- 1Division of Endocrinology and Metabolism, Department of Medicine, University of Alberta, Edmonton, AB, Canada
- 2Division of Diabetes, Endocrinology and Metabolism, Department of Medicine, University of Minnesota, Minneapolis, MN, United States
- 3Department of Genetics, Cell Biology, and Development, University of Minnesota, Minneapolis, MN, United States
- 4Stem Cell Institute, University of Minnesota, Minneapolis, MN, United States
Up to 35% of aggressive pituitary tumors recur and significantly affect mortality and quality of life. Management can be challenging and often requires multimodal treatment. Current treatment options, including surgery, conventional medical therapies such as dopamine agonists, somatostatin receptor agonists and radiotherapy, often fail to inhibit pituitary tumor growth. Recently, anti-tumor effects of chemotherapeutic drugs such as Temozolomide, Capecitabine, and Everolimus, as well as peptide receptor radionuclide therapy on aggressive pituitary tumors have been increasingly investigated and yield mixed, although sometimes promising, outcomes. The purpose of this review is to provide thorough information on non-surgical medical therapies and their efficacies and used protocols for aggressive pituitary adenomas from pre-clinical level to clinical use.
Introduction
Pituitary tumors are mostly benign and progress slowly. Most of them are non-invasive and cured by surgery or controlled by long-term pharmacologic treatment. However, some pituitary tumors exhibit continued growth despite conventional therapies, including multiple surgeries, radiotherapy, and medical treatment (1). Recent evidence suggests that Temozolomide (TMZ), an alkylating agent, can be used as a valuable first-line chemotherapy for treatment of aggressive pituitary tumors and carcinomas (1). However, some patients may not respond to TMZ. Moreover, many patients experience disease progression or disease recurrence after completion of TMZ treatment, although tumors and carcinomas regressed during the treatment (1–13). In order to ensure long-term positive outcomes in cases of aggressive pituitary tumors, alternative treatment options are needed. Recent case reports showed that TMZ in combination with capecitabine had an improved progression free survival in aggressive pituitary tumors (14, 15). Moreover, several new therapies have been reported. These novel therapies include peptide receptor radionuclide therapy (PRRT) (16–24) and treatment with mTOR inhibitors (11, 25–27), epidermal growth factor receptor (EGFR) inhibitors (28), immune checkpoint inhibitors (29–31), cyclin dependent kinase (Cdk) inhibitors (32), or vitamin A derivatives (33, 34). In this review, we describe the mechanisms, efficacies, and protocols of each treatment and summarize the studies and cases published in the literature.
Temozolomide (TMZ)
TMZ is an orally-administered alkylating agent that was originally used for treatment of glioblastomas combined with radiation therapy (35). TMZ was first used in 2006 to manage high-risk pituitary tumors (36–38). TMZ induces methylation of guanine residue at the O6 position in the DNA. O6-methylguanine incorrectly pairs with thymine and triggers the mismatch repair system, leading to the formation of double-strand breaks in the genome, which causes cell cycle arrest and induction of apoptosis (39).
TMZ was recommended as first-line chemotherapy for aggressive pituitary tumors and carcinomas by the European Society of Endocrinology in 2018 (1) and usually elicits an immediate response. The standard dose of TMZ is 150–200 mg/m2 for 5 consecutive days every 28 days (=1 cycle) for at least six cycles (1) (Figure 1A). TMZ is a generally well-tolerated therapy. The major side effects are fatigue and nausea. Twelve studies with greater than five patients have been published since the first case of TMZ use in the management of aggressive pituitary tumors in 2006 (2–13) (Table 1). The total cumulative number of patients is 366, including 113 with pituitary carcinomas. Although follow-up duration and definition of a response differed among studies, the overall response in the reduction of tumor size ranged from 33%–87%. In the largest study by the European Society of Endocrinology, 166 patients were treated with TMZ as first-line chemotherapy. 37% of the patients showed radiological response and 33% showed stable disease (11). Similarly, the second largest study with 47 patients, including 13 with pituitary carcinomas, reported that 37% of patients exhibited tumor size reduction following TMZ treatment (13). In a meta-analysis of 106 patients from 11 studies of aggressive pituitary tumors, 47% of the patients showed reduction in tumor size (1).
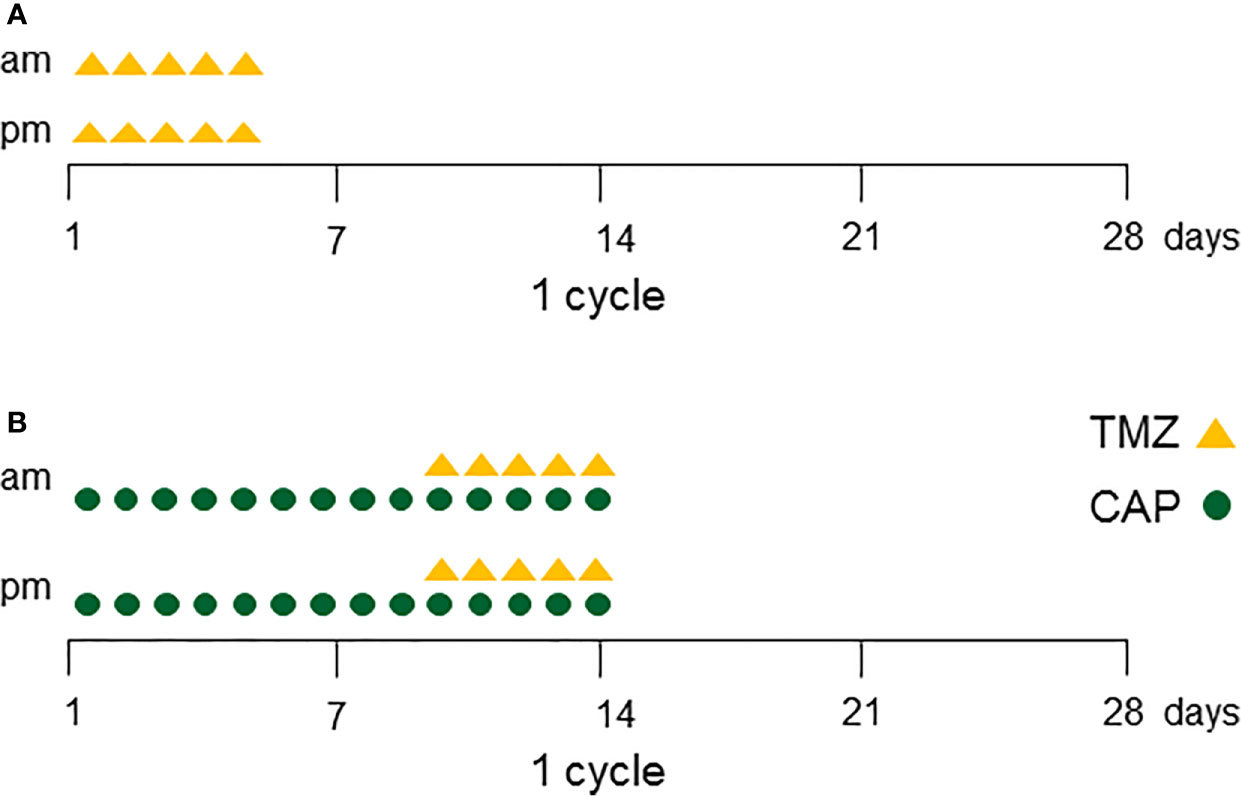
Figure 1 (A) One cycle of Temozolomide (TMZ) protocol. TMZ is given twice daily (150–200 mg/m2/day) for 5 consecutive days every 28 days (=1 cycle). After three cycles of TMZ, MRI is taken for treatment evaluation. Continue TMZ at least six cycles in total for responder patients. (B) One cycle of Capecitabine (pro-drug of 5-Fluorouracil) + Temozolomide (CAPTEM) protocol. Oral capecitabine (CAP) is given (1,500 mg/m2/day) on days 1 through 14 divided into two doses, and TMZ is given twice daily (150 to 200 mg/m2/day) on days 10 through 14. This 2-week regimen is followed by 2 weeks off treatment. Continue CAPTEM at least 12 cycles in total for responder patients.

Table 1 Temozolomide (TMZ) treatment response in pituitary tumors and carcinomas from published case series of 5 or more patients.
TMZ treatment outcome differs between hormone secreting and non-hormone secreting pituitary tumors. A review of 8 case series encompassing 100 pituitary tumor cases treated with TMZ showed that corticotroph adenomas and prolactinomas have about a 56% and 44% tumor reduction rate, respectively. In contrast, non-functioning pituitary adenomas have only a 22% of tumor response rate (40). Two other studies also showed that patients with functioning tumors exhibited better tumor size reduction after treatment with TMZ compared to those with NFPAs (2, 11).
As mentioned above, immediate responses to TMZ are mostly favorable; however, tumor progression and relapse frequently occur during long-term follow-up (33%–63%) (Table 1). In two large-scale studies with long-term follow-up, tumor relapse occurred in 46% (16 month follow up with 5 months after cessation) and 38% (21 months follow-up with 12 months after cessation) of patients, respectively (2, 11). In another study that focused on long-term effects of TMZ, up to 63% of the patients demonstrated disease progression after a median of 16 months following initiation of TMZ (13). Longer TMZ treatment duration (more than 12 cycles) was found to be associated with better survival free of tumor-progression (1, 2, 11, 41). A retrospective analysis showed higher survival rate in the long-term treatment group (longer than 12 months) compared to the short-term (1–12 months) treatment group [5-year overall survival 92% vs 54% (41)], suggesting that longer treatment cycles can increase the likelihood of sustained remission. However, reports from many cases showed that a second course of TMZ failed to induce tumor regression if tumor progression or systemic metastases occurred after an initial treatment (2, 8, 10, 42, 43). Therefore, re-challenging with TMZ is not recommended if an initial treatment fails to control disease progression. One of the reasons for such a relapse might be related to TMZ-induced hypermutations in genes such as MSH6, CDKN2A/B, and PIK3CA, which can be associated with TMZ resistance (29).
Several biomarkers that may predict response to TMZ have been identified. One such marker is O6-methylguanine DNA methyltransferase (MGMT), a DNA-repair enzyme, which counteracts TMZ-induced DNA damage (44). The expression of MGMT seems to correlate with response of a tumor to the TMZ therapy. A number of studies indicate an association between low expression levels of MGMT (determined by immunostaining) and better treatment response to TMZ (1, 5, 8, 11, 45–51). However, this association was not observed in several other studies (2–4, 6, 10, 13) (Table 1). This discrepancy could be, at least in part, derived from a lack of standardized scoring systems for MGMT immunostaining. In addition, the expression level of MGMT may increase during TMZ therapy (45), which could affect interpretation of MGMT expression. At present, the expression of MGMT is still considered a predictive marker for TMZ response. Another biomarker is MutS Homolog 6 (MSH6), a DNA mismatch repair protein. Mutations in the MSH6 gene in glioblastoma are known to be associated with TMZ resistance (52). One study showed that expression of MSH6 was positively correlated with pituitary tumor regression following treatment with TMZ (6); however, a follow-up study failed to confirm this correlation (8).
TMZ-Based Combination Therapies: Capecitabine (Pro-Drug of 5-Fluorouracil) + Temozolomide (CAPTEM)
Capecitabine (orally administered systemic pro-drug of 5-Fluorouracil) is an antimetabolite that incorporates 5-fluorodeoxyuridine triphosphate into genomic DNA. Capecitabine causes attenuation of MGMT DNA repair activity through thymidylate synthase inhibition and reduction of the thymidine level, which enhances the apoptotic effect of TMZ (53, 54). CAPTEM is a novel combination of capecitabine and TMZ. Two in vitro experiments investigating anti-tumor effects of CAPTEM have been reported. One study in human carcinoid cell lines showed synergistic cytotoxicity when 5-fluorouracil (5-FU) exposure was preceded by TMZ (55). The other study, conducted by the authors of this review, used mouse corticotroph tumor cells to demonstrate that 5-FU treatment in combination with TMZ had an additive effect both in decreasing cell viability and reducing the amount of ACTH released by the tumor cells in the culture medium (15).
The clinical protocol of CAPTEM consists of oral capecitabine 1,500 mg/m2/day (maximum daily dose of 2,500 mg on days 1 through 14 divided into two doses) and TMZ 150 to 200 mg/m2/day (oral divided into two doses) given on days 10 through 14. This 2-week regimen is followed by 2 weeks off-treatment [Figure 1B (14)]. Data obtained from in vitro experiments support the efficacy of two separate TMZ treatments (56). Specifically, the first treatment causes partial reduction of MGMT activities, whereas the second treatment is responsible for the methylation of guanine residues once repair activity by MGMT has been attenuated (53).
CAPTEM is generally well tolerated; among 13 patients with aggressive pituitary tumors/carcinomas, 12 patients were able to tolerate the treatment without discontinuing therapies (Table 2). Three patients developed thrombocytopenia but only one of these patients had to discontinue CAPTEM due to thrombocytopenia and poor tolerance (29). Another patient developed lymphopenia without discontinuing treatment, and one case reduced to 75% of the maximal dose of CAPTEM due to nausea (15). The first reported use of CAPTEM for pituitary tumors was for an aggressive corticotroph pituitary carcinoma in 2011 (57). To date, 13 TMZ naïve cases of aggressive pituitary tumors/carcinomas treated with CAPTEM (TMZ naïve cases) have been reported, of which seven were carcinomas (7/13) and 11 were ACTH positive (11/13) (11, 14, 15, 26, 29, 57–59) (Table 2). Clinical or radiological improvements were observed in 11 out of 13 cases, in which tumor progression stopped for up to 54 months. In seven patients with carcinomas (five ACTH positive, one PRL positive, and one PIT-1 positive), five patients (71%) initially showed partial reduction of tumor size in response to CAPTEM treatment and no tumor progression for as long as 39 months. These reports include two cases of pituitary tumors treated with CAPTEM: one is a corticotroph carcinoma and the other is an aggressive corticotroph tumor (15). Both patients had undergone previous surgical and radiologic therapies. The first patient with corticotroph carcinoma started to receive the CAPTEM treatment after developing leptomeningeal spread. Twelve cycles of the treatment resulted in tumor control associated with improvement of clinical and radiological tumor size reduction for 39 months. The patient restarted CAPTEM after recurrence of the tumor, and is currently under treatment. The second patient, with an aggressive corticotroph tumor, was treated with 12 cycles of CAPTEM, which led to tumor shrinkage with no tumor regrowth 22 months after cessation of therapy. As for the side effects of CAPTEM, the second patient had nausea, which was manageable by reducing the dose of CAPTEM to 75% of the maximal after the 6th cycle.
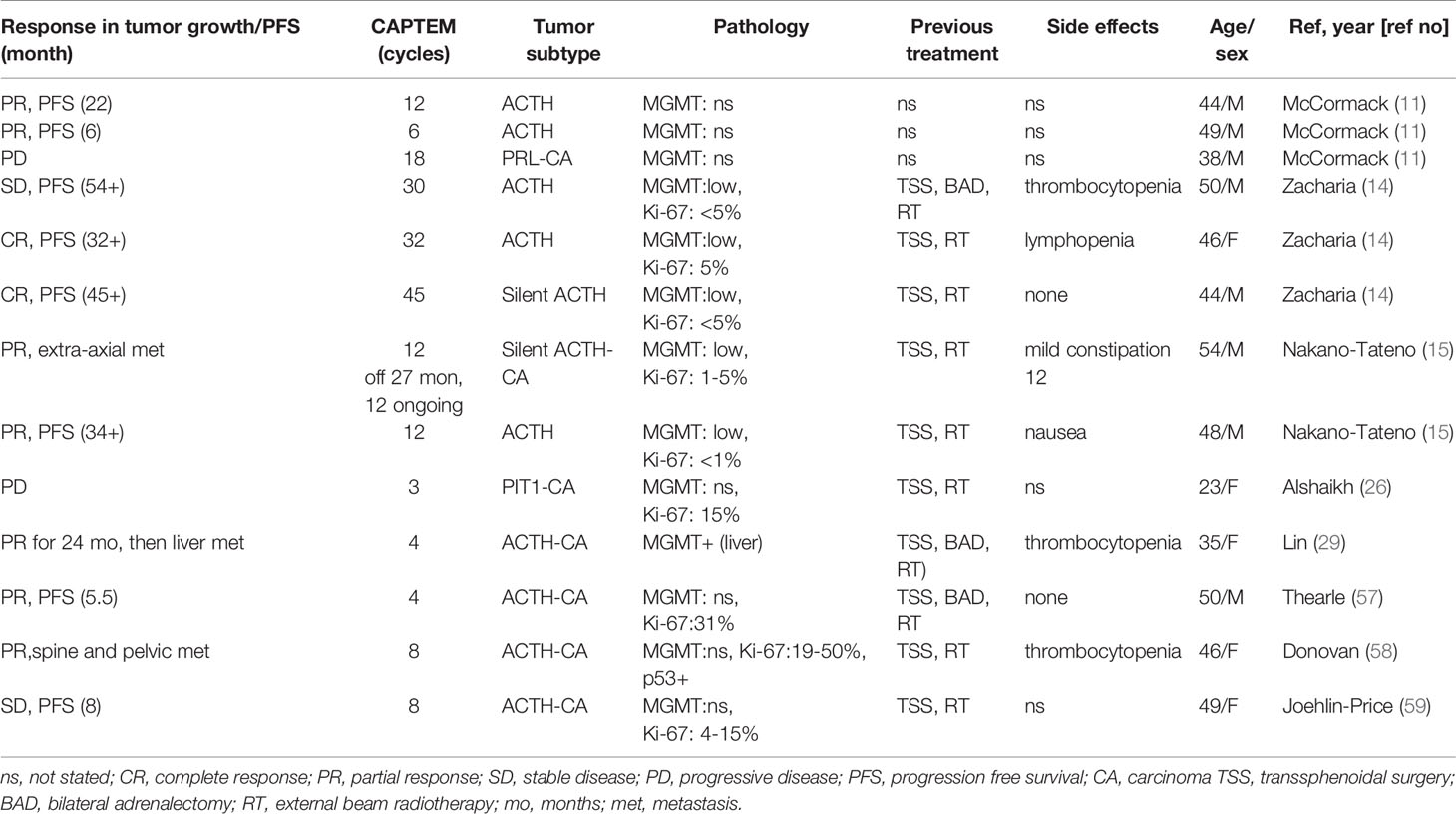
Table 2 Published cases of Capecitabine (pro-drug of 5-Fluorouracil) + Temozolomide (CAPTEM) (TMZ naïve) for aggressive pituitary tumors and carcinomas.
CAPTEM has been used in TMZ resistant cases as well. CAPTEM treatment followed by TMZ monotherapy (TMZ resistant cases) has been reported in eight cases (three ACTH secreting carcinomas, two PRL secreting carcinomas, one null-cell carcinoma, and two cases not stated) (8, 10–12, 42). However, the outcome was unfavorable in seven cases (88%), and only one (null- cell carcinoma) had a partial regression of carcinoma (42). From this limited dataset, CAPTEM seems to be more effective in TMZ naïve cases compared to those with TMZ resistance. Unlike TMZ monotherapy, it is unknown whether MGMT expression in tumors can be a predictive marker for response to CAPTEM. In six aggressive pituitary tumors treated with CAPTEM, five cases (83%) showed low MGMT expression levels, and one case had positive expression in the liver metastasis (14, 15, 29, 57). The outcome of these low MGMT cases varies, including cases of complete regression, partial regression, and stabilization of the tumor. Further studies are required to determine the role of MGMT expression in predicting response to CAPTEM therapy.
Other than CAPTEM, there are a few case reports of TMZ in combination with other therapeutic agents. Among them are the combinations of TMZ with VEGF-targeted therapy (Bevacizumab or Apatanib) (60–62) and a somatostatin receptor ligand (Pasireotide) (9, 63). In a survey by the European Society of Endocrinology, 1 case treated with TMZ in combination with bevacizumab achieved a partial tumor regression, 1 case with thalidomide showed no progression of the disease, and 1 case with Carmustine showed progressive disease (PD) (11). Although a prospective clinical trial is required to determine whether the treatment with CAPTEM is superior to the treatment with TMZ alone, CAPTEM appears to be a promising treatment option for aggressive pituitary tumors and carcinomas based on several case reports and our experimental data.
Peptide Receptor Radionuclide Therapy (PRRT)
Peptide Receptor Radionuclide Therapy (PRRT) is a form of targeted therapy that utilizes the delivery of radionuclide-bound somatostatin agonists to pituitary tumors expressing somatostatin receptors (SSTRs). PRRT has successfully treated neuroendocrine tumors, due to their high levels of SSTR expression (64–66). The use of PRRT for pituitary tumors was introduced after its success in treating neuroendocrine tumors (17). Radionuclides such as Yttrium 90 (Y-90), Lutetium 177 (Lu-177), and Indium-111 (In-111) are combined with peptides or somatostatin agonists (e.g., DOTATOC, DOTATATE) to deliver radiation to tumor cells. Because this is a targeted therapy, the risk of the systemic adverse effects is lower than conventional radiation therapy (18, 38).
There is still limited evidence whether PRRT is effective in the management of aggressive pituitary adenomas or carcinomas (8, 16–24) (Table 3). From the review of English literature between the years of 2012 and 2020, we found a total of 15 cases describing PRRT treatment in aggressive pituitary tumors (11 cases) and carcinomas (four cases). The treatment protocols used (dose, type of radionuclide and peptide, timing of radionuclide delivery) and measured outcomes across these cases vary greatly and are summarized in Table 3. Broadly, regarding tumor size, among 15 reported cases, six cases (43%) responded to PRRT. Decrease in hormone levels was reported in only three cases (20%, Table 3).
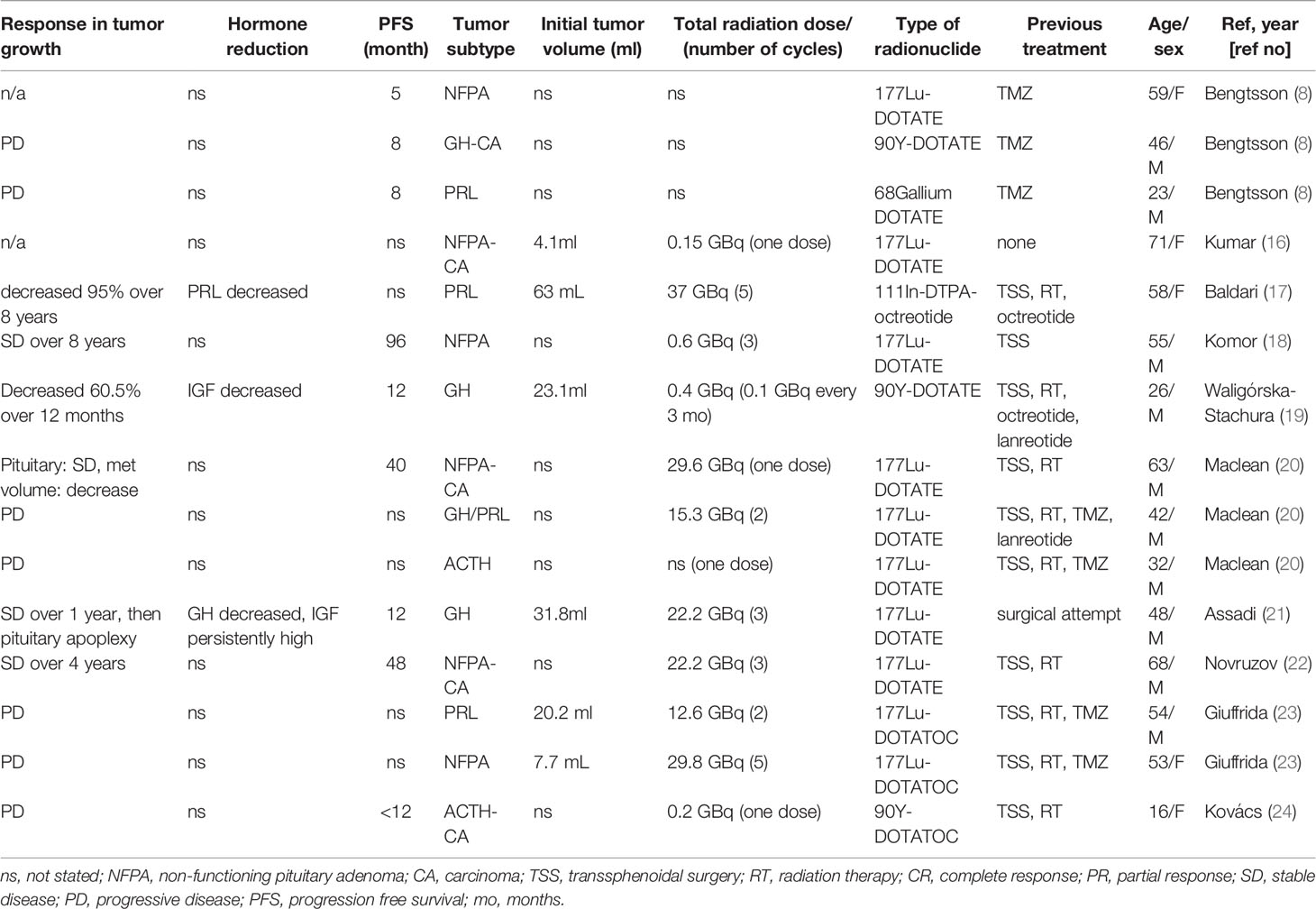
Table 3 Published cases of peptide receptor radionuclide therapy (PRRT) for pituitary tumors and carcinomas.
In general, PRRT treatment is tolerated well without major side effects. In a study of 30 patients with advanced cancers (mostly neuroendocrine tumors) receiving [111In-DTPA0] octreotide up to a high cumulative dose of 75Gbq, no major side effects were noted. Transient reduction in platelets and leukocytes can occur (67). From our review of 15 cases of aggressive pituitary tumors treated with PRRT, one patient developed transient grade 2 thrombocytopenia (20).
There is no clear evidence as to whether dosage or type of radionucleotide affects outcomes (23). From our review of 15 cases, total average radiation dose seems similar, with an average of 15.4 GBq in responders versus an average dose of 14.5 GBq in non-responders. However, dosages varied widely in both groups (from 0.4 GBq to 37 GBq), therefore it is difficult to assess whether dosage affects outcomes or not. It is also unknown whether the pre-treatment radioisotope uptake scan corresponds with treatment outcomes (18, 22, 23). In well-differentiated neuroendocrine tumors and medullary thyroid cancers, the total amount of pre-treatment radioisotope uptake did not reflect prognosis well, but heterogeneous uptake may correspond with poor prognosis after PRRT treatment (68, 69). In pituitary tumors, dosimetry analysis was reported in only 1 case, which showed a heterogeneous uptake pattern with progressed disease (20).
It is of interest to know the difference in efficacy between somatostatin analog (SSA) therapy and PRRT, however, at this point, there is no direct comparison study. At this stage, PRRT is a treatment option for aggressive pituitary tumors that express SSTR and are resistant to other therapies. The 2018 guidelines of the European Society of Endocrinology listed PRRT as an alternative treatment option for aggressive pituitary tumors (1). Taken together, current evidence does not suggest that PRRT is an impressively effective treatment for aggressive pituitary tumors, however it is still premature to conclude that PRRT is not an important treatment strategy since half of the reported cases were TMZ resistant which are usually difficult to control disease progressions.
mTOR Inhibitors
The phosphatidylinositol 3-kinase/mammalian target of rapamycin (mTOR) pathway is involved in the regulation of survival, growth, protein synthesis, and cellular metabolism (70–73). Everolimus (EVE) is an orally active mTOR inhibitor FDA-approved to treat neuroendocrine tumors. EVE forms a complex with mTORC1, affecting downstream cellular activities, and causes cell cycle arrest and protein synthesis inhibition. Many studies have shown that EVE is effective in human pituitary tumor cultures and murine cell lines in vitro (74–77). It is also effective in vivo in intracranial GH4 cell xenograft mouse models and reduced transplanted tumor cell viability and proliferation (78). Moreover, EVE synergized with SSAs, such as octreotide or pasireotide, and exhibited anti-proliferative effects in primary human pituitary tumor cells (79, 80).
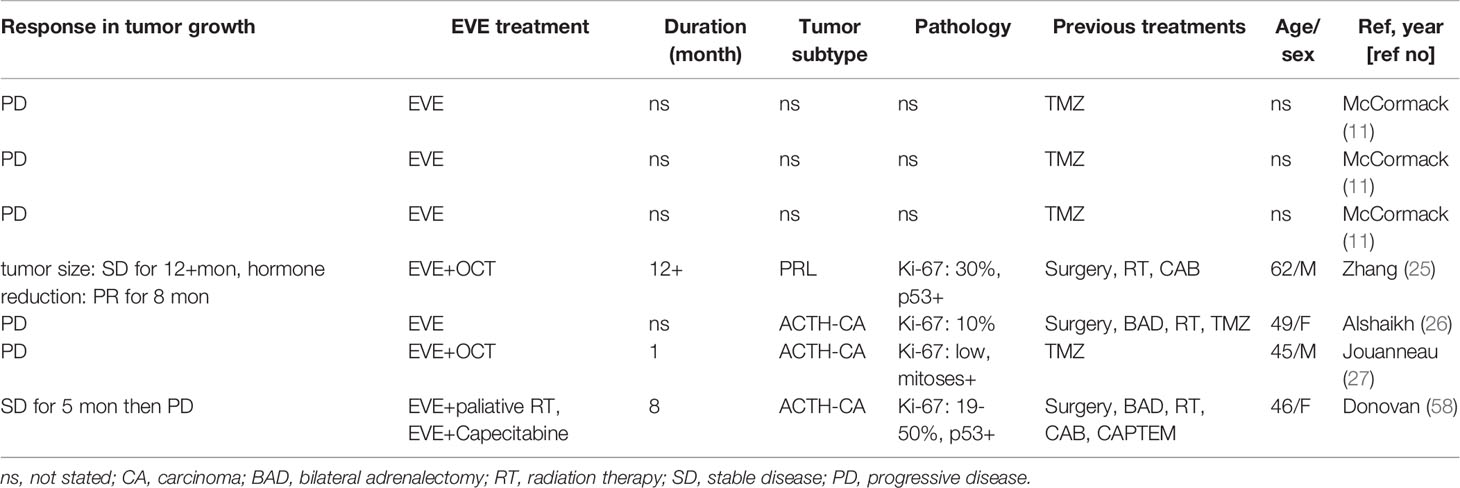
Although EVE has been widely used in the management of pancreatic and other neuroendocrine neoplasms, there are only seven published cases of pituitary tumors treated with EVE combination therapy (three ACTH secreting carcinomas, one PRL secreting adenoma, and three not stated) (11, 25–27, 58) (Table 4). Of note, five out of seven (71%) cases had disease progression, and tumors in all the failed cases were TMZ resistant. The protocol used for EVE treatment is variable. The reported dose of EVE is 5 to 10 mg daily for several months, either as a monotherapy or in combination with other therapies. Common side effects include stomatitis, rash, fatigue, diarrhea, infections, anemia, and hyperglycemia (25). Initial reduction in hormone secretion was observed in only one out of seven patients treated with EVE (25). A patient with a PRL secreting tumor treated with EVE 10mg + octreotide showed decreased PRL levels (454 ng/ml to 253 ng/ml; 55% reduction) after an initial 3 months of EVE therapy; however, PRL level gradually increased to its previous level by 5 months following initiation of EVE therapy (25). Among the seven published cases, only two out of seven cases (29%) reported no progression of tumor growth: one corticotroph carcinoma treated with EVE (7.5 mg) + Capecitabine followed by EVE (7.5–10 mg) + RT showed no increase in tumor volume for 5 months (58), and an aggressive PRL secreting tumor treated with EVE 10mg + octreotide showed stable tumor volume for 12 months (25). Currently, there is not a sufficient number of cases with EVE treatment for pituitary tumors to determine its effectiveness. In addition, the complexity of the reported cases may have biased the outcomes of EVE treatment. For example, three out of seven reported cases were pituitary carcinomas and six out of seven EVE treated cases were TMZ refractory cases. Further work will be required to define the role and effectiveness of EVE in management of pituitary tumors.
Immunotherapy
Immune checkpoint inhibitors upregulate the body’s immune response to fight against malignancy and have been used to treat melanomas, lung cancers, renal cancers and Hodgkin lymphomas (81). Pembrolizumab (PEM) and nivolumab (NIV) are checkpoint inhibitors that inhibit programmed cell death 1 (PD-1), which is a transmembrane protein expressed on immune cells that inhibits T-cell destruction of tumor cells. Ipilimumab (IPI) is a checkpoint inhibitor that inhibits the action of Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), which downregulates immune response (82). CTLA-4 and PD-1 are both expressed in pituitary adenomas (83–85). This is thought to be a mechanism by which immunotherapy can contribute to the treatment of pituitary tumors. However, the use of checkpoint inhibitors is associated with several adverse effects, including hypophysitis (86). For example, single-agent anti-PD-1/PD-L1 monoclonal antibody therapy is associated with incidence rate of hypophysitis between 1% to 6% (87). Combination of IPI (CTLA-4) plus NIV (PD-1) is associated with hypophysitis rate as high as 7.7% to 11.7 percent (88, 89). Other adverse effects by ICIs include thyroiditis (1%–6%) (90), primary adrenal insufficiency (0.7%) (91), dermatitis (34%–39%) (92), and hepatotoxicity (5%–10% with PD-1) (93).
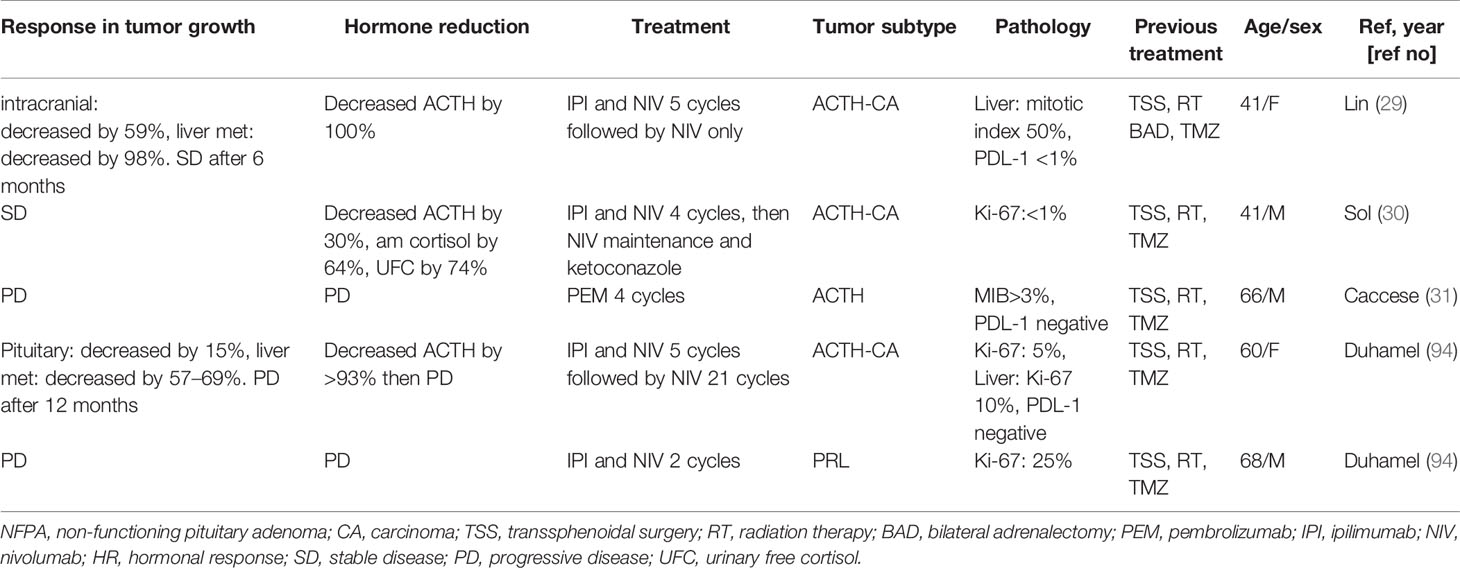
Thus far, checkpoint inhibitors have been used to treat pituitary adenomas in five cases (29–31, 94) (Table 5). Of these five cases, three cases were ACTH-producing carcinomas, one case was an ACTH-producing adenoma, and one case was a prolactinoma. All ACTH-producing carcinoma cases received a combination of IPI and NIV and showed decreased to stable sizes of pituitary carcinomas, ACTH levels, and the liver metastatic volume in two cases (29, 30, 94). However, one of these positively responding cases of pituitary carcinoma recurred over 1 year. Of note, two other cases of non-carcinomas [ACTH-producing adenoma (treated with PEM) and prolactinoma (treated with IPI and NIV)] had disease progression despite immunotherapy (31, 94).
Conventional Therapies
DAs Based Combination Therapies for Aggressive Prolactinomas
Although the dopamine agonists (DAs) (bromocriptine (BRC) and cabergoline (CAB)) are established first-line treatments for prolactinomas (95), DA resistance in prolactinomas occurs in 20%–30% of patients treated with BRC and 10% of patients treated with CAB (96). Possible mechanisms include decreased expression of dopamine receptor D2 (D2R), changes upstream or downstream of D2Rs signaling, increased angiogenic markers, and disruptions in the TGF- β1 pathway (97). It has been reported that higher dose of CAB therapy is successful in treating BRC resistant prolactinomas (96). Patients are generally able to tolerate high doses of DAs (98), but periodic echocardiogram may be required for prolonged high-dose use since cumulative dosage increases the risk of valvular heart disease (99). In addition to monotherapy, DA therapies in combination with tamoxifen (TAM) or octreotide (OCT) have been reported to treat DA-resistant prolactinomas with various response rates, summarized in Table 6 (100–105). Metformin was also used in combination therapies (103). A case series with DAs and metformin over 8-14 months have noted both PRL normalization and significant tumor reduction (103).
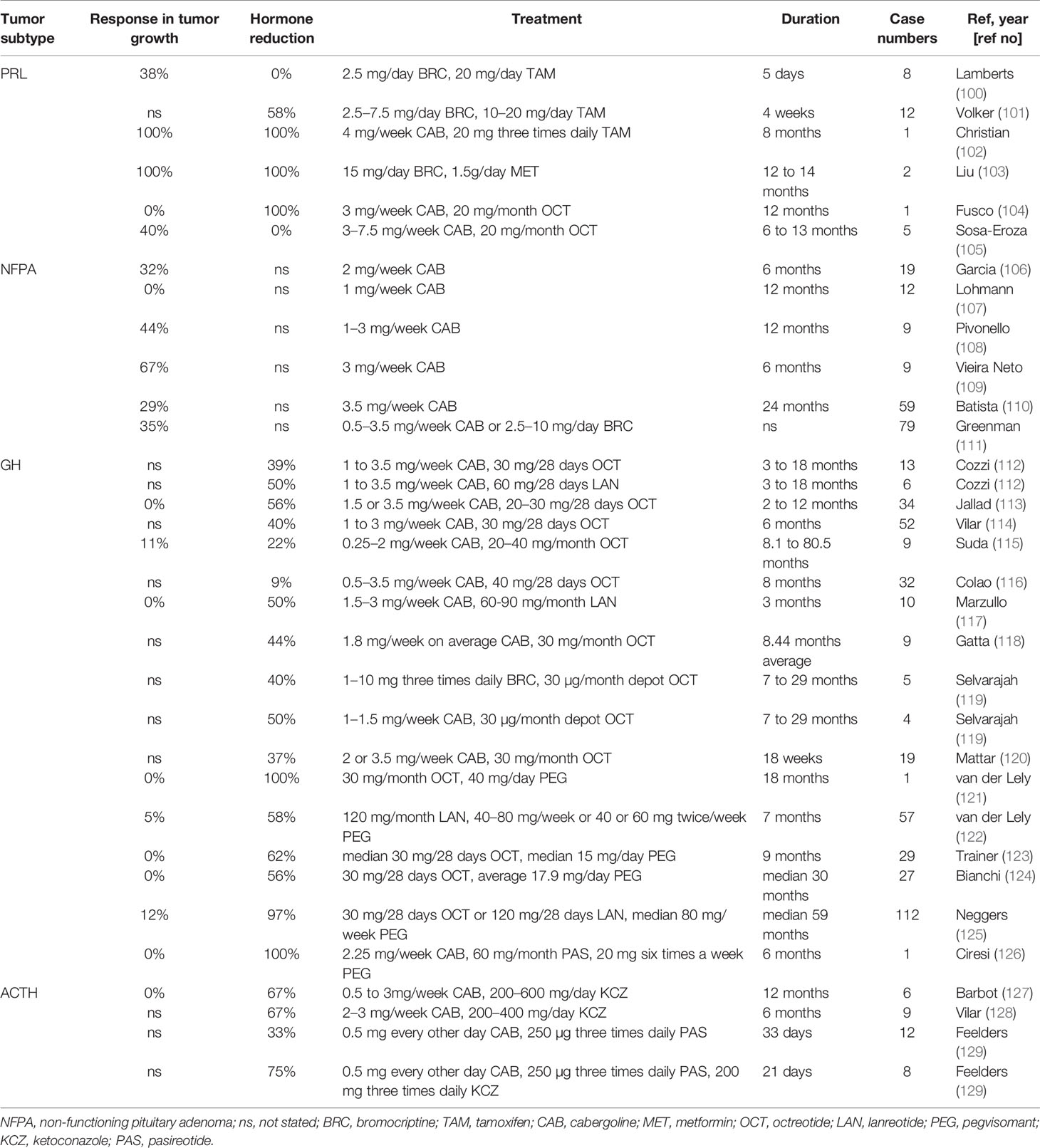
Table 6 Summary of conventional therapies for aggressive prolactinomas, Cushing’s disease, and acromegaly and non-functional pituitary adenomas.
DAs Based Therapies for Recurrent or Residual NFPAs
CAB monotherapy has been also used for recurrent or residual NFPAs (187 cases in six studies) (106–111). In these studies, in 33% of patients, CAB monotherapy resulted in more than 25% of tumor volume reduction or reduction of tumor size less than 2 mm in diameter, although the tumor reduction rate in these studies varies. The average dose of CAB was 2.95 mg/week and the average treatment duration was 8 months. The largest study, including 79 patients treated with DAs, demonstrated 35% reduction of tumor size on average when used against recurrent or residual NFPAs (111). It remains to be determined whether combinations of DAs with other drugs will be effective against refractory NFPAs.
SSA Based Combination Therapies for SSA-Resistant Acromegaly
OCT resistance is estimated to occur in approximately 30% of GH-secreting adenomas and is thought to be mediated by reduced expression of SSTRs (130). It is found that three combination therapies (DA, pegvisomant or both) with SSA are mildly to moderately effective against these SSA-resistant GH-secreting tumors. As summarized in Table 6, these conventional combination therapies have shown a wide range of efficacies for controlling hormone levels (9%–100%) (112–126). The combination of PEG and SSA seems more effective than CAB and SSA to control IGF1 levels (112–126). None of the combinations are effective in reducing tumor size (0%–12%) (112–120).
Combination Therapies for Aggressive Cushing Disease
Only a limited number of studies report treatment of aggressive Cushing disease with combinations of conventional therapies [Table 6 (127–129)]. Ketoconazole (KCZ) in combination with CAB or PAS showed a moderate normalizing effect on cortisol levels in KCZ monotherapy resistant cases (129). CAB and PAS combinations were reported, but were less effective for reducing cortisol levels (129). One study showed no tumor reduction and the others did not mention efficacies on tumor size reductions.
Epidermal Growth Factor Receptor (EGFR) Inhibitors
EGFR is a transmembrane receptor with the intracellular tyrosine kinase domain that regulates cell proliferation, migration, and survival. The EGFR inhibitors gefitinib and lapatinib are promising treatments for aggressive prolactinomas and aggressive corticotroph adenomas. EGFR signaling is detected in up to 82% of DA resistant prolactinomas. Activating EGFR signaling in rat lactosomatotroph cells causes elevation of PRL mRNA expression (131). EGFR signaling also upregulates the POMC (proopiomelanocortin) promoter and increases ACTH production in corticotrophs (132). Mutations in the ubiquitin specific peptidase 8 (USP8) gene, which causes gain-of-function of USP8 protein, are highly prevalent in Cushing disease (133). It is thought that the mutations in USP8 cause enhanced EGFR signaling and are closely associated with development of Cushing disease (133–136).
Gefitinib has been implemented for treatment of both aggressive prolactinomas and corticotroph adenomas (137). Gefitinib suppressed PRL secretion by approximately 50% in human prolactinoma primary cultures in vitro. Moreover, gefitinib suppressed serum PRL levels by 40%–50% and tumor volume by 30% in somatotroph adenoma xenografted rodent models (131, 138). In primary cultures of ACTH-secreting human, canine, and murine adenomas, POMC mRNA was significantly suppressed by 63%–95% following treatment with gefitinib. In corticotroph adenoma xenografted mice, a 10-day course of treatment with gefitinib resulted in 40% inhibition of tumor growth (132).
Lapatinib is a dual EGFR inhibitor that inhibits both EGFR and human epidermal growth factor receptor 2 (HER2) (139). It has mostly been used to treat aggressive prolactinomas. A comparative study between lapatinib and gefitinib has found that lapatinib suppressed PRL levels more strongly in human prolactinoma primary cultures (60% with lapatinib and 40% with gefitinib) (138). It has also been reported that lapatinib suppresses PRL secretion (by 72%) and cell proliferation (by 80%) in EGFR and HER2-expressing transgenic mice, which are models of prolactinomas (140). In addition, a recent clinical trial treating two patients with DA-resistant prolactinomas with lapatinib at a dosage of 1,250 mg/day has reported 78% and 42% PRL suppression respectively with a 22% tumor reduction in the former and a stabilized tumor in the latter (28). These studies suggest that targeting EGFR signaling is a promising therapeutic strategy to, at least, populations of pituitary tumors with elevated EGFR signaling.
Cyclin Dependent Kinase 2 (Cdk2) Inhibitor
CDK2 interacts with Cyclin E to facilitate entry into the S phase of the cell cycle and protects cells against apoptosis (141). The CDK2 inhibitor, roscovitine, has been tested in phase I and II clinical trials against various malignancies, including non-small cell lung cancer and nasopharyngeal and hepatocellular carcinomas (142). Roscovitine is currently being evaluated in preclinical studies for its ability to treat Cushing disease. Roscovitine targets the CDK2/Cyclin E complexes that are highly expressed specifically in corticotroph tumors (143). Roscovitine has dual effects; inhibition of corticotroph tumor growth and suppression of transcription of POMC, which encodes a precursor of ACTH (32, 143). It has been demonstrated that roscovitine can reduce pomc expression by over 50% in pituitary tumor transforming gene (PTTG) zebrafish model and reduced ACTH and corticosterone levels by 50% in corticotroph xenografted mice (143). In primary cells derived from human corticotroph tumors, roscovitine suppressed ACTH levels in five out of six tumors (83%) (32). These reports suggest promising results and may become a new avenue of treatment of Cushing disease.
Retinoic Acid (RA)
RA is a vitamin A derivative that interacts with RA receptors and retinoid X receptors and regulates transcription of downstream genes (144, 145). In terms of the effects on pituitary hormone genes, studies have found that the all-trans and 9-cis isomers of RA reduce the DNA-binding affinity of NURR1, a nuclear orphan receptor important for the transcription of POMC (146). 9-cis RA has also been found to activate the transcription of D2Rs, suggesting that RA may be effective against both corticotroph adenomas and prolactinomas (147). As of the publication of this review, studies of the application of RA in the treatment of pituitary tumors have focused on the treatment of corticotroph adenomas.
The majority of studies utilizing RA are pre-clinical. It has been shown that RA treatment inhibits ACTH production and tumor growth in murine and canine models of Cushing disease (148, 149). Two other clinical studies have been published using RA to treat aggressive Cushing disease (33, 34). In one prospective study, urinary free cortisol levels normalized in 4 of 16 patients after treatment with RA (33). In a recent clinical trial, over 50% cortisol suppression was observed in five of seven recurrent pituitary tumors, with normalization in three patients (34). Of note, one patient who was resistant to CAB therapy had normalized urinary free cortisol levels following the addition of RA in the treatment (33). Moreover, a study utilizing primary culture derived from 11 corticotroph adenomas found that the combination of RA+BRC inhibited POMC transcription and ACTH production at higher levels than either alone in five of 11 cultures, indicating that RA+DA combination therapy may be a possible treatment for Cushing disease (33, 150). At this point, the use of RA remains at pre-clinical level, and further evidence is required to determine its efficacy.
Conclusion
Aggressive pituitary tumors can be resistant to conventional therapies, including surgery, radiotherapy, and medical treatment. TMZ is recommended as the first-line chemotherapy for treatment of aggressive pituitary tumors. However, TMZ has a high rate of relapse in the long term. Several lines of evidence suggest the use of novel therapy, such as CAPTEM, PRRT, EVE, immunotherapy, EGFR inhibitors, CDK2 inhibitors, and RA, could be valuable strategies for long-term tumor control. These novel therapies could improve inhibition of pituitary tumor growth and/or control of excess hormone(s) compared to established treatment methods. Although information about the efficacy of such treatments is limited and few cases have utilized them so far, some treatments are showing promising outcomes. Further research will establish new treatment options and optimize treatment sequencing for aggressive pituitary tumors.
Author Contributions
TA and TT designed the project. TN-T, KL, and JW did literature searches and analyzed data. TA, TT, TN-T, KL, JW, CM, and YK wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study is supported by grants from the Graduate School, University of Minnesota (UMF0011528 to TA, Grant-in-Aid 212588 to TA), the University of Alberta (Start-up to TT), the University Hospital Foundation (Medical Research Competition 2019 to TT), and the US National Institutes of Health (AR064195 to YK).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Raverot G, Burman P, McCormack A, Heaney A, Petersenn S, Popovic V, et al. European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol (2018) 178(1):G1–g24. doi: 10.1530/EJE-17-0796
2. Lasolle H, Cortet C, Castinetti F, Cloix L, Caron P, Delemer B, et al. Temozolomide treatment can improve overall survival in aggressive pituitary tumors and pituitary carcinomas. Eur J Endocrinol (2017) 176(6):769–77. doi: 10.1530/EJE-16-0979
3. Bush ZM, Longtine JA, Cunningham T, Schiff D, Jane JA Jr, Vance ML, et al. Temozolomide treatment for aggressive pituitary tumors: correlation of clinical outcome with O(6)-methylguanine methyltransferase (MGMT) promoter methylation and expression. J Clin Endocrinol Metab (2010) 95(11):E280–90. doi: 10.1210/jc.2010-0441
4. Raverot G, Sturm N, de Fraipont F, Muller M, Salenave S, Caron P, et al. Temozolomide treatment in aggressive pituitary tumors and pituitary carcinomas: a French multicenter experience. J Clin Endocrinol Metab (2010) 95(10):4592–9. doi: 10.1210/jc.2010-0644
5. Losa M, Mazza E, Terreni MR, McCormack A, Gill AJ, Motta M, et al. Salvage therapy with temozolomide in patients with aggressive or metastatic pituitary adenomas: experience in six cases. Eur J Endocrinol (2010) 163(6):843–51. doi: 10.1530/EJE-10-0629
6. Hirohata T, Asano K, Ogawa Y, Takano S, Amano K, Isozaki O, et al. DNA mismatch repair protein (MSH6) correlated with the responses ofatypical pituitary adenomas and pituitary carcinomas to temozolomide: the national cooperative studyby the Japan Society for Hypothalamic and Pituitary Tumors. J Clin Endocrinol Metab (2013) 98(3):1130–6. doi: 10.1210/jc.2012-2924
7. Bruno OD, Juárez-Allen L, Christiansen SB, Manavela M, Danilowicz K, Vigovich C, et al. Temozolomide Therapy for Aggressive Pituitary Tumors: Results in a Small Series of Patients from Argentina. Int J Endocrinol (2015) 2015:587893. doi: 10.1155/2015/587893
8. Bengtsson D, Schrøder HD, Andersen M, Maiter D, Berinder K, Feldt Rasmussen U, et al. Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide. J Clin Endocrinol Metab (2015) 100(4):1689–98. doi: 10.1210/jc.2014-4350
9. Ceccato F, Lombardi G, Manara R, Emanuelli E, Denaro L, Milanese L, et al. Temozolomide and pasireotide treatment for aggressive pituitary adenoma: expertise at a tertiary care center. J Neurooncol (2015) 122(1):189–96. doi: 10.1007/s11060-014-1702-0
10. Losa M, Bogazzi F, Cannavo S, Ceccato F, Curtò L, De Marinis L, et al. Temozolomide therapy in patients with aggressive pituitary adenomas or carcinomas. J Neurooncol (2016) 126(3):519–25. doi: 10.1007/s11060-015-1991-y
11. McCormack A, Dekkers OM, Petersenn S, Popovic V, Trouillas J, Raverot G, et al. Treatment of aggressive pituitary tumours and carcinomas: results ofa European Society of Endocrinology (ESE) survey 2016. Eur J Endocrinol (2018) 178(3):265–76. doi: 10.1530/eje-17-0933
12. Santos-Pinheiro F, Prado M, Kamiya-Matsuoka C, Waguespack SG, Mahajan A, Brown PD, et al. Treatment and long-term outcomes in pituitary carcinoma: a cohort study. Eur J Endocrinol (2019) 181(4):397–407. doi: 10.1530/EJE-18-0795
13. Elbelt U, Schlaffer SM, Buchfelder M, Knappe UJ, Vila G, Micko A, et al. Efficacy of Temozolomide Therapy in Patients With Aggressive Pituitary Adenomas and Carcinomas-A German Survey. J Clin Endocrinol Metab (2020) 105(3). doi: 10.1210/clinem/dgz211
14. Zacharia BE, Gulati AP, Bruce JN, Carminucci AS, Wardlaw SL, Siegelin M, et al. High response rates and prolonged survival in patients with corticotroph pituitary tumors and refractory Cushing disease from capecitabine and temozolomide (CAPTEM): a case series. Neurosurgery (2014) 74(4):E447–55; discussion E455. doi: 10.1227/NEU.0000000000000251
15. Nakano-Tateno T, Satou M, Inoshita N, van Landeghem FKH, Easaw J, Mehta V, et al. Effects of CAPTEM (Capecitabine and Temozolomide) on a Corticotroph Carcinoma and an Aggressive Corticotroph Tumor. Endocr Pathol (2020). doi: 10.1007/s12022-020-09647-w
16. Kumar Gupta S, Singla S, Damle NA, Agarwal K, Bal C. Diagnosis of Men-I Syndrome on (68)Ga-DOTANOC PET-CT and Role of Peptide Receptor Radionuclide Therapy With (177)Lu-DOTATATE. Int J Endocrinol Metab (2012) 10(4):629–33. doi: 10.5812/ijem.4313
17. Baldari S, Ferraù F, Alafaci C, Herberg A, Granata F, Militano V, et al. First demonstration of the effectiveness of peptide receptor radionuclide therapy (PRRT) with 111In-DTPA-octreotide in a giant PRL-secreting pituitary adenoma resistant to conventional treatment. Pituitary (2012) 15 Suppl 1:S57–60. doi: 10.1007/s11102-011-0373-5
18. Komor J, Reubi JC, Christ ER. Peptide receptor radionuclide therapy in a patient with disabling non-functioning pituitary adenoma. Pituitary (2014) 17(3):227–31. doi: 10.1007/s11102-013-0494-0
19. Waligórska-Stachura J, Gut P, Sawicka-Gutaj N, Liebert W, Gryczyńska M, Baszko-Blaszyk D, et al. Growth hormone-secreting macroadenoma of the pituitary gland successfully treated with the radiolabeled somatostatin analog (90)Y-DOTATATE: case report. J Neurosurg (2016) 125(2):346–9. doi: 10.3171/2015.6.JNS15363
20. Maclean J, Aldridge M, Bomanji J, Short S, Fersht N. Peptide receptor radionuclide therapy for aggressive atypical pituitary adenoma/carcinoma: variable clinical response in preliminary evaluation. Pituitary (2014) 17(6):530–8. doi: 10.1007/s11102-013-0540-y
21. Assadi M, Nemati R, Shooli H, Rekabpour SJ, Nabipour I, Jafari E, et al. An aggressive functioning pituitary adenoma treated with peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging (2020) 47(4):1015–6. doi: 10.1007/s00259-019-04578-z
22. Novruzov F, Aliyev JA, Jaunmuktane F, Bomanji JB, Kayani I. The use of (68)Ga DOTATATE PET/CT for diagnostic assessment and monitoring of (177)Lu DOTATATE therapy in pituitary carcinoma. Clin Nucl Med (2015) 40(1):47–9. doi: 10.1097/RLU.0000000000000589
23. Giuffrida G, Ferraù F, Laudicella R, Cotta O, Messina E, Granata F, et al. Peptide receptor radionuclide therapy for aggressive pituitary tumors: a monocentric experience. Endocr Connect (2019) 8(5):528–35. doi: 10.1530/EC-19-0065
24. Kovács GL, Góth M, Rotondo F, Scheithauer BW, Carlsen E, Saadia A, et al. ACTH-secreting Crooke cell carcinoma of the pituitary. Eur J Clin Invest (2013) 43(1):20–6. doi: 10.1111/eci.12010
25. Zhang D, Way JS, Zhang X, Sergey M, Bergsneider M, Wang MB, et al. Effect of Everolimus in Treatment of Aggressive Prolactin-Secreting Pituitary Adenomas. J Clin Endocrinol Metab (2019) 104(6):1929–36. doi: 10.1210/jc.2018-02461
26. Alshaikh OM, Asa SL, Mete O, Ezzat S. An Institutional Experience of Tumor Progression to Pituitary Carcinoma in a 15-Year Cohort of 1055 Consecutive Pituitary Neuroendocrine Tumors. Endocr Pathol (2019) 30(2):118–27. doi: 10.1007/s12022-019-9568-5
27. Jouanneau E, Wierinckx A, Ducray F, Favrel V, Borson-Chazot F, Honnorat J, et al. New targeted therapies in pituitary carcinoma resistant to temozolomide. Pituitary (2012) 15(1):37–43. doi: 10.1007/s11102-011-0341-0
28. Cooper O, Mamelak A, Bannykh S, Carmichael J, Bonert V, Lim S, et al. Prolactinoma ErbB receptor expression and targeted therapy for aggressive tumors. Endocrine (2014) 46(2):318–27. doi: 10.1007/s12020-013-0093-x
29. Lin AL, Jonsson P, Tabar V, Yang TJ, Cuaron J, Beal K, Lin AL, et al. Marked Response of a Hypermutated ACTH-Secreting Pituitary Carcinoma to Ipilimumab and Nivolumab. J Clin Endocrinol Metab (2018) 103(10):3925–30. doi: 10.1210/jc.2018-01347
30. Sol B, de Filette JMK, Awada G, Raeymaeckers S, Aspeslagh S, Andreescu CE, et al. Immune checkpoint inhibitor therapy for ACTH-secreting pituitary carcinoma: a new emerging treatment? Eur J Endocrinol (2021) 184(1):K1–5. doi: 10.1530/EJE-20-0151
31. Caccese M, Barbot M, Ceccato F, Padovan M, Gardiman MP, Fassan M, et al. Rapid disease progression in patient with mismatch-repair deficiency pituitary ACTH-secreting adenoma treated with checkpoint inhibitor pembrolizumab. Anticancer Drugs (2020) 31(2):199–204. doi: 10.1097/CAD.0000000000000856
32. Liu NA, Araki T, Cuevas-Ramos D, Hong J, Ben-Shlomo A, Tone Y, et al. Cyclin E-Mediated Human Proopiomelanocortin Regulation as a Therapeutic Target for Cushing Disease. J Clin Endocrinol Metab (2015) 100(7):2557–64. doi: 10.1210/jc.2015-1606
33. Vilar L, Albuquerque JL, Lyra R, Trovão L, Diniz E, Rangel Filho F, et al. The Role of Isotretinoin Therapy for Cushing’s Disease: Results of a Prospective Study. Int J Endocrinol (2016) 2016:8173182. doi: 10.1155/2016/8173182
34. Pecori Giraldi F, Ambrogio AG, Andrioli M, Sanguin F, Karamouzis I, Corsello SM, et al. Potential role for retinoic acid in patients with Cushing’s disease. J Clin Endocrinol Metab (2012) 97(10):3577–83. doi: 10.1210/jc.2012-2328
35. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med (2005) 352(10):987–96. doi: 10.1056/NEJMoa043330
36. Lim S, Shahinian H, Maya MM, Yong W. Temozolomide: a novel treatment for pituitary carcinoma. Lancet Oncol (2006) 7(6):518–20. doi: 10.1016/S1470-2045(06)70728-8
37. Syro LV, Uribe H, Penagos LC, Ortiz LD, Fadul CE, Horvath E, et al. Antitumour effects of temozolomide in a man with a large, invasive prolactin-producing pituitary neoplasm. Clin Endocrinol (Oxf) (2006) 65(4):552–3. doi: 10.1111/j.1365-2265.2006.02653.x
38. Fadul CE, Kominsky AL, Meyer LP, Kingman LS, Kinlaw WB, Rhodes CH, et al. Long-term response of pituitary carcinoma to temozolomide. Report of two cases. J Neurosurg (2006) 105(4):621–6. doi: 10.3171/jns.2006.105.4.621
39. Liu L, Gerson SL. Targeted modulation of MGMT: clinical implications. Clin Cancer Res (2006) 12(2):328–31. doi: 10.1158/1078-0432.CCR-05-2543
40. Halevy C, Whitelaw BC. How effective is temozolomide for treating pituitary tumours and when should it be used? Pituitary (2017) 20(2):261–6. doi: 10.1007/s11102-016-0745-y
41. Ji Y, Vogel RI, Lou E. Temozolomide treatment of pituitary carcinomas and atypical adenomas: systematic review of case reports. Neurooncol Pract (2016) 3(3):188–95. doi: 10.1093/nop/npv059
42. Campderá M, Palacios N, Aller J, Magallón R, Martín P, Saucedo G, et al. Temozolomide for aggressive ACTH pituitary tumors: failure of a second course of treatment. Pituitary (2016) 19(2):158–66. doi: 10.1007/s11102-015-0694-x
43. Bilbao I, Egaña N, García C, Olaizola I, et al. Failure of a second temozolomide cycle in a patient with a prolactin-secreting pituitary carcinoma. Endocrinol Diabetes Nutr (2017) 64(10):564–6. doi: 10.1016/j.endien.2017.08.007
44. Syro LV, Rotondo F, Camargo M, Ortiz LD, Serna CA, Kovacs K, et al. Temozolomide and Pituitary Tumors: Current Understanding, Unresolved Issues, and Future Directions. Front Endocrinol (Lausanne) (2018) 9:318. doi: 10.3389/fendo.2018.00318
45. McCormack AI, Wass JA, Grossman AB. Aggressive pituitary tumours: the role of temozolomide and theassessment of MGMT status. Eur J Clin Invest (2011)41:1133–48. doi: 10.1111/j.1365-2362.2011.02520.x
46. Mohammed AI, Cusimano MD, Scheithauer BW, Rotondo F, Horvath E, Kovacs K. O-methylguanine-DNA methyltransferase immunoexpression in a double pituitary adenoma: case report. Neurosurgery (2010) 66:E421–2;discussion E422. doi: 10.1227/01.Neu.0000363852.77126.Ad
47. Kovacs K, Scheithauer BW, Lombardero M, McLendon RE, Syro LV, Uribe H, et al. MGMT immunoexpression predicts responsiveness of pituitary tumors to temozolomide therapy. Acta Neuropathol (2008) 115:261–2. doi: 10.1007/s00401-007-0279-5
48. McCormack AI, McDonald KL, Gill AJ, Clark SJ, Burt MG, Campbell KA, et al. Low O6-methylguanine-DNA methyltransferase (MGMT) expression and response to temozolomide in aggressive pituitary tumours. Clin Endocrinol (Oxf) (2009) 71:226–33. doi: 10.1111/j.1365-2265.2008.03487.x
49. Hagen C, Schroeder HD, Hansen S, Hagen C, Andersen M. Temozolomide treatment of a pituitary carcinoma and two pituitary macroadenomas resistant to conventional therapy. Eur J Endocrinol (2009) 161:631–7. doi: 10.1530/eje-09-0389
50. Whitelaw BC, Dworakowska D, Thomas NW, Barazi S, Riordan-Eva P, King AP, et al. Temozolomide in the management of dopamine agonist-resistant prolactinomas. Clin Endocrinol (Oxf) (2012) 76:877–86. doi: 10.1111/j.1365-2265.2012.04373.x
51. Annamalai AK, Dean AF, Kandasamy N, Kovacs K, Burton H, Halsall DJ, et al. Temozolomide responsiveness in aggressive corticotroph tumours: a case report and review of the literature. Pituitary (2012) 15:276–87. doi: 10.1007/s11102-011-0363-7
52. Yip S, Miao J, Cahill DP, Iafrate AJ, Aldape K, Nutt CL, et al. MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin Cancer Res (2009) 15(14):4622–9. doi: 10.1158/1078-0432.CCR-08-3012
53. Murakami J, Lee YJ, Kokeguchi S, Tsujigiwa H, Asaumi J, Nagatsuka H, et al. Depletion of O6-methylguanine-DNA methyltransferase by O6-benzylguanine enhances 5-FU cytotoxicity in colon and oral cancer cell lines. Oncol Rep (2007) 17(6):1461–7. doi: 10.3892/or.17.6.1461
54. Kulke MH, Hornick JL, Frauenhoffer C, Hooshmand S, Ryan DP, Enzinger PC, et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res (2009) 15(1):338–45. doi: 10.1158/1078-0432.CCR-08-1476
55. Fine RL, Gulati AP, Krantz BA, Moss RA, Schreibman S, Tsushima DA, et al. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: The Pancreas Center at Columbia University experience. Cancer Chemother Pharmacol (2013) 71(3):663–70. doi: 10.1007/s00280-012-2055-z
56. Spiro TP, Liu L, Majka S, Haaga J, Willson JK, Gerson SL. Temozolomide: the effect of once- and twice-a-day dosing on tumor tissue levels of the DNA repair protein O(6)-alkylguanine-DNA-alkyltransferase. Clin Cancer Res (2001) 7(8):2309–17.
57. Thearle MS, Freda PU, Bruce JN, Isaacson SR, Lee Y, Fine RL. Temozolomide (Temodar®) and capecitabine (Xeloda®) treatment of an aggressive corticotroph pituitary tumor. Pituitary (2011) 14(4):418–24. doi: 10.1007/s11102-009-0211-1
58. Donovan LE, Arnal AV, Wang SH, Odia Y. Widely metastatic atypical pituitary adenoma with mTOR pathway STK11(F298L) mutation treated with everolimus therapy. CNS Oncol (2016) 5(4):203–9. doi: 10.2217/cns-2016-0011
59. Joehlin-Price AS, Hardesty DA, Arnold CA, Kirschner LS, Prevedello DM, Lehman NL. Case report: ACTH-secreting pituitary carcinoma metastatic to the liver in a patient with a history of atypical pituitary adenoma and Cushing’s disease. Diagn Pathol (2017) 12(1):34. doi: 10.1186/s13000-017-0624-5
60. Rotman LE, Vaughan TB, Hackney JR, Riley KO. Long-Term Survival After Transformation of an Adrenocorticotropic Hormone-Secreting Pituitary Macroadenoma to a Silent Corticotroph Pituitary Carcinoma. World Neurosurg (2019) 122:417–23. doi: 10.1016/j.wneu.2018.11.011
61. Touma W, Hoostal S, Peterson RA, Wiernik A, SantaCruz KS, Lou E. Successful treatment of pituitary carcinoma with concurrent radiation, temozolomide, and bevacizumab after resection. J Clin Neurosci (2017) 41:75–7. doi: 10.1016/j.jocn.2017.02.052
62. Wang Y, He Q, Meng X, Zhou S, Zhu Y, Xu J. Apatinib (YN968D1) and temozolomide in recurrent invasive pituitary adenoma: case report and literature review. World Neurosurg (2019) 124:319–22. doi: 10.1016/j.wneu.2018.12.174
63. Bode H, Seiz M, Lammert A, Brockmann MA, Back W, Hammes HP. SOM230 (pasireotide) and temozolomide achieve sustained control of tumour progression and ACTH secretion in pituitary carcinoma with widespread metastases. Exp Clin Endocrinol Diabetes (2010) 118(10):760–3. doi: 10.1055/s-0030-1253419
64. Goglia U, Ferone D, Sidoti M, Spaziante R, Dadati P, Ravetti JL, et al. Treatment of a pituitary metastasis from a neuroendocrine tumour: case report and literature review. Pituitary (2008) 11(1):93–102. doi: 10.1007/s11102-007-0038-6
65. Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol (2011) 29(17):2416–23. doi: 10.1200/JCO.2010.33.7873
66. Kwekkeboom DJ, de Herder WW, Krenning EP. Somatostatin receptor-targeted radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am (2011) 40(1):173–85, ix. doi: 10.1016/j.ecl.2010.12.003
67. Krenning EP, de Jong M, Kooij PPM, Breeman WAP, Bakker WH, de Herder WW, et al. Radiolabelled somatostatin analogue(s) for peptide receptor scintigraphy and radionuclide therapy. Ann Oncol (1999) 10:S23–30. doi: 10.1093/annonc/10.suppl_2.S23
68. Lapa C, Werner RA, Schmid JS, Papp L, Zsótér N, Biko J, et al. Prognostic value of positron emission tomography-assessed tumor heterogeneity in patients with thyroid cancer undergoing treatment with radiopeptide therapy. Nucl Med Biol (2015) 42(4):349–54. doi: 10.1016/j.nucmedbio.2014.12.006
69. Werner RA, Lapa C, Ilhan H, Higuchi T, Buck AK, Lehner S, et al. Survival prediction in patients undergoing radionuclide therapy based on intratumoral somatostatin-receptor heterogeneity. Oncotarget (2017) 8(4):7039–49. doi: 10.18632/oncotarget.12402
70. Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet (2006) 7(8):606–19. doi: 10.1038/nrg1879
71. Monsalves E, Juraschka K, Tateno T, Agnihotri S, Asa SL, Ezzat S, et al. The PI3K/AKT/mTOR pathway in the pathophysiology and treatment of pituitary adenomas. Endocr Relat Cancer (2014) 21(4):R331–44. doi: 10.1530/ERC-14-0188
72. Musat M, Korbonits M, Kola B, Borboli N, Hanson MR, Nanzer AM, et al. Enhanced protein kinase B/Akt signalling in pituitary tumours. Endocr Relat Cancer (2005) 12(2):423–33. doi: 10.1677/erc.1.00949
73. Sajjad EA, Zieliński G, Maksymowicz M, Hutnik Ł, Bednarczuk T, Włodarski P. mTOR is frequently active in GH-secreting pituitary adenomas without influencing their morphopathological features. Endocr Pathol (2013) 24(1):11–9. doi: 10.1007/s12022-012-9230-y
74. Gorshtein A, Rubinfeld H, Kendler E, Theodoropoulou M, Cerovac V, Stalla GK, et al. Mammalian target of rapamycin inhibitors rapamycin and RAD001 (everolimus) induce anti-proliferative effects in GH-secreting pituitary tumor cells in vitro. Endocr Relat Cancer (2009) 16(3):1017–27. doi: 10.1677/ERC-08-0269
75. Lee M, Wiedemann T, Gross C, Leinhäuser I, Roncaroli F, Braren R, et al. Targeting PI3K/mTOR Signaling Displays Potent Antitumor Efficacy against Nonfunctioning Pituitary Adenomas. Clin Cancer Res (2015) 21(14):3204–15. doi: 10.1158/1078-0432.CCR-15-0288
76. Chanal M, Chevallier P, Raverot V, Fonteneau G, Lucia K, Monteserin Garcia JL, et al. Differential Effects of PI3K and Dual PI3K/mTOR Inhibition in Rat Prolactin-Secreting Pituitary Tumors. Mol Cancer Ther (2016) 15(6):1261–70. doi: 10.1158/1535-7163.MCT-15-0891
77. Pivonello C, Patalano R, Solari D, Auriemma RS, Frio F, Vitulli F, et al. Effect of combined treatment with a pan-PI3K inhibitor or an isoform-specific PI3K inhibitor and everolimus on cell proliferation in GH-secreting pituitary tumour in an experimental setting. Endocrine (2018) 62(3):663–80. doi: 10.1007/s12020-018-1677-2
78. Jalali S, Monsalves E, Tateno T, Zadeh G. Role of mTOR Inhibitors in Growth Hormone-Producing Pituitary Adenomas Harboring Different FGFR4 Genotypes. Endocrinology (2016) 157(9):3577–87. doi: 10.1210/en.2016-1028
79. Zatelli MC, Minoia M, Filieri C, Tagliati F, Buratto M, Ambrosio MR, et al. Effect of everolimus on cell viability in nonfunctioning pituitary adenomas. J Clin Endocrinol Metab (2010) 95(2):968–76. doi: 10.1210/jc.2009-1641
80. Cerovac V, Monteserin-Garcia J, Rubinfeld H, Buchfelder M, Losa M, Florio T, et al. The somatostatin analogue octreotide confers sensitivity to rapamycin treatment on pituitary tumor cells. Cancer Res (2010) 70(2):666–74. doi: 10.1158/0008-5472.CAN-09-2951
81. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med (2018) 50(12):1–11. doi: 10.1038/s12276-018-0191-1
82. Hui E. Immune checkpoint inhibitors. J Cell Biol (2019) 218(3):740–1. doi: 10.1083/jcb.201810035
83. Mei Y, Bi WL, Greenwald NF, Du Z, Agar NY, Kaiser UB, et al. Increased expression of programmed death ligand 1 (PD-L1) in human pituitary tumors. Oncotarget (2016) 7(47):76565–76. doi: 10.18632/oncotarget.12088
84. Uraki S, Ariyasu H, Doi A, Takeshima K, Morita S, Inaba H, et al. MSH6/2 and PD-L1 Expressions Are Associated with Tumor Growth and Invasiveness in Silent Pituitary Adenoma Subtypes. Int J Mol Sci (2020) 21(8). doi: 10.3390/ijms21082831
85. Wang Z, Guo X, Gao L, Deng K, Lian W, Bao X, et al. The Immune Profile of Pituitary Adenomas and a Novel Immune Classification for Predicting Immunotherapy Responsiveness. J Clin Endocrinol Metab (2020) 105(9):e3207–23. doi: 10.1210/clinem/dgaa449
86. Albarel F, Gaudy C, Castinetti F, Carré T, Morange I, Conte–Devolx B, et al. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur J Endocrinol (2015) 172(2):195–204. doi: 10.1530/EJE-14-0845
87. Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol (2015) 26(12):2375–91. doi: 10.1093/annonc/mdv383
88. Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med (2015) 372(21):2006–17. doi: 10.1056/NEJMoa1414428
89. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med (2015) 373(1):23–34. doi: 10.1056/NEJMoa1504030
90. Ferrari SM, Fallahi P, Galetta F, Citi E, Benvenga S, Antonelli A. Thyroid disorders induced by checkpoint inhibitors. Rev Endocr Metab Disord (2018) 19(4):325–33. doi: 10.1007/s11154-018-9463-2
91. Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, et al. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol (2018) 4(2):173–82. doi: 10.1001/jamaoncol.2017.3064
92. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med (2010) 363(8):711–23. doi: 10.1056/NEJMoa1003466
93. Tian Y, Abu-Sbeih H, Wang Y. Immune Checkpoint Inhibitors-Induced Hepatitis. Adv Exp Med Biol (2018) 995:159–64. doi: 10.1007/978-3-030-02505-2_8
94. Duhamel C, Ilie MD, Salle H, Nassouri AS, Gaillard S, Deluche E, et al. Immunotherapy in Corticotroph and Lactotroph Aggressive Tumors and Carcinomas: Two Case Reports and a Review of the Literature. J Pers Med (2020) 10(3). doi: 10.3390/jpm10030088
95. Colao A, Savastano S. Medical treatment of prolactinomas. Nat Rev Endocrinol (2011) 7(5):267–78. doi: 10.1038/nrendo.2011.37
96. Maiter D. Management of Dopamine Agonist-Resistant Prolactinoma. Neuroendocrinology (2019) 109(1):42–50. doi: 10.1159/000495775
97. Recouvreux MV, Camilletti MA, Rifkin DB, Díaz-Torga G. The pituitary TGFβ1 system as a novel target for the treatment of resistant prolactinomas. J Endocrinol (2016) 228(3):R73–83. doi: 10.1530/JOE-15-0451
98. Ono M, Miki N, Kawamata T, Makino R, Amano K, Seki T, et al. Prospective study of high-dose cabergoline treatment of prolactinomas in 150 patients. J Clin Endocrinol Metab (2008) 93(12):4721–7. doi: 10.1210/jc.2007-2758
99. Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N Engl J Med (2007) 356(1):39–46. doi: 10.1056/NEJMoa054830
100. Lamberts SW, Verleun T, Oosterom R. Effect of tamoxifen administration on prolactin release by invasive prolactin-secreting pituitary adenomas. Neuroendocrinology (1982) 34(5):339–42. doi: 10.1159/000123324
101. Völker W, Gehring WG, Berning R, Schmidt RC, Schneider J, von zur Mühlen A. Impaired pituitary response to bromocriptine suppression: reversal after bromocriptine plus tamoxifen. Acta Endocrinol (Copenh) (1982) 101(4):491–500. doi: 10.1530/acta.0.1010491
102. Christian ZK, Hatanpaa KJ, Auchus RJ, Hammes SR, Patel AR, Mickey BE. Dopamine agonist and tamoxifen combination therapy for a prolactin-secreting pituitary tumor resistant to dopamine agonist monotherapy: Case report and review. Interdiscip Neurosurg (2020) 21:100777. doi: 10.1016/j.inat.2020.100777
103. Liu X, Liu Y, Gao J, Feng M, Bao X, Deng K, et al. Combination Treatment with Bromocriptine and Metformin in Patients with Bromocriptine-Resistant Prolactinomas: Pilot Study. World Neurosurg (2018) 115:94–8. doi: 10.1016/j.wneu.2018.02.188
104. Fusco A, Lugli F, Sacco E, Tilaro L, Bianchi A, Angelini F, et al. Efficacy of the combined cabergoline and octreotide treatment in a case of a dopamine-agonist resistant macroprolactinoma. Pituitary (2011) 14(4):351–7. doi: 10.1007/s11102-008-0162-y
105. Sosa-Eroza E, Espinosa E, Ramírez-Rentería C, Mendoza V, Arreola R, Mercado M. Treatment of multiresistant prolactinomas with a combination of cabergoline and octreotide LAR. Endocrine (2018) 61(2):343–8. doi: 10.1007/s12020-018-1638-9
106. Garcia EC, Naves LA, Silva AO, de Castro LF, Casulari LA, Azevedo MF. Short-term treatment with cabergoline can lead to tumor shrinkage in patients with nonfunctioning pituitary adenomas. Pituitary (2013) 16(2):189–94. doi: 10.1007/s11102-012-0403-y
107. Lohmann T, Trantakis C, Biesold M, Prothmann S, Guenzel S, Schober R, et al. Minor tumour shrinkage in nonfunctioning pituitary adenomas by long-term treatment with the dopamine agonist cabergoline. Pituitary (2001) 4(3):173–8. doi: 10.1023/a:1015366923810
108. Pivonello R, Matrone C, Filippella M, Cavallo LM, Di Somma C, Cappabianca P, et al. Dopamine receptor expression and function in clinically nonfunctioning pituitary tumors: comparison with the effectiveness of cabergoline treatment. J Clin Endocrinol Metab (2004) 89(4):1674–83. doi: 10.1210/jc.2003-030859
109. Vieira Neto L, Wildemberg LE, Moraes AB, Colli LM, Kasuki L, Marques NV, et al. Dopamine receptor subtype 2 expression profile in nonfunctioning pituitary adenomas and in vivo response to cabergoline therapy. Clin Endocrinol (Oxf) (2015) 82(5):739–46. doi: 10.1111/cen.12684
110. Batista RL, Musolino NRC, Cescato VAS, da Silva GO, Medeiros RSS, Herkenhoff CGB, et al. Cabergoline in the Management of Residual Nonfunctioning Pituitary Adenoma: A Single-Center, Open-Label, 2-Year Randomized Clinical Trial. Am J Clin Oncol (2019) 42(2):221–7. doi: 10.1097/COC.0000000000000505
111. Greenman Y, Cooper O, Yaish I, Robenshtok E, Sagiv N, Jonas-Kimchi T, et al. Treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists. Eur J Endocrinol (2016) 175(1):63–72. doi: 10.1530/EJE-16-0206
112. Cozzi R, Attanasio R, Lodrini S, Lasio G. Cabergoline addition to depot somatostatin analogues in resistant acromegalic patients: efficacy and lack of predictive value of prolactin status. Clin Endocrinol (Oxf) (2004) 61(2):209–15. doi: 10.1111/j.1365-2265.2004.02082.x
113. Jallad RS, Bronstein MD. Optimizing medical therapy of acromegaly: beneficial effects of cabergoline in patients uncontrolled with long-acting release octreotide. Neuroendocrinology (2009) 90(1):82–92. doi: 10.1159/000218323
114. Vilar L, Azevedo MF, Naves LA, Casulari LA, Albuquerque JL, Montenegro RM, et al. Role of the addition of cabergoline to the management of acromegalic patients resistant to longterm treatment with octreotide LAR. Pituitary (2011) 14(2):148–56. doi: 10.1007/s11102-010-0272-1
115. Suda K, Inoshita N, Iguchi G, Fukuoka H, Takahashi M, Nishizawa H, et al. Efficacy of combined octreotide and cabergoline treatment in patients with acromegaly: a retrospective clinical study and review of the literature. Endocr J (2013) 60(4):507–15.
116. Colao A, Zgliczyński W, Komorowski J, Kos-Kudla B, Tabarin A, Kerlan V, et al. Efficacy and safety of high-dose long-acting repeatable octreotide as monotherapy or in combination with pegvisomant or cabergoline in patients with acromegaly not adequately controlled by conventional regimens: results of an open-label, multicentre study. Endokrynol Pol (2019) 70(4):305–12. doi: 10.5603/EP.a2019.0023
117. Marzullo P, Ferone D, Di Somma C, Pivonello R, Filippella M, Lombardi G, et al. Efficacy of combined treatment with lanreotide and cabergoline in selected therapy-resistant acromegalic patients. Pituitary (1999) 1(2):115–20. doi: 10.1023/a:1009932521242
118. Gatta B, Hau DH, Catargi B, Roger P, Tabarin A. Re-evaluation of the efficacy of the association of cabergoline to somatostatin analogues in acromegalic patients. Clin Endocrinol (Oxf) (2005) 63(4):477–8. doi: 10.1111/j.1365-2265.2005.02329.x
119. Selvarajah D, Webster J, Ross R, Newell-Price J. Effectiveness of adding dopamine agonist therapy to long-acting somatostatin analogues in the management of acromegaly. Eur J Endocrinol (2005) 152(4):569–74. doi: 10.1530/eje.1.01888
120. Mattar P, Alves Martins MR, Abucham J. Short- and long-term efficacy of combined cabergoline and octreotide treatment in controlling igf-I levels in acromegaly. Neuroendocrinology (2010) 92(2):120–7. doi: 10.1159/000317314
121. van der Lely AJ, Muller A, Janssen JA, Davis RJ, Zib KA, Scarlett JA, et al. Control of tumor size and disease activity during cotreatment with octreotide and the growth hormone receptor antagonist pegvisomant in an acromegalic patient. J Clin Endocrinol Metab (2001) 86(2):478–81. doi: 10.1210/jcem.86.2.7206
122. van der Lely AJ, Bernabeu I, Cap J, Caron P, Colao A, Marek J, et al. Coadministration of lanreotide Autogel and pegvisomant normalizes IGF1 levels and is well tolerated in patients with acromegaly partially controlled by somatostatin analogs alone. Eur J Endocrinol (2011) 164(3):325–33. doi: 10.1530/EJE-10-0867
123. Trainer PJ, Ezzat S, D'Souza GA, Layton G, Strasburger CJ. A randomized, controlled, multicentre trial comparing pegvisomant alone with combination therapy of pegvisomant and long-acting octreotide in patients with acromegaly. Clin Endocrinol (Oxf) (2009) 71(4):549–57. doi: 10.1111/j.1365-2265.2009.03620.x
124. Bianchi A, Valentini F, Iuorio R, Poggi M, Baldelli R, Passeri M, et al. Long-term treatment of somatostatin analog-refractory growth hormone-secreting pituitary tumors with pegvisomant alone or combined with long-acting somatostatin analogs: a retrospective analysis of clinical practice and outcomes. J Exp Clin Cancer Res (2013) 32(1):40. doi: 10.1186/1756-9966-32-40
125. Neggers SJ, Franck SE, de Rooij FW, Dallenga AH, Poublon RM, Feelders RA, et al. Long-term efficacy and safety of pegvisomant in combination with long-acting somatostatin analogs in acromegaly. J Clin Endocrinol Metab (2014) 99(10):3644–52. doi: 10.1210/jc.2014-2032
126. Ciresi A, Radellini S, Guarnotta V, Giordano C. Efficacy of combined treatment with pasireotide, pegvisomant and cabergoline in an acromegalic patient resistant to other treatments: a case report. BMC Endocr Disord (2018) 18(1):2. doi: 10.1186/s12902-018-0231-9
127. Barbot M, Albiger N, Ceccato F, Zilio M, Frigo AC, Denaro L, et al. Combination therapy for Cushing’s disease: effectiveness of two schedules of treatment: should we start with cabergoline or ketoconazole? Pituitary (2014) 17(2):109–17. doi: 10.1007/s11102-013-0475-3
128. Vilar L, Naves LA, Azevedo MF, Arruda MJ, Arahata CM, Moura ESL, et al. Effectiveness of cabergoline in monotherapy and combined with ketoconazole in the management of Cushing’s disease. Pituitary (2010) 13(2):123–9. doi: 10.1007/s11102-009-0209-8
129. Feelders RA, de Bruin C, Pereira AM, Romijn JA, Netea-Maier RT, Hermus AR, et al. Pasireotide alone or with cabergoline and ketoconazole in Cushing’s disease. N Engl J Med (2010) 362(19):1846–8. doi: 10.1056/NEJMc1000094
130. Peverelli E, Treppiedi D, Giardino E, Vitali E, Lania AG, Mantovani G. Dopamine and Somatostatin Analogues Resistance of Pituitary Tumors: Focus on Cytoskeleton Involvement. Front Endocrinol (Lausanne) (2015) 6:187. doi: 10.3389/fendo.2015.00187
131. Vlotides G, Siegel E, Donangelo I, Gutman S, Ren SG, Melmed S, et al. Rat prolactinoma cell growth regulation by epidermal growth factor receptor ligands. Cancer Res (2008) 68(15). doi: 10.1158/0008-5472.CAN-08-0508
132. Fukuoka H, Cooper O, Ben-Shlomo A, Mamelak A, Ren SG, Bruyette D, et al. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J Clin Invest (2011) 121(12):4712–21. doi: 10.1172/JCI60417
133. Reincke M, Sbiera S, Hayakawa A, Theodoropoulou M, Osswald A, Beuschlein F, et al. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat Genet (2015) 47(1):31–8. doi: 10.1038/ng.3166
134. Ma ZY, Song ZJ, Chen JH, Wang YF, Li SQ, Zhou LF, et al. Recurrent gain-of-function USP8 mutations in Cushing’s disease. Cell Res (2015) 25(3):306–17. doi: 10.1038/cr.2015.20
135. Weigand I, Knobloch L, Flitsch J, Saeger W, Monoranu CM, Höfner K, et al. Impact of USP8 Gene Mutations on Protein Deregulation in Cushing Disease. J Clin Endocrinol Metab (2019) 104(7):2535–46. doi: 10.1210/jc.2018-02564
136. Wanichi IQ, de Paula Mariani BM, Frassetto FP, Siqueira SAC, de Castro Musolino NR, Cunha-Neto MBC, et al. Cushing’s disease due to somatic USP8 mutations: a systematic review and meta-analysis. Pituitary (2019) 22(4):435–42. doi: 10.1007/s11102-019-00973-9
137. Nurwidya F, Takahashi F, Takahashi K. Gefitinib in the treatment of nonsmall cell lung cancer with activating epidermal growth factor receptor mutation. J Nat Sci Biol Med (2016) 7(2):119–23. doi: 10.4103/0976-9668.184695
138. Fukuoka H, Cooper O, Mizutani J, Tong Y, Ren SG, Bannykh S, et al. HER2/ErbB2 receptor signaling in rat and human prolactinoma cells: strategy for targeted prolactinoma therapy. Mol Endocrinol (2011) 25(1):92–103. doi: 10.1210/me.2010-0353
139. Opdam FL, Guchelaar HJ, Beijnen JH, Schellens JH. Lapatinib for advanced or metastatic breast cancer. Oncologist (2012) 17(4):536–42. doi: 10.1634/theoncologist.2011-0461
140. Liu X, Kano M, Araki T, Cooper O, Fukuoka H, Tone Y, et al. ErbB receptor-driven prolactinomas respond to targeted lapatinib treatment in female transgenic mice. Endocrinology (2015) 156(1):71–9. doi: 10.1210/en.2014-1627
141. Tadesse S, Caldon EC, Tilley W, Wang S. Cyclin-Dependent Kinase 2 Inhibitors in Cancer Therapy: An Update. J Med Chem (2019) 62(9):4233–51. doi: 10.1021/acs.jmedchem.8b01469
142. Cicenas J, Kalyan K, Sorokinas A, Stankunas E, Levy J, Meskinyte I, et al. Roscovitine in cancer and other diseases. Ann Transl Med (2015) 3(10):135. doi: 10.3978/j.issn.2305-5839.2015.03.61
143. Liu NA, Jiang H, Ben-Shlomo A, Wawrowsky K, Fan XM, Lin S, et al. Targeting zebrafish and murine pituitary corticotroph tumors with a cyclin-dependent kinase (CDK) inhibitor. Proc Natl Acad Sci USA (2011) 108(20):8414–9. doi: 10.1073/pnas.1018091108
144. Massaro F, Molica M, Breccia M. Current first- and second-line treatment options in acute promyelocytic leukemia. Int J Hematol Oncol (2016) 5(3):105–18. doi: 10.2217/ijh-2016-0010
145. Labeur M, Paez-Pereda M, Arzt E, Stalla GK. Potential of retinoic acid derivatives for the treatment of corticotroph pituitary adenomas. Rev Endocr Metab Disord (2009) 10(2):103–9. doi: 10.1007/s11154-008-9080-6
146. Kang HJ, Song MR, Lee SK, Shin EC, Choi YH, Kim SJ, et al. Retinoic acid and its receptors repress the expression and transactivation functions of Nur77: a possible mechanism for the inhibition of apoptosis by retinoic acid. Exp Cell Res (2000) 256(2):545–54. doi: 10.1006/excr.2000.4832
147. Bondioni S, Angioni AR, Corbetta S, Locatelli M, Ferrero S, Ferrante E, et al. Effect of 9-cis retinoic acid on dopamine D2 receptor expression in pituitary adenoma cells. Exp Biol Med (Maywood) (2008) 233(4):439–46. doi: 10.3181/0704-RM-94
148. Páez-Pereda M, Kovalovsky D, Hopfner U, Theodoropoulou M, Pagotto U, Uhl E, et al. Retinoic acid prevents experimental Cushing syndrome. J Clin Invest (2001) 108(8):1123–31. doi: 10.1172/JCI11098
149. Castillo V, Giacomini D, Páez-Pereda M, Stalla J, Labeur M, Theodoropoulou M, et al. Retinoic acid as a novel medical therapy for Cushing’s disease in dogs. Endocrinology (2006) 147(9):4438–44. doi: 10.1210/en.2006-0414
150. Occhi G, Regazzo D, Albiger NM, Ceccato F, Ferasin S, Scanarini M, et al. Activation of the dopamine receptor type-2 (DRD2) promoter by 9-cis retinoic acid in a cellular model of Cushing’s disease mediates the inhibition of cell proliferation and ACTH secretion without a complete corticotroph-to-melanotroph transdifferentiation. Endocrinology (2014) 155(9):3538–49. doi: 10.1210/en.2013-1820
Keywords: non-surgical therapy, Temozolomide, CAPTEM, PRRT (Peptide Receptor Radionuclide Therapy), aggressive pituitary tumors, pituitary carcinomas
Citation: Nakano-Tateno T, Lau KJ, Wang J, McMahon C, Kawakami Y, Tateno T and Araki T (2021) Multimodal Non-Surgical Treatments of Aggressive Pituitary Tumors. Front. Endocrinol. 12:624686. doi: 10.3389/fendo.2021.624686
Received: 31 October 2020; Accepted: 12 January 2021;
Published: 26 March 2021.
Edited by:
Adam Mamelak, Cedars Sinai Medical Center, United StatesReviewed by:
Thierry C. Brue, Aix-Marseille Université, FranceMaria Chiara Zatelli, University of Ferrara, Italy
Copyright © 2021 Nakano-Tateno, Lau, Wang, McMahon, Kawakami, Tateno and Araki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toru Tateno, dGF0ZW5vQHVhbGJlcnRhLmNh; Takako Araki, dGFyYWtpQHVtbi5lZHU=
†These authors have contributed equally to this work
‡These authors share last authorship
 Tae Nakano-Tateno
Tae Nakano-Tateno Kheng Joe Lau2†
Kheng Joe Lau2† Justin Wang
Justin Wang Toru Tateno
Toru Tateno Takako Araki
Takako Araki