- 1Department of Nuclear Medicine, National Taiwan University Hospital, Taipei, Taiwan
- 2Institute of Epidemiology and Preventive Medicine, College of Public Health, National Taiwan University, Taipei, Taiwan
- 3Division of Nephrology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
- 4Glickman Urological and Kidney Institute, Cleveland Clinic Lerner College of Medicine, Cleveland Clinic, Cleveland, OH, United States
- 5Department of Public Health, College of Public Health, National Taiwan University, Taipei, Taiwan
Purpose: Somatic KCNJ5 mutation occurs in half of unilateral primary aldosteronism (PA) and is associated with more severe phenotype. Mutation status can only be identified by tissue sample from adrenalectomy. NP-59 adrenal scintigraphy is a noninvasive functional study for disease activity assessment. This study aimed to evaluate the predictive value of NP-59 adrenal scintigraphy in somatic KCNJ5 mutation among PA patients who received adrenalectomy.
Methods: Sixty-two PA patients who had NP-59 adrenal scintigraphy before adrenalectomy with available KCNJ5 mutation status were included. Two semiquantitative parameters, adrenal to liver ratio (ALR) and lesion to contralateral ratio of bilateral adrenal glands (CON) derived from NP-59 adrenal scintigraphy, of mutated and wild-type patients were compared. Cutoff values calculated by receiver-operating characteristic (ROC) analysis were used as a predictor of KCNJ5 mutation.
Results: Twenty patients had KCNJ5 mutation and 42 patients were wild type. Patients harboring KCNJ5 mutation had both higher ALR and CON (p = 0.0031 and 0.0833, respectively) than wild-type patients. With ALR and CON cutoff of 2.10 and 1.95, the sensitivity and specificity to predict KCNJ5 mutation were 85%, 57% and 45%, 93%, respectively. Among 20 patients with KCNJ5 mutation, 16 showed G151R point mutation (KCNJ5- G151R) and 4 showed L168R point mutation (KCNJ5-L168R), which former one had significantly lower ALR (p=0.0471).
Conclusion: PA patients harboring somatic KCNJ5 mutation had significantly higher NP-59 uptake regarding to ALR and CON than those without mutation. APAs with KCNJ5-L168R point mutation showed significantly higher ALR than those with KCNJ5-G151R point mutation.
Introduction
Primary aldosteronism (PA) is the most common cause of secondary hypertension which is characterized by overproduction of aldosterone, leading to hypertension and sometimes hypokalemia (1). There are three concerns making PA an important issue. First, the prevalence is underestimated. Due to highly heterogeneity of each study, lack of uniform screening tests and cutoff values, it is difficult to conclude a definite prevalence. Roughly 10% is a commonly acceptable prevalence among hypertensive populations (1). However, prevalence of 30% has been reported, which may occur in severe hypertensive populations at tertiary referral center (2). Second, PA patients had significantly higher risk of cardiovascular events, diabetes and metabolic syndrome as compared with patients with essential hypertension (3). Third, PA is controllable and even curable through proper treatment, by means of mineralocorticoid antagonist for bilateral disease and adrenalectomy for unilateral disease (4). John W Funder has stated that PA is a public health issue on the basis of prior mentioned concerns and underlying great impact on medical care resources (5).
The major advance of understanding PA pathophysiology is the identification of KCNJ5 mutation in aldosterone-producing adenomas (APAs) (6). Mutated KCNJ5 leads to persistent cell depolarization turning out to aldosterone overproduction. Approximately 40% APAs harbored KCNJ5 mutation, while eastern countries had much lower mutation rate than Asian countries. KCNJ5 mutation is also associated with clinical phenotype. Younger age, higher plasma aldosterone, larger tumor, and female were more commonly seen with mutation (7). However, mutation status is only available by surgically resected specimen, which the mutation rate among PA patients will not be truly revealed.
NP-59 adrenal scintigraphy is a molecular imaging evaluating adrenal cortical function based on the activity of cholesterol uptake and transfer. It is able to correctly differentiate unilateral disease from bilateral disease and with excellent predictive value of postsurgical outcome (8, 9). Although adrenal venous sampling (AVS) is currently the gold standard for lateralization, NP-59 adrenal scintigraphy is an alternative method since AVS is technically dependent and invasive. Considering that more severe disease brings higher cholesterol demand to produce aldosterone, and KCNJ5 mutation is associated with higher plasma aldosterone, we assume that NP-59 adrenal scintigraphy may be an imaging biomarker to predict KCNJ5 mutation.
Materials and Methods
Patients
The study protocol was approved by the Institutional Review Board of National Taiwan University Hospital (approval No. 201002002R and 200912003R). Patients were retrospectively recruited from the Taiwan Primary Aldosteronism Investigation (TAIPAI) database with the following inclusion criteria: (1) clinically confirmed PA by either saline loading test or captopril test (a positive saline loading test is defined as post-test PAS higher than 10 ng/dl and a positive captopril test is defined as PAC suppression less than 30% of the baseline level concurrent with suppressed PRA or ARR > 35 ng/dl per ng/ml/h), (2) had NP-59 adrenal scintigraphy with single photon emission computed tomography (SPECT) and computed tomography (CT) before surgery, (3) underwent adrenalectomy within 1 year after NP-59 scintigraphy, and (4) available KCNJ5 mutation status from surgical specimen. The only exclusion criteria was known malignancy with adrenal gland involvement. Adrenalectomy was determined by a successful non-stimulated AVS (which is defined as a selective index greater than 2) with a lateralization index than 2. Clinical and biochemical profiles were acquired at initial evaluation and 1 year after adrenalectomy.
Protocol and Interpretation of NP-59 Adrenal Scintigraphy
Medications may alter NP-59 uptake such as glucocorticoids, diuretics, spironolactone, beta-blockers, alpha-blockers, and calcium channel blockers were postponed or switched to alternative medications (10). Oral dexamethasone suppression (8 mg daily) was carried out throughout the study for 8 days. One mCi NP-59 was slowly injected intravenously on the 4th day, and SPECT/CT was performed on the 96th hour after NP-59 injection. Patients were also given 1 ml of diluted Lugol’s solution daily to protect the thyroid from free 131I uptake.
Two semiquantitative parameters were used to evaluated adrenal cortical function as previously reported (9). Maximal count of the adrenal gland with lesion (defined as adrenal gland having adrenalectomy) divided by the mean count of the liver resulted in adrenal to liver ratio (ALR). Maximal count of adrenal gland with lesion to maximal count of contralateral adrenal gland is defined as CON.
KCNJ5 Sequencing
The specimens of APAs after adrenalectomy were collected and stored at −72°C until analysis. Genomic DNA were isolated from APAs using the QIAamp DNA mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The DNA regions containing the most frequently occurred point mutations of KCNJ5, p.Gly151Arg (G151R) and p.Leu168Arg (L168R), were amplified and sequenced using gene-specific primers (forward 5′-CTTCATTTGGTGGCTCATTGC-′3, reverse 5′-GGGACTTGATGAGCTTGGC-′3) as previously reported (11). The annealing temperature was 58°C. Direct sequencing of PCR products was performed using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, USA) with a 3730 DNA Analyzer (Applied Biosystems, Foster City, USA). Sequences were analyzed using DNAStar Lasergene SeqMan Pro 7.1.0 software. Standard protocol of sequencing in TAIPAI followed that which had been previously reported (12).
Statistical Analysis
Descriptive statistics were used for patients’ characteristics. Continues data were expressed as median with 25th percentile and 75th percentile. Mann-Whitney U test was used for data comparison. Difference of ALR and CON between mutated and wild-type patients were compared by Mann-Whitney U test. Receiver-operating characteristic (ROC) curves were plotted and areas under the curve (AUC) were calculated for ALR and CON. The sensitivity and specificity of ALR and CON to predict KCNJ5 mutation were calculated according to the optimal cutoff value selected by Youden’s index. A p value of less than 0.05 was deemed statistically significant. All statistical analyses were performed using MedCalc Statistical Software version 17.9.2 (MedCalc Software bvba, Ostend, Belgium).
Results
Patient Characteristics
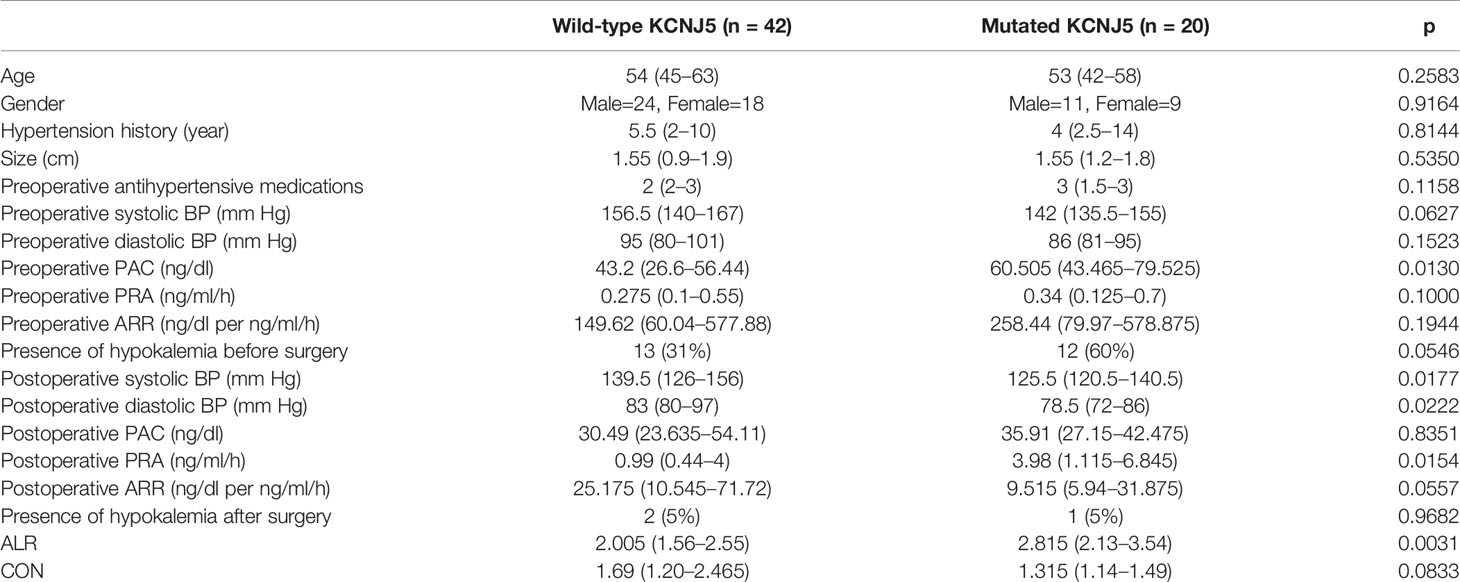
From October 2007 to October 2018, 64 patients were enrolled in this study. Two patients were excluded due to insufficient imaging information (one with missing data and the other with only SPECT imaging). Finally, 62 patients were enrolled for analysis, including 35 male (56%) and 27 female (44%), with a median age of 53 years (45–63). All patients received unilateral adrenalectomy and the median time interval between NP-59 adrenal scintigraphy and adrenalectomy was 134 days (75–249). The median ALR was 2.205 (1.69–2.98) and CON was 1.325 (1.14–1.83). The patients’ characteristics were listed in Table 1.
Correlation Between Clinical/Biochemical Profiles and KCNJ5 Mutation
Mutated patients had significantly higher preoperative plasma aldosterone concentration (PAC, 60.505 ng/dl [43.465–79.525] versus 43.2 ng/dl [26.6–56.44], p = 0.0130) and borderline higher portion of presence of hypokalemia before surgery (60% versus 13%, p = 0.0546). There were no differences regarding to age, gender, hypertension history, tumor size, preoperative antihypertensive medications, preoperative systolic blood pressure (BP), preoperative diastolic BP, preoperative plasma renin activity (PRA), and preoperative aldosterone to renin ratio (ARR).
There were significant differences regarding to postoperative clinical and biochemical profiles between mutated and wild-type patients. Significant lower postoperative systolic BP (125.5 mmHg [120.5–140.5] versus 139.5 mmHg [126–156], p = 0.02), diastolic BP (78.5 mmHg [72–86] versus 83 mmHg [80–97], p = 0.02), higher PRA (3.98 ng/ml/h [1.115–6.8456] versus 0.99 ng/ml/h [0.44–4], p = 0.02) and borderline lower ARR (9.515 ng/dl per ng/ml/h [5.94–31.875] versus 25.175 ng/dl per ng/ml/h [10.545–71.72], p = 0.06) were noted in mutated patients. Normalization of hypokalemia was seen in most patients except one mutated and two wild-type patients.
Correlation Between NP-59 Adrenal Scintigraphy and KCNJ5 Mutation
ALR were significantly higher in mutated patients than in wild-type patients (2.815 [2.13–3.54] versus 2.005 [1.56–2.55], p = 0.0031; Figure 1), while CON showed borderline higher in mutated patients (1.315 [1.14–1.49] versus 1.69 [1.20–2.456], p = 0.0833; Figure 1). ROC analysis showed that an ALR cutoff of 2.10 and a CON cutoff of 1.95 were the best values to predict KCNJ5 mutation. By ALR cutoff of 2.10, the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 85%, 57%, 49%, 89% and 66%, respectively. By CON cutoff of 1.95, the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 45%, 93%, 75%, 78%, and 77%, respectively. Among 12 patients with both ALR greater than 2.1 and CON greater than 1.95, 10 harbored KCNJ5 mutation (83%). Twenty-four of 27 patients (89%) having ALR less than 2.10 and CON less than 1.95 had wild-type KCNJ5. For patients with CON greater than 1.95, ALR was greater than 2.10 without exception. Representative SPECT/CT images of two patients with and without KCNJ5 mutations were shown in Figure 2.
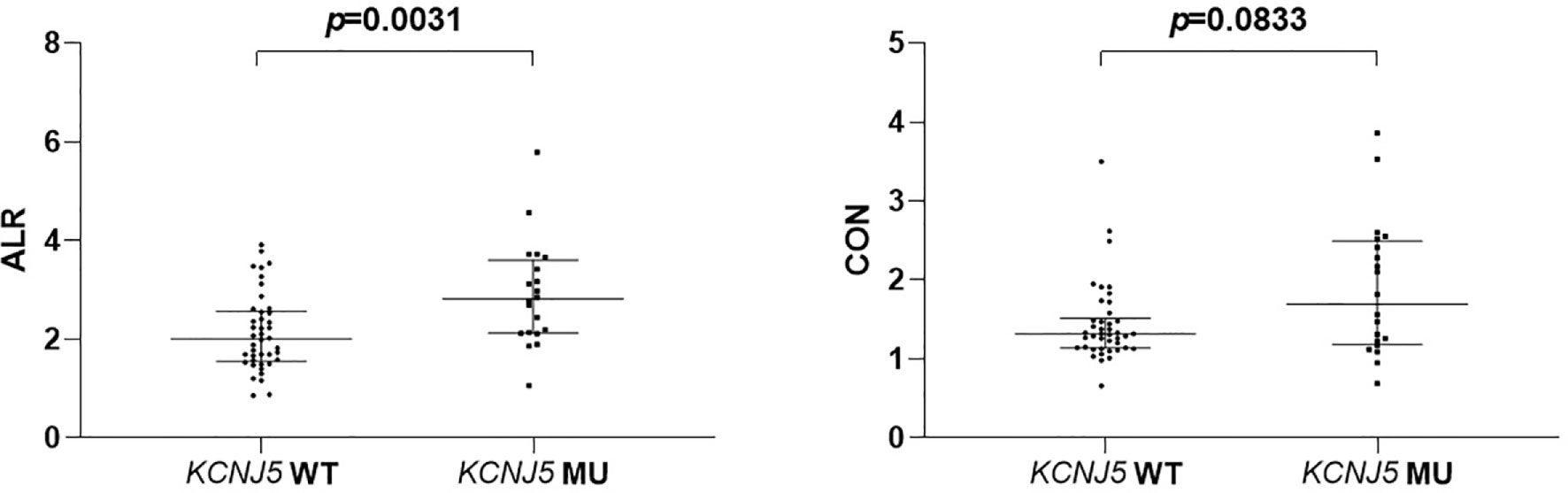
Figure 1 Comparison of semiquantitative parameters of NP-59 adrenal scintigraphy according to KCNJ5 mutation status.
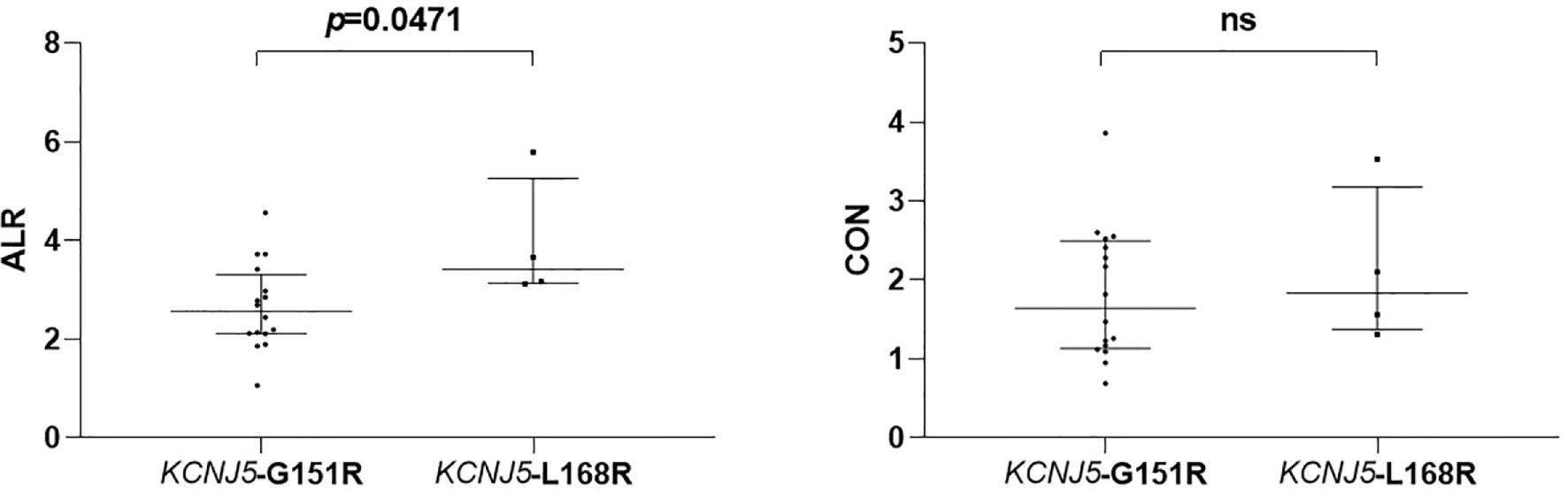
Figure 2 Comparison of semiquantitative parameters of NP-59 adrenal scintigraphy according to different point mutations of KCNJ5. ns, non-significant.
G151R and L168R Point Mutations
Among 20 patients harboring KCNJ5 mutation, 16 of them had G151R point mutation and 4 of them had L168R point mutation. There were no differences regarding to age, gender, hypertension history, tumor size, preoperative antihypertensive medications, preoperative systolic BP, preoperative diastolic BP, preoperative PAC, preoperative PRA and preoperative ARR. Postoperative clinical and biochemical profiles were similar except borderline lower PAC in patients with KCNJ5-L168R point mutation (20.05 ng/dl [12.24–32.15] versus 37.27 ng/dl [31.75–42.72], p = 0.07). ALR of KCNJ5-G151R was significantly lower than of KCNJ5-L168R point mutation (2.565 [2.115–3.20] versus 3.415 [3.145–4.725], p = 0.0471; Figure 3). There was no difference between these point mutations regarding to CON (1.645 [1.145–2.465] versus 1.83 [1.435–2.815], p = 0.5536; Figure 3). ROC analysis showed that with cutoff of 2.98, ALR had best ability to differentiate KCNJ5-G151R from KCNJ5-L168R point mutation. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 75%, 100%, 100%, 50%, 80%, respectively. The patients’ characteristics were listed in Table 2.
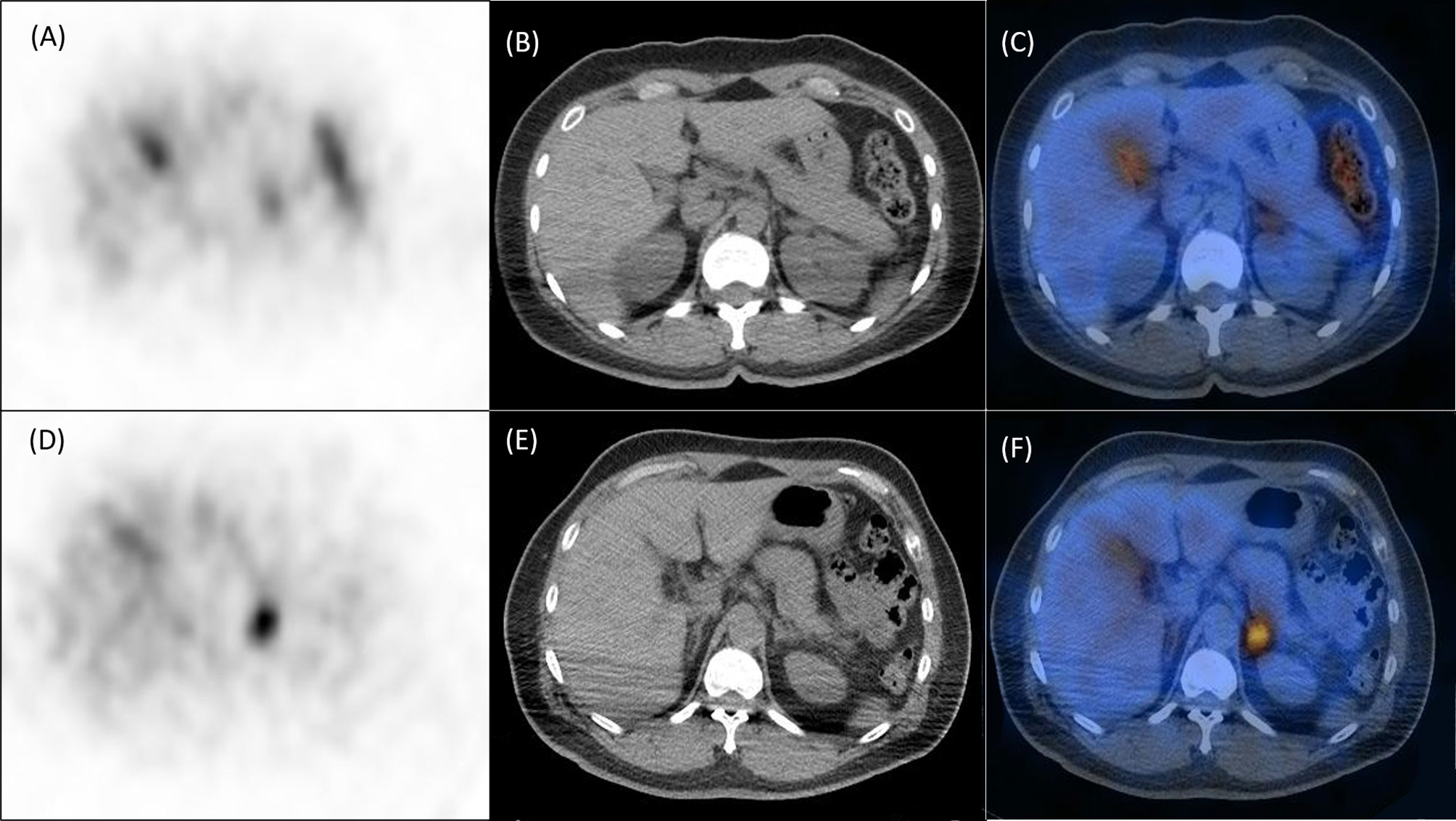
Figure 3 Representative NP-59 adrenal SPECT/CT and KCNJ5 mutation status. Upper panel, findings of a 40-year-old female without KCNJ5 mutation. SPECT (A), CT (B) and fusion SPECT/CT (C) showed moderate NP-59 uptake at left adrenal gland with ALR of 2.62 and CON of 1.38. Lower panel, findings of a 54-year-old male with KCNJ5 mutation. SPECT (D), CT (E) and fusion SPECT/CT (F) showed significant NP-59 uptake at left adrenal gland with ALR of 3.72 and CON of 2.41.
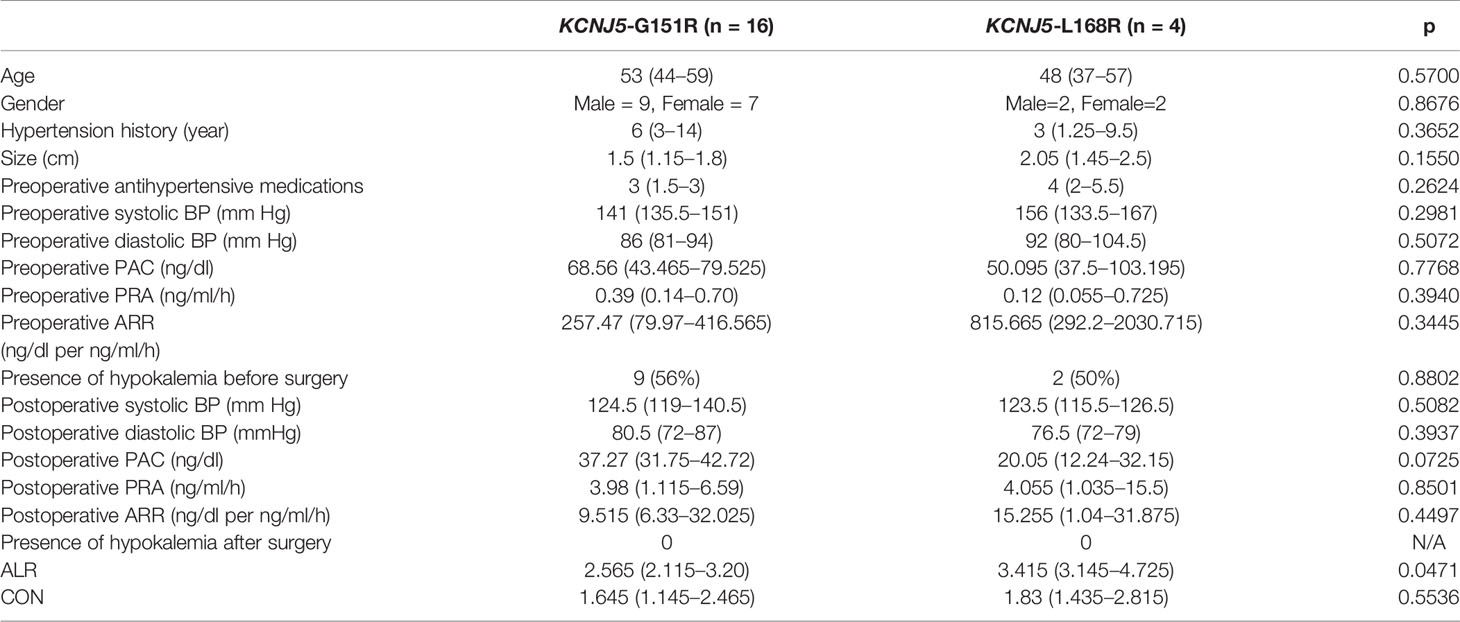
Table 2 Characteristics of clinical and NP-59 adrenal scintigraphy regarding to different point mutation of KCNJ5.
Discussion
In the present study, we found significant correlation between NP-59 uptake and KCNJ5 mutation. Adrenal glands with tumor harboring KCNJ5 mutation had higher NP-59 uptake than contralateral adrenal glands both by direct comparison (CON) and corrected by liver background (ALR). Furthermore, tumors with KCNJ5-G151R point mutation had significantly higher ALR than those with KCNJ5-L168R point mutation. To our knowledge, this is the first study analyzing SPECT/CT imaging to predict genetic mutation.
Five decades have passed since the first adrenal cortical imaging agent was developed (13). Basic concept is that cholesterol is the key component of hormones released from adrenal cortex, and radiolabeled cholesterol should be able to lead the way to imaging the factory. The original compound was 125I-19-iodochelesterol (14, 15) followed by first-in-human study by Beierwaltes et al. (16). Subsequent studies using 131I-19-iodochelesterol were applied to variety of adrenal diseases such as Cushing’s syndrome and primary aldosteronism (17–19). A chemical impurity 131I-6β-iodomethyl-19-norchelesterol (NP-59) was found during the synthesis of 19-iodochelesterol which showed three to five times higher adrenal uptake (20).
Criticism to NP-59 adrenal scintigraphy in PA lateralization also arose with long history, mainly based on limited imaging resolution resulting in poor ability to detect small APAs (21). Guideline by expert consensus has addressed that NP-59 adrenal scintigraphy has no role in subtype evaluation of PA (22). Great effort has been put on improving imaging quality by SPECT to illustrate adrenal lesions more clearly (23–25). We have previously reported the most comprehensive study using SPECT/CT to differentiate APA from idiopathic bilateral adrenal hyperplasia with sensitivity and specificity of 82% and 67%, respectively (8). Semiquantitative analysis could be easily performed from SPECT/CT imaging. We furthermore applied two semiquantitative parameters, ALR and CON, to predict postsurgical outcome which found that ALR and CON greater than the cutoff were significantly correlated with improvement of postsurgical outcome (9). In Taiwan mini-frontier of primary aldosteronism we suggested that NP-59 adrenal scintigraphy is an alternative method for lateralization when AVS is not available based on the above mentioned evidence (26).
Recognition of KCNJ5 mutation in APAs by Choi et al. in 2011 is the most tremendous progression to understand the pathophysiology of PA. KCNJ5 mutation altered the selectivity of encoding potassium channel which lead to cell membrane depolarization, influx of calcium ion and subsequent aldosterone production (6). KCNJ5 is the most frequent genetic mutations in APAs with overall prevalence of 43%, ranging from 12% to 80% and it is widely studied for the correlation with phenotype, mainly in female, with younger age, larger tumor and higher PAC (7). Moreover, KCNJ5 mutations were associated with postsurgical outcome. Kitamoto et al. found that patients with KCNJ5 mutation had higher rate of hypertension resolution and decreased left ventricular hypertrophy after adrenalectomy (27). Vilela et al. also represented that KCNJ5 mutation is the only independent predictor of hypertension remission (28). Change et al. recently demonstrated that mutation carriers had higher greater decrease in left ventricular mass index (LVMI) and inappropriately LVMI in a prospective cohort (29).
The importance of KCNJ5 mutation arises from its high prevalence, phenotype association and postsurgical outcome correlation. However, KCNJ5 mutation is only available with surgical specimen. Therefore, we aimed to utilize NP-59 adrenal scintigraphy as an imaging biomarker to predict KCNJ5 mutation. In fact, it is common to use nuclear medicine imaging to predict disease biomarkers since it is noninvasive and easily to manipulate. Radiomics extracts quantitative imaging data and associates these features to relevant clinical profiles. Several studies have addressed the correlation between mutations and semiquantitative parameters of nuclear medicine imaging and the most relevant example is to predict EGFR mutation in lung cancer by 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET). Maximal standard uptake value (SUVmax), which is the most commonly used semiquantitative parameter in PET, is associated with non-small cell lung cancer (NSCLC) with EGFR mutation and ALK positivity showing higher value (30). Radiomic features and conventional parameters (metabolic tumor volume and SUVmax) were proved to predictive of EGFR mutation in NSCLC (31). SUVmax is also shown to be correlated with KRAS mutation in colorectal cancer, BRAFV600E mutation in thyroid cancer and HER2 expression in gastric cancer (32–34).
In the present study we found that APA with KCNJ5 mutation expressed significantly higher NP-59 uptake than those without KCNJ5 mutation, in terms of ALR and CON. This correlation could be explained by the straightforward mechanism. Mutated channels alter the permeability of sodium which lead to depolarization of cell membrane and subsequent autonomous aldosterone production. Aldosterone production requires cholesterol as synthesis material and radiolabeled cholesterol, NP-59, is delivered to the overproduction side of adrenal gland. Mutated APAs produce more aldosterone than wild-type APAs which lead to more NP-59 uptake. In our cohort this genetic difference reflects both on the level of phenotype, preoperative PAC, and of molecular imaging, NP-59 adrenal scintigraphy.
AVS is currently the gold standard for lateralization and some studies have addressed the impact of KCNJ5 mutation on lateralization index (LI). In 170 PA patients Seccia et al. evaluated 40 of them with selective index of AVS greater than 2.0 at both side, and found that LI was significantly higher in APA with KCNJ5 mutations those without mutations (p = 0.02, detail value of LI not specified in the study) (35). Zheng et al. analyzed a larger Chinese cohort with 162 PA patients which revealed borderline higher LI in APA with KCNJ5 mutations than those without mutations (10.9 [7.5–22.7] versus 6.4 [3.0–10.6], p = 0.053) (36). In contrast, Oßwald et al. found 19 PA patients harboring KCNJ5 mutation had similar LI compared to 32 patients without KCNJ5 mutation (20.5, interquartile range 30.3 versus 16.0, interquartile range 41.9) (37). The conflict and nonreplicated results not only attract attention of necessity of larger study, but also face up the contentious issues of AVS such as unstandardized protocol, different interpretation criteria and variable failed rate. NP-59 adrenal scintigraphy is an ancient nuclear medicine study with well-established protocol and sufficient evidence for both interpretation, lateralization and prognosis. Considering noninvasive nature and beneficial both for lateralization and mutation prediction, it is reasonable to have NP-59 adrenal scintigraphy before further treatment.
It is important to conduct the correlation between genotype and imaging findings into clinical practice, mainly referring to treatment. Predicting EGFR mutation by SUVmax of PET could identify proper candidate who may be beneficial from target therapy. In the present study we expect to use NP-59 adrenal scintigraphy selecting patients who may beneficial from medical treatment other than mineralocorticoid receptor antagonist (MRA). MRA is the drug of choice for bilateral disease as well as for patients who have contraindications to surgery or are not willing to receive surgery (which is common in Eastern countries) (22). The first line MRA is spironolactone acting by direct antagonizing the receptors which effectively lowers PAC. Side effects come along with its affinity to androgen receptor leading to gynecomastia and erectile dysfunction in male and to progesterone receptor leading to menstrual irregularity in female (38). In the SPARTACUS trial 57% of the patients developed these side effects from spironolactone compared with 1% of the adrenaletomy patients. This dose-dependent side effect has indeed limited the clinical application (39). Eplerenone is an alternative MRA when spironolactone is not tolerated or optimal BP is not achieved (22). It is selective for mineralocorticoid receptor without significant interaction with androgen or progesterone receptor and therefore it has much lower side effect compared to spironolactone (40). However, Eplerenone has less affinity to mineralocorticoid receptor and is only approved for PA use in Japan and USA, not in Europe, Australia and Taiwan (41, 42). Moreover, the price of eplerenone is 26 times compared to spironolactone in Taiwan. Epithelial sodium channel antagonists, such as amiloride, are the second-line choice for medical treatment of PA. It works as potassium-sparing diuretic which improves hypertension and hypokalemia. Although less effective than spironolactone, amiloride is generally well-tolerated due to lack of androgenic effect (43, 44). Calcium channel blockers (CCBs) could be a choice for PA treatment with variable mechanisms and effects. Dihydropyridine CCBs reduced BP by competing aldosterone binding to mineralocorticoid receptor (45). However, the concentration of most dihydropyridine CCBs is not able to block mineralocorticoid receptor at regular doses in treating hypertension (46). Several studies have been published to illustrate the pharmacological effects of above mentioned alternative medications regarding to KCNJ5 mutation. Tauber et al. reported that amiloride and its analog EIPA blocked with Na+-permeable mutated KCNJ5 cells with L168R point mutation. Although nondihydropyridine CCBs have no effect on mineralocorticoid receptor, verapamil and diltiazem showed potent inhibition of KCNJ5-L168R cells (47). Physicians are not able to know the effects of these alternative medications before prescription. In our cohort, patients harboring KCNJ5-L168R mutation had significantly higher ALR compared to those with KCNJ5-G151R mutation. It is of great novelty that amiloride and verapamil/diltiazem may work on patients with stronger potency and less side effects by the mutation status provided by NP-59 adrenal scintigraphy.
Recently macrolide antibiotics is proved to inhibit HEK293T cells transfected with mutated KCNJ5 but not to wild-type cells without antibiotic activity (48). Thereafter Caroccia et al. proceeded the study which demonstrated that clarithromycin lowered CYP11B2 gene expression and aldosterone secretion in CD56+ cells ex vivo from KCNJ5 mutated APAs, but not in CD56+ cells from wild-type APAs (49). NP-59 adrenal scintigraphy provides noninvasive method to predict KCNJ5 mutation and may be able to identify patients beneficial to macrolide treatment. For patients intolerable to the dose-dependent side effect of spironolactone who may harboring KCNJ5 mutation predicted by NP-59 adrenal scintigraphy, macrolide could be a choice to treatment regimen in the future, and the key point is to identify KCNJ5 mutation in advance.
There are some limitations in our study. First, the retrospective, single-center design with relatively small sample size may lead to selection bias. Second, the patients in the present study were all Taiwanese. There is significant difference among races regarding to KCNJ5 mutation rate. Higher KCNJ5 mutation rate was noted in Eastern Asians (70%) compared to Caucasians (38%) (50). Validation of our results to general population requires more comprehensive survey. Third, other gene mutations such as ATP1A1, ATP2B3, CACNA1D, and CTNNB1 account for a certain portion of somatic mutations in APA (51). Although these mutations were not found in the present cohort, it is not negligible and further study should be conducted.
In conclusion, our study suggested that semiqualitative parameters from NP-59 adrenal scintigraphy could predict KCNJ5 mutations in PA patients. APAs harboring KCNJ5 mutations had significantly higher ALR and borderline higher CON than those without KCNJ5 mutations. Furthermore, APAs with KCNJ5-L168R mutation had significantly higher ALR than those with KCNJ5-G151R point mutation. Precision medicine, which individualized treatment based on distinct signature of each patient, is the trend of disease management. Our study is a proof-of-concept and the first study applying SPECT to predict mutation status which may be utilized in treatment plan of PA.
Data Availability Statement
The data sets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by the institutional review board of National Taiwan University Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
C-CL: data collection, imaging analysis, and manuscript writing. R-FY: imaging analysis. K-YP: genetic analysis. J-YH: statistical consult. K-DW: data collection. JC: data collection. W-YL: study design and correspondence. All authors contributed to the article and approved the submitted version.
Funding
The study is partly supported by the grant provided from National Taiwan University Hospital (110-S5009).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A Prospective Study of the Prevalence of Primary Aldosteronism in 1,125 Hypertensive Patients. J Am Coll Cardiol (2006) 48:2293–300. doi: 10.1016/j.jacc.2006.07.059
2. Kayser SC, Dekkers T, Groenewoud HJ, van der Wilt GJ, Carel Bakx J, van der Wel MC, et al. Study Heterogeneity and Estimation of Prevalence of Primary Aldosteronism: A Systematic Review and Meta-Regression Analysis. J Clin Endocrinol Metab (2016) 101:2826–35. doi: 10.1210/jc.2016-1472
3. Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, et al. Cardiovascular Events and Target Organ Damage in Primary Aldosteronism Compared With Essential Hypertension: A Systematic Review and Meta-Analysis. Lancet Diabetes Endocrinol (2018) 6:41–50. doi: 10.1016/S2213-8587(17)30319-4
4. Huang KH, Yu CC, Hu YH, Chang CC, Chan CK, Liao SC, et al. Targeted Treatment of Primary Aldosteronism - The Consensus of Taiwan Society of Aldosteronism. J Formos Med Assoc (2019) 118:72–82. doi: 10.1016/j.jfma.2018.01.006
5. Funder JW. Primary Aldosteronism as a Public Health Issue. Lancet Diabetes Endocrinol (2016) 4:972–3. doi: 10.1016/S2213-8587(16)30272-8
6. Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, et al. K+ Channel Mutations in Adrenal Aldosterone-Producing Adenomas and Hereditary Hypertension. Science (2011) 331:768–72. doi: 10.1126/science.1198785
7. Lenzini L, Rossitto G, Maiolino G, Letizia C, Funder JW. Rossi Gp. A Meta-Analysis of Somatic Kcnj5 K(+) Channel Mutations in 1636 Patients With an Aldosterone-Producing Adenoma. J Clin Endocrinol Metab (2015) 100:E1089–95. doi: 10.1210/jc.2015-2149
8. Yen RF, Wu VC, Liu KL, Cheng MF, Wu YW, Chueh SC, et al. 131I-6beta-Iodomethyl-19-Norcholesterol SPECT/CT for Primary Aldosteronism Patients With Inconclusive Adrenal Venous Sampling and CT Results. J Nucl Med (2009) 50:1631–7. doi: 10.2967/jnumed.109.064873
9. Lu CC, Wu VC, Wu KD, Liu KL, Lin WC, Cheng MF, et al. Prognostic Value of Semiquantification NP-59 SPECT/CT in Primary Aldosteronism Patients After Adrenalectomy. Eur J Nucl Med Mol Imaging (2014) 41:1375–84. doi: 10.1007/s00259-014-2692-z
10. Gross MD, Valk TW, Swanson DP, Thrall JH, Grekin RJ, Beirewaltes WH. The Role of Parmacologic Manipulation in Adrenal Cortical Scintigraphy. Semin Nucl Med (1981) 11:128–48. doi: 10.1016/S0001-2998(81)80042-6
11. Azizan EA, Murthy M, Stowasser M, Gordon R, Kowalski B, Xu S, et al. Somatic Mutations Affecting the Selectivity Filter of KCNJ5 are Frequent in 2 Large Unselected Collections of Adrenal Aldosteronomas. Hypertension (2012) 59:587–91. doi: 10.1161/HYPERTENSIONAHA.111.186239
12. Wu VC, Huang KH, Peng KY, Tsai YC, Wu CH, Wang SM, et al. Prevalence and Clinical Correlates of Somatic Mutation in Aldosterone Producing adenoma-Taiwanese Population. Sci Rep (2015) 5:11396. doi: 10.1038/srep11396
13. Nagai T, Solis BA, Koh CS. An Approach to Developing Adrenal-Gland Scanning. J Nucl Med (1968) 9:576–81.
14. Counsell RE, Ranade VV, Blair RJ, Beierwaltes WH, Weinhold PA. Tumor Localizing Agents. IX. Radioiodinated Cholesterol. Steroids (1970) 16:317–28. doi: 10.1016/S0039-128X(70)80116-7
15. Varma VM, Blair RJ, Beierwaltes WH, Lieberman CM, Boyd RE, Counsell RE, et al. Radiolabeled Cholesterol as an Adrenal Scanning Agent. J Nucl Med (1971) 12:176.
16. Beierwaltes WH, Lieberman LM, Ansari AN, Nishiyama H. Visualization of Human Adrenal Glands In Vivo by Scintillation Scanning. JAMA (1971) 216:275–7. doi: 10.1001/jama.216.2.275
17. Lieberman LM, Beierwaltes WH, Conn JW, Ansari AN, Nishiyama H. Diagnosis of Adrenal Disease by Visualization of Human Adrenal Glands With 131 I-19-Iodocholesterol. N Engl J Med (1971) 285:1387–93. doi: 10.1056/NEJM197112162852501
18. Beierwaltes WH, Sturman MF, Ryo U, Ice RD. Imaging Functional Nodules of the Adrenal Glands With 131-I-19-Iodocholesterol. J Nucl Med (1974) 15:246–51.
19. Hogan MJ, McRae J, Schambelan M, Biglieri EG. Location of Aldosterone-Producing Adenomas With 131I-19-Iodocholesterol. N Engl J Med (1976) 294:410–4. doi: 10.1056/NEJM197602192940802
20. Sarkar SD, Beierwaltes H, Ice RD, Basmadjian GP, Hetzel KR, Kennedy WP, et al. A New and Superior Adrenal Scanning Agent, NP-59. J Nucl Med (1975) 16:1038–42.
21. Nomura K, Kusakabe K, Maki M, Ito Y, Aiba M, Demura H. Iodomethylnorcholesterol Uptake in an Aldosteronoma Shown by Dexamethasone-Suppression Scintigraphy: Relationship to Adenoma Size and Functional Activity. J Clin Endocrinol Metab (1990) 71:825–30. doi: 10.1210/jcem-71-4-825
22. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2016) 101:1889–916. doi: 10.1210/jc.2015-4061
23. Ishimura J, Kawanaka M, Fukuchi M. Clinical Application of SPECT in Adrenal Imaging With iodine-131 6 Beta-Iodomethyl-19-Norcholesterol. Clin Nucl Med (1989) 14:278–81. doi: 10.1097/00003072-198904000-00009
24. Hwang I, Balingit AG, Georgitis WJ, Sisson JC, Shapiro B. Adrenocortical SPECT Using iodine-131 Np-59. J Nucl Med (1998) 39:1460–3.
25. La Cava G, Imperiale A, Olianti C, Gheri GR, Ladu C, Mannelli M, et al. SPECT Semiquantitative Analysis of Adrenocortical (131)I-6 Beta Iodomethyl-Norcholesterol Uptake to Discriminate Subclinical and Preclinical Functioning Adrenal Incidentaloma. J Nucl Med (2003) 44:1057–64.
26. Chang CC, Chen YY, Lai TS, Zeng YH, Chen CK, Tu KH, et al. Taiwan Mini-Frontier of Primary Aldosteronism: Updating Detection and Diagnosis. J Formos Med Assoc (2021) 120:121–9. doi: 10.1016/j.jfma.2020.08.001
27. Kitamoto T, Omura M, Suematsu S, Saito J, Nishikawa T. KCNJ5 Mutation as a Predictor for Resolution of Hypertension After Surgical Treatment of Aldosterone-Producing Adenoma. J Hypertens (2018) 36:619–27. doi: 10.1097/HJH.0000000000001578
28. Vilela LAP, Rassi-Cruz M, Guimaraes AG, Moises CCS, Freitas TC, Alencar NP, et al. Kcnj5 Somatic Mutation Is a Predictor of Hypertension Remission After Adrenalectomy for Unilateral Primary Aldosteronism. J Clin Endocrinol Metab (2019) 104:4695–702. doi: 10.1210/jc.2019-00531
29. Chang YY, Tsai CH, Peng SY, Chen CC, Chang BC, Lee CW, et al. Kcnj5 Somatic Mutations in Aldosterone-Producing Adenoma Are Associated With a Worse Baseline Status and Better Recovery of Left Ventricular Remodeling and Diastolic Function. Hypertension (2020) Hypertensionaha12015679. doi: 10.1161/HYPERTENSIONAHA.120.15679
30. Lv Z, Fan J, Xu J, Wu F, Huang Q, Guo M, et al. Value of (18)F-FDG PET/CT for Predicting EGFR Mutations and Positive ALK Expression in Patients With non-Small Cell Lung Cancer: A Retrospective Analysis of 849 Chinese Patients. Eur J Nucl Med Mol Imaging (2018) 45:735–50. doi: 10.1007/s00259-017-3885-z
31. Yip SS, Kim J, Coroller TP, Parmar C, Velazquez ER, Huynh E, et al. Associations Between Somatic Mutations and Metabolic Imaging Phenotypes in Non-Small Cell Lung Cancer. J Nucl Med (2017) 58:569–76. doi: 10.2967/jnumed.116.181826
32. Kawada K, Toda K, Nakamoto Y, Iwamoto M, Hatano E, Chen F, et al. Relationship Between 18f-Fdg PET/CT Scans and KRAS Mutations in Metastatic Colorectal Cancer. J Nucl Med (2015) 56:1322–7. doi: 10.2967/jnumed.115.160614
33. Nagarajah J, Ho AL, Tuttle RM, Weber WA, Grewal RK. Correlation of BRAFV600E Mutation and Glucose Metabolism in Thyroid Cancer Patients: An (1)(8)F-Fdg PET Study. J Nucl Med (2015) 56:662–7. doi: 10.2967/jnumed.114.150607
34. Chen R, Zhou X, Liu J, Huang G. Relationship Between 18f-Fdg PET/CT Findings and HER2 Expression in Gastric Cancer. J Nucl Med (2016) 57:1040–4. doi: 10.2967/jnumed.115.171165
35. Seccia TM, Mantero F, Letizia C, Kuppusamy M, Caroccia B, Barisa M, et al. Somatic Mutations in the KCNJ5 Gene Raise the Lateralization Index: Implications for the Diagnosis of Primary Aldosteronism by Adrenal Vein Sampling. J Clin Endocrinol Metab (2012) 97:E2307–13. doi: 10.1210/jc.2012-2342
36. Zheng FF, Zhu LM, Nie AF, Li XY, Lin JR, Zhang K, et al. Clinical Characteristics of Somatic Mutations in Chinese Patients With Aldosterone-Producing Adenoma. Hypertension (2015) 65:622–8. doi: 10.1161/HYPERTENSIONAHA.114.03346
37. Osswald A, Fischer E, Degenhart C, Quinkler M, Bidlingmaier M, Pallauf A, et al. Lack of Influence of Somatic Mutations on Steroid Gradients During Adrenal Vein Sampling in Aldosterone-Producing Adenoma Patients. Eur J Endocrinol (2013) 169:657–63. doi: 10.1530/EJE-13-0551
38. Lechner B, Lechner K, Heinrich D, Adolf C, Holler F, Schneider H, et al. Therapy OF Endocrine DISEASE: Medical Treatment of Primary Aldosteronism. Eur J Endocrinol (2019) 181:R147–53. doi: 10.1530/EJE-19-0215
39. Dekkers T, Prejbisz A, Kool LJS, Groenewoud H, Velema M, Spiering W, et al. Adrenal Vein Sampling Versus CT Scan to Determine Treatment in Primary Aldosteronism: An Outcome-Based Randomised Diagnostic Trial. Lancet Diabetes Endocrinol (2016) 4:739–46. doi: 10.1016/S2213-8587(16)30100-0
40. Parthasarathy HK, Menard J, White WB, Young WF Jr, Williams GH, Williams B, et al. A Double-Blind, Randomized Study Comparing the Antihypertensive Effect of Eplerenone and Spironolactone in Patients With Hypertension and Evidence of Primary Aldosteronism. J Hypertens (2011) 29:980–90. doi: 10.1097/HJH.0b013e3283455ca5
41. de Gasparo M, Joss U, Ramjoue HP, Whitebread SE, Haenni H, Schenkel L, et al. Three New Epoxy-Spirolactone Derivatives: Characterization In Vivo and In Vitro. J Pharmacol Exp Ther (1987) 240:650–6. doi: 10.1530/EJE-17-0990
42. Williams TA, Reincke M. Management OF Endocrine DISEASE: Diagnosis and Management of Primary Aldosteronism: The Endocrine Society Guideline 2016 Revisited. Eur J Endocrinol (2018) 179:R19–29. doi: 10.1530/EJE-17-0990
43. Eide IK, Torjesen PA, Drolsum A, Babovic A, Lilledahl NP. Low-Renin Status in Therapy-Resistant Hypertension: A Clue to Efficient Treatment. J Hypertens (2004) 22:2217–26. doi: 10.1097/00004872-200411000-00026
44. Lim PO, Young WF, MacDonald TM. A Review of the Medical Treatment of Primary Aldosteronism. J Hypertens (2001) 19:353–61. doi: 10.1097/00004872-200103000-00001
45. Dietz JD, Du S, Bolten CW, Payne MA, Xia C, Blinn JR, et al. A Number of Marketed Dihydropyridine Calcium Channel Blockers Have Mineralocorticoid Receptor Antagonist Activity. Hypertension (2008) 51:742–8. doi: 10.1161/HYPERTENSIONAHA.107.103580
46. Deinum J, Riksen NP, Lenders JW. Pharmacological Treatment of Aldosterone Excess. Pharmacol Ther (2015) 154:120–33. doi: 10.1016/j.pharmthera.2015.07.006
47. Tauber P, Penton D, Stindl J, Humberg E, Tegtmeier I, Sterner C, et al. Pharmacology and Pathophysiology of Mutated KCNJ5 Found in Adrenal Aldosterone-Producing Adenomas. Endocrinology (2014) 155:1353–62. doi: 10.1210/en.2013-1944
48. Scholl UI, Abriola L, Zhang C, Reimer EN, Plummer M, Kazmierczak BI, et al. Macrolides Selectively Inhibit Mutant KCNJ5 Potassium Channels That Cause Aldosterone-Producing Adenoma. J Clin Invest (2017) 127:2739–50. doi: 10.1172/JCI91733
49. Caroccia B, Prisco S, Seccia TM, Piazza M, Maiolino G, Rossi GP. Macrolides Blunt Aldosterone Biosynthesis: A Proof-of-Concept Study in KCNJ5 Mutated Adenoma Cells Ex Vivo. Hypertension (2017) 70:1238–42. doi: 10.1161/HYPERTENSIONAHA.117.10226
50. Williams TA, Monticone S, Mulatero P. KCNJ5 Mutations are the Most Frequent Genetic Alteration in Primary Aldosteronism. Hypertension (2015) 65:507–9. doi: 10.1161/HYPERTENSIONAHA.114.04636
Keywords: primary aldosteronism, NP-59 adrenal scintigraphy, KCNJ5, semiquantification, mutation prediction
Citation: Lu C-C, Yen R-F, Peng K-Y, Huang J-Y, Wu K-D, Chueh JS and Lin W-Y (2021) NP-59 Adrenal Scintigraphy as an Imaging Biomarker to Predict KCNJ5 Mutation in Primary Aldosteronism Patients. Front. Endocrinol. 12:644927. doi: 10.3389/fendo.2021.644927
Received: 22 December 2020; Accepted: 06 April 2021;
Published: 28 April 2021.
Edited by:
Ben Nephew, Worcester Polytechnic Institute, United StatesReviewed by:
Jinbo Hu, First Affiliated Hospital of Chongqing Medical University, ChinaYuh-Feng Wang, Dalin Tzu Chi Hospital, Taiwan
Copyright © 2021 Lu, Yen, Peng, Huang, Wu, Chueh and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wan-Yu Lin, bGlud3lAbnR1LmVkdS50dw==
 Ching-Chu Lu
Ching-Chu Lu Ruoh-Fang Yen
Ruoh-Fang Yen Kang-Yung Peng3
Kang-Yung Peng3 Jei-Yie Huang
Jei-Yie Huang Kwan-Dun Wu
Kwan-Dun Wu Wan-Yu Lin
Wan-Yu Lin