
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 30 August 2021
Sec. Bone Research
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.688269
 Yongquan Gao1
Yongquan Gao1 Xiaochen Liu2
Xiaochen Liu2 Yuan Gu1
Yuan Gu1 Deye Song1
Deye Song1 Muliang Ding1
Muliang Ding1 Lele Liao1
Lele Liao1 Junjie Wang1
Junjie Wang1 Jiangdong Ni1
Jiangdong Ni1 Guangxu He1*
Guangxu He1*Background: Osteoporosis is a common complication of acute fracture, which can lead to fracture delayed union or other complications and resulting in poor fracture healing. Bisphosphate is a common anti-osteoporosis drug, but its application in fracture patients is still controversial because of its inhibitory effect on bone resorption.
Method: Studies were acquired from literature databases in accordance with established inclusion criteria. Standard mean difference (SMD) and 95% confidence intervals (Cls) were calculated to evaluate the effectiveness of the bisphosphonates treatment in fracture patients. Data analysis was conducted with the Review Manager 5.4.1 software.
Results: A total of 16 studies involving 5022 patients obtained from selected databases were examined. As expected, bisphosphate had no significant effect on fracture healing time, but it could significantly increase BMD and prevent osteoporosis. Meanwhile, bisphosphate can inhibit both bone resorption and bone formation markers, resulting in low bone turnover state.
Conclusion: This meta-analysis showed that bisphosphonate have no significant effect on fracture healing time but they do increase the changes in BMD and reduce bone synthesis and resorption markers. Early application of bisphosphonates after injury in the appropriate patient population should be considered.
Osteoporosis is a common orthopedic process that increases the incidence of pathologic fractures. There are 8.9 million osteoporotic fractures per year worldwide (1). Disuse osteopenia of the affected extremity can occur within a few months of acute fractures. Most significant bone density loss is often found in the hips and vertebral bodies. Such bone density loss can result in osteoporosis in patients with pre-existing osteopenia or risk factors. Standard treatment for fractures include reduction, splinting, external fixator, and plate/screw fixation. Anticoagulation and nonsteroidal anti-inflammatory drugs (NSAIDs) are supplemented to prevent thromboembolic events and pain control.
Bisphosphonate (BP) prevent bone mass loss by inhibiting osteoclast resorption. While it is an effective anti-osteoporotic and is widely used in the world, it is limited by its side effects (2, 3) and is thus reserved for patients with pre-existing conditions including osteoporosis, metastatic bone disease, multiple myeloma, Paget’s disease, polyostotic fibrous dysplasia, total joint arthroplasty, early stage avascular necrosis, osteogenesis imperfecta, metastatic hypercalcemia (4). Recent studies have shown that due to its inhibitory effect on osteoclasts (5), BPs can down-regulate bone metabolism (6, 7), which can lead to a low bone turnover state. Medication compliance is also an issue and the noncompliance rate increases with length of use (8) and the rate of compliance decreases with time. A study of primary physicians, anti-osteoporosis treatments is more likely to be overlooked than treatment for cardiovascular diseases (9).
For fracture patients, the use of BPs can significantly reduce the recurrence rate of fractures (10). However, the role of BPs in fragility fracture healing is not well elucidated with study results ranging from improved healing, no effect, and inhibition of healing (4, 11–17). Earlier studies have shown that the incidence of low impact hip fractures and the rate of recurrent fractures is significantly increased after more than 5 years of long-term bisphosphonate treatment. However, some patients in the above studies are not simply osteoporotic patients. For instance, patient may also have Paget’s disease, which could have influenced the clinical outcome. In animal studies, BPs have been shown to cause low bone turnover (18). Other studies have shown that bisphosphonate can promote fracture healing in vivo and in vitro (14, 19), accelerating the formation of trabecular bone and the thickness of cortical bone callus (20). There is a lack of sufficient data and human research on the effect of BPs on fracture healing.
The main purpose of this article is to explore the effect of BPs on fracture healing. The main parameters include bone mineral density (BMD) changes, healing time, and bone metabolism indicators. The goal is to clarify if BPs can be used to promote fracture healing after fractures.
Meta-analysis was performed following Preferred Reporting Items for Meta-Analyses(PRISMA) criteria.
This meta analysis was conducted according to the Preferred Reporting Items for Meta-Analyses statement (21). PubMed was searched in June 2021 for studies published between 1987 (first used in clinical treatment) and June 2021 using the following combination of terms: (((((((((((Bisphosphate[Title]) OR (Alendronate[MeSH Terms])) OR (Zoledronic acid[MeSH Terms])) OR (neridronate[MeSH Terms])) OR (Olpadronic acid[MeSH Terms])) OR (Risedronic Acid[MeSH Terms])) OR (Ibandronic Acid[MeSH Terms])) OR (Clodronic Acid[MeSH Terms])) OR (Pamidronate[MeSH Terms])) OR (tiludronic acid[MeSH Terms])) OR (Etidronic Acid[MeSH Terms])) AND (((fracture healing[MeSH Terms]) OR (bone remodeling[MeSH Terms])) OR (fracture[MeSH Terms])). No language restrictions were applied. Two investigators (Yongquan Gao and Yuan Gu) independently completed the search and assessed the identified titles for relevance. Abstracts were screened for all potentially relevant titles, and full papers were obtained for all abstracts of potential relevance. In addition, for trials with several treatment groups, the eligibility of each individual group was assessed and only those relevant were included. The reference lists of the selected papers were also screened for articles that may have been overlooked in the initial search, and references cited in the identified articles were searched manually.
This meta-analysis followed a detailed, pre-specified protocol that set out the objectives, inclusion criteria for trials, data to be collected, and analyses to be completed.
Studies were considered for inclusion if they met the following criteria: (1) A controlled trial of BPs and placebo in the study; (2) participants were adults with acute fractures and were accepting BP therapy following surgical repair or manual reduction of the fracture; (3) the intervention was the initiation of BPs earlier than 3 months compared with the initiation of placebo at the same time, BPs begun later than 3 months after surgery, or no therapy; (4) trials provided the relevant data.
Studies were considered for exclusion if they met the following criteria: (1) participants previously used BPs or parathyroid hormone, unless patients had undergone a washout period; (2) participants involved in tumor, Paget`s disease, pregnancy, dialysis, organ transplantation, secondary fracture or other diseases that may affect bone healing; (3) the fracture treatment involved inserting prostheses, such as total hip arthroplasty (THA); (4) If multiple studies from the same series were available, the one including the most individuals was used in the analysis.
The following information from the article and the details of group allocation were reviewed: first author, year of publication, age, gender, type of fracture and treatment, type of Bisphosphate, length of BP used, and the body mass index (BMI) and bone mineral density (BMD). All data were thoroughly checked for consistency, plausibility, and integrity of randomization and follow-up. The two responsible trial investigators resolved any queries and verified the final database entries. The primary outcome was the time of fracture healing, BMD changes and bone metabolism biomarkers. Fracture healing is defined as fracture bridging by trabeculae or osseous bone in at least one cortex as seen on anteroposterior radiographs and one as seen on lateral radiographs, The above process requires at least 2 orthopedic or radiologists obtaining same diagnosis under double blindness. Part of the data is obtained by analyzing Figure from the full text, using GetData Graph Digitizer (version2.26.0.20).
The quality of evidence of outcomes was judged according to the Cochrane Scale (22). Cochrane Scale scores ranges from 0 to 7, with higher scores indicating better quality.
Data were analyzed using Review Manager Software (RevMan version 5.4.1; The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark). The SMD in continuous outcomes and risk ratios (RR) in dichotomous variables with 95% CI and P values were calculated to assess effects of study. In meta-analysis, SMD is applied as an aggregate statistics when all trials evaluated the same outcome, but assessed it with many kind of methods (i.e. different rating scales) (23). We used the inverse variance method in continuous variables with random effects model and/or fixed effects model to combine data and generate the overall effect estimate according the degree of heterogeneity. The degree of heterogeneity was assessed by a χ2 test combined with the I2 method (I2 < 25% representing low heterogeneity, and I2 > 75% representing high heterogeneity) (24). High heterogeneity is modeled with random effects, and vice versa with fixed effect models. The analysis was performed with Revman version 5.4.1. P < 0.05 represents statistically significant. Funnel plotting were used to assess publication bias with Revman version 5.4.1.
As of June 2021, a total of 2127 published articles were retrieved, in which 34 were considered potentially meaningful. By reviewing the full text, 18 articles were abandoned for the following reasons: absent of useful outcome data; unclear description; tumors; joint replacement surgery; Metabolic diseases other than osteoporosis; dialysis/transplantation; Paget disease. In the end, 16 articles were included to evaluate the relationship between BPs and fracture healing. The process is outlined in Figure 1.
All the studies were published from 1987 to 2021. All the characteristics of the studies were summarized in Table 1, including basic information of the paper (first author and year of publication), basic information of patients, detailed types of BPs, and fracture type.
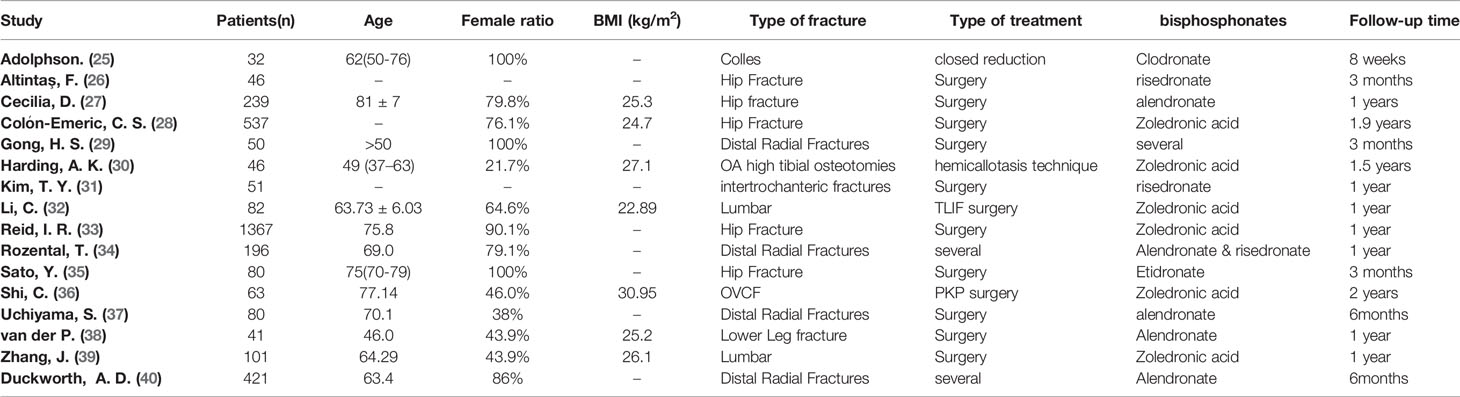
Table 1 Characteristics of patients included studies. “-” means that it cannot be obtained from the full text.
The patient characteristics are summarized in Table 1. This meta-analysis included a total of 5022 patients. Regarding the fracture types, four trials included fractures of the distal radius (25, 29, 34, 37, 40), six trials were hip fracture (26–28, 31, 33, 35), three trial were spinal fractures (32, 36, 39), one trial was lower leg fracture (38), and one trial was OA high tibial osteotomies (30). There was also variability in the type of treatment, surgical versus nonsurgical. There was no significant difference between cases and controls in all the included studies.
In four trials, patients received Alendronate (27, 37, 38, 40), six trials used zoledronic acid (28, 30, 32, 33, 36, 39), two trials used risedronate (26), one trial used Etidronate (35), one trial used Clodronate (25) and two trials used multiple bisphosphates (29, 34). Besides Bisphosphate, in most of study, patients also received other treatment, such as, vitamin D and calcium supplements.
All 16 studies were assessed by the Cochrane Scale (Figure 2). Green meant low risk, red meant high risk, and yellow meant unclear risk. Two studies scored 7 and most of articles scored at least 6. One article was written in Turkish, we used Google Translate (https://translate.google.cn/) to extract data and explore the study design.
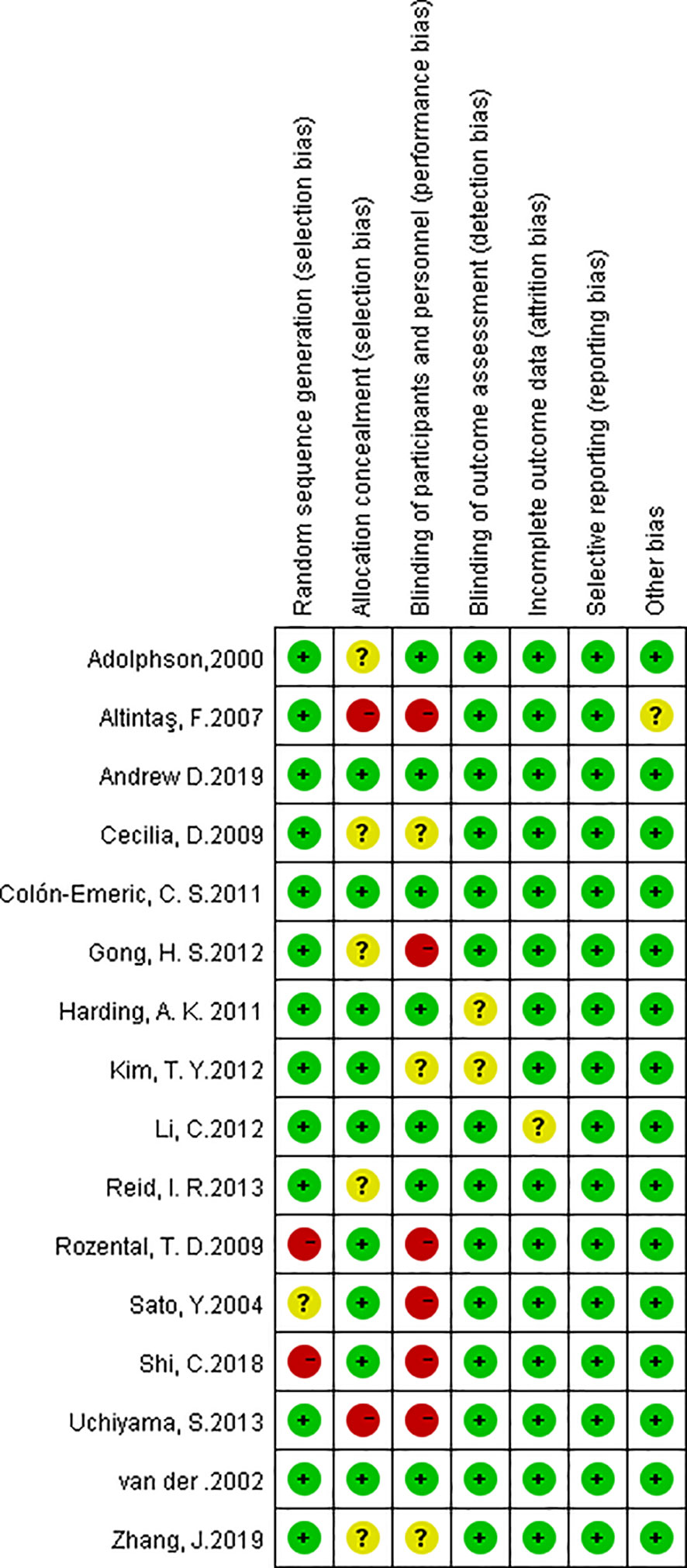
Figure 2 Risk of bias summary. The red with a minus means high risk of bias; the yellow with a question mark means equivocal; the green with a plus means low risk of bias.
All studies used medical imaging to determine the extent and time of fracture healing. Through expanded data analysis, we confirmed that the fracture healing time is not related to the use of BPs (Figure 3). 423 patients from five trials were eligible for the meta-analysis. (SMD 0.17, 95% CI −0.09 to 0.42; I2 = 59%, P=0.21; Radom effects model).
BMD changes reflects changes in bone metabolism which is critical for fracture healing. Unfortunately, the description of BMD and units in the various studies were not uniform and many studies lacked baseline data. In order to normalize the data, the changes in BMD are recalculated and expressed as a percentage of the initial BMD (41). Data from 9 trials (1140 patients) showed that there was a significant increase of BMD in the bisphosphonate group compared with the placebo treatment group (Figure 4). (SMD 2.31, 95% CI 0.38 to 2.39; I2 = 98%, P=0.007; Radom effects model).
In the subsequent subgroup analysis (Figure S2), it has been found that the trials using alendronate had higher degree of variability compared to zoledronic acid and chlorophosphate. Fracture types had no statistically significant effect on fracture healing.
Various types of bone turnover markers were used in different trials. In order to obtain sufficient and accurate data, the markers were summarized in our analysis. P1NP was used in Reid et al. (33) and Li et al. (32). ALP was used in Uchiyama et al. (29) and van der et al. (38). Sato et al. (35) used BGP. NTX was used as the bone resorption markers in all trials. CTX was used in Zhang et al. (39). Subgroup analysis found that different types of markers had no statistically significant effect on the results.
BPs can significantly inhibit bone resorption (SMD -4.86, 95% CI -6.97 to 2.75; I2 = 98%, P<0.00001; Radom effects model) and bone formation markers (SMD -1.34, 95% CI -1.68 to -1.01; I2 = 98%, P<0.00001; Fixed effects model). As expected, BPs causes low bone turnover (Figure 5) which means BP can increase BMD.
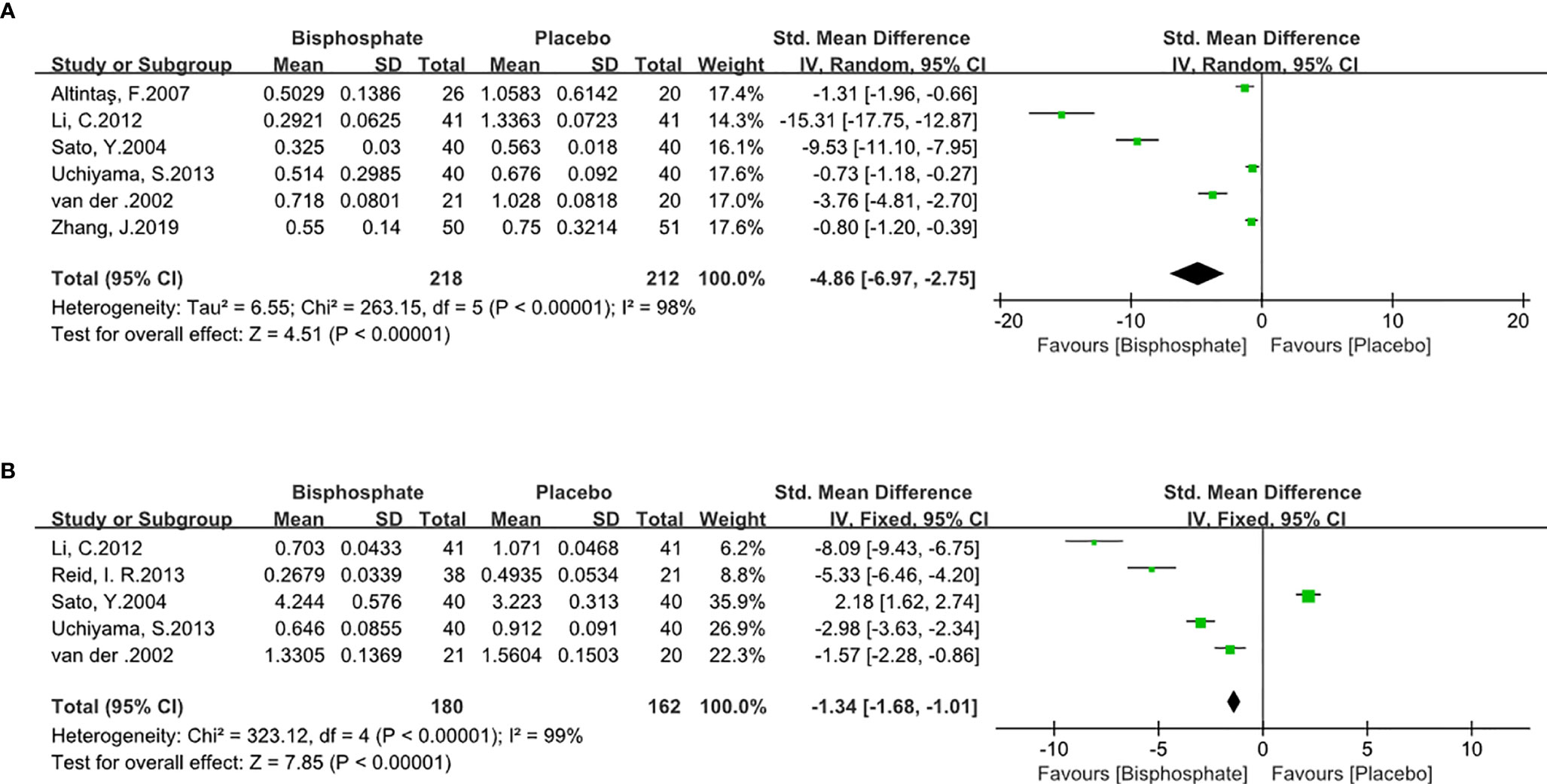
Figure 5 Forest Plot of Bone Turnover Markers (A). Bone resorption biomarker. (B). Bone formation biomarker.
In order to determine the contribution of individual studies, the results were pooled for sensitivity analysis. We removed each study from the analysis and determined pooled SMD. Significant change of I2 (98%→77%) and SMD (1.26→0.84) occurred in BMD change Forest plot with Reid et al. (33) deleted. Results showed BPs increased bone density. In other words, while the sensitivity of the study may have been affected, the results did not change.
Publication bias was assessed by generating and analyzing a funnel plot for the analysis of BMD changes (Figure 6) with Reid et al. (33) deleted, the funnel plot performed more symmetrical (Figure S1), which also means this study may be a deviation in the meta-analysis.
Fracture healing depends on the dynamic balance between bone formation and bone resorption. Any factors that changes the balance can affect the fracture healing time and prognosis of the injury. BPs inhibit osteoclast activities resulting in decreased bone resorption. It is commonly used for osteoporosis and metabolic bone diseases (42–44). The BPs all have two phosphonate groups that share a common carbon atom (P-C-P), which can inhibit the synthesis of ATP or the activity of FPPS (farnesyl pyrophosphate synthase) in osteoclast and induce apoptosis. BPs can also bind to bone mineral and inhibit the dissolution of hydroxyapatite(HAP).
Since BPs inhibit bone resorption (16, 45), it may have negative effects on the bone remodeling, thereby prolonging fracture healing. One study has shown the anti-fracture efficacy of intravenous zoledronic acid in patients with recent low trauma hip fractures (46).
Long-term use of BPs can have serious adverse reactions, including osteonecrosis of the jaw and low-stress fractures (47–49). It is unclear if BPs should be used in the early stage of fracture patients (12, 39, 50, 51). Animal experiments have shown that BPs will not inhibit bone healing in rats (11, 52, 53) and it can have a positive effect on the early healing of metaphyseal fractures in mice (15). Recent study has shown that pamidronic acid has significant effect on bone mineral content and strength recovery in fractured rats (54). Early application of BPs has been shown to significantly reduce pain (55) which is conducive to early rehabilitation exercise and improves quality of life.
A clear positive effect of BPs have been found on fracture healing in animal experiment (14, 15). Our analysis found that post traumatic/postoperative application of BPs has no significant effect on fracture healing time which differs from previous studies (56) (SMD=0.17, 95% CI : −0.09 to 0.42, p=0.21). This result also indicates that BPs have no negative effect on the fracture healing in human patients, and will not lead to delayed union and nonunion. Our meta-analysis showed that compared with placebo, BPs significantly increased the BMD in patients with fractures. In the subgroup analysis, we found that alendronate has a more significant effect on BMD changes compared with zoledronate or chlorophosphite. More large number randomized control trials are needed to further elucidate the effects of BPs on fracture healing time. The use of BPs showed no discernable negative effects on fracture healing.
Significant BMD reduction and low body weight can be observed within 1 year after fracture, which can result in recurrent fractures (2). Early application of BPs can effectively increase bone density which can improve patients’ quality of life and functional recovery. Previous studies have shown that different R substituents of bisphosphonate molecules have significantly influence on binding ability to bone minerals, which also affects its clinical effect. Third-generation BPs, such as, zoledronic has better binding ability to HAP compared to alendronate (57), while zoledronic has a stronger ability to inhibit FPPS, which result to stronger inhibition to osteoclast. In other studies, the N-H-O hydrogen bond formed by the zoledronic acid and FPPS enzyme can inhibit the effect of FPPS more compared with alendronate (58). This is consistent with our analysis results. In research of spine fractures and non-spine fractures, the onset time of zoledronic acid is also shorter than that of alendronate. BPs increases callus thickness and improves VAS and disability scores (13, 20, 55, 59–62). Hence, early application of BPs can effectively increase bone density, which can improve patients` quality of life and functional recovery.
Type I collagen is the main component of bone organic matter. Type I collagen is consistent of NTX and CTX, which have a similar metabolic process. NTX and CTX are often used as bone resorption markers. P1NP, ALP, and BGP are typical osteogenic markers. Although several biomarkers are used in our research, they all have significant changes and have no impact on inference. All of the bone metabolic markers have a significant impact on changes in bone density and bone mass (63–65). The forest plot showed that bisphosphonates have a clear inhibitory effect on the bone resorption markers and bone formation markers, resulting in a low bone turnover state. BPs is, therefore, not recommended after fracture because of the resultant low bone turnover state in these studies. Other studies, however, have shown that BPs do not inhibit fracture healing (12, 17, 66).
It should be clearly stated that anatomic reduction and fixation are still the gold standard treatment for fracture healing (67). BPs, other metabolically modifying drugs, and rehabilitation are adjunct options with beneficial effects in specific situations.
There are limitations to this meta-analysis. In order to obtain exact data, which are not directly available in some manuscripts, we used software to identify the Figure from full text. Through comparison with known data, some data have proportionable certain errors (less than 5%), which may affect the results and conclusions of the analysis. The bone density was normalized which may have introduced a degree heterogeneity in the meta-analysis. The data of the included studies are not uniform. For example, NTX was used as a bone resorption marker in most of the study whereas CTX was used in others (32, 39) due to the lack of NTX data. This may introduce a certain degree of errors and biases. This is also one of the possible reasons why I2 was high in multiple Forest Plots. Lastly, the types of BPs used in the studies were different, which may have different clinical effects.
This meta-analysis showed that bisphosphonate have no significant effect on fracture healing time but they do increase the changes in BMD and reduce bone synthesis and resorption markers. The application of bisphosphate in the early stage after injury is a correct choice for fracture healing.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YqG and YG reviewed the literature and extracted the data. DS, MD, and JN assessed the quality of included studies. YqG wrote the manuscript, and designed the figures and tables. XL and LL provided some key ideas for this manuscript. GH critically revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by National Nature Science Foundation of China (NSCF) (Grant No: 81802207), the Xiangya Famous Doctor Fund of Central South University(Grant 2014-68), and The Hunan province Science Fund for Distinguished Young Scholars (Grant 2018JJ1046).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.688269/full#supplementary-material.
Supplementary Figure 1 | Funnel plot of BMD changes with Reid, I. R. 2013 deleted.
Supplementary Figure 2 | subsequent subgroup analysis of Fracture Healing time.
1. Johnell O, Kanis JA. An Estimate of the Worldwide Prevalence and Disability Associated With Osteoporotic Fractures. Osteoporos Int (2006) 17(12):1726–33. doi: 10.1007/s00198-006-0172-4
2. Ensrud KE, Crandall CJ. Osteoporosis. Ann Intern Med (2017) 167(3):Itc17–itc32. doi: 10.7326/AITC201708010
3. Qaseem A, Forciea MA, McLean RM, Denberg TD. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann Intern Med (2017) 166(11):818–39. doi: 10.7326/M15-1361
4. Cheung WH, Miclau T, Chow SK, Yang FF, Alt V. Fracture Healing in Osteoporotic Bone. Injury (2016) 47(Suppl 2):S21–6. doi: 10.1016/S0020-1383(16)47004-X
5. von Knoch F, Jaquiery C, Kowalsky M, Schaeren S, Alabre C, Martin I, et al. Effects of Bisphosphonates on Proliferation and Osteoblast Differentiation of Human Bone Marrow Stromal Cells. Biomaterials (2005) 26(34):6941–9. doi: 10.1016/j.biomaterials.2005.04.059
6. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CYC. Severely Suppressed Bone Turnover: A Potential Complication of Alendronate Therapy. J Clin Endocrinol Metab (2005) 90(3):1294–301. doi: 10.1210/jc.2004-0952
7. Cao Y, Mori S, Mashiba T, Westmore MS, Ma L, Sato M, et al. Raloxifene, Estrogen, and Alendronate Affect the Processes of Fracture Repair Differently in Ovariectomized Rats. J Bone Mineral Res (2002) 17(12):2237–46. doi: 10.1359/jbmr.2002.17.12.2237
8. Rabenda V, Vanoverloop J, Fabri V, Mertens R, Sumkay F, Vannecke C, et al. Low Incidence of Anti-Osteoporosis Treatment After Hip Fracture. J Bone Joint Surg Am (2008) 90(10):2142–8. doi: 10.2106/JBJS.G.00864
9. Kuiper BW, Graybill S, Tate JM, Kaufman N, Bersabe D. After the Fall: Improving Osteoporosis Treatment Following Hip Fracture. Osteoporos Int (2018) 29(6):1295–301. doi: 10.1007/s00198-018-4416-x
10. Colon-Emeric CS, Caminis J, Suh TT, Pieper CF, Janning C, Magaziner J, et al. The HORIZON Recurrent Fracture Trial: Design of a Clinical Trial in the Prevention of Subsequent Fractures After Low Trauma Hip Fracture Repair. Curr Med Res Opin (2004) 20(6):903–10. doi: 10.1185/030079904125003683
11. Madsen JE, Berg-Larsen T, Kirkeby OJ, Falch JA, Nordsletten L. No Adverse Effects of Clodronate on Fracture Healing in Rats. Acta Orthop Scand (1998) 69(5):532–6. doi: 10.3109/17453679808997793
12. Eriksen EF, Lyles KW, Colón-Emeric CS, Pieper CF, Magaziner JS, Adachi JD, et al. Antifracture Efficacy and Reduction of Mortality in Relation to Timing of the First Dose of Zoledronic Acid After Hip Fracture. J Bone Miner Res (2009) 24(7):1308–13. doi: 10.1359/jbmr.090209
13. Tu CW, Huang KF, Hsu HT, Li HY, Yang SS, Chen YC. Zoledronic Acid Infusion for Lumbar Fusion in Osteoporosis Patients. J Surg Res (2014) 192(1):112–6. doi: 10.1016/j.jss.2014.05.034
14. Menzdorf L, Weuster M, Klüter T, Brüggemann S, Behrendt P, Fitchen-Oestern S, et al. Local Pamidronate Influences Fracture Healing in a Rodent Femur Fracture Model: An Experimental Study. BMC Musculoskelet Disord (2016) 17:255. doi: 10.1186/s12891-016-1113-9
15. Sandberg O, Bernhardsson M, Aspenberg P. Earlier Effect of Alendronate in Mouse Metaphyseal Versus Diaphyseal Bone Healing. J Orthop Res (2017) 35(4):793–9. doi: 10.1002/jor.23316
16. Cui P, Liu H, Sun J, Amizuka N, Sun Q, Li M. Zoledronate Promotes Bone Formation by Blocking Osteocyte-Osteoblast Communication During Bone Defect Healing. Histol Histopathol (2018) 33(1):89–99. doi: 10.14670/HH-11-893
17. Fung E. Early Alendronate can be Safe During Fracture Healing? J Bone Miner Res (2020) 35(1):214. doi: 10.1002/jbmr.3898
18. Hauser M, Siegrist M, Keller I, Hofstetter W. Healing of Fractures in Osteoporotic Bones in Mice Treated With Bisphosphonates - A Transcriptome Analysis. Bone (2018) 112:107–19. doi: 10.1016/j.bone.2018.04.017
19. Heino TJ, Alm JJ, Halkosaari HJ, Välimäki VV. Zoledronic Acid In Vivo Increases In Vitro Proliferation of Rat Mesenchymal Stromal Cells. Acta Orthop (2016) 87(4):412–7. doi: 10.1080/17453674.2016.1188258
20. Gong X, Yu W, Zhao H, Su J, Sheng Q. Skeletal Site-Specific Effects of Zoledronate on In Vivo Bone Remodeling and In Vitro Bmscs Osteogenic Activity. Sci Rep (2017) 7:36129. doi: 10.1038/srep36129
21. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ (2015) 350:g7647. doi: 10.1136/bmj.g7647
22. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
24. Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The Effects of Clinical and Statistical Heterogeneity on the Predictive Values of Results From Meta-Analyses. Clin Microbiol Infect (2014) 20(2):123–9. doi: 10.1111/1469-0691.12494
25. Adolphson P, Abbaszadegan H, Bodén H, Salemyr M, Henriques T. Clodronate Increases Mineralization of Callus After Colles’ Fracture: A Randomized, Double-Blind, Placebo-Controlled, Prospective Trial in 32 Patients. Acta Orthop Scand (2000) 71(2):195–200. doi: 10.1080/000164700317413193
26. Altintaş F, Ozkut AT, Beyzadeoğlu T, Eren A, Güven M. The Effect of Risedronate Treatment on Bone Turnover Markers in Patients With Hip Fracture. Acta Orthop Traumatol Turc (2007) 41(2):132–5.
27. Cecilia D, Jódar E, Fernández C, Resines C, Hawkins F. Effect of Alendronate in Elderly Patients After Low Trauma Hip Fracture Repair. Osteoporos Int (2009) 20(6):903–10. doi: 10.1007/s00198-008-0767-z
28. Colón-Emeric CS, Lyles KW, Su G, Pieper CF, Magaziner JS, Adachi JD, et al. Clinical Risk Factors for Recurrent Fracture After Hip Fracture: A Prospective Study. Calcif Tissue Int (2011) 88(5):425–31. doi: 10.1007/s00223-011-9474-4
29. Gong HS, Song CH, Lee YH, Rhee SH, Lee HJ, Baek GH. Early Initiation of Bisphosphonate Does Not Affect Healing and Outcomes of Volar Plate Fixation of Osteoporotic Distal Radial Fractures. J Bone Joint Surg Am (2012) 94(19):1729–36. doi: 10.2106/JBJS.K.01434
30. Harding AK, WD A, Geijer M, Toksvig-Larsen S, Tägil M. A Single Bisphosphonate Infusion Does Not Accelerate Fracture Healing in High Tibial Osteotomies. Acta Orthop (2011) 82(4):465–70. doi: 10.3109/17453674.2011.594231
31. Kim TY, Ha YC, Kang BJ, Lee YK, Koo KH. Does Early Administration of Bisphosphonate Affect Fracture Healing in Patients With Intertrochanteric Fractures? J Bone Joint Surg Br (2012) 94(7):956–60. doi: 10.1302/0301-620X.94B7.29079
32. Li C, Wang HR, Li XL, Zhou XG, Dong J. The Relation Between Zoledronic Acid Infusion and Interbody Fusion in Patients Undergoing Transforaminal Lumbar Interbody Fusion Surgery. Acta Neurochir (Wien) (2012) 154(4):731–8. doi: 10.1007/s00701-012-1283-7
33. Reid IR, Black DM, Eastell R, Bucci-Rechtweg C, Su G, Hue TF, et al. Reduction in the Risk of Clinical Fractures After a Single Dose of Zoledronic Acid 5 Milligrams. J Clin Endocrinol Metab (2013) 98(2):557–63. doi: 10.1210/jc.2012-2868
34. Rozental TD, Vazquez MA, Chacko AT, Ayogu N, Bouxsein ML. Comparison of Radiographic Fracture Healing in the Distal Radius for Patients on and Off Bisphosphonate Therapy. J Handb Surg Am (2009) 34(4):595–602. doi: 10.1016/j.jhsa.2008.12.011
35. Sato Y, Kanoko T, Yasuda H, Satoh K, Iwamoto J. Beneficial Effect of Etidronate Therapy in Immobilized Hip Fracture Patients. Am J Phys Med Rehabil (2004) 83(4):298–303. doi: 10.1097/01.PHM.0000122877.28631.23
36. Shi C, Zhang M, Cheng AY, Huang ZF. Percutaneous Kyphoplasty Combined With Zoledronic Acid Infusion in the Treatment of Osteoporotic Thoracolumbar Fractures in the Elderly. Clin Interv Aging (2018) 13:853–61. doi: 10.2147/CIA.S146871
37. Uchiyama S, Itsubo T, Nakamura K, Fujinaga Y, Sato N, Imaeda T, et al. Effect of Early Administration of Alendronate After Surgery for Distal Radial Fragility Fracture on Radiological Fracture Healing Time. Bone Joint J (2013) 95-b(11):1544–50. doi: 10.1302/0301-620X.95B11.31652
38. van der Poest Clement E, van Engeland M, Adèr H, Roos JC, Patka P, Lips P. Alendronate in the Prevention of Bone Loss After a Fracture of the Lower Leg. J Bone Miner Res (2002) 17(12):2247–55. doi: 10.1359/jbmr.2002.17.12.2247
39. Zhang J, Zhang T, Xu X, Cai Q, Zhao D. Zoledronic Acid Combined With Percutaneous Kyphoplasty in the Treatment of Osteoporotic Compression Fracture in a Single T12 or L1 Vertebral Body in Postmenopausal Women. Osteoporos Int (2019) 30(7):1475–80. doi: 10.1007/s00198-019-04896-w
40. Duckworth AD, McQueen MM, Tuck CE, Tobias JH, Wilkinson JM, Biant LC, et al. Effect of Alendronic Acid on Fracture Healing: A Multicenter Randomized Placebo-Controlled Trial. J Bone Mineral Res (2019) 34(6):1025–32. doi: 10.1002/jbmr.3679
41. Johnston CB, Dagar M. Osteoporosis in Older Adults. Med Clin North Am (2020) 104(5):873–84. doi: 10.1016/j.mcna.2020.06.004
42. Harris ST, Watts NB, Jackson RD, Genant HK, Wasnich RD, Ross P, et al. Four-Year Study of Intermittent Cyclic Etidronate Treatment of Postmenopausal Osteoporosis: Three Years of Blinded Therapy Followed by One Year of Open Therapy. Am J Med (1993) 95(6):557–67. doi: 10.1016/0002-9343(93)90350-X
43. Storm T, Thamsborg G, Steiniche T, Genant HK, Sørensen OH. Effect of Intermittent Cyclical Etidronate Therapy on Bone Mass and Fracture Rate in Women With Postmenopausal Osteoporosis. N Engl J Med (1990) 322(18):1265–71. doi: 10.1056/NEJM199005033221803
44. Davis S, Martyn-St James M, Sanderson J, Stevens J, Goka E, Rawdin A, et al. A Systematic Review and Economic Evaluation of Bisphosphonates for the Prevention of Fragility Fractures. Health Technol Assess (2016) 20(78):1–406. doi: 10.3310/hta20780
45. Wang M, Wang L, Ye R. Risedronate Reduces Postoperative Bone Resorption After Cementless Total Hip Arthroplasty: A Systematic Review and Meta-Analysis. Int J Surg (2018) 52:189–200. doi: 10.1016/j.ijsu.2018.02.007
46. Lyles KW, Colón-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, et al. Zoledronic Acid and Clinical Fractures and Mortality After Hip Fracture. N Engl J Med (2007) 357(18):1799–809. doi: 10.1056/NEJMoa074941
47. Sánchez A, Blanco R. Osteonecrosis of the Jaw (ONJ) and Atypical Femoral Fracture (AFF) in an Osteoporotic Patient Chronically Treated With Bisphosphonates. Osteoporos Int (2017) 28(3):1145–7. doi: 10.1007/s00198-016-3840-z
48. Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of Hip, Subtrochanteric, and Femoral Shaft Fractures Among Mid and Long Term Users of Alendronate: Nationwide Cohort and Nested Case-Control Study. BMJ (2016) 353:i3365. doi: 10.1136/bmj.i3365
49. Fink HA, MacDonald R, Forte ML, Rosebush CE, Ensrud KE, Schousboe JT, et al. Long-Term Drug Therapy and Drug Discontinuations and Holidays for Osteoporosis Fracture Prevention: A Systematic Review. Ann Intern Med (2019) 171(1):37–50. doi: 10.7326/M19-0533
50. Solomon DH, Hochberg MC, Mogun H, Schneeweiss S. The Relation Between Bisphosphonate Use and Non-Union of Fractures of the Humerus in Older Adults. Osteoporos Int (2009) 20(6):895–901. doi: 10.1007/s00198-008-0759-z
51. Tucci JR. Effect of Inappropriate and Continuous Therapy With Alendronate for Ten Years on Skeletal Integrity - Observations in Two Elderly Patients. J Endocrinol Invest (2008) 31(3):251–4. doi: 10.1007/BF03345598
52. Koivukangas A, Tuukkanen J, Kippo K, Jämsä T, Hannuniemi R, Pasanen I, et al. Long-Term Administration of Clodronate Does Not Prevent Fracture Healing in Rats. Clin Orthop Relat Res (2003) 408):268–78. doi: 10.1097/00003086-200303000-00036
53. Nyman MT, Paavolainen P, Lindholm TS. Clodronate Increases the Calcium Content in Fracture Callus. An Experimental Study in Rats. Arch Orthop Trauma Surg (1993) 112(5):228–31. doi: 10.1007/BF00451880
54. Amanat N, Brown R, Bilston LE, Little DG. A Single Systemic Dose of Pamidronate Improves Bone Mineral Content and Accelerates Restoration of Strength in a Rat Model of Fracture Repair. J Orthop Res (2005) 23(5):1029–34. doi: 10.1016/j.orthres.2005.03.004
55. Liu B, Gan F, Ge Y, Yu H. Clinical Efficacy Analysis of Percutaneous Kyphoplasty Combined With Zoledronic Acid in the Treatment and Prevention of Osteoporotic Vertebral Compression Fractures. J Invest Surg (2018) 31(5):425–30. doi: 10.1080/08941939.2017.1339151
56. Li YT, Cai HF, Zhang ZL. Timing of the Initiation of Bisphosphonates After Surgery for Fracture Healing: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Osteoporos Int (2015) 26(2):431–41. doi: 10.1007/s00198-014-2903-2
57. Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of Action of Bisphosphonates: Similarities and Differences and Their Potential Influence on Clinical Efficacy. Osteoporos Int (2008) 19(6):733–59. doi: 10.1007/s00198-007-0540-8
58. Rondeau JM, Bitsch F, Bourgier E, Geiser M, Hemmig R, Kroemer M, et al. Structural Basis for the Exceptional In Vivo Efficacy of Bisphosphonate Drugs. ChemMedChem (2006) 1(2):267–73. doi: 10.1002/cmdc.200500059
59. Adachi JD, Lyles KW, Colón-Emeric CS, Boonen S, Pieper CF, Mautalen C, et al. Zoledronic Acid Results in Better Health-Related Quality of Life Following Hip Fracture: The HORIZON-Recurrent Fracture Trial. Osteoporos Int (2011) 22(9):2539–49. doi: 10.1007/s00198-010-1514-9
60. Acott PD, Wong JA, Lang BA, Crocker JF. Pamidronate Treatment of Pediatric Fracture Patients on Chronic Steroid Therapy. Pediatr Nephrol (2005) 20(3):368–73. doi: 10.1007/s00467-004-1790-8
61. Laroche M, Cantogrel S, Jamard B, Constantin A, Zabraniecki L, Cantagrel A, et al. Comparison of the Analgesic Efficacy of Pamidronate and Synthetic Human Calcitonin in Osteoporotic Vertebral Fractures: A Double-Blind Controlled Study. Clin Rheumatol (2006) 25(5):683–6. doi: 10.1007/s10067-005-0159-0
62. Liu Z, Li CW, Mao YF, Liu K, Liang BC, Wu LG, et al. Study on Zoledronic Acid Reducing Acute Bone Loss and Fracture Rates in Elderly Postoperative Patients With Intertrochanteric Fractures. Orthop Surg (2019) 11(3):380–5. doi: 10.1111/os.12460
63. Rosen HN, Dresner-Pollak R, Moses AC, Rosenblatt M, Zeind AJ, Clemens JD, et al. Specificity of Urinary Excretion of Cross-Linked N-Telopeptides of Type I Collagen as a Marker of Bone Turnover. Calcif Tissue Int (1994) 54(1):26–9. doi: 10.1007/BF00316285
64. Gertz BJ, Clemens JD, Holland SD, Yuan W, Greenspan S. Application of a New Serum Assay for Type I Collagen Cross-Linked N-Telopeptides: Assessment of Diurnal Changes in Bone Turnover With and Without Alendronate Treatment. Calcif Tissue Int (1998) 63(2):102–6. doi: 10.1007/s002239900497
65. Rosen HN, Moses AC, Garber J, Iloputaife ID, Ross DS, Lee SL, et al. Serum CTX: A New Marker of Bone Resorption That Shows Treatment Effect More Often Than Other Markers Because of Low Coefficient of Variability and Large Changes With Bisphosphonate Therapy. Calcif Tissue Int (2000) 66(2):100–3. doi: 10.1007/PL00005830
66. Chen F, Dai Z, Kang Y, Lv G, Keller ET, Jiang Y. Effects of Zoledronic Acid on Bone Fusion in Osteoporotic Patients After Lumbar Fusion. Osteoporos Int (2016) 27(4):1469–76. doi: 10.1007/s00198-015-3398-1
Keywords: bisphosphonates, fracture healing, bone mass density, bone turnover markers, meta-analysis
Citation: Gao Y, Liu X, Gu Y, Song D, Ding M, Liao L, Wang J, Ni J and He G (2021) The Effect of Bisphosphonates on Fracture Healing Time and Changes in Bone Mass Density: A Meta-Analysis. Front. Endocrinol. 12:688269. doi: 10.3389/fendo.2021.688269
Received: 30 March 2021; Accepted: 03 August 2021;
Published: 30 August 2021.
Edited by:
Jonathan H. Tobias, University of Bristol, United KingdomReviewed by:
Nitin Kapoor, Christian Medical College & Hospital, IndiaCopyright © 2021 Gao, Liu, Gu, Song, Ding, Liao, Wang, Ni and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangxu He, aGVndWFuZ3h1MTk4N0Bjc3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.