- 1Endocrinology Unit, Department of Internal Medicine and Medical Specialties, School of Medical and Pharmaceutical Sciences, University of Genova, Genova, Italy
- 2Endocrinology Unit, IRCCS Policlinico San Martino, Genova, Italy
Growth hormone (GH), once the age of linear growth is completed, continues to play a fundamental role for the human body. In adulthood, GH contributes to regulate muscle, cardiovascular and bone metabolism. The same happens in old age, although there is less data on the effect of GH in the elderly. Regardless the age of onset, a reduced quality of life (QoL), an increased cardiovascular risk and an accelerated age-related decline in physical strength have been demonstrated in the elderly with GH deficiency (EGHD). In adults with GH deficiency (AGHD), recent studies suggest a role of GH replacement therapy (GHrt) in improving lean/fat mass ratio, blood pressure, lipid profile, bone metabolism and QoL. Despite these recent studies, there is still a lack of randomized controlled trials proving these positive effects in EGHD. Moreover, the lack of a long-term positive outcome on mortality, and the cost of GHrt could often impact on treatment decision-making and lead to postpone or avoid the prescription. The aim of this mini-review is to summarize the available data on GHrt in EGHD, in order to highlight its weaknesses and strengths and to provide directions to clinicians that will help in the management of this specific set of patients.
Introduction
The role of growth hormone (GH), which performs its most important functions through its peripheral mediator, the insulin-like growth factor (IGF-1), is certainly of primary importance in growth. In adult life, the pituitary production of GH physiologically decreases and its circulating levels are progressively reduced; however, the integrity of the GH/IGF-1 axis continues to ensure the maintenance of homeostasis of many organs and systems (1). Adult GH deficiency (AGHD) is a specific condition, diagnosed when GH levels in adults are pathologically reduced (2). It could be the continuation of a childhood-onset GH deficiency or begin in adulthood. AGHD commonly results from pituitary tumours, from the treatments of these disorders or traumatic brain injury (3–5). Apart from the physiological age-related decrease in GH and IGF-1 during life span, elderly patients may suffer from GHD (EGHD) (6). To date, several studies have shown that AGHD patients present physical deficiencies such as an increased cardiovascular risk and bone fragility, unfavourable fat/lean mass ratio, reduced muscle strength (2, 7–9), as well as psychological deficiencies, such as impaired quality of life (QoL) and social alienation (10, 11). It has been proved that GHrt results in stable improvement in these alterations (9, 12–14) and that it is safe in both the short and long term (3, 15, 16). The majority of scientific societies do not share a specific opinion on the identification of EGHD cases for which treatment is required, as there are no precise criteria for deciding whether to start therapy or when and if a previously initiated GHrt should be discontinued (17) once old age is reached (6, 16–18).
The aim of this review is to critically analyse the available peer reviewed papers on EGHD and the disease management in the elderly population. The definition of elderly population has undoubtedly changed over the years. Nonetheless, the majority of the analysed studies identify over-65 patients as elderly.
Diagnosis of Ghd in the Elderly: When To Suspect it and How To Carry it Out
The diagnosis of GHD in adults and in the elderly must be achieved through the use of standard stimulating tests, except for those patients in whom GHD arises from a non-modifiable structural brain defect, already producing partial hypopituitarism (coexistence of minimum 3 pituitary axes deficit) and low serum IGF-1 (< -2.0 SDS). The need to rely on a provocative test is based on the evidence that the simple measurement of the IGF-1 levels do not distinguish between normal and GHD subjects; in fact, a low IGF-1 is a reliable diagnostic indicator of GHD in the presence of hypopituitarism, but a normal IGF-1 does not rule out GHD (17–19). Current guidelines recommend to firstly determine the probability of an impaired pituitary function and to use further diagnostic investigation in those patients with other pituitary deficient axes especially if clinical signs such as dyslipidaemia, central obesity and loss of muscle mass are present. One or two positive stimulus tests are required to formulate the diagnosis of GHD, depending on the pre-test diagnostic suspicion (16, 18). It is recommended that the decision to carry out the diagnostic protocol for EGHD be corroborated by a strong suspicion to avoid false positive results, given the higher incidence of side effects of the GHrt in old age (11). At the same time, the symptoms and signs of GHD are often non-specific (asthenia, fatigue, reduced muscle strength, increased visceral fat, dyslipidaemia, osteoporosis). Therefore, especially in the elderly, formulating a clinical suspicion can be difficult and the diagnosis could be misrecognized. We can infer that establishing an accurate diagnosis of GHD in the elderly is challenging and even more so if considering the variability in response and interpretation of stimulus tests available (19, 20). In fact, these tests lack age-adjusted cut-offs, despite the well-known physiological decrease of GH and IGF-1 with age (21, 22).
The latest guidelines do not provide suggestions on the most appropriate test to diagnose EGHD and no dedicated studies have been performed to define it. Moreover, two of the most widely used tests for the diagnosis of GHD - the insulin tolerance test (ITT) and the glucagon stimulation test (GST) - are generally avoided in the elderly, due to their potential detrimental effect on patients with multiple comorbidities (23, 24). The GHrh plus arginine test seems to have the best accuracy/safety ratio in the elderly. Indeed, the side effects of this test are negligible, with the only limitation of unequal availability. The GH cut-off point after GHrh plus arginine test to determine AGHD is different, depending on the country and the effect of BMI is not always considered (25). In Italy it is established at 9 µg/l in the normal-weight and at 4.2 µg/l in obese people (BMI > 30 kg/m2) (26). In the United States, where GHrh was withdrawn from the market in 2008, the better test for EGHD diagnosis seems to be Macimorelin, which has excellent tolerability and minimal side effects (27), despite several pharmacological interferences (28).
Recombinant Gh Replacement Therapy (Ghrt) In The Elderly: When, How And Why
The effects of GHrt in AGHD have been widely studied and an improvement in most of the metabolic and psychological abnormalities associated with this condition has been recorded (10). Recent studies have suggested that most beneficial effects of GHrt basically last over the long term (29–32). In EGHD, as well as in AGHD, GHrt should be individually tailored and it is recommended that therapy is started at low doses and up titrated according to the clinical response, side effects, and IGF-1 levels. Periodic monitoring of both benefits and adverse events must be ensured in order to appropriately titrate the dose of therapy. Side effects consist primarily on fluid retention and increase in insulin resistance, typically seen at the beginning of therapy and/or after the increase of the dose. Adverse events are more common in the elderly and, generally, disappear with dose reduction or end of therapy (18, 28, 32).
As in AGHD, the goals of treatment in EGHD are an adequate clinical response, the achievement of IGF-1 levels within the normal range for age and the minimization of side effects (25, 33–35). Based on our clinical experience with EGHD, the treatment goal should be to maintain IGF-1 between -1 and +1 SD, in accordance with the findings of a study by Van Bunderen et al., specifically aimed at comparing different target IGF-1 therapeutics (36). However, clinical practice is not uniform (25, 37, 38) and the therapeutic goal is not univocal: for example, American guidelines suggest a wider range (IGF-1 between -2 and + 2 SD) (16).
In EGHD, initial doses of GHrt of 0.1 mg/day are recommended (34).
Toogood et al. conducted a dose-finding study to identify the minimum effective dose in EGHD, concluding that the majority of patients maintains an IGF-1 adequate level on a dose of 0.33 mg/day (39). In our clinical practice an average dose of 0.2 mg/day is generally sufficient to maintain IGF-1 between the normal range. Standard follow-up interval in treated EGHD is initially 1 or 2 months; the up-titration of GHrt dose is carried out with increments of 0.1 to 0.2 mg/day, based on the clinical response, IGF-1 levels, occurrence of side effects and individual considerations. In AGHD, once the maintenance dose is achieved, follow-up can be deferred to approximately 6 to 12 months. In EGHD, shorter follow-up and smaller dose increments are recommended, especially for those patients with other comorbidities such as diabetes mellitus (40). The parameters to be evaluated during treatment are circulating IGF-1, fasting glucose, glycosylated haemoglobin levels, lipid profile, BMI, waist circumference and waist-to-hip ratio.
It is known that GHrt influence thyroid, glucocorticoid and sex hormone requirements; hence, these hormones should be closely monitored during follow-up (16, 34).
Contraindications to GHrt in EGHD are the same ones identified for AGHD: active neoplasia and active proliferative or severe non-proliferative diabetic retinopathy. GHrt should be initiated with caution in pre-existing type II diabetic patients or with a strong family history of cancer (15, 16, 18) (Table 1).
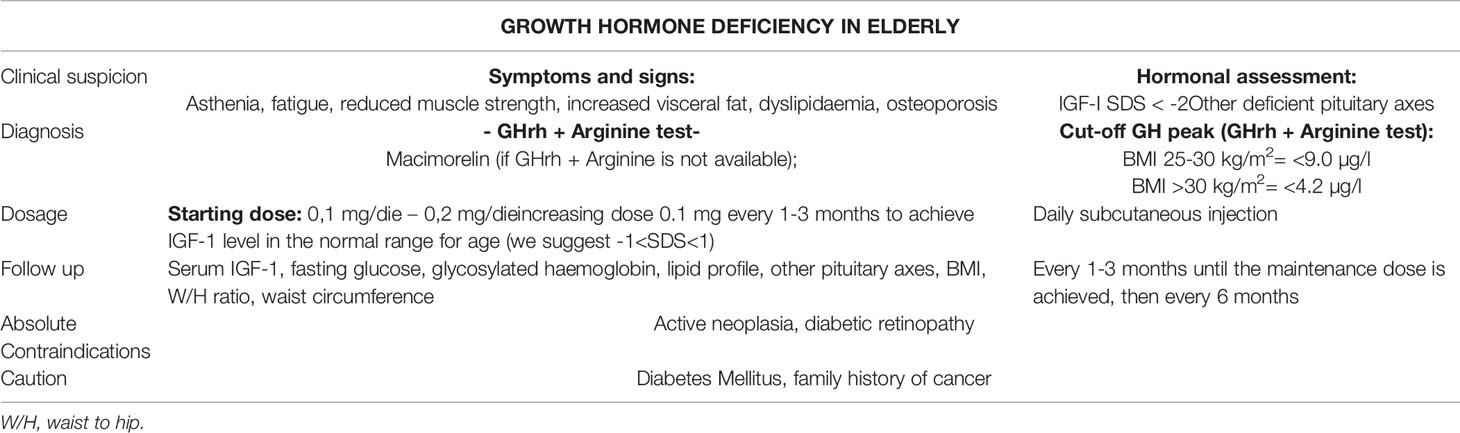
Table 1 A brief report about clinical suspicion, diagnosis, GH replacement therapy (GHrt) dose and titrating and follow up in elderly patients with GHD (EGHD).
To date, only few randomized placebo controlled trials have assessed the effects of GHrt in EGHD and there are no data on the efficacy and long-term safety in patients above 80 years of age (41). Given the benefits of GHrt in AGHD, and considering the similarity between some signs and symptoms of GHD and of aging, GHrt has been proposed in the past as anti-aging agent in healthy elderly subjects. However, the use of GHrt in this setting appears marginal and its benefits are offset by troublesome side effects (42).
Cardiovascular Effects
The effects of GHrt on the cardiovascular system in the elderly are the most studied to date. In EGHD patients, attention to cardiovascular risk should be a priority, given the demonstrated increase in cardiovascular morbidity and mortality from this cause in elderly patients and the supposed, although not yet unequivocally defined, increase in cardiovascular risk related to GHD itself (7, 14, 31, 43–45). Some studies show an improvement in HDL/LDL ratio after GHrt of up to 20% (11, 46, 47). In a study comparing treated EGHD and AGHD it was shown that, despite a lower dose of GHrt was used in EGHD, this group surprisingly displayed a more pronounced reduction in waist-to-hip ratio and LDL cholesterol levels (14). In 31 EGHD (age range 60-79 years) Elgzyri et al. demonstrated that GHrt leads to a transient increase in heart rate, an improvement in the resistance to a maximal exercise and an improved LDL/HDL ratio (44). These data suggest the importance to bring IGF-1 levels to the physiological threshold in order to reduce the cardiovascular risk in elderly patients with GHD. There were no clear consistent effects of GHrt on arterial blood pressure, as confirmed by many studies, especially in registries (46). Moreover, the reduction in waist-to-hip ratio after GHrt in EGHD appears controversial (11, 47–51). Considering the few existing studies and their heterogeneity in terms of patients, enrolment criteria and study design, it still remains difficult to establish the power of benefits in terms of clinical implications, such as reduced cardiovascular morbidity and mortality (41) (Figure 1).
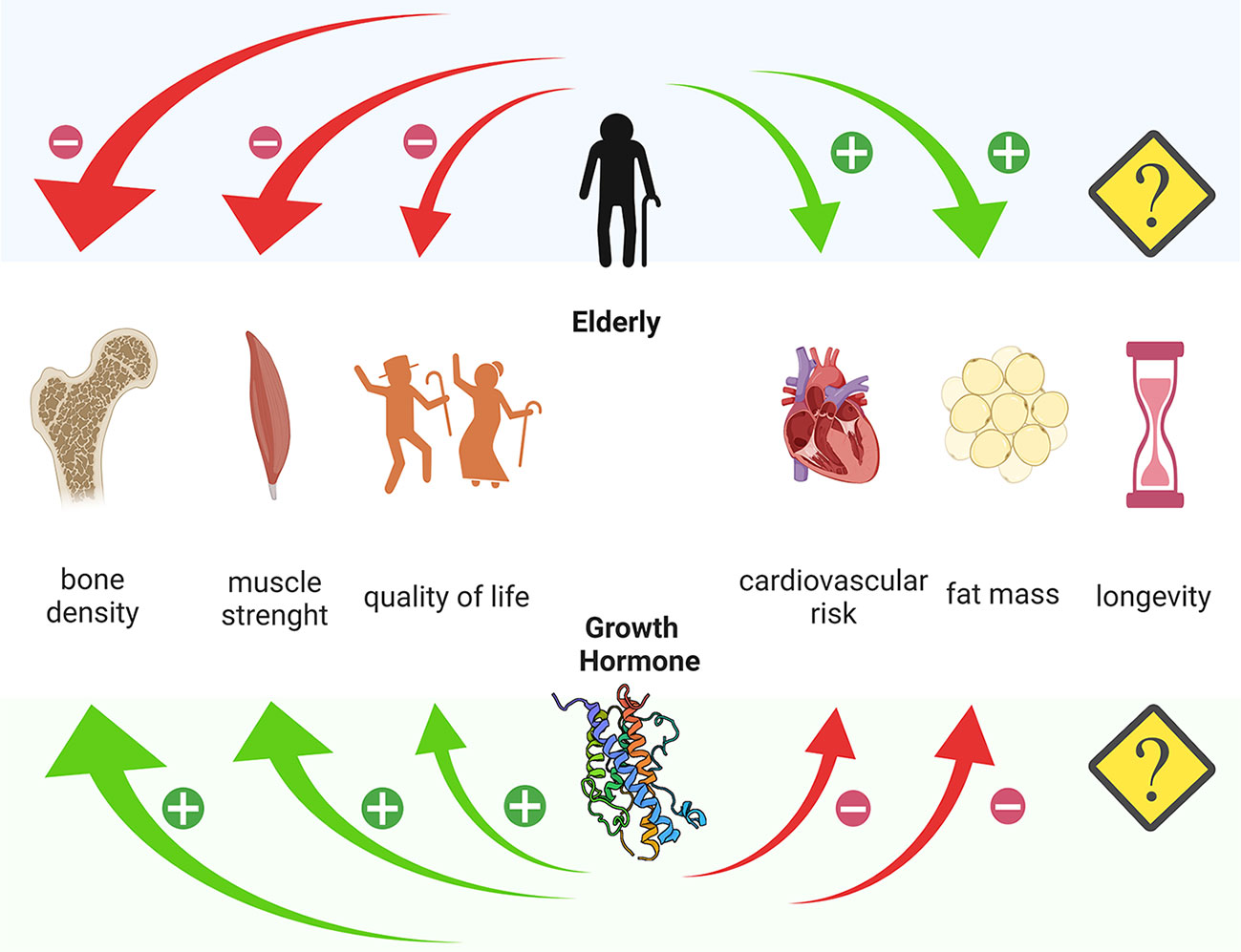
Figure 1 Different action of age and Growth Hormone on bone density, muscle strength, quality of life, cardiovascular risk and fat mass. Created with Biorender.com.
Effects on Cognitive Function
A higher incidence of mental disorders, more pronounced mental distress and cognitive dysfunctions are also symptoms of AGHD (10). However, the positive effect of GHrt, in terms of cognitive function, remains doubtful. In healthy elderly subjects, it has been shown that some cognitive aspects correlate inversely with IGF-1 levels (52, 53) and that regional cerebral blood flow, during the performance of memory tasks, increases more in healthy elderly with high circulating IGF-1 than in a group with “low” IGF-1 levels (54).
Sathiavageeswaran et al. in 2007 carried out the first double blind, randomized, placebo-controlled study to establish the effects of GH on cognition in EGHD. It has been found that certain aspects of cognitive function improved over the years in the GHrt group, while the placebo group deteriorated even further. There were no effects in patients without cognitive impairment at baseline. Therefore, the results of this study provide a basis for further investigations in this setting of patients (55).
Effects on Muscle Strength
Reduced levels of GH and IGF-1 are known to correlate with the grade of impairment of muscle strength (Figure 1). Indeed, a recent Chinese cross-sectional study of more than 3200 healthy elderly patients demonstrated how IGF-1 levels negatively correlate with the incidence of sarcopenia in both sexes (56). In AGDH, GHrt significantly improves muscle strength over the years (57). In EGHD, the correlation between GHD and muscle strength is still controversial, due to a very limited number of studies and assessed subjects. Based on the results of a prospective open-label study evaluating the effect of 10 years of GHrt on muscle strength in 24 EGHD (61-74 years), it seems that GHrt does not directly increase muscle strength, but it may reduce its age-related decline (58). Prospective studies in larger populations might be necessary.
Effects on Body Composition and Bone Metabolism
It is known that in AGHD, the imbalance between fat and lean masses is in favour of the former, (Figure 1) and there are some data suggesting an improvement in fat/lean mass ratio after GHrt. In EGHD, the data on this subject are still scarce. Toogood et al. demonstrated that EGHD show significant differences in body fat distribution but the response to treatment was not assessed (50). Moreover, more than one study, demonstrate a positive effect of GHrt on body composition (14, 39, 49), and the data extracted from the large KIMS database also support this evidence (51).
In AGHD, GHrt induces a progressive increase in bone mass and density (29), especially after 5–6 years of treatment (59), but results considerably differ according to age, gender, duration and schedule of treatment, including the dose (60, 61). According to the few studies available, GHrt in EGHD seems to improve bone metabolism (49, 62); however, given the lack of long-term prospective controlled studies, there is no clear evidence of a direct impact on the risk of fracture. This is a crucial point, because sarcopenia is an important risk factor for falls and fractures. Therefore, the maintenance of muscle strength (58) might have beneficial effects on reduction of falls and, indirectly, it could also reduce the fracture risk. We believe that targeted studies are needed to prove this assumption.
Effects on Quality of Life (Qol)
Reduced QoL in AGHD patients is one of the most consolidated evidence in the literature. GHrt improves QoL in AGHD patients, particularly by increasing energy and stabilizing emotionality (3, 30). Many studies have shown that the improvement in QoL seems more proportional to the degree of the baseline evaluation rather than the changes in IGF-1 levels (28). The assessment of QoL was proposed as a part of the clinical management in GHD patients, complementary to the measurement of surrogate biological markers or other clinical end points. In fact, in the NICE guidance the QoL score questionnaire is mandatory to decide whether to continue the GHrt (63) or not.
In a large recent study (64) including GHD patients older than 50 years old, 4 years of GHrt resulted in beneficial effects in terms of QoL, but no relevant differences were found in GHrt response between early or late initiation of treatment. Li Voon Chong et al. were among the first groups to study QoL in EGHD. They demonstrated that those patients had reduced energy, hypo-mobility, and lack of fulfilment in personal life, became socially isolated and suffered from mental fatigue (65). Remarkable improvements in QoL have been described after 6 months of GHrt and this has long been considered the goal of this therapy in patients with GHD (51). QoL should always be measured using validated questionnaires, such as the QoL-AGHDA (66) and we believe that in EGHD patients, an assessment of QoL, in addition to other clinical parameters, may be a valid determinant of whether to initiate replacement therapy or not.
Discussion
We can confirm that GHrt in EGHD can contribute to the restoration of a physiological state of health, without inducing significant adverse effects, in particular when the treatment is properly and individually titrated. The main, though few, evidences concern improvements in QoL, cardiovascular risk factors and metabolic features; however, targeted studies on this population are strongly recommended to confirm these results, to adequately test the effectiveness and safety of GHrt in old age and to optimize diagnostic aspects (i.e. to determine peak GH cut-offs stratified by age). It must also be considered that fat mass increases in the elderly and, therefore, correcting the cut-off for weight alone is not always reliable.
We believe it would be important to select suitable patients for treatment, taking into account their health status, comorbidities, life expectancy and on-going medications (67), as well as adherence to chronic treatment (i.e., cognitive status, presence of a caregiver, etc.). There are currently several on-going studies with long-acting recombinant GH preparations (68), which use a variety of technologies to prolong the action of GH by deferring administration over time (e.g., weekly). This is intended to improve patient compliance, especially among the elderly, who often take a high number of medications (28).
The evidence available to date suggests, in patients with hypopituitarism, GHD contributes to excess mortality and GHrt contributes to bring mortality rates to those of normal subjects, but a true relationship between mortality reduction and GHrt has not been conclusively established with long-term prospective controlled trials (69). However, it seems unlikely that such studies could be easily conducted, especially in EGHD (11, 49, 69).
We can conclude by stating that the main goal of GHrt in the elderly patient is to improve QoL, prolong independence, and avoid frailty (69, 70). The age-related decline in GH-IGF-1 levels does not justify GHrt supplementation, but patients with established GHD should be considered for treatment, regardless of age, but rather taking into account general conditions, comorbidities, and life expectancy, analysing each case individually (34, 37, 38). We add that considering the costs of GHrt and the mainly long-term effects, the cost/benefit ratio must always be carefully evaluated (38), especially in a population with a reduced life expectancy (71).
In our opinion, the main goal of GHrt in EGHD should be a significant improvement in QoL, which can only be achieved through the development of personalized treatment and careful follow-up. To achieve this, it is necessary to expand studies of GHrt in the elderly population.
Author Contributions
SR and MB conceived and wrote the manuscript. MF, MA, LV and FG realized the bibliography research. DF supervised the draft. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. van den Beld AW, Kaufman J-M, Zillikens MC, Lamberts SWJ, Egan JM, van der Lely AJ. The Physiology of Endocrine Systems With Ageing. Lancet Diabetes Endocrinol (2018) 6:647–58. doi: 10.1016/S2213-8587(18)30026-3
2. Salomon F, Cuneo RC, Hesp R, Sönksen PH. The Effects of Treatment With Recombinant Human Growth Hormone on Body Composition and Metabolism in Adults With Growth Hormone Deficiency. N Engl J Med (1989) 321:1797–803. doi: 10.1056/NEJM198912283212605
3. Appelman-Dijkstra NM, Claessen KMJA, Roelfsema F, Pereira AM, Biermasz NR. Therapy of Endocrine Disease: Long-term Effects of Recombinant Human GH Replacement in Adults With GH Deficiency: A Systematic Review. Eur J Endocrinol (2013) 169:R1–R14. doi: 10.1530/EJE-12-1088
4. Bates AS. The Effect of Hypopituitarism on Life Expectancy. J Clin Endocrinol Metab (1996) 81:1169–72. doi: 10.1210/jc.81.3.1169
5. Schneider HJ, Kreitschmann-Andermahr I, Ghigo E, Stalla GK, Agha A. Hypothalamopituitary Dysfunction Following Traumatic Brain Injury and Aneurysmal Subarachnoid Hemorrhage: A Systematic Review. JAMA (2007) 298:1429. doi: 10.1001/jama.298.12.1429
6. Toogood AA, O’Neill PA, Shalet SM. Beyond the Somatopause: Growth Hormone Deficiency in Adults Over the Age of 60 Years. J Clin Endocrinol Metab (1996) 81:460–5. doi: 10.1210/jcem.81.2.8636250
7. Colao A, Somma CD, Savanelli MC, Leo MD, Lombardi G. Beginning to End: Cardiovascular Implications of Growth Hormone (GH) Deficiency and GH Therapy. Growth Horm IGF Res (2006) 16:41–8. doi: 10.1016/j.ghir.2006.03.006
8. Gola M, Bonadonna S, Doga M, Giustina A. Growth Hormone and Cardiovascular Risk Factors. J Clin Endocrinol Metab (2005) 90:1864–70. doi: 10.1210/jc.2004-0545
9. Carroll PV, Christ ER, Sönksen PH. Growth Hormone Replacement in Adults With Growth Hormone Deficiency: Assessment of Current Knowledge. Trends Endocrinol Metab (2000) 11:231–8. doi: 10.1016/S1043-2760(00)00268-X
10. Koltowska-Häggström M, Mattsson AF, Shalet SM. Assessment of Quality of Life in Adult Patients With GH Deficiency: KIMS Contribution to Clinical Practice and Pharmacoeconomic Evaluations. Eur J Endocrinol (2009) 161:S51–64. doi: 10.1530/EJE-09-0266
11. Feldt-Rasmussen U, Wilton P, Jonsson P. Aspects of Growth Hormone Deficiency and Replacement in Elderly Hypopituitary Adults. Growth Horm IGF Res (2004) 14:51–8. doi: 10.1016/j.ghir.2004.03.013
12. Maison P, Griffin S, Nicoue-Beglah M, Haddad N, Balkau B, Chanson P. Impact of Growth Hormone (Gh) Treatment on Cardiovascular Risk Factors in GH-Deficient Adults: A Metaanalysis of Blinded, Randomized, Placebo-Controlled Trials. J Clin Endocrinol Metab (2004) 89:2192–9. doi: 10.1210/jc.2003-030840
13. Boschetti M, Agosti S, Albanese V, Casalino L, Teti C, Bezante GP, et al. One-Year GH Replacement Therapy Reduces Early Cardiac Target Organ Damage (TOD) in Adult GHD Patients. Endocrine (2017) 55:573–81. doi: 10.1007/s12020-016-0951-4
14. Franco C, Johannsson G, Bengtsson BA, Svensson J. Baseline Characteristics and Effects of Growth Hormone Therapy Over Two Years in Younger and Elderly Adults With Adult Onset GH Deficiency. J Clin Endocrinol Metab (2006) 91:4408–14. doi: 10.1210/jc.2006-0887
15. Svensson J, Bengtsson B-Å. Safety Aspects of GH Replacement. Eur J Endocrinol (2009) 161:S65–74. doi: 10.1530/EJE-09-0287
16. Yuen KCJ, Biller BMK, Radovick S, Carmichael JD, Jasim S, Pantalone KM, et al. American Association of Clinical Endocrinologists And American College of Endocrinology Guidelines For Management of Growth Hormone Deficiency in Adults And Patients Transitioning From Pediatric to Adult Care: 2019 AACE Growth Hormone Task Force. Endocr Pract (2019) 25:1191–232. doi: 10.4158/GL-2019-0405
17. Ghigo E, Aimaretti G, Corneli G. Diagnosis of Adult GH Deficiency. Growth Horm IGF Res (2008) 18:1–16. doi: 10.1016/j.ghir.2007.07.004
18. Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML. Evaluation and Treatment of Adult Growth Hormone Deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2011) 96:1587–609. doi: 10.1210/jc.2011-0179
19. Mancini A, Bruno C, Vergani E, Brunetti A, Palmisano G, Pontecorvi A. “Non-Classical” Indication for Provocative Testing of Growth Hormone: A Retrospective Cohort Study in Adult Patients Under Replacement Therapy. Endocr Metab Immune Disord - Drug Targets (2020) 20. doi: 10.2174/1871530320666200929141847
20. Fisker S, Jørgensen JOL, Christiansen JS. Variability in Growth Hormone Stimulation Tests. Growth Horm IGF Res (1998) 8:31–5. doi: 10.1016/S1096-6374(98)80006-3
21. Hilding A, Hall K, Wivall-Helleryd I-L, Sääf M, Melin A-L, Thorén M. Serum Levels of Insulin-Like Growth Factor I in 152 Patients With Growth Hormone Deficiency, Aged 19–82 Years, in Relation to Those in Healthy Subjects1. J Clin Endocrinol Metab (1999) 84:2013–9. doi: 10.1210/jcem.84.6.5793
22. Friedrich N, Wolthers OD, Arafat AM, Emeny RT, Spranger J, Roswall J, et al. Age- and Sex-Specific Reference Intervals Across Life Span for Insulin-Like Growth Factor Binding Protein 3 (Igfbp-3) and the IGF-I to IGFBP-3 Ratio Measured by New Automated Chemiluminescence Assays. J Clin Endocrinol Metab (2014) 99:1675–86. doi: 10.1210/jc.2013-3060
23. Tavares ABW, Seixas-da-Silva IA, Silvestre DHS, Paixão CM, Vaisman M, Conceição FL. Potential Risks of Glucagon Stimulation Test in Elderly People. Growth Horm IGF Res (2015) 25:53–6. doi: 10.1016/j.ghir.2014.11.002
24. Yuen KCJ. Growth Hormone Stimulation Tests in Assessing Adult Growth Hormone Deficiency, in: Endotext. South Dartmouth (MA: MDText.com, Inc. Available at: http://www.ncbi.nlm.nih.gov/books/NBK395585/ (Accessed January 10, 2021).
25. Martel-Duguech LM, Jorgensen JOL, Korbonits M, Johannsson G, Webb SM, Amadidou F, et al. ESE Audit on Management of Adult Growth Hormone Deficiency in Clinical Practice. Eur J Endocrinol (2020) 1:EJE–20-1180.R1. doi: 10.1530/EJE-20-1180
26. Corneli G, Di Somma C, Baldelli R, Rovere S, Gasco V, Croce CG, et al. The Cut-Off Limits of the GH Response to GH-releasing Hormone-Arginine Test Related to Body Mass Index. Eur J Endocrinol (2005) 153:257–64. doi: 10.1530/eje.1.01967
27. Garcia JM, Biller BMK, Korbonits M, Popovic V, Luger A, Strasburger CJ, et al. Sensitivity and Specificity of the Macimorelin Test for Diagnosis of AGHD. Endocr Connect (2020) 10(1):76–83. doi: 10.1530/EC-20-0491
28. Yuen KCJ, Llahana S, Miller BS. Adult Growth Hormone Deficiency: Clinical Advances and Approaches to Improve Adherence. Expert Rev Endocrinol Metab (2019) 14:419–36. doi: 10.1080/17446651.2019.1689119
29. Appelman-Dijkstra NM, Claessen KMJA, Hamdy NAT, Pereira AM, Biermasz NR. Effects of Up to 15 Years of Recombinant Human GH (rhGH) Replacement on Bone Metabolism in Adults With Growth Hormone Deficiency (Ghd): The Leiden Cohort Study. Clin Endocrinol (Oxf) (2014) 81:727–35. doi: 10.1111/cen.12493
30. Elbornsson M, Horvath A, Götherström G, Bengtsson B-Å, Johannsson G, Svensson J. Seven Years of Growth Hormone (GH) Replacement Improves Quality of Life in Hypopituitary Patients With Adult-Onset GH Deficiency. Eur J Endocrinol (2017) 176:99–109. doi: 10.1530/EJE-16-0875
31. Elbornsson M, Götherström G, Bosæus I, Bengtsson B-Å, Johannsson G, Svensson J. Fifteen Years of GH Replacement Improves Body Composition and Cardiovascular Risk Factors. Eur J Endocrinol (2013) 168:745–53. doi: 10.1530/EJE-12-1083
32. Olsson DS, Trimpou P, Hallén T, Bryngelsson I-L, Andersson E, Skoglund T, et al. Life Expectancy in Patients With Pituitary Adenoma Receiving Growth Hormone Replacement. Eur J Endocrinol (2017) 176:67–75. doi: 10.1530/EJE-16-0450
33. Fleseriu M, Hashim IA, Karavitaki N, Melmed S, Murad MH, Salvatori R. Et al. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2016) 101:3888–921. doi: 10.1210/jc.2016-2118
34. Ho KKY. Consensus Guidelines for the Diagnosis and Treatment of Adults With GH Deficiency II: A Statement of the GH Research Society in Association With the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol (2007) 157:695–700. doi: 10.1530/EJE-07-0631
35. Giustina A, Barkan A, Chanson P, Grossman A, Hoffman A, Ghigo E, et al. Guidelines for the Treatment of Growth Hormone Excess and Growth Hormone Deficiency in Adults. J Endocrinological Invest (2008) 31:820–38. doi: 10.1007/BF03349263
36. van Bunderen CC, Lips P, Kramer MH, Drent ML. Comparison of Low-Normal and High-Normal IGF-1 Target Levels During Growth Hormone Replacement Therapy: A Randomized Clinical Trial in Adult Growth Hormone Deficiency. Eur J Intern Med (2016) 31:88–93. doi: 10.1016/j.ejim.2016.03.026
37. van Bunderen CC, Glad C, Johannsson G, Olsson DS. Personalized Approach to Growth Hormone Replacement in Adults. Arch Endocrinol Metab (2020) 63:592–600. doi: 10.20945/2359-3997000000189
38. Shimon I, Badiu C, Bossowski A, Doknic M, Dzivite-Krisane I, Hána V, et al. Adult Growth Hormone Deficiency in CEE Region: Heterogeneity of the Patient Pathway. Growth Hormone IGF Res (2019) 46–47:44–9. doi: 10.1016/j.ghir.2019.06.001
39. Toogood AA, Shalet SM. Growth Hormone Replacement Therapy in the Elderly With Hypothalamic-Pituitary Disease: A Dose-Finding Study. J Clin Endocrinol Metab (1999) 84:131–6. doi: 10.1210/jcem.84.1.5408
40. van Bunderen CC, van Nieuwpoort IC, van Schoor NM, Deeg DJH, Lips P, Drent ML. The Association of Serum Insulin-Like Growth Factor-I With Mortality, Cardiovascular Disease, and Cancer in the Elderly: A Population-Based Study. J Clin Endocrinol Metab (2010) 95:4616–24. doi: 10.1210/jc.2010-0940
41. Kokshoorn NE, Biermasz NR, Roelfsema F, Smit JWA, Pereira AM, Romijn JA. GH Replacement Therapy in Elderly GH-deficient Patients: A Systematic Review. Eur J Endocrinol (2011) 164:657–65. doi: 10.1530/EJE-10-1170
42. Bartke A, Darcy J. GH and Ageing: Pitfalls and New Insights. Best Pract Res Clin Endocrinol Metab (2017) 31:113–25. doi: 10.1016/j.beem.2017.02.005
43. Abs R, Feldt-Rasmussen U, Mattsson AF, Monson JP, Bengtsson B-Å, Góth MI, et al. Determinants of Cardiovascular Risk in 2589 Hypopituitary GH-deficient Adults – a KIMS Database Analysis. Eur J Endocrinol (2006) 155:79–90. doi: 10.1530/eje.1.02179
44. Elgzyri T, Castenfors J, Hagg E, Backman C, Thoren M, Bramnert M. The Effects of GH Replacement Therapy on Cardiac Morphology and Function, Exercise Capacity and Serum Lipids in Elderly Patients With GH Deficiency. Clin Endocrinol (Oxf) (2004) 61:113–22. doi: 10.1111/j.1365-2265.2004.02080.x
45. Vasan RS, Sullivan LM, D’Agostino RB, Roubenoff R, Harris T, Sawyer DB, et al. Serum Insulin-like Growth Factor I and Risk for Heart Failure in Elderly Individuals Without a Previous Myocardial Infarction: The Framingham Heart Study. Ann Intern Med (2003) 139:642. doi: 10.7326/0003-4819-139-8-200310210-00007
46. Monson JP, Jönsson P. Aspects of Growth Hormone (Gh) Replacement in Elderly Patients With GH Deficiency: Data From KIMS. Horm Res Paediatr (2003) 60:112–20. doi: 10.1159/000071235
47. Gill MS. Serum Leptin Response to the Acute and Chronic Administration of Growth Hormone (GH) to Elderly Subjects With GH Deficiency. J Clin Endocrinol Metab (1999) 84:1288–95. doi: 10.1210/jc.84.4.1288
48. Gotherstrom G. A Prospective Study of 5 Years of GH Replacement Therapy in GH-Deficient Adults: Sustained Effects on Body Composition, Bone Mass, and Metabolic Indices. J Clin Endocrinol Metab (2001) 86:4657–65. doi: 10.1210/jc.86.10.4657
49. Fernholm R, Bramnert M, Hägg E, Hilding A, Baylink DJ, Mohan S, et al. Growth Hormone Replacement Therapy Improves Body Composition and Increases Bone Metabolism in Elderly Patients With Pituitary Disease1. J Clin Endocrinol Metab (2000) 85:4104–12. doi: 10.1210/jcem.85.11.6949
50. Toogood AA, Adams JE, O’Neill PA, Shalet SM. Body Composition in Growth Hormone Deficient Adults Over the Age of 60 Years. Clin Endocrinol (Oxf) (1996) 45:399–405. doi: 10.1046/j.1365-2265.1996.8310842.x
51. Monson JP, Abs R, Bengtsson B-Å, Bennmarker H, Feldt-Rasmussen U, Hernberg-Ståhl E, et al. Growth Hormone Deficiency and Replacement in Elderly Hypopituitary Adults: GH Replacement in Elderly Hypopituitarism. Clin Endocrinol (Oxf) (2000) 53:281–9. doi: 10.1046/j.1365-2265.2000.01104.x
52. Aleman A, Verhaar HJJ, de Haan EHF, de Vries WR, Samson MM, Drent ML, et al. Insulin-Like Growth Factor-I and Cognitive Function in Healthy Older Men. J Clin Endocrinol Metab (1999) 84:471–5. doi: 10.1210/jcem.84.2.5455
53. Sytze van Dam P, Aleman A. Insulin-Like Growth factor-I, Cognition and Brain Aging. Eur J Pharmacol (2004) 490:87–95. doi: 10.1016/j.ejphar.2004.02.047
54. Arwert LI, Deijen JB, Müller M, Drent ML. Long-Term Growth Hormone Treatment Preserves GH-induced Memory and Mood Improvements: A 10-Year Follow-Up Study in GH-deficient Adult Men. Horm Behav (2005) 47:343–9. doi: 10.1016/j.yhbeh.2004.11.015
55. Sathiavageeswaran M, Burman P, Lawrence D, Harris AG, Falleti MG, Maruff P, et al. Effects of GH on Cognitive Function in Elderly Patients With Adult-Onset GH Deficiency: A Placebo-Controlled 12-Month Study. Eur J Endocrinol (2007) 156:439–47. doi: 10.1530/eje.1.02346
56. Bian A, Ma Y, Zhou X, Guo Y, Wang W, Zhang Y, et al. Association Between Sarcopenia and Levels of Growth Hormone and Insulin-Like Growth Factor-1 in the Elderly. BMC Musculoskelet Disord (2020) 21:214. doi: 10.1186/s12891-020-03236-y
57. Cuneo RC, Salomon F, Wiles CM, Hesp R, Sonksen PH. Growth Hormone Treatment in Growth Hormone-Deficient Adults. I. Effects on Muscle Mass and Strength. J Appl Physiol (1991) 70:688–94. doi: 10.1152/jappl.1991.70.2.688
58. Götherström G, Elbornsson M, Stibrant-Sunnerhagen K, Bengtsson B-Å, Johannsson G, Svensson J. Muscle Strength in Elderly Adults With GH Deficiency After 10 Years of GH Replacement. Eur J Endocrinol (2010) 163:207–15. doi: 10.1530/EJE-10-0009
59. Clanget C, Seck T, Hinke V, Wüster C, Ziegler R, Pfeilschifter J. Effects of 6 Years of Growth Hormone (GH) Treatment on Bone Mineral Density in GH-deficient Adults: Long-term GH Effects on BMD in GH-deficient Adults. Clin Endocrinol (Oxf) (2001) 55:93–9. doi: 10.1046/j.1365-2265.2001.01284.x
60. Xue P, Wang Y, Yang J, Li Y. Effects of Growth Hormone Replacement Therapy on Bone Mineral Density in Growth Hormone Deficient Adults: A Meta-Analysis. Int J Endocrinol (2013) 2013:1–13. doi: 10.1155/2013/216107
61. Barake M, Klibanski A, Tritos NA. Effects of Recombinant Human Growth Hormone Therapy on Bone Mineral Density in Adults With Growth Hormone Deficiency: A Meta-Analysis. J Clin Endocrinol Metab (2014) 99:852–60. doi: 10.1210/jc.2013-3921
62. White HD, Ahmad AM, Durham BH, Patwala A, Whittingham P, Fraser WD, et al. Growth Hormone Replacement Is Important for the Restoration of Parathyroid Hormone Sensitivity and Improvement in Bone Metabolism in Older Adult Growth Hormone-Deficient Patients. J Clin Endocrinol Metab (2005) 90:3371–80. doi: 10.1210/jc.2004-1650
63. Postma MR, Burman P, van Beek AP. Early Versus Late Initiation of GH Replacement in Adult-Onset Hypopituitarism. Endocr Connect (2020) 9:687–95. doi: 10.1530/EC-20-0098
64. Doga M, Bonadonna S, Gola M, Mazziotti G, Giustina A. Growth Hormone Deficiency in the Adult. Pituitary (2006) 9:305–11. doi: 10.1007/s11102-006-0410-y
65. Li Voon Chong JSW, Benbow S, Foy P, Wallymahmed ME, Wile D, MacFarlane IA. Elderly People With Hypothalamic-Pituitary Disease and Growth Hormone Deficiency: Lipid Profiles, Body Composition and Quality of Life Compared With Control Subjects: Growth Hormone Deficiency in the Elderly. Clin Endocrinol (Oxf) (2000) 53:551–9. doi: 10.1046/j.1365-2265.2000.01140.x
66. Moock J, Friedrich N, Völzke H, Spielhagen C, Nauck M, Koltowska-Häggström M. Et alPrediction of Improvement in Quality of Life (QoL-AGHDA) in Adults With Growth Hormone Deficiency by Normative Reference Limits: Data of the German KIMS Cohort. Growth Horm IGF Res (2011) 21:272–8. doi: 10.1016/j.ghir.2011.07.005
67. Hoel RW, Giddings Connolly RM, Takahashi PY. Polypharmacy Management in Older Patients. Mayo Clin Proc (2021) 96:242–56. doi: 10.1016/j.mayocp.2020.06.012
68. Johannsson G, Gordon MB, Højby Rasmussen M, Håkonsson IH, Karges W, Sværke C, et al. Once-Weekly Somapacitan is Effective and Well Tolerated in Adults With GH Deficiency: A Randomized Phase 3 Trial. J Clin Endocrinol Metab (2020) 105:e1358–76. doi: 10.1210/clinem/dgaa049
69. Van Bunderen CC, Olsson DS. Growth Hormone Deficiency and Replacement Therapy in Adults: Impact on Survival. Rev Endocr Metab Disord (2021) 22:125–33. doi: 10.1007/s11154-020-09599-w
70. Besson A, Salemi S, Gallati S, Jenal A, Horn R, Mullis PS, et al. Reduced Longevity in Untreated Patients With Isolated Growth Hormone Deficiency. J Clin Endocrinol Metab (2003) 88:3664–7. doi: 10.1210/jc.2002-021938
Keywords: growth hormone, growth hormone deficiency (GDH), growth hormone replacement therapy, IGF-1, elderly, GHD diagnosis
Citation: Ricci Bitti S, Franco M, Albertelli M, Gatto F, Vera L, Ferone D and Boschetti M (2021) GH Replacement in the Elderly: Is It Worth It? Front. Endocrinol. 12:680579. doi: 10.3389/fendo.2021.680579
Received: 14 March 2021; Accepted: 02 June 2021;
Published: 15 June 2021.
Edited by:
Antonio Mancini, Catholic University of the Sacred Heart, Rome, ItalyReviewed by:
Marija Pfeifer, University of Ljubljana, SloveniaMoises Mercado, Mexican Social Security Institute (IMSS), Mexico
Copyright © 2021 Ricci Bitti, Franco, Albertelli, Gatto, Vera, Ferone and Boschetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mara Boschetti, bWFyYS5ib3NjaGV0dGlAdW5pZ2UuaXQ=
 Silvia Ricci Bitti
Silvia Ricci Bitti Marta Franco
Marta Franco Manuela Albertelli
Manuela Albertelli Federico Gatto
Federico Gatto Lara Vera2
Lara Vera2 Diego Ferone
Diego Ferone Mara Boschetti
Mara Boschetti