- Department of Thyroid Surgery, The Third Affiliated Hospital of Soochow University, Changzhou First People’s Hospital, Changzhou, China
Background: The status of lymph nodes in the central compartment is crucial to determining the surgical strategies for papillary thyroid carcinoma (PTC). We aimed to develop a nomogram for predicting central lymph node metastasis (CLNM).
Methods: A total of 886 PTC patients who underwent total thyroidectomy or lobectomy with central neck dissection (CND) from July 2019 to June 2020 were retrospectively retrieved. Clinical and ultrasound features were collected. Univariate and multivariate analysis were performed to determine risk factors of CLNM. A nomogram for predicting CLNM was developed, internal and external calibration was performed for the established model.
Results: Variables (sex, chronic lymphocytic thyroiditis, tumor size, the number of foci, tumor location, margin) significantly associated with CLNM were included in the nomogram. The nomogram showed excellent calibration in the training group and validation group, with area under curves of 0.806 (95% CI, 0.771 to 0.825), and 0.799 (95% CI, 0.778–0.813) respectively.
Conclusion: Through this accurate and easy-to-use nomogram, the possibility of CLNM can be objectively quantified preoperatively. Clinicians can use this nomogram to evaluate the status of lymph nodes in PTC patients and consider prophylactic CND for those with high scores.
Introduction
The incidence of thyroid cancer is rising worldwide, and more than 90% of all thyroid cancers are differentiated thyroid cancer (DTC) (1). Papillary thyroid carcinoma (PTC) is the most common type of DTC, and tends to metastasize to cervical lymph nodes. Central compartment lymph nodes are the first to be involved in PTC. According to the American Thyroid Association Surgery Working Group, the central compartment refers to level VI. The VI region extends from the lower edge of the hyoid to the upper edge of the sternum, and the bilateral boundary is the bilateral common carotid artery. The central neck compartment is subdivided into four zones for the dissection: prelaryngeal (delphian), pretracheal, right and left paratracheal regions. As reported, the risk of lymph node metastasis (LNM) in the central neck compartment was the highest, ranging from 18% to 80% (2–4). Some studies reported that central lymph node metastasis (CLNM) was even associated with an increased risk of regional recurrence (5, 6).
Preoperative detection techniques such as high-resolution ultrasonography (US) and US-guided fine-needle aspiration (FNA) biopsy can greatly improve the diagnosis of PTC. However, due to the limitations of imaging technology, the detection rate of CLNM is relatively low before surgery. For example, the diagnostic sensitivity of US for CLNM is only 51% to 58.3%, and the false negative rate is as high as 44.6% (7, 8). Currently, there is no uniform standard to measure the advantages and disadvantages of routine central neck dissection (CND). Hence, there has been controversy about the role of routine CND. Under these circumstances, an appropriate and noninvasive tool that could quantify the risk of CLNM may be helpful for the optimal treatment of PTC patients.
Different from previous studies that only determine the risk factors of CLNM, we aimed not only to identify risk factors for predicting CLNM, but also to develop and validate the nomogram via clinical and US variables. Through this accurate and easy-to-use nomogram, which has excellent user-friendliness and convenience in formulating personalized treatments for patients, the possibility of CLNM can be objectively quantified preoperatively.
Materials and Methods
Patients
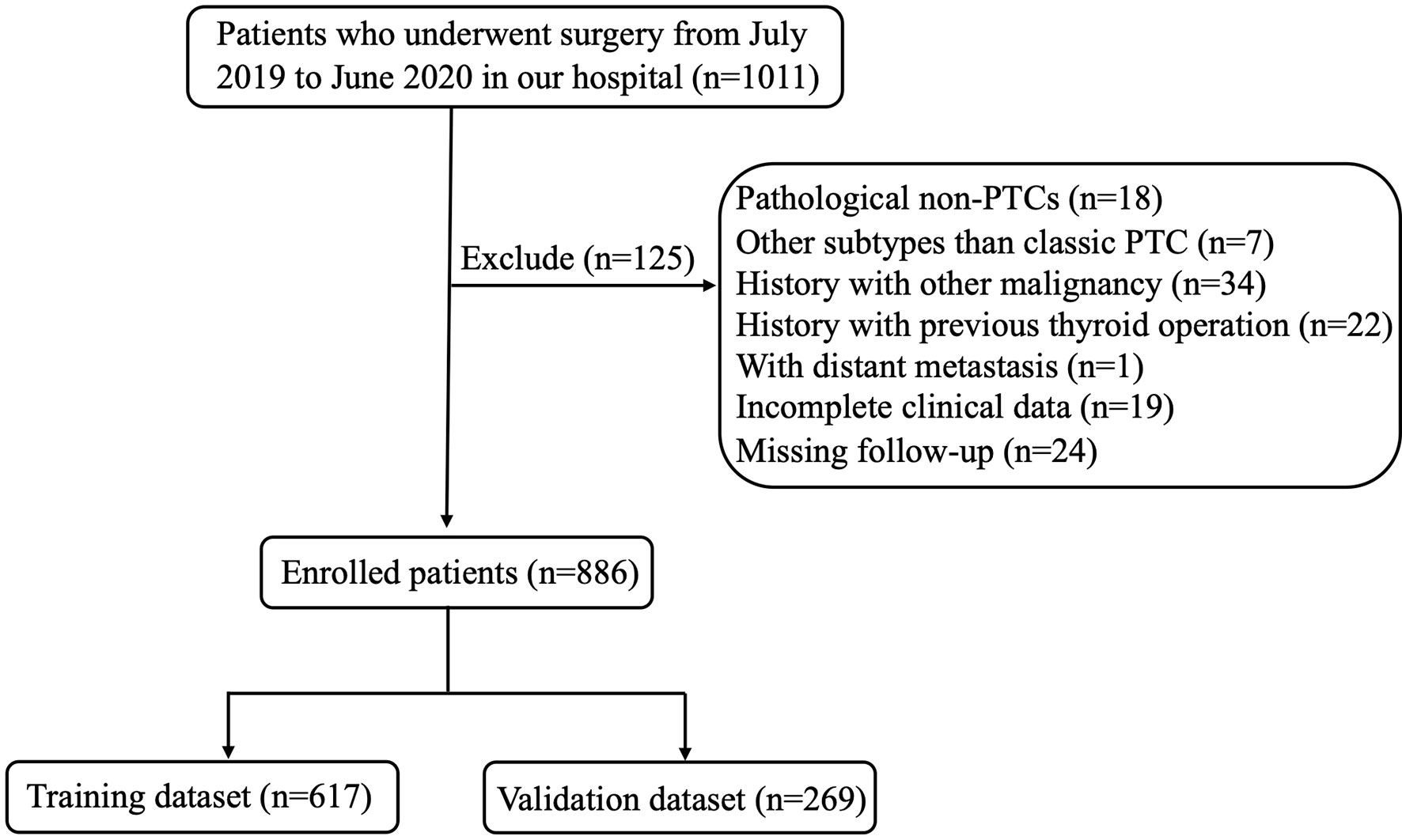
The study was approved by the Institutional Review Board of Changzhou First People’s Hospital. All participants gave written informed consent for their clinical records to be used in this study. The records of patients with PTC who underwent surgery from July 2019 to June 2020 at the Department of Thyroid Surgery of Changzhou First People’s Hospital were retrospectively reviewed. Patients were excluded from the study if they have any of the following factors: (1) non-PTCs (medullary/follicular/anaplastic) or other subtypes than classic PTC (such as mixed PTC and so on); (2) patients who underwent non-curable surgery or did not undergo CND; (3) patients with another malignancy before thyroidectomy; (4) patients with previous thyroid operation; (5) distant metastasis at diagnosis on pathological or clinical analysis; (6) history of neck radiation or familial cancer; (7) incomplete clinical data or missing follow-up. According to above criteria, 886 patients with PTC were enrolled in this study. Figure 1 showed the flow chart of the patients enrolled in our study.
Preoperative Examination and Surgical Procedures
With the discovery of thyroid nodule(s), a complete examination would be carried out. Apart from the routine measurement of thyroid hormone levels, US of the neck would be used to evaluate the primary lesions and cervical lymph nodes. We routinely conducted FNA to confirm the histopathologic diagnosis before surgery. The BRAF V600E mutation, which could help to diagnosis PTC, was also performed. Preoperative US characteristics of each nodule included the following features: aspect ratio (height divided by width on transverse views, A/T), tumor site (upper pole, upper part of the high plane of the isthmus; middle pole, parallel to the isthmus; and lower pole, lower part of the low plane of the isthmus), nodular composition (cystic or spongiform; mixed cystic and solid; solid), echogenicity (anechoic; hyperechoic or isoechoic; hypoechoic; very hypoechoic), margin (smooth; lobulated or irregular; extrathyroidal extension (ETE)), echogenic foci (none or large comet-tail artifacts; macrocalcifications; peripheral calcifications; punctate echogenic foci). Cervical lymph nodes were considered suspicious if they had one of the following characteristics: hyperechoic change, a round shape or necrosis, loss of the fatty hilum, microcalcifications.
Combined with FNA or imaging diagnosis, if patients have any of the following factors (tumor located in the thyroid isthmus, bilateral multifocality, tumor size >4.0 cm, or 1cm< tumor size ≤4.0 cm with risk factors of recurrence, presence of ETE), they would undergo the total thyroidectomy (TT). Otherwise, they would only undergo the lobectomy (9). CND was routinely performed in our institution. Bilateral CND was performed during TT, and ipsilateral CND was performed during lobectomy. TT was defined as the removal of two lobes, the isthmus, and the pyramidal lobe. Lobectomy was defined as the removal of the involved lobe, with the isthmus and the pyramidal lobe. The central compartment refers to level VI. Ipsilateral CND included the removal of prelaryngeal, pretracheal and ipsilateral paratracheal lymph nodes, whereas bilateral CND included the removal of prelaryngeal, pretracheal and bilateral paratracheal lymph nodes (10). All specimens were sent to the department of pathology for paraffin fixation and histological analysis.
Pathological Examination
All pathology specimens were reviewed and cross-checked by two or more experienced pathologists microscopically. Two or more PTC foci within the thyroid was defined as multifocality. Two or more PTC foci in a single lobe were unilateral multifocality, while 2 or more PTC foci in both lobes or one lobe plus isthmus were bilateral multifocality. The diameter of the largest tumor focus was taken as the primary tumor size in multifocal tumors. Papillary thyroid microcarcinoma (PTMC) was defined as PTC ≤1.0 cm in its maximum diameter while macro-PTC was PTC >1.0 cm in its maximum diameter. The location of the tumor was determined by the largest dominant lesion when the patient had multifocal lesions. The location of the tumor was determined by the portion containing more than two-thirds of the tumor volume when the dominant lesion occupied 2 adjacent parts. We used a holistic definition of chronic lymphocytic thyroiditis (CLT) that included (i) elevated antibodies to thyroid peroxidase level, and/or (ii) findings of diffuse heterogeneity on US, and/or (iii) diffuse lymphocytic thyroiditis on histopathology to avoid selection bias (11).
Statistical Analyses
All statistical analyses were performed using the SPSS v 25.0 software (Chicago, IL, USA), and R software version 3.5.3 (The R Foundation for Statistical Computing). Continuous variables were expressed as the means ± standard deviations (SD), categorical variables were reported as numbers and percentages. Patients were divided to a “training group” and “validation group” randomly. A t-test, Pearson’s chi-square test or Fisher’s exact test was used to compare the baseline characteristics of these two groups. Variables with a P<0.05 in the univariate analysis were included in the multivariate analysis, which were performed logistic regression analysis to assess risk factors for CLNM in PTC Patients. Variables with a P<0.05 in the multivariate analysis were then used to construct a risk prediction model – Nomogram, in R software. We used the receiver operating characteristic (ROC) curve to test the discriminative power and consensus of our established prediction model. The performance of the nomogram was further evaluated by the calibration chart, which plotted the predicted probability of the nomogram against the observed probability. According to our nomogram, the possibility of CLNM was quantified as a risk score, and each patient was divided into different subgroups through the calculated CLNM risk score. When there were total statistical differences between groups, Pearson’s chi-square test or Fisher’s exact test was used for pairwise comparison, and the P value of pairwise comparison was corrected by Bonferroni method.
Results
Baseline Clinical and US Characteristics of Patients With PTC
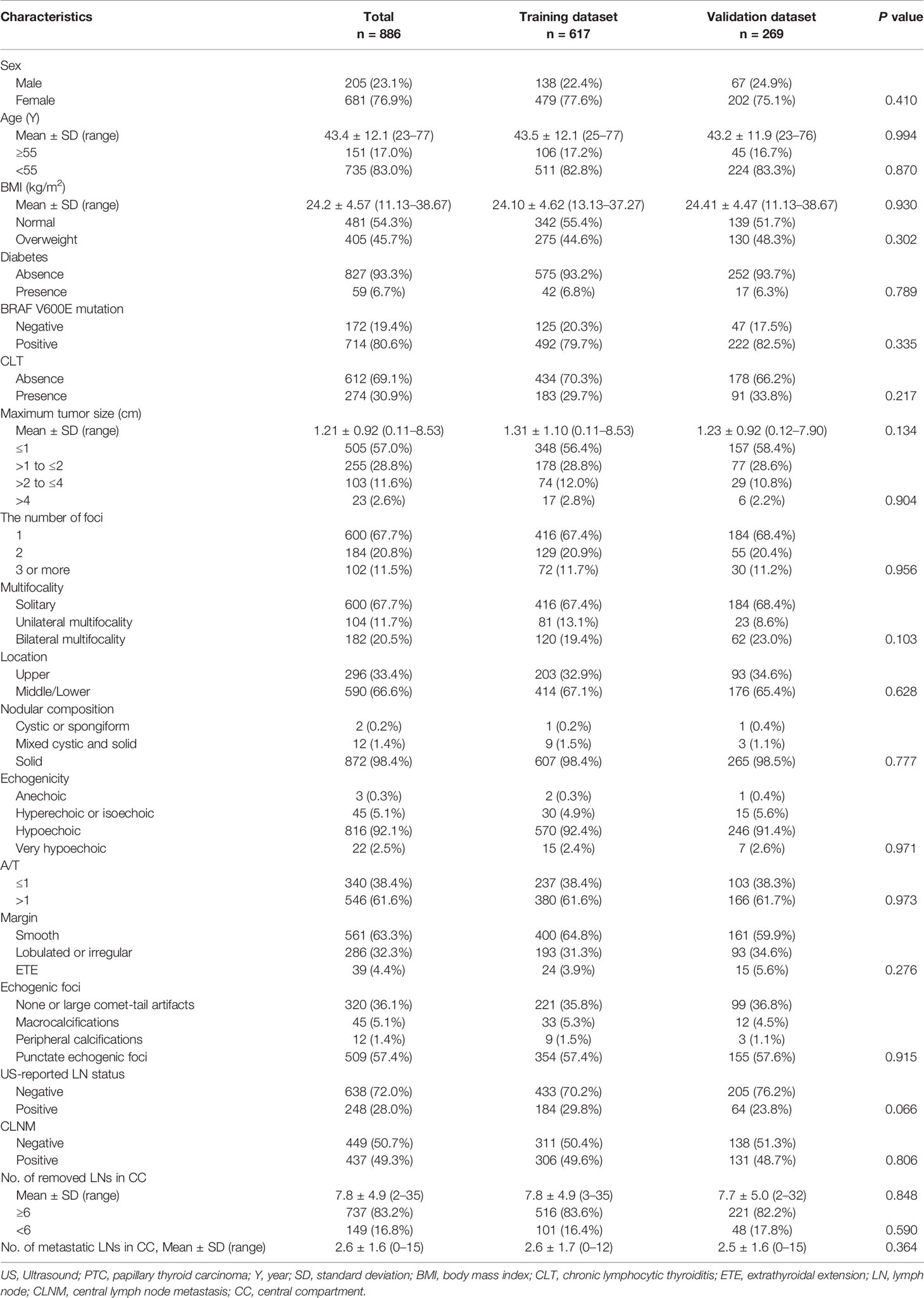
As summarized in Table 1, a total of 886 PTC patients including 205 males (23.1%) and 681 females (76.9%) underwent thyroidectomy plus CND in our institution. The average age at diagnosis was 43.4 ± 12.1 years (range from 23 to 77 years), the average BMI was 24.2 ± 4.57 kg/m2 (range from 11.13 to 38.67 kg/m2), and the average tumor size was 1.21 ± 0.92 cm (range from 0.11 to 8.53 cm). Diabetes was present in 59 patients (6.7%), and CLT was present in 274 patients (30.9%). A total of 714 patients (80.6%) were positive for BRAF V600E mutation, and 172 patients (19.4%) tested negative. Six hundred patients (67.7%) had solitary lesion, 184 patients (20.8%) had 2 foci, and 102 patients (11.5%) had 3 or more than 3 foci. Among 286 patients with multifocal lesions, 182 (20.5%) were confirmed to have bilateral multifocality, 104 (11.7%) were confirmed to have unilateral multifocality. Tumors located in the upper portion of the thyroid gland were detected in 296 (33.4%) patients, and tumors located in the middle/lower lobe of thyroid were detected in 590 (66.6%) patients. The detailed description of the tumor by US was shown in Table 1. There were 248 patients (28.0%) suspected of CLNM before surgery by US. And 437 (49.3%) were pathologically confirmed to have CLNM. The average number of removed lymph nodes in the central compartment was 7.8 ± 4.9 (range from 2 to 35); and the average number of metastatic lymph nodes was 2.6 ± 1.6 (range from 0 to 15). There were 737 patients (83.2%) with 6 or more lymph nodes removed during the operation and 149 patients (16.8%) with less than 6 lymph nodes removed during the operation.
There were 617 patients in the training dataset and 269 patients in the validation dataset. The training and validation groups had no significant differences in clinicopathological characteristics and US features of thyroid nodules (P>0.05 for all comparisons), which justified their use as training and validation cohorts.
Clinical and US Factors Associated With CLNM in the Training Group
In the univariate analysis, CLNM presented the significant association with sex, CLT, tumor size, multifocality, the number of foci, tumor location, A/T, margin, echogenic foci (all P<0.05) (Table 2). We further conducted the multivariate logistic regression modeling to screen for significant variables associated with CLNM.
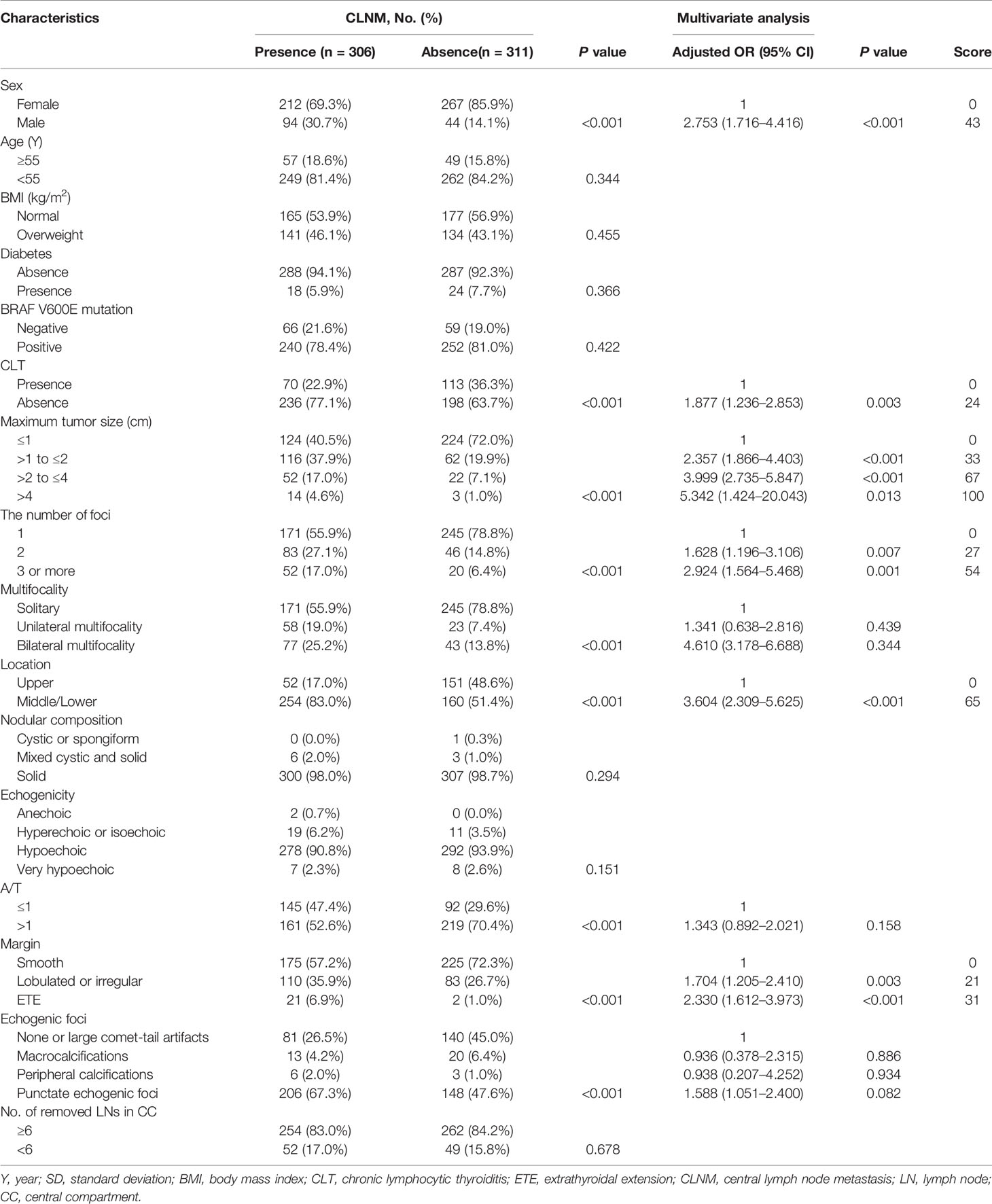
Table 2 Univariate analysis and multivariate analysis of factors associated with CLNM in the training dataset and score.
Multivariate analysis showed that male (OR: 2.735, 95% CI: 1.716–4.416, P<0.001), absence of CLT (OR: 1.877, 95% CI: 1.236–2.853, P=0.003), tumor size ranging between 1.0 and 2.0 cm (OR: 2.357, 95% CI: 1.866–4.403, P<0.001), tumor size ranging between 2.0 and 4.0 cm (OR: 3.999, 95% CI: 2.735–5.847, P<0.001), tumor size > 4.0 cm (OR: 5.342, 95% CI: 1.424–20.043, P=0.013), two tumor foci (OR: 1.628, 95% CI: 1.196–3.106, P=0.007), three or more tumor foci (OR: 2.924, 95% CI: 1.564–5.468, P=0.001), tumors located in the middle/lower pole (OR: 3.604, 95% CI: 2.309–5.625, P<0.001), lobulated or irregular margin (OR: 1.704, 95% CI: 1.205–2.410, P=0.003), and ETE (OR: 2.330, 95% CI: 1.612–3.973, P<0.001) remained independent predictive variables of CLNM, as shown in Table 2.
Development of the Nomogram for Predicting CLNM in PTC Patients
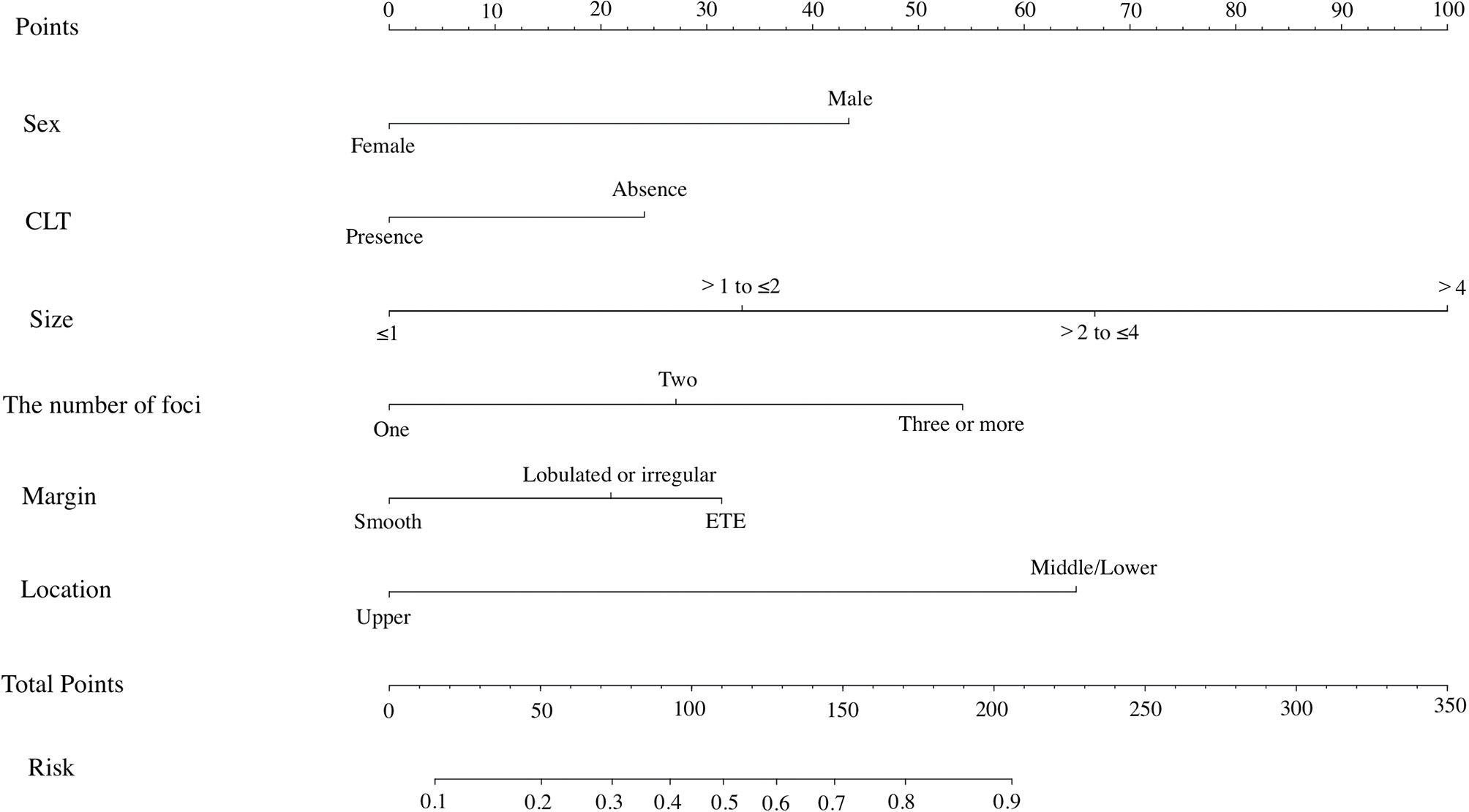
All risk factors that showed statistical significance in the logistic regression model were included in the nomogram, which could help estimate the metastasis risk of central compartment for individual patients with PTC (Figure 2). Each variable was proportionally assigned as the point on a scale from 0 to 100 in the nomogram based on the regression coefficient for CLNM. The nomogram confirmed tumor size as the largest contributor to scores. Detailed scores were listed in the Table 2. By adding the total score and positioning it on the scale of the total score, the corresponding probability of CLNM in each person can be determined.
Validation of the Prediction Nomogram
We then performed ROC analysis for the training and validation groups using this model (Figures 3A, B). The area under the curves (AUCs) in the training group and validation group were 0.806 (95% CI, 0.771 to 0.825), and 0.799 (95% CI, 0.778–0.813) respectively. Moreover, we calculated the AUC for preoperative US of predicting CLNM (Figure 3C). And the AUC was 0.558 (95% CI, 0.542–0.573) only, which was smaller than that of nomogram (P<0.001).
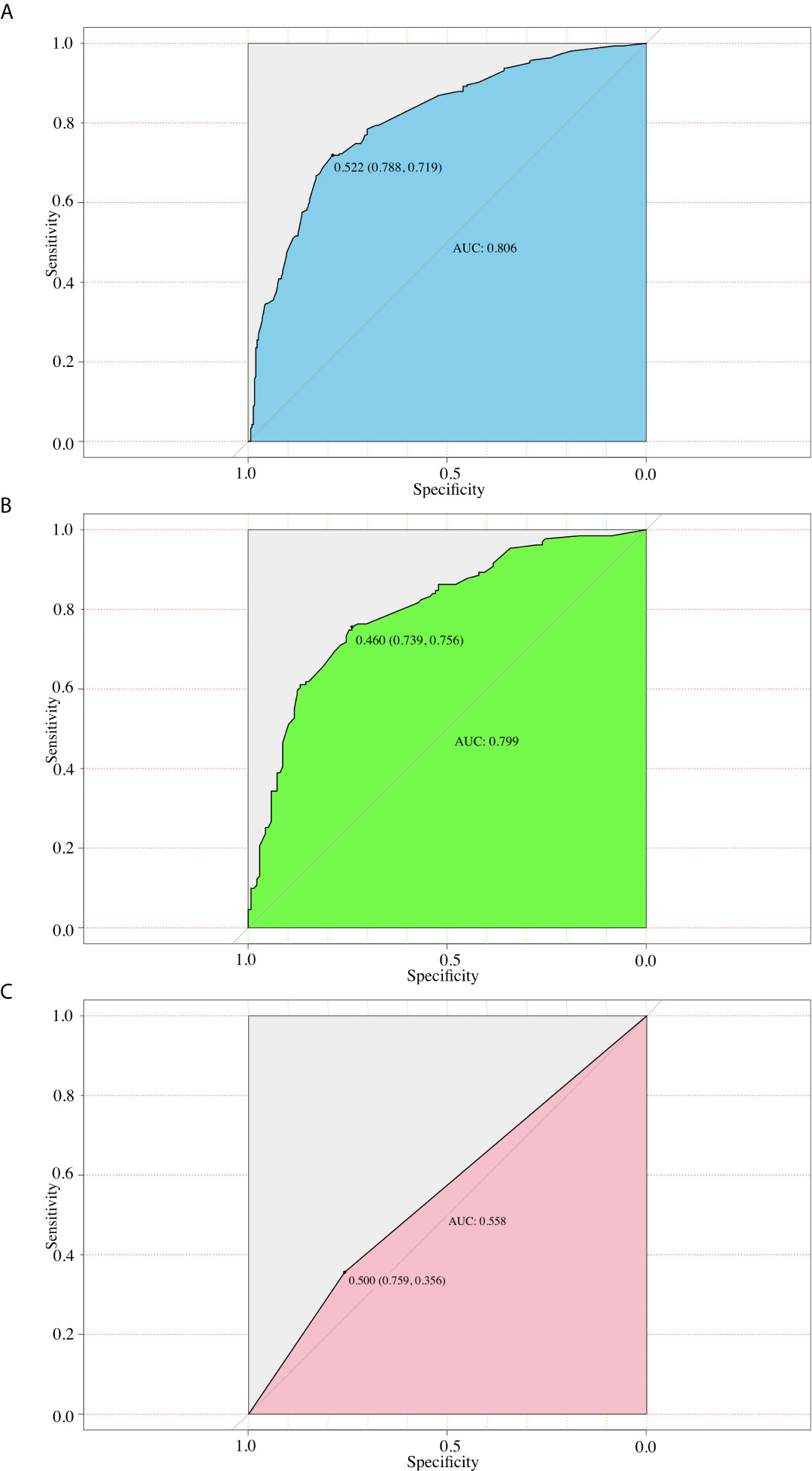
Figure 3 ROC curves for different models. (A) The ROC of the training group (AUC =0.806); (B) The ROC of the validation group (AUC =0.799); (C) The ROC of the preoperative US (AUC=0.558).
Furthermore, we used the similar bootstrap resampling procedure to conduct the internal and external calibration plot for the established model. Predicted and observed metastasis risks of CLNM were in good agreement. Moreover, the corrected risks also showed excellent agreement with observed metastasis risk after the adjustment for optimism, and only minor discrepancies were observed (Figures 4A, B).
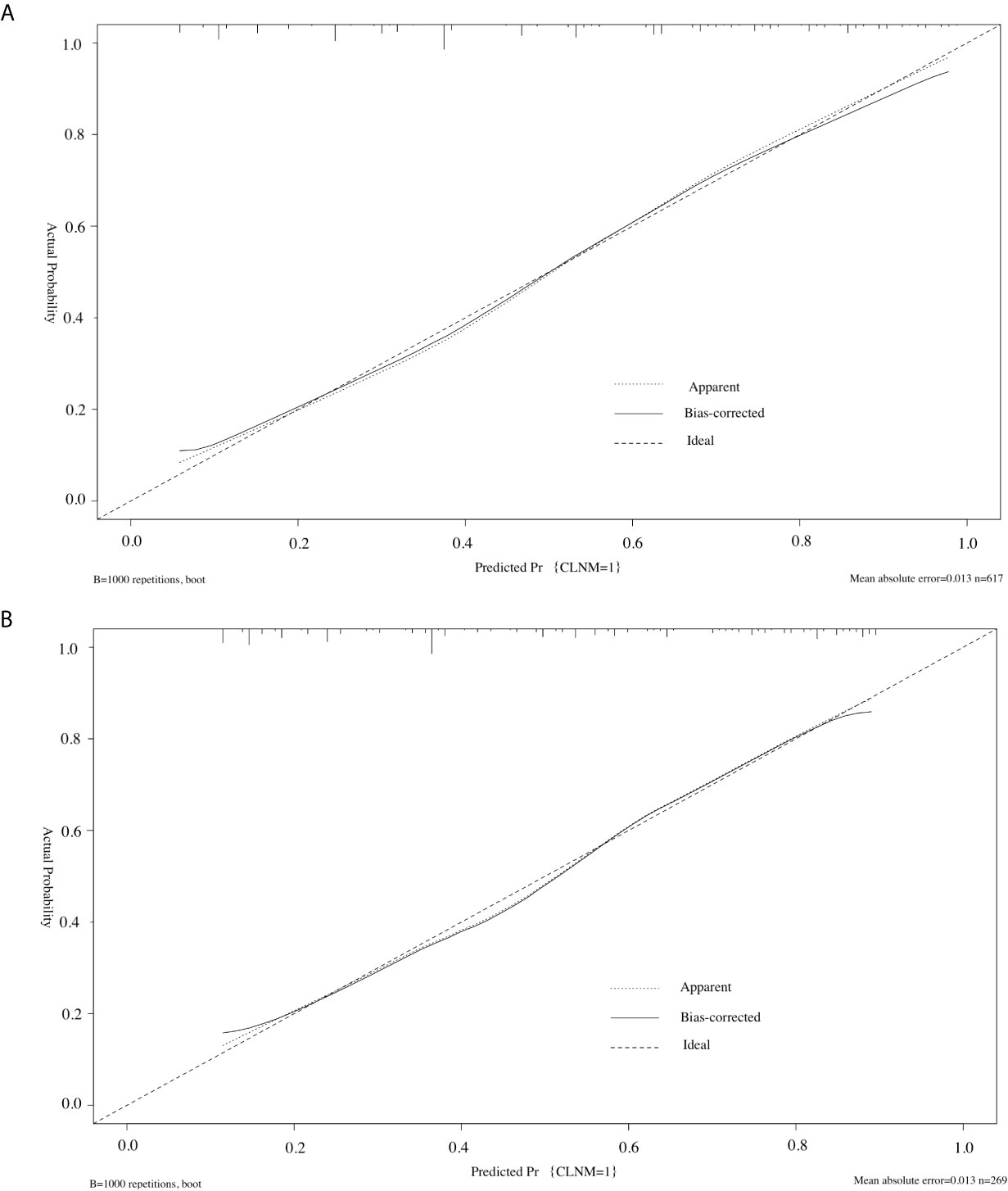
Figure 4 Calibration curve of the model in the training cohorts (A) and validation cohorts (B). The diagonal dashed line represents the ideal prediction by the perfect nomogram; the solid line represents the calibration estimate from internally validated model; the dotted line indicates the apparent predictive accuracy. The closer the solid line is to the dotted line, the stronger the predictive ability of the model.
Novel Risk Stratification Based on the Predictive Nomogram
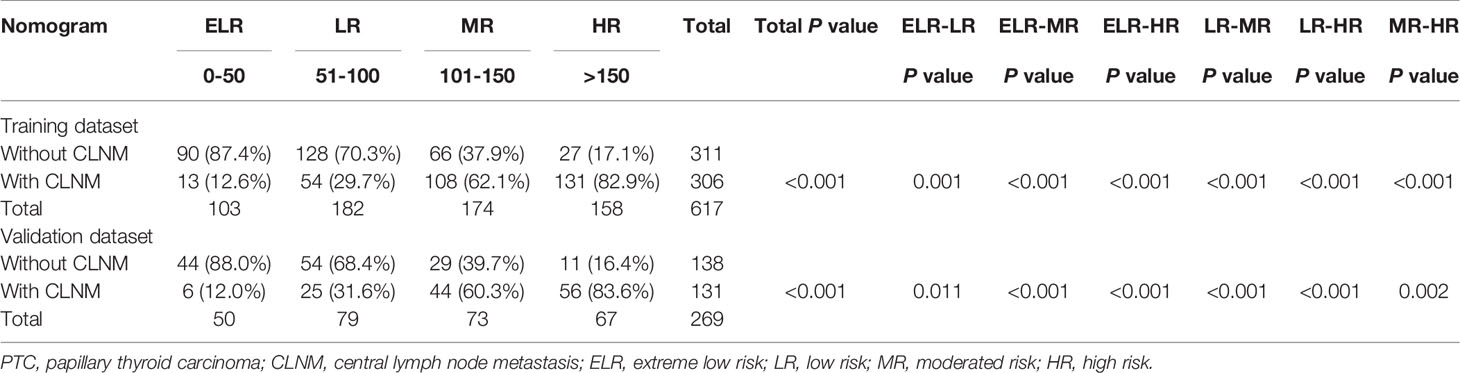
Considering that each variable contained in the nomogram has its corresponding risk point, and the total risk score calculated for all patients can quantitatively predict their respective CNM risk, we thereby determined three cut-off values (50, 100, 150) by using recursive partition analysis. As shown in Table 3, we established four subgroups as follows: (1) extreme low-risk group (patients with the nomogram score of ≤ 50), (2) low-risk group (50 < risk score ≤ 100), (3) moderate-risk group (100 < risk score ≤ 150), and (4) high-risk group (patients with the score of >150). In the training group, the rates of CLNM for extreme low, low, moderate, and high-risk groups were 12.6%, 29.7%, 62.1%, and 82.9%, respectively (P<0.001). Similarly, in the validation group, the rates of CLNM for extreme low, low, moderate, and high-risk groups were 12.0%, 31.6%, 60.3%, and 83.6%, respectively (P<0.001). We further studied whether the relative risk for CLNM in each risk category identified by the nomogram were significantly different from each other. After paired comparison, we found there were significant differences between all groups.
Discussion
With the increasing incidence of thyroid cancer, surgical resection is generally considered to be the most effective treatment for PTC. Decisions regarding the extent of surgery for the patient with PTC are mainly based on the preoperative assessment of lymph node status. But the role of prophylactic CND for clinically lymph node-negative (cN0) patients with PTC is still under debate. Supporters pointed that prophylactic CND not only eliminated potential recurrent sources, thereby reducing the risk of reoperation, but also improved the accuracy of staging (12, 13). Considering the potential complications of prophylactic CND, such as permanent hypoparathyroidism, recurrent laryngeal nerve injury and so on, opponents hold the view that prophylactic CND had the low prognostic benefits and many surgeons worldwide still preferred therapeutic CND only (14, 15). For cN0 PTC patients, the incidence of CLNM detected by histopathological examination ranged from 31% to 60.9% according to previous reports (16, 17). Therefore, routine CND is preferred for patients with PTC in our country due to the high risk of CLNM and unreliability of preoperative examinations in detecting CLNM.
The incidence of CLNM in our study was 49.3%, which was in accordance with the data of 24% to 58% reported in other studies (18, 19). We aimed to develop a nomogram, which could behave as a novel strategy to personalize and quantify the probability of CLNM in patients with PTC. Although some previous studies have also attempted to develop nomograms to predict CLNM for PTC patients, there were some limitations. For example, despite a nomogram with good discrimination (AUC=0.764) was built by Thompson et al. (13), only four variables were considered in this nomogram, which limited the clinical guidance. Moreover, these results were not reproducible in the external validation (AUC=0.615). Based on the 845 cN0 PTC patients with tumor size larger than 2 cm, Lang et al. (20) developed a nomogram, which showed a low discrimination (AUC=0.69) and was not validated in this study. Although enrolled larger patient cohorts, the AUC of 0.711 was not high for the nomogram established by Wang et al (21). In our study, we not only evaluated a large number of PTC patients, but also conducted both internal and external verification.
According to our findings, sex, CLT, tumor size, the number of foci, tumor location, margin were independent risk factors of CLNM among PTC patients by both univariate and multivariate analysis. Many clinicopathological factors related to CLNM have been reported previously, including sex (22–24), tumor size (13, 25), location (26), ETE (27), and the number of foci (28, 29). The incidence of PTC in women was significantly higher than that in men, and the ratio of women to men was approximately 3.7:1. However, the rate of CLNM in men was significantly higher than that in women (22–24). The relationship between multifocality and CLNM remains controversial (30). We divided the multifocality into unilateral multifocality and bilateral multifocality according to the location of tumors, and we found multifocality was not the independent risk factor of CLNM by multivariate logistic regression analysis. Instead of limited to investigating the difference between solitary and multifocal tumors, we further investigated the significance of the number of tumor foci on the incidence of CLNM. We found the proportion of CLNM increased with the number of foci, which was consistent with the study of Afif et al. (28) and Qu et al. (29). PTC cells from the upper region are more likely to be transported to the lateral lymph nodes through the lymph flow along the superior thyroid artery (31). Hence, tumor located in the upper pole of the thyroid lobe conferred a lower risk of CLNM and a higher risk of lateral cervical metastasis. It was known that larger tumor size was associated with more aggressive features in PTC. Interestingly, tumor size was the largest contributor to scores in our nomogram. Considering the guiding role of the nomogram before surgery, we took ETE diagnosed by preoperative US as variable instead of pathological ETE in this study. As reported, US showed high sensitivity (80%) for predicting minimal ETE of PTC (32). In addition, when US and magnetic resonance imaging (MRI) are combined, the diagnostic value of preoperative prediction of ETE would be greatly improved. Since our study is a retrospective study, MRI examinations were not performed for all patients. But we found lobulated or irregular margin and ETE detected by US were independent risk factors of CLNM. The pathogenetic mechanisms linking CLT and PTC are still poorly understood since1955 when the association between CLT and PTC was first proposed. Some mechanisms, such as elevated thyroid-stimulating hormone, RET/PTC rearrangement, and promoting tumor inflammation, have been proposed to explain the association between CLT and PTC (33). Our results showed that CLT was a protective factor against CLNM in PTC patients, which was in agreement with the meta-analysis of Lee et al. (34), that the lymphocytic infiltration counteracted tumor progression. Because the punctate echogenic foci were the strongest predictor of PTC in the US characteristics, the potential impact of microcalcification on CLNM should be discussed. In our study, echogenic foci were associated with the CLNM in the univariate analysis. But echogenic foci, especially punctate echogenic foci, were not the independent risk factors of CLNM by multivariate analysis. This may be due to other pathological structures, such as focal fibrosis of nodular goitres, which look similar to microcalcifications on US.
We incorporated the US characteristics and clinical risk factors into this easy-to-use nomogram, which may help individualized prediction of CLNM before surgery. The usage of nomogram is as follows: locate the patient’s sex on the sex axis. Draw a line straight upward to the point axis to establish how many points toward the probability of CLNM the patient may get. Repeat the process for each of the other variables. Calculate total points for each of the predictors. Pinpoint the final score on the total point axis. Draw a line straight down to determine the patient’s predicted probability of CLNM. For example, nomogram predicted a PTC male (43 points) patient with only one tumor (0 point) located in the middle portion (65 points), without CLT (24 points). According to US, the tumor had irregular margin (21 points), the size of tumor was 1.5cm (33 points). The total point was 186 for this patient. This patient had more than 80.0% chance of CLNM. By comparison with preoperative US, this nomogram showed a significant advantage over preoperative US (Figure 3). Apart from identifying the existence of CLNM, nomogram could also be used to guide surgeons to stratify patients so as to avoid unnecessary surgery. Based on the predictive nomogram, we proposed a risk stratification scheme and divided PTC patients into four quantified risk stratification (Table 3). For patients with different ratings, we can offer different treatment options. For example, for patients with extreme low risk or low risk of CLNM, prophylactic CND should be avoided to reduce surgical complications and damage; for patients with moderated risk of CLNM, prophylactic CND can be considered; for patients with high risk of CLNM, prophylactic CND is highly recommended to reduce the incidence of recurrence. In addition, for PTC patients who have not undergone CND, our nomogram may be helpful in detecting residual CLNM.
Despite some encouraging results were achieved, this study still had some limitations, which we would address in future studies. First, our study is a retrospective study. Compared with prospective studies, retrospective studies tend to have more errors and biases. For example, the criteria used to evaluate the US signature were subjective. Sonographers with insufficient experience may cause errors in a small sample. Nevertheless, the consensus of each feature among the sonographers in our study was consistent. The data we provided were extracted from the document and were not captured in the actual conversation. This model could also be improved by adding more useful technological parameters such as elastography and computer-aided diagnosis system. Second, the validation of the nomogram might be biased by institutional diagnostic patterns. Hence, strict external verification is required in prospective multi-center institutional trials to obtain more objective conclusions. Moreover, different surgeons were involved in performing thyroidectomy and lymph node dissection. Postoperative results, such as the number of metastasized lymph nodes may be affected by surgeon-specific factors.
In conclusion, our study found that CLNM was independently associated with sex, CLT, tumor size, the number of foci, tumor location, and margin. By using above variables, we constructed a nomogram that stratifies PTC patients into four groups that possess different CLNM risk levels. Clinicians can use these nomograms to evaluate the status of lymph nodes in PTC patients and consider prophylactic CND and meticulous postoperative evaluation for those with high scores.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study has been approved by the Institutional Review Board of Changzhou First People’s Hospital ethics committee, and has been performed according to the ethical standards laid down in the 1964 Declaration of Helsinki. Written informed consent was obtained from all individual participants included in the study.
Author Contributions
J-WF and L-ZH: writing - original draft, software, and data curation. S-YL: validation, formal analysis, and data curation. W-XW: conceptualization. FW and JH: validation and investigation. JY and YJ: writing - review & editing, visualization, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Lei Qin, the English language editor, was responsible for correcting language and grammar issues.
References
1. Roman BR, Morris LG, Davies L. The thyroid cancer epidemic, 2017 perspective. Curr Opin Endocrinol Diabetes Obes (2017) 24(5):332–6. doi: 10.1097/MED.0000000000000359
2. Yu XM, Wan Y, Sippel RS, Chen H. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg (2011) 254(4):653–60. doi: 10.1097/SLA.0b013e318230036d
3. Chow SM, Law SC, Chan JK, Au SK, Yau S, Lau WH. Papillary microcarcinoma of the thyroid-Prognostic significance of lymph node metastasis and multifocality. Cancer (2003) 98(1):31–40. doi: 10.1002/cncr.11442
4. Mehanna H, Al-Maqbili T, Carter B, Martin E, Campain N, Watkinson J, et al. Differences in the recurrence and mortality outcomes rates of incidental and nonincidental papillary thyroid microcarcinoma: a systematic review and meta-analysis of 21 329 person-years of follow-up. J Clin Endocrinol Metab (2014) 99(8):2834–43. doi: 10.1210/jc.2013-2118
5. Jiang LH, Yin KX, Wen QL, Chen C, Ge MH, Tan Z. Predictive Risk-scoring Model For Central Lymph Node Metastasis and Predictors of Recurrence in Papillary Thyroid Carcinoma. Sci Rep (2020) 10(1):710. doi: 10.1038/s41598-019-55991-1
6. Lee J, Song Y, Soh EY. Central lymph node metastasis is an important prognostic factor in patients with papillary thyroid microcarcinoma. J Korean Med Sci (2014) 29(1):48–52. doi: 10.3346/jkms.2014.29.1.48
7. Kim E, Park JS, Son KR, Kim JH, Jeon SJ, Na DG. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid (2008) 18(4):411. doi: 10.1089/thy.2007.0269
8. Guidoccio F, Grosso M, Orsini F, Boni G, Mariani G, Volterrani D. Thyroid Ultrasound and Other Imaging Procedures in the Pediatric Age. Curr Pediatr Rev (2016) 12(4):253–64. doi: 10.2174/1573396312666161031162436
9. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
10. Carty SE, Cooper DS, Doherty GM, Duh QY, Kloos RT, Mandel SJ, et al. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid (2009) 19(11):1153–8. doi: 10.1089/thy.2009.0159
11. Grani G, Carbotta G, Nesca A, D'Alessandri M, Vitale M, Del Sordo M, et al. A comprehensive score to diagnose Hashimoto’s thyroiditis: a proposal. Endocrine (2015) 49(2):361–5. doi: 10.1007/s12020-014-0441-5
12. Moo TA, McGill J, Allendorf J, Lee J, Fahey T 3rd, Zarnegar R. Impact of prophylactic central neck lymph node dissection on early recurrence in papillary thyroid carcinoma. World J Surg (2010) 34(6):1187–91. doi: 10.1007/s00268-010-0418-3
13. Thompson AM, Turner RM, Hayen A, Aniss A, Jalaty S, Learoyd DL, et al. A preoperative nomogram for the prediction of ipsilateral central compartment lymph node metastases in papillary thyroid cancer. Thyroid (2014) 24(4):675–82. doi: 10.1089/thy.2013.0224
14. Chen L, Wu YH, Lee CH, Chen HA, Loh EW, Tam KW. Prophylactic Central Neck Dissection for Papillary Thyroid Carcinoma with Clinically Uninvolved Central Neck Lymph Nodes: A Systematic Review and Meta-analysis. World J Surg (2018) 42(9):2846–57. doi: 10.1007/s00268-018-4547-4
15. Sancho JJ, Lennard TW, Paunovic I, Triponez F, Sitges-Serra A. Prophylactic central neck disection in papillary thyroid cancer: a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch Surg (2014) 399(2):155–63. doi: 10.1007/s00423-013-1152-8
16. Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg (2003) 237(3):399–407. doi: 10.1097/01.SLA.0000055273.58908.19
17. Lim YC, Choi EC, Yoon YH, Kim EH, Koo BS. Central lymph node metastases in unilateral papillary thyroid microcarcinoma. Br J Surg (2009) 96(3):253–7. doi: 10.1002/bjs.6484
18. Usluogullari CA, Onal ED, Ozdemir E, Ucler R, Kiyak G, Ersoy PE, et al. A retrospective analysis of prognostic factors predictive of lymph-node metastasis and recurrence in thyroid papillary microcarcinoma. Minerva Endocrinol (2015) 40(1):15–22.
19. Lee KE, Chung IY, Kang E, Koo do H, Kim KH, Kim SW, et al. Ipsilateral and contralateral central lymph node metastasis in papillary thyroid cancer: patterns and predictive factors of nodal metastasis. Head Neck (2013) 35(5):672–6. doi: 10.1002/hed.23016
20. Lang BH, Chai YJ, Cowling BJ, Min HS, Lee KE, Youn YK. Is BRAFV600E mutation a marker for central nodal metastasis in small papillary thyroid carcinoma? Endocr Relat Cancer (2014) 21(2):285–95. doi: 10.1530/ERC-13-0291
21. Wang Y, Guan Q, Xiang J. Nomogram for predicting central lymph node metastasis in papillary thyroid microcarcinoma: A retrospective cohort study of 8668 patients. Int J Surg (2018) 55:98–102. doi: 10.1016/j.ijsu.2018.05.023
22. Ardito G, Revelli L, Giustozzi E, Salvatori M, Fadda G, Ardito F, et al. Aggressive papillary thyroid microcarcinoma: prognostic factors and therapeutic strategy. Clin Nucl Med (2013) 38(1):25–8. doi: 10.1097/RLU.0b013e318279bc65
23. Jeon MJ, Chung MS, Kwon H, Kim M, Park S, Baek JH, et al. Features of papillary thyroid microcarcinoma associated with lateral cervical lymph node metastasis. Clin Endocrinol (Oxf) (2017) 86(6):845–51. doi: 10.1111/cen.13322
24. Liu LS, Liang J, Li JH, Liu X, Jiang L, Long JX, et al. The incidence and risk factors for central lymph node metastasis in cN0 papillary thyroid microcarcinoma: a meta-analysis. Eur Arch Otorhinolaryngol (2017) 274(3):1327–38. doi: 10.1007/s00405-016-4302-0
25. Kim SK, Woo JW, Lee JH, Park I, Choe JH, Kim JH, et al. Role of BRAF V600E mutation as an indicator of the extent of thyroidectomy and lymph node dissection in conventional papillary thyroid carcinoma. Surgery (2015) 158(6):1500–11. doi: 10.1016/j.surg.2015.05.016
26. Zhang L, Wei WJ, Ji QH, Zhu YX, Wang ZY, Wang Y, et al. Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1066 patients. J Clin Endocrinol Metab (2012) 97(4):1250–7. doi: 10.1210/jc.2011-1546
27. Giuseppe M, Andrea F, Corrado P, Debora F, Luigi R, Simonetta P, et al. Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid: results of a study in 445 patients. Thyroid (2009) 19(7):707–16. doi: 10.1089/thy.2008.0270
28. Al Afif A, Williams BA, Rigby MH, Bullock MJ, Taylor SM, Trites J, et al. Multifocal Papillary Thyroid Cancer Increases the Risk of Central Lymph Node Metastasis. Thyroid (2015) 25(9):1008–12. doi: 10.1089/thy.2015.0130
29. Qu N, Zhang L, Ji QH, Zhu YX, Wang ZY, Shen Q, et al. Number of tumor foci predicts prognosis in papillary thyroid cancer. BMC Cancer (2014) 14:914. doi: 10.1186/1471-2407-14-914
30. Iacobone M, Jansson S, Barczynski M, Goretzki P. Multifocal papillary thyroid carcinoma–a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch Surg (2014) 399(2):141–54. doi: 10.1007/s00423-013-1145-7
31. Lee YS, Shin SC, Lim YS, Lee JC, Wang SG, Son SM, et al. Tumor location-dependent skip lateral cervical lymph node metastasis in papillary thyroid cancer. Head Neck (2014) 36(6):887–91. doi: 10.1002/hed.23391
32. Kim H, Kim JA, Son EJ, Youk JH, Chung TS, Park CS, et al. Preoperative prediction of the extrathyroidal extension of papillary thyroid carcinoma with ultrasonography versus MRI: a retrospective cohort study. Int J Surg (2014) 12(5):544–8. doi: 10.1016/j.ijsu.2014.03.003
33. Vita R, Ieni A, Tuccari G, Benvenga S. The increasing prevalence of chronic lymphocytic thyroiditis in papillary microcarcinoma. Rev Endocr Metab Disord (2018) 19(4):301–9. doi: 10.1007/s11154-018-9474-z
Keywords: papillary thyroid carcinoma, central lymph node metastasis, central neck dissection, nomogram, surgery
Citation: Feng J-W, Hong L-Z, Wang F, Wu W-X, Hu J, Liu S-Y, Jiang Y and Ye J (2021) A Nomogram Based on Clinical and Ultrasound Characteristics to Predict Central Lymph Node Metastasis of Papillary Thyroid Carcinoma. Front. Endocrinol. 12:666315. doi: 10.3389/fendo.2021.666315
Received: 10 February 2021; Accepted: 29 March 2021;
Published: 28 April 2021.
Edited by:
Christoph Reiners, University Hospital Würzburg, GermanyReviewed by:
Jeffrey A. Knauf, Cleveland Clinic, United StatesChiara Mele, Università del Piemonte Orientale, Italy
Nicolas Schlegel, Julius Maximilian University of Würzburg, Germany
Copyright © 2021 Feng, Hong, Wang, Wu, Hu, Liu, Jiang and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Jiang, eWppYW5nODg4OEBob3RtYWlsLmNvbQ==; Jing Ye, Z2lmNTUxNzFAMTYzLmNvbQ==
 Jia-Wei Feng
Jia-Wei Feng Yong Jiang
Yong Jiang