- 1Department of Vascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Breast and Thyroid Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Department of Ultrasound, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 4Department of Pancreatic Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: Metaplastic breast cancer (MBC) is a rare and aggressive subtype of the breast. To understand the characteristics and prognosis of single hormone receptor-positive (HR+) MBC (estrogen receptor-positive [ER+]/progesterone receptor-negative [PR-] and ER-/PR+), we compared these tumors to double HR+ tumors as well as HR- tumors.
Patients and Methods: The Surveillance, Epidemiology, and End Results database was used to analyze MBC between 1975 and 2016. The effect of HR status was evaluated using a multivariate Cox regression model.
Results: We included 3369 patients with a median follow-up time of 42 months (range 0-322 months). In this study, 280 (8.3%) cases were double HR+ tumors, 2597 (77.1%) were double HR- tumors, and 492 (14.6%) cases were single HR+ tumors, of which 159 (4.7%) cases were ER-/PR+ tumors and 333 (9.9%) were ER+/PR- tumors. On multivariate Cox analysis, the prognosis was related to age, race/ethnicity, tumor grade, TNM stage, and surgery. HR status remained no impact on breast cancer-specific survival (BCSS). In the Kaplan-Meier curve, HR status was not associated with better BCSS or overall survival (OS). In patients without HER2 overexpression, the BCSS and OS of ER+/PR- and ER-/PR+ tumors were not significantly different from that of ER-/PR- and ER+/PR+ tumors. The difference remains no significant in patients with HER2 overexpression.
Conclusions: In comparison with both ER-/PR- and ER+/PR+ tumors, we have identified clinically and biologically distinct features of single HR+ tumors. In patients with or without HER2 overexpression, the prognosis of single HR+ tumors was similar to ER-/PR- and ER+/PR+ tumors.
Introduction
Metaplastic breast cancer (MBC) is a rare and aggressive subtype accounting for <1% of all breast cancers (1). Previous studies have reported histologic MBC characterized by either homogenous or mixed components (2–6). MBC was not identified as a unique pathological type by the World Health Organization until 2000 (7). Since then, as pathologists’ understanding of MBC has considerably improved, the incidence has also increased (8). However, given its rarity, the clinical characteristics and prognosis of single hormonal receptor-positive MBC (single HR+ MBC, ER+/PR-, and ER-/PR+) are unclear.
In the National Comprehensive Cancer Network (NCCN) breast cancer guidelines, the management of MBC is similar to that of invasive ductal carcinoma (IDC) (9). However, MBC is characterized by larger tumor size, lesser regional node involvement, and higher tumor grade than breast cancers with more common histology (10–12). The pathway of metaplastic cancer metastasis was hematogenous but not lymphatic spread (13). A previous study with data from 2001 to 2010 of the Surveillance, Epidemiology, and End Results (SEER) database found that patients with stage I–III MBC had significantly worse 5-year breast cancer-specific survival (BCSS) than those with synchronous IDC (14). Some studies reported that MBC is chemorefractory, regardless of whether the included patients received neoadjuvant or adjuvant settings (8, 15–17). Although the common molecular subtype is the triple-negative (TN) phenotype in MBC, HR+ and human epidermal growth receptor 2 positive (HER2+) tumors do exist (18). A population-based study reported that HR status was not associated with survival of metaplastic carcinoma, which was different from IDC and infiltrating lobular carcinomas (19).
Although the technique of immunohistochemistry has now considerably improved, the incidence of MBC with estrogen receptor-negative (ER-)/progesterone receptor-positive (PR+) phenotype has not decreased (20). Generally, HR+ breast cancers have a favorable prognosis. To understand the characteristics and prognosis of single HR+ MBC, we compared these tumors to double HR+ tumors (ER+/PR+) as well as HR- tumors (ER-/PR-) by using the database of the whole population.
Materials and Methods
Patients
Data were retrieved from the SEER database and included all cases of pathologically confirmed MBC diagnosed between 1975 and 2016. This database collects data on cancer incidence, demographics and clinicopathologic data, management, and survival from 18 population-based cancer registries. According to the third edition of the International Classification of Diseases for Oncology (ICD-0-3), carcinoma histology was identified in metaplastic cancers with ICD-0-3 codes: 8560, 8562, 8570–8572, 8575, and 8980–8982 (19). The inclusion criteria were as follows: female sex; age ≥18 years; breast cancer as first and the only cancer diagnosis; unilateral breast cancer; histologically or cytologically confirmed diagnosis (instead of autopsy-confirmed); available information regarding survival time and HR status; and stage exception of T0 and Tis. Accordingly, 3369 patients were finally enrolled.
Demographics and Clinicopathologic Features
The demographic parameters included age at diagnosis; race/ethnicity recorded in the SEER database (White, Black, other); and insurance status. The clinicopathologic parameters included tumor grade; tumor size (T1, T2, T3, T4); regional node status (N0, N1, N2, N3); chemotherapy (CT); radiotherapy (RT); type of surgery (no surgery, lumpectomy, mastectomy); and biomarker profile (ER, PR, HER2). The definition of TNM (T-tumor, N-node, and M-metastasis) stage was according to the sixth/seventh edition of the Union for International Cancer Control/American Joint Committee on Cancer Pathologic Staging System. According to the SEER, HR status was stratified as single HR+, double HR+ tumors, and double HR- tumors.
The primary clinical outcome was BCSS, defined as the date of diagnosis to the date of death from breast cancer. The secondary clinical outcome was overall survival (OS), defined as the date of diagnosis to the date of death from any cause.
Detection of ER, PR, and HER2
In the SEER database, in cases where ER/PR is reported on more than one tumor specimen, the highest value is recorded. If any sample is positive, that record as positive. If neoadjacent therapy was received, the assay was recorded from tumor specimens prior to neoadjuvant therapy. If neoadjuvant therapy was given and there were no ER/PR results from pre-treatment specimens, these findings were reported from post-treatment specimens. If ER/PR was positive on an in situ specimen and ER/PR was negative on all tested invasive specimens, code ER/PR was considered negative. If ≥1% cells stained positive, the test results were considered positive. HER2 positivity was defined as an intensity of 3+ by IHC, while a score of 2+ was interpreted as equivocal. A negative test was defined as staining with a score of 0/1+. For equivocal stating, silver in situ hybridization (SISH) or fluorescence in situ hybridization (FISH) were performed; the results were positive for HER2 amplification when the ratio of HER2 to CEP17 was >2.2. We provided four MBC patients with different ER/PR phenotype (Figure S1).
Statistical Analysis
The χ2 test was carried out to analyze the differences between groups. The Cox proportional hazards model was used to assess the risk factors related to BCSS. Survival curves were constructed using the Kaplan–Meier method. Hazard ratios were presented with 95% confidence intervals (CIs). All statistical analyses were performed using SPSS statistical software (version 24.0; IBM Corporation, Armonk, NY, USA), and P <0.05 was considered to indicate statistical significance.
Results
Patient Characteristics
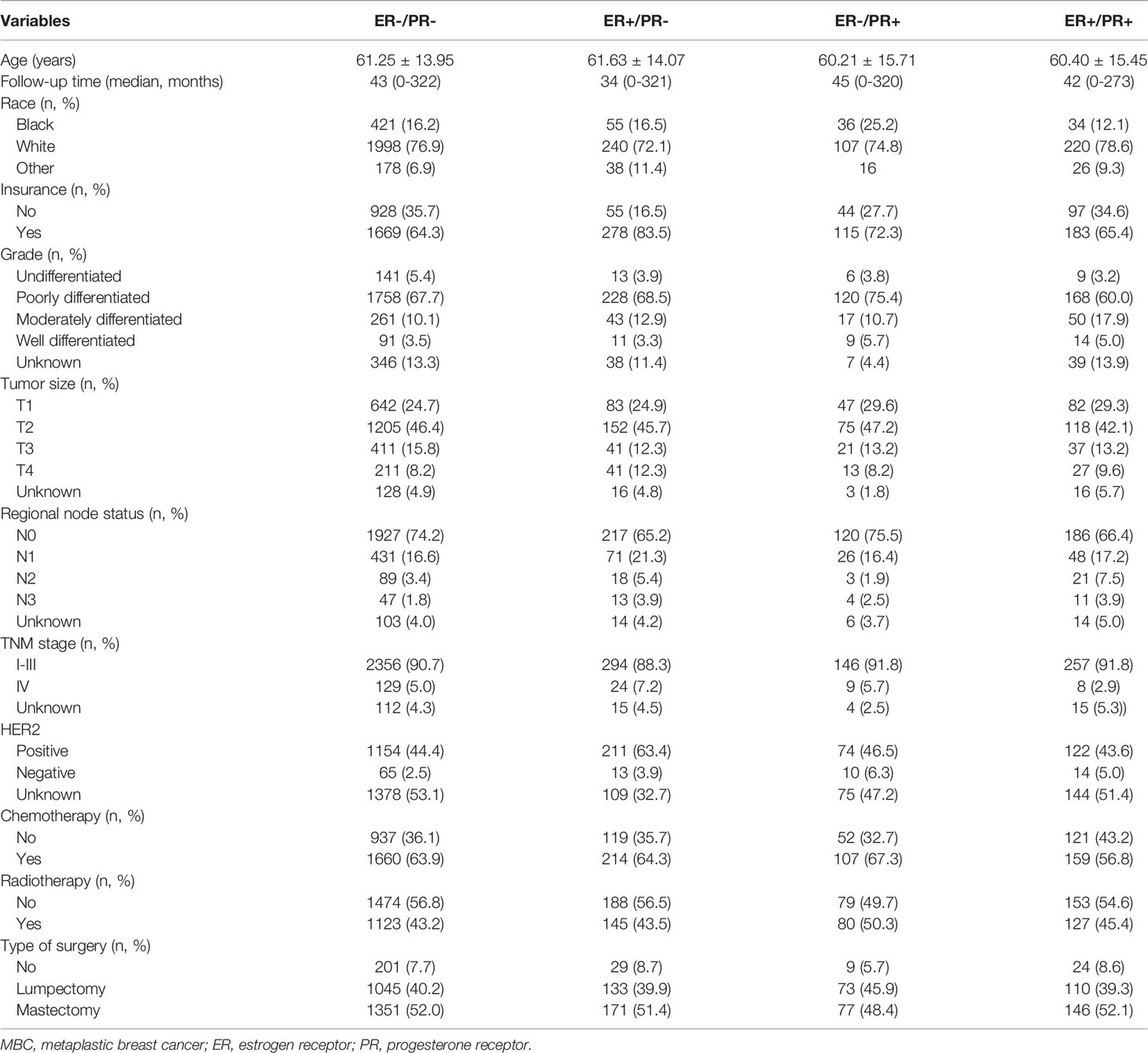
Of the 4672 MBC patients in the SEER registry, our final sample comprised 3369 patients. In this study, 280 (8.3%) patients had double HR+ tumors, 2597 (77.1%) had double HR- tumors, and 492 (14.6%) had single HR+ tumors, of which 159 (4.7%) cases were ER-/PR+ tumors and 333 (9.9%) were ER+/PR- tumors. The median age of the entire cohort was 61 years (range, 20–89 years). Most patients were white women (n=2565, 76.1%) and had poor differentiation (n=2274, 67.5%). In patients with available tumor size information, 46.0% were stage T2. A total of 3199 (95.0%) and 170 (5.0%) patients had stage I–III and stage IV disease, respectively. In addition, 2450 (72.7%), 576 (17.1%), 131 (3.9%), and 75 (2.2%) patients had N0, N1, N2, and N3 stage disease, respectively. A total of 1194 deaths were recorded, including 791 breast cancer related-deaths.
The clinicopathological characteristics of the four subtypes are summarized in Table 1. Compared with ER-/PR- tumors, ER+/PR- tumors were not significantly different with respect to ethnicity, tumor grade, tumor stage, and CT, but ER+/PR- tumors exhibited more regional node involvement (P = 0.004). However, compared with ER+/PR+ tumors, the clinicopathological characteristics of ER+/PR- tumors did not show a significant difference. ER-/PR+ tumors were found more in Black women (ER-/PR+ 25.2% vs. ER-/PR- 17.4%, P = 0.021) and had higher tumor grade (P = 0.010) than ER-/PR- tumors. Further, ER-/PR+ tumors were also found in more black women (ER-/PR+ 25.2% vs. ER-/PR- 13.4%, P = 0.012), had higher tumor grade (P = 0.003), and received more CT treatment (ER-/PR+ 67.3% vs. ER-/PR- 56.8%, P = 0.030) than ER+/PR+ tumors. There was no difference in stage (P = 0.139) or type of surgery (P = 0.288). Furthermore, there was no difference in the expression of HER2 (P = 0.831). Both ER-/PR+ and ER+/PR- tumors had similar HER2 overexpression to ER+/PR+ tumors (P = 0.831). However, ER-/ER+ tumors showed higher HER2 overexpression than ER-/PR- tumors (P = 0.028). The characteristics of single HR+ tumors were more distinct in HER2-negative tumors than in HER2 overexpressing tumors. (Tables S1 and S2)
Prognostic Factors for MBC
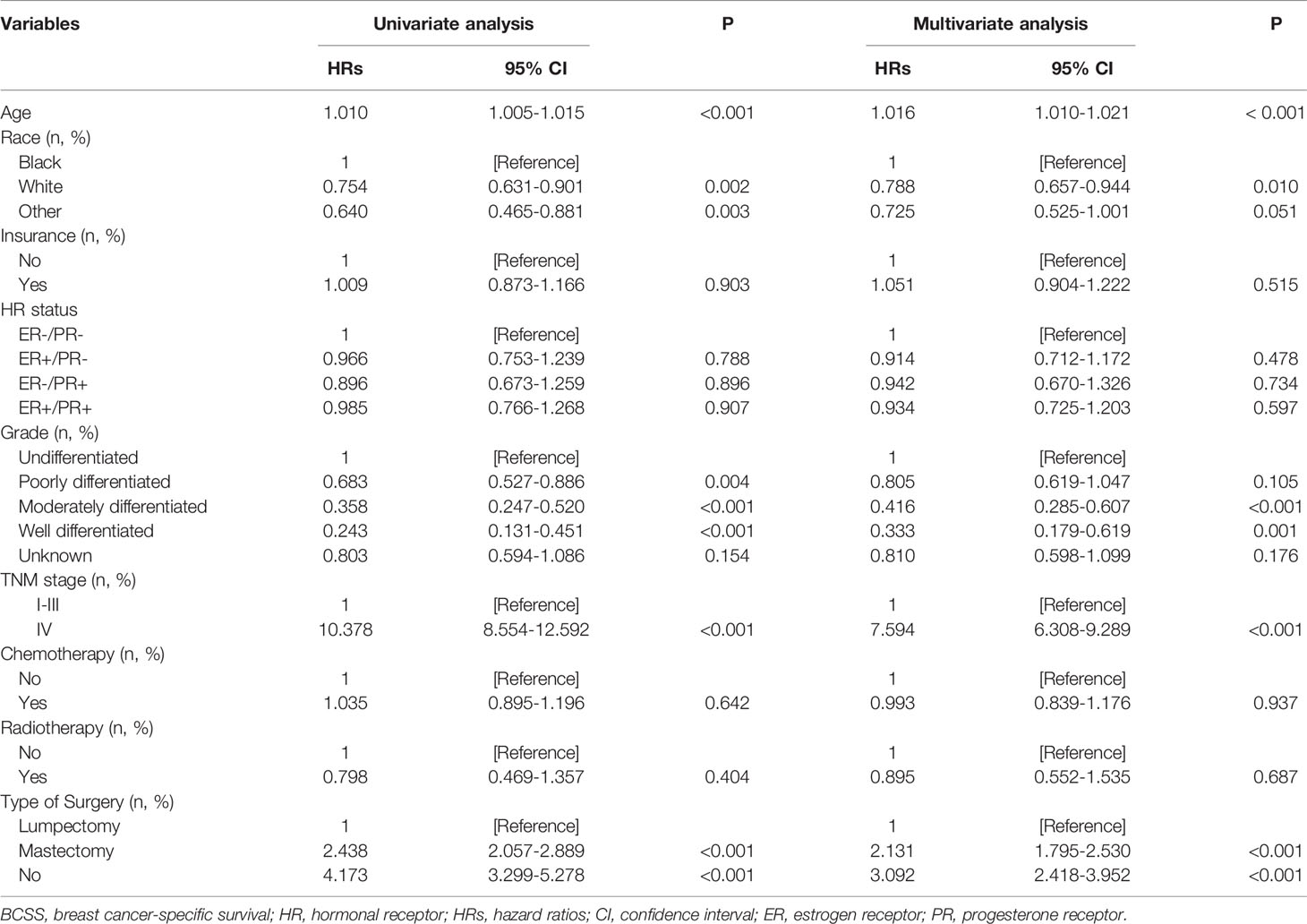
We further analyzed the independent prognostic factors associated with BCSS using the multivariate Cox proportional hazards model. HR status was not an independent prognostic factor related to better BCSS (hazard ratio: 0.839; 95%CI: 0.679–1.036; P = 0.102). Patients with stage IV disease had a worse prognosis than patients with stage I–III disease (hazard ratio: 7.594; 95%CI: 6.308–9.289; P < 0.001). In addition, patients could not benefit from CT (hazard ratio: 0.993; 95%CI: 0.839–1.176; P = 0.937) and RT (hazard ratio: 0.895; 95%CI: 0.552–1.535; P = 0.687). Patients who underwent mastectomy had worse prognosis than those who underwent lumpectomy (hazard ratio: 2.131; 95%CI: 1.795–2.530; P < 0.001). Furthermore, age, race/ethnicity, and tumor grade were independent indicators for BCSS (Table 2).
Survival Analysis of Single Hormone Receptor-Positive MBC
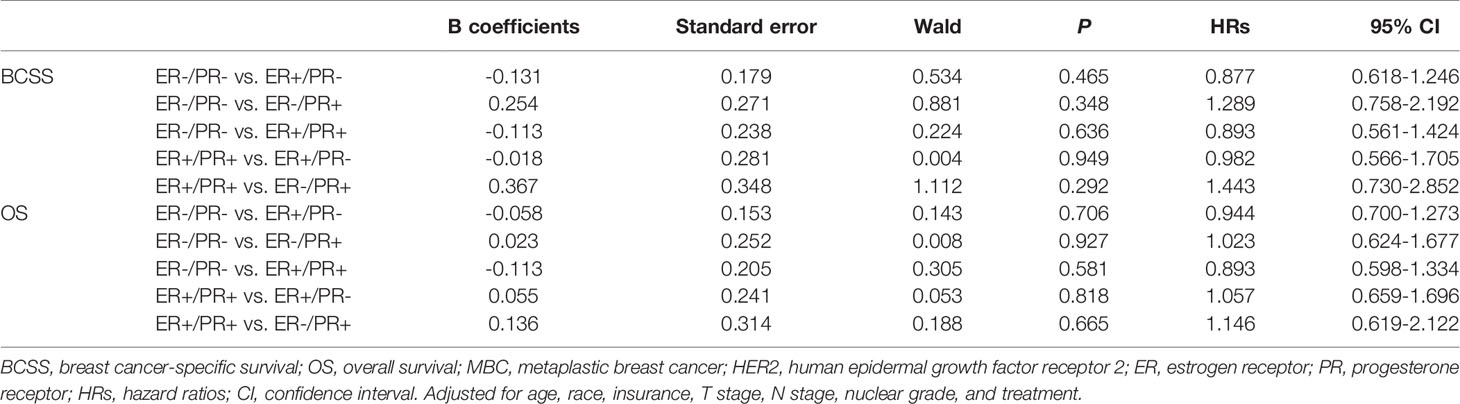
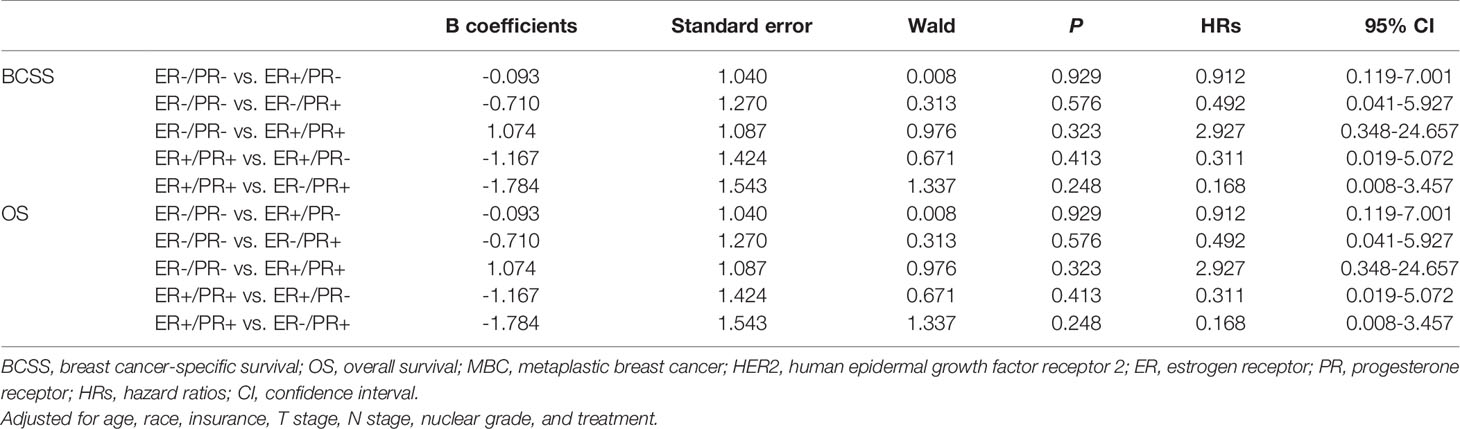
In multivariate analysis, in patients with or without HER2 overexpression, HR status was not associated with better BCSS or OS (Tables 3, 4). Survival curves were plotted using the Kaplan–Meier curve. HR status was neither associated with BCSS nor OS (Figures 1A, B). In patients without HER2 overexpression, the BCSS and OS of ER+/PR- and ER-/PR+ tumors were not significantly different from those of ER-/PR- and ER+/PR+ tumors (Figures 1C, D). In patients with HER2 overexpression, the prognosis of ER+/PR- and ER-/PR+ tumors was not significantly different from those of ER-/PR- and ER+/PR+ tumors (Figures 1E, F).
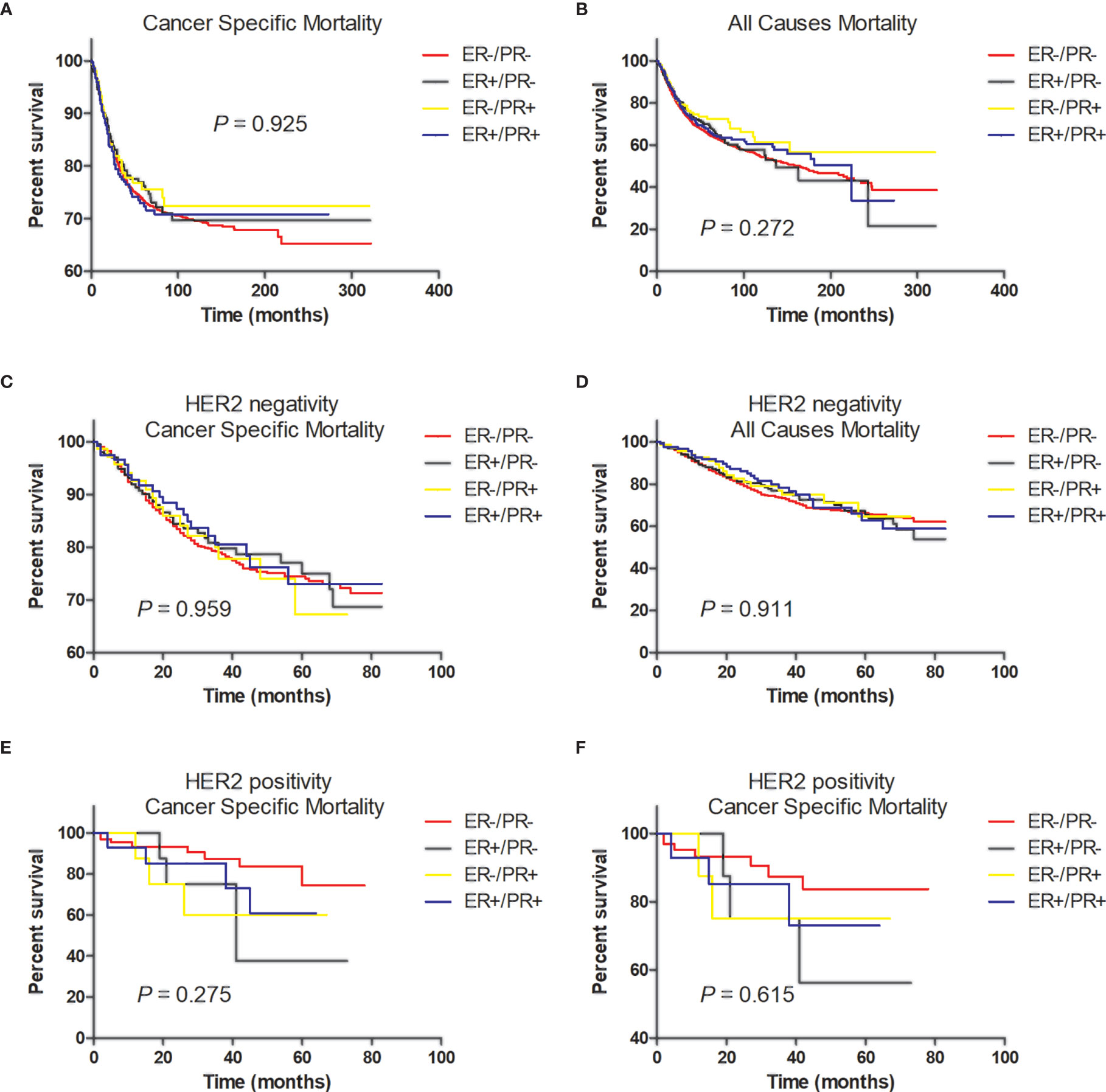
Figure 1 Tumor survival based on hormone receptor status. (A) Breast cancer-specific survival (BCSS) and (B) overall survival (OS) of all patients; (C) BCSS and (D) OS of patients with HER2-negative tumors; (E) BCSS and (F) OS of patients with HER2-positive tumors.
Discussion
In the current study, we evaluated tumor response to treatment with CT, RT, and surgery and compared differences in the clinical process, tumor characteristics, and prognosis among the four subtypes, namely ER-/PR-, ER+/PR-, ER-/PR+, and ER+/PR+. We found that CT and RT could not improve the prognosis of MBC. Patients that underwent mastectomy had a worse prognosis than those that underwent lumpectomy. Of concern was the finding that HR status was not associated with a better prognosis in the entire cohort. In patients with or without HER2 overexpression, the prognosis of single HR+ tumors was similar to that of ER-/PR- and ER+/PR+ tumors.
The data presented in this paper represent the largest cohort of patients with MBC, and this is the first descriptive report on the survival prognosis of MBC related to single HR status. For traditional breast cancer, patients with ER+/PR+ tumors had a better prognosis than those with ER+/PR- tumors, who in turn had a better prognosis than patients with ER-/PR- tumors (21). However, to our knowledge, no previous research has investigated the prognosis of single HR+ tumors in case of metaplastic carcinoma.
Ahmed et al. (20) reported that ER-/PR+ breast cancers exist, but are very rare. Itoh et al. (22) reported that among the ER-/PR+ patients, 65% of them were basal-like tumors. Bae et al. (23) pointed out that in single HR+ breast cancers, the ER+/PR- subtype accounts for 10%–15% of all breast cancers, while the ER-/PR+ subtype accounts for 2–4% of all breast cancers. Based on the SEER records, the frequency of the ER-/PR+ phenotype in our series was 4.7%. On the one hand, the results of immunohistochemistry from the SEER database were confirmed by pathologists. On the other hand, MBC tended to have poor differentiation accounting for 67.5% of all cases. In addition, Weigelt et al. (24) showed that MBCs are basal-like breast cancers. These reports propose that ER-/PR+ breast cancers are a biologically and clinically distinct subtype.
Although TN-subtype is the most common in MBC, the HR+ subtype also occurs (18). A study by Wright et al. (19) including 2,338 MBC cases concluded that contrary to traditional breast cancers, HR+ MBC did not have superior clinical outcomes. In our study, 2597 (77.1%), 333 (9.9%), 159 (4.7%), and 280 (8.3%) patients expressed ER-/PR-, ER+/PR-, ER-/PR+, and ER+/PR+, respectively. There was no difference in the prognosis among the four subtypes. In addition, He et al. (25) concluded that patients with TN-subtype had a worse prognosis than those with non-TN MBC. However, our study results showed that regardless of HER2 overexpression, the prognosis of ER+/PR- and ER-/PR+ tumors were not significantly different from those of ER-/PR- and ER+/PR+ tumors. The results using multivariate analysis may be more convincing than those obtained with Kaplan–Meier analysis that they using.
Although the rate of adjuvant CT was quite high (63.9% in ER-/PR-, 64.3% in ER+/PR-, 67.3% in ER-/PR+, and 56.8% in ER+/PR+; P=0.081), there was no significant difference among the four subtypes in the entire cohort or in patients with or without HER2 overexpression. However, previous research has shown that the response rate of MBC to CT regimens was relatively low. MBC might be a type of basal breast cancer, characterized by higher grade and more rapid growth (24, 26–28). The expression levels of ER, PR, and HER-2 receptor in MBC cells were lower than that of IDC, while the expression levels of Ki-67 and p-53 were higher (29, 30). In MBC patients, DNA repair pathways such as TOP2A, PTEN, and BRCA1 showed downregulation upon genomic profiling. These findings might explain the low incidence of lymph node metastasis and resistance to conventional CT regimens. This may be one of the causes of the poor prognosis of patients with MBC.
A recent retrospective analysis showed that RT was related to improvements in OS and BCSS (25). However, some authors pointed out that the role of RT in the prognosis of MBC was related to the types of surgical methods. As we know, post-lumpectomy RT is a standard component of lumpectomy for treating IDC to minimize local recurrence. Dave et al. (31) and Yu et al. (32) found that RT was beneficial for MBC patients undergoing a lumpectomy, but not a total mastectomy. Additionally, a few studies illustrated that the role of RT in prognosis was related to clinical characteristics of MBC besides the types of surgical methods. However, our study found that receipt of RT was not an independent factor for improved survival.
Notably, mastectomy was performed more often for patients with MBC, likely due to the presentation of larger tumors than those with other types of breast cancer. Tseng and Martinez explained that mastectomy or lumpectomy had no effect on OS or disease-specific survival for patients with MBC (33). In our study, the rate of patients receiving mastectomy was higher than that of patients receiving lumpectomy (51.8% vs. 40.4%), but mastectomy was an independent risk factor for BCSS. This may be another cause for poor prognosis with MBC.
Although detailed endocrine treatment strategies were not available in this analysis, previous studies have reported that the prognosis of HR+ patients receiving antiestrogen therapy showed no difference in outcome as compared to that of patients who did not receive antiestrogen therapy (8, 16, 34). The prognosis of single HR+ MBC is as poor as that of TN-MBC, which may be due to some factors.
Our study has some limitations. First, the retrospective nature of the study may have resulted in some selection bias. Second, detailed chemotherapy regimens, radiotherapy information, and endocrine treatment strategies could not be available from the SEER database; hence, a further case-control analysis could not be performed. However, we believe that our results will help researchers to understand the role of single hormonal receptor status in the prognosis of MBC.
Conclusion
We assessed a large cohort of patients with metaplastic breast cancer and found that HR status was not associated with prognosis. Furthermore, regardless of HER2 overexpression, the prognosis of ER+/PR- and ER-/PR+ tumors was not significantly different from those of ER-/PR- and ER+/PR+ tumors. When patients diagnosed with this rare and aggressive tumor were treated with surgery, physicians need to be careful with selecting the type of surgery. Furthermore, the role of anti-hormone therapy in HR+ MBC may need to be further investigated.
Data Availability Statement
The original contributions presented in the study are publicly available. This data can be found here: https://seer.cancer.gov/data
Ethics Statement
This study was exempt from the approval processes of the Institutional Review Boards because the SEER database patient information is de-identified. Also, a patient consent form was not applicable.
Author Contributions
XR, TH, and JMi contributed to the conception and design of the study. JH and JMa organized the database. YZ, JS, FD, and XZ performed the statistical analysis. JH wrote the first draft of the manuscript. JMa, YZ, JS, FD, and XZ wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81672611).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in providing high-quality open resources for researchers.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.628939/full#supplementary-material
Supplementary Table 1 | Clinicopathologic characteristics of patients with HER2-negative tumors.
Supplementary Table 2 | Clinicopathologic characteristics of patients with HER2-positive tumors.
Supplementary Figure 1 | Four MBC patients with different estrogen receptor (ER)/progesterone receptor (PR) phenotype. a, H&E, ×200; b, ER (-),×200; c, PR (-),×200; d, H&E, ×200; e, ER (+),×200; f, PR (+),×200; g, H&E, ×200; h, ER (-),×200; i, PR (+),×200; j, H&E, ×200; k, ER (+),×200; m, PR (-),×200.
References
1. Oberman HA. Metaplastic Carcinoma of the Breast. a Clinicopathologic Study of 29 Patients. Am J Surg Pathol (1987) 11(12):918–29. doi: 10.1097/00000478-198712000-00002
2. Wargotz ES, Deos PH, Norris HJ. Metaplastic Carcinomas of the Breast. II. Spindle Cell Carcinoma. Hum Pathol (1989) 20(8):732–40. doi: 10.1016/0046-8177(89)90065-8
3. Wargotz ES, Norris HJ. Metaplastic Carcinomas of the Breast. I. Matrix-Producing Carcinoma. Hum Pathol (1989) 20(7):628–35. doi: 10.1016/0046-8177(89)90149-4
4. Wargotz ES, Norris HJ. Metaplastic Carcinomas of the Breast. III. Carcinosarcoma. Cancer (1989) 64(7):1490–9. doi: 10.1002/1097-0142(19891001)64:7<1490::aid-cncr2820640722>3.0.co;2-l
5. Wargotz ES, Norris HJ. Metaplastic Carcinomas of the Breast. IV. Squamous Cell Carcinoma of Ductal Origin. Cancer (1990) 65(2):272–6. doi: 10.1002/1097-0142(19900115)65:2<272::aid-cncr2820650215>3.0.co;2-6
6. Wargotz ES, Norris HJ. Metaplastic Carcinomas of the Breast: V. Metaplastic Carcinoma With Osteoclastic Giant Cells. Hum Pathol (1990) 21(11):1142–50. doi: 10.1016/0046-8177(90)90151-t
7. Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International Classification of Diseases for Oncology, 3rd ed. (2000).
8. Lee H, Jung SY, Ro JY, Kwon Y, Sohn JH, Park IH, et al. Metaplastic Breast Cancer: Clinicopathological Features and Its Prognosis. J Clin Pathol (2012) 65(5):441–6. doi: 10.1136/jclinpath-2011-200586
9. Telli ML, Gradishar WJ, Ward JH. Nccn Guidelines Updates: Breast Cancer. J Natl Compr Canc Netw (2019) 17(5.5):552–5. doi: 10.6004/jnccn
10. Pezzi CM, Patel-Parekh L, Cole K, Franko J, Klimberg VS, Bland K. Characteristics and Treatment of Metaplastic Breast Cancer: Analysis of 892 Cases From the National Cancer Data Base. Ann Surg Oncol (2007) 14(1):166–73. doi: 10.1245/s10434-006-9124-7
11. Jung SY, Kim HY, Nam BH, Min SY, Lee SJ, Park C, et al. Worse Prognosis of Metaplastic Breast Cancer Patients Than Other Patients With Triple-Negative Breast Cancer. Breast Cancer Res Treat (2010) 120(3):627–37. doi: 10.1007/s10549-010-0780-8
12. Lim KH, Oh DY, Chie EK, Han W, Im SA, Kim TY, et al. Metaplastic Breast Carcinoma: Clinicopathologic Features and Prognostic Value of Triple Negativity. Jpn J Clin Oncol (2010) 40(2):112–8. doi: 10.1093/jjco/hyp139
13. Luini A, Aguilar M, Gatti G, Fasani R, Botteri E, Brito JAD, et al. Metaplastic Carcinoma of the Breast, an Unusual Disease With Worse Prognosis: The Experience of the European Institute of Oncology and Review of the Literature. Breast Cancer Res Treat (2007) 101(3):349–53. doi: 10.1007/s10549-006-9301-1
14. Nelson RA, Guye ML, Luu T, Lai LL. Survival Outcomes of Metaplastic Breast Cancer Patients: Results From a US Population-Based Analysis. Ann Surg Oncol (2015) 22(1):24–31. doi: 10.1245/s10434-014-3890-4
15. Bae SY, Lee SK, Koo MY, Hur SM, Choi MY, Cho DH, et al. The Prognoses of Metaplastic Breast Cancer Patients Compared to Those of Triple-Negative Breast Cancer Patients. Breast Cancer Res Treat (2011) 126(2):471–8. doi: 10.1007/s10549-011-1359-8
16. Hennessy BT, Giordano S, Broglio K, Duan Z, Trent J, Buchholz TA, et al. Biphasic Metaplastic Sarcomatoid Carcinoma of the Breast. Ann Oncol (2006) 17(4):605–13. doi: 10.1093/annonc/mdl006
17. Tzanninis IG, Kotteas EA, Ntanasis-Stathopoulos I, Kontogianni P, Fotopoulos G. Management and Outcomes in Metaplastic Breast Cancer. Clin Breast Cancer (2016) 16(6):437–43. doi: 10.1016/j.clbc.2016.06.002
18. Edenfield J, Schammel C, Collins J, Schammel D, Edenfield WJ. Metaplastic Breast Cancer: Molecular Typing and Identification of Potential Targeted Therapies At a Single Institution. Clin Breast Cancer (2017) 17(1):e1–10. doi: 10.1016/j.clbc.2016.07.004
19. Paul Wright G, Davis AT, Koehler TJ, Melnik MK, Chung MH. Hormone Receptor Status Does Not Affect Prognosis in Metaplastic Breast Cancer: A Population-Based Analysis With Comparison to Infiltrating Ductal and Lobular Carcinomas. Ann Surg Oncol (2014) 21(11):3497–503. doi: 10.1245/s10434-014-3782-7
20. Ahmed SS, Thike AA, Zhang K, Lim JC, Tan PH. Clinicopathological Characteristics of Oestrogen Receptor Negative, Progesterone Receptor Positive Breast Cancers: Re-Evaluating Subsets Within This Group. J Clin Pathol (2017) 70(4):320–6. doi: 10.1136/jclinpath-2016-203847
21. Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone Receptor Status Significantly Improves Outcome Prediction Over Estrogen Receptor Status Alone for Adjuvant Endocrine Therapy in Two Large Breast Cancer Databases. J Clin Oncol Off J Am Soc Clin Oncol (2003) 21(10):1973–9. doi: 10.1200/jco.2003.09.099
22. Itoh M, Iwamoto T, Matsuoka J, Nogami T, Motoki T, Shien T, et al. Estrogen Receptor (ER) Mrna Expression and Molecular Subtype Distribution in ER-Negative/Progesterone Receptor-Positive Breast Cancers. Breast Cancer Res Treat (2014) 143(2):403–9. doi: 10.1007/s10549-013-2763-z
23. Bae SY, Kim S, Lee JH, Lee HC, Lee SK, Kil WH, et al. Poor Prognosis of Single Hormone Receptor- Positive Breast Cancer: Similar Outcome as Triple-Negative Breast Cancer. BMC Cancer (2015) 15:138. doi: 10.1186/s12885-015-1121-4
24. Weigelt B, Kreike B, Reis-Filho JS. Metaplastic Breast Carcinomas are Basal-Like Breast Cancers: A Genomic Profiling Analysis. Breast Cancer Res Treat (2009) 117(2):273–80. doi: 10.1007/s10549-008-0197-9
25. He X, Ji J, Dong R, Liu H, Dai X, Wang C, et al. Prognosis in Different Subtypes of Metaplastic Breast Cancer: A Population-Based Analysis. Breast Cancer Res Treat (2019) 173(2):329–41. doi: 10.1007/s10549-018-5005-6
26. Kuroda N, Fujishima N, Inoue K, Ohara M, Hirouchi T, Mizuno K, et al. Basal-Like Carcinoma of the Breast: Further Evidence of the Possibility That Most Metaplastic Carcinomas May Be Actually Basal-Like Carcinomas. Med Mol Morphol (2008) 41(2):117–20. doi: 10.1007/s00795-007-0379-2
27. Reis-Filho J, Milanezi F, Steele D, Savage K, Simpson P, Nesland J, et al. Metaplastic Breast Carcinomas are Basal-Like Tumours. Histopathology (2006) 49(1):10–21. doi: 10.1111/j.1365-2559.2006.02467.x
28. Korsching E, Jeffrey S, Meinerz W, Decker T, Boecker W, Buerger H. Basal Carcinoma of the Breast Revisited: An Old Entity With New Interpretations. J Clin Pathol (2008) 61(5):553–60. doi: 10.1136/jcp.2008.055475
29. Barnes PJ, Boutilier R, Chiasson D, Rayson D. Metaplastic Breast Carcinoma: Clinical-Pathologic Characteristics and HER2/Neu Expression. Breast Cancer Res Treat (2005) 91(2):173–8. doi: 10.1007/s10549-004-7260-y
30. Tse GM, Tan PH, Putti TC, Lui PC, Chaiwun B, Law BK. Metaplastic Carcinoma of the Breast: A Clinicopathological Review. J Clin Pathol (2006) 59(10):1079–83. doi: 10.1136/jcp.2005.030536
31. Dave G, Cosmatos H, Do T, Lodin K, Varshney D. Metaplastic Carcinoma of the Breast: A Retrospective Review. Int J Radiat Oncol Biol Phys (2006) 64(3):771–5. doi: 10.1016/j.ijrobp.2005.08.024
32. Yu JI, Choi DH, Huh SJ, Ahn SJ, Lee JS, Shin KH, et al. Unique Characteristics and Failure Patterns of Metaplastic Breast Cancer in Contrast to Invasive Ductal Carcinoma: A Retrospective Multicenter Case-Control Study (KROG 13-07). Clin Breast Cancer (2015) 15(2):e105–15. doi: 10.1016/j.clbc.2014.10.002
33. Tseng WH, Martinez SR. Metaplastic Breast Cancer: to Radiate or Not to Radiate? Ann Surg Oncol (2011) 18(1):94–103. doi: 10.1245/s10434-010-1198-6
Keywords: metaplastic breast cancer, hormonal receptor, HER2, prognosis, SEER database
Citation: Mao J, Hu J, Zhang Y, Shen J, Dong F, Zhang X, Ming J, Huang T and Run X (2021) Single Hormone Receptor-Positive Metaplastic Breast Cancer: Similar Outcome as Triple-Negative Subtype. Front. Endocrinol. 12:628939. doi: 10.3389/fendo.2021.628939
Received: 13 November 2020; Accepted: 31 March 2021;
Published: 23 April 2021.
Edited by:
Pia Giovannelli, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Michihisa Umetani, University of Houston, United StatesGuan Chen, Medical College of Wisconsin, United States
Copyright © 2021 Mao, Hu, Zhang, Shen, Dong, Zhang, Ming, Huang and Run. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Ming, bWluZ2ppZXdoQDEyNi5jb20=; Tao Huang, aHVhbmd0YW93aEAxNjMuY29t; Xiaoqin Run, cnVueGlhb3Fpbnd1aGFuQDE2My5jb20=
†These authors have contributed equally to this work
 Jinqian Mao
Jinqian Mao Jin Hu2†
Jin Hu2†