
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 29 June 2021
Sec. Clinical Diabetes
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.645507
This article is part of the Research Topic Reviews and Novel Clinical Perspectives on Semaglutide: A GLP-1 Receptor Agonist with Both Injectable and Oral Formulations View all 6 articles
Early and effective glycemic control can prevent or delay the complications associated with type 2 diabetes (T2D). The benefits of glucagon-like peptide-1 receptor agonists (GLP-1RAs) are becoming increasingly recognized and they now feature prominently in international T2D treatment recommendations and guidelines across the disease continuum. However, despite providing effective glycemic control, weight loss, and a low risk of hypoglycemia, GLP-1RAs are currently underutilized in clinical practice. The long-acting GLP-1RA, semaglutide, is available for once-weekly injection and in a new once-daily oral formulation. Semaglutide is an advantageous choice for the treatment of T2D since it has greater efficacy in reducing glycated hemoglobin and body weight compared with other GLP-1RAs, has demonstrated benefits in reducing major adverse cardiovascular events, and has a favorable profile in special populations (e.g., patients with hepatic impairment or renal impairment). The oral formulation represents a useful option to help improve acceptance and adherence compared with injectable formulations for patients with a preference for oral therapy, and may lead to earlier and broader use of GLP-1RAs in the T2D treatment trajectory. Oral semaglutide should be taken on an empty stomach, which may influence the choice of formulation. As with most GLP-1RAs, initial dose escalation of semaglutide is required for both formulations to mitigate gastrointestinal adverse events. There are also specific dose instructions to follow with oral semaglutide to ensure sufficient gastric absorption. The evidence base surrounding the clinical use of semaglutide is being further expanded with trials investigating effects on diabetic retinopathy, cardiovascular outcomes, and on the common T2D comorbidities of obesity, chronic kidney disease, and non-alcoholic steatohepatitis. These will provide further information about whether the benefits of semaglutide extend to these other indications.
For patients with type 2 diabetes (T2D), early control of hyperglycemia after diagnosis is important to prevent debilitating long-term complications and to reduce diabetes-related mortality (1, 2). This is illustrated by the results from a recent registry analysis including 34,737 patients, which showed that glycated hemoglobin (HbA1c) levels between 7.0% and <8.0% (53 to <64 mmol/mol) for the first year after diagnosis were associated with a greater risk of future microvascular complications (hazard ratio [HR] 1.39; 95% confidence interval [CI] 1.23–1.58), macrovascular events (HR 1.29; 95% CI 1.20–1.38), and mortality (HR 1.29; 95% CI 1.10–1.51) compared with levels of <6.5% (<48 mmol/mol) (2).
Glycemic management in patients with T2D has become more individualized, and there are now several different treatment options available, with various factors influencing the most appropriate choice for individual patients. Glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs) are a well-established class of glucose-lowering agents that act on multiple pathophysiological defects in T2D, providing effective glycemic control, weight loss, and a low risk of hypoglycemia, with a well‑characterized safety profile (3). In addition, as described by Smits and van Raalte in this supplement (4), certain GLP-1RAs have also been shown to reduce the risk of cardiovascular (CV) events, as well as some renal-related endpoints, in CV outcomes trials (CVOTs) (5–8).
This article will review the place of GLP-1RAs in therapy and, within this class, specifically discuss some clinical considerations around the use of the long-acting GLP-1RA, semaglutide, when given subcutaneously or via its new oral formulation.
Metformin is the first-line therapy of choice for most patients with T2D; however, if patients do not achieve their individualized HbA1c target after 3–6 months, another glucose-lowering medication should be added (9). In 2018, the American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) consensus for the management of hyperglycemia in T2D presented a new decision algorithm and, as part of this, key patient characteristics should be assessed including the existence of comorbidities, such as atherosclerotic CV disease (CVD), chronic kidney disease (CKD), or heart failure (HF), which necessitate the preferential use of certain classes of glucose-lowering agents as second-line therapy (9, 10).
In patients who have established atherosclerotic CVD or evidence of high atherosclerotic CVD risk, the ADA/EASD consensus now recommends either a GLP-1RA or a sodium-glucose co-transporter-2 inhibitor (SGLT2i) (if estimated glomerular filtration rate [eGFR] is adequate) with proven efficacy to reduce the risk of CV events (9, 11). This change represents a shift in diabetes management beyond glycemic control alone and was based on CVOTs, which demonstrated that several GLP-1RAs and SGLT2is reduced the risk of major adverse CV events (MACE; CV death, nonfatal myocardial infarction, and nonfatal stroke) compared with placebo (5–8, 12, 13). A 2019 update to the ADA/EASD consensus, based on results from the REWIND CVOT with dulaglutide, suggests that a GLP-1RA or SGLT2i should also be considered in high-risk T2D patients without established CVD but with indicators of high CV risk, such as age ≥55 years with coronary, carotid, or lower-extremity artery stenosis >50%, left ventricular hypertrophy, an eGFR <60 mL/min/1.73 m2, or albuminuria (8, 11). Of note, beneficial outcomes observed in CVOTs do not appear to be restricted to patients with elevated HbA1c, and the 2019 update of the ADA/EASD consensus suggests that GLP-1RAs or SGLT2is should be considered independently of baseline HbA1c or the individualized HbA1c target in patients at high CV risk (11). In recent guidelines from the European Society of Cardiology on diabetes, prediabetes, and CVD, in collaboration with the EASD, a GLP-1RA or SGLT2i with proven CVD benefit is recommended as an add-on therapy to metformin and even as a first-line therapy in drug-naïve or metformin-intolerant patients with T2D and CVD or at high or very high CV risk (14).
For patients in which HF or CKD predominates, the ADA/EASD consensus recommends an SGLT2i with evidence of reducing HF and/or CKD progression, or if SGLT2is are not tolerated or contraindicated or if eGFR is less than adequate, a GLP-1RA with proven CV benefit can be added (11). If further treatment intensification is needed after second-line SGLT2i therapy, a GLP-1RA may be added (11). Recent results from a meta-analysis indicate greater reductions in HbA1c, body weight, and systolic blood pressure with a lower requirement of rescue therapy when a GLP-1RA was added in combination with an SGLT2i vs. SGLT2i monotherapy alone (15).
For patients without CVD, the ADA/EASD consensus advocates involving specific factors that could impact on the choice of treatment, including the need to avoid weight gain and/or hypoglycemia, in the decision cycle (9, 11). In addition, the importance of choosing treatment regimens to optimize adherence and persistence is emphasized (9). For patients without established CVD but with a compelling need to minimize weight gain or promote weight loss, either a GLP-1RA with good efficacy for weight loss or an SGLT2i is recommended (9, 11). For patients without established CVD but with a compelling need to minimize hypoglycemia, a GLP-1RA, an SGLT2i, a dipeptidyl peptidase-4 inhibitor, or a thiazolidinedione are the recommended options. A sulfonylurea or a thiazolidinedione should be considered when cost is a major issue.
Despite being effective glucose-lowering therapies with CV and renal benefits, GLP-1RAs are often underutilized. A nationwide analysis in Denmark found that, while the use of GLP-1RAs has increased since their introduction in 2005, they still only accounted for 8% of all glucose-lowering drugs used in 2017 (16). In a survey of patients who initiated a GLP-1RA in Northern Italy over the period 2010 to 2018 (N = 5,408), it appeared that over time GLP-1RAs were being prescribed to patients with progressively more advanced disease, with significant increases in baseline age, diabetes duration, presence of CVD, and insulin use in patients receiving GLP-1RA therapy during the study period (17).
This apparent delay in prescribing GLP-1RAs and intensifying treatment, despite poor glycemic control in a substantial proportion of patients, was also seen in a UK survey of 113 physicians who contributed data for 1,096 patients (18). The median time from diagnosis to GLP-1RA initiation was 6.1 years and patients had HbA1c values above 7.0% for a median of 13.5 months prior to switching from their last oral regimen to a GLP-1RA. In a UK physician perceptions survey completed in 2014, factors that most commonly caused hesitation when prescribing GLP-1RAs included that they were not considered first-line therapy according to guidelines, their injectable mode of administration, cost, and the potential for gastrointestinal (GI) adverse effects (19). The most common reasons reported for prescribing GLP-1RAs were weight loss, good efficacy, and low hypoglycemia risk.
Although GLP-1RAs act via the same overall mechanism, they vary structurally, and differ in their pharmacokinetics and clinical specifics (Table 1), with some degree of heterogeneity in respect to their ability to reduce HbA1c and body weight, and evidence of cardiorenal protection (27, 28). The first GLP-1RAs to be developed needed to be administered subcutaneously twice daily (exenatide (20)) or once daily (lixisenatide (21) and liraglutide (22)). Subsequent developments led to the approval of longer-acting GLP-1RAs that could be administered once weekly (exenatide extended release [ER] (23), dulaglutide (24), and semaglutide (25)) to reduce the injection burden and improve convenience. Indeed, once-weekly regimens have been associated with better adherence than more frequently dosed agents (exenatide vs. liraglutide) (29), and this may lead to improved outcomes.
Semaglutide has 94% sequence homology with native GLP-1, with three key structural differences that prolong its half-life to approximately one week, without compromising GLP-1 receptor binding (25, 30). In the SUSTAIN program, subcutaneous semaglutide consistently demonstrated superior and sustained glycemic control and weight loss compared with comparators across the T2D disease continuum (31). As reviewed by Meier in this supplement (32), in head-to-head trials with other long-acting GLP-1RAs, subcutaneous semaglutide 1.0 mg produced superior HbA1c and weight reductions compared with exenatide ER 2.0 mg (estimated treatment difference [ETD] –0.62% and 3.78 kg; both p < 0.0001, respectively) (33) and with dulaglutide 1.5 mg (ETD –0.41% and 3.55 kg; both p < 0.0001, respectively) (34). Since the approval of once-weekly subcutaneous semaglutide in 2017/2018, further information is being gathered through an ongoing series of prospective, noninterventional real-world studies across 10 different countries, which aim to determine its efficacy, safety, and treatment satisfaction in patients in local clinical practice over approximately 30 weeks of treatment (35–43).
It is known that some patients prefer oral over injectable medications (44, 45), and lower treatment adherence has been reported with more frequent administration or when patients perceive the treatment as difficult or inconvenient (45, 46). Oral medication may also help to overcome the clinical inertia seen in the frequent reluctance to initiate injectable medicines. For this reason, an oral formulation of semaglutide was developed and was approved for the treatment of adults with T2D by the U.S. Food and Drug Administration in September 2019 and by the European Medicines Agency in April 2020. In Europe, subcutaneous semaglutide and oral semaglutide are indicated as adjuncts to diet/exercise either as monotherapy, when metformin is considered inappropriate due to intolerance or contraindications, or in combination with other glucose-lowering medication(s), for patients who do not have sufficient glycemic control (25, 26). As the first oral formulation of a GLP-1RA, oral semaglutide represents a useful option to help improve acceptance and adherence compared with injectable formulations in those patients with a preference for oral therapy, and may contribute to the reversal of current underutilization, potentially leading to earlier initiation of GLP-1RAs in the T2D disease continuum.
As a class, GLP-1RAs have a well-defined safety profile. The most commonly reported adverse events (AEs) are GI-related effects, including nausea, diarrhea, and vomiting, which are generally mild-to-moderate in severity and transient in nature (47). In general, GI AEs are most frequent shortly after treatment initiation and therefore slow up-titration of the dose is recommended for most GLP-1RAs (Table 1). For subcutaneous semaglutide, the starting dose is 0.25 mg once weekly, and after 4 weeks, the dose should be increased to 0.5 mg once weekly (25). After at least 4 weeks on a dose of 0.5 mg once weekly, the dose can be increased to 1 mg once weekly to further improve glycemic control. For oral semaglutide, patients should start treatment with the 3 mg dose once daily for 1 month, then increase to 7 mg once daily (26). After at least 1 month on a dose of 7 mg once daily, the dose can be increased to a maintenance dose of 14 mg once daily if needed to further improve glycemic control. When starting semaglutide, patients should be reassured that GI AEs do not affect the majority of patients and are likely to be only mild-to-moderate in severity and transient (25, 26). To help minimize any nausea, patients could be advised to eat smaller meals and stop when they feel full, and to avoid meals with a high fat content (48–50).
Subcutaneous semaglutide can be dosed at any time on the day of the weekly injection, with or without meals (25). For oral semaglutide, the presence of food in the stomach impairs absorption (51, 52). Patients are advised to swallow the oral semaglutide tablet on an empty stomach, with a sip of water (up to half a glass of water equivalent to 120 mL), and to wait at least 30 minutes before eating, drinking, or taking other oral medications (26). This may be problematic for some patients, and may influence their preferred choice of formulation.
In pharmacokinetic studies, subcutaneous or oral semaglutide did not have clinically relevant effects on the exposure of other widely used medications, such as warfarin, metformin, digoxin, atorvastatin/rosuvastatin (53–55), or the combined oral contraceptive, ethinylestradiol/levonorgestrel (Figure 1) (56, 57). In addition, oral semaglutide did not have clinically relevant effects on the exposure of lisinopril or furosemide (53, 54). When tested with omeprazole, which increases gastric pH, no clinically relevant interactions were observed on the exposure of oral semaglutide (58).
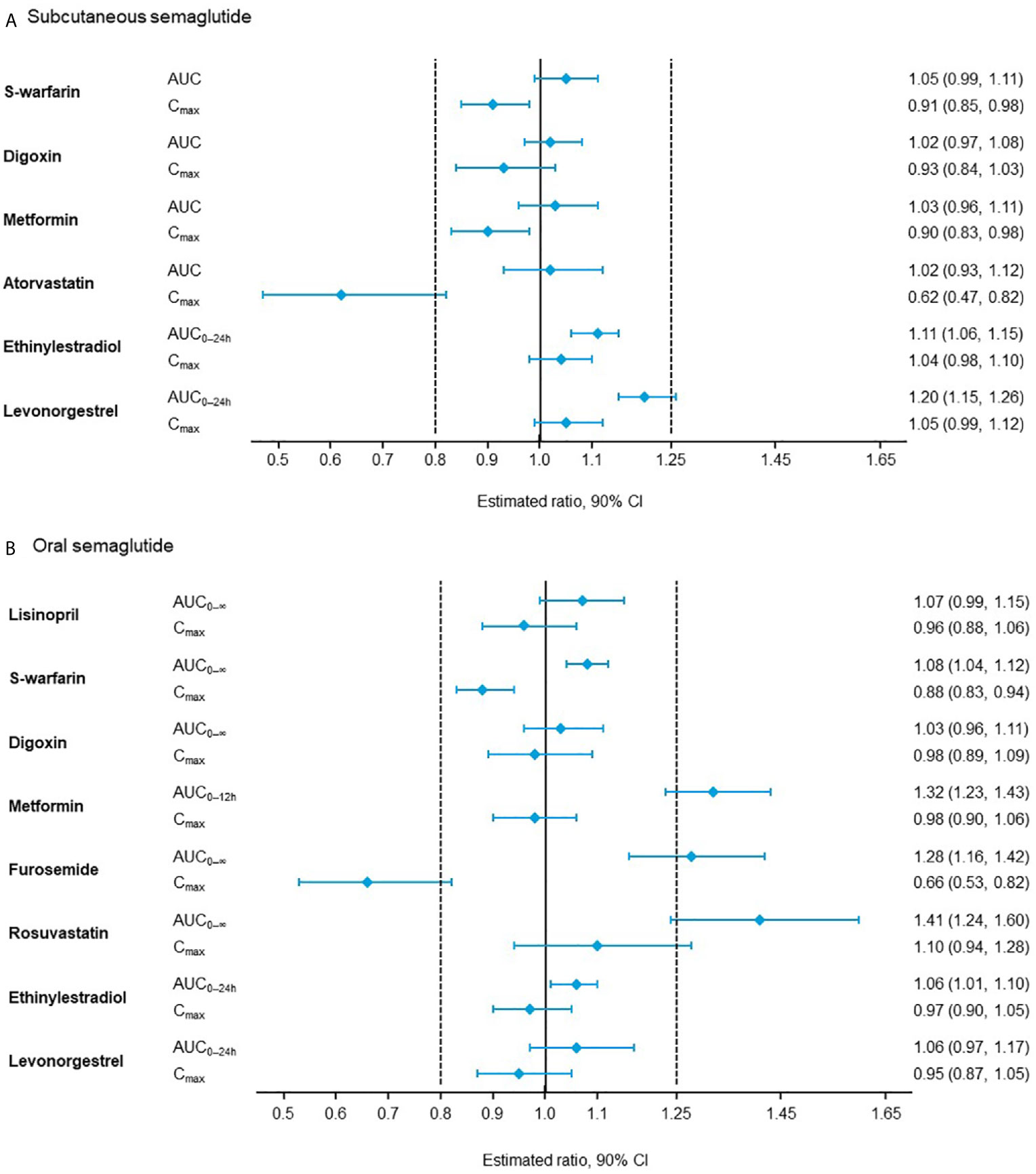
Figure 1 Effect of (A) subcutaneous semaglutide and (B) oral semaglutide on the pharmacokinetics of co-administered drugs (53–57). AUC, area under the curve; CI, confidence interval; Cmax, maximum concentration.
In a drug–drug interaction study, levothyroxine exposure was increased by 33% when co-administered with oral semaglutide 14 mg, which may be due to delayed gastric emptying and increased levothyroxine absorption (59). Monitoring of thyroid parameters should therefore be considered when treating patients with oral semaglutide at the same time as levothyroxine (26). When co-administering other oral medications, it is important to adhere to the administration instructions for oral semaglutide, and consider increased monitoring for medications that have a narrow therapeutic index or that require clinical monitoring (60).
In population pharmacokinetic and exposure−response analyses, the exposure range following oral semaglutide was wider than for subcutaneous dosing but with a considerable overlap between oral semaglutide 7 and 14 mg and subcutaneous semaglutide 0.5 and 1.0 mg (61). The effect of switching between oral and subcutaneous semaglutide cannot easily be predicted because of the high pharmacokinetic inter-individual variability of oral semaglutide; however, exposure after 14 mg oral semaglutide once daily appears comparable with 0.5 mg subcutaneous semaglutide once weekly (26). It is recommended that patients switching from once-weekly subcutaneous semaglutide at a dose of 0.5 mg can be transitioned onto oral semaglutide at a dose of 7 or 14 mg once daily, up to 7 days after their last injection of subcutaneous semaglutide; however, there is no equivalent oral dose for those switching from subcutaneous semaglutide 1 mg (60).
CKD is a common complication of T2D and a major cause of morbidity and mortality (62). The exendin-4-based GLP-1RAs, exenatide (immediate-release and ER) and lixisenatide are partially renally eliminated and are not recommended in patients with severe renal impairment (eGFR <30 mL/min/1.73 m2) (20, 21, 23) (Table 1). Furthermore, dose escalation of immediate-release exenatide should proceed conservatively in patients with moderate renal impairment (eGFR 30–50 mL/min/1.73 m2) (21). Results from pharmacokinetic studies have established that dose adjustments are not necessary when semaglutide (subcutaneous or oral) is used in patients with different levels of renal impairment (25, 26, 63, 64). Like all other GLP-1RAs, semaglutide is not recommended in patients with end-stage renal disease (ESRD) (eGFR <15 mL/min/1.73 m2) (25, 26).
To provide further data on the use of semaglutide in patients with renal dysfunction, the PIONEER 5 trial evaluated the efficacy and safety of once-daily oral semaglutide 14 mg vs. placebo in 324 patients with T2D and moderate renal impairment (eGFR 30–59 mL/min/1.73 m2) (65). Superior and significant reductions in HbA1c and body weight were observed with oral semaglutide vs. placebo over 26 weeks, and renal function was unchanged throughout the study in both treatment groups. Patients with CKD were also included in the SUSTAIN 6 and PIONEER 6 CVOTs (6, 66). Indeed, in SUSTAIN 6, the CKD-related endpoint of new or worsening nephropathy was found to occur in significantly fewer patients in the subcutaneous semaglutide group compared with the placebo group (3.8% vs. 6.1%; HR 0.64; 95% CI 0.46–0.88; p = 0.005) (6).
GLP-1RAs may exert beneficial actions on the kidneys through reductions in blood glucose, blood pressure, and weight, as well as via possible direct cardio-nephroprotective mechanisms, such as improved endothelial dysfunction, reduced oxidative stress, and reduced inflammation (62). The phase III FLOW trial (NCT03819153) is ongoing to determine the effect of once-weekly subcutaneous semaglutide 1.0 mg vs. placebo on the progression of renal impairment in over 3,000 patients with T2D and CKD (eGFR 50–75 mL/min/1.73 m2 and urinary albumin-to-creatinine ratio [UACR] >300–<5,000 mg/g or eGFR 25–50 mL/min/1.73 m2 and UACR >100–<5,000 mg/g) (67). The primary endpoint is the time to the first occurrence of a composite primary outcome event, defined as persistent eGFR decline of ≥50% from trial start, reaching ESRD, death from kidney disease, or death from CVD for up to 5 years.
There is a complex interplay between T2D and liver disease, particularly non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), which are common in patients with T2D (68). The mechanisms responsible for the link between NAFLD and T2D are not completely understood but could include genetic factors, insulin resistance, dysfunctional adipose tissue, chronic hyperglycemia, altered gut microbiome, and changes in hepatokines, among others (68, 69).
GLP-1RAs appear to be well-tolerated in patients with hepatic impairment, and dose adjustments are not necessary (Table 1) (20–26). Consistent with this, pharmacokinetic studies have established no apparent effect of hepatic impairment on the exposure of semaglutide when each formulation was tested (25, 26, 70, 71).
Novel therapies are in demand for the treatment of NAFLD, and early studies suggested that GLP-1RAs may reduce liver inflammation and fibrosis (72). Potential mechanisms for the GLP-1RAs’ benefit in the context of NAFLD include: reduced body weight and body fat through central regulation of satiety; reduced hepatic, skeletal muscle, and adipose tissue insulin resistance due to decrease in body weight; modified intestinal lipoprotein metabolism; and amelioration of dysfunctional adipose tissue and enhancement of insulin release (72, 73). The safety and efficacy of liraglutide 1.8 mg once daily for 48 weeks were tested in a phase II trial in 52 patients with NASH, in which this drug was found to be well-tolerated (74). Furthermore, there was evidence of histological resolution in the end-of-treatment biopsy in 39% of patients in the liraglutide group compared with only 9% in the placebo group.
A phase II trial recently evaluated the effects of once-daily subcutaneous semaglutide (0.1 mg, 0.2 mg, and 0.4 mg) vs. placebo in 320 patients with NASH (75). Treatment with semaglutide 0.4 mg resulted in a significantly higher percentage of patients achieving the primary endpoint of NASH resolution and no worsening of fibrosis than placebo after 72 weeks (59% vs. 17%; p < 0.001).
Given the lack of hepatic GLP-1 receptor expression, the potential mechanism of action by which semaglutide results in NASH resolution may be mediated via weight loss. However, semaglutide is also associated with improvements in insulin resistance, hepatic lipotoxicity, and hepatic inflammation. In pre-clinical models, improvements in inflammation with liraglutide were shown to be independent of weight reduction, as was prevention of initiation of fibrosis (76). Thus, it appears unlikely that improvements in NASH with GLP-1 receptor agonists are solely mediated via weight reduction.
Compared with other GLP-1RAs, the capability for weight loss appears to be higher with semaglutide, and the ADA/EASD consensus provides the following ranking for weight-loss efficacy: subcutaneous semaglutide > liraglutide > dulaglutide > exenatide > lixisenatide (9). The mechanisms responsible for weight loss have been investigated for both subcutaneous and oral semaglutide (77, 78). In 30 patients with obesity, ad libitum energy intake was substantially lower with once-weekly subcutaneous semaglutide (dose escalated to 1.0 mg) vs. placebo for 12 weeks, and this was associated with reduced appetite and food cravings, better control of eating, and lower preference for fatty, energy-dense food (77). Subcutaneous semaglutide induced a 5.0 kg reduction in mean body weight after 12 weeks, which was found to be derived predominantly from body fat mass reduction, assessed by air displacement plethysmography. Consistent results have been observed with once-daily oral semaglutide (dose escalated to 14 mg) vs. placebo in a similar study in 15 patients with T2D (78).
A phase II dose-finding trial evaluated the efficacy and safety of once-daily subcutaneous semaglutide in promoting weight loss (79). In total, 957 patients with obesity (body mass index [BMI] ≥30 kg/m2) but without T2D were randomized to once-daily subcutaneous semaglutide (dose escalated to 0.05 mg, 0.1 mg, 0.2 mg, 0.3 mg, or 0.4 mg), once-daily subcutaneous liraglutide (dose escalated to 3.0 mg), or placebo, in combination with dietary and physical activity counseling, with the primary endpoint of percentage weight loss at week 52. Estimated mean weight change was –2.3% for the placebo group and ranged from –6.0% with subcutaneous semaglutide 0.05 mg to –13.8% with subcutaneous semaglutide 0.4 mg after 52 weeks (all p ≤ 0.001). Furthermore, mean body weight reductions with semaglutide at a dose of 0.2 mg or higher were significantly greater than with liraglutide (–7.8%).
These findings paved the way for the phase III STEP (Semaglutide Treatment Effect in People with obesity) program, which is currently investigating body weight changes following treatment with once-weekly 2.4 mg subcutaneous semaglutide (80). This global clinical program has enrolled approximately 5,000 adults with overweight or obesity. The main eligibility criteria for weight in the STEP 1, 3, 4, and 5 trials were BMI ≥30 kg/m2 or BMI ≥27 kg/m2 with at least one weight-related comorbidity (hypertension, dyslipidemia, obstructive sleep apnea, or CVD), while patients in STEP 2 had to have a BMI ≥27 kg/m2 and T2D. The primary endpoint of STEP 1–5 is the change from baseline to end of treatment in body weight; the proportion of patients achieving a body weight reduction of ≥5% is a co-primary endpoint in STEP 1–3 and 5. In the completed STEP trials, semaglutide 2.4 mg as an adjunct to lifestyle intervention led to mean body weight losses of ~15–17% over 68 weeks in patients without T2D (STEP 1, 3 and 4), with a smaller mean weight loss of 9.6% seen in patients with T2D over the same period (STEP 2). At week 68, 86–89% of patients without T2D achieved ≥5% body weight loss (STEP 1, 3 and 4), with 69% of patients with T2D achieving this threshold (STEP 2). Across all studies, semaglutide 2.4 mg also demonstrated benefits beyond weight loss on cardiometabolic parameters and patient-reported outcomes (81–84).
In addition to the STEP program, the effect of semaglutide treatment on CV outcomes is being assessed in adults aged ≥45 years with overweight or obesity. The SELECT phase III trial (NCT03574597) is investigating whether once-weekly subcutaneous semaglutide (up to 2.4 mg) can reduce MACE vs. placebo in approximately 17,500 people with overweight or obesity and established CVD with a follow-up of approximately 5 years (85).
Following the phase III programs for subcutaneous and oral semaglutide, additional questions remain that are being investigated in ongoing studies. In the CVOT, SUSTAIN 6, subcutaneous semaglutide was associated with a higher risk of diabetic retinopathy complications than placebo after 2.1 years (6). Most events occurred early in the trial, and this has been suggested to be attributable to the magnitude and rapidity of the HbA1c reduction in patients with pre-existing diabetic retinopathy (86). Patients with proliferative retinopathy or maculopathy resulting in active treatment were excluded from the PIONEER 6 CVOT, in which no apparent imbalance was observed between oral semaglutide and placebo in the AE reporting of diabetic retinopathy over 16 months (66). The long-term FOCUS phase III trial (NCT03811561) is currently ongoing to specifically investigate the effects of subcutaneous semaglutide on diabetic retinopathy complications (87). Approximately 1,500 patients with T2D and Early Treatment Diabetic Retinopathy Study (ETDRS) level of 10–75 in both eyes and no ocular or intraocular treatment for diabetic retinopathy or diabetic macular edema in the 6 months prior to screening will receive once-weekly subcutaneous semaglutide 1.0 mg or placebo for up to 5 years, with the primary endpoint of progression of 3 steps or more in ETDRS level.
Subcutaneous semaglutide significantly reduced the rate of MACE vs. placebo in a post-hoc non-prespecified analysis of SUSTAIN 6, but it is unknown whether oral semaglutide can also reduce CV events (6). In PIONEER 6, oral semaglutide significantly reduced the rate of MACE and decreased all-cause mortality vs. placebo. However, while oral semaglutide was demonstrated to be noninferior to placebo in PIONEER 6, the trial was not powered to assess any potential CV benefit (66).
SOUL (NCT03914326) is an ongoing CVOT evaluating the effects of once-daily oral semaglutide (up to 14 mg) vs. placebo in 9,642 patients with T2D and CVD, cerebrovascular disease, symptomatic peripheral artery disease, or CKD (88). The primary endpoint is time to the first occurrence of MACE, with a follow-up of approximately 5 years. Secondary endpoints will explore the effects of oral semaglutide on other CV endpoints and assess any improvements in additional diabetic complications, including CKD and limb ischemia.
The benefits of GLP-1RAs are becoming increasingly recognized in international T2D recommendations and, along with other agents targeted at T2D pathophysiology, such as SGLT2is, their initiation early in the disease trajectory is advocated. The higher efficacy of semaglutide in reducing HbA1c and body weight compared with other GLP-1RAs and favorable clinical characteristics make semaglutide, either subcutaneous or oral, an advantageous choice for T2D treatment. Oral semaglutide provides an additional treatment option for patients and physicians who may be reluctant to initiate or intensify therapy by injection, and this may also help to increase earlier GLP-1RA utilization.
Where unanswered questions remain about the impact of semaglutide on outcomes, ongoing trials are underway to provide additional clarity. Effects on diabetic nephropathy and retinopathy are being assessed for subcutaneous semaglutide, and whether there are any positive CV benefits of oral semaglutide will also be determined. The management of comorbidities that are increasingly common in patients with T2D, such as obesity and liver disease, need to be better addressed; in this respect, ongoing trials will provide further information about whether the benefits of semaglutide extend to these other indications.
All authors contributed to the article and approved the submitted version.
This article was supported by Novo Nordisk, who was provided with the opportunity to perform a medical accuracy review.
FG has received research support from Eli Lilly, Lifescan, and Takeda, and has provided advisory services to AstraZeneca, Boehringer Ingelheim, Eli Lilly, Lifescan, Merck Sharp & Dohme, Novo Nordisk, Roche Diabetes Care, and Sanofi. BG has provided advisory services to AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, and Novo Nordisk, and has received lecture honoraria from Bristol Myers Squibb and the above-mentioned companies.
The authors declare that this article received funding from Novo Nordisk. The funder had the following involvement in the article: medical writing support.
Under the direction of the authors, medical writing and editorial support were provided by Andy Bond of Axis, a division of Spirit Medical Communications Group Limited (funded by Novo Nordisk).
1. Laiteerapong N, Ham SA, Gao Y, Moffet HH, Liu JY, Huang ES, et al. The Legacy Effect in Type 2 Diabetes: Impact of Early Glycemic Control on Future Complications (the Diabetes & Aging Study). Diabetes Care (2019) 42:416–26. doi: 10.2337/dc17-1144
2. Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in Treatment Intensification Increases the Risks of Cardiovascular Events in Patients With Type 2 Diabetes. Cardiovasc Diabetol (2015) 14:100. doi: 10.1186/s12933-015-0260-x
3. Romera I, Cebrián-Cuenca A, Álvarez-Guisasola F, Gomez-Peralta F, Reviriego J. A Review of Practical Issues on the Use of Glucagon-Like Peptide-1 Receptor Agonists for the Management of Type 2 Diabetes. Diabetes Ther (2019) 10:5–19. doi: 10.1007/s13300-018-0535-9
5. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med (2016) 375:311–22. doi: 10.1056/NEJMoa1603827
6. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes. N Engl J Med (2016) 375:1834–44. doi: 10.1056/NEJMoa1607141
7. Hernandez AF, Green JB, Janmohamed S, D’Agostino RB Sr, Granger CB, Jones NP, et al. Harmony Outcomes Committees and Investigators. Albiglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes and Cardiovascular Disease (Harmony Outcomes): A Double-Blind, Randomised Placebo-Controlled Trial. Lancet (2018) 392:1519–29. doi: 10.1016/S0140-6736(18)32261-X
8. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Rewind Investigators. Dulaglutide and Cardiovascular Outcomes in Type 2 Diabetes REWIND: A Double-Blind, Randomised Placebo-Controlled Trial. Lancet (2019) 394:121–30. doi: 10.1016/S0140-6736(19)31149-3
9. Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care (2018) 41:2669–701. doi: 10.2337/dci18-0033
10. Davies MJ, Bianchi C, Del Prato S. Use of Incretin-Based Medications: What do Current International Recommendations Suggest With Respect to GLP-1 Receptor Agonists and DPP-4 Inhibitors? Metabolism (2020) 107:154242. doi: 10.1016/j.metabol.2020.154242
11. Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care (2020) 43:487–93. doi: 10.2337/dci19-0066
12. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med (2015) 373:2117–28. doi: 10.1056/NEJMoa1504720
13. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med (2017) 377:644–57. doi: 10.1056/NEJMoa1611925
14. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. ESC Scientific Document Group. 2019 ESC Guidelines on Diabetes, Pre-Diabetes, and Cardiovascular Diseases Developed in Collaboration With the EASD. Eur Heart J (2020) 41:255–323. doi: 10.1093/eurheartj/ehz486
15. Castellana M, Cignarelli A, Brescia F, Perrini S, Natalicchio A, Laviola L, et al. Efficacy and Safety of GLP-1 Receptor Agonists as Add-on to SGLT2 Inhibitors in Type 2 Diabetes Mellitus: A Meta-Analysis. Sci Rep (2019) 9:19351. doi: 10.1038/s41598-019-55524-w
16. Bang C, Mortensen MB, Lauridsen KG, Bruun JM. Trends in Antidiabetic Drug Utilization and Expenditure in Denmark: A 22-Year Nationwide Study. Diabetes Obes Metab (2020) 22:167–72. doi: 10.1111/dom.13877
17. Fadini GP, Frison V, Rigato M, Morieri ML, Simioni N, Tadiotto F, et al. Trend 2010-2018 in the Clinical Use of GLP-1 Receptor Agonists for the Treatment of Type 2 Diabetes in Routine Clinical Practice: An Observational Study From Northeast Italy. Acta Diabetol (2020) 57:367–75. doi: 10.1007/s00592-019-01445-z
18. Boye KS, Stein D, Matza LS, Jordan J, Yu R, Norrbacka K, et al. Timing of GLP-1 Receptor Agonist Initiation for Treatment of Type 2 Diabetes in the UK. Drugs R D (2019) 19:213–25. doi: 10.1007/s40268-019-0273-0
19. Matza LS, Curtis SE, Jordan JB, Adetunji O, Martin SA, Boye KS. Physician Perceptions of GLP-1 Receptor Agonists in the UK. Curr Med Res Opin (2016) 32:857–64. doi: 10.1185/03007995.2016.1147025
20. Byetta® Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/byetta-epar-product-information_en.pdf (Accessed 2 June 2020).
21. Lyxumia® Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/lyxumia-epar-product-information_en.pdf (Accessed 2 June 2020).
22. Victoza® Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/victoza-epar-product-information_en.pdf (Accessed 2 June 2020).
23. Bydureon® Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/bydureon-epar-product-information_en.pdf (Accessed 2 June 2020).
24. Trulicity® Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/trulicity-epar-product-information_en.pdf (Accessed 2 June 2020).
25. Ozempic® Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/glossary/summary-product-characteristics (Accessed 2 June 2020).
26. Rybelsus® Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/rybelsus-epar-product-information_en.pdf (Accessed 19 August 2020).
27. Caruso I, Cignarelli A, Giorgino F. Heterogeneity and Similarities in GLP-1 Receptor Agonist Cardiovascular Outcomes Trials. Trends Endocrinol Metab (2019) 30:578–89. doi: 10.1016/j.tem.2019.07.004
28. Nauck MA, Meier JJ. Management of Endocrine Disease: Are All GLP-1 Agonists Equal in the Treatment of Type 2 Diabetes? Eur J Endocrinol (2019) 181:R211–34. doi: 10.1530/EJE-19-0566
29. Qiao Q, Ouwens MJ, Grandy S, Johnsson K, Kostev K. Adherence to GLP-1 Receptor Agonist Therapy Administered by Once-Daily or Once-Weekly Injection in Patients With Type 2 Diabetes in Germany. Diabetes Metab Syndr Obes (2016) 9:201–5. doi: 10.2147/DMSO.S99732
30. Lau J, Bloch P, Schäffer L, Pettersson I, Spetzler J, Kofoed J, et al. Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. J Med Chem (2015) 58:7370–80. doi: 10.1021/acs.jmedchem.5b00726
31. Aroda VR, Ahmann A, Cariou B, Chow F, Davies MJ, Jódar E, et al. Comparative Efficacy, Safety, and Cardiovascular Outcomes With Once-Weekly Subcutaneous Semaglutide in the Treatment of Type 2 Diabetes: Insights From the SUSTAIN 1-7 Trials. Diabetes Metab (2019) 45:409–18. doi: 10.1016/j.diabet.2018.12.001
33. Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, et al. Efficacy and Safety of Once-Weekly Semaglutide Versus Exenatide ER in Subjects With Type 2 Diabetes (SUSTAIN 3): A 56-Week, Open-Label, Randomized Clinical Trial. Diabetes Care (2018) 41:258–66. doi: 10.2337/dc17-0417
34. Pratley RE, Aroda VR, Lingvay I, Lüdemann J, Andreassen C, Navarria A, et al. SUSTAIN 7 Investigators. Semaglutide Versus Dulaglutide Once Weekly in Patients With Type 2 Diabetes (SUSTAIN 7): A Randomised, Open-Label, Phase 3b Trial. Lancet Diabetes Endocrinol (2018) 6:275–86. doi: 10.1016/S2213-8587(18)30024-X
35. ClinicalTrials.gov NCT03457012. A Research Study Looking at How Semaglutide Works in People With Type 2 Diabetes in Canada, as Part of Local Clinical Practice (SURE CANADA). Available at: https://clinicaltrials.gov/ct2/show/NCT03457012 (Accessed 24 June 2020).
36. ClinicalTrials.gov NCT03631186. A Research Study Looking at How Semaglutide Works in People With Type 2 Diabetes in Switzerland, as Part of Local Clinical Practice (SURE SWITZERLAND). Available at: https://clinicaltrials.gov/ct2/show/NCT03631186 (Accessed 24 June 2020).
37. ClinicalTrials.gov NCT03648281. A Research Study Looking at How Semaglutide Works in People With Type 2 Diabetes in Denmark and Sweden, as Part of Local Clinical Practice (SURE DENMARK/SWEDEN). Available at: https://clinicaltrials.gov/ct2/show/NCT03648281 (Accessed 24 June 2020).
38. ClinicalTrials.gov NCT03876015. A Research Study Looking at How Semaglutide Works in People With Type 2 Diabetes in United Kingdom, as Part of Local Clinical Practice (SURE UK). Available at: https://clinicaltrials.gov/ct2/show/NCT03876015 (Accessed 24 June 2020).
39. ClinicalTrials.gov NCT03929679. A Research Study Looking at How Semaglutide Works in People With Type 2 Diabetes in The Netherlands, as Part of Local Clinical Practice (SURE NETHERLANDS). Available at: https://clinicaltrials.gov/ct2/show/NCT03929679 (Accessed 24 June 2020).
40. ClinicalTrials.gov NCT04067999. A Research Study Looking at How Semaglutide Works in People With Type 2 Diabetes in Spain, as Part of Local Clinical Practice (SURE SPAIN). Available at: https://clinicaltrials.gov/ct2/show/NCT04067999 (Accessed 24 June 2020).
41. ClinicalTrials.gov NCT04083820. A Research Study Looking at How Semaglutide Works in People With Type 2 Diabetes in France, as Part of Local Clinical Practice (SURE FRANCE). Available at: https://clinicaltrials.gov/ct2/show/NCT04083820 (Accessed 24 June 2020).
42. ClinicalTrials.gov NCT04094415. A Research Study Looking at How Semaglutide Works in People With Type 2 Diabetes in Italy, as Part of Local Clinical Practice (SURE ITALY). Available at: https://clinicaltrials.gov/ct2/show/NCT04094415 (Accessed 24 June 2020).
43. ClinicalTrials.gov NCT04261933. A Research Study Looking at How Semaglutide Works in People With Type 2 Diabetes in Germany, as Part of Local Clinical Practice (SURE GERMANY). Available at: https://clinicaltrials.gov/ct2/show/NCT04261933 (Accessed 24 June 2020).
44. American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2020. Diabetes Care (2020) 43(suppl 1):S98–110. doi: 10.2337/dc20-S009
45. Polonsky WH, Henry RR. Poor Medication Adherence in Type 2 Diabetes: Recognizing the Scope of the Problem and its Key Contributors. Patient Prefer Adherence (2016) 10:1299–307. doi: 10.2147/PPA.S106821
46. Giorgino F, Penfornis A, Pechtner V, Gentilella R, Corcos A. Adherence to Antihyperglycemic Medications and Glucagon-Like Peptide 1-Receptor Agonists in Type 2 Diabetes: Clinical Consequences and Strategies for Improvement. Patient Prefer Adherence (2018) 12:707–19. doi: 10.2147/PPA.S151736
47. Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and Safety of Glucagon-Like Peptide-1 Receptor Agonists in Type 2 Diabetes: A Systematic Review and Mixed-Treatment Comparison Analysis. Diabetes Obes Metab (2017) 19:524–36. doi: 10.1111/dom.12849
48. Hinnen D. Glucagon-Like Peptide 1 Receptor Agonists for Type 2 Diabetes. Diabetes Spectr (2017) 30:202–10. doi: 10.2337/ds16-0026
49. Gomez-Peralta F, Abreu C. Profile of Semaglutide in the Management of Type 2 Diabetes: Design, Development, and Place in Therapy. Drug Des Devel Ther (2019) 13:731–8. doi: 10.2147/DDDT.S165372
50. Reid TS. Practical Use of Glucagon-Like Peptide-1 Receptor Agonist Therapy in Primary Care. Clin Diabetes (2013) 31:148–57. doi: 10.2337/diaclin.31.4.148
51. Bækdal TA, Borregaard J, Donsmark M, Breitschaft A, Søndergaard FL. Evaluation of the Effects of Water Volume With Dosing and Post-Dose Fasting Period on Pharmacokinetics of Oral Semaglutide. Diabetes (2017) 66(suppl 1):A315 (abstract 1179–P).
52. Buckley ST, Bækdal TA, Vegge A, Maarbjerg SJ, Pyke C, Ahnfelt-Rønne J, et al. Transcellular Stomach Absorption of a Derivatized Glucagon-Like Peptide-1 Receptor Agonist. Sci Transl Med (2018) 10(467):eaar7047. doi: 10.1126/scitranslmed.aar7047
53. Bækdal TA, Albayaty M, Maniigandan E, Anderson TW, Skibsted S. A Trial to Investigate the Effect of Oral Semaglutide on the Pharmacokinetics of Furosemide and Rosuvastatin in Healthy Subjects. Diabetologia (2018) 61(Suppl 1):S1–620 (abstract 714).
54. Bækdal TA, Borregaard J, Hansen CW, Thomsen M, Anderson TW. Effect of Oral Semaglutide on the Pharmacokinetics of Lisinopril, Warfarin, Digoxin, and Metformin in Healthy Subjects. Clin Pharmacokinet (2019) 58:1193–203. doi: 10.1007/s40262-019-00756-2
55. Hausner H, Derving Karsbøl J, Holst AG, Jacobsen JB, Wagner FD, Golor G, et al. Effect of Semaglutide on the Pharmacokinetics of Metformin, Warfarin, Atorvastatin and Digoxin in Healthy Subjects. Clin Pharmacokinet (2017) 56:1391–401. doi: 10.1007/s40262-017-0532-6
56. Kapitza C, Nosek L, Jensen L, Hartvig H, Jensen CB, Flint A. Semaglutide, a Once-Weekly Human GLP-1 Analog, Does Not Reduce the Bioavailability of the Combined Oral Contraceptive, Ethinylestradiol/Levonorgestrel. J Clin Pharmacol (2015) 55:497–504. doi: 10.1002/jcph.443
57. Jordy A, Breitschaft A, Christiansen E, Granhall C, Hansen CW, Houshmand-Øregaard A, et al. Oral Semaglutide Does Not Affect the Bioavailability of the Combined Oral Contraceptive, Ethinylestradiol/Levonorgestrel. Diabetologia (2018) 61(Suppl 1):S1–620 (abstract 713). doi: 10.2337/db18-1135-P
58. Bækdal TA, Breitschaft A, Navarria A, Hansen CW. A Randomized Study Investigating the Effect of Omeprazole on the Pharmacokinetics of Oral Semaglutide. Expert Opin Drug Metab Toxicol (2018) 14:869–77. doi: 10.1080/17425255.2018.1488965
59. Hauge C, Breitschaft A, Hartoft-Nielsen ML, Jensen S, Baekdal T. A Drug-Drug Interaction Trial of Oral Semaglutide With Levothyroxine and Multiple Coadministered Tablets. J Endo Soc (2019) 3(Suppl 1):SAT–140. doi: 10.1210/js.2019-SAT-140
60. Rybelsus® Prescribing Information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209637s003lbl.pdf (Accessed 2 June 2020).
61. Overgaard RV, Navarria A, Hertz CL, Ingwersen SH. Similar Efficacy and Gastrointestinal Tolerability Versus Exposure for Oral and Subcutaneous Semaglutide, in: Abstract 777 presented at the 55th Annual Meeting of the European Association for the Study of Diabetes, September 17–20, 2019. Barcelona, Spain.
62. Górriz JL, Soler MJ, Navarro-González JF, García-Carro C, Puchades MJ, D’Marco L, et al. GLP-1 Receptor Agonists and Diabetic Kidney Disease: A Call of Attention to Nephrologists. J Clin Med (2020) 9(4):pii: E947. doi: 10.3390/jcm9040947
63. Granhall C, Søndergaard FL, Thomsen M, Anderson TW. Pharmacokinetics, Safety and Tolerability of Oral Semaglutide in Subjects With Renal Impairment. Clin Pharmacokinet (2018) 57:1571–80. doi: 10.1007/s40262-018-0649-2
64. Marbury TC, Flint A, Jacobsen JB, Derving Karsbøl J, Lasseter K. Pharmacokinetics and Tolerability of a Single Dose of Semaglutide, a Human Glucagon-Like Peptide-1 Analog, in Subjects With and Without Renal Impairment. Clin Pharmacokinet (2017) 56:1381–90. doi: 10.1007/s40262-017-0528-2
65. Mosenzon O, Blicher TM, Rosenlund S, Eriksson JW, Heller S, Hels OH, et al. PIONEER 5 Investigators. Efficacy and Safety of Oral Semaglutide in Patients With Type 2 Diabetes and Moderate Renal Impairment (PIONEER 5): A Placebo-Controlled, Randomised, Phase 3a Trial. Lancet Diabetes Endocrinol (2019) 7:515–27. doi: 10.2337/db19-1004-P
66. Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. PIONEER 6 Investigators. Oral Semaglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes. N Engl J Med (2019) 381:841–51. doi: 10.1056/NEJMoa1901118
67. ClinicalTrials.gov NCT03819153. A Research Study to See How Semaglutide Works Compared to Placebo in People With Type 2 Diabetes and Chronic Kidney Disease (FLOW). Available at: https://clinicaltrials.gov/ct2/show/NCT03819153 (Accessed 3 June 2020).
68. Cusi K. A Diabetologist’s Perspective of non-Alcoholic Steatohepatitis (NASH): Knowledge Gaps and Future Directions. Liver Int (2020) 40 Suppl 1:82–8. doi: 10.1111/liv.14350
69. Ke Y, Xu C, Lin J, Li Y. Role of Hepatokines in Non-alcoholic Fatty Liver Disease. J Transl Int Med (2019) 7:143–8. doi: 10.2478/jtim-2019-0029
70. Bækdal TA, Thomsen M, Kupčová V, Hansen CW, Anderson TW. Pharmacokinetics, Safety, and Tolerability of Oral Semaglutide in Subjects With Hepatic Impairment. J Clin Pharmacol (2018) 58:1314–23. doi: 10.1002/jcph.1131
71. Jensen L, Kupcova V, Arold G, Pettersson J, Hjerpsted JB. Pharmacokinetics and Tolerability of Semaglutide in People With Hepatic Impairment. Diabetes Obes Metab (2018) 20:998–1005. doi: 10.1111/dom.13186
72. Seghieri M, Christensen AS, Andersen A, Solini A, Knop FK, Vilsbøll T. Future Perspectives on GLP-1 Receptor Agonists and GLP-1/Glucagon Receptor Co-agonists in the Treatment of NAFLD. Front Endocrinol (Lausanne) (2018) 9:649. doi: 10.3389/fendo.2018.00649
73. Zhou JY, Poudel A, Welchko R, Mekala N, Chandramani-Shivalingappa P, Rosca MG, et al. Liraglutide Improves Insulin Sensitivity in High Fat Diet Induced Diabetic Mice Through Multiple Pathways. Eur J Pharmacol (2019) 861:172594. doi: 10.1016/j.ejphar.2019.172594
74. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide Safety and Efficacy in Patients With Non-Alcoholic Steatohepatitis (LEAN): A Multicentre, Double-Blind, Randomised, Placebo-Controlled Phase 2 Study. Lancet (2016) 387:679–90. doi: 10.1016/S0140-6736(15)00803-X
75. Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med (2021) 384(12):1113–24. doi: 10.1056/NEJMoa2028395
76. Somm E, Montandon SA, Loizides-Mangold U, Gaïa N, Lazarevic V, De Vito C, et al. The GLP-1R Agonist Liraglutide Limits Hepatic Lipotoxicity and Inflammatory Response in Mice Fed a Methionine-Choline Deficient Diet. Transl Res (2021) 227:75–88. doi: 10.1016/j.trsl.2020.07.008
77. Blundell J, Finlayson G, Axelsen M, Flint A, Gibbons C, Kvist T, et al. Effects of Once-Weekly Semaglutide on Appetite, Energy Intake, Control of Eating, Food Preference and Body Weight in Subjects With Obesity. Diabetes Obes Metab (2017) 19:1242–51. doi: 10.1111/dom.12932
78. Gibbons C, Blundell J, Tetens Hoff S, Dahl K, Bauer R, Baekdal T. Effects of Oral Semaglutide on Energy Intake, Food Preference, Appetite, Control of Eating and Body Weight in Subjects With Type 2 Diabetes. Diabetes Obes Metab (2021) 23:581–8. doi: 10.1111/dom.14255
79. O’Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S, et al. Efficacy and Safety of Semaglutide Compared With Liraglutide and Placebo for Weight Loss in Patients With Obesity: A Randomised, Double-Blind, Placebo and Active Controlled, Dose-Ranging, Phase 2 Trial. Lancet (2018) 392:637–49. doi: 10.1016/S0140-6736(18)31773-2
80. Kushner RF, Calanna S, Davies M, Dicker D, Garvey WT, Goldman B, et al. Semaglutide 2.4 Mg for the Treatment of Obesity: Key Elements of the STEP Trials 1 to 5. Obesity (Silver Spring) (2020) 28:1050–61. doi: 10.1002/oby.22794
81. Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2·4 Mg Once a Week in Adults With Overweight or Obesity, and Type 2 Diabetes (STEP 2): A Randomised, Double-Blind, Double-Dummy, Placebo-Controlled, Phase 3 Trial. Lancet (2021) 397(10278):971–84. doi: 10.1016/S0140-6736(21)00213-0
82. Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, et al. For the STEP 4 Study Group. Effect of Continued Once-Weekly Semaglutide 2.4 Mg on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Maintenance Trial. JAMA (2021) 325(14):1414–25. doi: 10.1001/jama.2021.3224
83. Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, et al. For the STEP 3 Investigators. Effect on Body Weight of Semaglutide 2.4 Mg Versus Placebo as Adjunct to Intensive Behavioral Therapy in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA (2021) 325(14):1403–13. doi: 10.1001/jama.2021.1831
84. Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-Weekly Semaglutide in Adults With Overweight or Obesity. N Engl J Med (2021) 384(11):989. doi: 10.1056/NEJMoa2032183
85. ClinicalTrials.gov NCT03574597. Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity (SELECT). Available at: https://clinicaltrials.gov/ct2/show/NCT03574597 (Accessed 2 June 2020).
86. Vilsbøll T, Bain SC, Leiter LA, Lingvay I, Matthews D, Simó R, et al. Semaglutide, Reduction in Glycated Haemoglobin and the Risk of Diabetic Retinopathy. Diabetes Obes Metab (2018) 20:889–97. doi: 10.1111/dom.13172
87. ClinicalTrials.gov NCT03811561. A Research Study to Look at How Semaglutide Compared to Placebo Affects Diabetic Eye Disease in People With Type 2 Diabetes (FOCUS). Available at: https://clinicaltrials.gov/ct2/show/NCT03811561 (Accessed 2 June 2020).
88. ClinicalTrials.gov NCT03914326. A Heart Disease Study of Semaglutide in Patients With Type 2 Diabetes (SOUL). Available at: https://clinicaltrials.gov/ct2/show/NCT03914326 (Accessed 2 June 2020).
Keywords: glucagon-like peptide-1 receptor agonist (GLP-1RA), oral, subcutaneous, semaglutide, type 2 diabetes
Citation: Gallwitz B and Giorgino F (2021) Clinical Perspectives on the Use of Subcutaneous and Oral Formulations of Semaglutide. Front. Endocrinol. 12:645507. doi: 10.3389/fendo.2021.645507
Received: 23 December 2020; Accepted: 03 June 2021;
Published: 29 June 2021.
Edited by:
Erwin Dieter Schleicher, University of Tübingen, GermanyReviewed by:
Michael Nauck, Katholisches Klinikum Bochum, GermanyCopyright © 2021 Gallwitz and Giorgino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baptist Gallwitz, QmFwdGlzdC5nYWxsd2l0ekBtZWQudW5pLXR1ZWJpbmdlbi5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.