- 1Department of Breast Cancer, Cancer Center, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 2Shantou University Medical College, Shantou, China
- 3Phil Rivers Technology, Beijing, China
Peritoneal metastases from invasive lobular carcinoma (ILC) of breast are uncommon and usually related to poor prognosis due to difficulty of detection in clinical practice and drug resistance. Therefore, recognizing the entities of peritoneal metastases of ILC and the potential mechanism of drug resistance is of great significance for early detection and providing accurate management. We herein report a case of a 60-year-old female who presented with nausea and vomiting as the first manifestation after treated with abemaciclib (a CDK4/6 inhibitor) plus fulvestrant for 23 months due to bone metastasis of ILC. Exploratory laparotomy found multiple nodules in the peritoneum and omentum, and immunohistochemistry confirmed that the peritoneal metastatic lesions were consistent with ILC. Palliative therapy was initiated, but the patient died two months later due to disease progression with malignant ascites. Whole exome sequencing (WES) was used to detect the tumor samples and showed the peritoneal metastatic lesions had acquired ESR1 and PI3KCA mutations, potentially explaining the mechanism of endocrine therapy resistance. We argue that early diagnosis of peritoneal metastasis from breast cancer is crucial for prompt and adequate treatment and WES might be an effective supplementary technique for detection of potential gene mutations and providing accurate treatment for metastatic breast cancer patients.
Introduction
Invasive breast cancer is a histologically diverse disease that has several defined histological subtypes. Invasive breast carcinoma of no special type (IBC-NST), which presents in 70%-75% of the cases, is the most common histologic subtype of breast cancer, followed by invasive lobular carcinoma (ILC), which accounts for only 5%-15% of invasive mammary carcinomas (1, 2). ILC was more likely estrogen receptor positive, HER-2 negative and had a lower proliferative index compared to IBC-NST (3).
ILC, with the hallmark loss of E-cadherin expression, is characterized by its infiltrating growth behavior, which invades the surrounding tissue with a single-file pattern at histologic examination (3, 4). Compared with IBC-NST, ILC displays a predilection for distant metastasis to uncommon sites such as gastrointestinal (GI) tract, peritoneum and genitourinary system (5, 6), and has slightly worse prognosis (7). Peritoneal metastases of breast cancer are challenging for clinicians to diagnose promptly, and recognition of the entities is of great significance for early detection and providing accurate management. As for HR-positive metastatic breast cancer (MBC), hormonal therapy represents the backbone of treatment. Recently, a new class of molecular drug, cyclin-dependent kinase 4/6 (CDK4/6) inhibitors, has been proved to improve efficacy of the first- or second-line treatment of HR positive, HER2-negative MBC (8–13). Herein, we present a rare case of metastatic ILC with peritoneal metastases causing bowel obstruction during the treatment with abemaciclib (a CDK4/6 inhibitor) plus fulvestrant.
Case Presentation
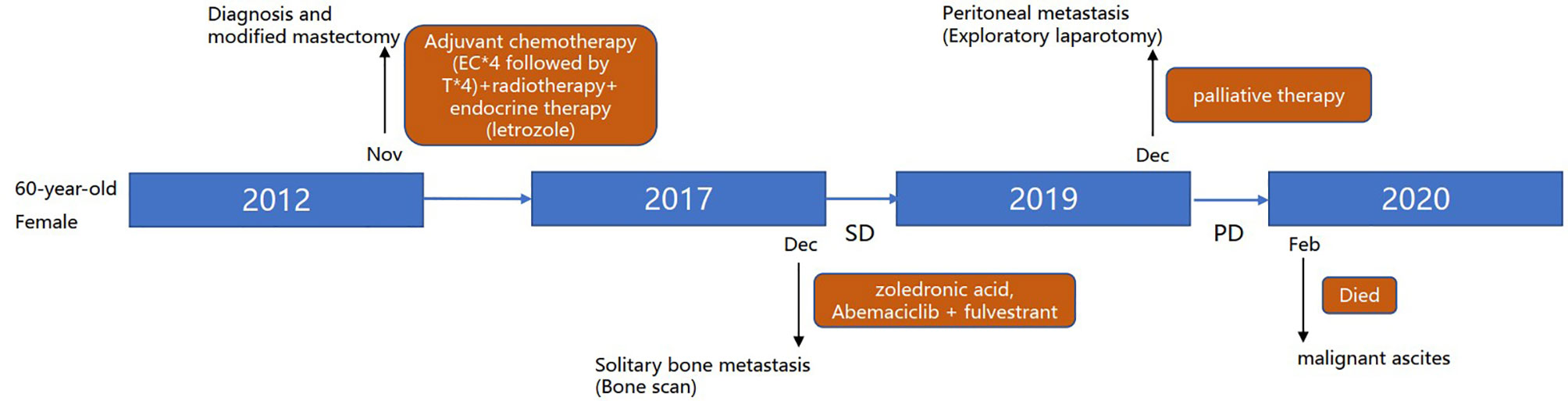
A 60-year-old female with no family history of cancer underwent left mastectomy with axillary lymph node dissection in November 2012 for stage IIIC (cT2N3M0) invasive lobular carcinoma. Histopathological examination demonstrated an invasive lobular carcinoma with positive estrogen receptor (ER+) and progesterone receptor (PgR+), negative human epidermal growth factor receptor 2 (HER2-), lymphovascular invasion and metastases to axillary lymph nodes (11/21). The pathological stage was pT2N3M0. She completed 4 cycles of epirubicin and cyclophosphamide (EC) followed by 4 cycles of paclitaxel. Then the patient underwent adjuvant radiotherapy; specifically, the left chest wall, infraclavicular and supraclavicular region, and internal mammary nodes were irradiated at a dose of 50.4 Gy in 5 weeks with a 1.8 Gy daily fraction. Meanwhile, she received once-daily regimen of letrozole 2.5 mg regularly. Follow-up was arranged every 3 months for 2 years in the breast clinic and there was no distinct evidence of recurrence. Then, the patient was followed up every 6 months in the next 3 years.
In December 2017 (5 years after surgery), bone scan detected solitary bone metastasis in the left ischium. She received intravenous zoledronic acid injections every month. She was subjected to abemaciclib plus fulvestrant with stable disease until her current presentation.
In November 2019, the patient complained of nausea. A contrasted abdominal Computed Tomography (CT) showed no distinct abnormalities. The patient took some prescribed medication and felt better thereafter. However, vomiting after meals occurred in December 2019. The patient came to our hospital for further treatment. She presented with jaundice and mild tenderness in the upper abdomen on admission. Blood chemistry tests showed elevated bilirubin and liver enzymes. The laboratory workup showed CA153 186.8U/ml, CA199 203.5U/ml, CA125 66.27U/ml, and CEA 13.2U/ml. An upper gastrointestinal X-ray (Figure 1A) and upper gastrointestinal endoscopy (Figure 1B) showed a stricture in the horizontal part of duodenum which had poor distension. A biopsy obtained from the duodenum did not detect any malignant cell. Positron Emission Tomography/Computed Tomography (PET/CT) showed thickening of duodenal wall, peritoneum and mesenteries, slightly larger lymph nodes in the mesenteric area, and varying degrees of increase in glucose metabolism (Figures 1C−G); combined with perirenal, duodenal and bladder lesions, peritonitis was highly suspected, while tuberculous peritonitis was supposed to be excluded. Thereafter, a decision was made to perform an exploratory laparotomy. In the operation, approximately 1 liter of yellow-brown ascitic fluid was drained and three nodules were seen in the peritoneal cavity, including one nodule on the ligamentum teres hepatis (Figure 1H) and the other two on the omentum (Figure 1I). Similar to the primary breast cancer (Figure 1J) and metastatic axially lymph node (Figure 1K), histological examination of the peritoneal nodule showed single-file strands of infiltrating tumor cells throughout the fibrous matrix, which were consistent with ILC (Figure 1L). The immunohistochemical (IHC) studies revealed the tumor cells were highly positive for gross cystic disease fluid protein-15 (GCDFP-15), Cytokeratin 7 (CK7), GATA-3 and ER, but negative for E-cadherin, PR and HER2 status; and the Ki67 index was 20%. The results of IHC staining were consistent with a diagnosis of peritoneal metastases from ILC. During the process of diagnosis, the patient manifested with severer nausea and vomiting and even abdominal distention, and she was received parenteral nutrition instead of oral feeding. Due to the poor condition of the patient, aggressive treatment such as chemotherapy was not considered for her, and finally palliative therapy was initiated. Unfortunately, the patient died two months later due to disease progression with malignant ascites (Figure 2).
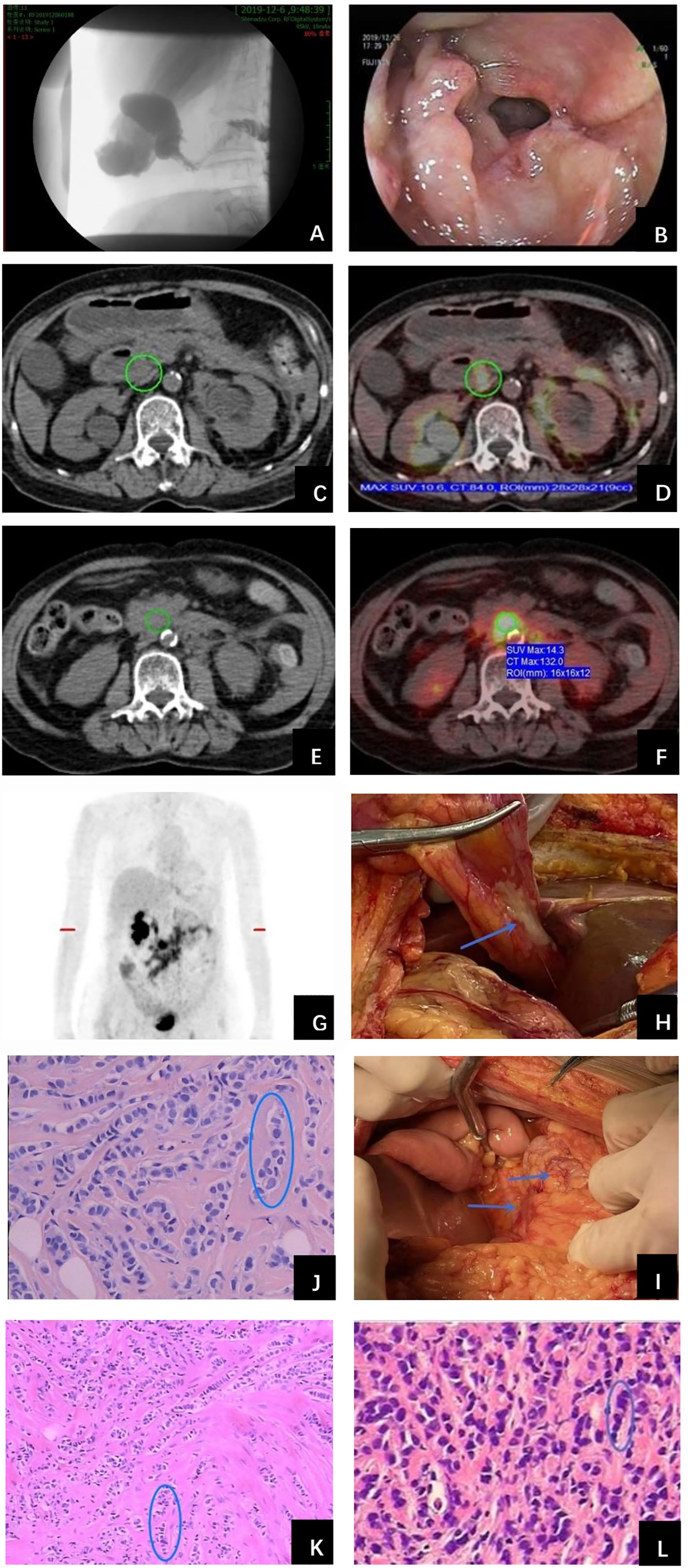
Figure 1 Examinations and histopathological results during work-up of the patient. (A) Upper gastrointestinal X-ray showed a stricture in the second portion of the duodenum. (B) Upper gastrointestinal endoscopy detected a stricture with circumferential edematous friable mucosa, extending from the duodenal bulb to the second portion of the duodenum. (C) PET/CT revealed duodenal wall was thickened and identified as metabolically active lesions (SUVmax=10.6) (D). (E) Thickened peritoneum and mesenteries and slightly larger lymph nodes in the mesenteries were found with intense FDG uptake (SUVmax=14.3) (F). (G) Holistic view of PET/CT: metabolic lesions in the duodenum, peritoneum and mesenteries. Exploratory laparotomy showed three metastatic nodules in the peritoneal cavity, including one nodule on the ligamentum teres hepatis (H) and the other two on the omentum (I) (arrows). Histopathological examination of primary breast cancer (J), metastatic axillary lymph node (K) and metastatic peritoneal nodule (L) all revealed single-file strands of infiltrating small tumor cells dispersed in the fibrous matrix (circle).
In order to know the patient’s genetic information and investigate the possible mechanisms of resistance to endocrine therapy (ET), we utilized whole exome sequencing (WES) to detect the tumor samples from primary lesion, regional lymph nodes and peritoneal metastatic lesions. The 3-way Venn Diagram showed that 47 common mutations were detected among primary lesion, lymph nodes and peritoneal metastatic lesions (Figure 3A); combined with somatic mutation heatmap (Figure 3B) and variation frequency (VAF) distribution (Figure 3C), it was implied that the three tumor samples may have the same origin. Mutation analysis of signal transduction enrichment showed that the PI3K-AKT signaling pathway was significantly enriched in the peritoneal metastatic lesions. All three tumor samples carried PIK3CA p.H1047R, which is one of the most common mutation of PIK3CA in breast cancer. What’ more, the sample of metastatic lesion was found to have acquired PIK3CA p.D959N (Figure 4A) and ESR1 p.E380Q mutation (Figure 4B), which were not detected in neither primary lesion nor lymph node.
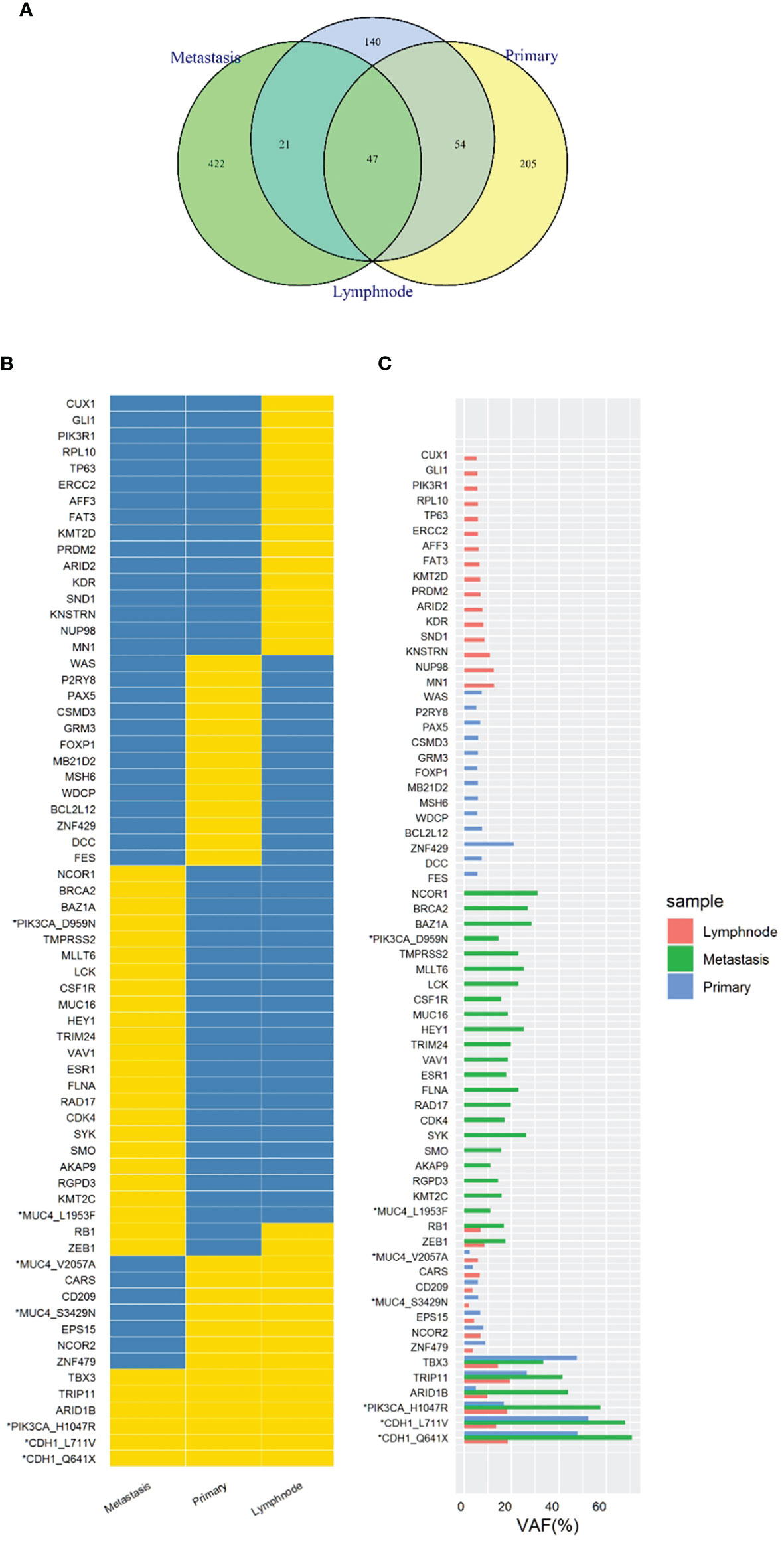
Figure 3 Whole exome sequencing (WES) of tumor samples from primary lesion, regional lymph nodes and peritoneal metastatic lesions. (A) 3-way Venn Diagram showed the mutational overlaps in the three samples. There were 47 common mutations in the three samples, while another 21 common mutations between lymph node and metastatic site, and another 54 common mutations between lymph node and primary site. (B) Somatic mutation heatmap. The mark “*” means that there are 2 or more mutations in the same gene, which was labelled with gene or amino acid changes. Yellow means there is variation, while blue means there is no variation. (C) Variation frequency (VAF) distribution. The mark “*” means that there are 2 or more mutations in the same gene, which was labelled with gene or amino acid changes.
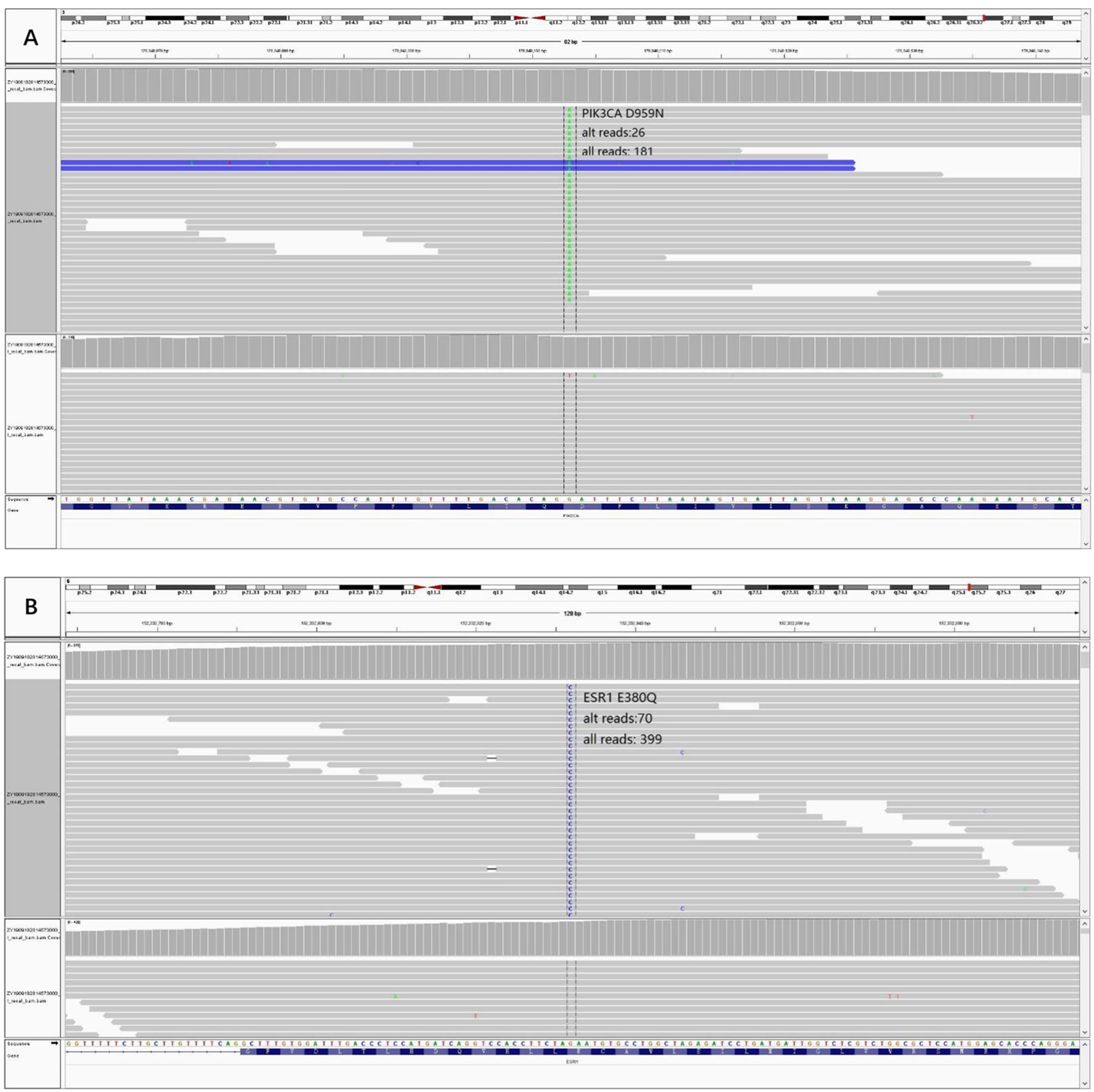
Figure 4 Acquired mutations were detected in the sample of peritoneal metastatic lesion and visualized through Integrative Genomics Viewer (IGV). (A) Variant PIK3CA p.D959N IGV plot (all reads: 181, alternative allele supported reads: 26). (B) Variant ESR1 p.E380Q IGV plot (all reads: 399, alternative allele supported reads: 70).
Discussion
The patient in this case was found to have peritoneal metastasis from ILC after diagnostic work-up for the presence of nausea and vomiting. Peritoneal carcinomatosis secondary to breast cancer has been reported in literatures (Table 1) (14–21), and some studies showed nearly 3% of ILC patients had peritoneal metastasis, which was higher than those of IBC-NST patients (22, 23).
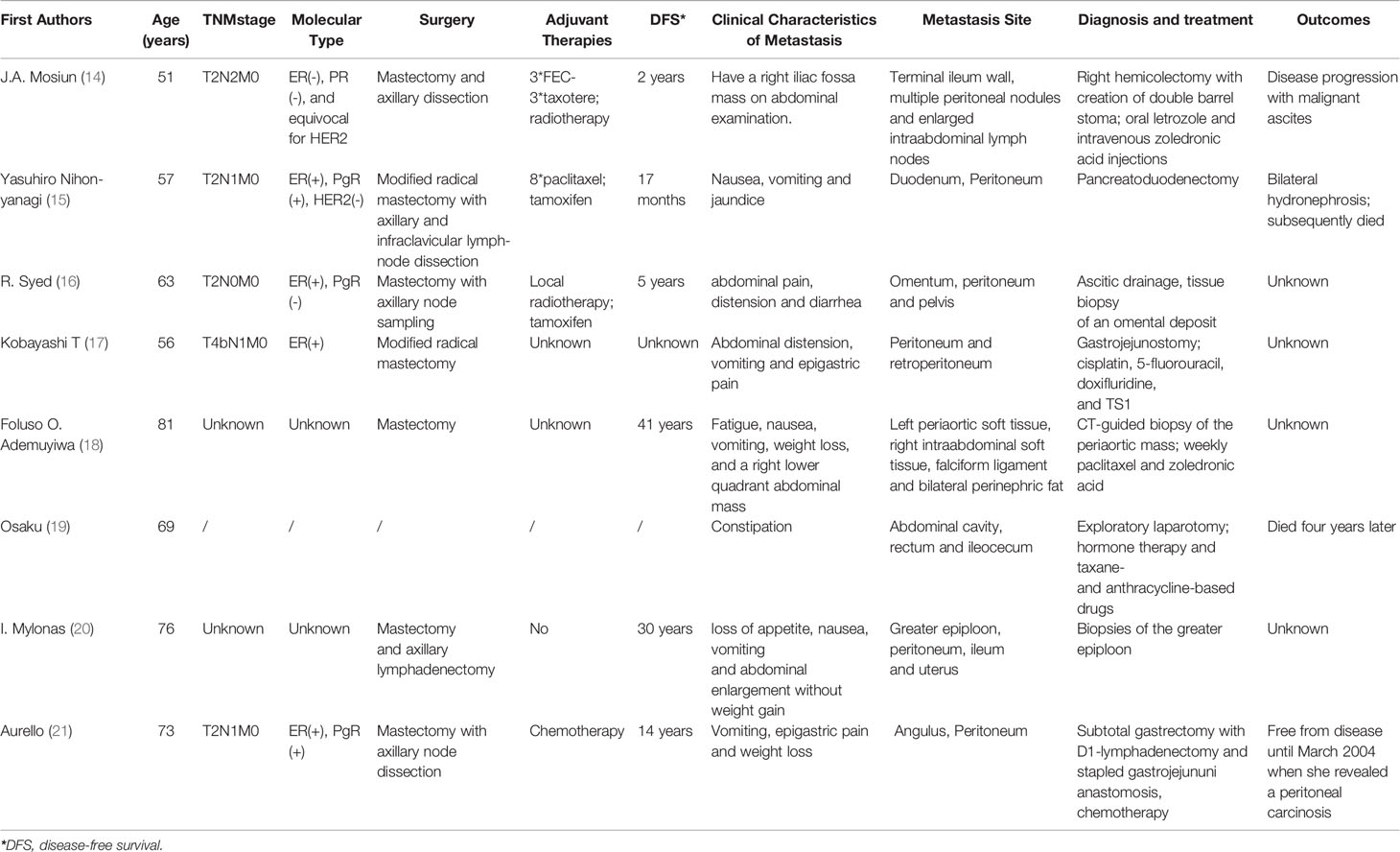
Table 1 Review of literature: characteristics and outcomes of breast cancer patients with peritoneal metastasis.
Clinical manifestations of peritoneal metastasis from ILC are variable and non-specific. Patients usually do not have any symptoms until later, even several days before death. Metastasis to the peritoneum or retroperitoneum leads to thickening and sclerosis of the surrounding tissues. A common finding of peritoneum metastasis from breast cancer is stenosis, frequently with presentation of abdominal pain, early satiety and obstructive symptoms. These patients might be misdiagnosed with a primary GI tumor or even not diagnosed with malignancy at all (24, 25). A case series of 12, 001 patients found 11% of the patients were not diagnosed with GI metastasis from breast carcinoma until an exploratory laparotomy was performed, as was the patient in our case (6). As the clinical presentation of peritoneal metastases is usually non-specific, histopathological and immunohistochemical examinations are the definitive diagnostic methods. Microscopically, single-file strands of infiltrating tumor cells invading the surrounding tissue can frequently be seen in metastases from ILC as observed in our study (26, 27). However, it is still quite challenging to come up with the definite diagnosis via histological examination because signet ring cell carcinoma can indeed arise from any tissue. Immunohistochemical markers are crucial for diagnosing metastatic lobular carcinoma of the breast. The most important markers for ILC are Cytokeratin 7 (CK7), GATA-3, gross cystic disease fluid protein-15 (GCDFP-15), ER and PR, all of which but not PR were highly positive in the biopsy specimens of peritoneal metastatic lesions in our patient (28, 29). There was also negative of HER2. Samples from distant sites often show features similar to that of primary breast cancer which is most commonly an ILC. The availability of IHC studies allowed clinicians to accurately diagnose metastatic lobular breast carcinoma (30, 31).
There is no consensus for the treatment of peritoneal carcinomatosis secondary to breast cancer, as there have not yet been any large-scale studies that compared the efficacy of different managements (30, 31). Palliative surgery is necessary in the treatment of patients with symptomatic obstruction, bleeding or perforation, even though no survival benefit may ensue (24, 30, 32). A few studies have been published describing the combination of surgical debulking and hyperthermic intraperitoneal chemotherapy (HIPEC) for patients with secondary peritoneal carcinomatosis due to breast cancer as well as other primary diseases, which showed improvement in morbidity and mortality (33, 34). A retrospective study (6), which included 73 breast cancer patients with GI or peritoneal metastasis, reported palliative surgical intervention conferred no survival benefit while systemic chemotherapy or hormone therapy might have improved survival of the patients. Late presentation of signs and symptoms of peritoneal metastasis was related to poor prognosis. However, there is no enough data in the best treatment and precise prognosis for those patients due to the limited number of case reports. Treatment should be tailored to the patient and their projected performance status along with quality-of-life consideration (35, 36).
In this case, ILC metastasized to the peritoneum and omentum in association with spread of many small nodules. It remains unclear concerning the mechanism of these metastatic patterns. Previous study (37) revealed that most of ILC lack cohesiveness because the E-cadherin was inactivated, which was a cell-to-cell adhesion protein. WES showed the patient had gene mutations in ESR1 and PIK3CA at the metastatic lesions, which was thought to be acquired due to chronic exposure of CDK4/6 inhibitor plus ET (38). As the most common mechanism of resistance to ET in MBC, acquired ESR1 mutations may have been existing in primary tumors and become enriched only when metastasis occurs (39). By enhancing coactivator recruitment, ESR1 mutations with altered structure conferred distinct mechanism of resistance to ER antagonists such as tamoxifen (40, 41). Previous studies demonstrated that MBC patients with ESR1 mutation are resistant to standard ET and have worse overall survival (42, 43), as seen in our patient. Currently, the best treatment for MBC patients with ESR1 mutations is fulvestrant combined with CDK4/6 inhibitor, which conferred significantly improved PFS in patients with ESR1 mutations (39). In this case, the patient was treated with abemaciclib plus fulvestrant after solitary bone metastasis, and the PFS was 23 months. Besides to ESR1 mutation, the PIK3CA mutation was also found in the metastatic lesions. Some studies (44, 45) demonstrated that the PI3K/mTOR pathway was upregulated in response to long-term use of CDK4/6 inhibitor, which drove cell cycle progression via upregulating cyclin D. Therefore, PIK3CA mutation and the subsequently activated PI3K-AKT signaling pathway might mediate resistance to CDK4/6 inhibitor for this patient. The SOLAR-1 trial (46) showed patients with PIK3CA mutation had double PFS after receiving PIK3CA inhibitor alpelisib plus fulvestrant compared with those receiving fulvestrant plus placebo (11.0 months and 5.7 months, respectively). In the subgroup of patients who had been treated with CDK4/6 inhibitors previously, receiving alpelisib reduced 52% risk in PFS compared with placebo (47). Hence, PIK3CA inhibitors may be used to overcome resistance to CDK4/6 inhibitor for MBC patients. However, PIK3CA inhibitors including alpelisib are not available in the mainland of China up to now. Meanwhile, ILC patients with peritoneal metastasis usually progress quickly and are easily misdiagnosed, which makes it difficult for these patients to acquire timely and effective treatment. Therefore, it is crucial that more efforts need to be put into early detection of ILC patients with peritoneal metastasis and availability of new drugs like PIK3CA inhibitors.
By using WES to detect the tumor samples, it is available for us to get access to the genomic information of this patient and investigate the possible mechanism of endocrine therapy resistance. WES showed the peritoneal metastatic lesions had acquired ESR1 and PI3KCA mutations, potentially explaining the mechanism of endocrine therapy resistance. Therefore, we argue that early diagnosis of peritoneal metastasis from breast cancer is crucial for prompt and adequate treatment and WES might be an effective supplementary technique for detection of potential gene mutations and providing accurate treatment for metastatic breast cancer patients.
Conclusion
All clinicians should realize that there is an unusual pattern of peritoneal metastasis from ILC. For patients with vomiting and previous history of ILC, it is necessary to highly suspect peritoneum metastasis. Early diagnosis is vital in ensuring prompt and adequate treatment. Our results suggest that ESR1 and PIK3CA mutations are acquired resistance mechanism of CDK4/6 inhibitor plus endocrine therapy and WES might be an effective supplementary technique for detection of potential gene mutations for MBC patients with drug resistance, thus ensuring timely and accurate salvage treatment. Nevertheless, further studies need to be conducted to investigate the mechanism and predictive factors of peritoneal metastasis of ILC.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
H-FG and J-SZ was mainly responsible for the article writing. Q-ZZ, and FJ was mainly responsible for the gene analysis. KW and GN were in charge of all study procedures. TZ, C-QY, L-LZ and MY were responsible for patient’s clinical data and analysis. J-QL and M-YC were responsible for consent from the patient and ethics committee. All authors gave final approval of the manuscript to be submitted and agreed to be accountable for all aspects of the work.
Funding
This study is supported by grants from National Natural Science Foundation of China (82171898, 82103093), Science and Technology Planning Project of Guangzhou City (202002030236), Beijing Medical Award Foundation (YXJL-2020-0941-0758), Guangdong Basic and Applied Basic Research Foundation (2020A1515010346, 2021A1515011570), Guangzhou Science and Technology Project (202102021055), Fundamental Research Funds for the Central Universities (2020ZYGXZR017), Science and Technology Special Fund of Guangdong Provincial People’s Hospital (2017zh01), CSCO-Hengrui Cancer Research Fund (Y-HR2016-067), and Guangdong Provincial Department of Education Characteristic Innovation Project (2015KTSCX080). Funding sources were not involved in the study design, data collection, analysis and interpretation, writing of the report, or decision to submit the article for publication.
Conflict of Interest
Author GN and Q-ZZ was employed by the company Phil Rivers Technology, China.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.659537/full#supplementary-material
References
1. Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell (2015) 163:506–19. doi: 10.1016/j.cell.2015.09.033
2. Cristofanilli M, Gonzalez-Angulo A, Sneige N, Kau SW, Broglio K, Theriault RL, et al. Invasive Lobular Carcinoma Classic Type: Response to Primary Chemotherapy and Survival Outcomes. J Clin Oncol (2005) 23(1):41–8. doi: 10.1200/JCO.2005.03.111
3. Biglia N, Maggiorotto F, Liberale V, Bounous VE, Sgro LG, Pecchio S, et al. Clinical-Pathologic Features, Long Term-Outcome and Surgical Treatment in a Large Series of Patients With Invasive Lobular Carcinoma (ILC) and Invasive Ductal Carcinoma (IDC). Eur J Surg Oncol (2013) 39(5):455–60. doi: 10.1016/j.ejso.2013.02.007
4. Moll R, Mitze M, Frixen UH, Birchmeier W. Differential Loss of E-Cadherin Expression in Infiltrating Ductal and Lobular Breast Carcinomas. Am J Pathol (1993) 143:1731–42.
5. Winston CB, Hadar O, Teitcher JB, Caravelli JF, Sklarin NT, Panicek DM, et al. Metastatic Lobular Carcinoma of the Breast: Patterns of Spread in the Chest, Abdomen, and Pelvis on CT. AJR Am J roentgenology (2000) 175:795–800. doi: 10.2214/ajr.175.3.1750795
6. McLemore EC, Pockaj BA, Reynolds C, Gray RJ, Hernandez JL, Grant CS, et al. Breast Cancer: Presentation and Intervention in Women With Gastrointestinal Metastasis and Carcinomatosis. Ann Surg Oncol (2005) 12:886–94. doi: 10.1245/ASO.2005.03.030
7. Metzger-Filho O, Ferreira AR, Jeselsohn R, Barry WT, Dillon DA, Brock JE, et al. Mixed Invasive Ductal and Lobular Carcinoma of the Breast: Prognosis and the Importance of Histologic Grade. Oncologist (2019) 24(7):e441–9. doi: 10.1634/theoncologist.2018-0363
8. Sledge GW Jr., Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol Off J Am Soc Clin Oncol (2017) 35:2875–84. doi: 10.1200/JCO.2017.73.7585
9. Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and Letrozole in Advanced Breast Cancer. New Engl J Med (2016) 375:1925–36. doi: 10.1056/NEJMoa1607303
10. Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant Plus Palbociclib Versus Fulvestrant Plus Placebo for Treatment of Hormone-Receptor-Positive, HER2-Negative Metastatic Breast Cancer That Progressed on Previous Endocrine Therapy (PALOMA-3): Final Analysis of the Multicentre, Double-Blind, Phase 3 Randomised Controlled Trial. Lancet Oncol (2016) 17:425–39. doi: 10.1016/S1470-2045(15)00613-0
11. Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib as Initial Therapy for Advanced Breast Cancer. J Clin Oncol Off J Am Soc Clin Oncol (2017) 35:3638–46. doi: 10.1200/JCO.2017.75.6155
12. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. New Engl J Med (2016) 375:1738–48. doi: 10.1056/NEJMoa1609709
13. Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36:2465–72. doi: 10.1200/JCO.2018.78.9909
14. Mosiun JA, Idris MSB, Teoh LY, Teh MS, Chandran PA, See MH. Gastrointestinal Tract Metastasis Presenting as Intussusception in Invasive Lobular Carcinoma of the Breast: A Case Report. Int J Surg Case Rep (2019) 64:109–12. doi: 10.1016/j.ijscr.2019.10.003
15. Nihon-Yanagi Y, Park Y, Ooshiro M, Aoki H, Suzuki Y, Hiruta N, et al. A Case of Recurrent Invasive Lobular Carcinoma of the Breast Found as Metastasis to the Duodenum. Breast Cancer (Tokyo Japan) (2009) 16:83–7. doi: 10.1007/s12282-008-0045-0
16. Syed R, Nazir SA, Lwin KY, Bose P, Evans P, Choji K. Occurrence of Synchronous Invasive Lobular Breast Carcinoma and Poorly Differentiated Ovarian Carcinoma in a Single Peritoneal Deposit. Oncology (2007) 73:136–40. doi: 10.1159/000121003
17. Kobayashi T, Adachi S, Matsuda Y, Tominaga S. A Case of Metastatic Lobular Breast Carcinoma With Detection of the Primary Tumor After Ten Years. Breast Cancer (Tokyo Japan) (2007) 14:333–6. doi: 10.2325/jbcs.14.333
18. Ademuyiwa FO, Khoury T, Warner J, Gannon J, Hwang H. An 81-Year-Old Patient With Distant Metastasis of Invasive Lobular Carcinoma Occurring 41 Years After Mastectomy. Clin Breast Cancer (2012) 12:293–5. doi: 10.1016/j.clbc.2012.03.012
19. Osaku T, Ogata H, Magoshi S, Kubota Y, Saito F, Kanazawa S, et al. Metastatic Nonpalpable Invasive Lobular Breast Carcinoma Presenting as Rectal Stenosis: A Case Report. J Med Case Rep (2015) 9:88–8. doi: 10.1186/s13256-015-0568-x
20. Mylonas I, Janni W, Friese K, Gerber B. Unexpected Metastatic Lobular Carcinoma of the Breast With Intraabdominal Spread and Subsequent Port-Site Metastasis After Diagnostic Laparoscopy for Exclusion of Ovarian Cancer. Gynecologic Oncol (2004) 95:405–8. doi: 10.1016/j.ygyno.2004.07.057
21. Aurello P, D'Angelo F, Cosenza G, Petrocca S, Stoppacciaro A, Ramacciato G, et al. Gastric Metastasis 14 Years After Mastectomy for Breast Lobular Carcinoma: Case Report and Literature Review. Am surgeon (2006) 72:456–60. doi: 10.1177/000313480607200518
22. Lamovec J, Bracko M. Metastatic Pattern of Infiltrating Lobular Carcinoma of the Breast: An Autopsy Study. J Surg Oncol (1991) 48:28–33. doi: 10.1002/jso.2930480106
23. Borst MJ, Ingold JA. Metastatic Patterns of Invasive Lobular Versus Invasive Ductal Carcinoma of the Breast. Surgery (1993) 114:637–41.
24. Tsujimura K, Teruya T, Kiyuna M, Higa K, Higa J, Iha K, et al. Colonic Metastasis From Breast Carcinoma: A Case Report. World J Surg Oncol (2017) 15:124–4. doi: 10.1186/s12957-017-1193-5
25. Sobinsky JD, Willson TD, Podbielski FJ, Connolly MM. Unusual Metastatic Patterns of Invasive Lobular Carcinoma of the Breast. Case Rep oncological Med (2013) 2013:986517–7. doi: 10.1155/2013/986517
26. Raju U, Ma CK, Shaw A. Signet Ring Variant of Lobular Carcinoma of the Breast: A Clinicopathologic and Immunohistochemical Study. Modern Pathol an Off J United States Can Acad Pathology Inc (1993) 6:516–20.
27. Signorelli C, Pomponi-Formiconi D, Nelli F, Pollera CF. Single Colon Metastasis From Breast Cancer: A Clinical Case Report. Tumori (2005) 91:424–7. doi: 10.1177/030089160509100509
28. Tot T. The Role of Cytokeratins 20 and 7 and Estrogen Receptor Analysis in Separation of Metastatic Lobular Carcinoma of the Breast and Metastatic Signet Ring Cell Carcinoma of the Gastrointestinal Tract. APMIS Acta Pathologica Microbiologica Immunologica Scandinavica (2000) 108:467–72. doi: 10.1034/j.1600-0463.2000.d01-84.x
29. Miettinen M, McCue PA, Sarlomo-Rikala M, Rys J, Czapiewski P, Wazny K, et al. GATA3: A Multispecific But Potentially Useful Marker in Surgical Pathology: A Systematic Analysis of 2500 Epithelial and Nonepithelial Tumors. Am J Surg Pathol (2014) 38:13–22. doi: 10.1097/PAS.0b013e3182a0218f
30. Franceschini G, Manno A, Mulè A, Verbo A, Rizzo G, Sermoneta D, et al. Gastro-Intestinal Symptoms as Clinical Manifestation of Peritoneal and Retroperitoneal Spread of an Invasive Lobular Breast Cancer: Report of a Case and Review of the Literature. BMC Cancer (2006) 6:193–3. doi: 10.1186/1471-2407-6-193
31. Nikkar-Esfahani A, Kumar BG, Aitken D, Wilson RG. Metastatic Breast Carcinoma Presenting as a Sigmoid Stricture: Report of a Case and Review of the Literature. Case Rep Gastroenterol (2013) 7:106–11. doi: 10.1159/000348760
32. López Deogracias M, Flores Jaime L, Arias-Camisón I, Zamacola I, Murillo Guibert J. Rectal Metastasis From Lobular Breast Carcinoma 15 Years After Primary Diagnosis. Clin Trans Oncol Off Publ Fed Spanish Oncol Societies Natl Cancer Institute Mexico (2010) 12:150–3. doi: 10.1007/S12094-010-0481-0
33. Cardi M, Sammartino P, Mingarelli V, Sibio S, Accarpio F, Biacchi D, et al. Cytoreduction and HIPEC in the Treatment of “Unconventional” Secondary Peritoneal Carcinomatosis. World J Surg Oncol (2015) 13:305–5. doi: 10.1186/s12957-015-0703-6
34. Cardi M, Sammartino P, Framarino ML, Biacchi D, Cortesi E, Sibio S, et al. Treatment of Peritoneal Carcinomatosis From Breast Cancer by Maximal Cytoreduction and HIPEC: A Preliminary Report on 5 Cases. Breast (Edinburgh Scotland) (2013) 22:845–9. doi: 10.1016/j.breast.2013.02.020
35. Mitra SK, Lim ST, Chi A, Schlaepfer DD. Intrinsic Focal Adhesion Kinase Activity Controls Orthotopic Breast Carcinoma Metastasis via the Regulation of Urokinase Plasminogen Activator Expression in a Syngeneic Tumor Model. Oncogene (2006) 25:4429–40. doi: 10.1038/sj.onc.1209482
36. Derksen PWB, Braumuller TM, van der Burg E, Hornsveld M, Mesman E, Wesseling J, et al. Mammary-Specific Inactivation of E-Cadherin and P53 Impairs Functional Gland Development and Leads to Pleomorphic Invasive Lobular Carcinoma in Mice. Dis Models Mech (2011) 4:347–58. doi: 10.1242/dmm.006395
37. Berx G, Cleton-Jansen AM, Strumane K, de Leeuw WJ, Nollet F, van Roy F, et al. E-Cadherin Is Inactivated in a Majority of Invasive Human Lobular Breast Cancers by Truncation Mutations Throughout Its Extracellular Domain. Oncogene (1996) 13:1919–25.
38. O'Leary B, Cutts RJ, Liu Y, Hrebien S, Huang X, Fenwick K, et al. The Genetic Landscape and Clonal Evolution of Breast Cancer Resistance to Palbociclib Plus Fulvestrant in the PALOMA-3 Trial. Cancer Discovery (2018) 8:1390–403. doi: 10.1158/2159-8290.CD-18-0264
39. Dustin D, Gu G, Fuqua SAW. ESR1 Mutations in Breast Cancer. Cancer (2019) 125:3714–28. doi: 10.1002/cncr.32345
40. Gelsomino L, Gu G, Rechoum Y, Beyer AR, Pejerrey SM, Tsimelzon A, et al. ESR1 Mutations Affect Anti-Proliferative Responses to Tamoxifen Through Enhanced Cross-Talk With IGF Signaling. Breast Cancer Res Treat (2016) 157:253–65. doi: 10.1007/s10549-016-3829-5
41. Robinson DR, Wu YM, Vats P, Su F, Lonigro RJ, Cao X, et al. Activating ESR1 Mutations in Hormone-Resistant Metastatic Breast Cancer. Nat Genet (2013) 45:1446–51. doi: 10.1038/ng.2823
42. Fribbens C, O'Leary B, Kilburn L, Hrebien S, Garcia-Murillas I, Beaney M, et al. Plasma ESR1 Mutations and the Treatment of Estrogen Receptor-Positive Advanced Breast Cancer. J Clin Oncol (2016) 34:2961–8. doi: 10.1200/JCO.2016.67.3061
43. Chandarlapaty S, Chen D, He W, Sung P, Samoila A, You D, et al. Prevalence of ESR1 Mutations in Cell-Free DNA and Outcomes in Metastatic Breast Cancer: A Secondary Analysis of the BOLERO-2 Clinical Trial. JAMA Oncol (2016) 2:1310–5. doi: 10.1001/jamaoncol.2016.1279
44. Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, et al. Early Adaptation and Acquired Resistance to CDK4/6 Inhibition in Estrogen Receptor-Positive Breast Cancer. Cancer Res (2016) 76:2301–13. doi: 10.1158/0008-5472.CAN-15-0728
45. Jansen VM, Bhola NE, Bauer JA, Formisano L, Lee KM, Hutchinson KE, et al. Kinome-Wide RNA Interference Screen Reveals a Role for PDK1 in Acquired Resistance to CDK4/6 Inhibition in ER-Positive Breast Cancer. Cancer Res (2017) 77:2488–99. doi: 10.1158/0008-5472.CAN-16-2653
46. André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med (2019) 380:1929–40. doi: 10.1056/NEJMoa1813904
Keywords: breast, lobular carcinoma, neoplasm metastasis, peritoneum, whole exome sequencing
Citation: Gao H-F, Zhang J-S, Zhang Q-Z, Zhu T, Yang C-Q, Zhang L-L, Yang M, Ji F, Li J-Q, Cheng M-Y, Niu G and Wang K (2021) Peritoneal Metastasis After Treated With Abemaciclib Plus Fulvestrant for Metastatic Invasive Lobular Breast Cancer: A Case Report and Review of the Literature. Front. Endocrinol. 12:659537. doi: 10.3389/fendo.2021.659537
Received: 11 February 2021; Accepted: 31 August 2021;
Published: 08 October 2021.
Edited by:
Penelope Dawn Ottewell, The University of Sheffield, United KingdomReviewed by:
Osama Shiraz Shah, University of Pittsburgh, United StatesPatrick Neven, University Hospitals Leuven, Belgium
Copyright © 2021 Gao, Zhang, Zhang, Zhu, Yang, Zhang, Yang, Ji, Li, Cheng, Niu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kun Wang, Z3p3YW5na3VuQDEyNi5jb20=
†These authors have contributed equally to this work
 Hong-Fei Gao1†
Hong-Fei Gao1† Qiang-Zu Zhang
Qiang-Zu Zhang Fei Ji
Fei Ji Kun Wang
Kun Wang