic risk factors, we noted isolated low HDL-C levels (28-34 mg/dl) in the proband’s father and 3 siblings (II-2,3,4). Since Apo A-1 is the main lipoprotein of HDL-C (11), we performed additional NGS of the Apo-A1 gene and detected co-segregation of the paternally-inherited mutation of p.Gln156AlafsTer11 in all siblings with low HDL-C levels. This novel mutation created a premature stop codon which can lead to nonsense mediated decay of the mRNA and is likely to be pathogenic by ACMG guidelines.
The two maternal aunts (I-3, I-4) with diabetes and deafness did not carry the GCK or SLC29A3 mutations or mtA3243G mutation. The mother of proband (I-2) had diabetes without deafness and was negative for all 3 mutations. Using the MODY panel, both maternal aunts were found to carry a mutation in the WFS1 (Wolfram syndrome type 1) known to be associated with diabetes and deafness (12). Both maternal aunts but not the proband’s mother were heterozygous for an amino-acid changing mutation (p.E830Q). This novel variant was predicted to damage protein functions and classified as pathogenic by the ACMG guidelines. No additional mutations of the 34 MODY genes were detected in the mother or maternal aunts. Using Sanger sequencing, the WFS1 mutation (p.E830Q) was detected in 5 of the 8 family members of the 2 maternal aunts which co-segregated with deafness. None of these family members reported history of diabetes.
Age, obesity, insulin resistance and other non-genetic factors can modify clinical presentation of MODY (2, 13). Using a MODY panel and NGS, we discovered co-segregation of 2 paternally-inherited mutations of GCK and SLC29A3 with diabetes and 1 paternally-inherited mutation of Apo-A1 with low HDL-C amongst the children. The presence of deafness and diabetes in the maternal aunts led to the discovery of a WFS-1 mutation in the maternal pedigree, although none of the members had diabetes. Meanwhile, the cause of diabetes in the mother remained uncertain.
There are many lessons learnt in this Thai family. Using a MODY panel, we discovered a paternally-inherited GCK variant to be the cause of diabetes in a non-obese patient with familial young-onset diabetes who subsequently discontinued metformin due to mild disease. However, the phenotypic variability amongst the family members raised the possibility of other modifying factors to influence the typical non-progressive clinical course of GCK-MODY. Although the missense variant (c.716 A>G) of GCK gene with amino acid change (Q239R) detected in this family was considered to be a VUS due to its high frequency in Asian population, this mutation had been reported in other Chinese families of young-onset diabetes (9). In this Thai family, despite all carrying the same mutation, both the father and index case had IGT (after discontinuing metformin for 1 year) while 2 siblings had diabetes with varying degrees of obesity and HBV status which might contribute towards the phenotypic heterogeneity. Other reports on correlations between molecular severity of GCK mutations and glucose tolerance yielded mixed results (13–15). In a study of Italian children with GCK-MODY, individual differences in insulin sensitivity but not molecular severity of GCK mutations affected the 2-hour post-load blood glucose level during OGTT (13).
Using a MODY panel and NGS, we discovered additional paternally-inherited mutations implicated in syndromic diabetes and lipid metabolism with potential pathogenic significance although these findings should be interpreted with caution. Homozygous carriers of SLC29A3 mutation were reported to have the rare H syndrome characterized by pigmented hypertrichotic dermatosis, insulin-dependent diabetes and neuroendocrine dysfunction (16). However, no report of familial diabetes had been reported in heterozygous carriers of SLC29A3 mutation and its significance remains unclear in this GCK-MODY family. Given the frequent co-occurrence of low HDL-C with diabetes (17), the co-segregation of the paternally-inherited rare mutant of the Apo-A1 gene (18) with lipid traits might be coincidental rather than causal. A recent mendelian randomization study did not show causal relationship between genetically-reduced HDL-C and increased risk of type 2 diabetes (19).
With increasing affordability of NGS, our study also revealed its utility in uncovering rare causes of maternally-inherited diabetes and deafness, traditionally considered due to mitochondrial mutations (20). Both maternal aunts with deafness and diabetes carried a novel mutation in the WFS1 gene (21). This gene encodes for the wolframin protein implicated in calcium metabolism and could explain the autosomal dominant inheritance of hearing loss in all affected family members in the maternal pedigree. However, we were not able to detect mutations in this MODY panel which could explain the young-onset diabetes with complications in the mother of the index case.
In this Thai family, we discovered a paternally-inherited GCK mutation with phenotypic heterogeneity possibly modified by other non-genetic factors including timing of diagnosis and treatment. The use of NGS has also led to the discovery of paternally-inherited mutations co-segregated with glucose and lipid traits and a novel mutation of WFS1 gene in the maternal pedigree with diabetes and deafness. Thus, apart from gene-environment-lifestyle-treatment interactions that can modify clinical presentation, the discovery of these rare variants through NGS requires careful interpretation by a multidisciplinary team including clinicians, geneticists, molecular biologists and data scientists in our advice of patients with or at risk of having monogenic diabetes often with partial penetrance and complex inheritance with regards to diagnosis, treatment and genetic counselling.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Theptarin Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
YT and JC wrote the manuscript. TT and CL conceived and performed genetic analysis. YT, EW, SN, and TH were involved in the care of patients. SK, CL, JC, and TH reviewed the manuscript. YT and JC are the co-guarantors of this work and, as such, have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the article and approved the submitted version.
This work was supported by a grant for promoting intramural research in Theptarin Hospital (Grant No. 1/2563).
CL is the Chief Scientific Officer at GemVCare, a diabetes genetic testing laboratory, which was established through support from the Technology Start-up Support Scheme for Universities (TSSSU) funded by the Hong Kong Government Innovation and Technology Commission (ITC). JC and CL are co-founders of GemVCare.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are extremely grateful to the patients and their families who have been instrumental in the conduct of this study. We also acknowledge the proofreading and editing by Dr. Tinapa Himathongkam.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.690343/full#supplementary-material
1. Chakera AJ, Steele AM, Gloyn AL, Shepherd MH, Shields B, Ellard S, et al. Recognition and Management of Individuals With Hyperglycemia Because of a Heterozygous Glucokinase Mutation. Diabetes Care (2015) 38(7):1383–92. doi: 10.2337/dc14-2769
2. Cuesta-Munoz AL, Tuomi T, Cobo-Vuilleumier N, Koskela H, Odili S, Stride A, et al. Clinical Heterogeneity in Monogenic Diabetes Caused by Mutations in the Glucokinase Gene (GCK-MODY). Diabetes Care (2010) 33(2):290–2. doi: 10.2337/dc09-0681
3. Calcaterra V, Martinetti M, Salina A, Aloi C, Larizza D. The Coexistence of Type 1 Diabetes, MODY2 and Metabolic Syndrome in a Young Girl. Acta Diabetol (2012) 49(5):401–4. doi: 10.1007/s00592-011-0300-2
4. Hattersley AT, Patel KA. Precision Diabetes: Learning From Monogenic Diabetes. Diabetologia (2017) 60(5):769–77. doi: 10.1007/s00125-017-4226-2
5. Cervin C, Liljeström B, Tuomi T, Heikkinen S, Tapanainen JS, Groop L, et al. Cosegregation of MIDD and MODY in a Pedigree: Functional and Clinical Consequences. Diabetes (2004) 53(7):1894–9. doi: 10.2337/diabetes.53.7.1894
6. Forlani G, Zucchini S, Di Rocco A, Di Luzio R, Scipione M, Marasco E, et al. Double Heterozygous Mutations Involving Both HNF1A/MODY3 and HNF4A/MODY1 Genes: A Case Report. Diabetes Care (2010) 33(11):2336–8. doi: 10.2337/dc10-0561
7. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function From Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia (1985) 28(7):412–9. doi: 10.1007/bf00280883
8. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med (2015) 17(5):405–24. doi: 10.1038/gim.2015.30
9. Ng MC, Lee SC, Ko GT, Li JK, So WY, Hashim Y, et al. Familial Early-Onset Type 2 Diabetes in Chinese Patients: Obesity and Genetics Have More Significant Roles Than Autoimmunity. Diabetes Care (2001) 24(4):663–71. doi: 10.2337/diacare.24.4.663
10. Li-Ng M, Tropp S, Danoff A, Bini EJ. Association Between Chronic Hepatitis B Virus Infection and Diabetes Among Asian Americans and Pacific Islanders. Dig Liver Dis (2007) 39(6):549–56. doi: 10.1016/j.dld.2007.02.014
11. Schonfeld G, Pfleger B. The Structure of Human High Density Lipoprotein and the Levels of Apolipoprotein A-I in Plasma as Determined by Radioimmunoassay. J Clin Invest (1974) 54(2):236–46. doi: 10.1172/jci107758
12. Bespalova IN, Van Camp G, Bom SJ, Brown DJ, Cryns K, DeWan AT, et al. Mutations in the Wolfram Syndrome 1 Gene (WFS1) Are a Common Cause of Low Frequency Sensorineural Hearing Loss. Hum Mol Genet (2001) 10(22):2501–8. doi: 10.1093/hmg/10.22.2501
13. Massa O, Meschi F, Cuesta-Munoz A, Caumo A, Cerutti F, Toni S, et al. High Prevalence of Glucokinase Mutations in Italian Children With MODY. Influence on Glucose Tolerance, First-Phase Insulin Response, Insulin Sensitivity and BMI. Diabetologia (2001) 44(7):898–905. doi: 10.1007/s001250100530
14. Tinto N, Zagari A, Capuano M, De Simone A, Capobianco V, Daniele G, et al. Glucokinase Gene Mutations: Structural and Genotype-Phenotype Analyses in MODY Children From South Italy. PloS One (2008) 3(4):e1870. doi: 10.1371/journal.pone.0001870
15. García-Herrero CM, Galán M, Vincent O, Flández B, Gargallo M, Delgado-Alvarez E, et al. Functional Analysis of Human Glucokinase Gene Mutations Causing MODY2: Exploring the Regulatory Mechanisms of Glucokinase Activity. Diabetologia (2007) 50(2):325–33. doi: 10.1007/s00125-006-0542-7
16. Cliffe ST, Kramer JM, Hussain K, Robben JH, de Jong EK, de Brouwer AP, et al. SLC29A3 Gene Is Mutated in Pigmented Hypertrichosis With Insulin-Dependent Diabetes Mellitus Syndrome and Interacts With the Insulin Signaling Pathway. Hum Mol Genet (2009) 18(12):2257–65. doi: 10.1093/hmg/ddp161
17. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation (2009) 120(16):1640–5. doi: 10.1161/circulationaha.109.192644
18. Haase CL, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Population-Based Resequencing of APOA1 in 10,330 Individuals: Spectrum of Genetic Variation, Phenotype, and Comparison With Extreme Phenotype Approach. PloS Genet (2012) 8(11):e1003063. doi: 10.1371/journal.pgen.1003063
19. Haase CL, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. HDL Cholesterol and Risk of Type 2 Diabetes: A Mendelian Randomization Study. Diabetes (2015) 64(9):3328–33. doi: 10.2337/db14-1603
20. van den Ouweland JM, Lemkes HH, Ruitenbeek W, Sandkuijl LA, de Vijlder MF, Struyvenberg PA, et al. Mutation in Mitochondrial tRNA(Leu)(UUR) Gene in a Large Pedigree With Maternally Transmitted Type II Diabetes Mellitus and Deafness. Nat Genet (1992) 1(5):368–71. doi: 10.1038/ng0892-368
Keywords: GCK-MODY, WFS1, Apo-A1, SLC29A3, deafness, heterogeneity
Citation: Thewjitcharoen Y, Wanothayaroj E, Krittiyawong S, Nakasatien S, Tsoi TF, Lim CKP, Chan JCN and Himathongkam T (2021) Phenotypic and Genetic Heterogeneity in a Thai Glucokinase MODY Family Reveals the Complexity of Young-Onset Diabetes. Front. Endocrinol. 12:690343. doi: 10.3389/fendo.2021.690343
Received: 02 April 2021; Accepted: 19 August 2021;
Published: 01 September 2021.
Edited by:
Hans Ulrich Häring, Tübingen University Hospital, GermanyReviewed by:
Aaron Hanukoglu, Tel Aviv University, IsraelCopyright © 2021 Thewjitcharoen, Wanothayaroj, Krittiyawong, Nakasatien, Tsoi, Lim, Chan and Himathongkam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yotsapon Thewjitcharoen, a2FtaWpvYUBob3RtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.