- 1Department of Endocrinology and Metabolism, The First Affiliated Hospital of China Medical University, Shenyang, China
- 2Department of Endocrinology, Chinese People’s Liberation Army (PLA) General Hospital, Beijing, China
- 3Department of Endocrinology, Southwest Hospital, Third Military Medical University, Chongqing, China
- 4Department of Endocrinology, First Affiliated Hospital, Dalian Medical University, Dalian, China
- 5Department of Endocrinology, Cardiovascular and Cerebrovascular Disease Hospital, General Hospital of Ningxia Medical University, Jinfeng, China
- 6Department of Endocrinology and Metabolism, Second Affiliated Hospital of Nanchang University, Nanchang, China
- 7Department of Endocrinology, First Affiliated Hospital of Harbin Medical University, Harbin, China
- 8Department of Endocrinology, Hohhot First Hospital, Hohhot, China
- 9Department of Endocrinology and Metabolism, Second Xiangya Hospital, Central South University, Changsha, China
- 10Research Center of Endocrine and Metabolic Diseases, Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, China
- 11Fujian Institute of Hematology, Union Hospital, Fujian Medical University, Fuzhou, China
- 12International Medical Center, The First Affiliated Hospital, Zhengzhou University, Zhengzhou, China
- 13Department of Endocrinology, First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 14Department of Endocrinology, Hainan General Hospital, Haikou, China
- 15Department of Endocrinology, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 16Department of Endocrinology, Wuhan Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 17Department of Endocrinology, First Hospital of Lanzhou University, Lanzhou, China
- 18State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu, China
- 19Department of Endocrinology and Metabolism, First Affiliated Hospital of Jilin University, Changchun, China
- 20Department of Endocrinology, Zhoupu Hospital, Shanghai University of Medicine and Health Sciences, Shanghai, China
- 21Department of Endocrinology, First Affiliated Hospital of Anhui Medical University, Hefei, China
- 22Department of Endocrinology, The First People’s Hospital of Yunnan Province, Kunming, China
- 23Department of Otolaryngology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
- 24Department of Endocrinology, First Hospital of Shanxi Medical University, Taiyuan, China
- 25Department of Endocrinology and Metabolism, People’s Hospital of Tibet Autonomous Region, Lhasa, China
- 26Department of Endocrinology, Qinghai Provincial People’s Hospital, Xining, China
- 27Zhejiang Center for Disease Control and Prevention (Zhejiang CDC), Hangzhou, China
- 28Department of Endocrinology and Metabolism, Affiliated Hospital of Guiyang Medical University, Guiyang, China
- 29Department of Endocrinology, Second Hospital of Hebei Medical University, Shijiazhuang, China
- 30Department of Endocrinology, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, China
- 31Department of Endocrinology and Metabolism, Tianjin Medical University General Hospital, Tianjin, China
Background: Universal salt iodization (USI) was implemented in mainland China in 1996. The prevalence of hyperthyroidism and its risk factors now require examination.
Methods: Data were acquired from a nationwide Thyroid, Iodine, and Diabetes Epidemiological survey (TIDE 2015–2017) of 78,470 subjects from 31 provinces. Iodine status, and thyroid hormones and antibodies were measured.
Results: After two decades of USI, the prevalence of overt hyperthyroidism (OH), Graves’ disease (GD), severe subclinical hyperthyroidism (severe SCH), and mild subclinical hyperthyroidism (mild SCH) in mainland China was 0.78%, 0.53%, 0.22%, and 0.22%, respectively. OH and GD prevalence were higher in women than in men (OH: 1.16% vs. 0.64%, P<0.001; GD: 0.65% vs. 0.37%, P<0.001).Prevalence was significantly decreased after 60 years-of-age compared with 30–39 years-of-age (OH:0.61% vs. 0.81%, P<0.001; GD: 0.38% vs. 0.57%, P<0.001).Excessive iodine(EI) and deficient iodine(DI) were both related to increased prevalence of OH (odds ratio [OR] 2.09, 95% confidence interval [CI] 1.68–2.59; OR1.35, 95%CI 1.07–1.72, respectively); however, only deficient iodine was associated with increased prevalence of GD (OR1.67, 95%CI 1.30–2.15). Increased thyroid peroxidase antibody and thyroglobulin antibody levels were significantly associated with prevalence of OH and GD, but not severe SCH and mild SCH. Although hyperthyroidism was more prevalent in women, the association disappeared after adjusting for other factors such as antibody levels.
Conclusion: OH and GD prevalences in mainland China are stable after two decades of USI. Iodine deficiency, elevated thyroid antibody levels, and middle age are the main risk factors for OH and GD. The severe SCH population, rather than the mild SCH population, shows similar characteristics to the OH population.
Introduction
Hyperthyroidism represents a group of clinical syndromes characterized by hypermetabolism and increased activation in the nervous, circulatory, and digestive systems caused by excessive thyroid hormone synthesis and secretion. The most common causes include Graves’ disease (GD), toxic multinodular goiter (TMNG), and toxic adenoma (TA) (1).Hyperthyroidism is divided into overt (overt hyperthyroidism [OH]) or subclinical (subclinical hyperthyroidism [SCH]). OH is characterized by a decrease in serum thyroid-stimulating hormone (TSH) and an increase in serum thyroxine (T4) and/or triiodothyronine (T3) levels. In SCH, serum TSH level is decreased, but serum T4 and T3 levels are in the normal range (2). According to the degree of inhibition of TSH, SCH can be further divided into severe SCH (TSH<0.1mIU/L) and mild SCH (TSH between 0.1mIU/L and lower limit of reference range) (3). As major regulators of cell proliferation and energy metabolism, thyroid hormones affect almost all cells in the human body (4). Studies have shown that OH increases the risk of fracture, stroke, atrial fibrillation, and cardiovascular events (5, 6). Although SCH has milder clinical symptoms than OH, the long-term effects on health and its potential for progression toward OH should not be underestimated (7).Hyperthyroidism prevalence is mainly influenced by thyroid autoimmunity levels and iodine status. It also varies with age, gender, and race (8). Studies have shown that the thyroid peroxidase antibody (TPOAb)-positive population with normal thyroid function has a two-fold higher risk of progression to hyperthyroidism within 6 years than the TPOAb-negative population (9).
The distribution of iodine, the most important element in thyroid hormone synthesis, is uneven throughout the world, with some regions adequately supplied with iodine supplemented and other regions iodine deficient (10). China was once an iodine-deficient country, with the prevalence of thyroid goiter as high as 20.4% (11). Because of this, China implemented a national universal salt iodization (USI) program in 1996. Since USI started, the national iodine status has changed along with prevalence of hyperthyroidism, which has decreased since the early years of the USI program from 1.68% to 0.89%, after gradual adaptation and several adjustments (12, 13). To clarify the status of thyroid-associated disease nationally after two decades of USI, we had implemented Thyroid, Iodine, Diabetes Epidemiology study (TIDE study) to investigate iodine and thyroid status in urban and rural areas in 31 provinces of mainland China from 2015 to 2017. The data in this study were obtained from the TIDE project, to analyze the prevalences of OH, GD, severe SCH, and mild SCH, as well as their associated risk factors.
Materials and Methods
Study Population
The TIDE study used a random sampling method across urban and rural areas (14). From the whole country, 31 cities were selected, and one district was randomly selected from each city. Two residential communities were then randomly selected from the selected district. Eligible individuals from these communities who met the inclusion criteria of being aged 18 years or older and not pregnant women were randomly selected and stratified by age and sex. A total of 78470 subjects were enrolled. Each one completed a questionnaire that included demographic information, family history of thyroid disease, current smoking status, family income, and education level. From each participant, samples of fasting blood and fasting spot urine were collected. Serums obtained by centrifugation of the blood samples were preserved at - 20 °C before being further processed. Upon the completion of the survey and specimen collection, all specimens were airlifted by the cold chain system to the central laboratory in Shenyang, China, for centralized tests. All participants underwent thyroid ultrasonography by qualified observers, who had trained and passed examination in the project center, using a portable instrument (LOGIQ 100 PRO, GE, Milwaukee, WI, USA with 7·5 MHz linear transducers). The research protocols were approved by the Medical Ethics Committee of China Medical University.
The content of the standard questionnaire included demographic characteristics, personal and family medical history of thyroid disorders, current smoking status, family income, education levels and household salt consumption. In each province, sixty 9 -11 years old school-children were sampled for B-mode ultrasonography examination on the thyroid gland and fasting urine collection.
Laboratory Tests
Electrochemiluminescence immunoassay on a Cobas 601 analyzer (Roche Diagnostic, Switzerland) was used to test serum TSH, thyroid peroxidase antibody (TPOAb), and thyroglobulin antibodies (TgAb) for each sample. Free thyroxine (FT4) and free triiodothyronine (FT3) levels, and TSH receptor antibodies (TRAb) were measured in subjects with TSH <0.27 mIU/L. The recommended ranges for TSH, FT4, FT3, TPOAb, TgAb, and TRAb provided by the test kitswere 0.27–4.2mIU/L, 12.0–22.0pmol/L, 3.1–6.8 pmol/L, ≤34IU/mL, ≤115IU/mL, and ≤1.75IU/L, respectively. The functional sensitivity of the serum TSH assay was 0.014mIU/L. The repeatability of the assays for serum TSH, FT4, FT3, TPOAb, TgAb, and TRAb was ensured by an inter-assay coefficient of variation (CV) of 1.1%–6.3% and intra-assay CV of 1.9%–9.5%. Urinary iodine concentration (UIC) derived from spot- urine was tested using inductively coupled plasma mass spectrometry (Agilent 7700x; Agilent Technologies, Santa Clara, CA). The target values of the standards were 70.8 ± 9.0µg/L, 143 ± 10µg/L, and 224 ± 14µg/L, for intra-assay CVs of 2.3%, 2.5%, and 2.4%, and intra-assay CVs of 2.7%, 1.4%, and 2.3%, respectively. All participants underwent thyroid ultrasonography by qualified observers, who had trained and passed examination in the project center, using a portable instrument (LOGIQ 100 PRO, GE, Milwaukee, WI, USA with 7•5 MHz linear transducers).
Clinical Diagnosis
The diagnostic criteria for OH were TSH<0.27mIU/L and FT4>22pmol/L or FT3>6.8pmol/L; mild SCH: TSH 0.1–0.26 mIU/L and FT3 and FT4 within normal range; severe SCH: TSH <0.1 mIU/L and FT3 and FT4 within normal range; GD: OH or SCH and TRAb>1.75IU/L, or a diffuse goiter on ultrasonography. Details of diagnostic criteria are listed in Supplementary Table 1.
Iodine and Antibody Groups
Subjects were classified into four groups according to UIC, using reference ranges adopted from World Health Organization recommendations (15). Those with UIC ≥300 μg/L, 200–299 μg/L, 100–199 μg/L, and <100 μg/L were defined as having an excessive iodine intake (EI), more-than-adequate iodine intake (MAI), adequate iodine intake (AI), and deficient iodine intake (DI), respectively. Thyroid autoimmune antibodies (TPOAb and TgAb) were grouped into <15 IU/mL, 15–34 IU/mL, 35–60 IU/mL, 61–115 IU/mL, 116–200 IU/mL, 201–400 IU/mL, and >400 IU/mL respectively, according to the multiple ratio of antibody titer.
Statistical Analysis
Calculations were performed using SUDAAN software (version 10.0) and SPSS software (version 20.0; SPSS, Inc. Chicago, IL, USA). The chi-square test and Fisher’s exact test were used in the statistical analysis and the statistical significance of differences between continuous variables was assessed using analysis of variance. Significance was defined as P<0.05. Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were calculated using multivariable logistic regression to examine the association between the risk factors and the prevalence of thyroid disorders. Three models with progressively increased adjustment of risk factors were applied.
Results
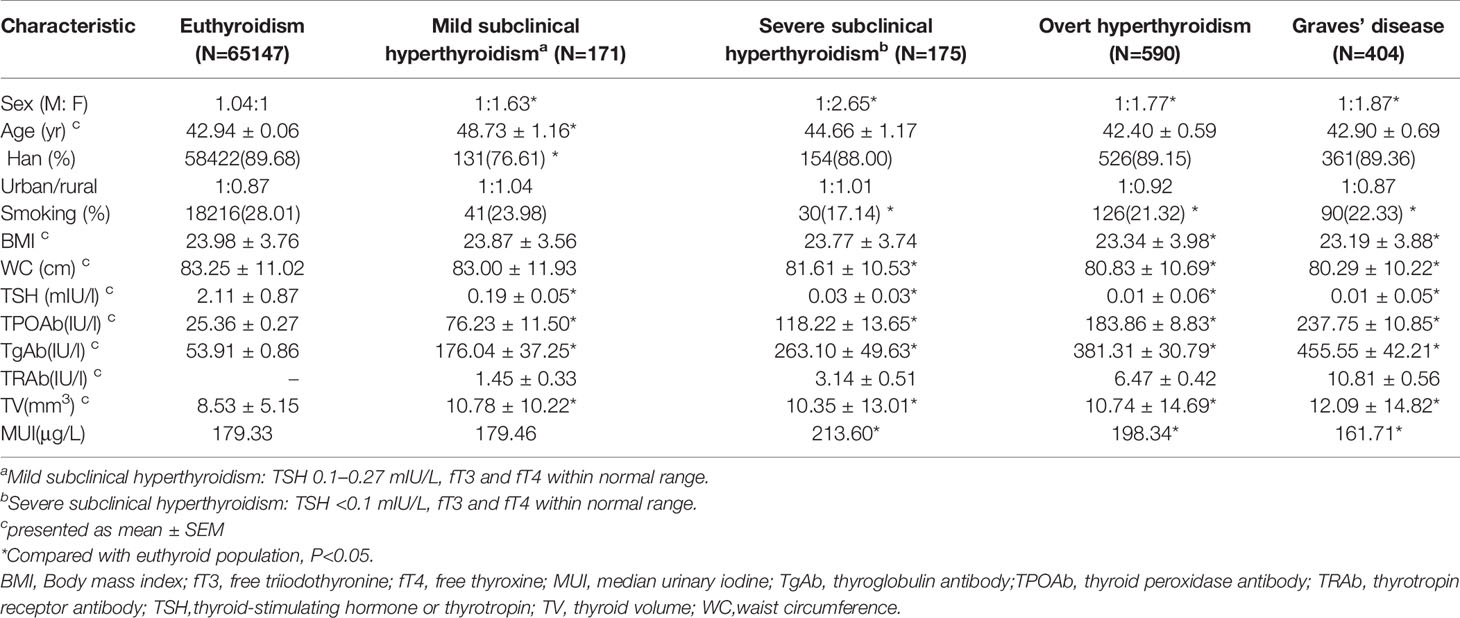
Full information was available for a total of 78470 subjects. The prevalence of low serum TSH (<0.27 mU/L) was 1.19% and among these subjects, 590 had OH, a prevalence of 0.78% of the total sample population. A total of 345 subjects had SCH, a prevalence of 0.44%; of these, the prevalence of mild SCH and severe SCH was 0.22% for each. GD was found in 404 subjects, a prevalence of 0.53% (Table 1). GD accounted for 53.89% of OH, 30.86% of severe SCH, and 18.13% of mild SCH.
Age
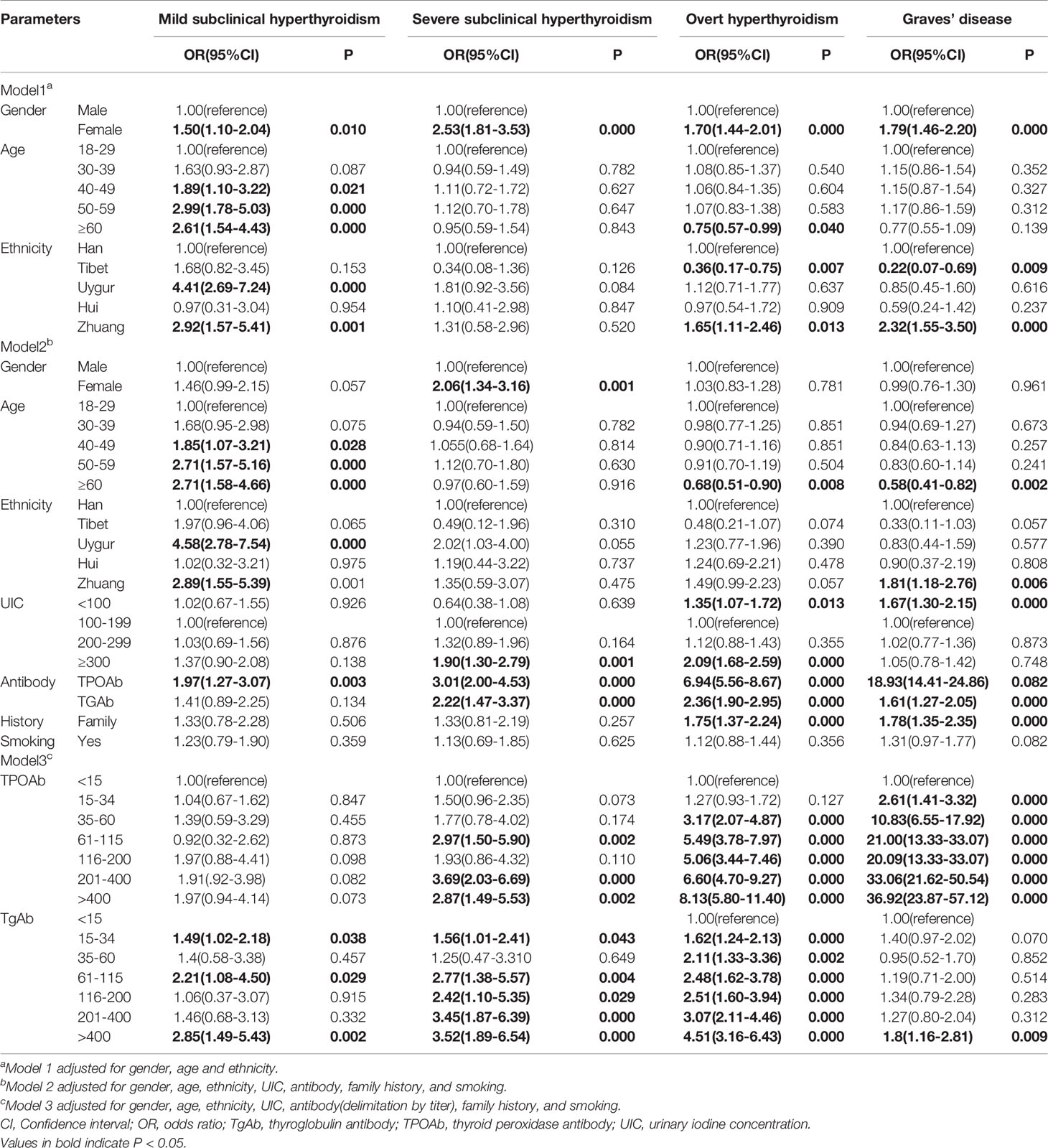
OH prevalence was highest in the 30-59 years and lowest in≥60 years age group (Table 2). GD prevalence showed a similar trend. No significant trends between age and severe SCH were observed. Mild SCH prevalence increased with age. Multifactorial regression analysis showed that after adjusting for gender, ethnic group, UIC, antibodies, family history, and smoking, OH risk (OR 0.68, 95% CI 0.51–0.90) and GD risk (OR 0.58, 95% CI 0.41–0.82) were significantly reduced above the age of 60 years. Mild SCH risk significantly increased above 40 years of age (Table 3).
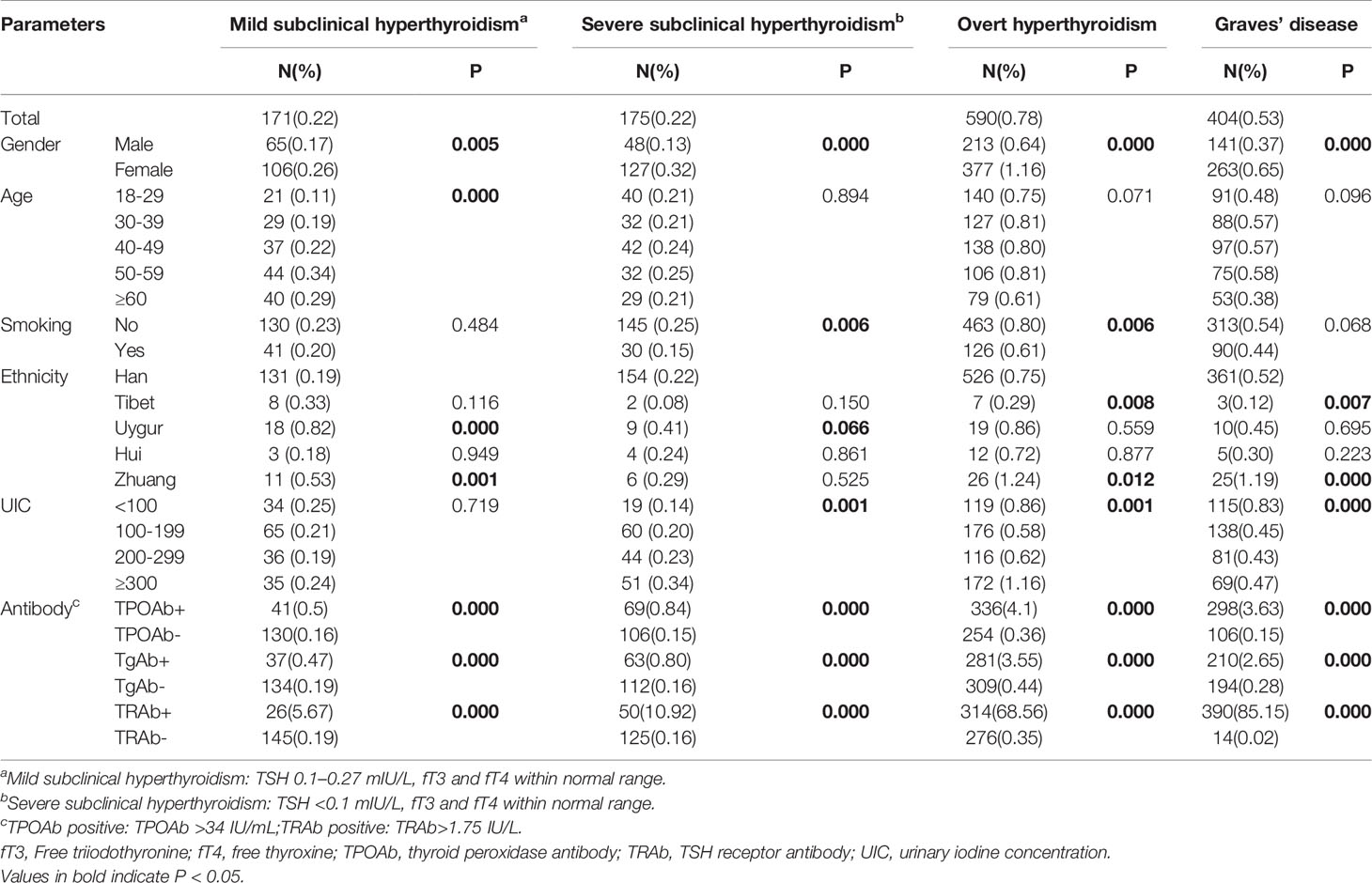
Table 2 The prevalence of subclinical hyperthyroidism, overt hyperthyroidism and Graves’ disease in different populations.
Sex
OH, GD, mild SCH, and severe SCH prevalences were significantly higher in women than in men (Table 2, Figure 1). Regression analysis showed that when adjusted only for age and ethnicity (Table 3, Model 1), the risk of every category of thyroid disease in women was significantly increased. Interestingly, after adjusting further for influencing factors (Table 3, Models 2 and 3), being female was the only independent risk factor for severe SCH (OR2.06, 95%CI1.34–3.16). However, in mild SCH, OH, and GD, no significant differences between women and men were found.

Figure 1 (A) refers to sex- and iodine-specific prevalence of mild subclinical hyperthyroidism; (B) refers to sex- and iodine-specific prevalence of severe subclinical hyperthyroidism; (C) refers to sex- and iodine-specific prevalence of Graves’ disease and (D) refers to sex- and iodine-specific prevalence of overt hyperthyroidism. Mild subclinical hyperthyroidism [thyroid-stimulating hormone (TSH) 0.1–0.26 mIU/L, free thyroxine (fT3) and free triiodothyronine (fT4) within the normal range]. Severe subclinical hyperthyroidism (TSH <0.1 mIU/L, fT3 and fT4 within the normal range). The black line refers to male, the gray line refers to female and the dotted line refers to total. UIC, Urinary iodine concentration.
Smoking and Family History
OH and severe SCH prevalences were significantly higher in nonsmokers than in smokers (Table 2), but no differences in GD prevalence was found in these two populations. Regression analysis revealed that, after adjusting for sex, age, ethnic group, antibodies, and UIC, no relationship existed between smoking and severe SCH, and OH prevalence (Table 3, Model 3). Family history of thyroid disease was significantly associated with OH and GD prevalence, but not with SCH prevalence (Table 3, Model 3).
Ethnicity
Compared with the Han group, OH, GD, and mild SCH prevalences in the Zhuang group were significantly higher (P<0.05) and SCH prevalence in the Uygur group was higher (P<0.05). OH and GD prevalences in the Tibetan group were significantly lower (P<0.05), and no significant differences in the prevalence of any thyroid diseases were found in the Hui group (Table 2).
Iodine Intake
OH prevalence showed a U-shaped trend with increase in iodine intake, and this trend was more obvious in women than in men (Figure 1D). The prevalence of OH in the deficient iodine and excessive iodine populations was much higher than in the adequate intake population, peaking in the UIC≥300 μg/L group (Table 2). The prevalence of GD was only influenced by deficient iodine, and peaked in the UIC ≤ 50μg/L group (Figure 1C). Excessive iodine had no relation with GD prevalence (Figure 1C).
In the SCH patients, the effects of iodine nutrition on the prevalence of mild SCH and severe SCH were quite different. With an increase in UIC, severe SCH prevalence showed an upward trend, peaking in the UIC≥300 μg/L group (Table 2). However, there was no clear relationship between iodine status and mild SCH prevalence (Figures 1A). Multifactorial regression analysis showed that deficient iodine was associated with the prevalence of OH (OR 1.35, 95% CI 1.07–1.72) and GD (OR 1.67 95%CI 1.30–2.15). Excessive iodine was associated with the prevalence of severe SCH (OR 1.90, 95% CI 1.30–2.79) and OH (OR 2.09, 95% CI (1.68–2.59).
The proportion of GD in OH and SCH varied in different iodine status regions, and with an increase in iodine intake, the proportion of GD in OH and SCH decreased accordingly (Figure 2A).
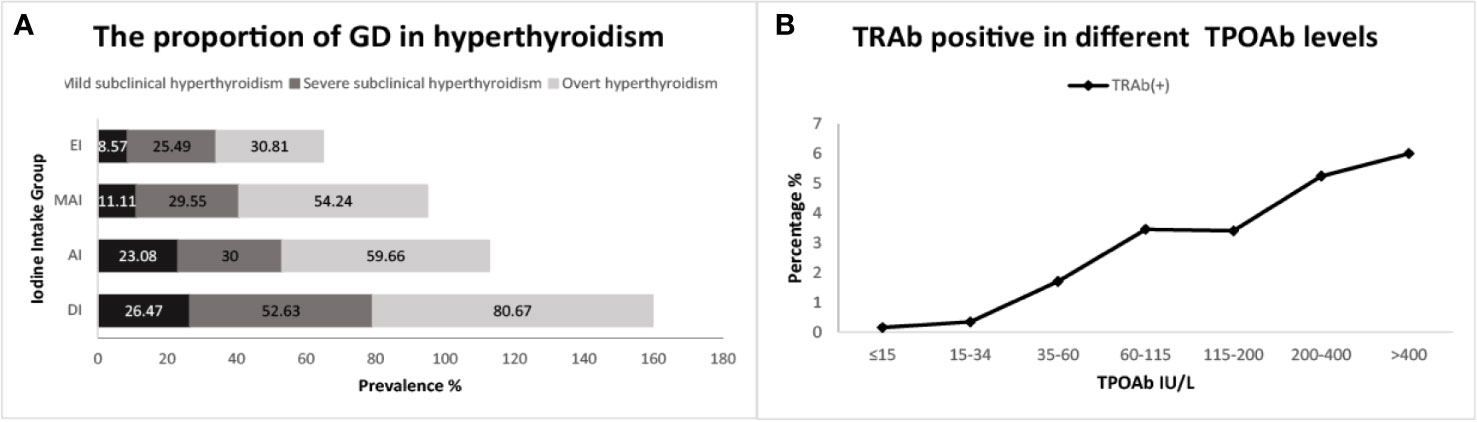
Figure 2 (A) refers to the proportion of subjects with Graves’ disease (GD) in hyperthyroidism; (B) refers to the proportion positive for TSH receptor antibodies (TRAb) across thyroid peroxidase antibody (TPOAb) groups.
Thyroid Autoimmune Antibodies
The proportions of positive TRAb was the highest in GD, and gradually decreased in OH, severe SCH and mild SCH. The proportions of positive TPOAb and TgAb were the highest in OH, and gradually decreased in GD, severe SCH and mild SCH (Table 2). After we stratified each antibody according to its titer, we could see that with an increase in antibodies, OH and GD prevalence increased significantly (Figure 3), with peaks for both occurring in the >400 IU/mL group. However, these trends were less significant in mild SCH and severe SCH. Multifactorial regression analysis showed that an increase in TPOAb had a strong influence on increasing prevalence of OH and GD (Table 3, Model3). These influence of TgAb on OH prevalence was similar to that of TPOAb, but the influence of TgAb on GD prevalence was different from that of TPOAb—only when TgAb was >400 IU/mL was there a positive influence on GD prevalence. The influence of antibody levels on severe SCH was more like that in OH. With increase of antibody titer, the influence gradually increased, but the influence was weaker than in OH. However, neither TPOAb nor TgAb had significant influence on mild SCH prevalence. Additionally, with an increase of TPOAb titer, the proportion of subjects with TRAb increased (Figure 2B).
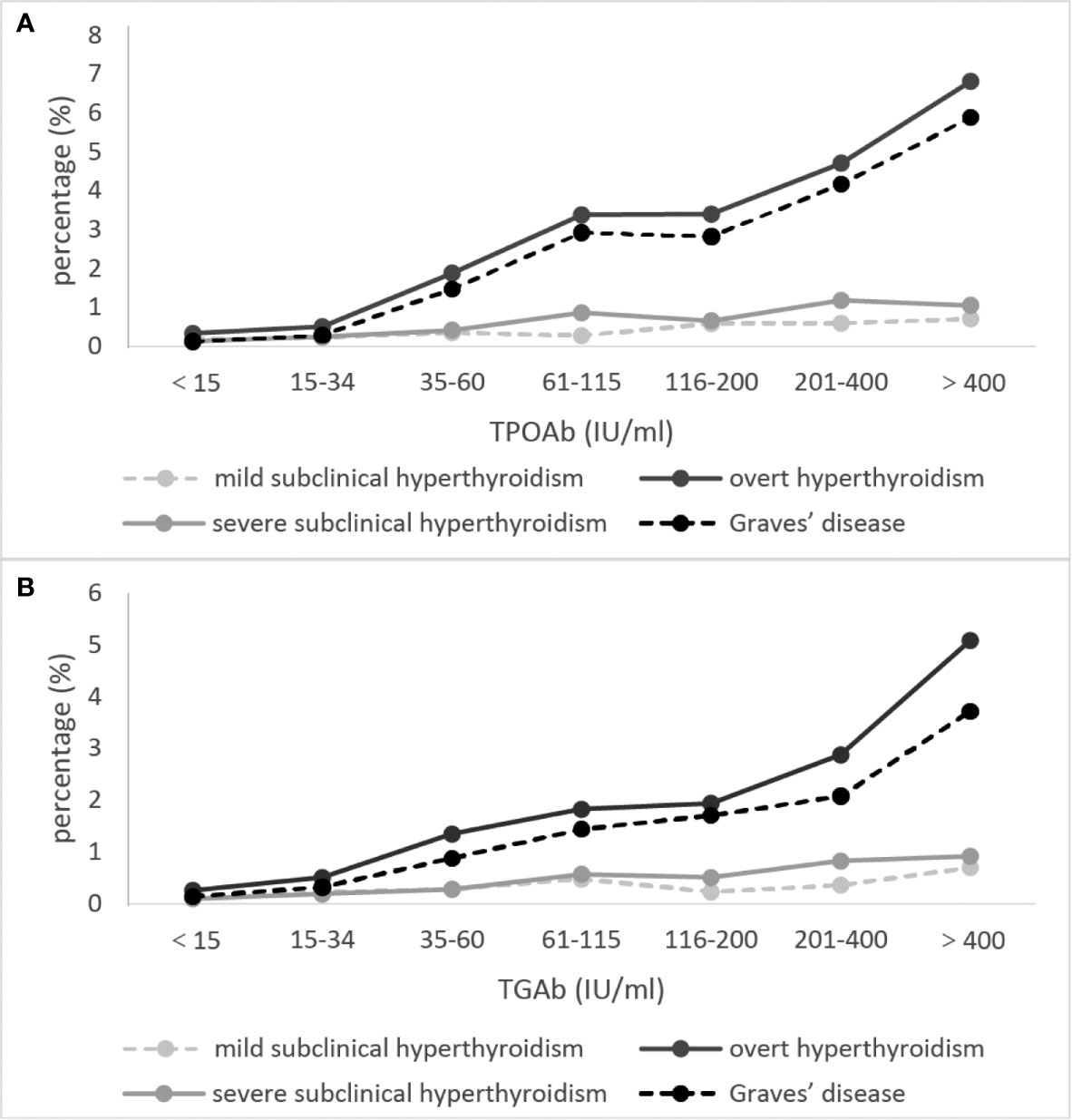
Figure 3 The proportion of subjects with overt hyperthyroidism and Graves’ disease at different autoimmune antibody levels. (A) refers to TPOAb, (B) refers to TgAb. Mild subclinical hyperthyroidism [thyroid-stimulating hormone (TSH) 0.1–0.26 mIU/L, free thyroxine (fT3) and free triiodothyronine (fT4) within the normal range]. Severe subclinical hyperthyroidism (TSH <0.1 mIU/L, fT3 and fT4 within the normal range). The concentrations of thyroid autoimmune antibodies (TPOAb and TgAb) were stratified by gradient in accordance with ratio.
Discussion
In this large-scale cross-sectional study, we found that OH and GD prevalence remained steady after more than two decades of USI. GD is the main cause of OH in China. Iodine intake has a U-shaped correlation with OH. With increasing thyroid autoimmune antibody concentration, the positive correlation between TPOAb and OH and GD grew stronger. But for TgAb this trend was only seen patients with OH. We also found a significant correlation between age and hyperthyroidism prevalence, but gender influenced the hyperthyroidism prevalence through other factors. Furthermore, we classified SCH into two subtypes, mild SCH and severe SCH, and found that the relationship between influencing factors (iodine status and antibody concentration) and severe SCH was consistent with OH. However, there was no significant correlation between these factors and mild SCH, indicating that severe SCH and mild SCH may differ in pathogenesis.
China previously had one of the highest rates of iodine deficiency disease in the world. Therefore, a USI program was implemented in 1996 (16). Since then the Chinese people have experienced iodine excess, a more-than-adequate iodine status, and, currently, adequate iodine status (14).With these changes to iodine status, hyperthyroidism prevalence has also changed. In the fourth year of USI implementation, the prevalences of OH and GD were as high as 1.68% and 1.25%, respectively (12), dropping to 0.89% and 0.61%, respectively, in the ninth year of USI (13).At present, USI has been implemented for two decades, and our study shows current OH and GD prevalence in China of 0.78% and 0.53%, respectively. Hyperthyroidism prevalence varies widely worldwide, from 0.34%–1.66% (17–21).The differences in prevalence among countries are related to various factors including iodine status, thyroid autoimmune antibodies, gender, age, and ethnicity.
Many factors influence OH prevalence, and iodine status has always been one of the most debatable. Some studies have shown that after excessive iodine exposure, hyperthyroidism prevalence may increase significantly (22). This phenomenon usually occurs 1–3 years after initial iodine supplementation in an area with iodine deficiency (23, 24). With the gradual adaptation of the body to iodine intake changes, this increased prevalence will gradually drop to normal (25). During the early years of USI implementation in China, hyperthyroidism prevalence indeed increased temporarily (12). However, as shown in our study, with iodine status gradually reaching an optimal level after several adjustments during USI over the two decades, hyperthyroidism prevalence in China has dropped to a relatively steady level.
Our study also found that the effect of iodine status on OH was bidirectional. Deficient iodine and excessive iodine can both lead to an increase in OH prevalence. There are two explanations for this phenomenon. First, in the deficient iodine population, to make enough thyroid hormone to meet the needs of normal metabolism, follicular cells switch into autonomous hyperfunction, eventually leading to hyperthyroidism (26).Second, deficient iodine can lead to thyroid nodules, and thyroid antigens are released from the abnormal thyroid tissue in the nodules, which can lead to an increase in circulating thyroid autoimmune antibodies (27). However, excessive iodine intake also has an effect on the production of thyroid autoantibodies, mainly because it can provoke strong immunogenicity of thyroglobulin, which may trigger the immune system response to thyroid tissue (28, 29).
In our study, the effect of iodine deficiency on GD was obvious. The reason may be related to thyroid autoimmunity. Our previous study has shown that iodine deficiency is a risk factor for thyroid autoimmunity, the prevalence of TRAb was significantly higher in iodine deficiency subjects than in iodine sufficient subjects, the prevalence of TPOAb decreased significantly with increased iodine intake (30).When we further analyzed the relationship between TPOAb and TRAb, and GD (Figure 2; Table 3, Model 3), it showed a significant positive correlation.
We also found that the influence of iodine nutrition on mild SCH and severe SCH was inconsistent. The influence of iodine intake on severe SCH was similar to that on OH. However, there was no obvious correlation between iodine status and mild SCH, suggesting that mild SCH and severe SCH may have a different pathogenesis.
In areas with adequate iodine status, autoimmune thyroid disease is the main cause of hyperthyroidism (31). Our results demonstrated that hyperthyroidism prevalence was increased in the population with positive thyroid autoimmune antibodies. Furthermore, when we stratified antibody concentration by gradient, we found that the increased TPOAb antibody titer exerted a more powerful influence on OH and GD prevalence (Table 3, Model 3; Figure 3). Studies have demonstrated a positive correlation between TPOAb and OH. Patients with positive TPOAb will progress to OH at a rate of 2.5% per year, whereas if patients with SCH are also positive for TPOAb, they will progress to OH at a rate of 4.5% per year (32). We further analyzed the significant positive correlation between TPOAb and TRAb. With a gradual increase of TPOAb concentration, the positive rate for TRAb also increased, which explains the increase in GD prevalence with the increase in TPOAb.
Differing from the strong correlation found between TPOAb and GD, the correlation between TgAb and GD was much weaker. When we graded TgAb according to its titer, only the group with TgAb>400IU/mL was significantly correlated with GD prevalence. Studies have shown that>90% of Hashimoto thyroiditis patients and 40%–70% of GD patients test positive for TgAb. However, about 20% of the general population are positive for TgAb (33). This indicates that the specificity of TgAb was relatively low when viewed with the previous results, since excessive iodine intake may lead to an increase in the immunogenicity of thyroglobulin, and the correlation between TgAb and GD was weak. This may explain why excess iodine intake did not have a significant influence on GD in our study. However, further research is needed to explain the relationship between iodine status, thyroid autoimmune antibodies, and hyperthyroidism and GD prevalence.
Additionally, we analyzed the relationship between thyroid autoimmune antibodies and SCH. The relationship seen between TPOAb and severe SCH was similar to that between TPOAb and OH. However, no significant relationship was found between TPOAb and mild SCH. These results also suggest a different pathogenesis for mild SCH and severe SCH, and may explain why severe SCH is more likely to progress to OH. Hence when considering whether SCH needs intervention in a clinical setting, severe SCH should be taken seriously (1).
An interesting phenomenon found in this study was that although OH, GD, and SCH prevalence differed between genders, as seen in previous studies (32, 34, 35), these differences disappeared after adjusting for thyroid autoimmune antibodies in the regression analysis. Previous studies have reported that thyroid autoimmune antibody positive rates are much higher in women than men (36).When we rule out the influence of antibodies, the difference between the genders also disappears. Hence, the differing prevalence between the genders may be caused by the difference in positive antibody rate between them, rather than a specific difference by gender.
We also found that advanced age had a negative relationship with OH and GD. This trend persisted even after adjusting for factors such as antibodies and iodine status. Opinion on the relationship between age and hyperthyroidism has been controversial in previous research. One study in Denmark involving 8,219 subjects indicated that OH prevalence increased with age (37).However, a larger study carried out in Scotland indicated that hyperthyroidism prevalence decreased with age, which agrees with our study findings (38). These conflicting results suggest the effect of advanced age on hyperthyroidism prevalence requires further study.
As China is a multiethnic country, we analyzed the relationship between the main ethnic groups and thyroid disease prevalence. Differences were found in OH, GD, and SCH prevalences between the Han group and the other ethnic groups. Regression analysis showed that after adjusting for iodine status, antibodies, and other factors, the Zhuang group still had high prevalences of OH and GD compared with the Han. The genetic polymorphism of each ethnic group could be the explanation. A European study has reported that hyperthyroidism prevalence in people who are ethnically white is slightly higher than that in other ethnic groups (38). Other studies have also suggested that thyroid-related disease incidence varies among ethnic groups (39–41).
Our study had limitations. It was cross-sectional, and although the sample was large, cause and effect and the mechanism of the findings cannot be demonstrated. Hence, further cohort studies are needed. In this study, we did not do the thyroid iodine-131 uptake rate examination, so the cause of thyrotoxicosis cannot be identified. In the epidemiologic analysis, the diagnosis of SCH was inconsistent with the guideline recommendation.
However, there are the advantages of the present study. In our previous studies two papers simply pointed out the prevalence of hyperthyroidism and mainly analyzed the influence of iodine on various thyroid diseases (12, 13). One paper discussed the changes in urinary iodine levels in Chinese people during the first 5 years after the implementation of USI, and pointed out that we had made significant progress in the goal of eliminating iodine deficiency disorders. But the prevalence of hyperthyroidism was not analyzed (16). Compared with general results of TIDE study (14), the present article specifically discusses the prevalence and influencing factors of overt hyperthyroidism, severe and mild hyperthyroidism, including age, sex, ethnicity, smoking, iodine, thyroid antibodies and BMI. The analysis of the influencing factors of hyperthyroidism and subclinical hyperthyroidism is more comprehensive, and the discussion is more in-depth.
Conclusion
In conclusion, OH and GD prevalences in mainland China remain stable two decades after USI was implemented. Iodine status, thyroid antibody levels, and age are the main risk factors for OH and GD. Finally, the severe SCH population, rather than the mild SCH population, shows similar characteristics to the OH population.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available because we promised that the data will not be provided to the third parties when reviewed by the ethics committee. But if there are reasonable request, please ask the corresponding author. Requests to access these datasets should be directed to Y211c2hhbnpob25neWFuQDE2My5jb20=.
Ethics Statement
The studies involving human participants were reviewed and approved by The research protocols were approved by the Medical Ethics Committee of China Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ZYS,WPT, CYW and YZL conceived and designed the study. ZYS and WPT supervised the study. ZYS, WPT, CYW and YZL performed the statistical analysis. All authors contributed to the analysis and interpretation of the data. CYW drafted the manuscript. All authors approved the final version of the manuscript prior to submission. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Research Fund for Public Welfare, National Health and Family Planning Commission of China (Grant No. 201402005).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the study subjects for their contribution to this research. We would also like to thank the following people for their continuous support, assistance, and cooperation: Wei Gong, Chenling Fan, Hong Wang, Hongmei Zhang, Shuangning Ding, Xiaochen Xie, and Tingting Liu (The First Hospital of China Medical University); Caiping Li and Jian Huangfu (The Affiliated Hospital of Inner Mongolia University); Nan Jin (Chinese PLA General Hospital); Wuquan Deng and Fang Deng (Third Military Medical University); Haicheng Zhou (The First Affiliated Hospital of Dalian Medical University); Qingling Lu (Cardiovascular and Cerebrovascular Disease Hospital of Ningxia Medical University); Yunfeng Shen (The Second Affiliated Hospital of Nanchang University); Guodong Liu (The First Affiliated Hospital of Harbin Medical University); Junxiu Hou and Zhiqiang Zhang (The Affiliated Hospital of Inner Mongolia Medical University); Hong Zhang (The Second Xiangya Hospital); Xiaodong Mao, Qifeng Wang, and Kun Wang (Nanjing University of Chinese Medicine); Yanping Wang (Fujian Medical University Union Hospital); Xiaojun Ma (The First Affiliated Hospital of Zhengzhou University); Liheng Meng (First Affiliated Hospital of Guangxi Medical University); Weihua Linle and Tuanyu Fang (Hainan General Hospital); Xingjun Liu and Yanru Zhao (The First Affiliated Hospital of Xi’an Jiaotong University); Lulu Chen, Jiaoyue Zhang, and Hanyu Wang (Huazhong University of Science and Technology); Jingfang Liu and Songbo Fu (The First Hospital of Lanzhou University); Qingguo Lv (West China Hospital); Chenglin Sun (The First Hospital of Jilin University); Qiuming Yao and Ronghua Song (Shanghai University of Medicine & Health Science Affiliated to Zhoupu Hospital); Tingting Chen (The First Hospital of An Hui Medical University); Ben Niu (The First People’s Hospital of Yunnan Province); Mingtong Xu and Feng Li (Sun Yat-sen Memorial Hospital); Lizhen Lan (The First Hospital of Shanxi Medical University); Jun Yue and Jia Song (People’s Hospital of Tibet Autonomous Region); Yanan Li and Wei Luo (Qinghai Provincial People’s Hospital); Xiaoming Lou and Zhe Mo (Zhejiang Provincial Center for Disease Control and Prevention); Nianchun Peng and Lixin Shi (Affiliated Hospital of Guiyang Medical University); Mian Wang, Qiuxiao Zhu, and Lingling Yuan (Second Hospital of Hebei Medical University); Haiqing Zhang (Shandong Provincial Hospital affiliated with Shandong University); Yong Fan (The First Affiliated Hospital of Xinjiang Medical University); and Hongyan Wei (Tianjin Medical University General Hospital).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.651534/full#supplementary-material
References
1. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid (2016) 26:1343–421. doi: 10.1089/thy.2016.0229
2. Cooper DS, Biondi B. Subclinical Thyroid Disease. Lancet (Lond Engl) (2012) 379:1142–54. doi: 10.1016/S0140-6736(11)60276-6
3. Biondi B, Cooper DS. Subclinical Hyperthyroidism. N Engl J Med (2018) 378:2411–9. doi: 10.1056/NEJMcp1709318
4. Maenhaut C, Christophe D, Vassart G, Dumont J, Roger PP, Feingold KR, et al. “Ontogeny, Anatomy, Metabolism and Physiology of the Thyroid”. In: Endotext. South Dartmouth (MA):MD Text.com, Inc (2000). 2000-2015 Jul 15.
5. Friberg L, Rosenqvist M, Lip GYH. Evaluation of Risk Stratification Schemes for Ischaemic Stroke and Bleeding in 182 678 Patients With Atrial Fibrillation: The Swedish Atrial Fibrillation Cohort Study. Eur Heart J (2012) 33:1500–10. doi: 10.1093/eurheartj/ehr488
6. Baumgartner C, da Costa BR, Collet T-H, Feller M, Floriani C, Bauer DC, et al. Thyroid Function Within the Normal Range, Subclinical Hypothyroidism, and the Risk of Atrial Fibrillation. Circulation (2017) 136:2100–16. doi: 10.1161/CIRCULATIONAHA.117.028753
7. Blum MR, Bauer DC, Collet TH, Fink HA, Cappola AR, da Costa BR, et al. Subclinical Thyroid Dysfunction and Fracture Risk: A Meta-Analysis. JAMA (2015) 313:2055–65. doi: 10.1001/jama.2015.5161
8. De Leo S, Lee SY, Braverman LE. Hyperthyroidism. Lancet (London England) (2016) 388:906–18. doi: 10.1016/S0140-6736(16)00278-6
9. Amouzegar A, Gharibzadeh S, Kazemian E, Mehran L, Tohidi M, Azizi F, et al. The Prevalence, Incidence and Natural Course of Positive Antithyroperoxidase Antibodies in a Population-Based Study: Tehran Thyroid Study. PLoS One (2017) 12(1):e0169283. doi: 10.1371/journal.pone.0169283
11. Wang J, Harris M, Amos B, Li M, Wang X, Zhang J, et al. A Ten Year Review of the Iodine Deficiency Disorders Program of the People's Republic of China. J Public Health Policy (1997) 18:219–41. doi: 10.2307/3343436
12. Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, et al. Effect of Iodine Intake on Thyroid Diseases in China. N Engl J Med (2006) 354:2783–93. doi: 10.1056/NEJMoa054022
13. Shan Z, Chen L, Lian X, Liu C, Shi B, Shi L, et al. Iodine Status and Prevalence of Thyroid Disorders After Introduction of Mandatory Universal Salt Iodization for 16 Years in China: A Cross-Sectional Study in 10 Cities. Thyroid (2016) 26:1125–30. doi: 10.1089/thy.2015.0613
14. Li YZ, Teng D, Ba J, Chen B, Du J, He L, et al. Efficacy and Safety of Long-Term Universal Salt Iodization on Thyroid Disorders: Epidemiological Evidence From 31 Provinces of Mainland China. Thyroid (2020) 30(4):568–79. doi: 10.1089/thy.2019.0067
15. WHO. (2017). Available at: http://wwwwhoint/vmnis/indicators/urinaryiodine/en/.
16. Zhao J, van der Haar F. Progress in Salt Iodization and Improved Iodine Nutrition in China, 1995–99. Food Nutr Bull (2004) 25:337–43. doi: 10.1177/156482650402500403
17. Delitala AP, Pilia MG, Ferreli L, Loi F, Curreli N, Balaci L, et al. Prevalence of Unknown Thyroid Disorders in a Sardinian Cohort. Eur J Endocrinol (2014) 171:143–9. doi: 10.1530/EJE-14-0182
18. Camargo RYA, Tomimori EK, Neves SC, Rubio IGS, Galrao AL, Knobel M, et al. Thyroid and the Environment: Exposure to Excessive Nutritional Iodine Increases the Prevalence of Thyroid Disorders in Sao Paulo, Brazil. Eur J Endocrinol (2008) 159:293–9. doi: 10.1530/EJE-08-0192
19. Kasagi K, Takahashi N, Inoue G, Honda T, Kawachi Y, Izumi Y, et al. Thyroid Function in Japanese Adults as Assessed by a General Health Checkup System in Relation With Thyroid-Related Antibodies and Other Clinical Parameters. Thyroid (2009) 19:937–44. doi: 10.1089/thy.2009.0205
20. Kwon H, Jung JH, Han KD, Park YG, Cho JH, Lee DY, et al. Prevalence and Annual Incidence of Thyroid Disease in Korea From 2006 to 2015: A Nationwide Population-Based Cohort Study. Endocrinol Metab (Seoul) (2018) 33:260–7. doi: 10.3803/EnM.2018.33.2.260
21. Azizi F, Hedayati M, Rahmani M, Sheikholeslam R, Allahverdian S, Salarkia N, et al. Reappraisal of the Risk of Iodine-Induced Hyperthyroidism: An Epidemiological Population Survey. J Endocrinol Invest (2005) 28:23–9. doi: 10.1007/BF03345525
22. Rhee CM, Bhan I, Alexander EK, Brunelli SM. Association Between Iodinated Contrast Media Exposure and Incident Hyperthyroidism and Hypothyroidism. Arch Intern Med (2012) 172:153–9. doi: 10.1001/archinternmed.2011.677
23. Martins MC, Lima N, Knobel M, Medeiros-Neto G. Natural Course of Iodine-Induced Thyrotoxicosis (Jodbasedow) in Endemic Goiter Area: A 5 Year Follow-Up. J Endocrinol Invest (1989) 12:239–44. doi: 10.1007/BF03349973
24. Elnagar B, Eltom M, Karlsson FA, Ermans AM, Gebre-Medhin M, Bourdoux PP. The Effects of Different Doses of Oral Iodized Oil on Goiter Size, Urinary Iodine, and Thyroid-Related Hormones. J Clin Endocrinol Metab (1995) 80:891–7. doi: 10.1210/jcem.80.3.7883848
25. Roti E, Uberti ED. Iodine Excess and Hyperthyroidism. Thyroid (2001) 11:493–500. doi: 10.1089/105072501300176453
26. Song Y, Driessens N, Costa M, Deken XD, Detours V, Corvilain B, et al. Roles of Hydrogen Peroxide in Thyroid Physiology and Disease. J Clin Endocrinol Metab (2007) 92:3764–73. doi: 10.1210/jc.2007-0660
27. Pedersen IB, Knudsen N, Jørgensen T, Perrild H, Ovesen L, Laurberg P. Thyroid Peroxidase and Thyroglobulin Autoantibodies in a Large Survey of Populations With Mild and Moderate Iodine Deficiency. Clin Endocrinol (Oxf) (2003) 58:36–42. doi: 10.1046/j.1365-2265.2003.01633.x
28. Burek CL, Talor MV. Environmental Triggers of Autoimmune Thyroiditis. J Autoimmun (2009) 33:183–9. doi: 10.1016/j.jaut.2009.09.001
29. Zimmermann MB, Boelaert K. Iodine Deficiency and Thyroid Disorders. Lancet Diabetes Endocrinol (2015) 3:286–95. doi: 10.1016/S2213-8587(14)70225-6
30. Teng D, Yang W, Shi X, Li Y, Ba J, Chen B. An Inverse Relationship Between Iodine Intake and Thyroid Antibodies: A National Cross-Sectional Survey in Mainland China. Thyroid (2020) 30(11):1656–65. doi: 10.1089/thy.2020.0037
31. Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM. Global Epidemiology of Hyperthyroidism and Hypothyroidism. Nat Rev Endocrinol (2018) 14:301–16. doi: 10.1038/nrendo.2018.18
32. Vanderpump MP. How Should We Manage Patients With Mildly Increased Serum Thyrotrophin Concentrations? Clin Endocrinol (Oxf) (2010) 72:436–40. doi: 10.1111/j.1365-2265.2009.03720.x
33. Rayman MP. Multiple Nutritional Factors and Thyroid Disease, With Particular Reference to Autoimmune Thyroid Disease. Proc Nutr Society (2019) 78:34–44. doi: 10.1017/S0029665118001192
34. Abraham-Nordling M, Byström K, Törring O, Lantz M, Berg G, Calissendorff J, et al. Incidence of Hyperthyroidism in Sweden. Eur J Endocrinol (2011) 165:899–905. doi: 10.1530/EJE-11-0548
35. Wémeau J-L, Klein M, Sadoul J-L, Briet C, Vélayoudom-Céphise FL. Graves' Disease: Introduction, Epidemiology, Endogenous and Environmental Pathogenic Factors. Ann Endocrinol (Paris) (2018) 79:599–607. doi: 10.1016/j.ando.2018.09.002
36. Belin RM, Astor BC, Powe NR, Ladenson PW. Smoke Exposure is Associated With a Lower Prevalence of Serum Thyroid Autoantibodies and Thyrotropin Concentration Elevation and a Higher Prevalence of Mild Thyrotropin Concentration Suppression in the Third National Health and Nutrition Examination Survey (Nhanes Iii). J Clin Endocrinol Metab (2004) 89:6077–86. doi: 10.1210/jc.2004-0431
37. Laurberg P, Jørgensen T, Perrild H, Ovesen L, Knudsen N, Pedersen IB, et al. The Danish Investigation on Iodine Intake and Thyroid Disease, DanThyr: Status and Perspectives. Eur J Endocrinol (2006) 155:219–28. doi: 10.1530/eje.1.02210
38. Garmendia Madariaga A, Santos Palacios S, Guillen-Grima F, Galofré JC. The Incidence and Prevalence of Thyroid Dysfunction in Europe: A Meta-Analysis. J Clin Endocrinol Metab (2014) 99:923–31. doi: 10.1210/jc.2013-2409
39. Kim SJ, Kim MJ, Yoon SG, Myong JP, Yu HW, Chai YJ, et al. Impact of Smoking on Thyroid Gland: Dose-Related Effect of Urinary Cotinine Levels on Thyroid Function and Thyroid Autoimmunity. Sci Rep (2019) 9:4213. doi: 10.1038/s41598-019-40708-1
40. Verma V, Kumar Y, Kotwal N, Upretiet V, Hari Kumar KVS, Singh Y, et al. Thyrotoxic Periodic Paralysis: A Retrospective, Observational Study From India. Indian J Med Res (2020) 151:42–6. doi: 10.4103/ijmr.IJMR_335_18
Keywords: thyroid autoimmune antibodies, cross-sectional study, Graves’ disease, iodine intake, hyperthyroidism
Citation: Wang C, Li Y, Teng D, Shi X, Ba J, Chen B, Du J, He L, Lai X, Li Y, Chi H, Liao E, Liu C, Liu L, Qin G, Qin Y, Quan H, Shi B, Sun H, Tang X, Tong N, Wang G, Zhang J-a, Wang Y, Xue Y, Yan L, Yang J, Yang L, Yao Y, Ye Z, Zhang Q, Zhang L, Zhu J, Zhu M, Shan Z and Teng W (2021) Hyperthyroidism Prevalence in China After Universal Salt Iodization. Front. Endocrinol. 12:651534. doi: 10.3389/fendo.2021.651534
Received: 10 January 2021; Accepted: 20 April 2021;
Published: 28 May 2021.
Edited by:
Bernadette Biondi, University of Naples Federico II, ItalyReviewed by:
Serena Ippolito, ASL Napoli 1 centro, ItalyAngela M. Leung, University of California, Los Angeles, United States
Copyright © 2021 Wang, Li, Teng, Shi, Ba, Chen, Du, He, Lai, Li, Chi, Liao, Liu, Liu, Qin, Qin, Quan, Shi, Sun, Tang, Tong, Wang, Zhang, Wang, Xue, Yan, Yang, Yang, Yao, Ye, Zhang, Zhang, Zhu, Zhu, Shan and Teng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongyan Shan, Y211c2hhbnpob25neWFuQDE2My5jb20=; Weiping Teng, dHdwQHZpcC4xNjMuY29t
†ORCID: Zhongyan Shan, orcid.org/0000-0002-2849-2380
Weiping Teng, orcid.org/0000-0002-6445-6192
 Chuyuan Wang
Chuyuan Wang Yongze Li
Yongze Li Di Teng1
Di Teng1 Guijun Qin
Guijun Qin Bingyin Shi
Bingyin Shi Xulei Tang
Xulei Tang Nanwei Tong
Nanwei Tong Guixia Wang
Guixia Wang Jin-an Zhang
Jin-an Zhang Jing Yang
Jing Yang Lihui Zhang
Lihui Zhang Weiping Teng
Weiping Teng