- 1Department of Molecular & Cellular Endocrinology, Arthur Riggs Diabetes and Metabolism Research Institute, Beckman Research Institute, City of Hope Medical Center, Duarte, CA, United States
- 2Comprehensive Cancer Center, Beckman Research Institute, City of Hope Medical Center, Duarte, CA, United States
Brown adipocyte in brown adipose tissue (BAT) specializes in expending energy through non-shivering thermogenesis, a process that produces heat either by uncoupling protein 1 (UCP1) dependent uncoupling of mitochondrial respiration or by UCP1 independent mechanisms. Apart from this, there is ample evidence suggesting that BAT has an endocrine function. Studies in rodents point toward its vital roles in glucose and lipid homeostasis, making it an important therapeutic target for treating metabolic disorders related to morbidities such as obesity and type 2 diabetes. The rediscovery of thermogenically active BAT depots in humans by several independent research groups in the last decade has revitalized interest in BAT as an even more promising therapeutic intervention. Over the last few years, there has been overwhelming interest in understanding brown adipocyte’s developmental lineages and how brown adipocyte uniquely utilizes energy beyond UCP1 mediated uncoupling respiration. These new discoveries would be leveraged for designing novel therapeutic interventions for metabolic disorders.
Introduction
Adipose tissue, one of the most plastic organs, is now widely accepted as an essential player in maintaining whole-body energy homeostasis (1, 2). Three different types of adipocytes that exist in mammals are white adipocytes, brown adipocytes, and beige or brite (stands for brown in white) adipocytes (3–6). Brown adipocytes and white adipocytes form a major part of brown adipose tissue (BAT) and white adipose tissue (WAT), respectively. In these adipose tissues, in addition to adipocytes, there are also stem cells, preadipocytes (committed adipocyte precursors), immune cells, fibroblasts, and endothelial cells. Both BAT and WAT appear as several discrete depots located throughout the body (7). Metabolically, WAT specializes in storing energy in the form of triglycerides. BAT expends energy via non-shivering thermogenesis, a process that involves dissipation of heat generated via uncoupling of mitochondrial respiration mediated by uncoupling protein 1 (UCP1) (8, 9). Morphologically, white adipocytes have unilocular lipid droplets, fewer mitochondria, and no expression of UCP1. Brown adipocytes have multilocular lipid droplets, high mitochondria content, and high expression of UCP1 (3–5). We recently showed that there are two subpopulations of brown adipocytes which co-exist in the BAT of mice (10). One subpopulation has high thermogenic activity with high UCP1 expression, and the other one has low thermogenic activity with low UCP1 expression. Conversely, beige adipocytes usually appear in the WAT depots in response to external cues such as cold exposure, exercise, or adrenergic stimulation. Although similar to white adipocytes they have low UCP1 expression, with external stimuli they can be activated to increase both UCP1 expression as well as respiration rate. Furthermore, they show a molecular signature that is distinct from either white or brown adipocytes (11, 12).
It is now evident that heterogeneity exists within the thermogenic adipocytes in both rodents and humans. Studying the developmental lineages of this heterogeneous cell population along with the investigation of the key mechanisms involved in the activation and thermoregulation of these adipocytes will enable us to identify novel therapeutic targets to treat metabolic disorders. In this review, we will discuss the developmental origins of BAT along with its heterogeneity followed by its developmental timeline. We will also summarize the recent works on BAT metabolism and fuel selection.
Developmental Origins and Heterogeneity of BAT
Early lineage tracing studies suggested that brown adipocytes are closer to skeletal muscles in developmental origin than white adipocytes. Atit et al. showed that cells from mouse embryo central dermomyotome, which express the homeobox transcription factor Engrailed 1 at E9.5 give rise to interscapular BAT (iBAT), dermis, and skeletal muscles (13). After this observation, several other studies were published supporting the same notion that BAT and skeletal muscles share the common progenitors. Using a Myf5Cre driver crossed to a cytoplasmic reporter, the Spiegelman group elegantly showed that Myf5+ cells contribute to iBAT and skeletal muscles but not to any WAT depots (14). They also demonstrated that PR domain zinc-finger protein 16 (PRDM16) acts as a molecular switch between myoblast and brown fat cell lineages (14). Another study using an inducible Cre under the control of the Pax7 promoter also showed that Pax7+ progenitors labeled at E9.5 give rise to iBAT. They also found that the myogenic restriction of the Pax7+ lineage occurs at the later stage in between E9.5 and E12.5 (15). Also, microarray analysis demonstrated that brown preadipocytes show myogenic transcription signature (16). Furthermore, when compared at transcriptome as well as proteome levels, brown fat mitochondria were found to share many similarities with muscle mitochondria (17). Remarkably, several factors including Ewing Sarcoma (EWS), Zinc Finger Protein 516 (ZFP516), Euchromatic Histone Lysine Methyltransferase 1 (EHMT1), early B cell factor-2 (EBF2), TATA-Box Binding Protein Associated Factor 7 Like (TAF7L), and some microRNAs (MyomiR-133, Mir193b-365) have been shown to affect cell fate decision between brown fat and muscle (14, 18–25). Using Myf5Cre crossed to R26R-mTmG reporter, the Guertin group suggested that brown adipocytes are not exclusively derived from Myf5 lineage, and this lineage also contributes to white and beige adipocyte populations (26, 27). They also traced a couple of other myoblast-specific markers viz. Pax3 and MyoD1 and found that Pax3 lineage was represented more broadly in the global brown adipocyte population, while MyoD1Cre did not trace any of the brown adipocyte population confirming that they do not arise from MyoD1 lineage (26). Furthermore, some other studies have also reported the heterogeneous adipocyte labeling with Myf5 lineage (28, 29). Altogether, these studies suggest that brown adipocytes from different depots or even within the same depots could derive from different embryonic lineages. Moreover, another recent report described that only 50% of adipocytes in iBAT derive from Pax7+ lineage originating from the central dermomyotome (30); which initially was thought to be a sole source of iBAT.
Several groups have reported the heterogeneous UCP1 expression as well as mitochondrial potential in brown adipocytes (31–34). Spaethling et al. using a single-cell RNA sequencing (scRNA-seq) of nine handpicked mature brown adipocytes showed a transcriptome variability of brown adipocytes. In addition to variability in the expression of well-known brown adipocyte markers such as UCP1 and Adrb3, they also identified differential expression of various transporters as well as receptors for neurotransmitters, cytokines, and hormones (32). Similarly, recent work from our lab reported a high degree of functional diversity in the iBAT of mice. More precisely, using a scRNA-seq of mature brown adipocytes we identified two metabolically distinct brown adipocytes; high thermogenic (BA-H) and low thermogenic (BA-L). For thermogenic activity/function, we refer to the ability of the brown adipocyte to get activated to significantly increase substrates oxidization. BA-H is the classical thermogenic population with high UCP1 expression, high mitochondrial content as well as high respiration rate. On the contrary, a novel BA-L population had low UCP1 expression, low mitochondrial content, a respiratory rate that is intermediate between white adipocytes and BA-H, but similar respiration potential (Figure 1) (10). Further investigation of the lineage and metabolic functions of this newly identified novel BA-L population will help uncover new cellular mechanisms of thermogenic regulation in BAT. Interestingly, a very recent report, using single nucleus RNA-sequencing (snRNA-seq), identified a unique, rare subpopulation of regulatory brown adipocytes (35). This novel subpopulation that increases in abundance at higher environmental temperature modulates the thermogenic activity of neighboring adipocytes. The higher number of these cells in humans as compared to mice may explain the lower thermogenic capacity of human BAT. It is important to note here that none of these studies (except one by Bertholet et al.) including ours measured mitochondrial respiration in presence of GDP which represents a direct UCP1 dependent respiration. Seahorse Xfe mitochondrial stress test is utilized commonly by researchers to report the thermogenic or UCP1 function in brown adipocytes. However, it is not a direct measure of UCP1 activity. Although BA-L population has lower UCP1 protein level, it is not determined if these UCP1 protein has similar activity as UCP1 in the BA-H cells. Our future plan is to use GDP as a direct inhibitor of UCP1 in the mitochondrial stress test to measure UCP1 dependent thermogenic activity (36).
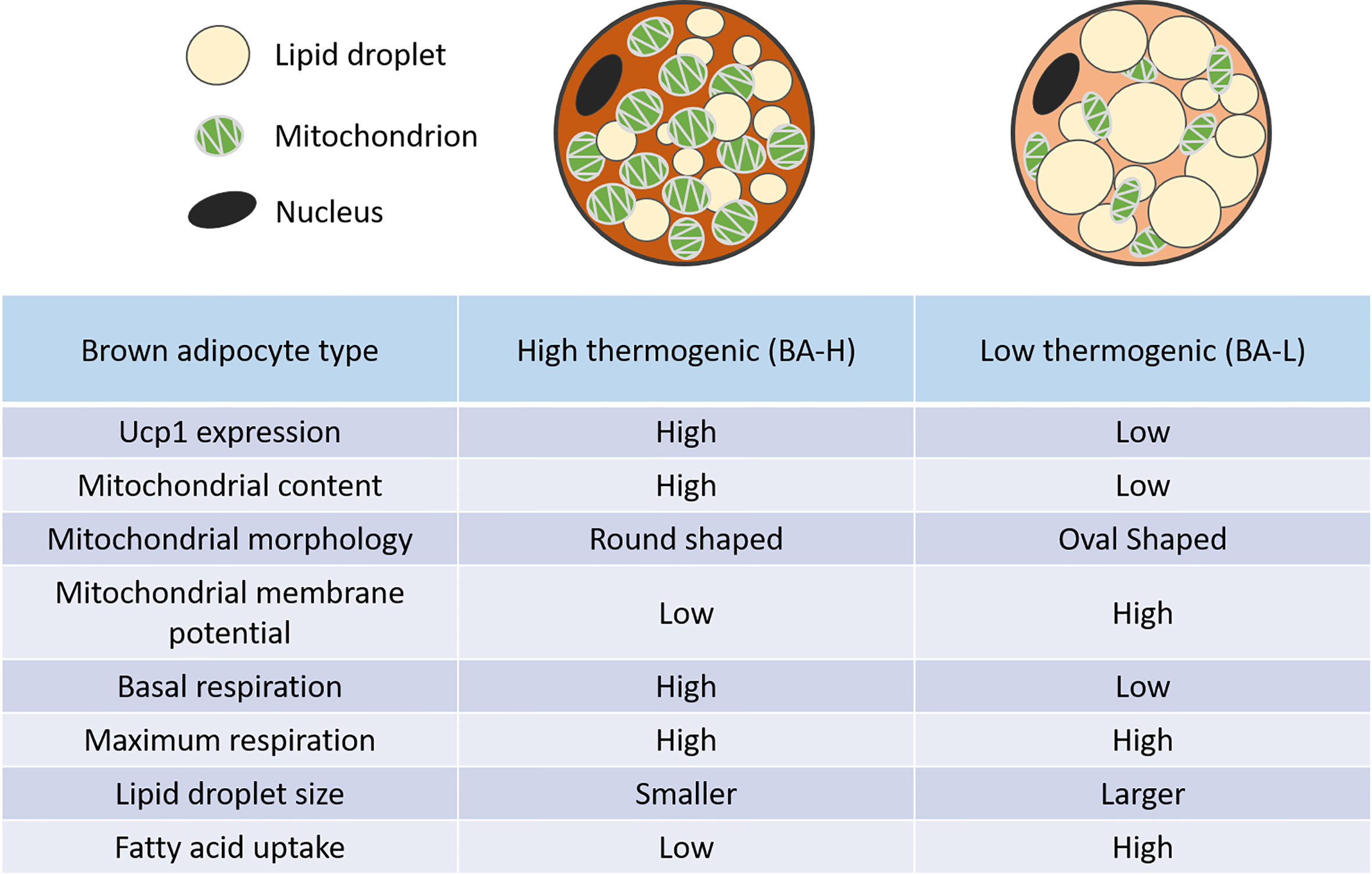
Figure 1 Distinct features of high and low thermogenic adipocytes. Relative to the high thermogenic brown adipocytes (BA-H), the low thermogenic brown adipocytes (BA-L) have lower UCP1 expression, low mitochondrial content, high mitochondrial membrane potential, and distinct mitochondrial morphology. These cells have a lower basal respiration rate, but a similar maximum respiration rate. They also have larger lipid droplets, and a higher fatty acid uptake rate (10).
The biggest disadvantage of scRNA-seq is the possible alterations in gene expression because of dissociation and cell isolation procedures used while making single-cell suspensions. Although single-cell/nucleus sequencing technologies can dissect the cellular heterogeneity of the tissue at high resolution, the spatial information however is lost in the process. To help retain such information recent technologies such as spatial transcriptomics (37) and multiplexed in situ hybridization (38, 39), or visium spatial gene expression analysis (40) should be utilized along with sn/scRNA-seq. At the moment, even if spatial transcriptomics technologies do not provide a resolution at a single-cell level, progress is being made to achieve it by every passing day. Its application to frozen tissues is one important advantage though, making it a valuable resource for precious samples such as banked human biopsies. Lastly, integrating other omics technologies with sn/scRNA-seq to quantify proteins (41), cell surface epitopes (42) and chromatin accessibility (43) simultaneously will allow us a better understanding not only of the heterogeneity of these cells but also the cellular interactions present in the tissue. Finally, whether these distinct adipocyte types really represent distinct cell types or whether they are just the same cell types under distinct metabolic states remains to be further studied.
In humans, BAT was initially thought to exist only in infants to cope with the cold conditions during and after birth and eventually become metabolically inactive and disappear during adulthood. However, the presence of active BAT has been reported in outdoor workers from northern Finland as early as 1981 (44). Furthermore, there are several reports based on PET/CT scans of pheochromocytoma patients suggesting the presence of active BAT in adult humans (45–47). Later on other dedicated cold exposure as well as retrospective studies confirmed these observations (48–53). A recent report has defined several additional novel brown fat depots in mice, which are anatomically comparable and share molecular similarities with humans (54). An earlier study found the overlap between brown and beige molecular markers in human supraclavicular BAT (55). Later, the Kajimura group, using genetic profiling of clonally derived human brown adipocytes, elegantly showed that their molecular signatures were closely associated with those of mouse beige adipocytes (56, 57). This also led to the identification of human brown adipocyte molecular markers such as potassium channel K3 and mitochondrial tumor suppressor 1, which were found to be essential for beige adipogenesis in mice (56, 57). Using genetic profiling approach for preadipocytes derived from human neck fat, the Tseng group identified CD29 as a surface marker that specifically marks preadipocytes with high thermogenic potential (58). Furthermore, human beige adipocytes derive from the progenitors residing in the capillary network and have been shown to proliferate in response to pro-angiogenic factors (59). Interestingly, a recent study suggested that brown adipocytes, but not beige adipocytes of the physiologically humanized mouse had the thermogenic potential. They also found that the BAT of these mice closely resembles that of adult humans both morphologically as well as transcriptionally (60). It is important to note here that the difference between this study and the earlier study (57) is that the earlier study used young mice housed in standard conditions and were fed a normal chow diet. In the recent study by the Petrovic laboratory, they used physiologically humanized mice that are middle-aged, have been fed a high-fat diet, and are housed at thermoneutral temperatures. Lately, the housing temperature of mice for the metabolic studies has been questionable as standard conditions represent higher basal metabolic rate (BMR) in mice than that humans show at thermoneutrality. A recent report by Fischer et al. suggested housing mice at higher temperatures such as 30°C to achieve BMR comparable to resting humans (61), however as per Keijer et al. the best temperatures to achieve comparable BMR are between 25.5 – 27.6°C (62). So, it is important to consider housing temperatures and diets while planning and even comparing different mouse studies involving metabolic analyses. Lastly, the fact that only brown adipocytes and not the beige adipocytes of humanized mice retain thermogenic capacity, suggests the decreased thermocapacity of beige adipocytes during aging, thus making BAT an attractive target for therapeutics of metabolic disorders. Nevertheless, these data provide important insights into the heterogeneous nature of both rodent and human thermogenic adipocytes.
In summary, brown adipocytes within BAT represent high heterogeneity, and characterization of these distinct subpopulations will enable us to elucidate BAT thermogenic functions and regulations in detail. BAT’s heterogeneous nature offers new critical aspects to consider for future attempts that pursue novel therapeutic targets that activate BAT thermogenesis.
Timeline of BAT Development
BAT depots appear earlier than WAT depots during embryogenesis (3). Also, most studies reporting such information have studied only a classic BAT depot iBAT. Early studies in rodents like mice and rats using mRNA measurements of mitochondrial markers such as cytochrome oxidase, UCP1, and ATP synthase found clusters of brown adipocytes appearing in the interscapular region around E15-16. UCP1 expression in these cells was hardly detectable during early embryogenesis and was found to be abruptly increased just before the birth around E18-19. This suggests the functional transformation of these cells into thermogenic competent brown adipocytes around E18-19 (63, 64). Another study used immunostaining of master regulator of adipogenesis, PPARγ (65, 66), to identify iBAT depots and found differentiating brown adipocytes as early as E14.5 (28). In a very recent report, in-situ hybridization of another critical factor involved in adipogenesis, C/EBPα (67, 68), demonstrated that the differentiation of adipocytes in iBAT starts at E12.5 and is functionally complete at E17.5 in the mouse embryo (69). Using the AdipoChaser mouse model based on adiponectin promoter (70), we recently suggested that the development of brown adipocytes in the iBAT starts as early as E10, and is most active at E14 (10). The generation of new brown adipocytes finishes by E16, as there were no new brown adipocytes labeled beyond this point. Furthermore, we showed that the heterogeneity in the iBAT cell population is achieved shortly after birth, around two weeks postnatally (10). Apart from the classic iBAT depot, several other BAT depots analogous to those observed in humans have been recently identified and characterized (54, 71). However, it remains unknown if they share a similar developmental timeline.
Postnatally, the BAT is a very plastic tissue and has been shown to undergo dramatic morphological alterations when exposed to different environmental temperatures. When exposed to thermoneutral temperature (30° C) as compared to standard housing conditions (22-24° C), brown adipocytes in mice undergo hypertrophic expansion with lipid droplets coalescing into a unilocular lipid droplet, leading to WAT like morphology as well gene expression profile. However, these cells maintain their molecular identity and are ready to go back to the classical morphology (72, 73). The Granneman group showed that the cold exposure at 4°C could induce brown adipogenesis in mice and their genetic lineage tracing model demonstrated that new adipocytes are derived from PDGFRα+ progenitors. Such de novo brown adipogenesis was restricted to the dorsal edge region of the iBAT (74). It remains unknown if long-term cold exposure can induce brown adipocyte recruitment in other BAT depots. Our work showed that cold exposure within a few days alters brown adipocytes’ heterogeneity, converting low-thermogenic brown adipocytes into high-thermogenic brown adipocytes (10). Both obesity and aging have been associated with the reduction of BAT thermogenic capacity (75, 76). Interestingly, the phenomenon of dynamic interconversion of BA-L and BA-H was not affected by the high-fat diet (HFD) feeding, but it declined with age (10). These observations provide important insights into the mechanisms of BAT thermoregulation and might help explaining its reduced thermogenic capacity during aging. It will be interesting to investigate the molecular mechanisms that regulate and balance the equilibrium between de novo brown adipogenesis and interconversion of high and low thermogenic brown adipocytes after cold exposure.
Non-Shivering Thermogenesis in BAT
Adaptive thermogenesis is the most important metabolic function of BAT. There are two types of adaptive thermogenesis: cold-induced thermogenesis (77) and diet-induced thermogenesis (78). Skeletal muscle-based shivering accounts for a portion of cold-induced thermogenesis. However, with the cold adaptation non-shivering thermogenesis in BAT becomes prominent (79). The primary mechanism of BAT non-shivering thermogenesis involves the uncoupling of the mitochondrial respiratory chain, which is mediated via UCP1; a proton transporter located on the inner mitochondrial membrane (8, 9, 80–82). This uncoupling results in increased substrate oxidation and dissipation of energy in the form of heat (83, 84). The rich vascularity of BAT helps in redistributing the generated heat across the body for temperature homeostasis (84). BAT is also highly innervated by sympathetic neurons (85). During cold exposure, activation of β3-adrenergic receptors by norepinephrine leads to lipid catabolism liberating free fatty acids and also induces the expression of UCP1 as well as other pro-thermogenic genes (3, 86). Apart from catecholamines some other non-sympathetic molecules such as triiodothyronine (T3), hepatic bile acids, and various retinoids have also been shown to play a role in the thermogenic activation of brown adipocytes (3). Classical studies performed using isolated BAT mitochondria have suggested an essential role of fatty acids as a regulator of brown adipocyte mitochondrial respiration (87, 88). A recent study using a patch-clamp measurement of UCP1 currents in BAT mitochondria found that fatty acids serve as an anion transporter to transport protons across the inner mitochondrial membrane. Both short-chain fatty acids and long-chain fatty acids are associated with UCP1. However, long-chain fatty acids are unable to dissociate because of hydrophobic interaction (89). Moreover, cold exposure has been shown to increase the activities of several antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidases, and glutathione reductase in rat BAT suggesting elevated oxidative stress in BAT during thermogenesis (90).
Interestingly, several recent studies revealed that elevating the levels of reactive oxygen species (ROS), using genetic or pharmacological approaches, increased whole-body energy expenditure and protected against diet-induced obesity, along with increased brown adipocyte mitochondrial respiration (91–94). These observations suggest a critical role of elevated ROS in the thermogenic regulation of brown adipocytes. Furthermore, Cys253 of UCP1 has been recently shown to be sulfenylated by increased mitochondrial ROS in activated brown adipocytes, and this modification was found to be an important regulatory mechanism of UCP1-dependent thermogenesis (95). Moreover, succinylation of lysine residues (K56 and K151) of UCP1 by Sirtuin 5 has been suggested to modulate its stability and activity (96). When there is no thermal stress, UCP1 is usually thought to be functionally inhibited by purine nucleotides (97, 98). A recent report showed that the activation of brown adipocytes by adrenergic stimulation resulted in the degradation of these purine nucleotides in brown adipocytes both in vivo and in vitro, leading to the activation of UCP1 (99). These observations emphasize an essential role of UCP1 in BAT thermogenesis and further understanding of the regulation of its activity might uncover novel mechanisms of thermoregulation.
Genetic ablation of UCP1 in mice led to fatal hypothermia after cold exposure (100). Interestingly, the cold sensitivity of these mice was dependent on the genetic background. Mice with congenic C57BL/6J and 129/SvImJ backgrounds were cold-sensitive, whereas those on F1 hybrid background were found to be resistant to cold (101). Also, the effects of diet-induced obesity on these mice were found to be temperature-dependent (102, 103). Moreover, when UCP1-null mice were gradually exposed to reduced environmental temperatures, they could regain their ability to acclimatize to cold (104). These findings suggest that UCP1-independent mechanisms of thermogenesis may exist. As an alternate thermogenic mechanism, creatine cycling was initially discovered in the murine beige adipocyte mitochondria (34, 105). Ablation of the creatine synthesis rate-limiting enzyme glycine amidinotransferase in an adipocyte-specific manner in mice resulted in reduced BAT creatine concentration and mild cold intolerance (106). Recently, creatine kinase B (CKB), as the only isoenzyme in brown adipocytes, is proven to be indispensable for the futile creatine cycle-related thermogenesis (107). These studies revealed the critical role of creatine cycling as an alternate thermoregulatory mechanism in brown adipocytes.
Brown Adipocyte Energy Metabolism
As BAT has exceptionally high energy expenditure, it is not surprising that it is an essential player in whole-body metabolic regulation. As mentioned above, studies done using BAT transplantation approaches suggested a major role of BAT in glucose homeostasis in mice (108–110). Similarly, activating BAT by cold exposure in humans led to improved whole-body glucose homeostasis and insulin sensitivity in both healthy individuals and individuals with type 2 diabetes (111, 112). With Seahorse Analyzers, for the first time, we were able to measure oxygen consumption in primary mature brown adipocytes, which is largely different from BAT tissue chucks, or in vitro cultured brown adipocyte differentiated from primary stromal vascular fraction (10). These primary mature brown adipocytes had 5-10 folds higher basal respiration, per cell, compared to the cells in the stromal vascular fraction of the same BAT, or mature white adipocytes from the same mouse (10). This result further validated that brown adipocytes would utilize energy at a much higher rate than other cell types, even at the basal, unstimulated status.
Although BAT is known to have a high rate of glucose uptake, fatty acids are commonly viewed as the primary fuel for mitochondrial uncoupling respiration (49, 51, 52, 54, 113–118). The Jiang group’s most recent work used in vivo [U-13C]glucose tracing and demonstrated that BAT activation by chronic cold exposure leads to increased glucose oxidation and enhances glucose flux to mitochondrial tricarboxylic acid cycle (119). Such increase in glucose uptake by adrenergic stimulation is, however, found to be independent of UCP1 presence or activity (120). Moreover, BAT has been shown to express pyruvate carboxylase; which can further enhance the glucose uptake by promoting anaplerosis (121). Mitochondrial pyruvate carrier (MPC) connects cytosolic glycolysis and mitochondrial glucose oxidation (122, 123). Most importantly, inhibition of MPC in mice resulted in blockade of cold-induced glucose oxidation in BAT, thereby impairing the body temperature homeostasis (119). In line with this, another group also showed that BAT-selective ablation of mitochondrial pyruvate carrier 1 (MPC1) in mice led to impaired cold sensitivity and glucose handling (124). Moreover, MPC inhibition in in vitro differentiated brown adipocytes, without any adrenergic stimulation, resulted in increased mitochondrial fatty acid oxidation and lipid cycling; thereby increasing the energy expenditure (125). Thus, limiting pyruvate uptake in brown adipocyte mitochondria could be an effective way of increasing the energy expenditure in the absence of adrenergic stimulation. These observations point toward an important role of glucose oxidation in BAT thermogenesis.
Fatty acids and lipolytic agents have been shown to stimulate respiration in brown adipocytes (126). Stimulation of β3-adrenoreceptor results in increased uptake as well as utilization of FFAs in BAT, but not in WAT (127). BAT uses circulating FFAs after hydrolysis of triacylglycerol-rich lipoproteins (TRLs) as a substrate for thermogenesis (128). Notably, lipoprotein lipase (LPL), an enzyme that is required for this hydrolysis, was induced several folds in BAT during cold acclimatization (129). Moreover, this induction has been shown to contribute to vascular lipoprotein homeostasis during cold exposure by channeling TRLs to BAT (130). This effect can also be partly attributed to the downregulation of a secreted protein angiopoietin-like 4 which inhibits LPL activity. This downregulation further potentiates the LPL activity, thereby increasing the uptake of TRLs in BAT, leading to increased systemic triglyceride clearance (131). Intriguingly, BAT volume during thermoneutral or cold exposures was found to be positively correlated to lipolysis, FFA cycling as well as oxidation, and adipose insulin sensitivity in humans (132). Global deletion of adipose triglyceride lipase (ATGL); a rate-limiting enzyme involved in lipolysis of lipid droplet triglycerides, resulted in defective cold adaptation in mice. This suggested an essential role of ATGL in fueling thermogenesis by locally derived FFAs (133). Indeed, adipocyte-specific ablation of ATGL in mice led to the conversion of BAT to WAT-like tissue and resulted in severely impaired thermogenesis (134). However, recent studies using a BAT-specific inhibition of lipolysis using genetic approaches suggested that BAT lipolysis is not essential for cold-induced thermogenesis (135, 136). Cold intolerance previously observed in global ATGL KO mice was attributed to the impaired cardiac function. Another recent study that impaired the triglyceride synthesis and storage in the BAT lipid droplets by BAT-specific deletion of triglyceride synthesis enzymes also suggested that BAT lipid droplets are dispensable for cold-induced thermogenesis (137). While there is no doubt that BAT could utilize a large amount of glucose and fatty acids, it would be fascinating to explore further how brown adipocyte selects the primary fuel for thermogenesis, and how this selection would have a dynamic impact on whole-body glucose and lipid homeostasis.
Apart from FFAs and glucose, branched-chain amino acids (BCAAs) have also been shown to fuel BAT thermogenesis in mice and humans (138). Interestingly, increased blood levels and impaired metabolism of BCAAs, have been linked to the etiology of type 2 diabetes (139, 140). These observations may provide a novel link between impaired BAT thermogenesis and metabolic disorders. Furthermore, the Kajimura group suggested that stimulation of BCAA catabolism by activating BAT may protect against insulin resistance development by preventing the activation of mTOR signaling (138). Moreover, 13C-labeled isotope tracing of preadipocytes and differentiated adipocytes showed increased BCAA catabolism in differentiated adipocytes as compared to proliferating cells, which used glucose and glutamine to fuel the mitochondrial oxidation. Inhibition of BCAA catabolism resulted in impaired adipogenesis, suggesting an important functional role of BCAAs in adipocyte differentiation (141). Additionally, the Chouchani group found a significant accumulation of succinate (92), a tricarboxylic acid cycle intermediates in cold-activated brown adipocytes, which was independent of adrenergic signaling. Interestingly, the administration of succinate to mice also led to the activation of BAT thermogenesis. Mechanistically this effect was dependent on succinate dehydrogenase generated ROS (92). Another recent report showed that FFAs, released from white adipocytes in response to cold exposure, induced hepatocyte nuclear factor 4 alpha mediated acylcarnitine production in the liver (142). This led to increased plasma concentration of acylcarnitines, which were taken up by BAT to fuel the thermogenesis. Most importantly, supplementation with L-carnitine or palmitoylcarnitine rescued the age-dependent cold sensitivity in mice, suggesting an essential role of acylcarnitine metabolism in age-induced impairment of thermogenesis (142). Altogether, brown adipocytes utilize multiple substrates as fuels for thermogenesis (Figure 2). Again, how brown adipocytes perform fuel selection among all these substrates remains mostly unknown, and yet it is unclear if there are switches of fuel selections during aging or the development of metabolic disorders.
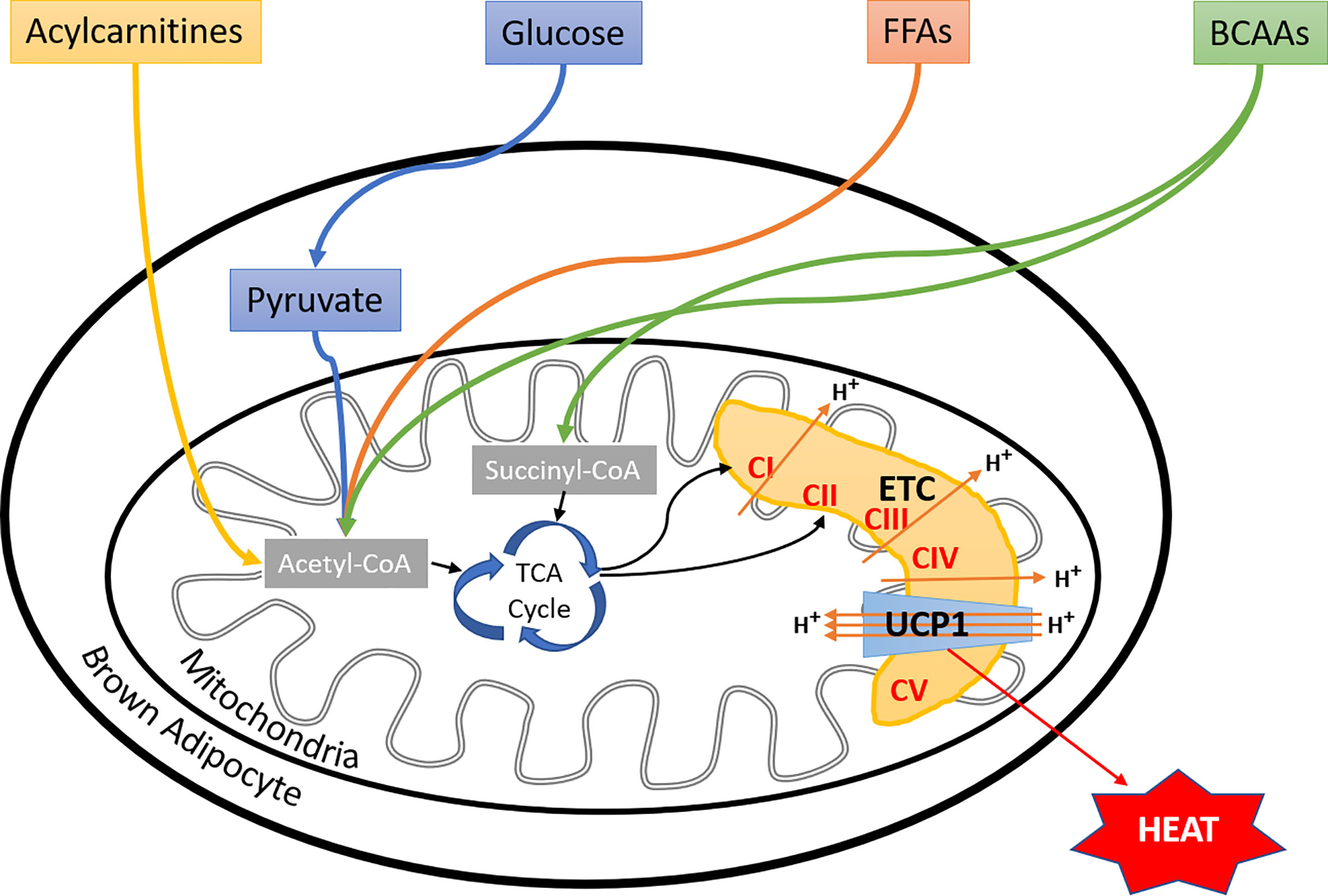
Figure 2 Fuel selection by brown adipocyte. Besides glucose and fatty acids, BAT utilizes a variety of substrates, including BCAAs, succinate, and liver-derived acylcarnitines to fuel thermogenesis, more substrates to be discovered in the near future. However, the regulatory mechanisms of brown adipocyte fuel selection, especially upon environmental temperature changes, and whether aging or metabolic disorders affect these processes remain unknown. FFAs, free fatty acids; BCAAs, branched-chain amino acids; ETC, electron transport chain; CI, complex I; CII, complex II; CIII, complex III; CIV, complex IV; CV, complex V; UCP1, uncoupling protein I.
Lastly, as mentioned earlier, it is important to perform BAT metabolic studies in rodents at thermoneutral temperatures to generate data that is comparable to humans. Although studies in humans have confirmed the presence of thermoactive BAT, one should note that the prevalence of BAT was increased only after cold exposure. In warm conditions little to no BAT was detected in these subjects (50–52). Moreover, overweight, and obese subjects showed significantly lower BAT activity (52). Also, such cold-induced BAT activation was higher during winter as compared to summer (50). Furthermore, Yoneshiro et al. showed that the cold-induced thermoactivation of BAT reduced during aging; as the incidence of cold-activated BAT fell from about 50% in the twenties to less than 10% in the fifties and sixties (75). So, taken together factors such as temperature, age, and dietary compositions should be carefully considered while performing metabolic studies related to BAT or as a matter of fact related to any other metabolically active organ in both rodents and humans.
BAT as an Endocrine Organ
The WAT is well established as an endocrine organ, secreting adipokines such as adiponectin (143) and leptin (144). There is recently ample evidence supporting the fact that BAT may act as a unique endocrine organ by secreting some factors, so-referred to as “batokines” (145). In the 1980s, Silva and Larsen found that the enzyme type 2 iodothyronine deiodinase (DIO2) is specifically expressed in BAT, and it converts thyroxine (T4) to triiodothyronine (T3) (146). They also showed that its activity is strongly induced during thermogenesis, and BAT serves as an important site for both local and systemic T3 generation (147). Both DIO2 and T3 have essential functions in regulating BAT thermogenesis (148, 149). Fibroblast growth factor-21 (FGF21), an essential player in glucose oxidation in multiple organs, was found to be upregulated in BAT in response to cold exposure as well as adrenergic stimulation (150, 151). Furthermore, cytokine interleukin-6 (IL-6) is induced during thermogenesis in mouse brown adipocytes (152). BAT from healthy mice, when implanted in HFD fed mice, improved glucose homeostasis and insulin sensitivity. This effect was found to be mediated via endocrine actions of IL-6 as BAT implantation from IL-6 KO mice failed to show such improvements (108). Likewise, the insulin-independent reversal of type I diabetes (T1D) was achieved when BAT from healthy mice was transplanted in the streptozotocin-induced diabetic mouse model. Such transplantation, if done before the induction, was even able to prevent or significantly delay the development of T1D. This antidiabetic effect of BAT implantation was attributed to insulin-like growth factor-1 (IGF-1), which was upregulated in the tissue transplants. It is supposed to mediate its effects via improving the WAT inflammation, adipogenesis, and direct effect on insulin receptors (109, 110). Like many other cell types, brown adipocytes also secrete Vascular endothelial growth factor-A (VEGF-A), a signaling protein that promotes the growth of new blood vessels. VEGF-A is essential for the activation and expansion of BAT (153). Importantly, another brown adipocyte enriched factor neuregulin 4 (Nrg4) has been demonstrated by the Lin laboratory to protect against diet-induced insulin resistance as well as hepatic steatosis in mice. This is achieved by negatively regulating the de novo lipogenesis in the liver and by activating hepatic fatty acid oxidation (154, 155). Nrg4 transgenic mice also showed increased energy expenditure and improvement of whole-body glucose metabolism (154, 155). The Kahn laboratory recently discovered BAT-derived circulating miRNAs, which control the gene expression in the liver, especially that of FGF21. Mice lacking miRNA processing enzyme, Dicer, specifically in adipose tissue, had improved glucose tolerance (156). Furthermore, some lipid-derived lipokines, such as 12,13-dihydroxy-9Z-octadecenoic acid (12,13-diHOME) and 12-hydroxyeicosapentaenoic acid (12-HEPE) are secreted specifically by BAT. 12,13-diHOME promotes fatty acid uptake in skeletal muscle and BAT, leading to enhanced cold tolerance and improved systemic triglyceride clearing (157, 158). 12-HEPE, on the other hand, improved the whole-body glucose homeostasis by increasing glucose uptake in skeletal muscle and adipocytes (159). Lastly, secretome analyses of brown adipocytes using modern-day proteomics and transcriptomics approaches have identified several novel batokine candidates including ependymin-related protein 1 (EPDR1) and phospholipid transfer protein (PLTP) (160–162). EPDR1 was found to be an important commitment factor for brown adipogenesis (161). Whereas, PLTP improved glucose and lipid homeostasis via the regulation of liver lipoproteins and bile acids (160). Lastly, recruitment of immune cells in BAT; especially that of activated macrophages has been shown to be associated with the thermogenic activation (163). Chemokine C-X-C motif chemokine ligand-14 (CXCL14) is another example of batokine secreted by BAT in response to adrenergic stimulation and has been shown to play an important role in activation and recruitment of macrophages to BAT during thermogenic activation (164). Taken together, these recent studies highlighted the function of BAT as a unique endocrine organ, playing essential functions in regulating whole-body metabolic homeostasis.
BAT Centered Therapeutic Approaches and Future Perspectives
BAT plays an essential role in energy homeostasis. Upon activation, BAT can function as an effective energy sink, burning and disposing excess lipids and glucose. Unfortunately, BAT activity declines during aging or the development of metabolic disorders (49, 75, 165). Therefore, enhancing BAT thermogenic activity has been an attractive strategy for the treatment of obesity and type 2 diabetes. Indeed, thermogenic activation of BAT either by cold exposure (111, 113, 166, 167) or by adrenergic stimulation via β3-adrenoreceptor (AR) agonist Mirabegron (168) showed beneficial metabolic effects such as increased BAT glucose uptake, improved insulin sensitivity, and weight loss in humans. Furthermore, several synthetic molecules acting via different mechanisms have recently been shown to activate BAT in mice, increasing whole-body energy expenditure (169–172). The clinical applications of these compounds are being actively evaluated. Although activating BAT might seem an exciting target for treating metabolic disorders, it is worth noting that humans have various responses to the same stimulation regarding BAT activity. BAT mass is negatively correlated with age as well as diabetic status (173), making this approach more challenging in aged as well as diabetic individuals. BAT transplantation studies in mice have shown the vital role of BAT in the regulation of adiposity, glucose homeostasis, and insulin resistance (108–110, 174, 175). Interestingly, brown adipocytes engineered from human fibroblasts or stem cells from human WAT stromal vascular fraction were transplanted in mice in multiple studies (176–178). In general, these transplantations showed beneficial metabolic effects, such as protection from diet-induced adiposity and insulin resistance. A similar approach can be used in humans, in theory, to increase functional BAT mass. Moreover, common dietary supplements such as L-arginine and capsinoids have been shown to increase BAT recruitment and activation, leading to beneficial effects with respect to glucose homeostasis and insulin sensitivity in both mice and humans (179–181). Lastly, as mentioned above several secretory factors having endocrine functions have been recently identified from BAT. These batokines may be considered emerging therapeutic targets for metabolic disorders. For instance, a recent report by Baruch et al. showed that FGF21 mimetic antibody BFKB8488A when injected subcutaneously in overweight/obese human subjects, resulted in a reduction in body weight, improved cardiometabolic parameters, and reduced carbohydrate intake (182). Taking together, the rediscovery of functional BAT depots in humans has undoubtedly sparked a new era of research about the therapeutic targeting of this tissue for its amazing metabolic health benefits. However, a detailed understanding of the basic biology of its development, heterogeneity, and metabolic regulation will surely further aid this cause.
Author Contributions
ABS, QW, and AS wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
QAW was supported by US National Institutes of Health grants R01AG063854, R01HD096152, American Diabetes Association Junior Faculty Development Award 1-19-JDF-023, and City of Hope Caltech-COH Initiative Award.
Conflict of Interest
The authors declare that the review was construed in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Lei Jiang (COH) and Zhichao Wang (COH) for discussions and comments.
References
1. Scherer E. The many secret lives of adipocytes: implications for diabetes. Diabetologia (2019) 62(2):223–32. doi: 10.1007/s00125-018-4777-x
2. Kusminski CM, Bickel PE, Scherer PE. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat Rev Drug Discovery (2016) 15(9):639–60. doi: 10.1038/nrd.2016.75
3. Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell (2014) 156(1-2):20–44. doi: 10.1016/j.cell.2013.12.012
4. Ikeda K, Maretich P, Kajimura S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol Metab (2018) 29(3):191–200. doi: 10.1016/j.tem.2018.01.001
5. Pfeifer A, Hoffmann LS. Brown, beige, and white: the new color code of fat and its pharmacological implications. Annu Rev Pharmacol Toxicol (2015) 55:207–27. doi: 10.1146/annurev-pharmtox-010814-124346
6. Waldén TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab (2012) 302(1):E19–31. doi: 10.1152/ajpendo.00249.2011
7. Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell (2007) 131(2):242–56. doi: 10.1016/j.cell.2007.10.004
8. Aquila H, Link TA, Klingenberg M. The uncoupling protein from brown fat mitochondria is related to the mitochondrial ADP/ATP carrier. Analysis of sequence homologies and of folding of the protein in the membrane. EMBO J (1985) 4(9):2369–76. doi: 10.1002/j.1460-2075.1985.tb03941.x
9. Heaton GM, Wagenvoord RJ, Kemp A Jr, Nicholls DG. Brown-adipose-tissue mitochondria: photoaffinity labelling of the regulatory site of energy dissipation. Eur J Biochem (1978) 82(2):515–21. doi: 10.1111/j.1432-1033.1978.tb12045.x
10. Song A, Dai W, Jang MJ, Medrano L, Li Z, Zhao H, et al. Low- and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue. J Clin Invest (2020) 130(1):247–57. doi: 10.1172/JCI129167
11. Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem (2010) 285(10):7153–64. doi: 10.1074/jbc.M109.053942
12. Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell (2012) 150(2):366–76. doi: 10.1016/j.cell.2012.05.016
13. Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL, et al. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol (2006) 296(1):164–76. doi: 10.1016/j.ydbio.2006.04.449
14. Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature (2008) 454(7207):961–7. doi: 10.1038/nature07182
15. Lepper C, Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis (2010) 48(7):424–36. doi: 10.1002/dvg.20630
16. Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA (2007) 104(11):4401–6. doi: 10.1073/pnas.0610615104
17. Forner F, Kumar C, Luber CA, Fromme T, Klingenspor M, Mann M, et al. Proteome differences between brown and white fat mitochondria reveal specialized metabolic functions. Cell Metab (2009) 10(4):324–35. doi: 10.1016/j.cmet.2009.08.014
18. Yin H, Pasut A, Soleimani VD, Bentzinger CF, Antoun G, Thorn S, et al. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab (2013) 17(2):210–24. doi: 10.1016/j.cmet.2013.01.004
19. Trajkovski M, Ahmed K, Esau CC, Stoffel M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat Cell Biol (2012) 14(12):1330–5. doi: 10.1038/ncb2612
20. Sun L, Xie H, Mori MA, Alexander R, Yuan B, Hattangadi SM, et al. Mir193b-365 is essential for brown fat differentiation. Nat Cell Biol (2011) 13(8):958–65. doi: 10.1038/ncb2286
21. Zhou H, Wan B, Grubisic I, Kaplan T, Tjian R. TAF7L modulates brown adipose tissue formation. Elife (2014) 3:e02811. doi: 10.7554/eLife.02811
22. Rajakumari S, Wu J, Ishibashi J, Lim HW, Giang AH, Won KJ, et al. EBF2 determines and maintains brown adipocyte identity. Cell Metab (2013) 17(4):562–74. doi: 10.1016/j.cmet.2013.01.015
23. Ohno H, Shinoda K, Ohyama K, Sharp LZ, Kajimura S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature (2013) 504(7478):163–7. doi: 10.1038/nature12652
24. Dempersmier J, Sambeat A, Gulyaeva O, Paul SM, Hudak CS, Raposo HF, et al. Cold-inducible Zfp516 activates UCP1 transcription to promote browning of white fat and development of brown fat. Mol Cell (2015) 57(2):235–46. doi: 10.1016/j.molcel.2014.12.005
25. Park JH, Kang HJ, Kang SI, Lee JE, Hur J, Ge K, et al. A multifunctional protein, EWS, is essential for early brown fat lineage determination. Dev Cell (2013) 26(4):393–404. doi: 10.1016/j.devcel.2013.07.002
26. Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun (2014) 5:4099. doi: 10.1038/ncomms5099
27. Sanchez-Gurmaches J, Hung CM, Sparks CA, Tang Y, Li H, Guertin DA, et al. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab (2012) 16(3):348–62. doi: 10.1016/j.cmet.2012.08.003
28. Wang W, Kissig M, Rajakumari S, Huang L, Lim HW, Won KJ, et al. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci USA (2014) 111(40):14466–71. doi: 10.1073/pnas.1412685111
29. Shan T, Liang X, Bi P, Zhang P, Liu W, Kuang S, et al. Distinct populations of adipogenic and myogenic Myf5-lineage progenitors in white adipose tissues. J Lipid Res (2013) 54(8):2214–24. doi: 10.1194/jlr.M038711
30. Sebo ZL, Jeffery E, Holtrup B, Rodeheffer MS. A mesodermal fate map for adipose tissue. Development (2018) 145(17):dev166801. doi: 10.1242/dev.166801
31. Wikstrom JD, Mahdaviani K, Liesa M, Sereda SB, Si Y, Las G, et al. Hormone-induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J (2014) 33(5):418–36. doi: 10.1002/embj.201385014
32. Spaethling JM, Sanchez-Alavez M, Lee J, Xia FC, Dueck H, Wang W, et al. Single-cell transcriptomics and functional target validation of brown adipocytes show their complex roles in metabolic homeostasis. FASEB J (2016) 30(1):81–92. doi: 10.1096/fj.15-273797
33. Cinti S, Cancello R, Zingaretti MC, Ceresi E, De Matteis R, Giordano A, et al. CL316,243 and cold stress induce heterogeneous expression of UCP1 mRNA and protein in rodent brown adipocytes. J Histochem Cytochem (2002) 50(1):21–31. doi: 10.1177/002215540205000103
34. Bertholet AM, Kazak L, Chouchani ET, Bogaczynska MG, Paranjpe I, Wainwright GL, et al. Mitochondrial Patch Clamp of Beige Adipocytes Reveals UCP1-Positive and UCP1-Negative Cells Both Exhibiting Futile Creatine Cycling. Cell Metab (2017) 25(4):811–822.e4 doi: 10.1016/j.cmet.2017.03.002
35. Sun W, Dong H, Balaz M, Slyper M, Drokhlyansky E, Colleluori G, et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature (2020) 587(7832):98–102. doi: 10.1038/s41586-020-2856-x
36. Porter C. Quantification of UCP1 function in human brown adipose tissue. Adipocyte (2017) 6(2):167–74. doi: 10.1080/21623945.2017.1319535
37. Ståhl PL, Salmén F, Vickovic S, Lundmark A, Navarro JF, Magnusson J, et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science (2016) 353(6294):78–82. doi: 10.1126/science.aaf2403
38. Anderson R. Multiplex fluorescence in situ hybridization (M-FISH). Methods Mol Biol (2010) 659:83–97. doi: 10.1007/978-1-60761-789-1_6
39. Eng CL, Lawson M, Zhu Q, Dries R, Koulena N, Takei Y, et al. Transcriptome-scale super-resolved imaging in tissues by RNA seqFISH. Nature (2019) 568(7751):235–9. doi: 10.1038/s41586-019-1049-y
40. Maynard KR, Collado-Torres L, Weber LM, Uytingco C, Barry BK, Williams SR, et al. Transcriptome-scale spatial gene expression in the human dorsolateral prefrontal cortex. Nat Neurosci (2021) 24(3):425–36. doi: 10.1038/s41593-021-00817-5
41. Peterson VM, Zhang KX, Kumar N, Wong J, Li L, Wilson DC, et al. Multiplexed quantification of proteins and transcripts in single cells. Nat Biotechnol (2017) 35(10):936–9. doi: 10.1038/nbt.3973
42. Stoeckius M, Hafemeister C, Stephenson W, Houck-Loomis B, Chattopadhyay PK, Swerdlow H, et al. Simultaneous epitope and transcriptome measurement in single cells. Nat Methods (2017) 14(9):865–8. doi: 10.1038/nmeth.4380
43. Liu L, Liu C, Quintero A, Wu L, Yuan Y, Wang M, et al. Deconvolution of single-cell multi-omics layers reveals regulatory heterogeneity. Nat Commun (2019) 10(1):470. doi: 10.1038/s41467-018-08205-7
44. Huttunen P, Hirvonen J, Kinnula V. The occurrence of brown adipose tissue in outdoor workers. Eur J Appl Physiol Occup Physiol (1981) 46(4):339–45. doi: 10.1007/BF00422121
45. Yamaga LY, Thom AF, Wagner J, Baroni RH, Hidal JT, Funari MG. The effect of catecholamines on the glucose uptake in brown adipose tissue demonstrated by (18)F-FDG PET/CT in a patient with adrenal pheochromocytoma. Eur J Nucl Med Mol Imaging (2008) 35(2):446–7. doi: 10.1007/s00259-007-0538-7
46. Hadi M, Chen CC, Whatley M, Pacak K, Carrasquillo JA. Brown fat imaging with (18)F-6-fluorodopamine PET/CT, (18)F-FDG PET/CT, and (123)I-MIBG SPECT: a study of patients being evaluated for pheochromocytoma. J Nucl Med (2007) 48(7):1077–83. doi: 10.2967/jnumed.106.035915
47. Kuji I, Imabayashi E, Minagawa A, Matsuda H, Miyauchi T. Brown adipose tissue demonstrating intense FDG uptake in a patient with mediastinal pheochromocytoma. Ann Nucl Med (2008) 22(3):231–5. doi: 10.1007/s12149-007-0096-x
48. Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab (2007) 293(2):E444–52. doi: 10.1152/ajpendo.00691.2006
49. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. New Engl J Med (2009) 360(15):1509–17. doi: 10.1056/NEJMoa0810780
50. Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio- Kobayashi J, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes (2009) 58(7):1526–31. doi: 10.2337/db09-0530
51. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med (2009) 360(15):1518–25. doi: 10.1056/NEJMoa0808949
52. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med (2009) 360(15):1500–8. doi: 10.1056/NEJMoa0808718
53. Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J (2009) 23(9):3113–20. doi: 10.1096/fj.09-133546
54. Zhang F. An Adipose Tissue Atlas: An Image-Guided Identification ofand Beige Depots in Rodents. Cell Metab (2018) 27(1):252–62.e3. doi: 10.1016/j.cmet.2017.12.004
55. Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homoe P, Loft A, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab (2013) 17(5):798–805. doi: 10.1016/j.cmet.2013.04.011
56. Shinoda K, Luijten IH, Hasegawa Y, Hong H, Sonne SB, Kim M, et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med (2015) 21(4):389–94. doi: 10.1038/nm.3819
57. Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PloS One (2012) 7(11):e49452. doi: 10.1371/journal.pone.0049452
58. Xue R, Lynes MD, Dreyfuss JM, Shamsi F, Schulz TJ, Zhang H, et al. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nat Med (2015) 21(7):760–8. doi: 10.1038/nm.3881
59. Min SY, Kady J, Nam M, Rojas-Rodriguez R, Berkenwald A, Kim JH, et al. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat Med (2016) 22(3):312–8. doi: 10.1038/nm.4031
60. de Jong JMA, Sun W, Pires ND, Frontini A, Balaz M, Jespersen NZ, et al. Human brown adipose tissue is phenocopied by classical brown adipose tissue in physiologically humanized mice. Nat Metab (2019) 1(8):830–43. doi: 10.1038/s42255-019-0101-4
61. Fischer AW, Cannon B, Nedergaard J. Optimal housing temperatures for mice to mimic the thermal environment of humans: An experimental study. Mol Metab (2018) 7:161–70. doi: 10.1016/j.molmet.2017.10.009
62. Keijer J, Li M, Speakman JR. What is the best housing temperature to translate mouse experiments to humans? Mol Metab (2019) 25:168–76. doi: 10.1016/j.molmet.2019.04.001
63. Houstek J, Kopecky J, Rychter Z, Soukup T. Uncoupling protein in embryonic brown adipose tissue–existence of nonthermogenic and thermogenic mitochondria. Biochim Biophys Acta (1988) 935(1):19–25. doi: 10.1016/0005-2728(88)90103-X
64. Giralt M, Martin I, Iglesias R, Vinas O, Villarroya F, Mampel T. Ontogeny and perinatal modulation of gene expression in rat brown adipose tissue. Unaltered iodothyronine 5’-deiodinase activity is necessary for the response to environmental temperature at birth. Eur J Biochem (1990) 193(1):297–302. doi: 10.1111/j.1432-1033.1990.tb19336.x
65. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell (1994) 79(7):1147–56. doi: 10.1016/0092-8674(94)90006-X
66. Wang QA, Zhang F, Jiang L, Ye R, An Y, Shao M, et al. Peroxisome Proliferator-Activated Receptor γ and Its Role in Adipocyte Homeostasis and Thiazolidinedione-Mediated Insulin Sensitization. Mol Cell Biol (2018) 38(10):e00677–17. doi: 10.1128/MCB.00677-17
67. Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, et al. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell (1999) 3(2):151–8. doi: 10.1016/S1097-2765(00)80306-8
68. Wang QA, Tao C, Jiang L, Shao M, Ye R, Zhu Y, et al. Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat Cell Biol (2015) 17(9):1099. doi: 10.1038/ncb3217
69. Mayeuf-Louchart A, Lancel S, Sebti Y, Pourcet B, Loyens A, Delhaye S, et al. Glycogen Dynamics Drives Lipid Droplet Biogenesis during Brown Adipocyte Differentiation. Cell Rep (2019) 29(6):1410–1418 e6. doi: 10.1016/j.celrep.2019.09.073
70. Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med (2013) 19(10):1338–44. doi: 10.1038/nm.3324
71. Mo Q, Salley J, Roshan T, Baer LA, May FJ, Jaehnig EJ, et al. Identification and characterization of a supraclavicular brown adipose tissue in mice. JCI Insight (2017) 2(11):e93166. doi: 10.1172/jci.insight.93166
72. Cui X, Nguyen NL, Zarebidaki E, Cao Q, Li F, Zha L, et al. Thermoneutrality decreases thermogenic program and promotes adiposity in high-fat diet-fed mice. Physiol Rep (2016) 4(10):e12799. doi: 10.14814/phy2.12799
73. Roh HC, Tsai LTY, Shao M, Tenen D, Shen Y, Kumari M, et al. Warming Induces Significant Reprogramming of Beige, but Not Brown, Adipocyte Cellular Identity. Cell Metab (2018) 27(5):1121–1137 e5. doi: 10.1016/j.cmet.2018.03.005
74. Lee YH, Petkova AP, Konkar AA, Granneman JG. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J (2015) 29(1):286–99. doi: 10.1096/fj.14-263038
75. Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y, et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obes (Silver Spring) (2011) 19(9):1755–60. doi: 10.1038/oby.2011.125
76. Jung RT, Shetty PS, James WP, Barrand MA, Callingham BA. Reduced thermogenesis in obesity. Nature (1979) 279(5711):322–3. doi: 10.1038/279322a0
77. Davis TR, Johnston DR, Bell FC, Cremer BJ. Regulation of shivering and non-shivering heat production during acclimation of rats. Am J Physiol (1960) 198:471–5. doi: 10.1152/ajplegacy.1960.198.3.471
78. Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature (1979) 281(5726):31–5. doi: 10.1038/281031a0
79. Foster DO, Frydman ML. Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol (1979) 57(3):257–70. doi: 10.1139/y79-039
80. Bouillaud F, Weissenbach J, Ricquier D. Complete cDNA-derived amino acid sequence of rat brown fat uncoupling protein. J Biol Chem (1986) 261(4):1487–90. doi: 10.1016/S0021-9258(17)35962-8
81. Jacobsson A, Stadler U, Glotzer MA, Kozak LP. Mitochondrial uncoupling protein from mouse brown fat. Molecular cloning, genetic mapping, and mRNA expression. J Biol Chem (1985) 260(30):16250–4. doi: 10.1016/S0021-9258(17)36228-2
82. Matthias A, Ohlson KB, Fredriksson JM, Jacobsson A, Nedergaard J, Cannon B. Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid-induced thermogenesis. J Biol Chem (2000) 275(33):25073–81. doi: 10.1074/jbc.M000547200
83. Bargmann W, von Hehn G, Lindner E. [On the cells of the brown fatty tissue and their innervation]. Z Zellforsch Mikrosk Anat (1968) 85(4):601–13. doi: 10.1007/BF00324749
84. Smith RE, Roberts JC. Thermogenesis of Brown Adipose Tissue in Cold-Acclimated Rats. Am J Physiol (1964) 206:143–8. doi: 10.1152/ajplegacy.1964.206.1.143
85. Wirsen C. Distribution of adrenergic nerve fibers in brown and white adipose tissue. In: Renold AE, Cahill GFJ, editors. Adipose Tissue Am Physiol Soc. Washington, DC: Wiley Publishing (1965). p. 197–9.
86. Cannon B, Cannon B, Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev (2004) 84(1):277–359. doi: 10.1152/physrev.00015.2003
87. Hittelman KJ, Lindberg O, Cannon B. Oxidative phosphorylation and compartmentation of fatty acid metabolism in brown fat mitochondria. Eur J Biochem (1969) 11(1):183–92. doi: 10.1111/j.1432-1033.1969.tb00759.x
88. Locke RM, Rial E, Scott ID, Nicholls DG. Fatty acids as acute regulators of the proton conductance of hamster brown-fat mitochondria. Eur J Biochem (1982) 129(2):373–80. doi: 10.1111/j.1432-1033.1982.tb07060.x
89. Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell (2012) 151(2):400–13. doi: 10.1016/j.cell.2012.09.010
90. Barja de Quiroga G, Lopez-Torres M, Perez-Campo R, Abelenda M, Paz Nava M, Puerta ML. Effect of cold acclimation on GSH, antioxidant enzymes and lipid peroxidation in brown adipose tissue. Biochem J (1991) 277( Pt 1):289–92. doi: 10.1042/bj2770289
91. Schneider K, Valdez J, Nguyen J, Vawter M, Galke B, Kurtz TW, et al. Increased Energy Expenditure, Ucp1 Expression, and Resistance to Diet-induced Obesity in Mice Lacking Nuclear Factor-Erythroid-2-related Transcription Factor-2 (Nrf2). J Biol Chem (2016) 291(14):7754–66. doi: 10.1074/jbc.M115.673756
92. Mills EL, Pierce KA, Jedrychowski MP, Garrity R, Winther S, Vidoni S, et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature (2018) 560(7716):102–6. doi: 10.1038/s41586-018-0353-2
93. Lee SJ, Kim SH, Park KM, Lee JH, Park JW. Increased obesity resistance and insulin sensitivity in mice lacking the isocitrate dehydrogenase 2 gene. Free Radic Biol Med (2016) 99:179–88. doi: 10.1016/j.freeradbiomed.2016.08.011
94. Han YH, Buffolo M, Pires KM, Pei S, Scherer PE, Boudina S. Adipocyte-Specific Deletion of Manganese Superoxide Dismutase Protects From Diet-Induced Obesity Through Increased Mitochondrial Uncoupling and Biogenesis. Diabetes (2016) 65(9):2639–51. doi: 10.2337/db16-0283
95. Chouchani ET, Kazak L, Jedrychowski MP, Lu GZ, Erickson BK, Szpyt J, et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature (2016) 532(7597):112–6. doi: 10.1038/nature17399
96. Wang G, Meyer JG, Cai W, Softic S, Li ME, Verdin E, et al. Regulation of UCP1 and Mitochondrial Metabolism in Brown Adipose Tissue by Reversible Succinylation. Mol Cell (2019) 74(4):844–57.e7. doi: 10.1016/j.molcel.2019.03.021
97. Rafael J, Ludolph HJ, Hohorst HJ. [Mitochondria from brown adipose tissue: uncoupling of respiratory chain phosphorylation by long fatty acids and recoupling by guanosine triphosphate]. Hoppe Seylers Z Physiol Chem (1969) 350(9):1121–31.
98. Klingenberg M, Winkler E. The reconstituted isolated uncoupling protein is a membrane potential driven H+ translocator. EMBO J (1985) 4(12):3087–92. doi: 10.1002/j.1460-2075.1985.tb04049.x
99. Fromme T, Kleigrewe K, Dunkel A, Retzler A, Li Y, Maurer S, et al. Degradation of brown adipocyte purine nucleotides regulates uncoupling protein 1 activity. Mol Metab (2018) 8:77–85. doi: 10.1016/j.molmet.2017.12.010
100. Enerbäck S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature (1997) 387(6628):90–4. doi: 10.1038/387090a0
101. Hofmann WE, Liu X, Bearden CM, Harper ME, Kozak LP. Effects of genetic background on thermoregulation and fatty acid-induced uncoupling of mitochondria in UCP1-deficient mice. J Biol Chem (2001) 276(15):12460–5. doi: 10.1074/jbc.M100466200
102. Liu X, Rossmeisl M, McClaine J, Riachi M, Harper ME, Kozak LP. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest (2003) 111(3):399–407. doi: 10.1172/JCI200315737
103. Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab (2009) 9(2):203–9. doi: 10.1016/j.cmet.2008.12.014
104. Golozoubova V, Hohtola E, Matthias A, Jacobsson A, Cannon B, Nedergaard J. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J (2001) 15(11):2048–50. doi: 10.1096/fj.00-0536fje
105. Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell (2015) 163(3):643–55. doi: 10.1016/j.cell.2015.09.035
106. Kazak L, Chouchani ET, Lu GZ, Jedrychowski MP, Bare CJ, Mina AI, et al. Genetic Depletion of Adipocyte Creatine Metabolism Inhibits Diet-Induced Thermogenesis and Drives Obesity. Cell Metab (2017) 26(4):660–71.e3. doi: 10.1016/j.cmet.2017.08.009
107. Rahbani JF, Roesler A, Hussain MF, Samborska B, Dykstra CB, Tsai L, et al. Creatine kinase B controls futile creatine cycling in thermogenic fat. Nature (2021) 590:480–5. doi: 10.1038/s41586-021-03221-y
108. Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest (2013) 123(1):215–23. doi: 10.1172/JCI62308
109. Gunawardana SC, Piston DW. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes (2012) 61(3):674–82. doi: 10.2337/db11-0510
110. Gunawardana SC, Piston DW. Insulin-independent reversal of type 1 diabetes in nonobese diabetic mice with brown adipose tissue transplant. Am J Physiol Endocrinol Metab (2015) 308(12):E1043–55. doi: 10.1152/ajpendo.00570.2014
111. Hanssen MJ, Hoeks J, Brans B, van der Lans AA, Schaart G, van den Driessche JJ, et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med (2015) 21(8):863–5. doi: 10.1038/nm.3891
112. Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes (2014) 63(12):4089–99. doi: 10.2337/db14-0746
113. Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest (2012) 122(2):545–52. doi: 10.1172/JCI60433
114. Blondin DP, Labbe SM, Noll C, Kunach M, Phoenix S, Guerin B, et al. Selective Impairment of Glucose but Not Fatty Acid or Oxidative Metabolism in Brown Adipose Tissue of Subjects With Type 2 Diabetes. Diabetes (2015) 64(7):2388–97. doi: 10.2337/db14-1651
115. Wang X, Minze LJ, Shi ZZ. Functional imaging of brown fat in mice with 18F-FDG micro-PET/CT. J Vis Exp (2012) 2012(69):4060. doi: 10.3791/4060
116. Townsend KL, Tseng YH. Brown fat fuel utilization and thermogenesis. Trends Endocrinol Metab (2014) 25(4):168–77. doi: 10.1016/j.tem.2013.12.004
117. Labbe SM, Caron A, Chechi K, Laplante M, Lecomte R, Richard D. Metabolic activity of brown, “beige,” and white adipose tissues in response to chronic adrenergic stimulation in male mice. Am J Physiol Endocrinol Metab (2016) 311(1):E260–8. doi: 10.1152/ajpendo.00545.2015
118. Hankir MK, Klingenspor M. Brown adipocyte glucose metabolism: a heated subject. EMBO Rep (2018) 19(9):e46404. doi: 10.15252/embr.201846404
119. Wang Z, Ning T, Song A, Rutter J, Wang QA, Jiang L. Chronic cold exposure enhances glucose oxidation in brown adipose tissue. EMBO Rep (2020) 21(11):e50085. doi: 10.15252/embr.202050085
120. Olsen JM, Csikasz RI, Dehvari N, Lu L, Sandström A, Öberg AI, et al. β3-Adrenergically induced glucose uptake in brown adipose tissue is independent of UCP1 presence or activity: Mediation through the mTOR pathway. Mol Metab (2017) 6(6):611–9. doi: 10.1016/j.molmet.2017.02.006
121. Cannon B, Nedergaard J. The physiological role of pyruvate carboxylation in hamster brown adipose tissue. Eur J Biochem (1979) 94(2):419–26. doi: 10.1111/j.1432-1033.1979.tb12909.x
122. Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science (2012) 337(6090):96–100. doi: 10.1126/science.1218099
123. Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science (2012) 337(6090):93–6. doi: 10.1126/science.1218530
124. Panic V, Pearson S, Banks J, Tippetts TS, Velasco-Silva JN, Lee S, et al. Mitochondrial pyruvate carrier is required for optimal brown fat thermogenesis. Elife (2020) 9:e52558. doi: 10.7554/eLife.52558
125. Veliova M, Ferreira CM, Benador IY, Jones AE, Mahdaviani K, Brownstein AJ, et al. Blocking mitochondrial pyruvate import in brown adipocytes induces energy wasting via lipid cycling. EMBO Rep (2020) 12):e49634. doi: 10.1101/841551
126. Reed N, Fain JN. Potassium-dependent stimulation of respiration in brown fat cells by fatty acids and lipolytic agents. J Biol Chem (1968) 243(23):6077–83. doi: 10.1016/S0021-9258(18)94462-5
127. Warner A, Kjellstedt A, Carreras A, Bottcher G, Peng XR, Seale P, et al. Activation of beta3-adrenoceptors increases in vivo free fatty acid uptake and utilization in brown but not white fat depots in high-fat-fed rats. Am J Physiol Endocrinol Metab (2016) 311(6):E901–10. doi: 10.1152/ajpendo.00204.2016
128. Khedoe PP, Hoeke G, Kooijman S, Dijk W, Buijs JT, Kersten S, et al. Brown adipose tissue takes up plasma triglycerides mostly after lipolysis. J Lipid Res (2015) 56(1):51–9. doi: 10.1194/jlr.M052746
129. Carneheim C, Nedergaard J, Cannon B. Beta-adrenergic stimulation of lipoprotein lipase in rat brown adipose tissue during acclimation to cold. Am J Physiol (1984) 246(4 Pt 1):E327–33. doi: 10.1152/ajpendo.1984.246.4.E327
130. Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med (2011) 17(2):200–5. doi: 10.1038/nm.2297
131. Dijk W, Heine M, Vergnes L, Boon MR, Schaart G, Hesselink MK, et al. ANGPTL4 mediates shuttling of lipid fuel to brown adipose tissue during sustained cold exposure. Elife (2015) 4:e08428. doi: 10.7554/eLife.08428
132. Chondronikola M, Volpi E, Borsheim E, Porter C, Saraf MK, Annamalai P, et al. Brown Adipose Tissue Activation Is Linked to Distinct Systemic Effects on Lipid Metabolism in Humans. Cell Metab (2016) 23(6):1200–6. doi: 10.1016/j.cmet.2016.04.029
133. Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science (2006) 312(5774):734–7. doi: 10.1126/science.1123965
134. Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab (2011) 13(6):739–48. doi: 10.1016/j.cmet.2011.05.002
135. Shin H, Ma Y, Chanturiya T, Cao Q, Wang Y, Kadegowda AKG, et al. Lipolysis in Brown Adipocytes Is Not Essential for Cold-Induced Thermogenesis in Mice. Cell Metab (2017) 26(5):764–77.e5. doi: 10.1016/j.cmet.2017.09.002
136. Schreiber R, Diwoky C, Schoiswohl G, Feiler U, Wongsiriroj N, Abdellatif M, et al. Cold-Induced Thermogenesis Depends on ATGL-Mediated Lipolysis in Cardiac Muscle, but Not Brown Adipose Tissue. Cell Metab (2017) 26(5):753–63.e7. doi: 10.1016/j.cmet.2017.09.004
137. Chitraju C, Fischer AW, Farese RV Jr., Walther TC. Lipid Droplets in Brown Adipose Tissue Are Dispensable for Cold-Induced Thermogenesis. Cell Rep (2020) 33(5):108348. doi: 10.1016/j.celrep.2020.108348
138. Yoneshiro T, Wang Q, Tajima K, Matsushita M, Maki H, Igarashi K, et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature (2019) 572(7771):614–9. doi: 10.1038/s41586-019-1503-x
139. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med (2011) 17(4):448–53. doi: 10.1038/nm.2307
140. Lotta LA, Scott RA, Sharp SJ, Burgess S, Luan J, Tillin T, et al. Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis. PloS Med (2016) 13(11):e1002179. doi: 10.1371/journal.pmed.1002179
141. Green CR, Wallace M, Divakaruni AS, Phillips SA, Murphy AN, Ciaraldi TP, et al. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat Chem Biol (2016) 12(1):15–21. doi: 10.1038/nchembio.1961
142. Simcox J, Geoghegan G, Maschek JA, Bensard CL, Pasquali M, Miao R, et al. Global Analysis of Plasma Lipids Identifies Liver-Derived Acylcarnitines as a Fuel Source for Brown Fat Thermogenesis. Cell Metab (2017) 26(3):509–22.e6. doi: 10.1016/j.cmet.2017.08.006
143. Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A Novel Serum Protein Similar to C1q, Produced Exclusively in Adipocytes (*). J Biol Chem (1995) 270(45):26746–9. doi: 10.1074/jbc.270.45.26746
144. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–32 (1994). doi: 10.1038/372425a0
145. Villarroya F, Cereijo R, Villarroya J, Giralt M. Brown adipose tissue as a secretory organ. Nat Rev Endocrinol (2017) 13(1):26–35. doi: 10.1038/nrendo.2016.136
146. Silva JE, Larsen PR. Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature (1983) 305(5936):712–3. doi: 10.1038/305712a0
147. Silva JE, Larsen PR. Potential of brown adipose tissue type II thyroxine 5’-deiodinase as a local and systemic source of triiodothyronine in rats. J Clin Invest (1985) 76(6):2296–305. doi: 10.1172/JCI112239
148. Bianco AC, Silva JE. Intracellular conversion of thyroxine to triiodothyronine is required for the optimal thermogenic function of brown adipose tissue. J Clin Invest (1987) 79(1):295–300. doi: 10.1172/JCI112798
149. de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest (2001) 108(9):1379–85. doi: 10.1172/JCI200113803
150. Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, et al. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem (2011) 286(15):12983–90. doi: 10.1074/jbc.M110.215889
151. Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, Papavassiliou AG. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol Med (2011) 17(7-8):736–40. doi: 10.2119/molmed.2011.00075
152. Burysek L, Houstek J. beta-Adrenergic stimulation of interleukin-1alpha and interleukin-6 expression in mouse brown adipocytes. FEBS Lett (1997) 411(1):83–6. doi: 10.1016/S0014-5793(97)00671-6
153. Sun K, Kusminski CM, Luby-Phelps K, Spurgin SB, An YA, Wang QA, et al. Brown adipose tissue derived VEGF-A modulates cold tolerance and energy expenditure. Mol Metab (2014) 3(4):474–83. doi: 10.1016/j.molmet.2014.03.010
154. Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med (2014) 20(12):1436–43. doi: 10.1038/nm.3713
155. Chen Z, Wang GX, Ma SL, Jung DY, Ha H, Altamimi T, et al. Nrg4 promotes fuel oxidation and a healthy adipokine profile to ameliorate diet-induced metabolic disorders. Mol Metab (2017) 6(8):863–72. doi: 10.1016/j.molmet.2017.03.016
156. Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature (2017) 542(7642):450–5. doi: 10.1038/nature21365
157. Stanford KI, Lynes MD, Takahashi H, Baer LA, Arts PJ, May FJ, et al. 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab (2018) 27(6):1357. doi: 10.1016/j.cmet.2018.04.023
158. Lynes MD, Leiria LO, Lundh M, Bartelt A, Shamsi F, Huang TL, et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat Med (2017) 23(5):631–7. doi: 10.1038/nm.4297
159. Leiria LO, Wang CH, Lynes MD, Yang K, Shamsi F, Sato M, et al. 12-Lipoxygenase Regulates Cold Adaptation and Glucose Metabolism by Producing the Omega-3 Lipid 12-HEPE from Brown Fat. Cell Metab (2019) 30(4):768–83.e7. doi: 10.1016/j.cmet.2019.07.001
160. Sponton CH, Hosono T, Taura J, Jedrychowski MP, Yoneshiro T, Wang Q, et al. The regulation of glucose and lipid homeostasis via PLTP as a mediator of BAT-liver communication. EMBO Rep (2020) 21(9):e49828. doi: 10.15252/embr.201949828
161. Deshmukh AS, Peijs L, Beaudry JL, Jespersen NZ, Nielsen CH, Ma T, et al. Proteomics-Based Comparative Mapping of the Secretomes of Human Brown and White Adipocytes Reveals EPDR1 as a Novel Batokine. Cell Metab (2019) 30(5):963–75.e7. doi: 10.1016/j.cmet.2019.10.001
162. Ali Khan A, Hansson J, Weber P, Foehr S, Krijgsveld J, Herzig S, et al. Comparative Secretome Analyses of Primary Murine White and Brown Adipocytes Reveal Novel Adipokines. Mol Cell Proteomics (2018) 17(12):2358–70. doi: 10.1074/mcp.RA118.000704
163. Villarroya J, Cereijo R, Gavaldà-Navarro A, Peyrou M, Giralt M, Villarroya F. New insights into the secretory functions of brown adipose tissue. J Endocrinol (2019) 243(2):R19–27. doi: 10.1530/JOE-19-0295
164. Cereijo R, Gavaldà-Navarro A, Cairó M, Quesada-López T, Villarroya J, Morón- Ros S, et al. CXCL14, a Brown Adipokine that Mediates Brown-Fat-to-Macrophage Communication in Thermogenic Adaptation. Cell Metab (2018) 28(5):750–63.e6. doi: 10.1016/j.cmet.2018.07.015
165. Leitner BP, Huang S, Brychta RJ, Duckworth CJ, Baskin AS, McGehee S, et al. Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci USA (2017) 114(32):8649–54. doi: 10.1073/pnas.1705287114
166. van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest (2013) 123(8):3395–403. doi: 10.1172/JCI68993
167. Lee P, Smith S, Linderman J, Courville AB, Brychta RJ, Dieckmann W, et al. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes (2014) 63(11):3686–98. doi: 10.2337/db14-0513
168. Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elia E, Kessler SH, Kahn PA, et al. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab (2015) 21(1):33–8. doi: 10.1016/j.cmet.2014.12.009
169. Galmozzi A, Sonne SB, Altshuler-Keylin S, Hasegawa Y, Shinoda K, Luijten IHN, et al. ThermoMouse: an in vivo model to identify modulators of UCP1 expression in brown adipose tissue. Cell Rep (2014) 9(5):1584–93. doi: 10.1016/j.celrep.2014.10.066
170. Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes (2014) 63(10):3346–58. doi: 10.2337/db14-0302
171. Reilly SM, Chiang SH, Decker SJ, Chang L, Uhm M, Larsen MJ, et al. An inhibitor of the protein kinases TBK1 and IKK-varepsilon improves obesity-related metabolic dysfunctions in mice. Nat Med (2013) 19(3):313–21. doi: 10.1038/nm.3082
172. Hoffmann LS, Etzrodt J, Willkomm L, Sanyal A, Scheja L, Fischer AWC, et al. Stimulation of soluble guanylyl cyclase protects against obesity by recruiting brown adipose tissue. Nat Commun (2015) 6:7235. doi: 10.1038/ncomms8235
173. Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab (2011) 96(1):192–9. doi: 10.1210/jc.2010-0989
174. Zhu Z, Spicer EG, Gavini CK, Goudjo-Ako AJ, Novak CM, Shi H. Enhanced sympathetic activity in mice with brown adipose tissue transplantation (transBATation). Physiol Behav (2014) 125:21–9. doi: 10.1016/j.physbeh.2013.11.008
175. Liu X, Zheng Z, Zhu X, Meng M, Li L, Shen Y, et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res (2013) 23(6):851–4. doi: 10.1038/cr.2013.64
176. Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature (2009) 460(7259):1154–8. doi: 10.1038/nature08262
177. Kishida T, Ejima A, Yamamoto K, Tanaka S, Yamamoto T, Mazda O. Reprogrammed Functional Brown Adipocytes Ameliorate Insulin Resistance and Dyslipidemia in Diet-Induced Obesity and Type 2 Diabetes. Stem Cell Rep (2015) 5(4):569–81. doi: 10.1016/j.stemcr.2015.08.007
178. Ahfeldt T, Schinzel RT, Lee YK, Hendrickson D, Kaplan A, Lum DH, et al. Programming human pluripotent stem cells into white and brown adipocytes. Nat Cell Biol (2012) 14(2):209–19. doi: 10.1038/ncb2411
179. Jobgen W, Meininger CJ, Jobgen SC, Li P, Lee MJ, Smith SB, et al. Dietary L-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr (2009) 139(2):230–7. doi: 10.3945/jn.108.096362
180. Lucotti P, Setola E, Monti LD, Galluccio E, Costa S, Sandoli EP, et al. Beneficial effects of a long-term oral L-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am J Physiol Endocrinol Metab (2006) 291(5):E906–12. doi: 10.1152/ajpendo.00002.2006
181. Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest (2013) 123(8):3404–8. doi: 10.1172/JCI67803
Keywords: brown adipocyte development, brown adipocyte heterogeneity, thermogenesis, brown adipocyte energy metabolism, obesity
Citation: Shinde AB, Song A and Wang QA (2021) Brown Adipose Tissue Heterogeneity, Energy Metabolism, and Beyond. Front. Endocrinol. 12:651763. doi: 10.3389/fendo.2021.651763
Received: 10 January 2021; Accepted: 18 March 2021;
Published: 19 April 2021.
Edited by:
Xinran Ma, East China Normal University, ChinaReviewed by:
Craig Porter, University of Arkansas for Medical Sciences, United StatesRubén Cereijo, University of Barcelona, Spain
Lawrence Kazak, McGill University, Canada
Copyright © 2021 Shinde, Song and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiong A. Wang, cXdhbmdAY29oLm9yZw==
 Abhijit Babaji Shinde
Abhijit Babaji Shinde Anying Song
Anying Song Qiong A. Wang
Qiong A. Wang