- 1Department of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, China
- 2Department of Pharmacy, Mindong Hospital of Ningde City, Fu’an, China
- 3The School of Pharmacy, Fujian Medical University, Fuzhou, China
Purpose: Dipeptidylpeptidase-4 (DPP-4) inhibitors, including linagliptin, alogliptin, saxagliptin, sitagliptin, and vildagliptin, are used for the treatment of type 2 diabetes mellitus (T2DM) patients in China. This study assessed the economic outcomes of different DPP-4 inhibitors in patients with T2DM inadequately controlled with metformin in the Chinese context.
Materials and Methods: In this study, the validated Chinese Outcomes Model for T2DM (COMT) was conducted to project economic outcomes from the perspective of Chinese healthcare service providers. Efficacy and safety, medical expenditure, and utility data were derived from the literature, which were assigned to model variables. The primary outputs of the model included the lifetime costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratio (ICER). One-way and probability sensitivity analysis was conducted to assess the potential uncertainties of parameters.
Results: Of the five competing strategies, alogliptin 25 mg strategy yielded the most significant health outcome, which associated with improvements in discounted QALY of 0.007, 0.014, 0.011, and 0.022 versus linagliptin 5 mg, saxagliptin 5 mg, sitagliptin 100 mg and vildagliptin50 mg, respectively. The sitagliptin 100 mg strategy was the cheapest option. The ICER of alogliptin 25 mg against sitagliptin 100 mg strategy was $6,952 per additional QALY gained, and the rest of the strategies were dominated or extended dominated. The most influential parameters were the cost of DPP-4 inhibitors and their treatment efficacy.
Conclusions: These results suggested that alogliptin was a preferred treatment option compared with other DPP-4 inhibitors for Chinese patients whose T2DM are inadequately controlled on metformin monotherapy.
Introduction
Type 2 diabetes is a chronic disease that leads to significant morbidity and mortality in poorly controlled patients (1). According to the international diabetes federation, 1 in 11 adults has diabetes. This equals 424.9 million people at a global level (2). The loss of productivity due to diabetes and its health consequences imposes an economic burden on patients, healthcare providers, and national economies. The World Health Organization (WHO) estimates that the cost of lost productivity for diabetic patients may exceed five times the direct cost of the disease (3). According to statistics, the economic burden caused by diabetes in 2018 reached 1.8% of global gross domestic product (GDP) and 12% of global health expenditures (4). In addition, more than 80% of the deaths caused by diabetes each year occur in developing countries, so the economic burden of these countries is more serious than that of developed countries (5). China also has a huge disease burden related to diabetes; the financial burden caused by diabetes on the Chinese economy increased from 2.216 billion Chinese yuan in 1993 to 200 billion Chinese yuan in 2007 (6, 7). Based on Chinese Type 2 Diabetes Prevention Guidelines (8), metformin is used as the first-line drug. There is a debate about the best second-line therapy after the failure of metformin monotherapy due to the increasing availability of antidiabetic drugs and the lack of comparative clinical trials of secondary treatment options. Sulfonylureas (SU) are a common second-line treatment because they have a fast onset of hypoglycemic effect (9). However, there are safety-related issues, including the risk of hypoglycemia and weight gain (9). In contrast, newer drug classes [e.g., dipeptidylpeptidase 4 (DPP-4) inhibitors] have shown clinical benefits in combination therapy (10).
By inhibiting the DPP-4 enzyme, which rapidly degrades two major incretin hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide, the DPP-4 inhibitors have been approved as the recent class of therapies for managing T2DM (11, 12). In several clinical trials with T2DM, DPP-4 inhibitors, such as linagliptin, alogliptin, saxagliptin, sitagliptin, and vildagliptin, are found to decrease HbA1c, fasting plasma glucose (FPG) levels, and body weight in a variety of designs involving monotherapies and combination therapies (13). In China, linagliptin, alogliptin, saxagliptin, sitagliptin, and vildagliptin have been approved for treating T2DM. According to the expert consensus on the clinical application of DPP-4 inhibitors in China (14), these five types of DPP-4 inhibitors combined with metformin have little difference in clinical indications. They can all be used in combination with diet and exercise therapy for type 2 diabetes patients who still have poor blood glucose control after metformin monotherapy or are receiving the combined treatment of the two. As a chronic and progressive disease, the financial burden of T2DM over the long run is considerable, so it is necessary to provide a reference basis for health policy makers to use health resources rationally through decision analysis. Previously, there have been economic evaluations of DPP-4 compared to other antidiabetic agents [e.g., sodium-glucose cotransporter-2 (SGLT2) inhibitors, GLP-1 receptor agonists, and SU] (15, 16). However, there have been only a paucity of reports of head-to-head cost-effectiveness evaluations of DPP-4 inhibitors. This study aims to provide cost-effectiveness evidence for the use of different DDP-4 inhibitors to treat adult patients with T2DM uncontrolled on background metformin therapy by using the Chinese Outcomes Model for T2DM (COMT) (17, 18).
Methods
Model Overview
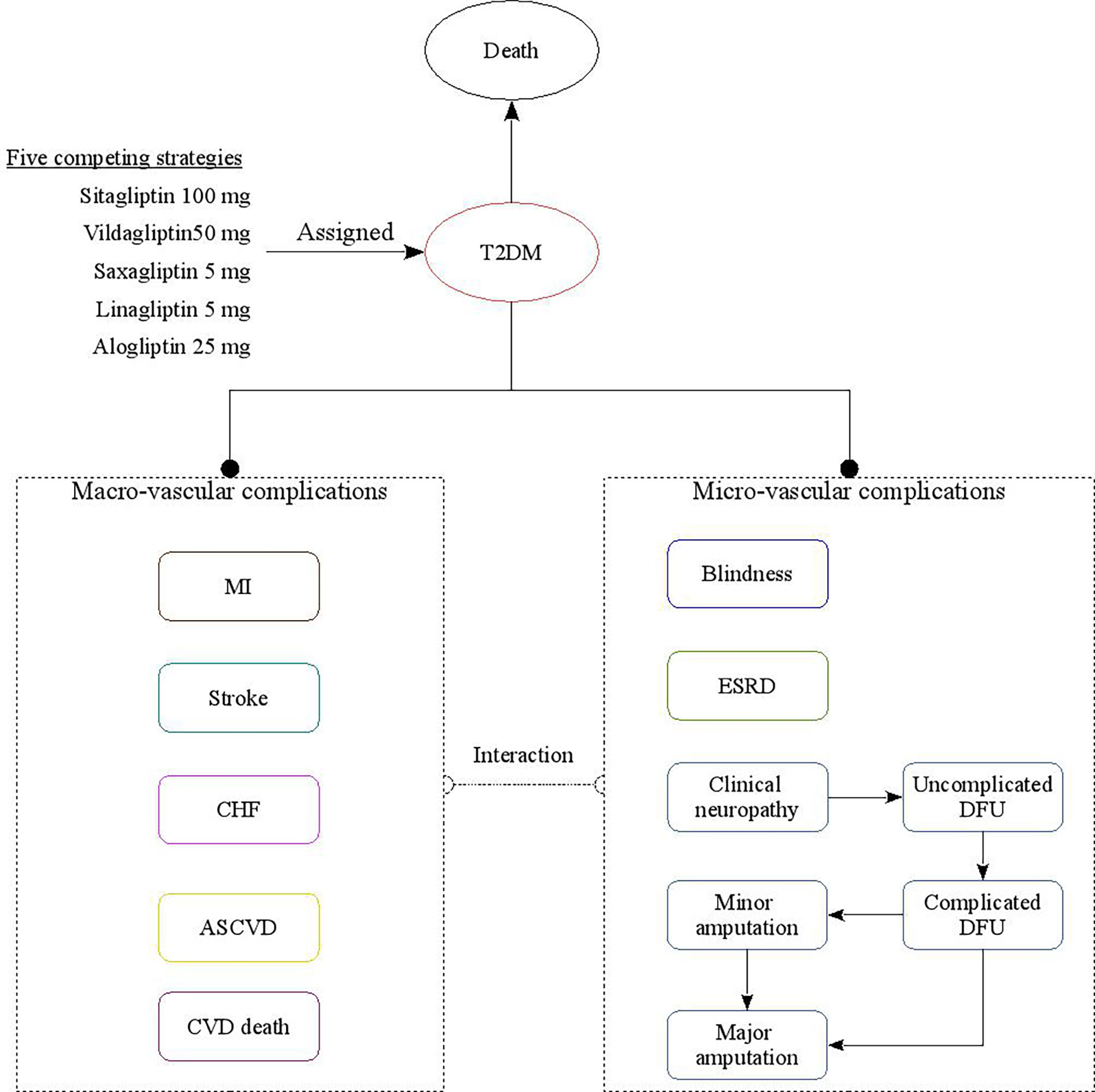
The subjects of this study are Chinese patients with T2DM aged between 50 and 60 who were additively treated with DPP-4 inhibitors in the face of poor metformin monotherapy response. This study evaluated the economics of patients receiving five different strategies for DPP-4 inhibitors (including linagliptin 5 mg, saxagliptin 5 mg, alogliptin 25 mg, sitagliptin 100 mg, and vildagliptin 50 mg strategies). The analysis was conducted using a validated China diabetes policy analysis model called COMT (17–21) (Figure 1); this model can simulate a series of complications during treatment of a T2DM patient, including myocardial infarction (MI), congestive heart failure (CHF), cardiovascular disease (CVD), stroke, blindness, end-stage renal disease (ESRD), clinical neuropathy, foot ulcer, and minor and major amputation. The time horizon of the model is lifetime. All-cause mortality will be adjusted based on the treatment effect and disease status; long-term mortality is adjusted for the risk of all-cause death from diabetes. After entering the COMT model, patients will be assigned to different states according to diabetes complications. Transition probabilities in the model are derived from published literature. During the model simulation, interconnectivity and interaction among submodels of individual complication were permitted to allow the complication risks to be updated by using tracker parameters. The annual disease progression of the hypothetical cohorts with T2DM is determined based on demographic characteristics, disease history, disease-related clinical indicators, drug use history, etc. During the simulation, risk parameters might be adjusted based on the treatment transition, thereby resulting in the likelihood of complication incidence.
To keep with other economic reports associated with T2DM therapies (22), the primary outputs of the model included costs, cumulative probabilities of diabetic complications, life year (LY), quality-adjusted life year (QALY), and incremental cost-effectiveness ratios (ICERs). All costs and health output are discounted at a discount rate of 5%. In accordance with the recommendations of the World Health Organization (23) China’s per capita GDP in 2019 is used as the threshold ($10,276) in this study.
Patient Profile and Treatment Effects
The patient characteristic profiles of receiving DPP-4 inhibitors were assumed to be similar to the SMART trial, which was an open-label Phase IV study comparing saxagliptin with acarbose in 488 Chinese patients with T2DM inadequately controlled with metformin monotherapy (24). When data pertaining to a specific parameter that was used for estimating the complications (25), such as history of smoking and anticoagulation usage, were not available, information from Chinese national cross-sectional were used as a reference (26). In the literature review by searching PubMed and Web of Knowledge, we electronically searched randomized controlled trials (≥24 weeks) including sitagliptin, vildagliptin, saxagliptin, linagliptin, and alogliptin that were published up to March 1, 2020. However, no head-to-head comparisons of sitagliptin, vildagliptin, saxagliptin, linagliptin, and alogliptin were reached. Thus, the clinical data was derived from a Bayesian mixed treatment comparison meta-analyses, which synthesized the treatment efficacy data reported by clinical trials (13). In this mixed treatment comparisons, the weighted absolute HbA1c changes from baseline (95% confidence interval) were −0.99 (−1.17 to −0.82), −1.03 (−1.21 to −0.85), −1.10 (−1.38 to −0.82), −1.06 (−1.22 to −0.91), and −1.02 (−1.18 to −0.86) in linagliptin 5 mg, saxagliptin 5 mg, alogliptin 25 mg, sitagliptin 100 mg, and vildagliptin 50 mg strategies, respectively. The mean absolute changes from baseline in HbA1c levels were employed in the first year of therapy. In the subsequent year, HbA1c was mimicked to rise naturally (nonlinear fashion) due to progressive nature of the disease, according to the HbA1c trajectories analysis. Because the adverse events with gliptin treatment, such as hypoglycemia, were at placebo level (27, 28), the current analysis did not consider the cost and disutilities related to adverse events.
Costs and Utilities
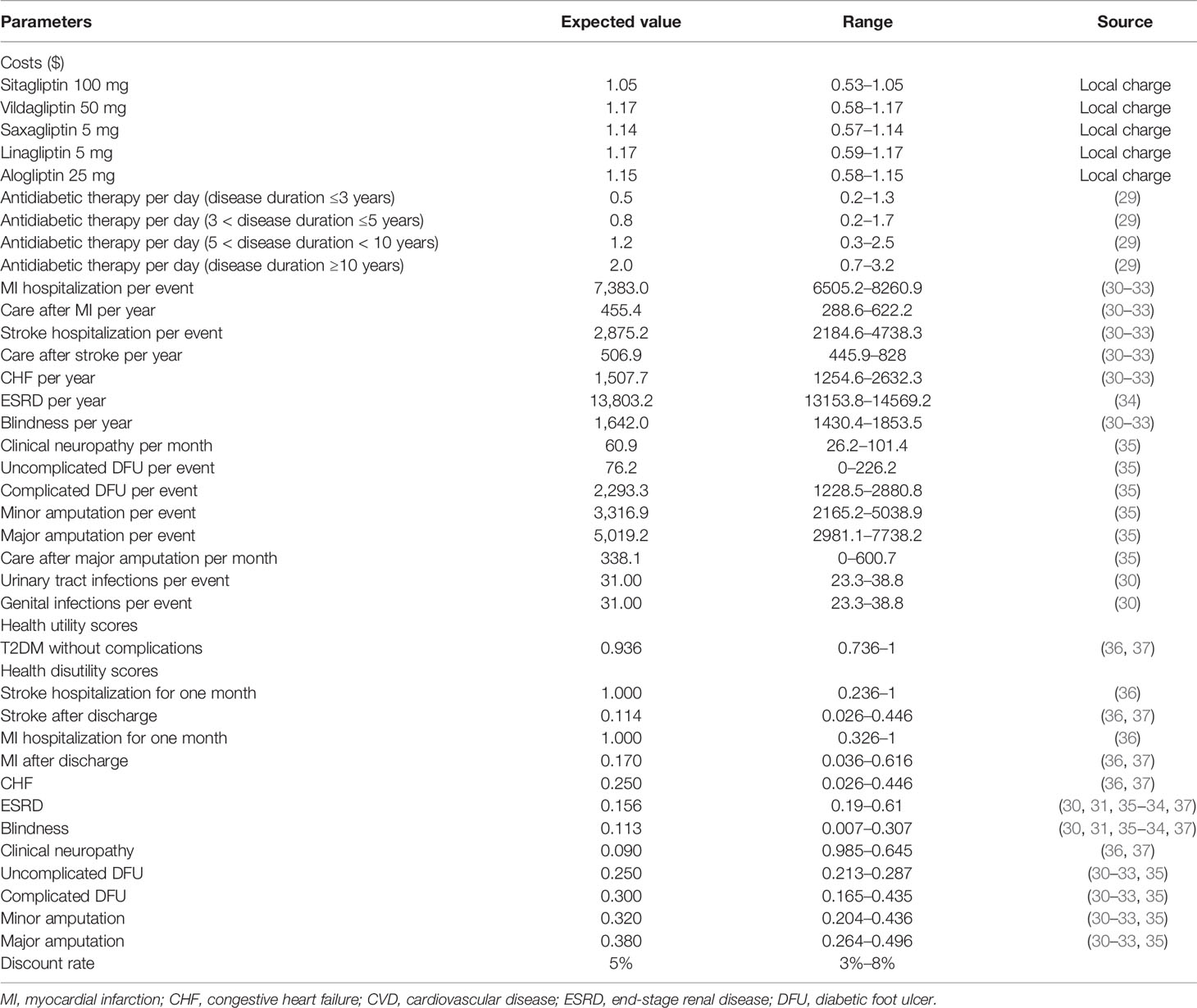
Cost estimation from the perspective of China Health System shows that the study mainly addresses direct medical costs related to T2DM and its complications (Table 1). All cost data are discounted to 2019, shown as 2019 US dollars (1 US $ = 7.0 Chinese Yuan). The prices of sitagliptin, vildagliptin, saxagliptin, linagliptin, and alogliptin were derived from local Chinese database. The treatment regimens were administered as alogliptin 25 mg QD, linagliptin 5 mg QD, saxagliptin 5 mg QD, sitagliptin 100 mg/day, and vildagliptin 50 mg BID, respectively. The cost of antidiabetic treatment and blood glucose test strips related to T2DM was collected from a large screening study based on the national population, which interviewed 1,482 adult patients with T2DM in China (29). The costs of adverse events, including hypoglycemic events were derived from Chinese cost studies (30, 38). Other potential health resource consumption, such as outpatient and hospital expenses related to diabetes complications, are directly extracted from published literature or other local sources (30–35).
The Health Utility Score was collected from a report of 12,583 Chinese patients with T2DM, and a validated Chinese EQ-5D-5L instrument was used to investigate the health status utility score for diabetes mellitus, neuropathy, and cardio-cerebovascular disease without complications (36, 37). Other utility scores that were not reported in this study, such as ESRD and minor and major amputations, were retrieved from published studies (30–35, 37).
Sensitivity Analyses
The impact of parameter uncertainty was explored by one-way sensitivity analysis on each model parameter. Results of the one-way sensitivity analysis were expressed as tornado charts. The ranges of the parameters used in the one-way sensitivity analyses were obtained from the published literature (Table 1). When reported data were not available, a range of ±25% of the base-case value was used. Random values were drawn from the chosen distributions as a second-order Monte-Carlo simulation of 1,000 patients to estimate the mean and 95% confidential intervals (CI) of costs and life-years gained. Beta distribution were attached to the probability, proportions, and utility and disutility scores; triangle distribution to cost estimates; and normal distribution to hazard ratio and patient characteristic profile. A cost-effectiveness acceptability curve (CEAC) is constructed to summarize the uncertainty of cost-effectiveness evaluation under different thresholds of willingness to pay.
Results
Base-Case Outcomes
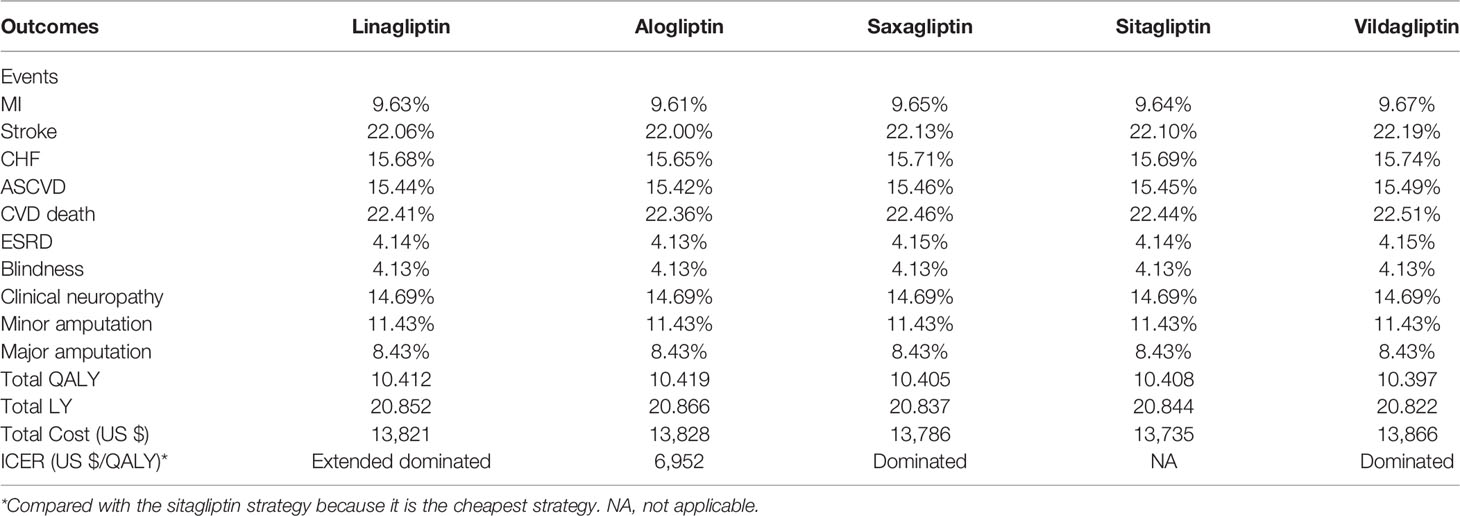
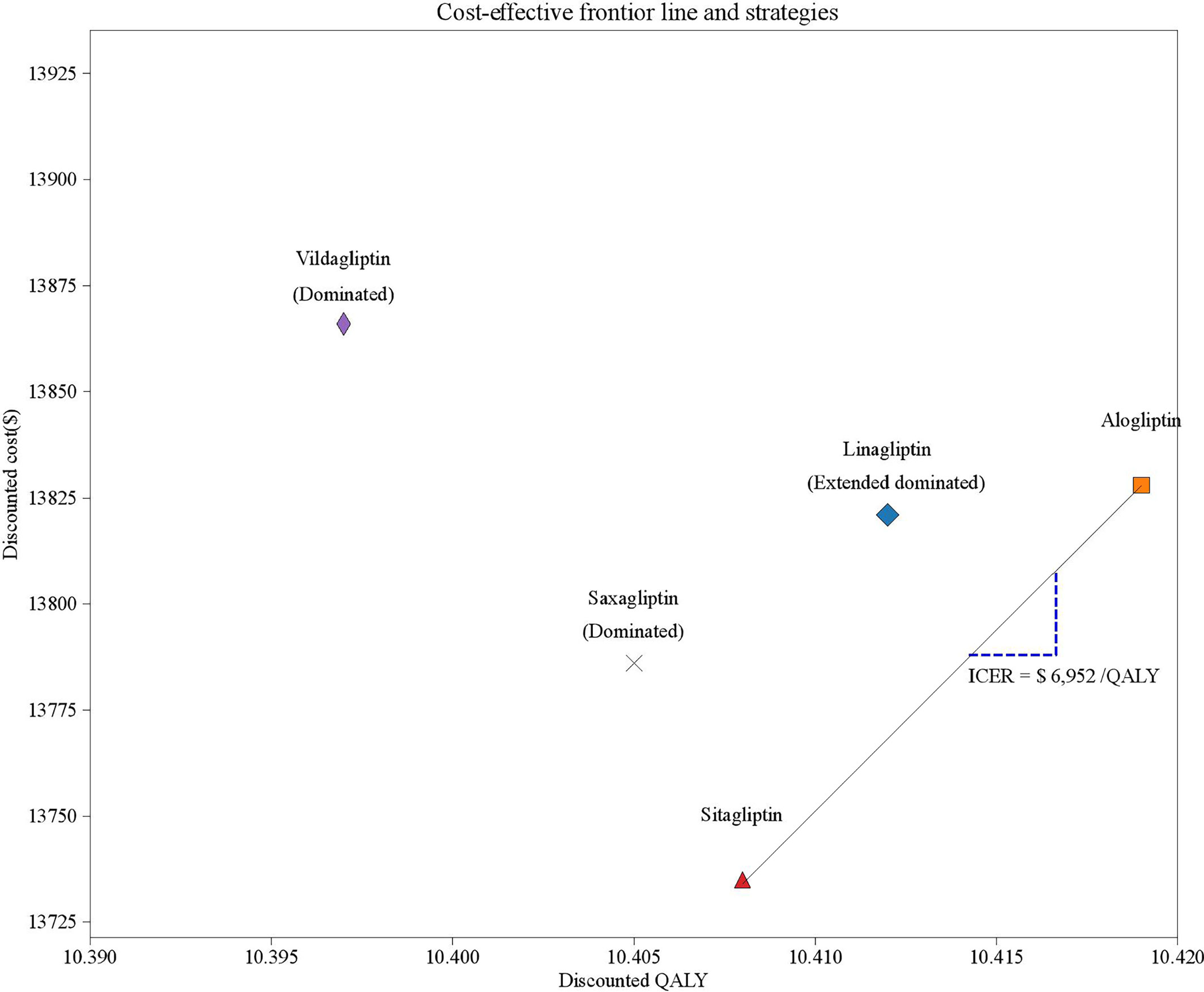
Long-term projections of clinical outcomes indicated that alogliptin 25 mg was associated with improvements in LY of 0.014, 0.029, 0.022, and 0.044 years and improvements in discounted QALY of 0.007, 0.014, 0.011 and 0.022 QALYs versus linagliptin 5 mg, saxagliptin 5 mg, sitagliptin 100 mg, and vildagliptin50 mg, respectively (Table 2). The better health outcomes in the alogliptin 25 mg treatment arm arose from the reduced cumulative diabetes-related complications. In five competing strategies, sitagliptin 100 mg was associated with a cost of $13,735, which was lower than that of the other four strategies. Due to their relatively higher costs and lower health outcomes, both vildagliptin50 mg and saxagliptin 5 mg were dominated by the sitagliptin 100 mg strategies. The linagliptin 5 mg strategy was extended by the alogliptin 25 mg strategy. The cost-effectiveness efficiency frontier included the alogliptin 25 mg and sitagliptin 100 mg strategies (Figure 2).The ICER of alogliptin 25 mg against sitagliptin 100 mg strategy was $6,952 per additional QALY gained. The ICER is less than China’s per capita GDP in 2019, indicating that alogliptin 25 mg is cost-effective.
Sensitivity Outcomes
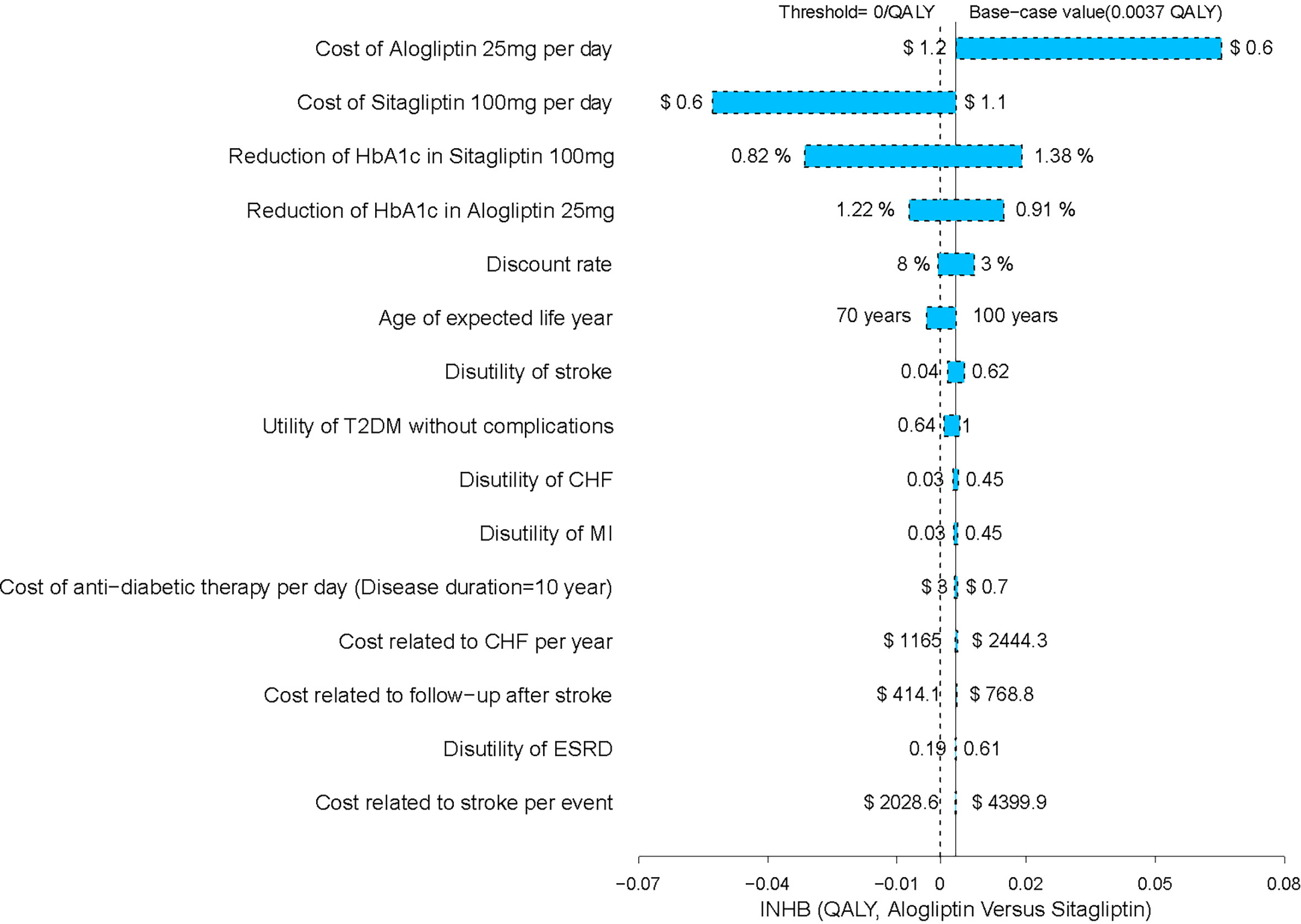
The one-way sensitivity analysis of alogliptin 25 mg against sitagliptin 100 mg strategy revealed that the results of the model were more sensitive to the cost of alogliptin and sitagliptin and the reductions in HbA1c in sitagliptin and alogliptin treatments. However, the variation does not exceed the threshold, that is, the result will not be reversed (Figure 3). We have also performed a one-way sensitivity analysis for the comparison of other drugs, and the results are robust.
As shown in the CEAC (Figure 4), the alogliptin 25 mg strategy produced a nearly half of the probability of cost-effectiveness compared with the other four competing strategies at an acceptable willingness-to-pay threshold of $10,276 (the GDP per capita of China in 2019).
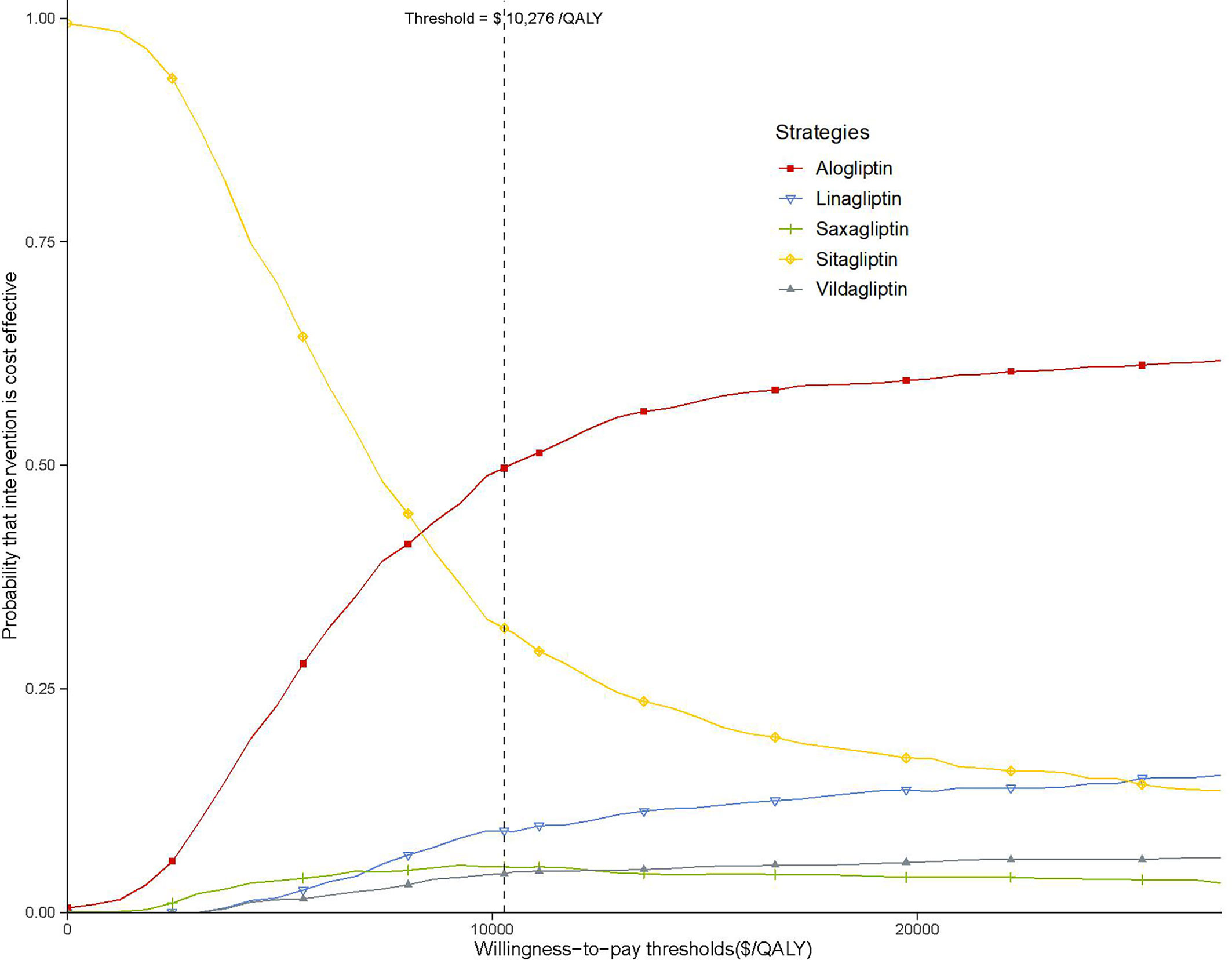
Figure 4 Acceptability curves comparing the cost-effectiveness of five competing strategies. The threshold is US$10,276 (the gross domestic product per capita of China in 2019).
Discussion
Relevant clinical trials have shown that for those patients who have not reached the A1C target of metformin, in the context of metformin, the use of DPP-4 inhibitors as a second-line treatment is clinically effective. Due to the large number of T2DM patients in China and the limited health resources, the need to find the most cost-effective DPP-4 inhibitors has become urgent. The current economic analysis indicates that alogliptin 25 mg was a preferred treatment option as add-on to metformin in comparison with linagliptin 5 mg, saxagliptin 5 mg, sitagliptin 100 mg, and vildagliptin50 mg from the perspective of Chinese healthcare service providers. Compared with the other four DPP-4 inhibitors, alogliptin 25 mg has more health benefits and lower cost. As far as we know, this is the first cost-effectiveness analysis of the results of adding five DPP-4 inhibitors to adult T2DM patients with poor efficacy of metformin. One of the strengths of this study is the application of the COMT model, which shows good model validity for the established effects of interventions such as glucose and other intermediate endpoints in Chinese patients (17–21).
Several economic studies have reported the cost-effectiveness of DPP-4 inhibitors among adults with T2DM (15, 39–41). By using a third-party payer perspective of high- and middle-income countries and randomized clinical trials to measure effectiveness, sitagliptin, saxagliptin, and vildagliptin had an ICER below 25,000 €/QALY, as second-line and as add-ons to metformin, in comparison to sulfonylureas. An improvement in HbA1c, an intermediate biomarker, was indicated as a key driver of these cost-effective outcomes. Other economic evaluations also provided data to compare DPP-4 inhibitors versus insulin, and the results favored the use of DPP-4 inhibitors as second-line therapy (39). These findings generally show to be coherent across analytical models, payer perspective, and nations of analysis. However, there are few economic analyses that assessed the economic outcomes among DPP-4 inhibitors. In the Japanese context, a pharmacoeconomic analysis suggested that vildagliptin provides a superior cost–benefit compared with sitagliptin and alogliptin (42), which was distinguished with ours. The economic endpoint used in Teramachi and colleague’s study was the cost required for a 0.1% decrease in HbA1c for 12 weeks. This approach might not project the long-term outcomes. This might have contributed to the different findings of the present study, which used the lifetime COMT model and adopted the incremental cost per additional QALY gained as the endpoint.
A sensitivity analysis was conducted to identify the major influencing factors, verifying the stability of the model and the reliability of the advantage strategy. The results show that in the comparison between alogliptin and sitagliptin strategy that made up of the cost-effectiveness efficiency frontier, the most sensitive variables were the cost of alogliptin and sitagliptin. When the cost of alogliptin is reduced, the economic outcomes of alogliptin become more favorable. The reduction in the cost of alogliptin enlarges the gaps of incremental net health benefit (INHB) between sitagliptin and alogliptin strategy. However, when the cost of alogliptin is reduced, the INHB of sitagliptin might become lower than zero. The results from the probabilistic sensitivity analyses were similar to those of the base-case results, which produced relatively high probabilities of cost-effectiveness at the predefined Chinese threshold.
The current analysis has several limitations. First, given that there are no clinical trials that directly compare all DPP-4 inhibitors, the treatment effect data used in this study is the result of a Bayesian network meta-analysis (13). Fortunately, probability sensitivity analysis shows that the results of cost-effectiveness analysis are robust, and the uncertainty of each parameter will not have a significant impact on the results. Second, our results may only be applicable to T2DM patients in China but not to T2DM patients in other countries. All cost parameters are set for China and may be different from other countries. Third, due to the absence of available long-term health outcomes data of DPP-4 inhibitors as an add-on to metformin on the progression of diabetes-related complications, long-term comparisons may be uncertain. Finally, our decision analysis model is a simplification of actual disease results. The treatment strategy and clinical practice are based on Chinese guidelines and the recommendations of clinical metabolism experts; individual treatment decisions are not reflected in the model.
Conclusion
This economic analysis found that alogliptin 25 mg strategy is likely to be more cost-effective in the Chinese healthcare system, as an alternative compared with linagliptin 5 mg, saxagliptin 5 mg, sitagliptin 100 mg, and vildagliptin 50 mg strategies for T2DM patients treated with DPP-4 inhibitors after failure of metformin treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material.. Further inquiries can be directed to the corresponding authors.
Ethics Statement
This study was based on literature review and modeling techniques; this study did not require approval by an institutional research ethics board.
Author Contributions
WL and ZC contributed to the conception and design of the study; collecting data, analyzing and interpreting data, and drafting manuscripts. TC contributed to collecting data, analyzing and interpreting data. ML contributed to analyzing data and providing recommendations. BZ and NL contributed to the conception and design of the study. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from Joint Funds for the innovation of science and Technology, Fujian province (grant number 2017Y9098), Science and Technology Department of Fujian Province (grant number 2020R0052), the National Natural Science Foundation of China (number 71373160), and Shanghai Municipal Health Commission (Evidence-based Public Health and Health Economics, number 15GWZK0901). The funding agency had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bhutani J, Bhutani S. Worldwide Burden of Diabetes. Indian J Endocrinol Metab (2014) 18(6):868–70. doi: 10.4103/2230-8210.141388
2. IDF. International Diabetes Federation Diabetes Atlas-8th Edition. (2018). Available at: http://www.diabetesatlas.org/.
3. World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation. (2006).
4. Zhang P, Gregg E. Global Economic Burden of Diabetes and Its Implications. Lancet Diabetes Endocrinol (2017) 5(6):404–5. doi: 10.1016/S2213-8587(17)30100-6
5. Khuwaja AK, Khowaja LA, Cosgrove P. The Economic Costs of Diabetes in Developing Countries: Some Concerns and Recommendations. Diabetologia (2010) 53(2):389–90. doi: 10.1007/s00125-009-1581-7
6. Wang H, Lin X, Zhang Z, Wang Q, Chen J-M, Liu J, et al. The Economic Burden of Inpatients With Type 2 Diabetes: A Case Study in a Chinese Hospital. Asia Pac J Public Health (2015) 27:49S–54S. doi: 10.1177/1010539515572220
7. Chan JCN, Zhang Y, Ning G. Diabetes in China: A Societal Solution for a Personal Challenge. Lancet Diabetes Endocrinol (2014) 2:969–79. doi: 10.1016/S2213-8587(14)70144-5
8. Chinese Diabetes Society. 2013 China Guideline for Prevention and Treatment of Type 2 Diabetes. Chin J Endocrinol Metab (2014) 30:893–942.
9. Göke B, Gallwitz B, Eriksson J, Hellqvist A, Gause-Nilsson I, D1680C00001 Investigators. Saxagliptin Is Non-Inferior to Glipizide in Patients With Type 2 Diabetes Mellitus Inadequately Controlled on Metformin Alone: A 52-Week Randomised Controlled Trial. Int J Clin Pract (2010) 64(12):1619–31. doi: 10.1111/j.1742-1241.2010.02510.x
10. Eriksson SE, Olsson JE. Survival and Recurrent Strokes in Patients With Different Subtypes of Stroke: A Fourteen-Year Follow-Up Study. Cerebrovasc Dis (2001) 12(3):171–80. doi: 10.1159/000047700
11. Dai D, Mao Y, Jin H, Zhang W. Efficacy and Hypoglycemic Risk of Sitagliptin in Obese/Overweight Patients With Type 2 Diabetes Compared With GLP-1 Receptor Agonists: A Meta-Analysis. Med (Baltimore) (2019) 98:e17081. doi: 10.1097/MD.0000000000017081
12. Mega C, Teixeira-de-Lemos E, Fernandes R, Reis F. Renoprotective Effects of the Dipeptidyl Peptidase-4 Inhibitor Sitagliptin: A Review in Type 2 Diabetes. J Diabetes Res (2017) 2017:5164292. doi: 10.1155/2017/5164292
13. Craddy P, Palin H-J, Johnson KI. Comparative Effectiveness of Dipeptidylpeptidase-4 Inhibitors in Type 2 Diabetes: A Systematic Review and Mixed Treatment Comparison. Diabetes Ther (2014) 5:1–41. doi: 10.1007/s13300-014-0061-3
14. Chinese Association of Endocrinology and Metabolism. Expert Consensus on the Clinical Application of DPP-4 Inhibitors. Chin J Endocrinol Metab (2018) 34(11):899–903.
15. Hong D, Si L, Jiang M, Shao H, Ming W-K, Zhao Y, et al. Cost Effectiveness of Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors, Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists, and Dipeptidyl Peptidase-4 (DPP-4) Inhibitors: A Systematic Review. Pharmacoeconomics (2019) 37:777–818. doi: 10.1007/s40273-019-00774-9
16. Kwon CS, Seoane-Vazquez E, Rodriguez-Monguio R. Cost-Effectiveness Analysis of Metformin+Dipeptidyl Peptidase-4 Inhibitors Compared to Metformin+Sulfonylureas for Treatment of Type 2 Diabetes. BMC Health Serv Res (2018) 18(1):78. doi: 10.1186/s12913-018-2860-0
17. Wu B, Ma J, Zhang S, Zhou L, Wu H. Development and Validation of a Health Policy Model of Type 2 Diabetes in Chinese Setting. J Comp Eff Res (2018) 7:749–63. doi: 10.2217/cer-2018-0001
18. Li T, Wan X, Ma J, Wu B. Cost-Effectiveness of Primary Prevention With Statin Treatment for Chinese Patients With Type 2 Diabetes. Adv Ther (2018) 35:2214–23. doi: 10.1007/s12325-018-0823-9
19. Nian H, Wan X, Ma J, Jie F, Wu B. Economic Evaluation of Dapagliflozin Versus Metformin in Chinese Patients Whose Diabetes is Inadequately Controlled With Diet and Exercise. Cost Eff Resour Alloc (2020) 18:12. doi: 10.1186/s12962-020-00208-w
20. Hou X, Wan X, Wu B. Cost-Effectiveness of Canagliflozin Versus Dapagliflozin Added to Metformin in Patients With Type 2 Diabetes in China. Front Pharmacol (2019) 10:480. doi: 10.3389/fphar.2019.00480
21. Cheng H, Wan X, Ma J, Wu B. Cost-Effectiveness of Insulin Degludec Versus Insulin Glargine in Insulin-Naive Chinese Patients With Type 2 Diabetes. Clin Ther (2019) 41:445–55. doi: 10.1016/j.clinthera.2019.01.003
22. Charokopou M, Sabater FJ, Townsend R, Roudaut M, McEwan P, Verheggen BG. Methods Applied in Cost-Effectiveness Models for Treatment Strategies in Type 2 Diabetes Mellitus and Their Use in Health Technology Assessments: A Systematic Review of the Literature From 2008 to 2013. Curr Med Res Opin (2016) 32:207–18. doi: 10.1185/03007995.2015.1102722
23. Wang X, Wang Z-F, Xie Y-M, Zhang W, Liao X, Chang Y-P, et al. Guideline for Postmarketing Chinese Medicine Pharmacoeconomic Evaluation. Chin J Integr Med (2015) 21:473–80. doi: 10.1007/s11655-014-1749-y
24. Du J, Liang L, Fang H, Xu F, Li W, Shen L, et al. Efficacy and Safety of Saxagliptin Compared With Acarbose in Chinese Patients With Type 2 Diabetes Mellitus Uncontrolled on Metformin Monotherapy: Results of a Phase IV Open-Label Randomized Controlled Study (the SMART Study). Diabetes Obes Metab (2017) 19:1513–20. doi: 10.1111/dom.12942
25. Basu S, Sussman JB, Berkowitz SA, Hayward RA, Yudkin JS. Development and Validation of Risk Equations for Complications of Type 2 Diabetes (Recode) Using Individual Participant Data From Randomised Trials. Lancet Diabetes Endocrinol (2017) 5:788–98. doi: 10.1016/S2213-8587(17)30221-8
26. Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of Diabetes Among Men and Women in China. N Engl J Med (2010) 362:1090–101. doi: 10.1056/NEJMoa0908292
27. Gooßen K, Gräber S. Longer Term Safety of Dipeptidyl Peptidase-4 Inhibitors in Patients With Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis. Diabetes Obes Metab (2012) 14:1061–72. doi: 10.1111/j.1463-1326.2012.01610.x
28. Wu D, Li L, Liu C. Efficacy and Safety of Dipeptidyl Peptidase-4 Inhibitors and Metformin as Initial Combination Therapy and as Monotherapy in Patients With Type 2 Diabetes Mellitus: A Meta-Analysis. Diabetes Obes Metab (2014) 16:30–7. doi: 10.1111/dom.12174
29. Yang W, Zhao W, Xiao J, Li R, Zhang P, Kissimova-Skarbek K, et al. Medical Care and Payment for Diabetes in China: Enormous Threat and Great Opportunity. PloS One (2012) 7:e39513. doi: 10.1371/journal.pone.0039513
30. Shao H, Zhai S, Zou D, Mir MU, Zawadzki NK, Shi Q, et al. Cost-Effectiveness Analysis of Dapagliflozin Versus Glimepiride as Monotherapy in a Chinese Population With Type 2 Diabetes Mellitus. Curr Med Res Opin (2017) 33:359–69. doi: 10.1080/03007995.2016.1257978
31. Li T, Liu M, Ben H, Xu Z, Zhong H, Wu B. Clopidogrel Versus Aspirin in Patients With Recent Ischemic Stroke and Established Peripheral Artery Disease: An Economic Evaluation in a Chinese Setting. Clin Drug Investig (2015) 35:365–74. doi: 10.1007/s40261-015-0290-x
32. Wang H, Lin X, Zhang Z, Wang Q, Chen J-M, Liu J, et al. The Economic Burden of Inpatients With Type 2 Diabetes: A Case Study in a Chinese Hospital. Asia Pac J Public Health (2015) 27:49S–54S. doi: 10.1177/1010539515572220
33. Gu S, Mu Y, Zhai S, Zeng Y, Zhen X, Dong H. Cost-Effectiveness of Dapagliflozin Versus Acarbose as a Monotherapy in Type 2 Diabetes in China. PloS One (2016) 11:e0165629. doi: 10.1371/journal.pone.0165629
34. Wu B, Zhang S, Lin H, Mou S. Prevention of Renal Failure in Chinese Patients With Newly Diagnosed Type 2 Diabetes: A Cost-Effectiveness Analysis. J Diabetes Investig (2018) 9:152–61. doi: 10.1111/jdi.12653
35. Wu B, Wan X, Ma J. Cost-Effectiveness of Prevention and Management of Diabetic Foot Ulcer and Amputation in a Health Resource-Limited Setting. J Diabetes (2018) 10:320–7. doi: 10.1111/1753-0407.12612
36. Pan C-W, Sun H-P, Zhou H-J, Ma Q, Xu Y, Luo N, et al. Valuing Health-Related Quality of Life in Type 2 Diabetes Patients in China. Med Decis Making (2016) 36:234–41. doi: 10.1177/0272989X15606903
37. Li C, Zhou H, Wang P. Health Utility of Type 2 Diabetes Patients Using Basal Insulin in China: Results From the BEYOND II Study. Acta Diabetol (2021) 58:329–39. doi: 10.1007/s00592-020-01618-1
38. Ya-ming Z WUJ, Incidence XIEK. And Cost of Hypoglycemia Episode in Patients With Type 2 Diabetes Mellitus (T2DM). Chin Rural Health Service Administration (2012) 32:1195–8.
39. Geng J, Yu H, Mao Y, Zhang P, Chen Y. Cost Effectiveness of Dipeptidyl Peptidase-4 Inhibitors for Type 2 Diabetes. Pharmacoeconomics (2015) 33:581–97. doi: 10.1007/s40273-015-0266-y
40. Baptista A, Teixeira I, Romano S, Carneiro AV, Perelman J. The Place of DPP-4 Inhibitors in the Treatment Algorithm of Diabetes Type 2: A Systematic Review of Cost-Effectiveness Studies. Eur J Health Econ (2017) 18:937–65. doi: 10.1007/s10198-016-0837-7
41. Kwon CS, Seoane-Vazquez E, Rodriguez-Monguio R. Cost-Effectiveness Analysis of Metformin+Dipeptidyl Peptidase-4 Inhibitors Compared to Metformin+Sulfonylureas for Treatment of Type 2 Diabetes. BMC Health Serv Res (2018) 18:78. doi: 10.1186/s12913-018-2860-0
Keywords: dipeptidylpeptidase-4 inhibitors, cost-effectiveness, type 2 diabetes mellitus, incremental cost-effectiveness ratio, quality-adjusted life-year
Citation: Lin W-Q, Cai Z-j, Chen T, Liu M-B, Li N and Zheng B (2021) Cost-Effectiveness of Dipeptidylpeptidase-4 Inhibitors Added to Metformin in Patients With Type 2 Diabetes in China. Front. Endocrinol. 12:684960. doi: 10.3389/fendo.2021.684960
Received: 24 March 2021; Accepted: 23 July 2021;
Published: 13 August 2021.
Edited by:
Bin Wu, Shanghai JiaoTong University, ChinaCopyright © 2021 Lin, Cai, Chen, Liu, Li and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Li, Zmp4aGxpbmExOTgzQGZqbXUuZWR1LmNu; Bin Zheng, emhlbmdiNUBmam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Wen-Qiang Lin1†
Wen-Qiang Lin1† Mao-Bai Liu
Mao-Bai Liu Bin Zheng
Bin Zheng