- 1Center for Reproductive Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Center for Reproductive Medicine, The First People’s Hospital of Shangqiu, Shangqiu, China
The mechanisms underlying poor ovarian response (POR) in assisted reproductive technology remain unclear, there is no consensus on the management of poor responders, the POSEIDON stratification classifies infertility patients into “expected” or “unexpected” groups to provide a more nuanced picture of POR, but few researchers have discussed the independent predictive factors (smoothed plots and the threshold effect) for live birth in POR patients classified by the new criteria. We conducted a retrospective cohort study using clinical data from 6,580 POR patients classified by the POSEIDON criteria in the First Affiliated Hospital of Zhengzhou University, and explored the live birth based on the results before and after the threshold inflection point of each independent influencing factor. Among 6,580 poor ovarian reserve patients classified by the POSEIDON criteria, 1,549 (23.54%) had live births, and 5,031 (76.46%) did not have live births. Multivariate logistic regression analysis showed that female age (OR 0.901; 95% CI 0.887~0.916; P < 0.001), body mass index (OR 0.963; 95% CI 0.951~0.982; P < 0.001), antral follicle counting (OR 1.049; 95% CI 1.009~1.042; P < 0.001) and controlled ovarian hyperstimulation protocol were independent factors predicting live birth in patients with POR. The threshold effect analysis found that the inflection point of female age was 34 years old, and when age was > 34 years old, the probability of live birth in POR patients dropped sharply (OR 0.7; 95% CI 0.7~0.8; P < 0.001). The inflection point of BMI was 23.4 kg/m2, and BMI had a negative correlation with live birth (OR 0.963; 95% CI 0.951~0.982; P < 0.001). The threshold inflection point of AFC was 8n. Female age, BMI, AFC and COH protocol were independent predictive factors associated with live birth in POR patients classified by the POSEIDON criteria. The smooth curve fit and threshold effect analyses provide clinical management strategies for these patients. In addition, the early-follicular-phase long-acting GnRH-agonist long protocol seems to have a higher live birth rates than other protocols. It is worth highlighting that BMI should be considered as well in the POSEIDON criteria.
Introduction
The Patient Oriented Strategies Encompassing Individualized Oocyte Number (POSEIDON) group proposed a new stratification method for poor ovarian response (POR) patients in 2016 (1, 2). The new stratification classifies infertility patients into “expected” or “unexpected” groups to provide a more nuanced picture of POR (3). Different from the failure of the Bologna criteria, which reflect the significantly variable profiles and biological characteristics of POR patients (4), the POSEIDON criteria consider clinical recommendations with a new pragmatic endpoint: the number of oocytes needed to obtain one euploid embryo for transfer in each patient that results in a live birth (5, 6). To date, the POSEIDON criteria have been well accepted by infertility specialists and reproductive endocrinologists worldwide (7, 8). The POSEIDON criteria seem to be useful for identifying and classifying patients with impaired ovarian reserves or PORs, but few researchers have discussed the independent predictive factors for live birth in POR patients classified by the POSEIDON criteria (6), there is still a lot of work to verify whether it is better than the Bologna standard.
Accurate predictive factors of ovarian reserve and pregnancy outcome in infertile women with poor ovarian response (POR) remain unknown and are one of the main puzzles of assisted reproductive technology treatments. Although many studies have been performed to identify predictors of IVF/ICSI outcome, there is still no real consensus (9, 10). This may be because diagnostic criteria and mechanisms underlying POR in assisted reproductive technology remain unclear (11), making it difficult for clinicians to provide guidance regarding outcome prediction and management in POR patients (12, 13). Therefore, a large and comprehensive study on the probability of live birth in POR patients is necessary. Our study retrospectively analyzed the clinical data of 6,580 POR patients classified by the POSEIDON criteria who underwent IVF/ICSI in the reproductive medical center of the First Affiliated Hospital of Zhengzhou University from June 2013 to December 2018. This study aimed to explore the independent predictive factors associated with live birth in POR to provide a reference and help for clinical work.
Today, large data and evidence-based medicine have progressively developed as the standard approach for clinical management strategies in the field of reproductive medicine (3, 14). At our reproductive medical center, > 10000 IVF/ICSI cycles are performed each year, which can help us more conveniently screen out independent predictive factors for the “unexpected” and “expected” POSEIDON groups, and to our knowledge, this could be the first study to establish smoothed plots and perform a threshold effect analysis to systematically evaluate independent factors associated with live birth in POR patients classified by the POSEIDON criteria, with the goal of providing guidance for predicting the probability of live birth and managing such patients in future clinical practice.
Material and Methods
This retrospective cohort study evaluated clinical data from 6,580 POR patients classified by the POSEIDON criteria who underwent IVF/ICSI in the reproductive medical center of the First Affiliated Hospital of Zhengzhou University from June 2013 to December 2018. All the included case data were recorded and sorted by dedicated personnel. The quality of data entry is strictly controlled to ensure the completeness and authenticity of the data. This study strictly followed the relevant requirements of the Declaration of Helsinki of the World Medical Association. The study was a retrospective study approved by the Medical Ethics Committee and did not require the informed consent of patients.
We compared the experimental data of patients in the “live birth group” and “non-live birth group” and determined the statistically significant influencing factors for the two groups of patients. Based on these results, further single-factor and multifactor logistic regression analysis was performed to screen for independent influencing factors for live birth in POR patients; then, a smooth fitting curve model was constructed based on independent influencing factors, and the linearity between these influencing factors and the occurrence of live birth was observed. The relationship was further analyzed based on the model observation results, and finally, the probability of live birth was explored in detail based on the results before and after the threshold inflection point of each independent influencing factor.
There patients were put on either the early-follicular-phase long-acting GnRH-agonist long protocol, Mid-luteal phase short-acting GnRH-a long protocol, GnRH antagonist protocol, GnRH agonist short protocol or natural cycle for IVF/ICSI followed by embryo transfer in the same cycle. The starting dose of gonadotropin (Puregon, Organon, The Netherlands) were administrated on the basis of patient`s antral follicle count, age, BMI and previous ovarian response to stimulation. The dosage was also adjusted constantly according to patient`s response. When dominant follicles measuring > 16mm account for 60% or a follicle reached 20mm in mean diameter, trigger was normally performed using 250ug r-hCG (Livzon Pharmaceuticals, China) in combination with 2000IU u-HCG (Merck Schlano, Italy). Oocyte retrieval under the guidance of transvaginal ultrasound was performed 37 hours after trigger. The diagnosis of spontaneous abortion is after the pregnancy is confirmed, the clinical history, physical examination, female endocrine and vaginal ultrasound confirm that the embryo stops developing before 20 weeks of pregnancy (7, 15, 16). Live birth was defined as a delivery of any birth event in which at least one baby was born alive, and the live fetus must be after 20 completed weeks of gestational age.
POSEIDON “unexpected” group: age < 35 years, AFC ≥ 5, AMH ≥ 1.2 ng/mL and ≤ 9 oocytes retrieved in the first stimulation cycle; age ≥ 35 years, AFC ≥ 5, AMH ≥ 1.2 ng/mL and ≤ 9 oocytes retrieved in the first stimulation cycle; POSEIDON “expected” group: age < 35 years, AFC < 5, AMH < 1.2 ng/mL; age ≥ 35 years, AFC < 5, AMH < 1.2 ng/mL (8, 17, 18).
Statistical Analysis
All analyses were performed using the statistical packages R (The R Foundation; http://www.r-project.org; version 3.6.1), EmpowerStats (http://www.empowerstats.com) and SPSS 19.0 (IBM, Armonk, NY, USA). The continuous variable data are expressed as the mean ± standard deviation (Mean ± SD) and were compared using Student’s t test or the Wilcoxon rank sum test; categorical variables are expressed as frequencies (percentages) and were compared using the chi-square test. We then applied multiple regression analysis to estimate the independent relationship between live birth and female age, BMI, AFC and COH protocol with an adjustment for potential confounders. P < 0.05 indicated that a difference was statistically significant, to avoid inflating the probability of a Type I error, P-values were adjusted with the Holm-Bonferroni method.
For the independent influencing factors (female age, BMI, AFC and COH protocols, the association remained statistically significant after Holm-Bonferroni correction for multiple testing, the R 3.6.1 software package was used to further apply a two piecewise-linear regression model to examine the threshold effect of the influencing factors on live birth using a smoothing function curve, and the threshold level was determined using trial and error. We also conducted a log-likelihood ratio test comparing the one-line linear regression model with a two piecewise-linear model and calculated before and after odds ratios (ORs) and 95% confidence intervals (Cis) for the threshold turning points of the independent influencing factors. P < 0.05 indicated that a difference was statistically significant.
Results
We collected effective experimental data from 6,580 POR patients classified by the POSEIDON criteria, the “unexpected” group (n=3639) and the “expected” group (n=2951), and 1,549 (23.54%) had live births, 5,031 (76.46%) did not have live births, and 1309 of these women (35.97%) succeeded had live births in “unexpected” group, 240 of these women (8.13%) succeeded had live births in “expected” group. There were statistically significant differences in female age, primary infertility, infertility years, BMI, pasal P, AMH, PRL, AFC, number of treatment cycles, initiation dosage of Gn used, duration of Gn used, oocyte number, and use of a controlled ovarian hyperstimulation protocol between the two groups (Table 1).
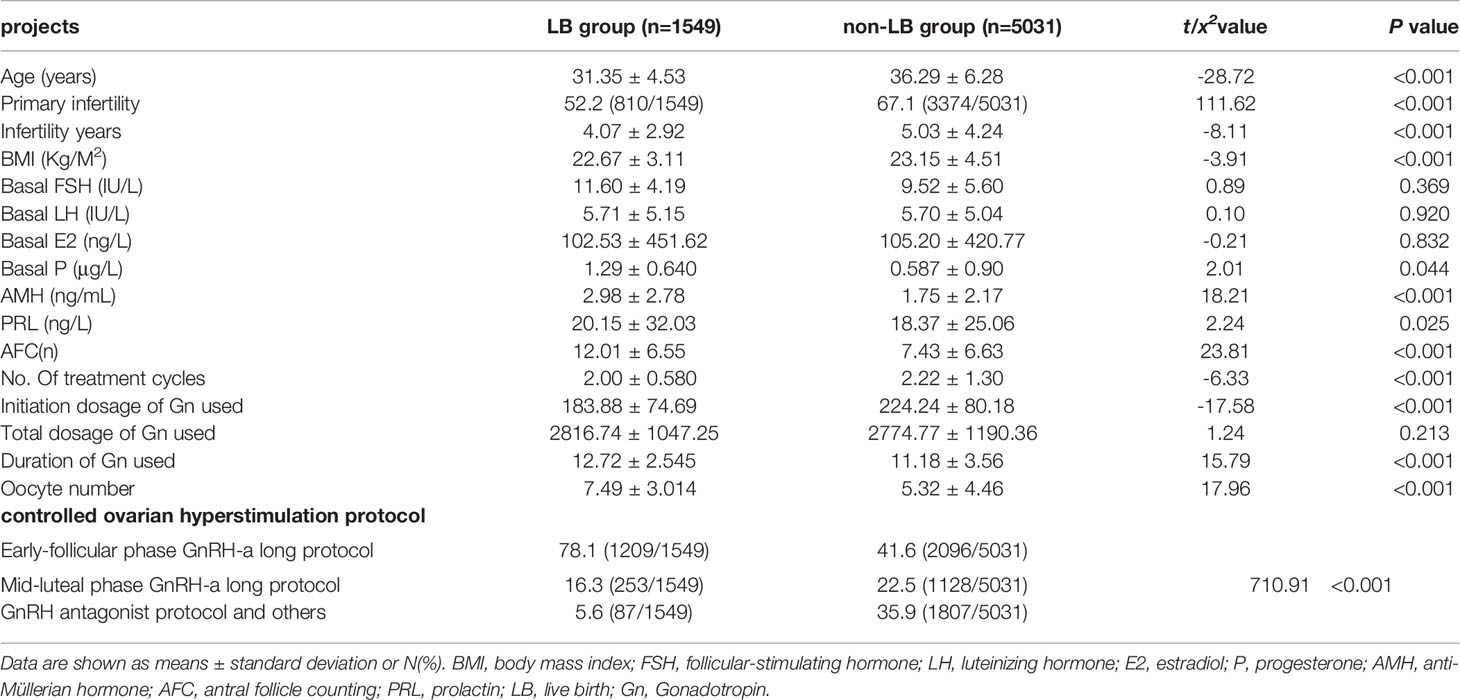
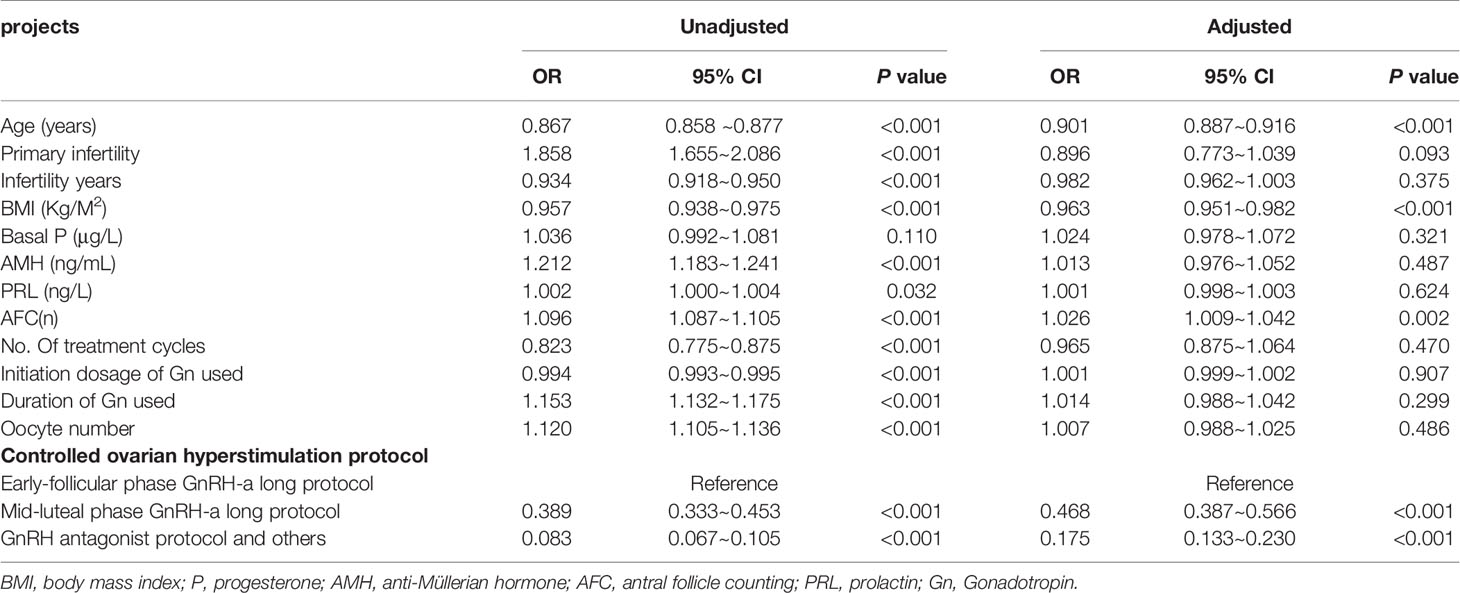
Table 1 Baseline characteristics of patients with LB and non-LB and independent risk factors for the pregnancy results.
Univariate logistic regression analysis showed that female age (OR 0.867; 95% CI 0.858 ~0.877; P < 0.001), primary infertility (OR 1.858; 95% CI 1.655~2.086; P < 0.001), infertility years (OR 0.934; 95% CI 0.918~0.950; P < 0.001), BMI (OR 0.957; 95% CI 0.938~0.975; P < 0.001), AMH (OR 1.212; 95% CI 1.183~1.241; P < 0.001), PRL (OR 1.002; 95% CI 1.000~1.004; P = 0.032), AFC (OR 1.096; 95% CI 1.087~1.105; P < 0.001), No. of treatment cycles (OR 0.823; 95% CI 0.775~0.875; P <0.001), initiation dosage of Gn used (OR 0.994; 95% CI 0.993~0.995; P < 0.001), duration of Gn used (OR 1.153; 95% CI 1.132~1.175; P < 0.001), oocyte number (OR 1.120; 95% 1.105~1.136; P < 0.001) and COH protocol were factors for predicting live birth in patients with POR. Multivariate logistic regression analysis showed that female age (OR 0.901; 95% CI 0.887~0.916; P < 0.001), BMI (OR 0.963; 95% CI 0.951~0.982; P < 0.001), AFC (OR 1.049; 95% CI 1.009~1.042; P < 0.001) and COH protocol were independent factors for predicting live birth in patients with POR (Table 2).
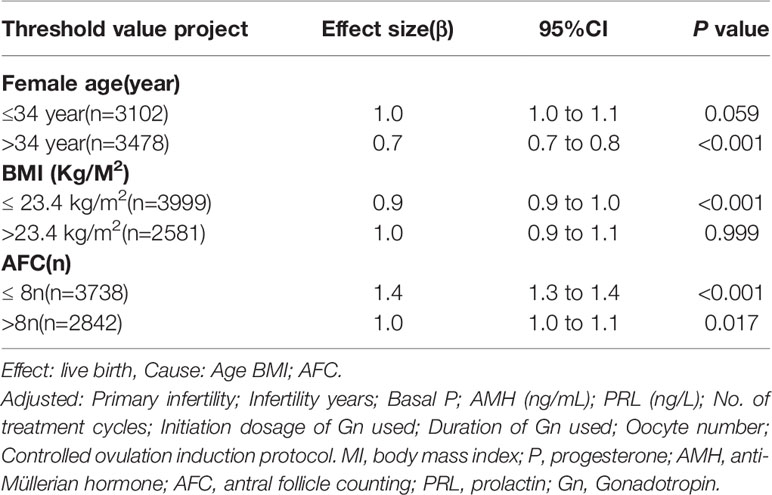
Multivariate logistic regression analysis showed that female age, BMI, and AFC were independent predictive factors associated with live birth in POR patients classified by the POSEIDON criteria. The adjusted smooth curve fit showed a nonlinear correlation between them. Age and BMI had a negative correlation with live birth, and AFC had a positive correlation with live birth. However, these influencing factors did not have a simple linear relationship with live birth (Figure 1-3), and further threshold effect analysis was needed.
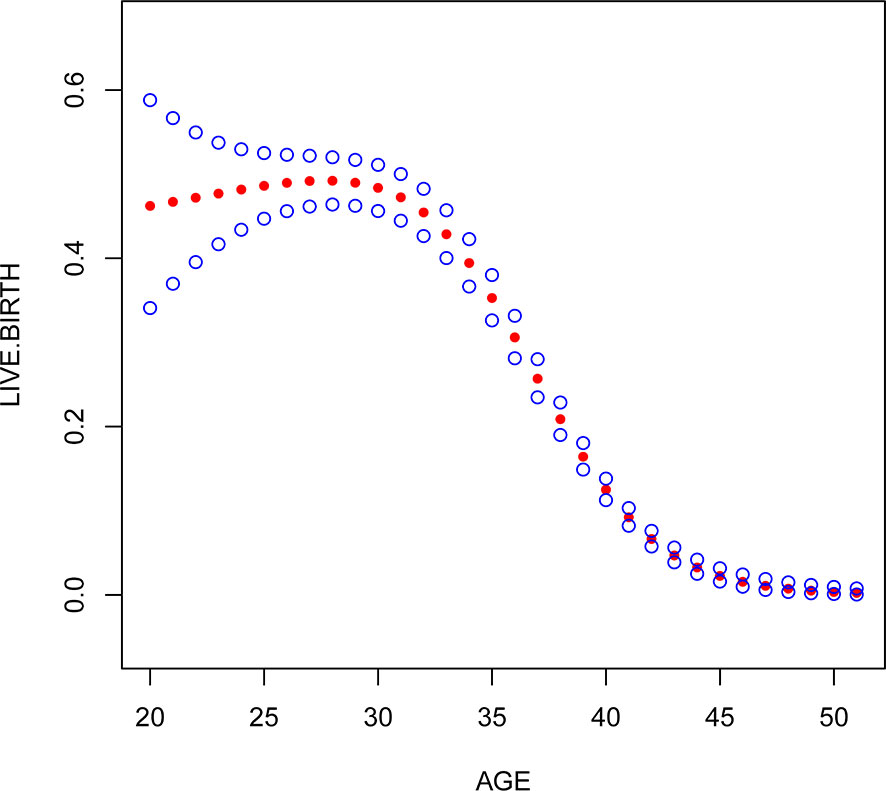
Figure 1 Association between live birth and female age. A threshold, nonlinear association between live birth and these independent predictive factors was found in a generalized additive model (GAM). Solid rad line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit.
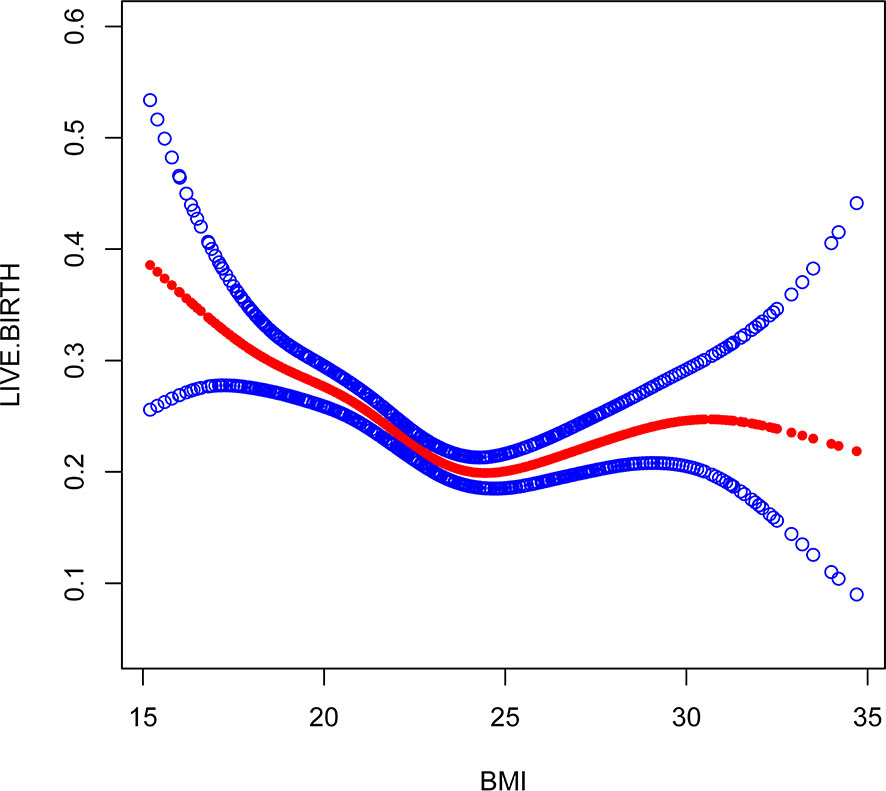
Figure 2 Association between live birth and BMI. A threshold, nonlinear association between live birth and these independent predictive factors was found in a generalized additive model (GAM). Solid rad line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit.
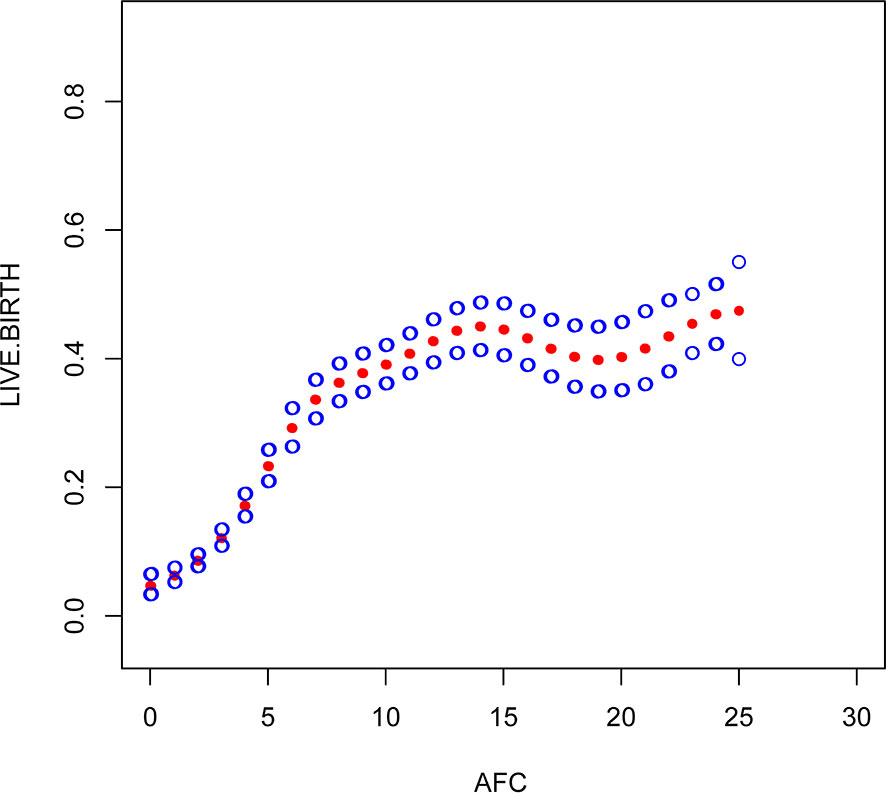
Figure 3 Association between live birth and AFC. A threshold, nonlinear association between live birth and these independent predictive factors was found in a generalized additive model (GAM). Solid rad line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit.
Through the threshold effect analysis, we found that the inflection point of female age was 34 years old, and when age was > 34 years old, the probability of live birth in POR patients dropped sharply (OR 0.7; 95% CI 0.7~0.8; P < 0.001). When age ≤ 32 years of age, the probability of spontaneous miscarriage and female age were not significantly related (OR 1.0; 95% 1.0~1.1; P=0.059). The inflection point of BMI was 23.4 kg/m2; when BMI > 23.4 kg/m2, it had a negative correlation with live birth (OR 0.9; 95% CI 0.9~1.0; P < 0.001), but when BMI > 23.4 kg/m2, there was no significant relationship between them (OR 1.0; 95% 0.9~1.1; P=0.999). The threshold inflection point of AFC was 8n. However, there was still a roughly linear positive relationship between AFC and live birth before and after the inflection point (Table 3).
Discussion
Our study first analyzed the independent factors predicting the possibility of live birth in POR patients using smooth curve fit and threshold effect analyses. The results confirm that the POSEIDON classification criteria are indeed very accurate in predicting the live birth rate for POR patients, which is very similar to our results, however, it seems that the results indicate that BMI should be considered as well in the POSEIDON criteria, and AFC threshold of 8 instead of 5 may be more relevant. AMH was not an independent factor, as this is also included in the POSEIDON criteria. We all know that various advances in assisted reproductive technologies have led to a significant improvement in the efficacy of treatment for couples who with infertility in recent decades, and various embryo manipulation techniques and controlled ovarian stimulation specifications continue to be improved (19, 20). However, the mechanisms underlying POR in ART remain unclear (21), and it has been difficult to develop clinical management strategies based on the characteristics and prognoses of POR patients. At the same time, research on independent factors predicting the live birth of POR patients is scarce, especially for patients classified by the POSEIDON criteria. For these independent predictors, a smoothed plot fit and threshold effect analyses were successfully conducted. These research results can provide a more detailed estimate of the probability of live birth in POR patients and guide preventive and personalized interventions for pregnant couples.
Our study found that the older the female was, the lower the probability of live birth in POR patients. In fact, some studies have suggested that the blastocyst euploidy rate drops from 60% before 35 years to 30% after 40 years and that the embryo euploidy rate decreases by 2.4% per year with increasing female age. These studies suggest a negative correlation between age and the live birth rate (7, 22). Different from these studies, we found that there was no simple linear relationship between these two factors according to the smoothed curve plots. The threshold effect of female age showed that the inflection point was 34 years old, which means that when age is > 34 years old, the probability of live birth in POR patients drops sharply; when age ≤ 34 years of age, the probability of spontaneous miscarriage and female age were not significantly related. A large number of studies have shown that female age is very important in the quality of embryos and endometrial receptivity (10, 23). With increasing age, the number and quality of oocytes are significantly reduced, the number of mitochondria and the ATP content in the cytoplasm significantly decrease, the proportion of abnormal embryo chromosome structure increases, and live birth is closely related to embryo chromosomal abnormalities (24, 25). Higher rates of single chromatid abnormalities in oocytes, as well as aneuploidy in preimplantation embryos and ongoing pregnancies, have been observed in older women, which is a major cause of increased miscarriage and decreased live birth rates in patients of advanced reproductive age (26). Given that aging is a complex process, it is difficult to find a clear critical point for age because the various risks caused by the age effect will be influenced by external factors, and it is difficult to define a cutoff point that clearly identifies all events that have the same result. However, research on the possible relationship between age and live birth can guide genetic counseling and the prediction of live birth before pregnancy. Therefore, we encourage POR patients aged > 34 to receive assisted pregnancy guidance as early as possible.
This study found that AFC and BMI are other important factors for predicting live birth in patients with POR. AFC is a good indicator of reactive ovarian reserve function in infertile patients (27, 28). Bunnewell SJ et al. showed that low AFC levels could predict higher odds of pregnancy loss (OR 2.45; 95% CI, 1.16-5.19), which leads to a drop in live birth rates (29). However, Bishop LA et al. showed that AFC was not significantly associated with pregnancy loss at any age (30). Sermondade N et al.’s meta-analysis showed a decreased probability of live birth in obese (BMI ≥ 30 kg/m2) women compared with normal weight (BMI 18.5-24.9 kg/m2) women: risk ratio (OR 0.85; 95% CI 0.82-0.87) (31). Through the establishment of a smooth plot curves, we found that AFC is an independent predictor of live birth and is positively correlated with it, suggesting that the higher the AFC, the higher the probability of live birth is. The threshold inflection point of AFC was 8n. However, there was still a roughly linear positive relationship between AFC and live birth before and after the inflection point. The inflection point of BMI was 23.4 kg/m2; when BMI ≤ 23.4 kg/m2, it had a negative correlation with live birth (OR 0.9; 95% CI 0.9~1.0; P < 0.001), but when BMI > 23.4 kg/m2, there was no significant relationship between them (OR 1.0; 95% 0.9~1.1; P=0.999). Through our research, it seems that BMI should be considered as well in the POSEIDON criteria, and AFC threshold of 8 instead of 5 may be more relevant, However, it needs to prospective, large-scale and multicenter clinical trials to confirm in the future.
The early-follicular-phase long-acting GnRH-agonist long protocol seems to have a higher live birth rates than other protocols for POR patients. This protocol is designed to suppress the pituitary gland for 28 days, and a standard full dose of GnRH-a before ovarian stimulation in IVF-ET might improve the pregnancy and live birth rates per fresh ET (7, 32). Some studies have suggested that a full-dose depot GnRH-a injection before ovarian stimulation in IVF-ET might improve endometrial receptivity and live birth rates (32–34). Some meta-analyses on live birth revealed that the GnRH-a protocol was more effective than the GnRH-ant protocol (34, 35). However, we want to emphasize that retrospective trials are always associated with selection bias issues (36). Despite our attempts to screen eligible subjects according to the POSEIDON criteria and remove confounding factors, patients with good ovarian responses may have been more likely to be assigned to the GnRH-ant protocol group. We will conduct randomized controlled trials to confirm this hypothesis in the future.
In summary, our study can more accurately predict the probability of a live birth and provide clinical management strategies for these patients. However, the main limitation of our study is that it is a retrospective study that could not exclude all potential biases, and pregnancy is a process of continuous change, during which there are many confounding factors. For example, maternal infection factors, environmental factors, emotional factors, etc., were not taken into account in this study, and prospective, large-scale and multicenter clinical trials are still needed to confirm our findings in the future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of The First Affiliated Hospital of Zhengzhou University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
GL and FL conceived of and designed the experiments. GL, TY and LH selected and supervised suitable patients. GL, JL, HJ, and LF obtained basic clinical data including age, body mass index, FSH, LH, estradiol, progesterone and AMH levels, total dosage of gonadotropin used, duration of gonadotropin use, oocyte number, and live birth rate per transfer. GL provided overall supervision. GL, GH and FL drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81771534), the Key Science and Technology Foundation of Henan Province (SBGL202002048), the Key Research Projects of Henan Higher Education Institutions (18A320057) and the Medical Science and Technology Project of Henan Province (212102310049).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the women who participated in this study and all the physicians and nurses at the Reproductive Medicine Center, the First Affiliated Hospital of Zhengzhou University, China, for their support in collecting the data.
References
1. Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, Esteves SC, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril (2016) 105(6):1452–3. doi: 10.1016/j.fertnstert.2016.02.005
2. Grynberg M, Labrosse J. Understanding Follicular Output Rate (FORT) and its Implications for POSEIDON Criteria. Front Endocrinol (2019) 10:246. doi: 10.3389/fendo.2019.00246
3. Haahr T, Dosouto C, Alviggi C, Esteves SC, Humaidan P. Management Strategies for POSEIDON Groups 3 and 4. Front Endocrinol (2019) 10: (undefined):614. doi: 10.3389/fendo.2019.00614
4. Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod (Oxford England) (2011) 26(7):1616–24. doi: 10.1093/humrep/der092
5. Drakopoulos P, Blockeel C, Stoop D, Camus M, de Vos M, Tournaye H, et al. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod (Oxford England) (2016) 31(2):370–6. doi: 10.1093/humrep/dev316
6. Esteves SC, Alviggi C, Humaidan P, Fischer R, Andersen CY, Conforti A, et al. The POSEIDON Criteria and Its Measure of Success Through the Eyes of Clinicians and Embryologists. Front Endocrinol (2019) 10(undefined):814. doi: 10.3389/fendo.2019.00814
7. Li F, Ye T, Kong H, Li J, Hu L, Jin H, et al. Efficacies of different ovarian hyperstimulation protocols in poor ovarian responders classified by the POSEIDON criteria. Aging (2020) 12(10):9354–64. doi: 10.18632/aging.103210
8. Grisendi V, Mastellari E, La Marca A. Ovarian Reserve Markers to Identify Poor Responders in the Context of Poseidon Classification. Front Endocrinol (2019) 10(undefined):281. doi: 10.3389/fendo.2019.00281
9. Lamazou F, Genro V, Fuchs F, Grynberg M, Frydman R. Serum AMH level is not a predictive value for IVF in modified natural cycle: analysis of 342 cycles). J Gynecol Obstet Biol Reprod (2011) 40(3):205–10. doi: 10.1016/j.jgyn.2011.02.002
10. Scheffer JB, Scheffer BB, de Carvalho RF, Rodrigues J, Mendez Lozano DH. Age as A Predictor of Embryo Quality Regardless of The Quantitative Ovarian Response. Int J Fertil Steril (2017) 11(1):40–6. doi: 10.22074/ijfs.2016.4579
11. Patrizio P, Vaiarelli A, Levi Setti PE, Tobler KJ, Shoham G, Leong M, et al. How to define, diagnose and treat poor responders? Responses from a worldwide survey of IVF clinics. Reprod Biomed Online (2015) 30(6):581–92. doi: 10.1016/j.rbmo.2015.03.002
12. Xu Y, Nisenblat V, Lu C, Li R, Qiao J, Zhen X, et al. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: a randomized controlled trial. Reprod Biol Endocrinol (2018) 16(1):29. doi: 10.1186/s12958-018-0343-0
13. Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update (2006) 12(6):685–718. doi: 10.1093/humupd/dml034
14. Schisterman EF, Sjaarda LA, Clemons T, Carrell DT, Perkins NJ, Johnstone E, et al. Effect of Folic Acid and Zinc Supplementation in Men on Semen Quality and Live Birth Among Couples Undergoing Infertility Treatment: A Randomized Clinical Trial. JAMA (2020) 323(1):35–48. doi: 10.1001/jama.2019.18714
15. Bar Hava I, Blueshtein M, Ganer Herman H, Omer Y, Ben David G. Gonadotropin-releasing hormone analogue as sole luteal support in antagonist-based assisted reproductive technology cycles. Fertil Steril (2017) 107(1):130–5.e1. doi: 10.1016/j.fertnstert.2016.10.011
16. Dan W, Jing G, Liangbin X, Ting Z, Ying Z. Association of follicle stimulating hormone receptor promoter with ovarian response in IVF-ET patients. Iran J Reprod Med (2015) 13(11):715–20.
17. Polyzos NP, Drakopoulos P. Management Strategies for POSEIDON’s Group 1. Front Endocrinol (2019) 10:679. doi: 10.3389/fendo.2019.00679
18. Humaidan P, Alviggi C, Fischer R, Esteves SC. The novel POSEIDON stratification of ‘Low prognosis patients in Assisted Reproductive Technology’ and its proposed marker of successful outcome. F1000Research (2016) 5:2911. doi: 10.12688/f1000research.10382.1
19. SenGupta SB, Dhanjal S, Harper JC. Quality control standards in PGD and PGS. Reprod Biomed Online (2016) 32(3):263–70. doi: 10.1016/j.rbmo.2015.11.020
20. Toftager M, Sylvest R, Schmidt L, Bogstad J, Løssl K, Prætorius L, et al. Quality of life and psychosocial and physical well-being among 1,023 women during their first assisted reproductive technology treatment: secondary outcome to a randomized controlled trial comparing gonadotropin-releasing hormone (GnRH) antagonist and GnRH agonist protocols. Fertil Steril (2018) 109(1):154–64. doi: 10.1016/j.fertnstert.2017.09.020
21. Esteves SC, Roque M, Bedoschi GM, Conforti A, Humaidan P, Alviggi C. Defining Low Prognosis Patients Undergoing Assisted Reproductive Technology: POSEIDON Criteria-The Why. Front Endocrinol (2018) 9:461:461. doi: 10.3389/fendo.2018.00461
22. Özkan ZS. Ovarian stimulation modalities in poor responders. Turk J Med Sci (2019) 49(4):959–62. doi: 10.3906/sag-1905-179
23. Abu-Musa A, Haahr T, Humaidan P. Novel Physiology and Definition of Poor Ovarian Response; Clinical Recommendations. Int J Mol Sci (2020) 21(6):211. doi: 10.3390/ijms21062110
24. Du Y, Chen L, Lin J, Zhu J, Zhang N, Qiu X, et al. Chromosomal karyotype in chorionic villi of recurrent spontaneous abortion patients. Biosci Trends (2018) 12(1):32–9. doi: 10.5582/bst.2017.01296
25. Scheffer JAB, Scheffer B, Scheffer R, Florencio F, Grynberg M, Lozano DM. Are age and anti-Müllerian hormone good predictors of ovarian reserve and response in women undergoing IVF? JBRA Assist Reprod (2018) 22(3):215–20. doi: 10.5935/1518-0557.20180043
26. Maignien C, Santulli P, Gayet V, Lafay-Pillet MC, Korb D, Bourdon M, et al. Prognostic factors for assisted reproductive technology in women with endometriosis-related infertility. Am J Obstet Gynecol (2017) 216(3):280.e1–.e9. doi: 10.1016/j.ajog.2016.11.1042
27. Aflatoonian A, Eftekhar M, Mohammadian F, Yousefnejad F. Outcome of assisted reproductive technology in women aged 40 years and older. Iran J Reprod Med (2011) 9(4):281–4.
28. Vaegter KK, Lakic TG, Olovsson M, Berglund L, Brodin T, Holte J. Which factors are most predictive for live birth after in vitro fertilization and intracytoplasmic sperm injection (IVF/ICSI) treatments? Analysis of 100 prospectively recorded variables in 8,400 IVF/ICSI single-embryo transfers. Fertil Steril (2017) 107(3):641–8.e2. doi: 10.1016/j.fertnstert.2016.12.005
29. Bunnewell SJ, Honess ER, Karia AM, Keay SD, Al Wattar BH, Quenby S. Diminished ovarian reserve in recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril (2020) 113(4):818–27.e3. doi: 10.1016/j.fertnstert.2019.11.014
30. Bishop LA, Richter KS, Patounakis G, Andriani L, Moon K, Devine K. Diminished ovarian reserve as measured by means of baseline follicle-stimulating hormone and antral follicle count is not associated with pregnancy loss in younger in vitro fertilization patients. Fertil Steril (2017) 108(6):980–7. doi: 10.1016/j.fertnstert.2017.09.011
31. Sermondade N, Huberlant S, Bourhis-Lefebvre V, Arbo E, Gallot V, Colombani M, et al. Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta-analysis. Hum Reprod Update (2019) 25(4):439–51. doi: 10.1093/humupd/dmz011
32. Ren J, Sha A, Han D, Li P, Geng J, Ma C. Does prolonged pituitary down-regulation with gonadotropin-releasing hormone agonist improve the live-birth rate in in vitro fertilization treatment? Fertil Steril (2014) 102(1):75–81. doi: 10.1016/j.fertnstert.2014.03.030
33. Casper RF. Basic understanding of gonadotropin-releasing hormone-agonist triggering. Fertil Steril (2015) 103(4):867–9. doi: 10.1016/j.fertnstert.2014.12.129
34. Wang R, Lin S, Wang Y, Qian W, Zhou L. Comparisons of GnRH antagonist protocol versus GnRH agonist long protocol in patients with normal ovarian reserve: A systematic review and meta-analysis. PloS One (2017) 12(4):e0175985. doi: 10.1371/journal.pone.0175985
35. Haouzi D, Assou S, Dechanet C, Anahory T, Dechaud H, De Vos J, et al. Controlled ovarian hyperstimulation for in vitro fertilization alters endometrial receptivity in humans: protocol effects. Biol Reprod (2010) 82(4):679–86. doi: 10.1095/biolreprod.109.081299
Keywords: poor ovarian response, live birth, POSEIDON criteria, smooth fitting curve, threshold effect
Citation: Li F, Ye T, Kong H, Li J, Hu L, Jin H, Guo Y and Li G (2021) Predictive Factors for Live Birth in Fresh In Vitro Fertilization/Intracytoplasmic Sperm Injection Treatment in Poor Ovarian Reserve Patients Classified by the POSEIDON Criteria. Front. Endocrinol. 12:630832. doi: 10.3389/fendo.2021.630832
Received: 18 November 2020; Accepted: 19 March 2021;
Published: 12 April 2021.
Edited by:
Carlo Alviggi, University of Naples Federico II, ItalyReviewed by:
Kok-Min Seow, Shin Kong Wu Ho-Su Memorial Hospital, TaiwanAndrea Di Nisio, University of Padua, Italy
Yonglun Fu, Shanghai First Maternity and Infant Hospital, China
Copyright © 2021 Li, Ye, Kong, Li, Hu, Jin, Guo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Li, bGd2aWdvckAxMjYuY29t
 Fei Li
Fei Li Tian Ye1
Tian Ye1 Linli Hu
Linli Hu Gang Li
Gang Li