
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 01 July 2021
Sec. Cellular Endocrinology
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.678309
This article is part of the Research Topic The Effects of Circulating non-Sex Hormones in Cardiovascular Disease View all 7 articles
 Wen-Lu Ou-Yang1
Wen-Lu Ou-Yang1 Bei Guo1
Bei Guo1 Feng Xu1
Feng Xu1 Xiao Lin2
Xiao Lin2 Fu-Xing-Zi Li1
Fu-Xing-Zi Li1 Su-Kang Shan1
Su-Kang Shan1 Feng Wu3
Feng Wu3 Yi Wang1
Yi Wang1 Ming-Hui Zheng1
Ming-Hui Zheng1 Qiu-Shuang Xu1
Qiu-Shuang Xu1 Ling-Qing Yuan1*
Ling-Qing Yuan1*Irisin, a PGC1α-dependent myokine, was once believed to have beneficial effects induced by exercise. Since its first discovery of adipose browning in 2012, multiple studies have been trying to explore the metabolic functions of irisin, such as glucose and lipid metabolism. However, recently many studies with irisin concentration measuring were doubt for methodological problems, which may account for the continuous inconsistencies. New tools like recombinant irisin and gene-knockout mice are required to reconfirm the questioned functions of irisin. In this paper, we make a critical introduction to the latest researches concerning the relationship between irisin and coronary heart disease, which includes atherosclerosis, stable angina pectoris and acute coronary syndromes. These studies provided various controversial evidence of short and long-term monitoring and therapeutic effect from molecular cellular mechanisms, in vivo experiments and epidemiological investigation. But with ambiguities, irisin still has a long way to go to identify its functions in the clinical management.
Irisin is a relatively newly discovered myokine, a small protein with 112 amino acids. Its precursor, fibronectin type III domain-containing protein 5 (FNDC5), is regulated by the transcription factor PGC1α (1). In human, irisin is mainly secreted by cardiomyocytes and skeletal muscle cells, and a small amount is also distributed in adipose tissue, brain, liver, spleen, testis and other tissue (2). Irisin is highly conserved among different species, homology of which between mouse and human irisin is almost 100% (1). However, Raschke et al. found that start codon of FNDC5 gene is different, and doubted whether it’s appropriate to translate all the beneficial effect observed in mice to humans (3).
The production level of irisin is affected by many factors. Exercise, cold, age, BMI, glucose, lipid metabolism level, and other cytokines are all related to its serum concentration (4–8). It was initially widely considered as an exercise-induced myokine, whose circulating level was affected by type and frequency of physical activity (1, 9, 10). Jedrychowski’s study (11) validated it by quantitation mass spectrometry (MS). However, inconsistencies emerged to doubt whether the stimulation of exercise worked. Browning of white adipose tissue (WAT), a major effect of irisin, was observed only in inguinal WAT of training mice and attenuated in FNDC5 knockout mice (12, 13), while cold exposure was effective for all adipose depots in murine (14). When it comes to human, current evidence are not qualified enough to confirm the irisin’s responses to exercise (15–20). The disagreement may blame to the insufficient detection accuracy considering the minor amounts of irisin’s expression (0.3 ng/mL in mice (21) and 3-4 ng/mL in humans (11) of serum level measured by MS) (15, 22–24).
In recent years, thousands of papers are trying to find out irisin’s engages in multiple biological processes of our body (Figure 1). First of all, browning the white adipose tissue was recognized at the same time irisin was discovered in 2012 (1). However, as the discussion above, exercise has little effect on the stimulation of irisin’s browning function in human (15–20). Despite the disputes on stimulators like exercise, primary mouse adipocytes were observed browning effect when directly incubated with recombinant C-terminal FNDC5 peptide (25). Then in brown adipose tissue, the uncoupling protein 1 (UCP1) could release the storage of energy generated by oxidation in the form of heat and reduce the production of ATP simultaneously (26). These metabolic effects could be achieved by activating p38 MAPK and ERK pathways (27). The discovery of the PGC1α-FNDC5-irisin axis was the theoretical basis for the latter studies concerning energy metabolism mechanisms, which made irisin closely related to obesity, T2DM, and other metabolic syndromes (1, 28–30) (Figure 2). While the browning function is now facing challenges, similar questions would also be raised in related area such as fat storage, energy consumption and insulin sensitivity.
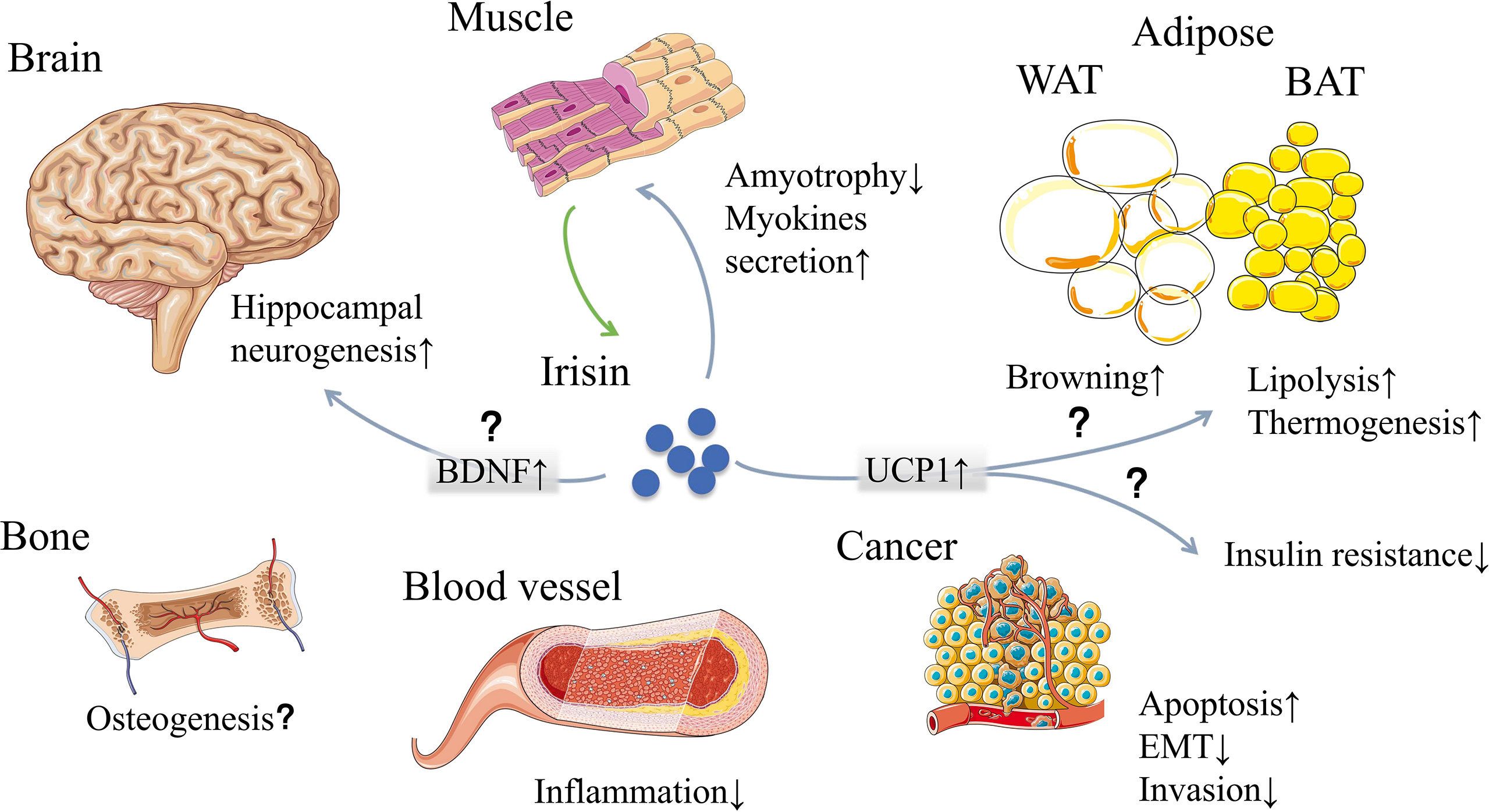
Figure 1 Irisin, mainly secreted by muscle, may have effects on multiple organs and tissues. The classic functions of irisin are browning white adipose tissue and generating heat. Other effects include muscle feedback, neurogenesis, osteogenesis, inflammation reduction, cancer suppression and so on. However, some of the functions are now facing challenges due to the measuring problems and interspecies genetic differences. WAT, white adipose tissue; BAT, brown adipose tissue; BDNF, brain-derived neurotrophic factor; UCP1, uncoupling protein 1; EMT, epithelial-mesenchymal transition. [The elements were produced using Servier Medical Art (https://smart.servier.com/) with some adaption].
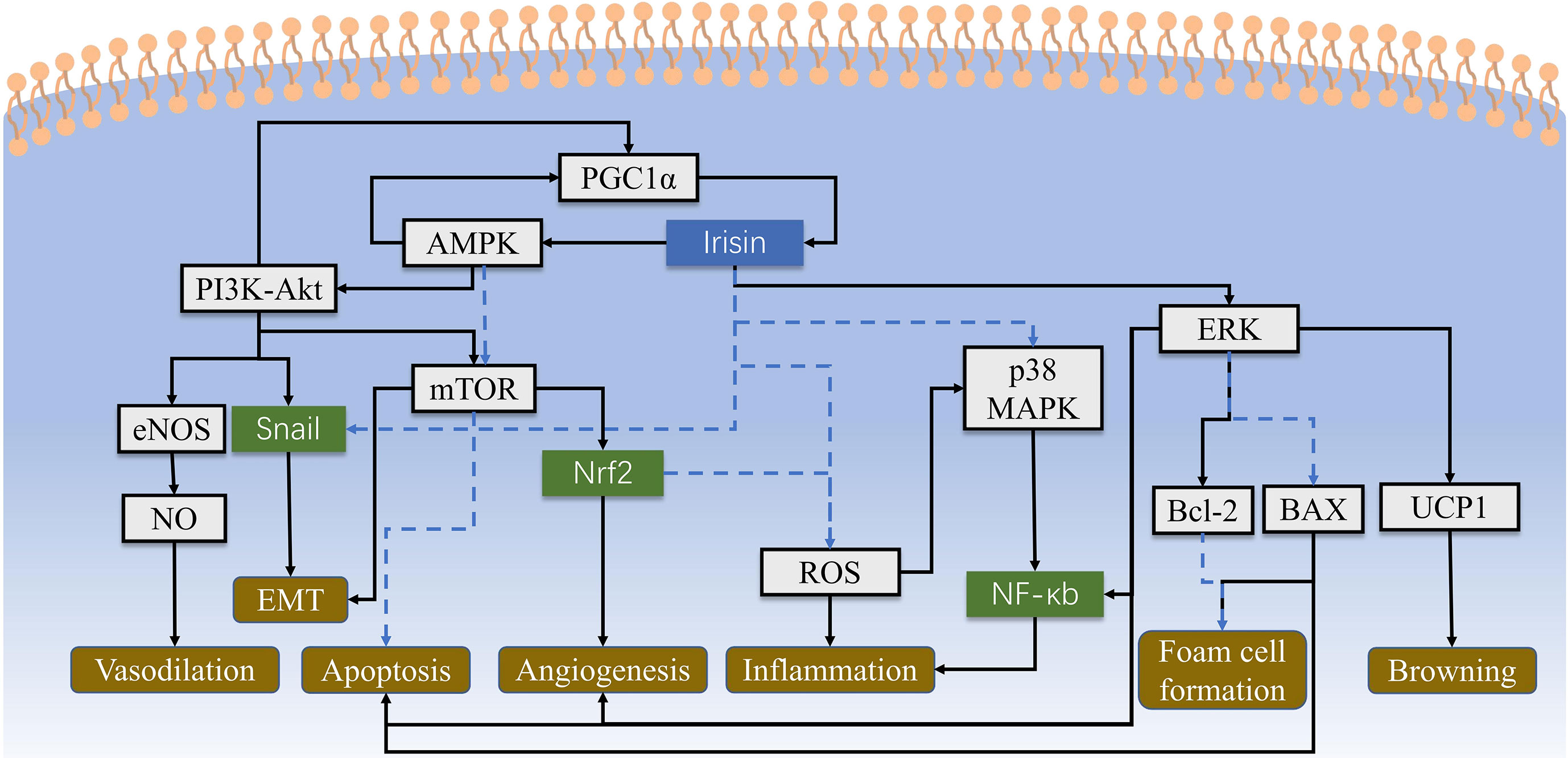
Figure 2 The cell signaling pathways of irisin are intricate and haven’t been fully identified yet. This figure shows main cellular biological processes concerning atherosclerosis and CHD, which include AMPK-PI3K-Akt-eNOS, AKT/mTOR, p38 MAPK and ERK pathways to regulate inflammation, angiogenesis and other physiological activities. Irisin could also alleviate the damage of ROS directly. Solid lines represent promotion, and dashed lines represent suppression.
In addition, FNDC5 are associated with cognitive function of the brain through brain-derived neurotrophic factor (BDNF). BDNF is the main mediator of exercise effect on the hippocampus, which can promote the growth, differentiation and repair of neurons (31). Wrann showed a positive correlation between FNDC5 and BDNF in exercising mice (31). Increasing the brain level of FNDC5/irisin can enhance synaptic plasticity and memory in Alzheimer’s disease mouse models (32), and stimulate the STAT3 signal transduction pathway required for sensory neuron development (33). However, the studies didn’t explain the mechanism of serum irisin to cross the blood-brain barrier, and applied much higher concentration of irisin than physiological level, which later showed no obvious effects on mouse hippocampal neuronal cells (33). Thus, evidence is not enough to demonstrate the direct relationship of irisin and BDNF. Besides, in terms of bone metabolism, irisin were reported to activate proliferation and differentiation of osteoblast through p38 and ERK (34–36), and reduce the extent of osteoporosis and muscle atrophy (37, 38). But according to H Kim, the increase of trabecular bone volume and BMD were observed in female FNDC5-KO mice (39). So, we still couldn’t figure out whether irisin helps in bone formation or not. Also, the increase of irisin can be detected in a variety of tumors. In cell assays, irisin showed its potential to inhibit epithelial-mesenchymal transition and cancer cell invasion via PI3K/Akt pathway, enhance the activity of caspase-3 and caspase-7 to induce cancer cell apoptosis, and inhibit the activity of NF-κb to reduce inflammation (40, 41). Moreover, irisin produced by cardiomyocytes is much more than that of skeletal muscle cells (42). Thus, studies on treatment of irisin showed its effects on cardiac hypertrophy, mainly through mTOR activation to prompt protective autophagy (43–45). In the development of cardiovascular disease, irisin may participate in this process through various non-metabolic ways, which will be discussed in the latter part.
However, there has been a big shadow cast on the measurement of irisin. In 2015, Albrecht E showed that the antibodies used in commercial ELISA tests are unspecific with a huge variation in plasma concentrations (46), which could explain the huge difference between the early rough estimate by ELISA kits (1) and recent measurement by quantitative MS from the same group (21). Calibrated MS was regarded as a “gold standard” to detect irisin (47), but Albrecht further doubt the repeatability of LC/MS method for detection of extremely low plasma concentrations (48). Additionally, due to the extremely low amounts of irisin in samples (11, 21), it’s hard to purify it for gain-of-function experiments. Yet new approaches like recombinant irisin and FNDC5 knockout mice could provide more direct evidence to verify the functions of irisin. Most studies creating FNDC5 knockout mice found no obvious difference of multiple biological process under normal conditions (21, 43, 49, 50). But they would suffer a lot more in several abnormal conditions compared to wild type, such as fasting and high-fat diet (13, 43, 49, 51). Thus, we would put more focus on the studies with new approaches to draw a rather reliable picture of irisin.
CHD is one of the most common cardiovascular diseases. The prevalence and fatality rate has been increasing consecutively year after year, seriously endangering people’s health (52). Due to coronary artery spasm or atherosclerosis, myocardial cells suffer from ischemia and hypoxia, leading to functional or structural pathological changes of heart. As early as irisin was first discovered, some scholars (53) suggested that there may be certain crosstalk between irisin and the cardiovascular system. As time passes by, a number of evidences accumulated to support this speculation. Irisin has participated in the development of CHD in a variety of ways, which is likely to lay a theoretical foundation for the clinical application of irisin as a biomarker or treatment.
Atherosclerosis, a chronic inflammatory disease, is the most common pathogenesis of CHD and a variety of vascular diseases. The etiology has not been clear yet, usually considered to be closely associated with lifestyle and caused by multiple factors such as lipid metabolism disorder and endothelial injury (54). Irisin participates in the regulation of this process through direct or indirect ways (such as glucose and lipid metabolism).
In many studies conducted in different sample sizes and different populations, the relationship between serum irisin levels and atherosclerosis is not consistent. Deng W et al. (55) conducted a cross-sectional study on the serum irisin concentration and coronary atherosclerosis index (CAI) of 350 coronary artery disease (CAD) patients and 214 healthy participants, finding that the serum irisin concentration was significantly different in the two groups of subjects. The lower the serum irisin concentration in CAD patients, the higher the CAI. Parallel to this result, in the study of cardiovascular complications, Khorasani (56) and Saadeldin (57) respectively confirmed that the serum irisin concentration was negatively correlated with the degree of coronary atherosclerosis in patients with type 2 diabetes. Besides, in some special populations [such as patients with dialysis (58) or Behcet’s Syndrome (59)], the detection of carotid artery intima-media thickness (IMT) was also found in line with this pattern. Irisin’s regulation on glucose and lipid metabolism is speculated to be the main contributor to this negative relationship, since hyperglycemia, insulin resistance and obesity are closely associated with atherosclerosis (55, 59, 60). Another possible explanation is the cross-talks between irisin and other molecules such as sclerostin or adipocytokines (57, 58).
However, some studies stood on the opposite side. As early as 2014, in a study of 192 European ancestry healthy people, it was found that irisin was positively correlated with the subjects’ carotid artery IMT (60). Moreover, a 25-year cohort study by Kwaniewska M (61) and a cross-sectional study of men with HIV infection (62) supported this result. In this regard, they explained that irisin may indirectly participate in the formation of atherosclerosis through fat or insulin (60, 62). And as the disease advances, the target cell’s sensitivity to irisin or insulin decreases, so that muscle/fat tissue “compensatively” releases more irisin to achieve the same effect as before. There is also an opinion, arguing that the difference in baseline between different studies leads to the opposite result: the various degrees of obesity of the study subjects may interfere with the results. As in non-obese subject, the serum irisin is mainly derived from muscle, while in the obese subject the proportion of fat source increases (63). Similar to the degrees of obesity, Moreno found that the high value of plasminogen activator inhibitor-1 (PAI-1) at baseline could also have a significant impact on the results, which could even count as an independent biomarker related to the thickness of the carotid artery IMT in patients (62). In a study based on a Japanese male population (64), the authors found that if cardiometabolic risk factors are taken into account, irisin had nothing to do with the prevalence of coronary artery calcification, but was related to progression. They believed that irisin may affect other unknown ways to cause this result except for traditional cardiometabolic risk factors such as fat and blood glucose. The inaccuracy measurement of irisin due to the questioned commercial ELISA may also count for the inconsistencies (46).
In short, the current studies lack consistency in the relationship between the level of irisin and the degree of atherosclerosis. And the correlation in multivariate analysis as well as the measurement is not sufficiently valid, which makes it difficult for irisin to be a predictive or clinical indicator of CHD.
The cellular biological processes related to atherosclerosis mainly involves vascular endothelial damage and foam cell formation, which then leads to wall thickening, endothelial narrowing, and eventually fibrous cap rupture (54).
As mentioned above, irisin is surmised to participate in atherosclerosis by some indirect ways such as influencing insulin release. In recent years, a series of studies have found that irisin can also directly regulate endothelial function though suppressing the inflammation and oxidation and encouraging the proliferation of cells. On one hand, Lu J (65) proved that irisin can activate AMPK-PI3K-Akt-eNOS signaling pathway in diabetic mice, inhibit high glucose-induced apoptosis in human umbilical vein endothelial cells (HUVECs), and increase the expression of antioxidant enzymes to reduce inflammation and oxidative stress of endothelial cells. Furthermore, Han F et al. (66) conducted similar experiments on healthy obese mice fed by a high-cholesterol diet and found that the activation of AMPK-eNOS signaling pathway by irisin can be dependent or independent of adiponectin. On the other hand, Zhang Y et al. (67) elaborated in mice that irisin could reduce the vascular damage induced by oxLDL. In animal experiment, irisin can regulate the p38 MAPK/NF-κB signaling pathway by inhibiting the generation of reactive oxygen species (ROS) and the nuclear translocation of NF-κB. It can also inhibit PKC-β/NADPH oxidase, down-regulate inflammatory factors to reduce oxidative/nitrative stresses, thus alleviating vascular endothelial inflammation (67, 68). In addition, Zhang M et al. found that irisin can increase cell viability, cell migration and capillary formation in apolipoprotein E (ApoE) deficient mice. This may be achieved by the up-regulation of microRNA126-5p in the ERK signaling pathway so as to maintain the stability of endothelial cells and promote proliferation (69). In an in vitro experiment exploring diabetes complicated with vascular diseases, it reported that irisin can inhibit ROS-NLRP3 inflammasome signaling, thereby slowing down the process of diabetes-related endothelial inflammation and other impairment (70). Protective functions of irisin are also found to lessen endothelial damage at an early stage. Irisin could suppress the expression of TNFα-induced VCAM-1 in HUVECs (71). A recent survey in obese children found that irisin can inhibit the expression of hsCPR, ICAM-1 and E-selectin in endothelial cells, indicating that irisin may have an effect on cardiovascular disease before symptoms show up (72).
Besides endothelial cell, irisin could also act on macrophages to slow down the formation of plaques. By up-regulating Bcl-2, down-regulating the expression of Bax and caspase-3, irisin could prevent macrophages from turning into foam cells, and inhibit cell apoptosis (67). Zheng G et al. (73) further found lipid accumulation in macrophages cultured in vitro and oxLDL-related apoptosis were reduced under the interference of irisin, which may be related to the inhibition of PERK/eIF2α/CHOP and ATF6/CHOP endoplasmic reticulum stress signaling pathways. All of these demonstrated that irisin does engage in multiple mechanisms in atherosclerosis, either by acting on endotheliocyte or on macrophage. However, some of the experiments were conducted on mice, which may not be qualified enough to translate the protective effects to human. And it must be noted that the serum irisin level is much lower than the applied dose in experiment, thus challenging the physiological functions of irisin under normal circumstances.
Various cell experiments have confirmed that irisin has a positive effect on vascular endothelial cells, while the therapeutic effect of irisin have shine a light on atherosclerosis in animal experiments. In apolipoprotein E-Null diabetic mice (69) or obese mice induced by high-cholesterol diet (66), the endothelium-dependent dilation function (EDV) was improved with the injection of irisin, while the endothelial cell apoptosis and the area of atherosclerotic plaque were pleasantly found to have a significant reduction. In the carotid artery partial ligation model (67, 69), systemic application of irisin can also inhibit the formation of the new carotid artery intima, for the reason that irisin may promote endothelial cell proliferation and inhibit monocyte recruitment and lipid deposition. These evidences show that irisin has potentiality to contribute to the therapy of atherosclerosis, while further researches are compulsory to provide sufficient clinical support.
CHD can be divided into two categories: stable angina pectoris (SAP) and acute coronary syndromes (CHD) according to its pathogenesis and clinical manifestations. Based on current studies, there is controversy over the role of irisin in the pathogenesis and repair of myocardial cells, and the specific mechanism is still unclear. But when it comes to treatment, most studies tend to support the protective effects of irisin on CHD.
SAP is the most common clinical type of CHD and is at the initial stage of CHD development. For many reasons, the insufficient blood supply of the coronary arteries leads to transient ischemia and hypoxia in the myocardium, often accompanied by cardiac dysfunction, but myocardial necrosis rarely occurs (74). There are not enough studies conducted on irisin in SAP, the quality of which are at different levels. In a small cross-sectional study, Efe et al. (75) found that the serum irisin level of stable coronary artery disease patients with a higher SYNTAX score (≥23) was significantly decreased compared to the group with low SYNTAX score or the healthy control group. They believed that serum irisin level can be used as an independent indicator to observe the severity of SAP, which is related to the degree of coronary stenosis in patients (76). Some previous researches (42, 77) also partly agreed with it. However, 24 hours after SAP patients having percutaneous coronary intervention (PCI), the serum irisin concentration was found to be lower than that of the control group (76). And the results of Park et al. (77) showed that compared with other groups, SAP patients with low preoperative serum irisin levels had a significantly higher proportion of no CHD events within 12 months after PCI. It should be noted that the measurement of irisin in those studies were all conducted by commercial ELISA, which reduced credibility of the potential connection between irisin and SAP, emphasizing the need for more clinical research.
ACS happens after plaque rupture or erosion on the basis of coronary atherosclerosis, which leads to vascular embolism, a sharp decrease in oxygen supply to the myocardium, and then damage. The degree of risk and prognosis are related to the location, area and speed of the establishment of collateral circulation (78–80). Many studies believe that irisin is involved in the occurrence of ACS and can be used as a therapeutic drug.
Several studies reported that the concentration fluctuation of irisin had a common pattern in different stages of myocardial infarction (MI). Kuloglu et al. (81) used isoproterenol on rats to induce myocardial infarction. The serum irisin level of MI rats decreased significantly in the first 2 hours, and slowly increased afterwards, but never returned to the baseline within 24 hours during monitoring. In the meanwhile, the synthesis level in tissue like skeletal muscle, liver and kidney decreased within 1-4 hours after MI, and recovered after 6 hours. Further monitoring of myocardial infarction rats showed that the serum irisin level was highly positively correlated with QRS duration, amplitude and TAS, while highly negatively correlated with ST-elevation, QTc, CK-MB, troponin and MDA (82). Similar changing pattern can be observed in human that the concentration of irisin fluctuates during ACS, but the point of time was delayed (83, 84). Similarly, the serum irisin measured 6 hours after PCI also decreased from baseline (76). However, during coronary artery bypass surgery, the serum irisin concentration increased from induction to the removal of the cross-clamp, and then gradually decreased as the patient rewarmed (85).
According to this phenomenon, Kuloglu (81) believed that it had something to do with the uncoupling effect of irisin. and may have a protective effect on cardiomyocytes. In addition, excessive irisin will lead to increased mitochondrial respiration and fatty acid oxidation, which leads to increased oxygen consumption and ROS, and up-regulates the level of caspase 9 which enhances cell apoptosis (1). On the contrary, the appropriate amount of irisin can activate Opa1-induced mitochondrial autophagy, alleviate oxidative stress and maintain the vitality of cardiomyocytes after myocardial infarction (86). Irisin can also lead to bradycardia through effects on the nucleus ambiguous (87), which helps reduce the energy consumption of the heart.
However, different perspectives interpret the possible mechanisms behind the changes of irisin during ACS. One point is that the reduction of irisin is a passive performance rather than an active regulation. When cardiomyocytes were treated with TNFα or IL-1β, the expression of FNDC5 protein decreased significantly, indicating that inflammatory factors could inhibit the secretion of irisin (6). And MI can restrain the expression of PPAR-α and PPAR-γ (nuclear receptors), thereby preventing the interaction of PGC1-α with a variety of transcription factors, resulting in a decrease in the synthesis of FNDC5 (irisin precursor) (88). Another explanation suggested that the decrease in irisin level is the reason rather than the result of the decrease in coronary blood flow. Since previous animal experiments have proven that irisin can regulate endothelial function and induce vasodilation (89), low levels of irisin may lead to decreased vasodilatation then reduced blood flow, forming a vicious circle.
It should be noticed that the commercial ELISA kits used to detect irisin in those studies were lack of specificity (46). Besides the shadow of questioned measuring methods, the mechanism of this fluctuation still remains controversial. Sharp decrease of irisin may protect mitochondria and allow cardiomyocytes to save energy in such an ischemic environment, which seems to be a self-protection mechanism of our body. But it may be also a consequence of damage and even assist in the progression of ACS. While there is still no reliable method of irisin measurement, irisin is blocked to become a marker showing the process of ACS.
For the long-term stage after the occurrence of ACS, the level and function of circulating irisin are inconclusive. In terms of surgery, the level of irisin at 6 months after PCI was higher than the baseline (53). The relatively high level of irisin was positively correlated with the incidence of postoperative major adverse cardiovascular events (MACE), especially angina, the risk of which increased by almost 4 times (hazard ratio=3.96) (83). On the contrary, a meta-analysis involving 867 patients and 700 controls showed that patients with cardiovascular disease or atherosclerosis had significantly lower levels of irisin (90). In 2014, a cross-sectional study by Emanuele et al. (91) found that serum irisin in young subjects who had suffered from MI was significantly lower than healthy controls. However, in an age-related study (53), there was no significant difference in irisin levels among people in their sixties, regardless of whether they had had ACS. The measurement problem may be the main reason to rationale the difference (46). Moreover, The circulating level of irisin in the body is influenced by fat, exercise, diet and so many other factors, and the level of irisin tends to increase randomly due to aging (92). Meanwhile, systematic errors such as inconsistent experimental design and detection levels cannot be ruled out.
There are also studies concerning the distribution of irisin genotypes. The G allele of rs3480 and the A allele of rs726344 are significantly related to cardiometabolic risk factors. Besides, the two SNPs are associated with each other (93). Dyslipidemia is a major initiator of atherosclerosis, and ApoE is particularly important to maintain the normal metabolism of lipoprotein (94, 95). Fuku et al. reported that FNDC5 rs16835198 was associated with APOE ϵ2/ϵ4 allele (96), while direct evidence towards atherosclerosis needed to be further explored. Hence future investigations are needed to explore genetic risk factors for myocardial infarction.
Nevertheless, limited researches still couldn’t provide sufficient materials to make a conclusion, which suggests that a more critical experimental design and use of validated measurement are required to draw a rounded picture for prognosis of irisin.
There is an interesting phenomenon in the distribution of irisin during myocardial infarction. Although serum irisin level decreases in the acute phase, the expression of irisin contrarily increases in connective tissues around the heart (81, 97), suggesting that irisin may participate in the follow-up process of MI such as restoration. As described above (67), we have mentioned that irisin can reduce the damage of vascular endothelial cells by inhibiting the production of ROS. Similar events may also occur in cardiomyocytes. Peng Q et al. (98) found that irisin can promote cell proliferation while reducing the production of HO-induced ROS and cell apoptosis in cardiomyocytes, which may be achieved by the miR-19b/AKT/mTOR signaling pathway. Zhao et al. (99) tried to find the connection between irisin and HDAC4 in vitro. HDAC4 is produced by H9c2 cardioblasts and is up-regulated in oxidative stress. Its overexpression can lead to mitochondrial dysfunction and even cell death (100). And irisin can induce HDAC4 degradation through ubiquitination modification to protect cardiomyocytes. Partially different from the results above (99), Moscoso et al. (97) only observed the protective effect of irisin under hypoxic conditions. They believed that under the condition of myocardial infarction, hypoxia and the compensatory adrenergic response would cause a lipotoxic environment and create conditions for apoptosis (101). Consequently, irisin could activate the Akt signaling pathway to resist apoptosis induced by lipotoxicity.
When the blood flow of myocardial tissue recanalized after ischemic injury, ischemia/reperfusion injury (I/R) often occurs. Irisin has shown its ability to reduce I/R injury in vitro and in vivo experiments. Mitochondrial dysfunction leads to the release of a large amount of ROS, and this oxidative stress response is one of the core steps of I/R. Irisin could inhibit the opening of mPTP(mitochondrial permeability transition pore), reduce mitochondrial swelling and increase the activity of superoxide dismutase 2 (SOD2) by upregulating mitochondrial ubiquitin ligase (MITOL/MARCH5), so as to protect mitochondria and reduce the oxidative stress response induced by mitochondria in vitro (99, 102, 103).
Studies on the therapeutic effect of irisin are still ongoing. Irisin was used for treatment in mice with coronary artery obstruction caused by ligation. It indicated that irisin could activate the ERK signaling pathway and promote endothelial cell migration, thereby accelerating angiogenesis at the infarct border zone, reducing infarct size and fibrosis and improving ventricular diastolic function in animal model (104, 105), which may involve the AKT/mTOR/S6K1/Nrf2 pathway (106). The lack of those protective effects was also observed in FNDC5-knockout mice (13). Irisin can also assist the cardiac repair process. Deng et al. (107) used bone marrow mesenchymal stem cells (BM-MSCs) pretreated with FNDC5 to transplant into myocardial infarction mice. They reported that compared with non-pretreatment transplantation, irisin promoted the effect of BM-MSCs in inducing proliferation of myocardial cell and vascular, and significantly reduced myocardial remodeling and fibrosis as well as apoptosis signals. In addition, they also observed an increase in the survival rate of BM-MSCs and the secretion of exosomes. Chen et al. (100) believed that the repair effect may be related to HDAC4 degradation and p38 activation. This may show the possibility that irisin would improve the success rate of cell transplantation and provide a new direction for the treatment of MI.
However, the concentration used in experiments were much higher than physiological state, suggesting that irisin may not function in the restoration of heart itself, yet might be promising in medical treatment to reduce the damage of cardiomyocytes after infarction and help with cell transplantation of heart. New tools like recombinant irisin should be encouraged for higher credibility. More importantly, since the current researches concerning treatment are all conducted on cells or animals, whether irisin helps in human remains unknown.
Since irisin was discovered, people have conducted a lot of in-depth research on its relationship with metabolic diseases due to its most widely recognized uncoupling effect. In recent years, as the anti-inflammatory, anti-oxidant and anti-apoptotic effects of irisin have been exposed, more and more researchers are trying to explore the maximum value of this newfound molecule. In this review, studies have explored its potential in cardiovascular system (Table 1). Some in vitro and in vivo experiments have shown that irisin can regulate endothelial cell function and reduce the damage of atherosclerosis through various ways, which could be a sign of therapeutic use. During ACS, it was believed that an appropriate amount of irisin’s serum concentration can protect the myocardium from damage. The serum concentration drops first and then rises after MI, which may be related to the reduction of energy consumption and the protection of mitochondrial function. Nevertheless, irisin would enhance the repairing effect in the process of cell transplantation of heart, which showed its therapeutic potential in a new way.
Yet there has been a big limitation of irisin’s development in recent years. With the questioning of traditional measuring methods, lots of findings of irisin call for reconfirmation. The huge range of irisin level caused by inaccurate measurement led to the inconsistency of cross-sectional studies, making it hard for irisin to become a detection indicator (90). Besides, the restoration function of irisin in an infarcted heart is also facing challenges. The effective concentration applied to cells or mice was 10- to 8000-fold higher than physiological level in the gain-of-function experiments, which is not convincing enough to prove the physiological functions of irisin (108). In addition, due to the extremely low amount of irisin, purification is not easy to meet the demand.
With all those setbacks and a relative scarcity of preclinical and clinical data, we failed to draw a consistent conclusion of irisin’s function in CHD. Thus, future researches need to be careful of selecting measuring techniques as well as observation subjects in order to control the baseline. New methods like recombinant irisin and gene-knockout mice would be the development direction in function experiments. In addition, the studies have been too scattered, each team working own its own perspective. It would push forward a lot if a general framework would form. And the experiments at present still remained at the stage of small-scale animal experiments, indicating that higher levels need to be performed.
In brief, while irisin hasn’t been qualified enough yet as an indicator of CHD, it is quite promising in treatment based on current studies. Only with more considerations in experimental design and larger-scale studies will irisin show its true colors in the near future.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was supported by funding from the National Natural Science Foundation of China (Nos. 81770881 and 82070910) and Key R&D Plan of Hunan Province (2020SK2078).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-Alpha-Dependent Myokine That Drives Brown-Fat-Like Development of White Fat and Thermogenesis. Nature (2012) 481(7382):463–8. doi: 10.1038/nature10777
2. Aydin S, Kuloglu T, Aydin S, Kalayci M, Yilmaz M, Cakmak T, et al. A Comprehensive Immunohistochemical Examination of the Distribution of the Fat-Burning Protein Irisin in Biological Tissues. Peptides (2014) 61:130–6. doi: 10.1016/j.peptides.2014.09.014
3. Raschke S, Elsen M, Gassenhuber H, Sommerfeld M, Schwahn U, Brockmann B, et al. Evidence Against a Beneficial Effect of Irisin in Humans. PloS One (2013) 8(9):e73680. doi: 10.1371/journal.pone.0073680
4. Dong J, Dong Y, Dong Y, Chen F, Mitch WE, Zhang L. Inhibition of Myostatin in Mice Improves Insulin Sensitivity via Irisin-Mediated Cross Talk Between Muscle and Adipose Tissues. Int J Obes (Lond) (2016) 40(3):434–42. doi: 10.1038/ijo.2015.200
5. Rodriguez A, Becerril S, Mendez-Gimenez L, Ramirez B, Sainz N, Catalan V, et al. Leptin Administration Activates Irisin-Induced Myogenesis via Nitric Oxide-Dependent Mechanisms, But Reduces its Effect on Subcutaneous Fat Browning in Mice. Int J Obes (Lond) (2015) 39(3):397–407. doi: 10.1038/ijo.2014.166
6. Matsuo Y, Gleitsmann K, Mangner N, Werner S, Fischer T, Bowen TS, et al. Fibronectin Type III Domain Containing 5 Expression in Skeletal Muscle in Chronic Heart Failure-Relevance of Inflammatory Cytokines. J Cachexia Sarcopenia Muscle (2015) 6(1):62–72. doi: 10.1002/jcsm.12006
7. Kurdiova T, Balaz M, Vician M, Maderova D, Vlcek M, Valkovic L, et al. Effects of Obesity, Diabetes and Exercise on Fndc5 Gene Expression and Irisin Release in Human Skeletal Muscle and Adipose Tissue: In Vivo and In Vitro Studies. J Physiol (2014) 592(5):1091–107. doi: 10.1113/jphysiol.2013.264655
8. Tang S, Zhang R, Jiang F, Wang J, Chen M, Peng D, et al. Circulating Irisin Levels Are Associated With Lipid and Uric Acid Metabolism in a Chinese Population. Clin Exp Pharmacol Physiol (2015) 42(9):896–901. doi: 10.1111/1440-1681.12439
9. Fox J, Rioux BV, Goulet EDB, Johanssen NM, Swift DL, Bouchard DR, et al. Effect of an Acute Exercise Bout on Immediate Post-Exercise Irisin Concentration in Adults: A Meta-Analysis. Scand J Med Sci Sports (2018) 28(1):16–28. doi: 10.1111/sms.12904
10. Qiu S, Cai X, Sun Z, Schumann U, Zugel M, Steinacker JM. Chronic Exercise Training and Circulating Irisin in Adults: A Meta-Analysis. Sports Med (2015) 45(11):1577–88. doi: 10.1007/s40279-014-0293-4
11. Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, et al. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab (2015) 22(4):734–40. doi: 10.1016/j.cmet.2015.08.001
12. Lehnig AC, Dewal RS, Baer LA, Kitching KM, Munoz VR, Arts PJ, et al. Exercise Training Induces Depot-Specific Adaptations to White and Brown Adipose Tissue. iScience (2019) 11:425–39. doi: 10.1016/j.isci.2018.12.033
13. Xiong Y, Wu Z, Zhang B, Wang C, Mao F, Liu X, et al. Fndc5 Loss-of-Function Attenuates Exercise-Induced Browning of White Adipose Tissue in Mice. FASEB J (2019) 33(5):5876–86. doi: 10.1096/fj.201801754RR
14. Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The Adipose Organ of Obesity-Prone C57BL/6J Mice Is Composed of Mixed White and Brown Adipocytes. J Lipid Res (2012) 53(4):619–29. doi: 10.1194/jlr.M018846
15. Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, et al. The Effects of Acute and Chronic Exercise on PGC-1alpha, Irisin and Browning of Subcutaneous Adipose Tissue in Humans. FEBS J (2014) 281(3):739–49. doi: 10.1111/febs.12619
16. Camera DM, Anderson MJ, Hawley JA, Carey AL. Short-Term Endurance Training Does Not Alter the Oxidative Capacity of Human Subcutaneous Adipose Tissue. Eur J Appl Physiol (2010) 109(2):307–16. doi: 10.1007/s00421-010-1356-3
17. Stinkens R, Brouwers B, Jocken JW, Blaak EE, Teunissen-Beekman KF, Hesselink MK, et al. Exercise Training-Induced Effects on the Abdominal Subcutaneous Adipose Tissue Phenotype in Humans With Obesity. J Appl Physiol (1985) (2018) 125(5):1585–93. doi: 10.1152/japplphysiol.00496.2018
18. Tsiloulis T, Carey AL, Bayliss J, Canny B, Meex RCR, Watt MJ. No Evidence of White Adipocyte Browning After Endurance Exercise Training in Obese Men. Int J Obes (Lond) (2018) 42(4):721–7. doi: 10.1038/ijo.2017.295
19. Vosselman MJ, Hoeks J, Brans B, Pallubinsky H, Nascimento EB, van der Lans AA, et al. Low Brown Adipose Tissue Activity in Endurance-Trained Compared With Lean Sedentary Men. Int J Obes (Lond) (2015) 39(12):1696–702. doi: 10.1038/ijo.2015.130
20. Pillon NJ, Gabriel BM, Dollet L, Smith JAB, Sardon Puig L, Botella J, et al. Transcriptomic Profiling of Skeletal Muscle Adaptations to Exercise and Inactivity. Nat Commun (2020) 11(1):470. doi: 10.1038/s41467-019-13869-w
21. Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y, Nagano K, et al. Irisin Mediates Effects on Bone and Fat via alphaV Integrin Receptors. Cell (2019) 178(2):507–8. doi: 10.1016/j.cell.2019.06.028
22. Bettini S, Favaretto F, Compagnin C, Belligoli A, Sanna M, Fabris R, et al. Resting Energy Expenditure, Insulin Resistance and UCP1 Expression in Human Subcutaneous and Visceral Adipose Tissue of Patients With Obesity. Front Endocrinol (Lausanne) (2019) 10:548. doi: 10.3389/fendo.2019.00548
23. Lim J, Park HS, Kim J, Jang YJ, Kim JH, Lee Y, et al. Depot-Specific UCP1 Expression in Human White Adipose Tissue and Its Association With Obesity-Related Markers. Int J Obes (Lond) (2020) 44(3):697–706. doi: 10.1038/s41366-020-0528-4
24. Timmons JA, Baar K, Davidsen PK, Atherton PJ. Is Irisin a Human Exercise Gene? Nature (2012) 488(7413):E9–10; discussion E-1. doi: 10.1038/nature11364
25. Shan T, Liang X, Bi P, Kuang S. Myostatin Knockout Drives Browning of White Adipose Tissue Through Activating the AMPK-PGC1alpha-Fndc5 Pathway in Muscle. FASEB J (2013) 27(5):1981–9. doi: 10.1096/fj.12-225755
26. Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, et al. Brown Adipose Tissue Oxidative Metabolism Contributes to Energy Expenditure During Acute Cold Exposure in Humans. J Clin Invest (2012) 122(2):545–52. doi: 10.1172/JCI60433
27. Zhang Y, Xie C, Wang H, Foss RM, Clare M, George EV, et al. Irisin Exerts Dual Effects on Browning and Adipogenesis of Human White Adipocytes. Am J Physiol Endocrinol Metab (2016) 311(2):E530–41. doi: 10.1152/ajpendo.00094.2016
28. Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F, et al. Irisin Is Expressed and Produced by Human Muscle and Adipose Tissue in Association With Obesity and Insulin Resistance. J Clin Endocrinol Metab (2013) 98(4):E769–78. doi: 10.1210/jc.2012-2749
29. Yang M, Chen P, Jin H, Xie X, Gao T, Yang L, et al. Circulating Levels of Irisin in Middle-Aged First-Degree Relatives of Type 2 Diabetes Mellitus - Correlation With Pancreatic Beta-Cell Function. Diabetol Metab Syndr (2014) 6(1):133. doi: 10.1186/1758-5996-6-133
30. Chang CL, Huang SY, Soong YK, Cheng PJ, Wang CJ, Liang IT. Circulating Irisin and Glucose-Dependent Insulinotropic Peptide Are Associated With the Development of Polycystic Ovary Syndrome. J Clin Endocrinol Metab (2014) 99(12):E2539–48. doi: 10.1210/jc.2014-1180
31. Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, et al. Exercise Induces Hippocampal BDNF Through a PGC-1alpha/FNDC5 Pathway. Cell Metab (2013) 18(5):649–59. doi: 10.1016/j.cmet.2013.09.008
32. Lourenco MV, Frozza RL, de Freitas GB, Zhang H, Kincheski GC, Ribeiro FC, et al. Exercise-Linked FNDC5/irisin Rescues Synaptic Plasticity and Memory Defects in Alzheimer’s Models. Nat Med (2019) 25(1):165–75. doi: 10.1038/s41591-018-0275-4
33. Moon HS, Dincer F, Mantzoros CS. Pharmacological Concentrations of Irisin Increase Cell Proliferation Without Influencing Markers of Neurite Outgrowth and Synaptogenesis in Mouse H19-7 Hippocampal Cell Lines. Metabolism (2013) 62(8):1131–6. doi: 10.1016/j.metabol.2013.04.007
34. Ma Y, Qiao X, Zeng R, Cheng R, Zhang J, Luo Y, et al. Irisin Promotes Proliferation But Inhibits Differentiation in Osteoclast Precursor Cells. FASEB J (2018) 32(11): 5813–23. doi: 10.1096/fj.201700983RR
35. Qiao X, Nie Y, Ma Y, Chen Y, Cheng R, Yin W, et al. Irisin Promotes Osteoblast Proliferation and Differentiation via Activating the MAP Kinase Signaling Pathways. Sci Rep (2016) 6:18732. doi: 10.1038/srep21053
36. Colaianni G, Cuscito C, Mongelli T, Oranger A, Mori G, Brunetti G, et al. Irisin Enhances Osteoblast Differentiation In Vitro. Int J Endocrinol (2014) 2014:902186. doi: 10.1155/2014/902186
37. Colaianni G, Mongelli T, Cuscito C, Pignataro P, Lippo L, Spiro G, et al. Irisin Prevents and Restores Bone Loss and Muscle Atrophy in Hind-Limb Suspended Mice. Sci Rep (2017) 7(1):2811. doi: 10.1038/s41598-017-02557-8
38. Anastasilakis AD, Polyzos SA, Makras P, Gkiomisi A, Bisbinas I, Katsarou A, et al. Circulating Irisin Is Associated With Osteoporotic Fractures in Postmenopausal Women With Low Bone Mass But Is Not Affected by Either Teriparatide or Denosumab Treatment for 3 Months. Osteoporos Int (2014) 25(5):1633–42. doi: 10.1007/s00198-014-2673-x
39. Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y, Nagano K, et al. Irisin Mediates Effects on Bone and Fat via alphaV Integrin Receptors. Cell (2018) 175(7):1756–68 e17. doi: 10.1016/j.cell.2018.10.025
40. Shao L, Li H, Chen J, Song H, Zhang Y, Wu F, et al. Irisin Suppresses the Migration, Proliferation, and Invasion of Lung Cancer Cells via Inhibition of Epithelial-to-Mesenchymal Transition. Biochem Biophys Res Commun (2017) 485(3):598–605. doi: 10.1016/j.bbrc.2016.12.084
41. Gannon NP, Vaughan RA, Garcia-Smith R, Bisoffi M, Trujillo KA. Effects of the Exercise-Inducible Myokine Irisin on Malignant and non-Malignant Breast Epithelial Cell Behavior In Vitro. Int J Cancer (2015) 136(4):E197–202. doi: 10.1002/ijc.29142
42. Park SE, Park CY, Sweeney G. Biomarkers of Insulin Sensitivity and Insulin Resistance: Past, Present and Future. Crit Rev Clin Lab Sci (2015) 52(4):180–90. doi: 10.3109/10408363.2015.1023429
43. Li RL, Wu SS, Wu Y, Wang XX, Chen HY, Xin JJ, et al. Irisin Alleviates Pressure Overload-Induced Cardiac Hypertrophy by Inducing Protective Autophagy via mTOR-Independent Activation of the AMPK-ULK1 Pathway. J Mol Cell Cardiol (2018) 121:242–55. doi: 10.1016/j.yjmcc.2018.07.250
44. Yu Q, Kou W, Xu X, Zhou S, Luan P, Xu X, et al. FNDC5/Irisin Inhibits Pathological Cardiac Hypertrophy. Clin Sci (Lond) (2019) 133(5):611–27. doi: 10.1042/CS20190016
45. Zhou X, Xu M, Bryant JL, Ma J, Xu X. Exercise-Induced Myokine FNDC5/irisin Functions in Cardiovascular Protection and Intracerebral Retrieval of Synaptic Plasticity. Cell Biosci (2019) 9:32. doi: 10.1186/s13578-019-0294-y
46. Albrecht E, Norheim F, Thiede B, Holen T, Ohashi T, Schering L, et al. Irisin - a Myth Rather Than an Exercise-Inducible Myokine. Sci Rep (2015) 5:8889. doi: 10.1038/srep08889
47. Polyzos SA, Mathew H, Mantzoros CS. Irisin: A True, Circulating Hormone. Metabolism (2015) 64(12):1611–8. doi: 10.1016/j.metabol.2015.09.001
48. Albrecht E, Schering L, Buck F, Vlach K, Schober HC, Drevon CA, et al. Irisin: Still Chasing Shadows. Mol Metab (2020) 34:124–35. doi: 10.1016/j.molmet.2020.01.016
49. Liu TY, Xiong XQ, Ren XS, Zhao MX, Shi CX, Wang JJ, et al. FNDC5 Alleviates Hepatosteatosis by Restoring AMPK/mTOR-Mediated Autophagy, Fatty Acid Oxidation, and Lipogenesis in Mice. Diabetes (2016) 65(11):3262–75. doi: 10.2337/db16-0356
50. Trevellin E, Scorzeto M, Olivieri M, Granzotto M, Valerio A, Tedesco L, et al. Exercise Training Induces Mitochondrial Biogenesis and Glucose Uptake in Subcutaneous Adipose Tissue Through eNOS-Dependent Mechanisms. Diabetes (2014) 63(8):2800–11. doi: 10.2337/db13-1234
51. Zhou B, Ling L, Zhang F, Liu TY, Zhou H, Qi XH, et al. Fibronectin Type III Domain-Containing 5 Attenuates Liver Fibrosis Via Inhibition of Hepatic Stellate Cell Activation. Cell Physiol Biochem (2018) 48(1):227–36. doi: 10.1159/000491722
52. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation (2020) 141(9):e139–596. doi: 10.1161/CIR.0000000000000757
53. Aronis KN, Moreno M, Polyzos SA, Moreno-Navarrete JM, Ricart W, Delgado E, et al. Circulating Irisin Levels and Coronary Heart Disease: Association With Future Acute Coronary Syndrome and Major Adverse Cardiovascular Events. Int J Obes (Lond) (2015) 39(1):156–61. doi: 10.1038/ijo.2014.101
54. Hopkins PN. Molecular Biology of Atherosclerosis. Physiol Rev (2013) 93(3):1317–542. doi: 10.1152/physrev.00004.2012
55. Deng W. Association of Serum Irisin Concentrations With Presence and Severity of Coronary Artery Disease. Med Sci Monit (2016) 22:4193–7. doi: 10.12659/MSM.897376
56. Khorasani ZM, Bagheri RK, Yaghoubi MA, Chobkar S, Aghaee MA, Abbaszadegan MR, et al. The Association Between Serum Irisin Levels and Cardiovascular Disease in Diabetic Patients. Diabetes Metab Syndr (2019) 13(1):786–90. doi: 10.1016/j.dsx.2018.11.050
57. Saadeldin MK, Elshaer SS, Emara IA, Maged M, Abdel-Aziz AK. Serum Sclerostin and Irisin as Predictive Markers for Atherosclerosis in Egyptian Type II Diabetic Female Patients: A Case Control Study. PloS One (2018) 13(11):e0206761. doi: 10.1371/journal.pone.0206761
58. Lee MJ, Lee SA, Nam BY, Park S, Lee SH, Ryu HJ, et al. Irisin, a Novel Myokine Is an Independent Predictor for Sarcopenia and Carotid Atherosclerosis in Dialysis Patients. Atherosclerosis (2015) 242(2):476–82. doi: 10.1016/j.atherosclerosis.2015.08.002
59. Icli A, Cure E, Cumhur Cure M, Uslu AU, Balta S, Arslan S, et al. Novel Myokine: Irisin May Be An Independent Predictor for Subclinic Atherosclerosis in Behcet’s Disease. J Investig Med (2016) 64(4):875–81. doi: 10.1136/jim-2015-000044
60. Sesti G, Andreozzi F, Fiorentino TV, Mannino GC, Sciacqua A, Marini MA, et al. High Circulating Irisin Levels Are Associated With Insulin Resistance and Vascular Atherosclerosis in a Cohort of Nondiabetic Adult Subjects. Acta Diabetol (2014) 51(5):705–13. doi: 10.1007/s00592-014-0576-0
61. Kwasniewska M, Kostka T, Jegier A, Dziankowska-Zaborszczyk E, Leszczynska J, Rebowska E, et al. Regular Physical Activity and Cardiovascular Biomarkers in Prevention of Atherosclerosis in Men: A 25-Year Prospective Cohort Study. BMC Cardiovasc Disord (2016) 16:65. doi: 10.1186/s12872-016-0239-x
62. Moreno-Perez O, Reyes-Garcia R, Munoz-Torres M, Merino E, Boix V, Reus S, et al. High Irisin Levels in Nondiabetic HIV-Infected Males Are Associated With Insulin Resistance, Nonalcoholic Fatty Liver Disease, and Subclinical Atherosclerosis. Clin Endocrinol (Oxf) (2018) 89(4):414–23. doi: 10.1111/cen.13800
63. Perakakis N, Triantafyllou GA, Fernandez-Real JM, Huh JY, Park KH, Seufert J, et al. Physiology and Role of Irisin in Glucose Homeostasis. Nat Rev Endocrinol (2017) 13(6):324–37. doi: 10.1038/nrendo.2016.221
64. Hisamatsu T, Miura K, Arima H, Fujiyoshi A, Kadota A, Kadowaki S, et al. Relationship of Serum Irisin Levels to Prevalence and Progression of Coronary Artery Calcification: A Prospective, Population-Based Study. Int J Cardiol (2018) 267:177–82. doi: 10.1016/j.ijcard.2018.05.075
65. Lu J, Xiang G, Liu M, Mei W, Xiang L, Dong J. Irisin Protects Against Endothelial Injury and Ameliorates Atherosclerosis in Apolipoprotein E-Null Diabetic Mice. Atherosclerosis (2015) 243(2):438–48. doi: 10.1016/j.atherosclerosis.2015.10.020
66. Han F, Zhang S, Hou N, Wang D, Sun X. Irisin Improves Endothelial Function in Obese Mice Through the AMPK-eNOS Pathway. Am J Physiol Heart Circ Physiol (2015) 309(9):H1501–8. doi: 10.1152/ajpheart.00443.2015
67. Zhang Y, Mu Q, Zhou Z, Song H, Zhang Y, Wu F, et al. Protective Effect of Irisin on Atherosclerosis via Suppressing Oxidized Low Density Lipoprotein Induced Vascular Inflammation and Endothelial Dysfunction. PloS One (2016) 11(6):e0158038. doi: 10.1371/journal.pone.0158038
68. Zhu D, Wang H, Zhang J, Zhang X, Xin C, Zhang F, et al. Irisin Improves Endothelial Function in Type 2 Diabetes Through Reducing Oxidative/Nitrative Stresses. J Mol Cell Cardiol (2015) 87:138–47. doi: 10.1016/j.yjmcc.2015.07.015
69. Zhang Y, Song H, Zhang Y, Wu F, Mu Q, Jiang M, et al. Irisin Inhibits Atherosclerosis by Promoting Endothelial Proliferation Through Microrna126-5p. J Am Heart Assoc (2016) 5(9):e004031. doi: 10.1161/JAHA.116.004031
70. Deng X, Huang W, Peng J, Zhu TT, Sun XL, Zhou XY, et al. Irisin Alleviates Advanced Glycation End Products-Induced Inflammation and Endothelial Dysfunction via Inhibiting ROS-NLRP3 Inflammasome Signaling. Inflammation (2018) 41(1):260–75. doi: 10.1007/s10753-017-0685-3
71. Shimba Y, Togawa H, Senoo N, Ikeda M, Miyoshi N, Morita A, et al. Skeletal Muscle-Specific PGC-1alpha Overexpression Suppresses Atherosclerosis in Apolipoprotein E-Knockout Mice. Sci Rep (2019) 9(1):4077. doi: 10.1038/s41598-019-40643-1
72. Yin C, Hu W, Wang M, Lv W, Jia T, Xiao Y. Irisin as a Mediator Between Obesity and Vascular Inflammation in Chinese Children and Adolescents. Nutr Metab Cardiovasc Dis (2020) 30(2):320–9. doi: 10.1016/j.numecd.2019.09.025
73. Zheng G, Li H, Zhang T, Yang L, Yao S, Chen S, et al. Irisin Protects Macrophages From Oxidized Low Density Lipoprotein-Induced Apoptosis by Inhibiting the Endoplasmic Reticulum Stress Pathway. Saudi J Biol Sci (2018) 25(5):849–57. doi: 10.1016/j.sjbs.2017.08.018
74. Kannel WB, Feinleib M. Natural History of Angina Pectoris in the Framingham Study. Prognosis and Survival. Am J Cardiol (1972) 29(2):154–63. doi: 10.1016/0002-9149(72)90624-8
75. Efe TH, Acar B, Ertem AG, Yayla KG, Algul E, Yayla C, et al. Serum Irisin Level Can Predict the Severity of Coronary Artery Disease in Patients With Stable Angina. Korean Circ J (2017) 47(1):44–9. doi: 10.4070/kcj.2016.0079
76. Anastasilakis AD, Koulaxis D, Kefala N, Polyzos SA, Upadhyay J, Pagkalidou E, et al. Circulating Irisin Levels Are Lower in Patients With Either Stable Coronary Artery Disease (CAD) or Myocardial Infarction (MI) Versus Healthy Controls, Whereas Follistatin and Activin A Levels Are Higher and can Discriminate MI From CAD With Similar to CK-MB Accuracy. Metabolism (2017) 73:1–8. doi: 10.1016/j.metabol.2017.05.002
77. Park KH, Zaichenko L, Brinkoetter M, Thakkar B, Sahin-Efe A, Joung KE, et al. Circulating Irisin in Relation to Insulin Resistance and the Metabolic Syndrome. J Clin Endocrinol Metab (2013) 98(12):4899–907. doi: 10.1210/jc.2013-2373
78. Arbustini E, Burke A, Dal Bello B, Morbini P, Specchia G, Virmani R. Plaque Erosion Is a Major Substrate for Coronary Thrombosis in Acute Myocardial Infarction. J Am Coll Cardiol (1998) 31(3):269. doi: 10.1136/hrt.82.3.269
79. Higuma T, Soeda T, Abe N, Yamada M, Yokoyama H, Shibutani S, et al. A Combined Optical Coherence Tomography and Intravascular Ultrasound Study on Plaque Rupture, Plaque Erosion, and Calcified Nodule in Patients With ST-Segment Elevation Myocardial Infarction: Incidence, Morphologic Characteristics, and Outcomes After Percutaneous Coronary Intervention. JACC Cardiovasc Interv (2015) 8(9):1166–76. doi: 10.1016/j.jcin.2015.02.026
80. Davies MJ, Thomas A. Thrombosis and Acute Coronary-Artery Lesions in Sudden Cardiac Ischemic Death. N Engl J Med (1984) 310(18):1137–40. doi: 10.1056/NEJM198405033101801
81. Kuloglu T, Aydin S, Eren MN, Yilmaz M, Sahin I, Kalayci M, et al. Irisin: A Potentially Candidate Marker for Myocardial Infarction. Peptides (2014) 55:85–91. doi: 10.1016/j.peptides.2014.02.008
82. Bashar SM, Samir El-Sherbeiny SM, Boraie MZ. Correlation Between the Blood Level of Irisin and the Severity of Acute Myocardial Infarction in Exercise-Trained Rats. J Basic Clin Physiol Pharmacol (2018) 30(1):59–71. doi: 10.1515/jbcpp-2018-0090
83. Hsieh IC, Ho MY, Wen MS, Chen CC, Hsieh MJ, Lin CP, et al. Serum Irisin Levels Are Associated With Adverse Cardiovascular Outcomes in Patients With Acute Myocardial Infarction. Int J Cardiol (2018) 261:12–7. doi: 10.1016/j.ijcard.2017.11.072
84. Aydin S, Kobat MA, Kalayci M, Eren MN, Yilmaz M, Kuloglu T, et al. Decreased Saliva/Serum Irisin Concentrations in the Acute Myocardial Infarction Promising for Being a New Candidate Biomarker for Diagnosis of This Pathology. Peptides (2014) 56:141–5. doi: 10.1016/j.peptides.2014.04.002
85. Aydin S, Catak Z, Eren MN, Topal AE, Aydin S. Irisin in Coronary Bypass Surgery. Cardiovasc Hematol Disord Drug Targets (2018) 18(3):208–14. doi: 10.2174/1871529X18666180511141151
86. Xin T, Lu C. Irisin Activates Opa1-Induced Mitophagy to Protect Cardiomyocytes Against Apoptosis Following Myocardial Infarction. Aging (Albany NY) (2020) 12(5):4474–88. doi: 10.18632/aging.102899
87. Brailoiu E, Deliu E, Sporici RA, Brailoiu GC. Irisin Evokes Bradycardia by Activating Cardiac-Projecting Neurons of Nucleus Ambiguus. Physiol Rep (2015) 3(6):e12419. doi: 10.14814/phy2.12419
88. Tao L, Bei Y, Lin S, Zhang H, Zhou Y, Jiang J, et al. Exercise Training Protects Against Acute Myocardial Infarction via Improving Myocardial Energy Metabolism and Mitochondrial Biogenesis. Cell Physiol Biochem (2015) 37(1):162–75. doi: 10.1159/000430342
89. Jiang M, Wan F, Wang F, Wu Q. Irisin Relaxes Mouse Mesenteric Arteries Through Endothelium-Dependent and Endothelium-Independent Mechanisms. Biochem Biophys Res Commun (2015) 468(4):832–6. doi: 10.1016/j.bbrc.2015.11.040
90. Guo W, Zhang B, Wang X. Lower Irisin Levels in Coronary Artery Disease: A Meta-Analysis. Minerva Endocrinol (2020) 45(1):61–9. doi: 10.23736/S0391-1977.17.02663-3
91. Emanuele E, Minoretti P, Pareja-Galeano H, Sanchis-Gomar F, Garatachea N, Lucia A. Serum Irisin Levels, Precocious Myocardial Infarction, and Healthy Exceptional Longevity. Am J Med (2014) 127(9):888–90. doi: 10.1016/j.amjmed.2014.04.025
92. Belviranli M, Okudan N. Exercise Training Increases Cardiac, Hepatic and Circulating Levels of Brain-Derived Neurotrophic Factor and Irisin in Young and Aged Rats. Horm Mol Biol Clin Investig (2018) 36(3):20180053. doi: 10.1515/hmbci-2018-0053
93. Badr EA, Mostafa RG, Awad SM, Marwan H, Abd El-Bary HM, Shehab HE, et al. A Pilot Study on the Relation Between Irisin Single-Nucleotide Polymorphism and Risk of Myocardial Infarction. Biochem Biophys Rep (2020) 22:100742. doi: 10.1016/j.bbrep.2020.100742
94. Martins IJ, Hone E, Foster JK, Sunram-Lea SI, Gnjec A, Fuller SJ, et al. Cholesterol Metabolism, Diabetes, and the Convergence of Risk Factors for Alzheimer’s Disease and Cardiovascular Disease. Mol Psychiatry (2006) 11(8):721–36. doi: 10.1038/sj.mp.4001854
95. Heeren J, Beisiegel U, Grewal T. Apolipoprotein E Recycling: Implications for Dyslipidemia and Atherosclerosis. Arterioscler Thromb Vasc Biol (2006) 26(3):442–8. doi: 10.1161/01.ATV.0000201282.64751.47
96. Fuku N, Diaz-Pena R, Arai Y, Abe Y, Zempo H, Naito H, et al. Epistasis, Physical Capacity-Related Genes and Exceptional Longevity: FNDC5 Gene Interactions With Candidate Genes FOXOA3 and APOE. BMC Genomics (2017) 18(Suppl 8):803. doi: 10.1186/s12864-017-4194-4
97. Moscoso I, Cebro-Marquez M, Rodriguez-Manero M, Gonzalez-Juanatey JR, Lage R. FNDC5/Irisin Counteracts Lipotoxic-Induced Apoptosis in Hypoxic H9c2 Cells. J Mol Endocrinol (2019) 63(2):151–9. doi: 10.1530/JME-19-0123
98. Peng Q, Wang X, Wu K, Liu K, Wang S, Chen X. Irisin Attenuates H2O2-Induced Apoptosis in Cardiomyocytes via microRNA-19b/AKT/mTOR Signaling Pathway. Int J Clin Exp Pathol (2017) 10(7):7707–17.
99. Zhao YT, Wang H, Zhang S, Du J, Zhuang S, Zhao TC. Irisin Ameliorates Hypoxia/Reoxygenation-Induced Injury Through Modulation of Histone Deacetylase 4. PloS One (2016) 11(11):e0166182. doi: 10.1371/journal.pone.0166182
100. Chen HP, Denicola M, Qin X, Zhao Y, Zhang L, Long XL, et al. HDAC Inhibition Promotes Cardiogenesis and the Survival of Embryonic Stem Cells Through Proteasome-Dependent Pathway. J Cell Biochem (2011) 112(11):3246–55. doi: 10.1002/jcb.23251
101. Wende AR, Abel ED. Lipotoxicity in the Heart. Biochim Biophys Acta (2010) 1801(3):311–9. doi: 10.1016/j.bbalip.2009.09.023
102. Wang Z, Chen K, Han Y, Zhu H, Zhou X, Tan T, et al. Irisin Protects Heart Against Ischemia-Reperfusion Injury Through a SOD2-Dependent Mitochondria Mechanism. J Cardiovasc Pharmacol (2018) 72(6):259–69. doi: 10.1097/FJC.0000000000000608
103. Lu L, Ma J, Tang J, Liu Y, Zheng Q, Chen S, et al. Irisin Attenuates Myocardial Ischemia/Reperfusion-Induced Cardiac Dysfunction by Regulating ER-Mitochondria Interaction Through a Mitochondrial Ubiquitin Ligase-Dependent Mechanism. Clin Transl Med (2020) 10(5):e166. doi: 10.1002/ctm2.166
104. Liao Q, Qu S, Tang LX, Li LP, He DF, Zeng CY, et al. Irisin Exerts a Therapeutic Effect Against Myocardial Infarction via Promoting Angiogenesis. Acta Pharmacol Sin (2019) 40(10):1314–21. doi: 10.1038/s41401-019-0230-z
105. Xin C, Zhang Z, Gao G, Ding L, Yang C, Wang C, et al. Irisin Attenuates Myocardial Ischemia/Reperfusion Injury and Improves Mitochondrial Function Through AMPK Pathway in Diabetic Mice. Front Pharmacol (2020) 11:565160. doi: 10.3389/fphar.2020.565160
106. Zhang M, Xu Y, Jiang L. Irisin Attenuates Oxidized Low-Density Lipoprotein Impaired Angiogenesis Through AKT/mTOR/S6K1/Nrf2 Pathway. J Cell Physiol (2019) 234(10):18951–62. doi: 10.1002/jcp.28535
107. Deng J, Zhang N, Wang Y, Yang C, Wang Y, Xin C, et al. FNDC5/irisin Improves the Therapeutic Efficacy of Bone Marrow-Derived Mesenchymal Stem Cells for Myocardial Infarction. Stem Cell Res Ther (2020) 11(1):228. doi: 10.1186/s13287-020-01746-z
108. Rabiee F, Lachinani L, Ghaedi S, Nasr-Esfahani MH, Megraw TL, Ghaedi K. New Insights Into the Cellular Activities of Fndc5/Irisin and Its Signaling Pathways. Cell Biosci (2020) 10:51. doi: 10.1186/s13578-020-00413-3
109. Song H, Wu F, Zhang Y, Zhang Y, Wang F, Jiang M, et al. Irisin Promotes Human Umbilical Vein Endothelial Cell Proliferation Through the ERK Signaling Pathway and Partly Suppresses High Glucose-Induced Apoptosis. PloS One (2014) 9(10):e110273. doi: 10.1371/journal.pone.0110273
Keywords: irisin, myokine, coronary heart disease, infarction, atherosclerosis
Citation: Ou-Yang W-L, Guo B, Xu F, Lin X, Li F-X-Z, Shan S-K, Wu F, Wang Y, Zheng M-H, Xu Q-S and Yuan L-Q (2021) The Controversial Role of Irisin in Clinical Management of Coronary Heart Disease. Front. Endocrinol. 12:678309. doi: 10.3389/fendo.2021.678309
Received: 09 March 2021; Accepted: 17 June 2021;
Published: 01 July 2021.
Edited by:
Rita De Matteis, University of Urbino Carlo Bo, ItalyReviewed by:
Michael Lichtenauer, Paracelsus Medical University, AustriaCopyright © 2021 Ou-Yang, Guo, Xu, Lin, Li, Shan, Wu, Wang, Zheng, Xu and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling-Qing Yuan, YWxsZW55bHFAY3N1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.