- 1Department of Endocrinology and Metabolism, Shanghai Tenth People’s Hospital, School of Medicine Tongji University, Shanghai, China
- 2Shanghai Center of Thyroid Disease, Shanghai, China
Aims: To investigate the predictive value of baseline serum triglyceride (TG) levels for improvements of metabolism after laparoscopic sleeve gastrectomy (LSG).
Methods: 112 obese patients [body mass index (BMI) ≥ 35 kg/m2] underwent LSG and with complete information of anthropometric and metabolic parameters were divided into normal TG group (group A) and high TG group (group B), while group A had TG levels ≤ 1.7 mmol/L, and group B had TG levels > 1.7 mmol/L. The post-operative changes (Δ) in metabolic parameters between the two groups were compared.
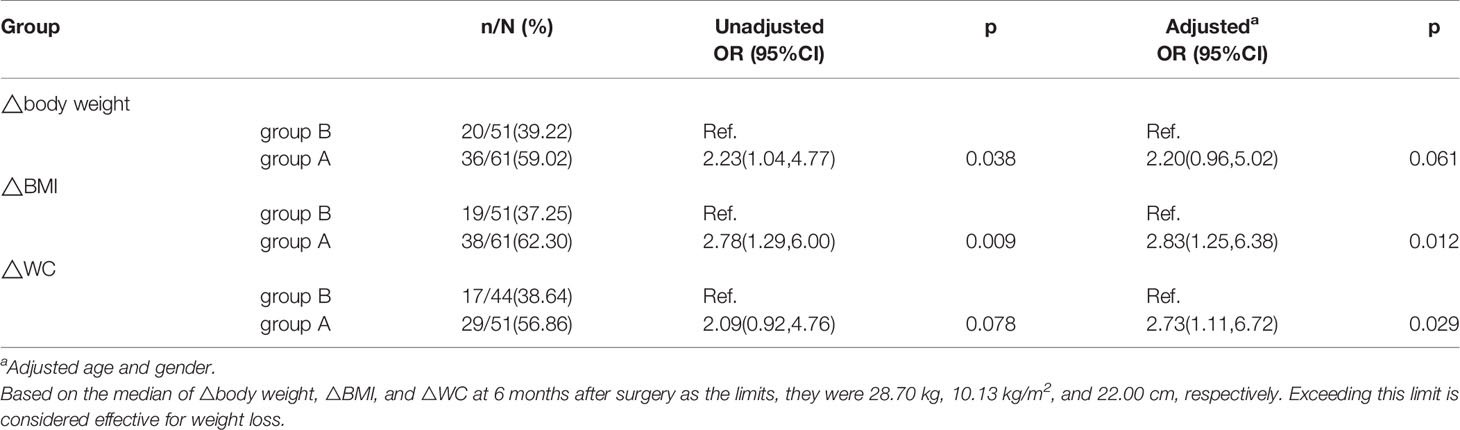
Results: In the whole cohort, the metabolic parameters were significantly improved at 6 months after LSG. BMI and waist circumference (WC) decreased significantly in the two groups. The ΔBMI among group A and group B were 11.42±3.23 vs 9.13±2.77 kg/m2 (p<0.001), respectively. ΔBMI was positively correlated with ΔWC (r=0.696, p<0.001), Δfasting insulin level (r=0.440, p=0.002), Δfasting serum C peptide level (r=0.453, p=0.002), and Δhomeostasis model assessment insulin resistance index (r=0.418, p=0.004) in group A. Compared with group B, group A had a significantly higher odds ratio (OR) of 2.83 (95% confidence interval [CI]1.25–6.38, p=0.012)and 2.73 (95% CI 1.11–6.72, p=0.029) for ΔBMI and ΔWC after adjustment for age and gender, respectively.
Conclusions: Obese patients with baseline TG levels under 1.7 mmol/L had greater loss of weight at six months follow-up later LSG. This finding suggests that baseline TG level may have a predictive value for weight loss, at least in the short-term follow-up.
Introduction
Obesity is a major public health concern globally (1). Based on adipose tissue distribution in the body (upper body and lower body), obesity can be divided into abdominal obesity and gluteofemoral obesity (2). Abdominal obesity is one of the symptoms of metabolic syndrome (MS), and is also a risk factor for cardiovascular disease (CVD) and diabetes mellitus (3, 4). Healthcare professionals have employed several treatment methods to improve weight loss management. Recently, metabolic surgery has become an internationally recognized method for long-term and effective weight control and for improvement in metabolic disorders (5). Laparoscopic sleeve gastrectomy (LSG) is one of the most commonly used methods and has been employed by clinicians globally for many years (6–9). Triglyceride (TG) is an important clinical feature of MS and associated with metabolic abnormalities in nonalcoholic fatty liver disease and abdominal obesity (10, 11). TG is also an independent risk factor for CVD (10). In an analysis of a 23-year cohort study, the first CVD events occurred in half of the patients in the high TG group, and the incidence of having first CVD events was approximately two-fold higher in the high TG group than in the normal TG group (12). However, the prognostic value of TG in obese patients treated with LSG remains unknown.
We aimed to investigate the potential predictive value of baseline TG level on several metabolic outcomes after LSG, and a retrospective analysis of a cohort of obese patients with different TG levels was performed.
Materials and Methods
Subjects
In this retrospective study, we recruited obese patients who underwent standard LSG at the Department of Gastrointestinal Surgery in Shanghai Tenth People’s Hospital affiliated with Tongji University from May 2015 to July 2019, and the selection criteria were as follows:
(1) BMI ≥ 35 kg/m2;
(2) Good liver and kidney function;
(3) Good cardiopulmonary function;
(4) The follow-up can be completed on time. That is, regular follow-up can be carried out in 1 month, 3 months, and 6 months after surgery;
(5) The TG values were measured before and after surgery.
Patients with a history of any malignant tumor, genetic disease, hypogonadism, renal dysfunction, severe liver dysfunction, preexisting heart disease, and inability to understand and observe the study protocol were excluded. Written informed consent was obtained from each participant before enrolment, and the study protocol was approved by the hospital ethics committee (Clinical Registration Number: ChiCTR-OCS-12002381).
Anthropometric Assessment and Laboratory Analyses
The height (H), body weight (BW), neck circumference (NC), waist circumference (WC), hip circumference (HC), and BMI were measured at time 0 (pre-operative) and time 6 (6 months after LSG) by trained physicians. Morning venous blood was drawn from the study participants after a 12-h overnight fast, and general biochemical data, including glucose levels, lipid profiles, and liver function markers, were measured at the same period (time 0 and time 6). The levels of FPG, 2-hour plasma glucose (2hPG), fasting insulin (FINS), 2-hour insulin, fasting serum C peptide (FCP), 2-hour serum C peptide, hemoglobin Alc (HbA1c), total cholesterol (TC), TG, HDL-C, low-density lipoprotein cholesterol (LDL-C), and free fatty acid (FFA) were measured using the electrochemiluminescence immunoassay method, as well as the levels of thyroid-stimulating hormone (TSH), free thyroxine, free triiodothyronine (FT3), interleukin 6 (IL-6), interleukin 8 (IL-8), and C-reactive protein (CRP). The homeostasis model assessment insulin resistance index (HOMA-IR) was calculated as following: FPG (mmol/L) ×FINS (mU/L)/22.5.
Patients were divided into two groups based on their serum levels of TG at baseline; group A with TG levels ≤ 1.7 mmol/L, and group B with TG levels > 1.7 mmol/L.
Statistical Analysis
All continuous data were presented as means ± standard deviation (SD). Independent Student’s t-tests were used to compare all parameters between groups, and paired sample t-tests were used to analyze differences between continuous variables before and after surgery. Pearson’s correlation analysis was used to evaluate the correlation between BMI and metabolic parameters. A binary logistic regression was performed to analyze the predictive indicators of the weight loss effect. All statistical analyses were performed using SPSS 22.0 software (SPSS, Inc., New York, NY, USA), and p-values <0.05 were considered statistically significant.
Results
Comparisons Between Metabolic Parameters in Patients With Different TG Levels at Baseline and 6 Months After LSG
A total of 112 (53 males, and 59 females) obese patients with a mean age of 31.15 ± 11.07 years were investigated in the present study. 61 (27 males, and 34 females) patients with a mean age of 27.97 ± 9.60 years were in group A, while 51 (26 males, and 25 females) patients with a mean age of 34.96 ± 11.59 years were in group B.
Great improvements in TG and HDL levels of the two groups were obtained in 6 months after LSG (p<0.01). Similarly, the BMI, NC, WC, WHR, and levels of FPG, FINS, FCP, HbA1c, and HomA-IR were also significantly improved after LSG in both groups (p<0.01).
The pre-operative TC levels in group B was higher than those in group A (4.81±1.06 vs 4.29±0.96 mmol/L, p=0.009); however, there was no difference between the two groups after surgery (4.49±0.90 vs 4.37±0.76 mmol/L, p=0.465). The HDL levels in group B was lower than those in group A before surgery (0.93±0.18 vs 1.04±0.21 mmol/L, p=0.005), and there was no difference after surgery (1.14±0.25 vs 1.20±0.32 mmol/L, p=0.253). Furthermore, pre-operative levels of LDL and FFA did not significantly differ between the two groups, while FFA decreased in group B 6 months after surgery (p<0.05, Table 1).
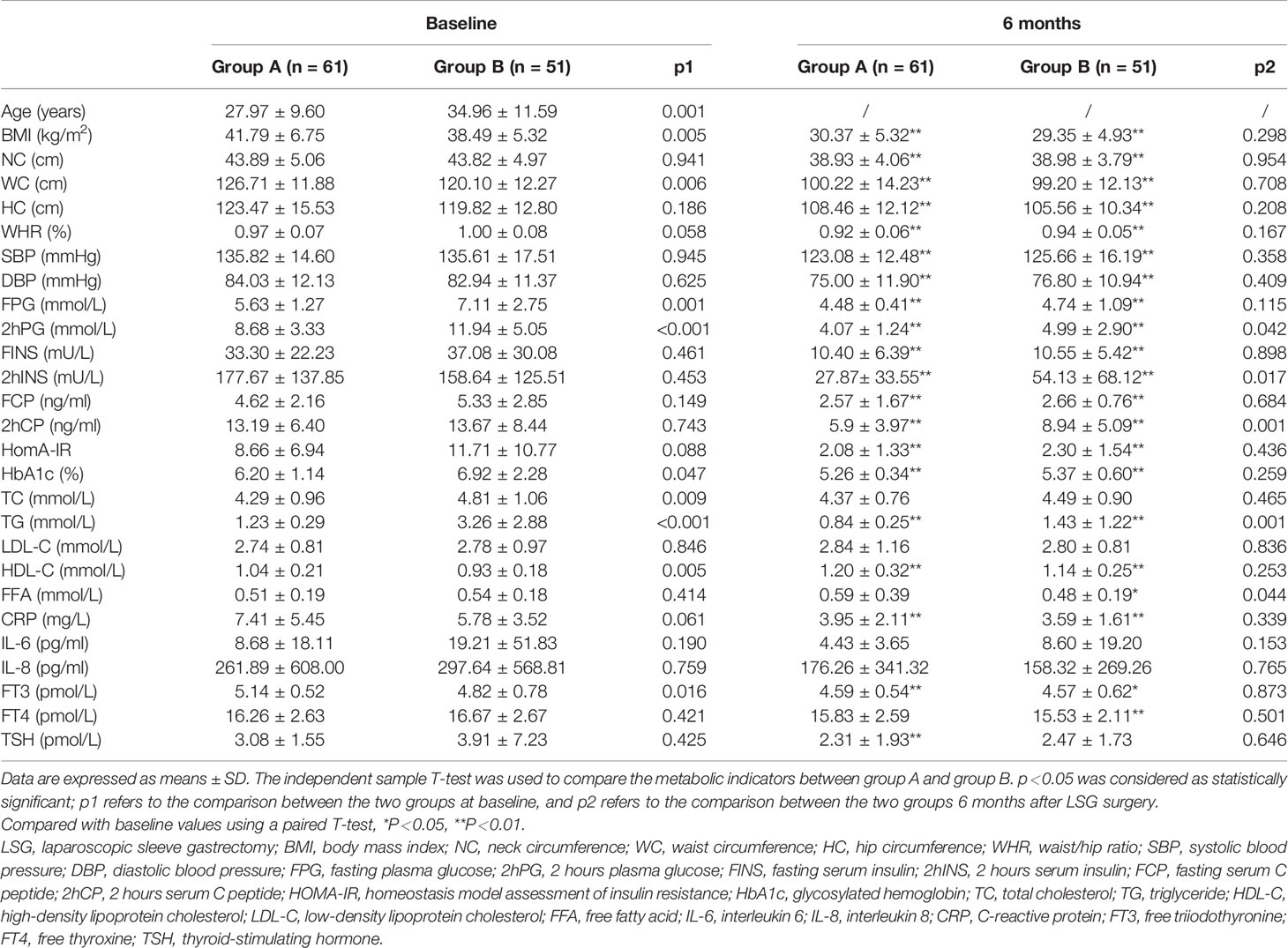
Table 1 Baseline clinical characteristics and metabolic parameters at 6 months after laparoscopic sleeve gastrectomy (LSG) in patients with different triglyceride (TG) Levels.
As Table 1 showed, group A had lower preoperative levels of FPG (p<0.01), 2hPG (p<0.01), and HbA1c (p<0.05) than group B. Significant improvements in blood glucose and islet function in both groups were observed after LSG (p<0.01).
Comparison of the Changes (Δ) in Metabolic Parameters Between Group A and Group B After LSG
Six months after LSG, we found that ΔBMI, ΔWC, and ΔHC in group A were significantly improved than group B (Table 2). However, the improvements in TC, TG, FFA, FPG, and 2hPG levels were greater in group B than in group A (p<0.05, Table 2).
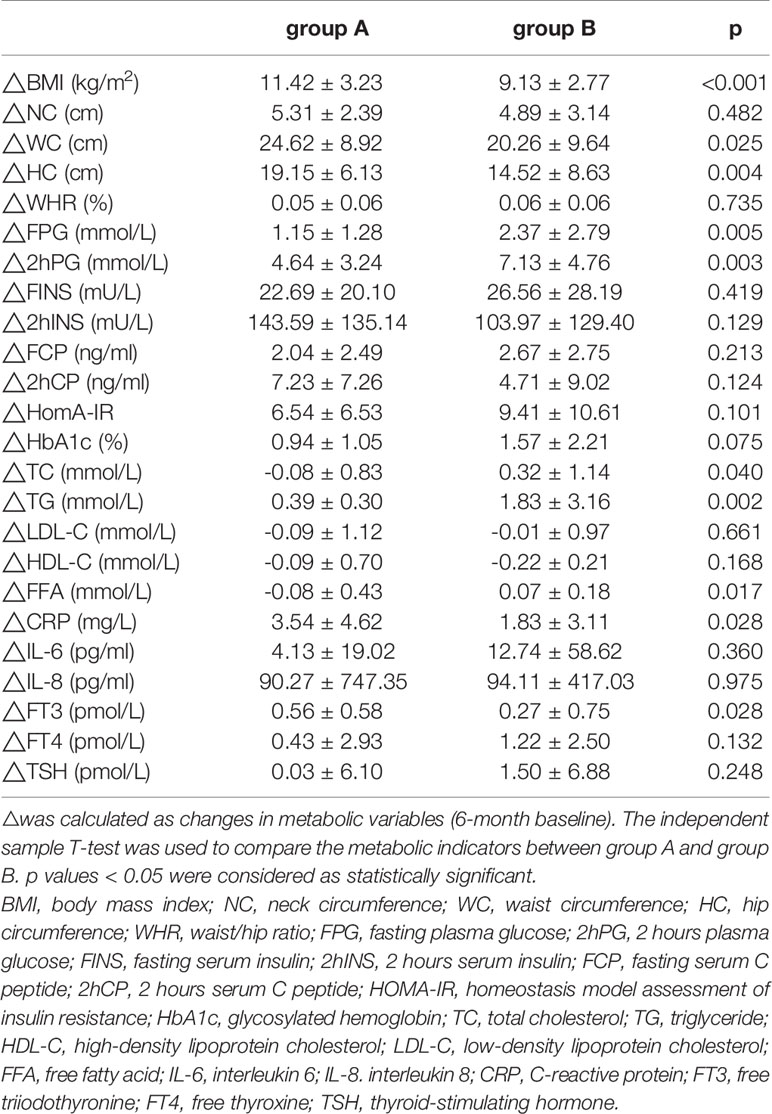
Table 2 Comparison of the improvements in metabolic parameters in patients with different triglyceride (TG) Levels.
Correlations Between ΔTG, as well as ΔBMI and Metabolic Parameters’ Changes in Patients After LSG
The ΔTG level was positively correlated with ΔFPG (r=0.410, p=0.001), Δ2hPG (r=0.349, p=0.008), and ΔHbA1c levels (r=0.267, p=0.045) in group A (Table 3). ΔTG level was positively correlated with the ΔFPG (r=0.562, p<0.001), Δ2hPG (r=0.435, p=0.003), ΔHbA1c (r=0.341, p=0.020), and ΔFFA levels (r=0.412, p=0.004), while the ΔTG was negatively correlated with ΔHDL-C (r= -0.307, p=0.034) and ΔLDL-C levels (r= - 0.294, p=0.043) in group B. Similarly, ΔBMI was positively correlated with ΔFPG (r=0.410, p=0.016), ΔFINS (r=0.423, p=0.013), ΔFCP (r=0.572, p<0.001) levels, and ΔHOMA-IR (r=0.540, p=0.001) in group B (Table 4).
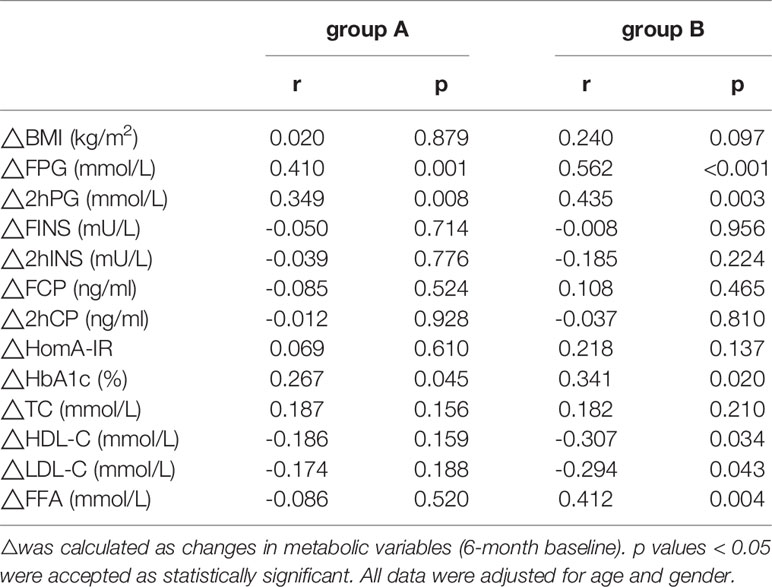
Table 3 Correlations between ΔTG and metabolic parameters’ changes in patients with different triglyceride (TG) Levels.
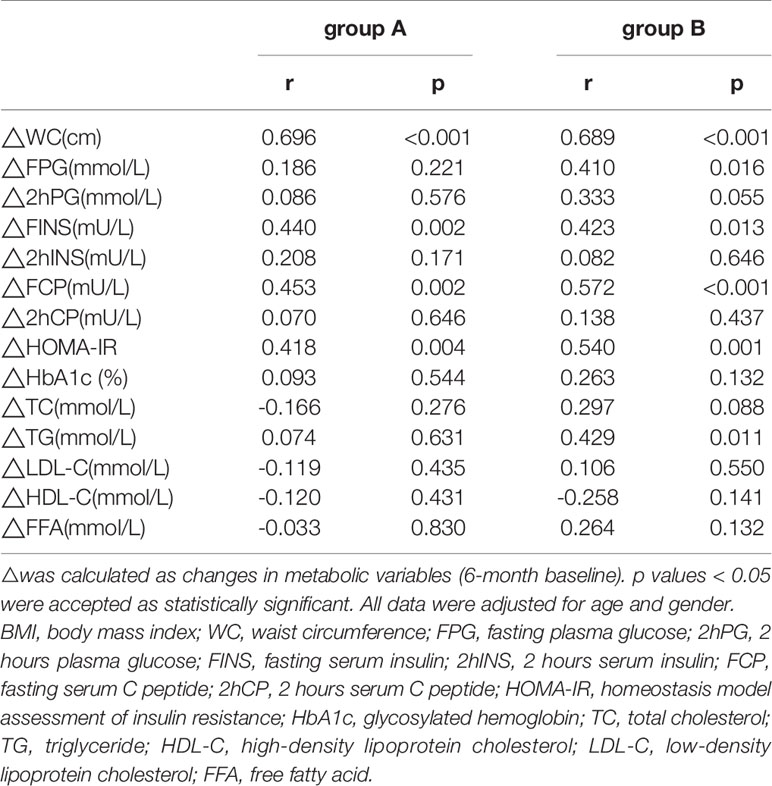
Table 4 Correlations between ΔBMI and metabolic parameters’ changes in patients with different triglyceride (TG) Levels.
The Relationship Between TG Levels and Effectiveness of LSG by Binary Logistic Regression Analysis
The median values (13) for the ΔBW, ΔBMI, and ΔWC 6 months after LSG were used as the limits; these were 28.70 kg, 10.13 kg/m2, and 22.00 cm, respectively. Compared with patients in group B, the odds ratios (ORs) for the ΔBW, ΔBMI, and ΔWC were 2.23(95% confidence interval [CI] 1.04–4.77, p=0.038), 2.78 (95%CI 1.29–6.00, p=0.009), and 2.09(95%CI 0.92–4.76, p=0.078) for group A patients. After adjustment for age and gender, the ORs for ΔBMI, ΔWC, and ΔBW were 2.83(95% CI 1.25–6.38, p=0.012), 2.73(95% CI 1.11–6.72, p=0.029), and 2.20(95% CI 0.96–5.02, p=0.061), respectively (Table 5).
Discussion
The TG level is an important indicator of the metabolic status in patients with obesity, and it is a component of the MS diagnostic criteria (14, 15). However, the association between baseline TG levels and LSG in patients with obesity remains unknown. In this study, we investigated the differences in patients’ metabolic parameters as a result of different baseline TG levels after LSG. The present study demonstrated that the levels of the blood glucose and lipids in both group A and B were significantly improved at 6 months after LSG, but it is worth highlighting that patients with a normal TG had greater improvements in BMI and WC.
Weight-loss surgery has been found to regulate BW safely and effectively, and improve metabolic parameters such as blood glucose and lipid levels (16, 17), as was also demonstrated in this study. BMI and WC, blood pressure, blood glucose and, blood lipids levels, insulin resistance, and HbA1c level were significantly improved after LSG. LSG has shown good efficacy and a low rate of complications and therefore, it is widely used worldwide (18–21). While LSG primarily exerts its restrictive effect by reducing stomach volume, and other metabolic and hormonal effects, it also improves serum lipid levels (22). Varlik et al. (23) found that TG and TC levels decreased significantly compared with preoperative levels in the dyslipidemia group, but not in the groups with normal lipid levels. They also found that LDL-C levels were significantly decreased, while HDL-C levels were significantly increased in both groups. Other research by our team has also extensively confirmed the benefits of LSG surgery for obese patients. The results demonstrate that 6 months after LSG surgery, total testosterone level increases and, fat mass decreases in all regions in obese male patients (24); moreover, the increased testosterone level negatively correlated with FINS and HOMA-IR, especially with IL-6 in acanthosis nigricans (AN) patients. Thus, LSG surgery can improve the skin condition of obese patients with AN (25). Similarly, 12 months after LSG, the subclinical hypothyroidism incidence, TSH levels, and inflammatory markers such as IL-6, TNF-α, and CRP also decrease significantly (5). In this study, patients significantly improved BMI, NC, WC, HC, waist-hip rate(WHR), and FPG, 2hPG, FINS, 2hINS, FCP, 2hCP, HbA1c, TG, HDL-C, CRP, and FT3 levels, and HOMA-IR in both groups.
The decrease in WC and BMI in group A is more significant, suggesting that the initial TG level has a certain prognostic value for the weight loss effect after LSG. To explore the effect of TG on weight loss, we performed regression models for the two groups of patients to evaluate the predictive effect of TG on the ΔBW, ΔBMI, and ΔWC. After adjustment for age and gender, the BMI and WC of patients in group A decreased greater than those in group B.
High TG levels are toxic to the body, resulting in poor blood glucose control, as well as disordered lipids and inflammatory factors levels. Studies have shown that high TG in mouse models can cause an increased level of reactive oxygen species (ROS), and increased activity of myeloperoxidase and adenosine deaminase, leading to inflammation (26). In this study, we observed a trend suggesting that group B patients had high levels of serum inflammatory markers, such as IL-6 and IL-8, than those in group A. Moreover, compared with the baseline values, the IL-6 and IL-8 serum levels also decreased. Zhu et al. (25) found that the IL-6 and IL-8 levels also decreased significantly after LSG in obese Chinese men with AN.
Additionally, a study by Pia Lundman et al. (27) showed that in patients with high TG levels, their plasma IL-6 levels were increased, and this was accompanied by a rise in other inflammation-related biochemical markers and the activation of endothelial cells. Similarly, another cross-sectional study also found that MS patients with hypertriglyceridemia had a significant increase in plasma IL-6 levels, and that IL-6 was positively correlated with HOMA-IR (28). We also found that the ΔTG level was positively correlated with the ΔFFA level in the high-TG group. Coincidentally, a study by Limin Wang et al. (29) found that triglyceride-rich lipoprotein can release neutral and oxidized FFA fragments during the degradation process, thereby, activating the NADPH enzyme and inducing the expression of cytochrome 450-mediated ROS products, which can cause endothelial cell inflammation. Interestingly, an in vitro experiment found that 17β-estradiol can decrease the level of TGs in adipocytes, but this effect can be attenuated by the large amount of IL-6 produced by LPS. In turn, this indicates that the inflammation state can also affect TG levels, which may be induced by the weakened activity of adipose TG lipase (30). When the body’s pro-inflammatory effect is stronger than the anti-inflammatory effect, the glucose and lipid metabolism in the body will be affected and related diseases will occur.
Obesity is associated with a chronic low-grade inflammatory state (31). Therefore, patients with higher TG levels had a more severe inflammatory state. Therefore, compared with obese patients with normal TG levels, the first step for patients with high TG levels is to improve the body’s inflammatory state and then, lose weight. This might explain our findings in which the BMI and WC among patients in group B did not show the same improvement as those among patients in group A after LSG surgery. However, the mechanism by which LSG could improve the inflammatory condition is yet to be elucidated.
Lowering TG levels can effectively reduce the risk of CVD, especially in the context of insulin resistance or low HDL levels (32, 33). Moreover, a TG reduction of ≥30% will produce a reduction in 1-year medical expenditure (34). Based on our study, baseline TG level may have a predictive value for weight loss in the short-term follow-up. In addition, obese patients with high TG levels can be treated to lower TG before LSG to improve their inflammatory state and increase the benefits of LSG for this kind of patient.
Patients with obesity or MS accompanied by insulin resistance tend to have TG enrichment and low HDL-C level due to decreased lipoprotein lipase activity. HDL can interchange proteins and lipids under the action of cholesterol ester transfer protein (CETP) and phospholipid transfer protein, thus maintaining the balance between proteins and lipids. In the presence of high TG, HDL level will further decrease, and TG will increase due to CETP (35). In fact, in our study, the HDL-C level in group B was lower than that of group A. A prospective cross-sectional study by Meryem Abi-Ayad et al. (36) found that in patients with MS, lower levels of HDL-C are associated with higher levels of VLDL-TG. Additionally, they found that HDL-C levels (<0.35 g/l), VLDL-TG levels (>0.656 g/l) can predict the presence of atherosclerotic plaque. In recent years, emerging studies have shown that the TG:HDL ratio can replace HOMA-IR and become a sensitive biomarker for early prediction of insulin resistance and cardiometabolic risk in obese people (37–39). Studies have highlighted that HDL can inhibit lipid oxidation and rebuild endothelial cells’ function, and has shown anti-inflammatory and anti-apoptotic activities in animal models (40). Another in vitro experiment confirmed that under LPS stimulation, HDL exhibits a wide range of anti-inflammatory activity. Its early anti-inflammatory activity is mainly achieved by reducing the level of Toll-like receptor 4, while the late anti-inflammatory activity is induced by reducing signals related to the interferon receptor pathway (41). From the perspective of anti-inflammatory activity, the decrease in HDL-C level may be closely related to the higher inflammatory state in the high TG group when compared to the normal TG group. Indeed, in our study, correlation analysis showed that the decrease in TG was positively correlated with the increase of HDL-C in group B.
Conclusions
In conclusion, TG levels play an important role in endocrine dysfunction in patients with obesity, and patients with high TG levels demonstrate slower decreases in body weight parameters (i.e., BMI and, WC), but exhibit rapid improvement in blood glucose and lipid levels. Hence, pre-operative serum TG levels can be used as a predictor of short-term weight loss following LSG.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of Shanghai Tenth People’s Hospital, Tongji University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conception and design: XH, GL, BX, YH, and SQ. Acquisition, statistical analysis, or interpretation of the data: all authors. Drafting of the manuscript: all authors. Revision of the English language: XC and MJ. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (2018YFC1314100), sponsored by the Shanghai Pujiang Program (2019PJD040, 2018PJD038), the Natural Science Foundation of China (81970677), the Shanghai Committee of Science and Technology, China (18411951803, 17DZ1910603), and the New Exploration of Blood Glucose Management Mode in Patients with Diabetes Mellitus in Chongming Area of Shanghai (CKY2018-19).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are thankful for the work of all the endocrinologists, surgeons, and nutritionists in National Metabolic Management Center in our hospital.
References
1. Abarca-Gómez L, Abdeen ZA, Hamid ZA, Abu-Rmeileh NM, Acosta-Cazares B, Acuin C, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet (London England) (2017) 390:2627–42. doi: 10.1016/s0140-6736(17)32129-3
2. Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue–link to whole-body phenotypes. Nat Rev Endocrinol (2015) 11:90–100. doi: 10.1038/nrendo.2014.185
3. Park S, Lee S, Kim Y, Lee Y, Kang MW, Han K, et al. Altered Risk for Cardiovascular Events With Changes in the Metabolic Syndrome Status: A Nationwide Population-Based Study of Approximately 10 Million Persons. Ann Internal Med (2019) 171:875–84. doi: 10.7326/m19-0563
4. Hudish LI, Reusch JE, Sussel L. β Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J Clin Invest (2019) 129:4001–8. doi: 10.1172/jci129188
5. Zhu C, Gao J, Mei F, Lu L, Zhou D, Qu S. Reduction in Thyroid-Stimulating Hormone Correlated with Improved Inflammation Markers in Chinese Patients with Morbid Obesity Undergoing Laparoscopic Sleeve Gastrectomy. Obes Surg (2019) 29:3954–65. doi: 10.1007/s11695-019-04063-4
6. English WJ, DeMaria EJ, Brethauer SA, Mattar SG, Rosenthal RJ, Morton JM. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis Off J Am Soc Bariatr Surg (2018) 14:259–63. doi: 10.1016/j.soard.2017.12.013
7. Sheetz KH, Woodside KJ, Shahinian VB, Dimick JB, Montgomery JR, Waits SA. Trends in Bariatric Surgery Procedures among Patients with ESKD in the United States. Clin J Am Soc Nephrol (2019) 14:1193–9. doi: 10.2215/cjn.01480219
8. Clapp B, Wynn M, Martyn C, Foster C, O’Dell M, Tyroch A. Long term (7 or more years) outcomes of the sleeve gastrectomy: a meta-analysis. Surg Obes Relat Dis Off J Am Soc Bariatr Surg (2018) 14:741–7. doi: 10.1016/j.soard.2018.02.027
9. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. New Engl J Med (2017) 376:641–51. doi: 10.1056/NEJMoa1600869
10. Aristizabal JC, Barona J, Gonzalez-Zapata LI, Deossa GC, Estrada A. Fatty Acid Content of Plasma Triglycerides May Contribute to the Heterogeneity in the Relationship Between Abdominal Obesity and the Metabolic Syndrome. Metab Syndr Relat Disord (2016) 14:311–7. doi: 10.1089/met.2015.0168
11. Bril F, Barb D, Portillo-Sanchez P, Biernacki D, Lomonaco R, Suman A, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatol (Baltimore Md) (2017) 65:1132–44. doi: 10.1002/hep.28985
12. Wang J, Shen X, He S, An Y, Gong Q, Li H, et al. Hypertriglyceridaemia predicts subsequent long-term risk of cardiovascular events in Chinese adults: 23-year follow-up of the Daqing Diabetes Study. Diabetes Metab Res Rev (2019) 35:e3163. doi: 10.1002/dmrr.3163
13. Austin PC, Merlo J. Intermediate and advanced topics in multilevel logistic regression analysis. Stat Med (2017) 36:3257–77. doi: 10.1002/sim.7336
14. Agyemang-Yeboah F, Eghan BAJ, Annani-Akollor ME, Togbe E, Donkor S, Oppong Afranie B. Evaluation of Metabolic Syndrome and Its Associated Risk Factors in Type 2 Diabetes: A Descriptive Cross-Sectional Study at the Komfo Anokye Teaching Hospital, Kumasi, Ghana. Biomed Res Int (2019) 2019:4562904. doi: 10.1155/2019/4562904
15. Sherling DH, Perumareddi P, Hennekens CH. Metabolic Syndrome. J Cardiovasc Pharmacol Ther (2017) 22:365–7. doi: 10.1177/1074248416686187
16. Zhang Y, Zhu C, Wen X, Wang X, Li L, Rampersad S, et al. Laparoscopic sleeve gastrectomy improves body composition and alleviates insulin resistance in obesity related acanthosis nigricans. Lipids Health Dis (2017) 16:209. doi: 10.1186/s12944-017-0598-z
17. Heffron SP, Parikh A, Volodarskiy A, Ren-Fielding C, Schwartzbard A, Nicholson J, et al. Changes in Lipid Profile of Obese Patients Following Contemporary Bariatric Surgery: A Meta-Analysis. Am J Med (2016) 129:952–9. doi: 10.1016/j.amjmed.2016.02.004
18. Azagury DE, Morton JM. Bariatric surgery: Overview of procedures and outcomes. Endocrinol Metab Clin North Am (2016) 45:647–56. doi: 10.1016/j.ecl.2016.04.013
19. Alsumali A, Eguale T, Bairdain S, Samnaliev M. Cost-Effectiveness Analysis of Bariatric Surgery for Morbid Obesity. Obes Surg (2018) 28:2203–14. doi: 10.1007/s11695-017-3100-0
20. Javanainen M, Penttilä A, Mustonen H, Juuti A, Scheinin T, Leivonen M. A Retrospective 2-Year Follow-up of Late Complications Treated Surgically and Endoscopically After Laparoscopic Roux-en-Y Gastric Bypass (LRYGB) and Laparoscopic Sleeve Gastrectomy (LSG) for Morbid Obesity. Obes Surg (2018) 28:1055–62. doi: 10.1007/s11695-017-2967-0
21. Yemini R, Nesher E, Carmeli I, Winkler J, Rahamimov R, Mor E, et al. Bariatric Surgery Is Efficacious and Improves Access to Transplantation for Morbidly Obese Renal Transplant Candidates. Obes Surg (2019) 29:2373–80. doi: 10.1007/s11695-019-03925-1
22. Morshed G, Fathy SM. Impact of post-laparoscopic sleeve gastrectomy weight loss on C-reactive protein, lipid profile and CA-125 in morbidly obese women. Wideochir Inne Tech Maloinwazyjne = Videosurgery Other Miniinvasive Tech (2016) 10:521–6. doi: 10.5114/wiitm.2015.56480
23. Erol V, Yılmaz TH, Tuncalı B, Arslan B, Gülay H. Changes in serum lipid levels after laparoscopic sleeve gastrectomy in morbidly obese dyslipidemic and normolipidemic patients. Acta Chir Belg (2018) 118:233–8. doi: 10.1080/00015458.2017.1417104
24. Gao J, Zhang M, Zhu C, Zhang Y, Liu Q, Wang X, et al. The Change in the Percent of Android and Gynoid Fat Mass Correlated with Increased Testosterone After Laparoscopic Sleeve Gastrectomy in Chinese Obese Men: a 6-Month Follow-Up. Obes Surg (2018) 28:1960–5. doi: 10.1007/s11695-018-3116-0
25. Zhu C, Mei F, Gao J, Zhou D, Lu L, Qu S. Changes in inflammatory markers correlated with increased testosterone after laparoscopic sleeve gastrectomy in obese Chinese men with acanthosis nigricans. J Dermatol (2019) 46:338–42. doi: 10.1111/1346-8138.14783
26. Manzoni AG, Passos DF, da Silva JLG, Bernardes VM, Bremm JM, Jantsch MH, et al. Rutin and curcumin reduce inflammation, triglyceride levels and ADA activity in serum and immune cells in a model of hyperlipidemia. Blood Cells Mol Dis (2019) 76:13–21. doi: 10.1016/j.bcmd.2018.12.005
27. Lundman P, Eriksson MJ, Silveira A, Hansson LO, Pernow J, Ericsson CG, et al. Relation of hypertriglyceridemia to plasma concentrations of biochemical markers of inflammation and endothelial activation (C-reactive protein, interleukin-6, soluble adhesion molecules, von Willebrand factor, and endothelin-1). Am J Cardiol (2003) 91:1128–31. doi: 10.1016/s0002-9149(03)00165-6
28. Zafar U, Khaliq S, Ahmad HU, Lone KP. Serum profile of cytokines and their genetic variants in metabolic syndrome and healthy subjects: a comparative study. Biosci Rep (2019) 39:BSR20181202. doi: 10.1042/bsr20181202
29. Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res (2009) 50:204–13. doi: 10.1194/jlr.M700505-JLR200
30. Luo F, Huang WY, Guo Y, Ruan GY, Peng R, Li XP. 17β-estradiol lowers triglycerides in adipocytes via estrogen receptor α and it may be attenuated by inflammation. Lipids Health Dis (2017) 16:182. doi: 10.1186/s12944-017-0575-6
31. Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract (2014) 105:141–50. doi: 10.1016/j.diabres.2014.04.006
32. Maki KC, Guyton JR, Orringer CE, Hamilton-Craig I, Alexander DD, Davidson MH. Triglyceride-lowering therapies reduce cardiovascular disease event risk in subjects with hypertriglyceridemia. J Clin Lipidol (2016) 10:905–14. doi: 10.1016/j.jacl.2016.03.008
33. Vrablík M, Češka R. Treatment of hypertriglyceridemia: a review of current options. Physiol Res (2015) 64:S331–40. doi: 10.33549/physiolres.933199
34. Nichols GA, Reynolds K, Olufade T, Kimes TM, O’Keeffe-Rosetti M, Sapp DS, et al. Effect of Combination Cholesterol-Lowering Therapy and Triglyceride-Lowering Therapy on Medical Costs in Patients With Type 2 Diabetes Mellitus. Am J Cardiol (2017) 119:410–5. doi: 10.1016/j.amjcard.2016.10.029
35. Girona J, Amigó N, Ibarretxe D, Plana N, Rodríguez-Borjabad C, Heras M, et al. HDL Triglycerides: A New Marker of Metabolic and Cardiovascular Risk. Int J Mol Sci (2019) 20:3151. doi: 10.3390/ijms20133151
36. Abi-Ayad M, Abbou A, Abi-Ayad FZ, Behadada O, Benyoucef M. HDL-C, ApoA1 and VLDL-TG as biomarkers for the carotid plaque presence in patients with metabolic syndrome. Diabetes Metab Syndr (2018) 12:175–9. doi: 10.1016/j.dsx.2017.12.017
37. Behiry EG, El Nady NM, AbdEl Haie OM, Mattar MK, Magdy A. Evaluation of TG-HDL Ratio Instead of HOMA Ratio as Insulin Resistance Marker in Overweight and Children with Obesity. Endocr Metab Immune Disord Drug Targets (2019) 19:676–82. doi: 10.2174/1871530319666190121123535
38. Nur Zati Iwani AK, Jalaludin MY, Wan Mohd Zin RM, Fuziah MZ, Hong JYH, Abqariyah Y, et al. TG : HDL-C Ratio Is a Good Marker to Identify Children Affected by Obesity with Increased Cardiometabolic Risk and Insulin Resistance. Int J Endocrinol (2019) 2019:8586167. doi: 10.1155/2019/8586167
39. Krawczyk M, Rumińska M, Witkowska-Sędek E, Majcher A, Pyrżak B. Usefulness of the Triglycerides to High-Density Lipoprotein Cholesterol ratio (TG/HDL-C) in prediction of metabolic syndrome in Polish obese children and adolescents. Acta Biochim Pol (2018) 65:605–11. doi: 10.18388/abp.2018_2649
40. Rosenson RS, Brewer HB Jr, Ansell BJ, Barter P, Chapman MJ, Heinecke JW, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol (2016) 13:48–60. doi: 10.1038/nrcardio.2015.124
41. Fotakis P, Kothari V, Thomas DG, Westerterp M, Molusky MM, Altin E, et al. Anti-Inflammatory Effects of HDL (High-Density Lipoprotein) in Macrophages Predominate Over Proinflammatory Effects in Atherosclerotic Plaques. Arterioscler Thromb Vasc Biol (2019) 39:e253–e72. doi: 10.1161/atvbaha.119.313253
Keywords: obesity, laparoscopic sleeve gastrectomy, triglyceride, body mass index, waist circumference
Citation: Huang X, Li G, Xu B, Zhang J, Wang X, Cheng X, Jayachandran M, Huang Y and Qu S (2021) Lower Baseline Serum Triglyceride Levels Are Associated With Higher Decrease in Body Mass Index After Laparoscopy Sleeve Gastrectomy Among Obese Patients. Front. Endocrinol. 12:633856. doi: 10.3389/fendo.2021.633856
Received: 26 November 2020; Accepted: 07 January 2021;
Published: 22 February 2021.
Edited by:
Marwan El Ghoch, Beirut Arab University, LebanonReviewed by:
Barbara Zanini, University of Brescia, ItalyFrancesca Abbatini, Sapienza University of Rome, Italy
Copyright © 2021 Huang, Li, Xu, Zhang, Wang, Cheng, Jayachandran, Huang and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shen Qu, cXVzaGVuY25AaG90bWFpbC5jb20=; Yueye Huang, aHVhbmd5dWV5ZTE5ODkwNjAzQDE2My5jb20=
†These authors have contributed equally to this work
 Xiu Huang1†
Xiu Huang1† Junyi Zhang
Junyi Zhang Shen Qu
Shen Qu