
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 26 August 2021
Sec. Clinical Diabetes
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.668447
This article is part of the Research Topic Achieving Efficient Diabetes Care Now Through Understanding the Risk Factors, Markers, and Patient's Experiences View all 10 articles
Women in the Middle East and North Africa (MENA) region are burdened with several risk factors related to gestational diabetes mellitus (GDM) including overweight and high parity. We systematically reviewed the literature and quantified the weighted prevalence of GDM in MENA at the regional, subregional, and national levels. Studies published from 2000 to 2019 reporting the prevalence of GDM in the MENA region were retrieved and were assessed for their eligibility. Overall and subgroup pooled prevalence of GDM was quantified by random-effects meta-analysis. Sources of heterogeneity were investigated by meta-regression. The risk of bias (RoB) was assessed by the National Heart, Lung, and Blood Institute’s tool. One hundred and two research articles with 279,202 tested pregnant women for GDM from 16 MENA countries were included. Most of the research reports sourced from Iran (36.3%) and Saudi Arabia (21.6%), with an overall low RoB. In the 16 countries, the pooled prevalence of GDM was 13.0% (95% confidence interval [CI], 11.5–14.6%, I2, 99.3%). Nationally, GDM was highest in Qatar (20.7%, 95% CI, 15.2–26.7% I2, 99.0%), whereas subregionally, GDM was highest in Gulf Cooperation Council (GCC) countries (14.7%, 95% CI, 13.0–16.5%, I2, 99.0%). The prevalence of GDM was high in pregnant women aged ≥30 years (21.9%, 95% CI, 18.5–25.5%, I2, 97.1%), in their third trimester (20.0%, 95% CI, 13.1–27.9%, I2, 98.8%), and who were obese (17.2%, 95% CI, 12.8–22.0%, I2, 93.8%). The prevalence of GDM was 10.6% (95% CI, 8.1–13.4%, I2, 98.9%) in studies conducted before 2009, whereas it was 14.0% (95% CI, 12.1–16.0%, I2, 99.3%) in studies conducted in or after 2010. Pregnant women in the MENA region are burdened with a substantial prevalence of GDM, particularly in GCC and North African countries. Findings have implications for maternal health in the MENA region and call for advocacy to unify GDM diagnostic criteria.
Systematic Review Registration: PROSPERO CRD42018100629
Gestational diabetes mellitus (GDM) (1) is usually diagnosed during the second and third trimesters of pregnancy (2). Risk factors of GDM include excessive body weight, low level of physical activity, consanguineous marriage, previous history of GDM, glycated hemoglobin >5.7%, and history of cardiovascular disease (3). As the toll of overweight and obese reproductive-age females soars, the risk of developing hyperglycemia in pregnancy increases (4).
GDM has a global public health burden (5) with both short- and long-term consequences on health. The short-term ramifications of GDM include adverse perinatal outcomes for the affected women (e.g., preeclampsia, polyhydramnios, and increased cesarean section [“C-section”] risk) and their neonates (e.g., macrosomia and shoulder dystocia) (1, 6), whereas the long-term complications of GDM incorporate the risk of type 2 diabetes mellitus (T2DM) for the mother and the risk of childhood obesity, impaired glucose tolerance, and/or metabolic syndrome for their neonates (6). Since increased blood glucose levels are associated with certain perinatal complications, gestational blood glucose control is vital (7).
Understanding population-specific healthcare needs at specific points of time is essential, and prevalence estimates are ideal for such purposes (8). Unfortunately, the global GDM prevalence estimates (<1%–28%) show a wide variation due to ethnicity, ethnic variation among various populations, and inconsistent use of screening and diagnostic criteria (4, 9). To precisely estimate the burden of GDM of a particular geographic area, it is essential to determine the region-specific prevalence estimate. There is scant literature on the prevalence of GDM in the Middle East and North Africa (MENA) region, although two of the main risk factors [physical inactivity and above-normal body mass index (BMI)] are identified as being highly prevalent in this region (10). Moreover, three of the world’s top ten most prevalent countries for diabetes mellitus belong to this region: Saudi Arabia (24%), Kuwait (23%), and Qatar (23%) (11). For the entire Eastern Mediterranean region, the existing prevalence estimate of GDM is 14.5%, although this includes only cases diagnosed according to the World Health Organization (WHO) 1999 criteria (4). One previous survey showed that physicians and hospitals in this region use different criteria to diagnose GDM (12).
A systematic review and meta-analysis of prevalence studies is considered to be an ideal method to understand the burden of GDM at regional and national levels. In this systematic review, meta-analysis, and meta-regression, we estimated the weighted pooled prevalence of GDM in the MENA region, at the regional, subregional, and national levels, based on literature published between January 2000 and December 2019.
This review follows the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2009 guidelines (13). The PRISMA checklist is provided elsewhere (Supplementary File 1). Following our published protocol, we report here “systematic review 2” (14). We implemented minor amendments whenever needed, including an updated database search.
To identify eligible studies reporting the prevalence of GDM in the MENA countries, we conducted a comprehensive search of five electronic databases (MEDLINE, EMBASE, Web of Science, SCOPUS, and Cochrane library) from January 1, 2000, to December 31, 2019, using variant Medical Subject Headings and free-text terms. Restricting the literature search to 2000 was to estimate changes in the GDM prevalence over the past two decades (before and after 2010), at national, sub-regional, and regional levels, whenever enough data is available for the meta-analysis. The literature search strategy was developed in consultation with an expert librarian at the National Medical Library at the United Arab Emirates University. The full search strategy available in the published protocol (14). Retrieved references were imported to the Covidence software (Covidence, Melbourne, Australia) (15). Deduplication of similar references was performed automatically by the Covidence software.
To identify and select studies for inclusion, we followed the PECO(T) framework: participants, exposure, comparator, outcome(s), and type of study (16). However, we considered only participants and outcomes because the focus of this review was on studies reporting the prevalence of GDM. Study eligibility criteria are presented in Table 1.
Titles and abstracts were screened by RHA, NMA, and MSP to detect eligible research reports on the prevalence of GDM. For studies that appeared eligible, the full text was reviewed (RHA, NMA, and MSP). Screening of all titles and abstracts and full text articles was performed independently by two reviewers. Disagreements among reviewers were resolved by discourse. We also searched the reference lists of eligible studies for studies that might have been missed. Figure 1 shows the PRISMA flowchart of study selection.
In this review, the term “research report” is used to refer to a full published research document. The term “study” is used to refer to a single study on a specific population group. One big observational study (one research report) provides GDM data stratified into four age groups (four studies). Hence, one research report could contribute several studies on GDM prevalence.
Relevant data from eligible studies were extracted into a predesigned Excel sheet using a predefined list of numerical and string variables. The outcome of interest was the weighted prevalence of GDM in pregnant women in the MENA countries, according to various characteristics including, but not limited to, age, BMI, trimester, and time period. We extracted author names, publication year, country, city, and study setting. In addition, data on the implemented methodology (design, data collection period, sampling strategy, and GDM diagnosis and ascertainment methodology) and characteristics of the studied pregnant women (age, pregnancy trimester, sample size, number of women with GDM and GDM prevalence) were extracted whenever available.
In addition to the overall prevalence of GDM, some research reports also reported the prevalence of GDM stratified according to different characteristics, such as age, parity, comorbidity, pregnancy trimester, and BMI. In such reports, data extraction was performed for the stratified GDM prevalence, following the rule that the study had to have at least ten tested subjects per strata; otherwise, information on the entire tested sample was extracted. A predefined sequential order was established when extracting stratified GDM prevalence estimates as follows: GDM stratified first according to comorbidities followed by parity, age, and BMI. This prioritization was used to identify the strata with more information on the tested pregnant women. When there was no stratification for the prevalence of GDM, we extracted the overall GDM prevalence measured.
For each research report reporting the stratified prevalence of GDM according to more than one category (i.e., age and BMI), one category per research report was considered and included based on the aforementioned prioritization scheme, to avoid double counting. In studies in which GDM was ascertained using different guidelines, the most sensitive and reliable ascertainment assay was considered (i.e., prioritizing fasting blood glucose over self-reported) or was based on the most recent and updated criteria (i.e., prioritizing WHO 2010 over 2006 criteria).
The risk of bias (RoB) assessment was performed at the level of the research report rather than the study. The quality of each research report was evaluated according to criteria of the National Heart, Lung, and Blood Institute (18). Six of 14 items from the quality assessment tool for prevalence studies were used (18). The six quality-related items assessed the research question/objectives, studied population, sample size justification, and outcome measures and assessment. Eight items were not used because they are applicable only to follow-up cohort studies. For additional quality assessment, we also assessed the robustness of the implemented methodology using three additional quality-of-evidence criteria: sampling methodology, GDM ascertainment methodology, and precision of the estimate. Studies were considered to have “high” precision if at least 100 women were tested for GDM. We computed the overall proportion of research reports with potentially low RoB across each of these nine quality criteria and also computed the proportion (out of nine) of quality items with a potentially low RoB for each of the included research reports.
Data abstraction and quality assessment were performed independently by two reviewers (NA and MP) and cross-checked for disagreements. Any discrepancies in the extraction phase or in the quality assessment between the reviewers were discussed and resolved with a consultation of a senior reviewer (RA-R).
To estimate the weighted pooled prevalence of GDM and the corresponding 95% confidence interval (CI), we performed meta-analyses of the extracted data. The Freeman–Tukey double arcsine transformation method was applied to stabilize the variances of the prevalence measures (19). The inverse variance method was used to weight the estimated pooled prevalence measures (20). Dersimonian–Laird random-effects model was used to estimate the overall pooled GDM prevalence (21). Cochran’s Q statistic and the inconsistency index, I2, were calculated to measure heterogeneity (22). Along with the pooled estimates, ranges and median were also reported to describe the dispersion of the GDM prevalence measures reported in the literature. The prediction interval, which estimates the 95% interval in which the true effect size in a new prevalence study will lie, was also quantified and reported (22).
For the subgroup meta-analysis, country-level pooled estimates were generated overall and based on time period. In addition, to estimate the change in GDM both at the country level and overall, the data collection period was stratified into two time periods: 2000–2009 and 2010–2019. For studies in which the data collection period overlapped, the collection period was defined as “overlap” so as not to miss any important data when estimating country-level, subregional, and regional prevalence. The median (~2 years) was used in studies with an unclear data collection period. In these studies, the median was subtracted from the year of publication to estimate the year of data collection.
The weighted pooled prevalence, regardless of country, was also estimated according to the age of the pregnant women, trimester, BMI, study period, GDM ascertainment guidelines, and sample size (<100 or ≥100). The provision of pooled estimates regardless of the ascertainment guidelines was justified by the fact that the women were defined and treated as GDM patients following each specific ascertainment guideline.
Accumulated evidence has shown that GDM is associated with an increased risk of C-section (23, 24) and maternal mortality (4). Independent of the research report and the characteristics of the tested pregnant women for GDM, we estimated the pooled GDM prevalence according to the C-section rate and maternal mortality ratio (MMR). Information on the C-section rate (25, 26) and MMR were retrieved from various resources (27). Depending on data availability, information on C-section rate and MMR was extracted in the same or the closest year to the estimated GDM prevalence. For every GDM study, the rate of C-section was then categorized as <15%, 15–29%, >30%, or unclear, whereas the MMR was categorized as either ≤100/100,000 live births, >100/100,000 live births, or unclear.
To provide prevalence estimates at a subregional level, we regrouped MENA countries into four subregions, namely, North Africa, Gulf Cooperation Council (GCC) countries, Levant, and Iran/Iraq region. We estimated the overall pooled prevalence in these subregions and according to patient age, trimester, BMI, study period, GDM ascertainment guidelines, rate of C-section, and MMR.
Random-effects univariate and multivariable meta-regression models were implemented to identify sources of between-study heterogeneity and to quantify their contribution to variability in the prevalence of GDM. In univariate meta-regression models, analysis was performed by country, age, pregnancy trimester, BMI, and sample size. All variables with a p-value <0.1 in the univariate models were included in the multivariable model. In the final multivariable model, a p-value ≤0.05 was considered statistically significant, which contributed to the heterogeneity in prevalence estimates.
A funnel plot was generated to explore the small-study effect on the pooled GDM prevalence estimates. The funnel plot was created by plotting each GDM prevalence measure against its standard error. The asymmetry of the funnel plot was tested using Egger’s test (28).
All analyses were performed using the metaprop (29) and metareg packages in Stata/SE v15 (30).
The study is registered with PROSPERO, number CRD42018100629.
Of the 13,139 citations retrieved from the 5 databases, 102 research reports were deemed eligible and included in this review (Figure 1).
The research reports were from 16 countries in the MENA region: Algeria (one), Bahrain (two), Egypt (four), Iraq (three), Iran (37), Jordan (four), Lebanon (two), Libya (one), Morocco (one), Oman (five), Qatar (six), Saudi Arabia (22), Sudan (two), Tunisia (one), United Arab Emirates (UAE) (eight), and Yemen (one). The prevalence data for both decades (time periods) were available from six countries (Bahrain, Iran, Oman, Qatar, Saudi Arabia, and the UAE); for the other countries, data were available for the time period 2010–2019 (Table 2). Self-reported GDM status was documented in five research reports (31, 73, 83, 90, 119). The predominantly used GDM diagnostic criteria in the MENA region were from the American Diabetes Association and the International Association of Diabetes and Pregnancy Study Group (ADA/IADPSG; 48.5% of studies).
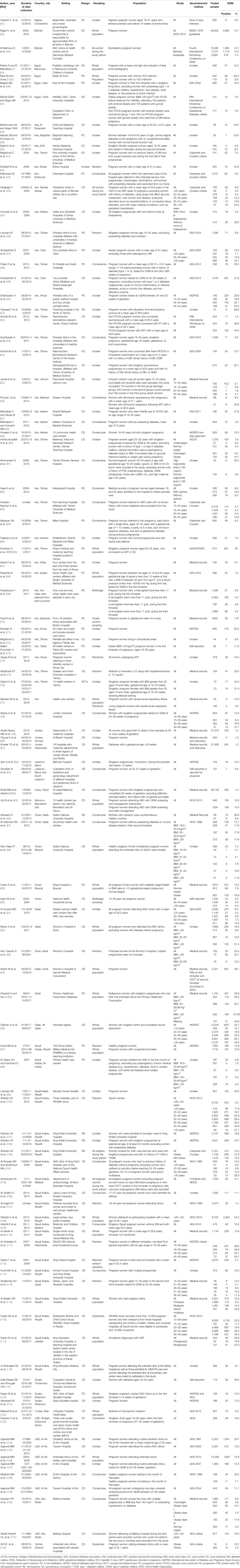
Table 2 Summary of the included studies reporting the prevalence of GDM in pregnant women in the MENA region, 2000–2019, stratified by country (102 reports with 198 prevalence measures).
The 102 research reports (31–67, 69–132) yielded 198 GDM prevalence studies. Iran (32.3%) (41, 43–67, 69–77) and Saudi Arabia (24.2%) (97–118) contributed to most of the prevalence studies, followed by Qatar (9.7%). In these prevalence studies, a total of 279,202 pregnant women were tested for GDM between 2000 and 2019, and the crude GDM prevalence was estimated to be about 11.0%. The prevalence of GDM ranged from 0.0% in three studies (60, 98, 104) to 50.7% in pregnant women aged 40–49 years in Saudi Arabia tested between 2007 and 2009 (111). The GDM prevalence range was identical in studies reported in the two decades (Tables 2 and 3).
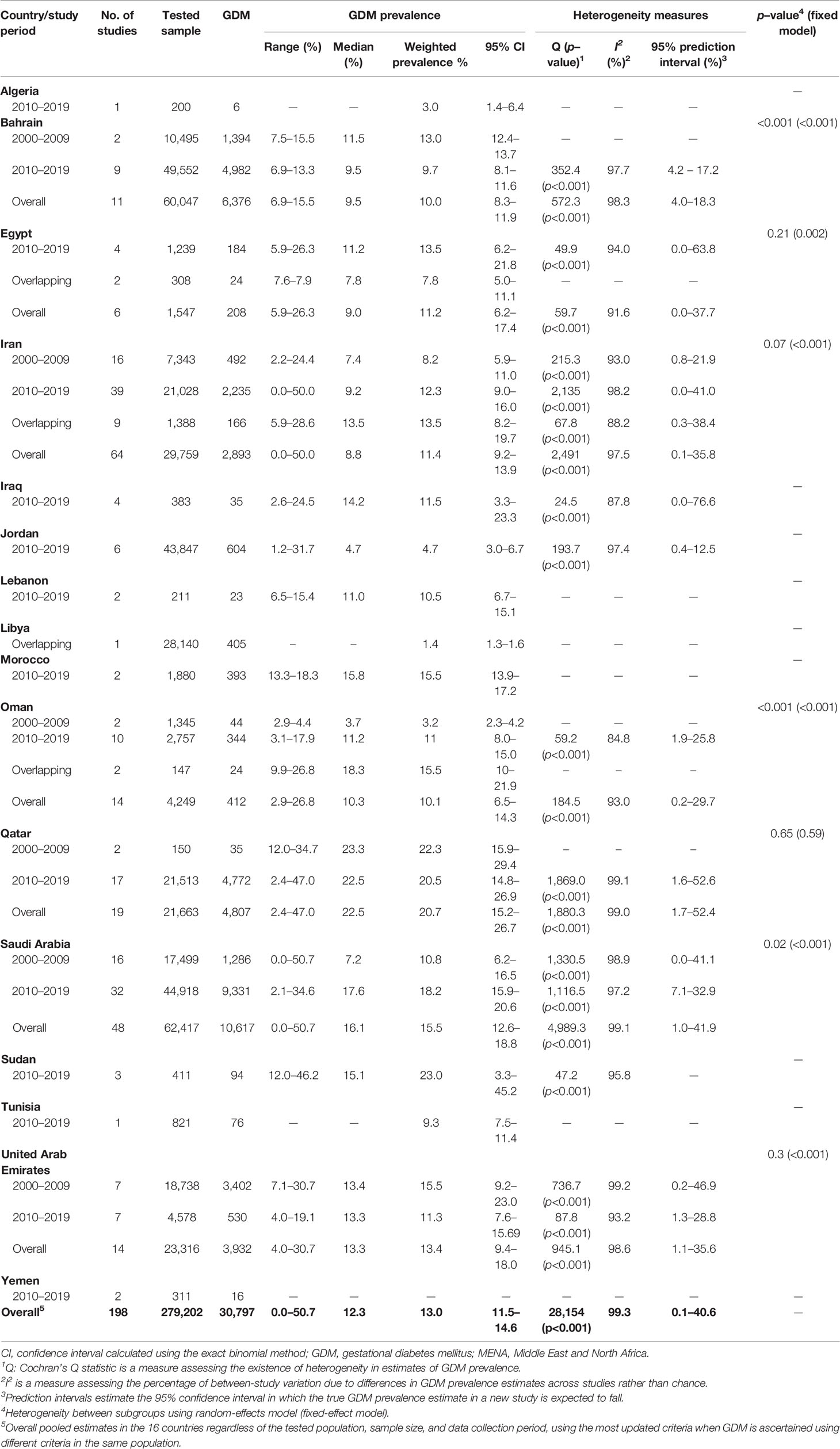
Table 3 Weighted national prevalence of GDM in pregnant women in 16 MENA countries by study period and overall.
The overall pooled weighted GDM prevalence in the MENA region was 13.0% (95% CI, 11.5–14.6%, I2, 99.3%; Table 3; Figure 2). The highest GDM prevalence was observed in Qatar (20.7%, 95% CI, 15.2–26.7%; 19 studies), followed by 15.5% in Saudi Arabia (95% CI, 12.6–18.8%; 48 studies) and 13.4% in the UAE (95% CI, 9.4–18.0%; 14 studies; Table 3). The lowest pooled GDM prevalence was 4.7% in Jordan (95% CI, 3.0–6.7%; six studies) reported between 2010 and 2019. In the studies conducted between 2000 and 2009, the prevalence estimates ranged from 3.2% in Oman (95% CI, 2.3–4.2%) to 22.3% in Qatar (95% CI, 15.9–29.4%), and in the studies conducted between 2010 and 2019, it ranged from 3.0% in Algeria (95% CI, 1.4–6.4%) to 23.0% in Sudan (95% CI, 3.3–45.2%; Table 3).
For the six countries reporting data on both decades, the overall GDM prevalence was estimated separately for each decade. There was a rise in the prevalence of GDM by 4% to 8% in Iran, Oman, and Saudi Arabia and a decrease of 2% to 4% in Bahrain, Qatar, and the UAE from 2000–2009 to 2010–2019 periods. The largest increase in prevalence occurred in Oman: from 3.2% in 2000 (95% CI, 2.3–4.2%) to 11.0% in 2019 (95% CI, 8.0–15.0%, I2, 84.2%). An appreciable reduction in the prevalence of GDM was observed in the UAE: from 15.5% in 2000 (95% CI, 9.2–23.0%, I2, 99.2%) to 11.3% in 2019 (95% CI, 7.6–15.69, I2, 93.2%; Tables 2 and 3).
The prevalence of GDM in pregnant women aged ≥30 years was 2.26 times higher (21.9%, 95% CI, 18.5–25.5%, I2, 97.1%) than that estimated in younger (15–29 years) pregnant women (9.7%, 95% CI, 6.7–13.2%, I2, 98.0%). A trend was observed between GDM and pregnancy trimester. The weighted GDM prevalence increased by 45.0%, from 8.9% in the first trimester to 12.9% in the second trimester, and by 55.0% in the third trimester (20.0%, 95% CI, 13.1–27.9%, I2, 98.8%) compared with the second trimester. It was also noticeable that, as the BMI increased, the prevalence of GDM increased by 54% in overweight (12.0%, 95% CI, 5.7–20.1%, I2, 96.7) and by 120% in obese (17.2%, 95% CI, 12.8–22.0%, I2, 93.8%) compared with normal-weight pregnant women (7.8%, 95% CI, 4.1–12.4%, I2, 95.0%). No GDM cases were reported in two studies that included underweight women (Table 4).
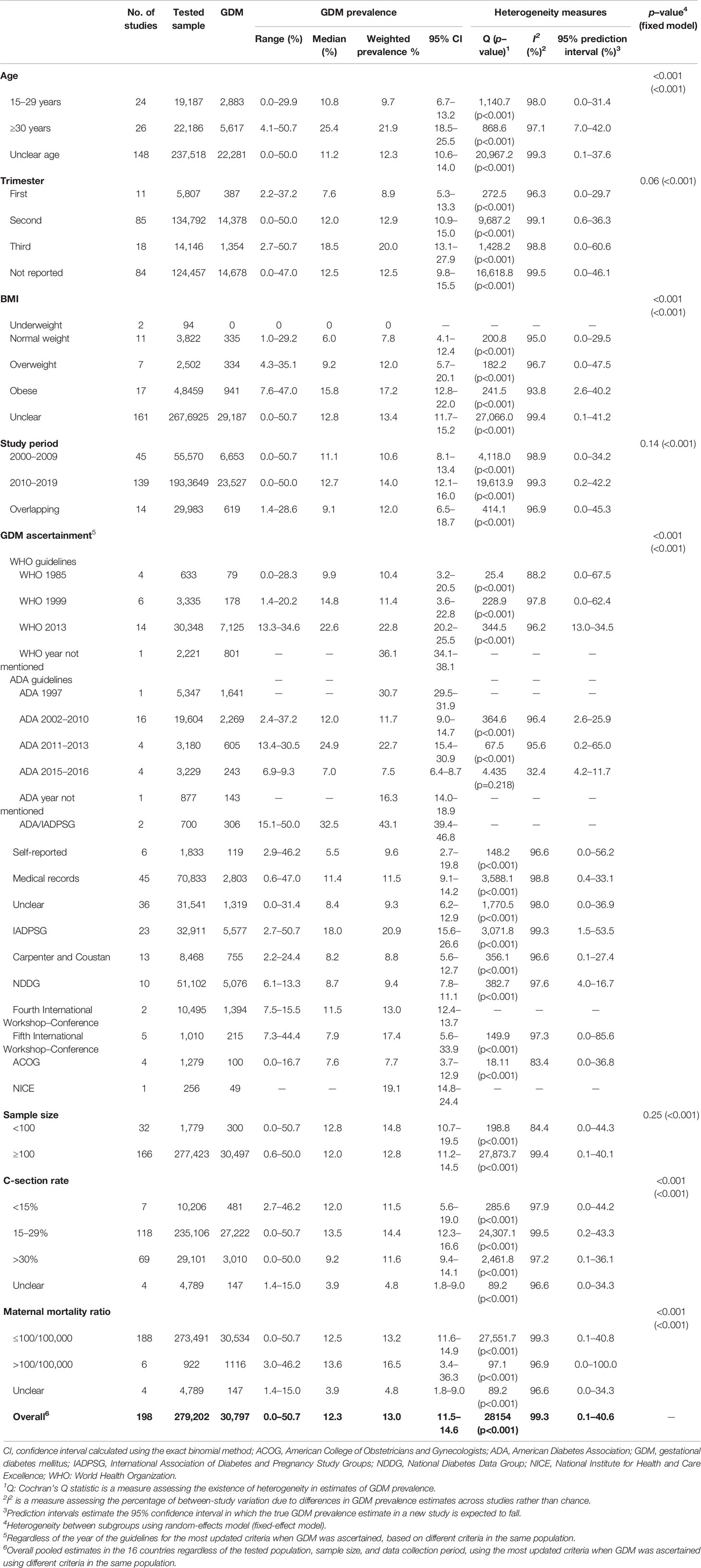
Table 4 Subgroup weighted prevalence of GDM in pregnant women in 16 MENA countries by age, pregnancy trimester, body mass index, study period, ascertainment methodology, tested sample, C-section, and maternal mortality ratio.
From the 137 studies conducted between 2010 and 2019, the pooled GDM prevalence (14.0%, 95% CI, 12.1–16.0%) was 32.0% higher than that reported in the 45 studies conducted in the previous decade (2000–2009; 10.6%, 95% CI, 8.1–13.4%). The pooled GDM prevalence was relatively higher in 32 studies with a sample size of <100 pregnant women (14.8%, 95% CI, 10.7–19.5%) compared with that in 164 studies with a sample size of ≥100 pregnant women (12.8%, 95% CI, 11.2–14.8%; Table 4).
The prevalence of GDM was 25.2% higher in countries with a C-section rate of 15–29% (weighted estimate of 14.4%, 95% CI, 12.3–16.6%, I2, 99.5%) than countries with a C-section rate of <15% (weighted estimate of 11.5%, 95% CI, 5.36–19.0%, I2, 97.9%; Table 4). In addition, in four studies in countries with high MMR (i.e., >100 per 100,000 live births), the prevalence of GDM was 25.0% higher than in countries with MMR ≤100 per 100,000 live births (weighted estimates of 16.5%, 95% CI, 3.4–36.3%, and 14.4%, 95% CI, 12.3–16.6%, respectively; Table 4).
In Sudan, one of the North African countries with a C-section rate of 15–29%, a lower GDM prevalence (weighted prevalence of 7.9%) was observed compared with countries with a C-section rate of <15% (weighted prevalence of 23.0%). In North African countries with an MMR of >100/100,000 live births, the prevalence of GDM was 32.0% higher than in countries with an MMR of ≤100/100,000 live births (Supplementary File 2).
The highest weighted GDM prevalence was in the GCC countries (14.7%, 95% CI, 13.0–16.5%, I2, 99.0%), followed by North African countries (13.5%, 95% CI, 7.4–20.9%, I2, 98.9%) and Iran/Iraq 11.2% (95% CI, 9.0–13.5%, I2, 97.4%), whereas the lowest prevalence was estimated in the Levant region countries (5.8%, 95% CI, 3.9–7.9%, I2, 97.1%; Supplementary File 3).
In GCC countries, the prevalence of GDM rose from 11.9% to 15.9% over the two successive decades. Overweight (12.5%) and obese (18.5%) pregnant women and pregnant women with a C-section rate of 15–29% (15.5%) were burdened with high GDM prevalence (Supplementary File 3). In these countries, pregnant women aged ≥30 years were burdened with higher GDM prevalence than the other subregions. As compared with the first decade, the weighted GDM prevalence in the subsequent decade increased by almost 4% in Iraq.
Tables 2–4 in the appendix provide additional weighted GDM prevalence estimates in each subregion according to different measured characteristics (Supplementary Files 2–5).
In the univariate meta-regression models, country, age, pregnancy trimester, BMI, and sample size were associated with variability in the prevalence of GDM at p<0.1. In the multivariate meta-regression model, only pregnancy trimester was retained, with no significant association with the prevalence of GDM at p<0.05. Compared with Saudi Arabia, the adjusted GDM prevalence was 135% (adjusted odds ratio [aOR], 2.35, 95% CI, 1.39–3.95) and 122% (aOR, 2.22, 95% CI, 1.30–3.76) higher in Qatar and Morocco, respectively, but lower in Libya (aOR, 0.09, 95% CI, 0.02–0.52) and Jordan (aOR, 0.38, 95% CI, 0.18–0.80). Pregnant women aged ≥30 years had a 152% higher prevalence of GDM (aOR, 2.52, 95% CI, 1.51–4.21) relative to younger pregnant women. Obese pregnant women were burdened with a 192% higher prevalence of GDM relative to normal-weight pregnant women (aOR, 2.92, 95% CI, 1.50–5.69; Supplementary File 6).
Both the visual (funnel plot asymmetry) and statistical assessment (Egger’s test, p<0.001) of publication bias suggested the role of a small-study effect (Supplementary File 7).
Supplementary Figure 2 presents the findings of the research report-specific quality assessment for relevant GDM prevalence studies. In all 102 research reports, the research question(s) and/or objective(s) were clearly stated, and the study population group was clearly specified and defined. Half of the research reports (49.5%) did not provide information on the sample size calculation or justification. Most (79.2%) of the research reports used biological assays or extracted data from medical records to ascertain GDM, whereas the GDM status was self-reported in only five reports. In more than half (58.4%) of the 102 research reports, the tested sample size was at least 100 pregnant women. Overall, the research reports were judged to be of potentially low RoB, with an average of seven of the nine measured assessment items. Four (4.0%) of the reports (70, 85, 105, 120) were of low RoB in all of the assessed RoB items (Supplementary File 8).
A total of 102 eligible research reports comprising 198 GDM prevalence studies were reported in 16 countries in the MENA region between 2000 and 2019. Most of these reports (58.41%) were from Iran and Saudi Arabia. The pooled prevalence of GDM in the 16 MENA countries was appreciably high (13.0%, 95% CI, 11.5–14.6%, I2, 99.3%), particularly in the GCC and North African countries. The prevalence of GDM increased with maternal age, gestational age, and BMI. It was also high in countries with a C-section rate of 15–29% and an MMR of >100/100,000 live births.
The pooled GDM prevalence (13.0%) was alarmingly higher than that of European countries (2–6%) (133) but was similar to the sub-Saharan Africa region (14.0%). In contrast to the pooled prevalence estimates of Asia (11.5%) (134), the prevalence estimated in the present meta-analysis was slightly higher. The Asian meta-analysis included prevalence estimates from Saudi Arabia, Iran, and Qatar, and when compared with our estimates, they were 3.5% and 7.4% lower for Iran and Saudi Arabia, respectively, and 7.4% higher for Qatar (134). Such variations might be due to the differences in the literature search dates and languages, eligible sample size, GDM ascertainment criteria, and differences in the type of observational studies used for the prevalence estimation.
Our overall weighted GDM prevalence estimate depicted substantial heterogeneity (I2, 99.3%). This could be attributable to the less restrictive inclusion criteria in this review. In addition, the prevalence estimates of GDM can significantly differ with the variation in the GDM diagnostic criteria (135, 136). We noted clinical inconsistency in GDM diagnostic criteria used in the prevalence studies we reviewed (Table 4). This corresponds to the common use of existing nonuniform GDM diagnostic criteria in different countries (12, 134). Given the importance of the prevalence of GDM in meaningful intervention development, its estimation can be affected by the inclusion of studies that use different GDM diagnosing criteria (137, 138). The prevalence of GDM estimated based on the IADPSG criteria is usually high due to the low threshold for fasting blood glucose level relevant to other criteria. In our study, more than 25% of the studies used IADPSG criteria. To obtain homogenous and comparable prevalence estimates and to avoid confusion in practices of screening, diagnosis, and follow-up of GDM, health authorities should consider implementing uniform GDM diagnostic criteria nationally and across the MENA region.
The GDM prevalence estimates in our analysis suggested an increasing trend, parallel to the increase in BMI, correlating with the known fact that overweight and obesity are risk factors of GDM (139, 140). Although this does not prove a causal link between these parameters, it inevitably might significantly reflect the impact of the high burden of overweight and obesity in several countries in the MENA region, such as Egypt and the six GCC countries (141). This highlights the importance of investigating dietitians’ role in ensuring the appropriate caloric intake of GDM patients based on their BMI as per the recommendations of the ADA (142) and promoting exercise, especially among those with increased BMI (143).
GDM can have devastating maternal and birth consequences. Mothers with GDM are at higher risk of developing T2DM, dying, and undergoing C-section (23, 24, 144). Children born to mothers with untreated GDM face an increased risk of neonatal death and long-term disability (145, 146). Notably, diabetes in pregnancy is a neglected cause of maternal mortality globally, affecting one of every sixth pregnancy in the world, and some of the known GDM morbidities that may cause maternal death are postpartum hemorrhage, obstructed labor, and preeclampsia (147). In our analysis, although the prevalence of GDM was higher (16.35%) in countries with high MMR (>100/100,000 live births), it was also substantial in countries with lower MMR (≤100/100,000 live births). Although this does not prove temporality, it highlights the importance of researching complications of GDM (if any) leading to maternal deaths, to help healthcare providers in the MENA region establish protocols to prevent these anticipated adversities. GCC countries with the highest GDM prevalence, as presented in this study, are also burdened with high T2DM (148). There is no doubt that controlling GDM would have multiple benefits in avoiding unfavorable health consequences for both mothers and their babies.
The strengths of our review included its comprehensive characterization of the burden of GDM among pregnant women in several MENA countries. The review provides several weighted estimates in different population groups of the pregnant women at national, subregional, and regional levels that could be used, in addition to future work, to guide the planning, implementation, and evaluation of programs to prevent and control GDM. The overall and national-based pooled prevalence estimates might help policy makers of the respective MENA countries to contrast and quantify the local burden of GDM and introduce better policy initiatives regarding the flow of resources and funds for GDM care and management. Moreover, the finding of higher GDM prevalence corresponding to higher BMI categories might help in developing BMI-specific dietary and exercise guidelines. Furthermore, health authorities and organizations in the region are encouraged to review and consider standardizing the GDM diagnostic criteria at least at the national levels to improve the measurability and comparability of GDM rates and burden across the country and over time. Since we found a wide range of GDM diagnostic criteria used in the MENA region, health organizations across this region might consider moving toward the use of uniform GDM diagnostic criteria to produce better comparable statistical estimates in the future. For instance, in the UAE, different hospitals within the country use different GDM screening and ascertainment criteria (12). Having different GDM diagnostic criteria will preclude understanding the exact burden of the GDM.
Limitations included that our review did not provide any prevalence estimate for about 29% of the MENA region countries, as no prevalence data were available. This might have compromised the comprehensiveness of our prevalence estimates at the regional level. Since we believe that this study is the first to determine the prevalence of GDM in the MENA region, a comparison with previous similar estimates was not possible. This study offers scarce help regarding the prevalence of GDM with its associated comorbidities, such as gestational hypertension, preterm birth, and traumatic vaginal delivery (149), and separate review articles are warranted. The prevalence of GDM can also vary depending on several sociodemographic and maternal characteristics as well as within [urban or rural setting (150, 151)] and between countries and regions; however, our study does not provide such distinction on the prevalence data. In some of the reviewed studies, detailed information on the methodology and GDM measurement procedures was missing, and this limits the category-based generalizability of the measured pooled GDM prevalence. For instance, the 3.35-times increase in the prevalence of GDM in studies reported before 2009 compared with studies reported after 2009 should be cautiously interpreted, as there was an overlap in the time period in 14 studies that tested 29,983 women. The various thresholds for fasting blood glucose level to diagnose GDM, applied on the several criteria considered from the studies, might suggest a bias in the estimated GDM prevalence. Unless estimated by rigorous comparable survey and testing methodology in individual population-based studies, the burden of GDM at the country, subregional, or regional level should not be interpreted as the burden of the measured outcomes at the population level. Moroever, this review did not explore the associations between various maternal and neaonatal characterstics and GDM. Therefore, future systematic reviews and meta-analyses studies focusing on the burden of GDM according to different maternal and neonatal characteristics as well as on the strength of association between various maternal characteristics and GDM are warranted.
Pregnant women in the MENA region are burdened with a relatively high GDM prevalence. Particularly, in the GCC and North African countries, the observed high burden of GDM may be mainly driven by the high prevalence of several risk factors for DM including overweight and obesity, parity, and late maternal age. To avoid maternal and newborn consequences, vigilant risk factor prevention programs and screening and management programs are necessary in the context of GDM. Moreover, unifying the GDM screening and diagnostic criteria, at least at the country level, is warranted to understand the precise burden of GDM. In countries that lack GDM burden data, high-quality research and surveillance programs are also warranted.
The data sets used and/or analyzed in the current study and the supplementary information files are available from the corresponding author on reasonable request.
Conceptualization, RHA. Methodology, RHA, NMA and MSP. Software, RHA. Validation, RHA. Formal analysis, RHA, NMA, and LAA. Resources, RHA. Writing—original draft preparation, SS and MSP. Writing—review and editing, NMA, MSP, SS, and LAA. Supervision, RHA. Project administration, RHA. Funding acquisition, RHA. All authors contributed to the article and approved the submitted version.
This systematic review was funded by the Summer Undergraduate Research Experience (SURE) PLUS-Grant of the United Arab Emirates University, 2017 (research grant 31M348). The funding source had no role in the study design, collection, analysis, or interpretation of the data, nor in writing or the decision to submit this article for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.668447/full#supplementary-material
Supplementary File 1 | PRISMA checklist.
Supplementary File 2 | Overall weighted prevalence of GDM in pregnant women in North African countries (Morocco, Algeria, Tunisia, Libya, Egypt) by pregnancy trimester, body mass index, study period, ascertainment methodology, rate of cesarean section deliveries, and maternal mortality.
Supplementary File 3 | Overall weighted prevalence of GDM in pregnant women in GCC countries and Yemen (the UAE, Qatar, Saudi Arabia, Kuwait, Yemen, Oman, Bahrain) by pregnancy trimester, body mass index, study period, ascertainment methodology, rate of cesarean section deliveries, and maternal mortality.
Supplementary File 4 | Overall weighted prevalence of GDM in pregnant women in Iran/Iraq by pregnancy trimester, body mass index, study period, ascertainment methodology, rate of cesarean section deliveries, and maternal mortality.
Supplementary File 5 | Overall weighted prevalence of GDM in pregnant women in the Levant region (Jordan, Syria, Palestine, Lebanon) by pregnancy trimester, body mass index, study period, ascertainment methodology, rate of cesarean section deliveries, and maternal mortality.
Supplementary File 6 | Univariate and multivariable meta-regression analyses to identify sources of heterogeneity in studies reporting on the prevalence of GDM in pregnant women by different measured characteristics.
Supplementary Figure 7 | Funnel plots (A) and Egger’s publication bias plot (B) examining small-study effects on the pooled GDM prevalence among pregnant women in the MENA region, 2000–2019.
Supplementary Figure 8 | Risk of bias assessment of the 102 reviewed research reports on GDM.
1. Quintanilla Rodriguez BS, Mahdy H. Gestational Diabetes. In: StatPearls. Treasure Island (FL) (2020).
2. Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Di Renzo GC, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on Gestational Diabetes Mellitus: A Pragmatic Guide for Diagnosis, Management, and Care. Int J Gynaecol Obstet (2015) 131(Suppl 3):S173–211. doi: 10.1016/S0020-7292(15)30033-3
3. Spaight C, Gross J, Horsch A, Puder JJ. Gestational Diabetes Mellitus. Endocr Dev (2016) 31:163–78. doi: 10.1159/000439413
4. Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Di Renzo GC, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on Gestational Diabetes Mellitus: A Pragmatic Guide for Diagnosis, Management, and Care(). Int J Gynaecol Obstet (2015) 131(Suppl 3):S173–211. doi: 10.1016/S0020-7292(15)30033-3
5. Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global Estimates of the Prevalence of Hyperglycaemia in Pregnancy. Diabetes Res Clin Pract (2014) 103(2):176–85. doi: 10.1016/j.diabres.2013.11.003
6. Mack LR, Tomich PG. Gestational Diabetes: Diagnosis, Classification, and Clinical Care. Obstet Gynecol Clin North Am (2017) 44(2):207–17. doi: 10.1016/j.ogc.2017.02.002
7. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of Treatment of Gestational Diabetes Mellitus on Pregnancy Outcomes. N Engl J Med (2005) 352(24):2477–86. doi: 10.1056/NEJMoa042973
8. Ward MM. Estimating Disease Prevalence and Incidence Using Administrative Data: Some Assembly Required. J Rheumatol (2013) 40(8):1241–3. doi: 10.3899/jrheum.130675
9. Jiwani A, Marseille E, Lohse N, Damm P, Hod M, Kahn JG. Gestational Diabetes Mellitus: Results From a Survey of Country Prevalence and Practices. J Matern Fetal Neonatal Med (2012) 25(6):600–10. doi: 10.3109/14767058.2011.587921
10. World health Organization. Global Health Observatory (GHO) Data. Overweight and Obesity. Noncommunicable Diseases. Obesity Among Adults (2016). Available at: https://www.who.int/gho/ncd/risk_factors/overweight/en/ (Accessed 10.06.2020).
11. International Diabetes Federation. IDF Diabetes Atlas 6th Edition (2013). Available at: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/19-atlas-6th-edition.html (Accessed June 3, 2020).
12. Agarwal MM, Shah SM, Al Kaabi J, Saquib S, Othman Y. Gestational Diabetes Mellitus: Confusion Among Medical Doctors Caused by Multiple International Criteria. J Obstet Gynaecol Res (2015) 41(6):861–9. doi: 10.1111/jog.12661
13. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
14. Al-Rifai RH, Aziz F. Prevalence of Type 2 Diabetes, Prediabetes, and Gestational Diabetes Mellitus in Women of Childbearing Age in Middle East and North Africa, 2000-2017: Protocol for Two Systematic Reviews and Meta-Analyses. Syst Rev (2018) 7(1):96. doi: 10.1186/s13643-018-0763-0
15. Covidence. Covidence – Better Systematic Review Management. Melbourne, Victoria, Australia: Covidence (2018). Available at: https://www.covidence.org/home.
16. Woodruff TJ, Sutton P. The Navigation Guide Systematic Review Methodology: A Rigorous and Transparent Method for Translating Environmental Health Science Into Better Health Outcomes. Environ Health Perspect (2014) 122(10):1007–14. doi: 10.1289/ehp.1307175
17. World Bank Definition of Middle East and North Africa Countries. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519.
18. National Heart, Lung, and Blood Institute. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
19. Tukey JW, Murray FF. Transformations Related to the Angular and the Square Root. Ann Math Stat (1950) 21(4):607–11. doi: 10.1214/aoms/1177729756
20. Miller JJ. The Inverse of the Freeman – Tukey Double Arcsine Transformation. Am Stat (1978) 32(4):138. doi: 10.2307/2682942
21. DerSimonian R, Laird N. Meta-Analysis in Clinical Trials Revisited. Contemp Clin Trials (2015) 45(Pt A):139–45. doi: 10.1016/j.cct.2015.09.002
22. Borenstein M, Hedges LV, Higgins J, Rothstein HR. Introduction to Meta-Analysis. Chichester, U.K: John Wiley & Sons, Ltd. (2009). doi: 10.1002/9780470743386
23. Billionnet C, et al. Gestational Diabetes and Adverse Perinatal Outcomes From 716,152 Births in France in 2012. Diabetologia (2017) 60(4):636–44. doi: 10.1007/s00125-017-4206-6
24. Bawah AT, Ngala RA, Alidu H, Seini MM, Wumbee JDK, Yeboah FA. Gestational Diabetes Mellitus and Obstetric Outcomes in a Ghanaian Community. Pan Afr Med J (2019) 32:94. doi: 10.11604/pamj.2019.32.94.17334
25. Betran AP, Ye J, Moller A, Zhang J, Gülmezoglu AM, Torloni MR. The Increasing Trend in Caesarean Section Rates: Global, Regional and National Estimates: 1990-2014. PloS One (2016) 11(2):e0148343. doi: 10.1371/journal.pone.0148343
26. World Health Organization. World Health Statistics 2015. Available at: https://apps.who.int/iris/bitstream/handle/10665/170250/9789240694439_eng.pdf;jsessionid=EF2A3F289024FCB434BE9A04B2C617DA?sequence=1 (Accessed February 2020).
27. Global Health Observatory (GHO). Maternal Mortality Country Profiles. World Health Organization. Available at: https://www.who.int/gho/mdg/maternal_health/countries/en/ (Accessed February 2020).
28. Sterne JA, Egger M. Funnel Plots for Detecting Bias in Meta-Analysis: Guidelines on Choice of Axis. J Clin Epidemiol (2001) 54(10):1046–55. doi: 10.1016/S0895-4356(01)00377-8
29. Nyaga VN, Arbyn M, Aerts M. Metaprop: A Stata Command to Perform Meta-Analysis of Binomial Data. Arch Public Health (2014) 72(1):39. doi: 10.1186/2049-3258-72-39
31. Tebbani F, Oulamara H, Agli A. Effects of Gestational Weight Gain on Pregnancy Complications. Nutr Clinique Metabolisme (2017) 32:27 – 32. doi: 10.1016/j.nupar.2017.09.011
32. Rajab KE, Issa AA, Hasan ZA, Rajab E, Jaradat AA. Incidence of Gestational Diabetes Mellitus in Bahrain From 2002 to 2010. Int J Gynaecol Obstet (2012) 117(1):74–7. doi: 10.1016/j.ijgo.2011.11.013
33. Al Mahroos S, Nagalla DS, Yousif W, Sanad H. A Population-Based Screening for Gestational Diabetes Mellitus in non-Diabetic Women in Bahrain. Ann Saudi Med (2005) 25(2):129–33. doi: 10.5144/0256-4947.2005.129
34. Rakha S, El Marsafawy H. Sensitivity, Specificity, and Accuracy of Fetal Echocardiography for High-Risk Pregnancies in a Tertiary Center in Egypt. Arch Pediatr (2019) 26(6):337–41. doi: 10.1016/j.arcped.2019.08.001
35. Rezk M, Omar Z. Deleterious Impact of Maternal Hepatitis-C Viral Infection on Maternal and Fetal Outcome: A 5-Year Prospective Study. Arch Gynecol Obstet (2017) 296(6):1097–102. doi: 10.1007/s00404-017-4550-2
36. Maged AM, Moety GAF, Mostafa WA, Hamed DA. Comparative Study Between Different Biomarkers for Early Prediction of Gestational Diabetes Mellitus. J Matern Fetal Neonatal Med (2014) 27(11):1108–12. doi: 10.3109/14767058.2013.850489
37. Elkholi DGEY, Nagy HM. The Effects of Adipocytokines on the Endocrino-Metabolic Features and Obstetric Outcome in Pregnant Obese Women With Polycystic Ovary Syndrome. Middle East Fertil Soc J (2014) 19:292–302. doi: 10.1016/j.mefs.2014.02.001
38. Mohammed AK, Algani VHA. The Correlation Between Serum Ferritin and Fasting Blood Sugar in Iraqi Women With Gestational Diabetes. J Pharm Sci (2017) 9(9):1654–8.
39. Alawad ZM, Al-Omary HL. Maternal and Cord Blood Prolactin Level and Pregnancy Complications. Pak J Med Sci (2019) 35(4):1122–7. doi: 10.12669/pjms.35.4.558
40. Safari K, Piro TJ, Ahmad HM. Perspectives and Pregnancy Outcomes of Maternal Ramadan Fasting in the Second Trimester of Pregnancy. BMC Pregnancy Childbirth (2019) 19(1):128. doi: 10.1186/s12884-019-2275-x
41. Maghbooli Z, Hossein-Nezhad A, Karimi F, Shafaei A, Larijani B. Correlation Between Vitamin D3 Deficiency and Insulin Resistance in Pregnancy. Diabetes Metab Res Rev (2008) 24(1):27–32. doi: 10.1002/dmrr.737
42. Abolfazl M, Hamidreza TS, Narges M, Maryam Y. Gestational Diabetes and its Association With Unpleasant Outcomes of Pregnancy. Pak J Med Sci (2008) 24(4):566–70.
43. Keshavarz M, Cheung NW, Babaee GR, Moghadam HK, Ajami ME, Shariati M. Gestational Diabetes in Iran: Incidence, Risk Factors and Pregnancy Outcomes. Diabetes Res Clin Pract (2005) 69(3):279–86. doi: 10.1016/j.diabres.2005.01.011
44. Hadaegh F, Tohidi M, Harati H, Kheirandish M, Rahimi S. Prevalence of Gestational Diabetes Mellitus in Southern Iran (Bandar Abbas City). Endocr Pract (2005) 11(5):313–8. doi: 10.4158/EP.11.5.313
45. Amooee S, Samsami A, Jahanbakhsh J, Karimi M. The Pregnancy Outcome in Patients With Minor Beta-Thalassemia. Iran J Reprod Med (2011) 9(1):9–14.
46. Lamyian M, Hosseinpour-Niazi S, Mirmiran P, Banaem LM, Goshtasebi A, Azizi F, et al. Pre-Pregnancy Fast Food Consumption Is Associated With Gestational Diabetes Mellitus Among Tehranian Women. Nutrients (2017) 9(3):216. doi: 10.3390/nu9030216
47. Soheilykhah S, Mogibian M, Rahimi-Saghand S, Rashidi M, Soheilykhah S, Piroz M. Incidence of Gestational Diabetes Mellitus in Pregnant Women. Iran J Reprod Med (2010) 8(1):24–8.
48. Pirjani R, Shirzad N, Qorbani M, Phelpheli M, Nasli-Esfahani E, Bandarian F, et al. Gestational Diabetes Mellitus Its Association With Obesity: A Prospective Cohort Study. Eat Weight Disord (2017) 22(3):445–50. doi: 10.1007/s40519-016-0332-2
49. Soheilykhah S, Mojibian M, Jannati Moghadam M. Serum Ferritin Concentration in Early Pregnancy and Risk of Subsequent Development of Gestational Diabetes: A Prospective Study. Int J Reprod BioMed (Yazd) (2017) 15(3):155–60. doi: 10.29252/ijrm.15.3.155
50. Shahbazian H, Nouhjah S, Shahbazian N, Jahanfar S, Latifi SM, Aleali A, et al. Gestational Diabetes Mellitus in an Iranian Pregnant Population Using IADPSG Criteria: Incidence, Contributing Factors and Outcomes. Diabetes Metab Syndr (2016) 10(4):242–6. doi: 10.1016/j.dsx.2016.06.019
51. Yassaee F, Eskandari R, Amiri Z. Pregnancy Outcomes in Women With Idiopathic Thrombocytopenic Purpura. Iran J Reprod Med (2012) 10(5):489–92.
52. Ashrafi M, Sheikhan F, Arabipoor A, Hosseini R, Nourbakhsh F, Zolfaghari Z. Gestational Diabetes Mellitus Risk Factors in Women With Polycystic Ovary Syndrome (PCOS). Eur J Obstet Gynecol Reprod Biol (2014) 181:195–9. doi: 10.1016/j.ejogrb.2014.07.043
53. Goshtasebi A, Hosseinpour-Niazi S, Mirmiran P, Lamyian M, Banaem LM, Azizi F. Pre-Pregnancy Consumption of Starchy Vegetables and Legumes and Risk of Gestational Diabetes Mellitus Among Tehranian Women. Diabetes Res Clin Pract (2018) 139:131–8. doi: 10.1016/j.diabres.2018.02.033
54. Ashrafi M, Gosili R, Hosseini R, Arabipoor A, Ahmadi J, Chehrazi M. Risk of Gestational Diabetes Mellitus in Patients Undergoing Assisted Reproductive Techniques. Eur J Obstet Gynecol Reprod Biol (2014) 176:149–52. doi: 10.1016/j.ejogrb.2014.02.009
55. Jamali S, Sh J, RakeshJahromi A, Haghbeen M. Teenage Versus Adult Pregnancy: Maternal and Neonatal Outcomes. J Fundam Appl Sci (2017) 9(2):1170–82. doi: 10.4314/jfas.v9i2.36
56. Pourali L, Ayati S, Jelodar S, Zarifian A, Andalibi MSS. Obstetrics and Perinatal Outcomes of Dichorionic Twin Pregnancy Following ART Compared With Spontaneous Pregnancy. Int J Reprod BioMed (Yazd) (2016) 14(5):317–22.
57. Mehrabian F, Rezae M. Sex Hormone Binding Globulin Measurement Before Conception as a Predictor of Gestational Diabetes in Women With Polycystic Ovarian Syndrome. J Res Med Sci (2013) 18(8):637–40.
58. Mehrabian F, Hosseini SM. Comparison of Gestational Diabetes Mellitus and Pre-Eclampsia in Women With High Hemoglobin in the First Trimester of Pregnancy: A Longitudinal Study. Pak J Med Sci (2013) 29(4):986–90. doi: 10.12669/pjms.294.4012
59. Hosseini E, Janghorbani M, Aminorroaya A. Incidence, Risk Factors, and Pregnancy Outcomes of Gestational Diabetes Mellitus Using One-Step Versus Two-Step Diagnostic Approaches: A Population-Based Cohort Study in Isfahan, Iran. Diabetes Res Clin Pract (2018) 140:288–94. doi: 10.1016/j.diabres.2018.04.014
60. Hantoushzadeh S, Sheikh M, Bosaghzadeh Z, Ghotbizadeh F, Tarafdari A, Panahi Z, et al. The Impact of Gestational Weight Gain in Different Trimesters of Pregnancy on Glucose Challenge Test and Gestational Diabetes. Postgrad Med J (2016) 92(1091):520–4. doi: 10.1136/postgradmedj-2015-133816
61. Niromanesh S, Shirazi M, Dastgerdy E, Sharbaf FR, Shirazi M, Khazaeipour Z. Association of Hypertriglyceridaemia With Pre-Eclampsia, Preterm Birth, Gestational Diabetes and Uterine Artery Pulsatility Index. Natl Med J India (2012) 25(5):265–7.
62. Vaezi A, Haghighi L, Beigmohammadi F, Nojomi M. Maternal Asthma, Pregnancy, Delivery and Birth Outcomes: A Retrospective Cohort Study. Iran J Allergy Asthma Immunol (2017) 16(2):92–8.
63. Hossein-Nezhad A, Maghbooli Z, Vassigh A, Larijani B. Prevalence of Gestational Diabetes Mellitus and Pregnancy Outcomes in Iranian Women. Taiwan J Obstet Gynecol (2007) 46(3):236–41. doi: 10.1016/S1028-4559(08)60026-1
64. Nastaran SA, Nourossadat K, Abbas HF, Hamid AM. Hemoglobin Level During the First Trimester of Pregnancy in Gestational Diabetes. Ginekol Pol (2012) 83(12):929–33.
65. Talebian A, Soltani B, Sehat M, Ai Z, Noorian A, Talebian M. Incidence and Risk Factors of Neural Tube Defects in Kashan, Central Iran. Iran J Child Neurol (2015) 9(3):50–6.
66. Kouhkan A, Khamseh ME, Pirjani R, Moini A, Arabipoor A, Maroufizadeh S, et al. Obstetric and Perinatal Outcomes of Singleton Pregnancies Conceived via Assisted Reproductive Technology Complicated by Gestational Diabetes Mellitus: A Prospective Cohort Study. BMC Pregnancy Childbirth (2018) 18(1):495. doi: 10.1186/s12884-018-2115-4
67. Abedi P, Bagheri R, Qorbani M, Ansari S. Is There a Relationship Between Restless Legs Syndrome and Medical Problems in Pregnant Women? A Cross-Sectional Study in Iran. Acta Neurol Belg (2018) 120(5):1091–6. doi: 10.1007/s13760-018-01062-7
68. Pezeshki B, Chiti H, Arasteh P, Mazloomzadeh S. Early Screening of Gestational Diabetes Mellitus Using Hemoglobin A1C: Revising Current Screening Guidelines. Caspian J Intern Med (2019) 10(1):16–24.
69. Heydarpour F, Soltani M, Najafi F, Tabatabaee HR, Etemad K, Hajipour M, et al. Maternal Anemia in Various Trimesters and Related Pregnancy Outcomes: Results From a Large Cohort Study in Iran. Iran J Pediatr (2019) 29(1):e69741. doi: 10.5812/ijp.69741
70. Fazel N, Kundi M, Jensen-Jarolim E, Pali-Schöll I, Kazemzadeh, et al. Prospective Cohort Study of Pregnancy Complications and Birth Outcomes in Women With Asthma. Arch Gynecol Obstet (2018) 298(2):279–87. doi: 10.1007/s00404-018-4800-y
71. Nouhjah S, Shahbazian H, Shahbazian N, Jahanfar S, Jahanshahi A, Cheraghian B, et al. Early Postpartum Metabolic Syndrome in Women With or Without Gestational Diabetes: Results From Life After Gestational Diabetes Ahvaz Cohort Study. Diabetes Metab Syndr (2018) 12(3):317–23. doi: 10.1016/j.dsx.2017.12.027
72. Maghbooli Z, Hossein-Nezhad A, Adabi E, Asadollah-Pour E, Sadeghi M, Mohammad-Nabi S, et al. Air Pollution During Pregnancy and Placental Adaptation in the Levels of Global DNA Methylation. PloS One (2018) 13(7):e0199772. doi: 10.1371/journal.pone.0199772
73. Salehi-Pourmehr H, Mohammad-Alizadeh S, Jafarilar-Agdam N, Rafiee S, Farshbaf-Khalili A, et al. The Association Between Pre-Pregnancy Obesity and Screening Results of Depression for All Trimesters of Pregnancy, Postpartum and 1 Year After Birth: A Cohort Study. J Perinat Med (2018) 46(1):87–95. doi: 10.1515/jpm-2016-0277
74. Zargar M, Razmkhah N, Nikbakht R. Evaluating the Factors Associated With Pregnancy Loss in Pregnant Women Undergoing Assisted Reproductive Techniques. Middle East Fertil Soc J (2018) 23:342 – 345. doi: 10.1016/j.mefs.2018.04.009
75. Mojtahedi SY, Izadi A, Seirafi G, Khedmat L, Tavakolizadeh R, et al. Risk Factors Associated With Neonatal Jaundice: A Cross-Sectional Study From Iran. Open Access Maced J Med Sci (2018) 6(8):1387–93. doi: 10.3889/oamjms.2018.319
76. Eslami E, Charandabi SMA, Khalili AF, Jafarabadi MA, Mirghafourvand M. The Effect of a Lifestyle-Based Training Package on Weight Gain and Frequency of Gestational Diabetes in Obese and Over Weight Pregnant Females. Iran Red Crescent Med J (2018) 20(S1):e62576. doi: 10.5812/ircmj.62576
77. Mardani M, Yazdani A, Rezaei F, Gachkar L, Shokouhi S, Pourkaveh B, et al. Risk Factors Associated With Outcomes of Seasonal Influenza in Pregnant Women Referring to Health Care Centers in Iran in 2015-2016. Arch Clin Infect Dis (2019) 14(3):e96403. doi: 10.5812/archcid.96403
78. Basha AS, Fram KM, Thekrallah FM, Irshaid ZA, Maswady AM, Obeidat ZN. Prevalence of Gestational Diabetes and Contributing Factors Among Pregnant Jordanian Women Attending Jordan University Hospital. Int J Diabetes Developing Countries (2018) 9(9):132–8. doi: 10.1007/s13410-018-0635-0
79. Abdel Razeq NM, Khader YS, Batieha AM. The Incidence, Risk Factors, and Mortality of Preterm Neonates: A Prospective Study From Jordan (2012-2013). Turk J Obstet Gynecol (2017) 14(1):28–36. doi: 10.4274/tjod.62582
80. Clouse K, Shehabi A, Suleimat AM, Faouri S, Khuri-Bulos N, Al Jammal A, et al. High Prevalence of Group B Streptococcus Colonization Among Pregnant Women in Amman, Jordan. BMC Pregnancy Childbirth (2019) 19(1):177. doi: 10.1186/s12884-019-2317-4
81. Khader YS, Batieha A, Al-Njadat RA, Hijazi SS. Preeclampsia in Jordan: Incidence, Risk Factors, and its Associated Maternal and Neonatal Outcomes. J Matern Fetal Neonatal Med (2018) 31(6):770–6. doi: 10.1080/14767058.2017.1297411
82. Zein S, Rachidi S, Awada S, Osman M, Al-Hajje A, Shami N, et al. High Iron Level in Early Pregnancy Increased Glucose Intolerance. J Trace Elem Med Biol (2015) 30:220–5. doi: 10.1016/j.jtemb.2014.09.004
83. Ghaddar N, Roz AE, Ghssein G, Ibrahim J. Emergence of Vulvovaginal Candidiasis Among Lebanese Pregnant Women: Prevalence, Risk Factors, and Species Distribution. Infect Dis Obstet Gynecol (2019) 2019:5016810. doi: 10.1155/2019/5016810
84. Khalil MM, Alzahra E. Fetal Gender and Pregnancy Outcomes in Libya: A Retrospective Study. Libyan J Med (2013) 8. doi: 10.3402/ljm.v8i0.20008
85. Utz B, Assarag B, Smekens T, Ennassiri H, Lekhal T, El Ansari N, et al. Detection and Initial Management of Gestational Diabetes Through Primary Health Care Services in Morocco: An Effectiveness-Implementation Trial. PloS One (2018) 13(12):e0209322. doi: 10.1371/journal.pone.0209322
86. Abdwani R, Al Shaqsi L, Al-Zakwani I. Neonatal and Obstetrical Outcomes of Pregnancies in Systemic Lupus Erythematosus. Oman Med J (2018) 33(1):15–21. doi: 10.5001/omj.2018.04
87. Al-Hakmani FM, Al-Fadhil FA, Al-Balushi LH, Al-Harthy NA, Al-Bahri ZA, Al-Rawahi NA, et al. The Effect of Obesity on Pregnancy and Its Outcome in the Population of Oman, Seeb Province. Oman Med J (2016) 31(1):12–7. doi: 10.5001/omj.2016.03
88. Abu-Heija AT, Al-Bash MR, Al-Kalbani MA. Effects of Maternal Age, Parity and Pre-Pregnancy Body Mass Index on the Glucose Challenge Test and Gestational Diabetes Mellitus. J Taibah Univ Med Sci (2017) 12(4):338342. doi: 10.1016/j.jtumed.2017.01.005
89. Zutshi A, Santhosh J, Sheikh J, Naeem F, Al-Hamedi A, Khan S, et al. Implications of Early Pregnancy Obesity on Maternal, Fetal and Neonatal Health: Retrospective Cohort Study From Oman. Sultan Qaboos Univ Med J (2018) 18(1):e47–53. doi: 10.18295/squmj.2018.18.01.008
90. Islam MM, Bakheit CS. Advanced Maternal Age and Risks for Adverse Pregnancy Outcomes: A Population-Based Study in Oman. Health Care Women Int (2015) 36(10):1081–103. doi: 10.1080/07399332.2014.990560
91. Al-Kuwari MG, Al-Kubaisi BS. Prevalence and Predictors of Gestational Diabetes in Qatar. Diabetol Croatica (2011) 40(3):65–70.
92. Bener A, Saleh NM, Al-Hamaq A. Prevalence of Gestational Diabetes and Associated Maternal and Neonatal Complications in a Fast-Developing Community: Global Comparisons. Int J Womens Health (2011) 3:367–73. doi: 10.2147/IJWH.S26094
93. Abu Yaacob S, Saad FA, Sharara HA, Khalifa L, Manther AA, Rashed YA. The Effect of Obesity in Pregnancy on Perinatal Outcome in Qatar. Qatar Med J (2002) 11(2):32–5. doi: 10.5339/qmj.2002.2.16
94. Bashir M, Aboulfotouh M, Dabbous Z, Mokhtar M, Siddique M, Wahba R, et al. Metformin-Treated-GDM has Lower Risk of Macrosomia Compared to Diet-Treated GDM- A Retrospective Cohort Study. J Matern Fetal Neonatal Med (2018) 1–141. doi: 10.1080/14767058.2018.1550480
95. Shaukat S, Nur U. Effect of Prepregnancy Maternal BMI on Adverse Pregnancy and Neonatal Outcomes: Results From a Retrospective Cohort Study of a Multiethnic Population in Qatar. BMJ Open (2019) 9(9):e029757. doi: 10.1136/bmjopen-2019-029757
96. Soliman A, Salama H, Rifai HA, Sanctis VD, Al-Obaidly S, Qubasi MA, et al. The Effect of Different Forms of Dysglycemia During Pregnancy on Maternal and Fetal Outcomes in Treated Women and Comparison With Large Cohort Studies. Acta BioMed (2018) 89(S5):11–21. doi: 10.23750/abm.v89iS4.7356
97. Kurdi AM, Majeed-Saidan MA, Rakaf MSA, AlHashem AM, Botto LD, Baaqeel HS, et al. Congenital Anomalies and Associated Risk Factors in a Saudi Population: A Cohort Study From Pregnancy to Age 2 Years. BMJ Open (2019) 9(9):e026351. doi: 10.1136/bmjopen-2018-026351
98. El-Gilany AH, Hammad S. Body Mass Index and Obstetric Outcomes in Pregnant in Saudi Arabia: A Prospective Cohort Study. Ann Saudi Med (2010) 30(5):376–80. doi: 10.4103/0256-4947.67075
99. Lasheen AE, Abdelbasit OB, Seidahmed MZ, Hussein KA, Miqdad AM, Zahrani MHA, et al. Infants of Diabetic Mothers. A Cohort Study. Saudi Med J (2014) 35(6):572–7.
100. Wahabi H, Fayed A, Esmaeil S, Mamdouh H, Kotb R. Prevalence and Complications of Pregestational and Gestational Diabetes in Saudi Women: Analysis From Riyadh Mother and Baby Cohort Study (RAHMA). BioMed Res Int (2017) 2017:6878263. doi: 10.1155/2017/6878263
101. Wahabi HA, Esmaeil SA, Fayed A, Al-Shaikh G, Alzeidan RA. Pre-Existing Diabetes Mellitus and Adverse Pregnancy Outcomes. BMC Res Notes (2012) 5:496. doi: 10.1186/1756-0500-5-496
102. Wahabi HA, Esmaeil SA, Fayed A, Alzeidan RA. Gestational Diabetes Mellitus: Maternal and Perinatal Outcomes in King Khalid University Hospital, Saudi Arabia. J Egypt Public Health Assoc (2013) 88(2):104–8. doi: 10.1097/01.EPX.0000430392.57811.20
103. Wahabi HA, Esmaeil SA, Fayed A, Mandil AA. The Independent Effects of Maternal Obesity and Gestational Diabetes on the Pregnancy Outcomes. BMC Endocr Disord (2014) 14:47. doi: 10.1186/1472-6823-14-47
104. Al-Rowaily MA, Abolfotouh MA. Predictors of Gestational Diabetes Mellitus in a High-Parity Community in Saudi Arabia. East Mediterr Health J (2010) 16(6):636–41. doi: 10.26719/2010.16.6.636
105. Almarzouki AA. Pregnancy Outcome With Controlled Gestational Diabetes: A Single Centre Experience. Pak J Med Sci (2012) 28(5):887–90.
106. Al-Shaikh GK, Ibrahim GH, Fayed AA, Al-Mandeel H. Impact of Vitamin D Deficiency on Maternal and Birth Outcomes in the Saudi Population: A Cross-Sectional Study. BMC Pregnancy Childbirth (2016) 16:119. doi: 10.1186/s12884-016-0901-4
107. Al-Daghri NM, Al-Attas OS, Alokail MS, Alkharfy KM, Yousef M, Sabico SL, et al. Diabetes Mellitus Type 2 and Other Chronic non-Communicable Diseases in the Central Region, Saudi Arabia (Riyadh Cohort 2): A Decade of an Epidemic. BMC Med (2011) 9:76. doi: 10.1186/1741-7015-9-76
108. Wahabi H, Fayed A, Esmaeil S, Alzeidan R, Elawad M, Tabassum R, et al. Riyadh Mother and Baby Multicenter Cohort Study: The Cohort Profile. PloS One (2016) 11(3):e0150297. doi: 10.1371/journal.pone.0150297
109. Alfadhli EM, Osman EN, Basri TH, Mansuri NS, Youssef MH, Assaaedi SA, et al. Gestational Diabetes Among Saudi Women: Prevalence, Risk Factors and Pregnancy Outcomes. Ann Saudi Med (2015) 35(3):222–30. doi: 10.5144/0256-4947.2015.222
110. Serehi AA, Ahmed AM, Shakeel F, Alkhatani K, El-Bakri NK, Buhari BAM, et al. A Comparison on the Prevalence and Outcomes of Gestational Versus Type 2 Diabetes Mellitus in 1718 Saudi Pregnancies. Int J Clin Exp Med (2015) 8(7):11502–7.
111. Al-Rubeaan K, Al-Manaa HA, Khoja TA, Youssef AM, Al-Sharqawi AH, Siddiqui K, et al. A Community-Based Survey for Different Abnormal Glucose Metabolism Among Pregnant Women in a Random Household Study (SAUDI-Dm). BMJ Open (2014) 4(8):e005906. doi: 10.1136/bmjopen-2014-005906
112. Gasim T. Gestational Diabetes Mellitus: Maternal and Perinatal Outcomes in 220 Saudi Women. Oman Med J (2012) 27(2):140–4. doi: 10.5001/omj.2012.29
113. Kurdi AM, Mesleh RA, Al-Hakeem MM, Khashoggi TY, Khalifa HM. Multiple Pregnancy and Preterm Labor. Saudi Med J (2004) 25(5):632–7.
114. Abdelmola AO, Mahfouz MS, Gahtani MAM, Mouharrq YJ, Hakami BHO, Daak OI, et al. Gestational Diabetes Prevalence and Risk Factors Among Pregnant Women — Jazan Region, Saudi Arabia. Clin Diabetol (2017) 6(5):172–7. doi: 10.5603/DK.2017.0028
115. Al-Shaikh GK, Ibrahim GH, Fayed AA, Al-Mandeel H. Grand Multiparity and the Possible Risk of Adverse Maternal and Neonatal Outcomes: A Dilemma to be Deciphered. BMC Pregnancy Childbirth (2017) 17(1):310. doi: 10.1186/s12884-017-1508-0
116. Fayed AA, Wahabi H, Mamdouh H, Kotb R, Esmaeil S. Demographic Profile and Pregnancy Outcomes of Adolescents and Older Mothers in Saudi Arabia: Analysis From Riyadh Mother (RAHMA) and Baby Cohort Study. BMJ Open (2017) 7(9):e016501. doi: 10.1136/bmjopen-2017-016501
117. Subki AH, Algethami HR, Baabdullah WM, Alnefaie MN, Alzanbagi MA, Alsolami RM, et al. Prevalence, Risk Factors, and Fetal and Maternal Outcomes of Hypertensive Disorders of Pregnancy: A Retrospective Study in Western Saudi Arabia. Oman Med J (2018) 33(5):409–15. doi: 10.5001/omj.2018.75
118. Al Shanqeeti SA, Alkhudairy YN, Alabdulwahed AA, Ahmed AE, Al-Adham MS, Mahmood NM. Prevalence of Subclinical Hypothyroidism in Pregnancy in Saudi Arabia. Saudi Med J (2018) 39(3):254–60. doi: 10.15537/smj.2018.3.21621
119. Dafa Elseed EB, Khougali HS. Preconceptions and Awareness of Good Glycemic Control, Pregnancy Contraindications, and Maternal and Fetal Adverse Events in Sudan. Int J Gynaecol Obstet (2019) 144(2):233–5. doi: 10.1002/ijgo.12720
120. Naser W, Adam I, Rayis DA, Ahmed MA, Hamdan HZ. Serum Magnesium and High-Sensitivity C-Reactive Protein as a Predictor for Gestational Diabetes Mellitus in Sudanese Pregnant Women. BMC Pregnancy Childbirth (2019) 19(1):301. doi: 10.1186/s12884-019-2429-x
121. Alshareef SA, Rayis DA, Adam I, Gasim GI. Helicobacter Pylori Infection, Gestational Diabetes Mellitus and Insulin Resistance Among Pregnant Sudanese Women. BMC Res Notes (2018) 11(1):517. doi: 10.1186/s13104-018-3642-9
122. Mallouli M, Derbel M, Ingrid A, Sahli J, Zedini C, Ajmi T, et al. Associated Outcomes to Fetal Macrosomia: Effect of Maternal Diabetes. Tunis Med (2017) 95(2):120–5.
123. Radwan H, Hashim M, Obaid RS, Hasan H, Naja F, Ghazal HA, et al. The Mother-Infant Study Cohort (MISC): Methodology, Challenges, and Baseline Characteristics. PloS One (2018) 13(5):e0198278. doi: 10.1371/journal.pone.0198278
124. Agarwal MM, Punnose J, Dhatt GS. Gestational Diabetes: Implications of Variation in Post-Partum Follow-Up Criteria. Eur J Obstet Gynecol Reprod Biol (2004) 113(2):149–53. doi: 10.1016/j.ejogrb.2003.09.021
125. Agarwal MM, Dhatt GS, Othman Y. Gestational Diabetes Mellitus Prevalence: Effect of the Laboratory Analytical Variation. Diabetes Res Clin Pract (2015) 109(3):493–9. doi: 10.1016/j.diabres.2015.06.001
126. Agarwal MM, Dhatt GS, Shah SM. Gestational Diabetes Mellitus: Simplifying the International Association of Diabetes and Pregnancy Diagnostic Algorithm Using Fasting Plasma Glucose. Diabetes Care (2010) 33(9):2018–20. doi: 10.2337/dc10-0572
127. Agarwal MM, Dhatt GS, Othman Y, Gupta R. Gestational Diabetes: Fasting Capillary Glucose as a Screening Test in a Multi-Ethnic, High-Risk Population. Diabetes Med (2009) 26(8):760–5. doi: 10.1111/j.1464-5491.2009.02765.x
128. Mirghani HM, Hamud OA. The Effect of Maternal Diet Restriction on Pregnancy Outcome. Am J Perinatol (2006) 23(1):21–4. doi: 10.1055/s-2005-923435
129. Agarwal MM, Dhatt GS, Punnose J, Koster G. Gestational Diabetes: A Reappraisal of HBA1c as a Screening Test. Acta Obstet Gynecol Scand (2005) 84(12):1159–63. doi: 10.1111/j.0001-6349.2005.00650.x
130. Vaswani PR, Balachandran L. Pregnancy Outcomes in a Population With High Prevalence of Obesity: How Bad Is It? Clin Epidemiol Global Health I (2013), 5–11. doi: 10.1016/j.cegh.2012.11.006
131. Abdel-Wareth LO, Kumari AS, Haq A, Bakir A, Sainudeen A, Sedaghatian MR, et al. An Evaluation of the Latest ADA Criteria for Screening and Diagnosing Gestational Diabetes at a Tertiary Care Hospital in the United Arab Emirates. Int J Diabetes Metab (2006) 14:55–60. doi: 10.1159/000497593
132. Ali AD, Mehrass AAO, Al-Adhroey AH, Al-Shammakh AA, Amran AA. Prevalence and Risk Factors of Gestational Diabetes Mellitus in Yemen. Int J Womens Health (2016) 8:35–41. doi: 10.2147/IJWH.S97502
133. Buckley BS, Harreiter J, Damm P, Corcoy R, Chico A, Simmons D, et al. Gestational Diabetes Mellitus in Europe: Prevalence, Current Screening Practice and Barriers to Screening. A Review. Diabetes Med (2012) 29(7):844–54. doi: 10.1111/j.1464-5491.2011.03541.x
134. Lee KW, Ching SM, Ramachandran V, Hoo AYFK, Chia YC, et al. Prevalence and Risk Factors of Gestational Diabetes Mellitus in Asia: A Systematic Review and Meta-Analysis. BMC Pregnancy Childbirth (2018) 18(1):494. doi: 10.1186/s12884-018-2131-4
135. Harper LM, Mele L, Landon MB, Carpenter MW, Ramin SM, Reddy UM, et al. Carpenter-Coustan Compared With National Diabetes Data Group Criteria for Diagnosing Gestational Diabetes. Obstet Gynecol (2016) 127(5):893–8. doi: 10.1097/AOG.0000000000001383
136. American Diabetes, A. Standards of Medical Care in Diabetes–2012. Diabetes Care (2012) 35(Suppl 1):S11–63. doi: 10.2337/dc12-s011
137. Olarinoye JK, Ohwovoriole AE, Ajayi GO. Diagnosis of Gestational Diabetes Mellitus in Nigerian Pregnant Women–Comparison Between 75G and 100G Oral Glucose Tolerance Tests. West Afr J Med (2004) 23(3):198–201. doi: 10.4314/wajm.v23i3.28120
138. Ranchod HA, Vaughan JE, Jarvis P. Incidence of Gestational Diabetes at Northdale Hospital, Pietermaritzburg. S Afr Med J (1991) 80(1):14–6.
139. Kim SY, Saraiva C, Curtis M, Wilson HG, Troyan J, Sharma AJ. Fraction of Gestational Diabetes Mellitus Attributable to Overweight and Obesity by Race/Ethnicity, California, 2007-2009. Am J Public Health (2013) 103(10):e65–72. doi: 10.2105/AJPH.2013.301469
140. Kim SY, England L, Wilson HG, Bish C, Satten GA, Dietz P. Percentage of Gestational Diabetes Mellitus Attributable to Overweight and Obesity. Am J Public Health (2010) 100(6):1047–52. doi: 10.2105/AJPH.2009.172890
141. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, Regional, and National Prevalence of Overweight and Obesity in Children and Adults During 1980-2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet (2014) 384(9945):766–81.
142. American Diabetes, A. 13. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2018. Diabetes Care (2018) 41(Suppl 1):S137–43. doi: 10.2337/dc18-S013
143. Committee on Practice, B.-O. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol (2018) 131(2):e49–64. doi: 10.1097/AOG.0000000000002501
144. Zhu Y, Zhang C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr Diabetes Rep (2016) 16(1):7. doi: 10.1007/s11892-015-0699-x
145. Alfadhli EM. Gestational Diabetes Mellitus. Saudi Med J (2015) 36(4):399–406. doi: 10.15537/smj.2015.4.10307
146. Guillen-Sacoto MA, Barquiel B, Hillman N, Burgos MA, Herranz L. Gestational Diabetes Mellitus: Glycemic Control During Pregnancy and Neonatal Outcomes of Twin and Singleton Pregnancies. Endocrinol Diabetes Nutr (2018) 65(6):319–27. doi: 10.1016/j.endien.2018.01.007
147. Iversen K. Diabetes in Pregnancy—A Neglected Cause of Maternal Mortality In: BMJ Open (2017). Available at: https://blogs.bmj.com/bmj/2017/05/02/katja-iversen-diabetes-in-pregnancy-a-neglected-cause-of-maternal-mortality/ (Accessed June 1, 2020).
148. Al-Rifai RH, Majeed M, Qambar MA, Ibrahim A, AlYammahi KM, Aziz F. Type 2 Diabetes and Pre-Diabetes Mellitus: A Systematic Review and Meta-Analysis of Prevalence Studies in Women of Childbearing Age in the Middle East and North Africa, 2000-2018. Syst Rev (2019) 8(1):268. doi: 10.1186/s13643-019-1187-1
149. Mirghani Dirar A, Doupis J. Gestational Diabetes From A to Z. World J Diabetes (2017) 8(12):489–511. doi: 10.4239/wjd.v8.i12.489
150. Seshiah V, Balaji V, Balaji MS, Paneerselvam A, Arthi T, Thamizharasi M, et al. Prevalence of Gestational Diabetes Mellitus in South India (Tamil Nadu)–a Community Based Study. J Assoc Phys India (2008) 56:329–33.
Keywords: gestational diabetes mellitus, MENA region, prevalence, meta-analysis, systematic review
Citation: Al-Rifai RH, Abdo NM, Paulo MS, Saha S and Ahmed LA (2021) Prevalence of Gestational Diabetes Mellitus in the Middle East and North Africa, 2000–2019: A Systematic Review, Meta-Analysis, and Meta-Regression. Front. Endocrinol. 12:668447. doi: 10.3389/fendo.2021.668447
Received: 16 February 2021; Accepted: 30 July 2021;
Published: 26 August 2021.
Edited by:
Boon-How Chew, Putra Malaysia University, MalaysiaReviewed by:
Abdullah Alkandari, Dasman Diabetes Institute, KuwaitCopyright © 2021 Al-Rifai, Abdo, Paulo, Saha and Ahmed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rami H. Al-Rifai, cnJpZmFpQHVhZXUuYWMuYWU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.