- Department of Ultrasound, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China
Ultrasonography (US) is one of the most important methods for the management of thyroid nodules, which can be classified as solid, partially cystic, or cystic by composition. The various Thyroid Imaging Reporting and Data System classifications pay more attention to solid nodules and have reported pertinent US features associated with malignancy. However, the likelihood of malignancy of partially cystic thyroid nodules (PCTNs) is 3.3–17.6%, and few studies have systematically discussed the value of US in differentiating such entities. Therefore, we deemed it necessary to perform a systematic evaluation of US features in recognizing malignant PCTNs. Our systematic review and meta-analysis aimed to assess the value of US features in predicting malignant PCTNs. We searched the PubMed/MEDLINE, Web of Science, and Cochrane Library databases to find studies that researched US features of PCTNs and that were published before June 2020. Review Manager 5.3 was used to summarize suspicious US features and calculate the sensitivity, specificity, and likelihood ratios. MetaDiSc 1.4 was used to estimate receiver operating characteristic curves and calculate areas under the curves (AUCs). Our review included eight studies with a total of 2,004 PCTNs. Seven features were considered to be associated with malignancy. High specificity (>0.9) was found in nodules with a taller-than-wide shape, those that were spiculated/microlobulated or with an ill-defined margin, those with microcalcification, and a non-smooth rim. Among US features, eccentric configuration, microcalcification, and marked or mild hypoechogenicity were more reliable in predicting malignancy (AUC: 0.9592, 0.8504, and 0.8092, respectively). After meta-analysis, we recommend combining PCTN US features including an eccentric internal solid portion, marked or mild hypoechogenicity, and presence of microcalcification to better identify malignant nodules. More studies are needed to explore and improve the diagnostic value of US in PCTNs.
Introduction
Ultrasonography (US) is one of the most important methods for the management of thyroid nodules (TNs). In clinical practice, a nodule can be classified as solid, partially cystic, or cystic based on the internal cystic components (1). The various Thyroid Imaging Reporting and Data Systems (TI-RADS) classifications have paid more attention to solid nodules and have reported pertinent US features associated with malignancy (1–5). Several studies reported that nodules with microcalcification, hypoechogenicity (mild or marked), a taller-than-wide shape, or a spiculated/microlobulated margin are more likely to be carcinoma (6–9). However, the likelihood of malignancy of partially cystic thyroid nodules (PCTNs) is 3.3–17.6%, and few studies have systematically reported the US features associated with malignant PCTNs and discussed the value of US in differentiating such entities. As a matter of fact, malignant PCTNs can be easily missed due to their low prevalence (10–14). Therefore, we consider that more attention should be paid to the diagnosis of malignant PCTNs. Our systematic review and meta-analysis aimed to identify US risk factors indicative of malignant PCTNs and to assess the diagnostic performance of these features.
Materials and Methods
Search Strategy
This meta-analysis was referred to Perfected Reporting Items for Systematic Review and Meta-analysis guideline (15). We searched the PubMed/MEDLINE and Web of Science databases to obtain relevant literature for this review. In the PubMed/MEDLINE database, the following search terms were conducted: (partially cystic thyroid nodules [MeSH Major Topic]) AND (ultrasonograph* OR sonograph* OR ultrasound OR US [MeSH Major Topic]). The advanced search terms “TS=[(partially cystic thyroid nodules) AND (ultrasoundgraph* OR sonograph* OR ultrasound OR US)]” were used in the Web of Science database. We also checked the Cochrane Library with “partially cystic thyroid” AND “ultraso*.” We did not screen according to language. From a search up to June 2020, 56 articles (31 in Web of Science and 25 in PubMed) in total were identified. There were no relevant studies registered in the Cochrane Library. All articles were managed with NoteExpress V3.0 and duplicated studies were manually deleted.
Inclusion and Exclusion Criteria
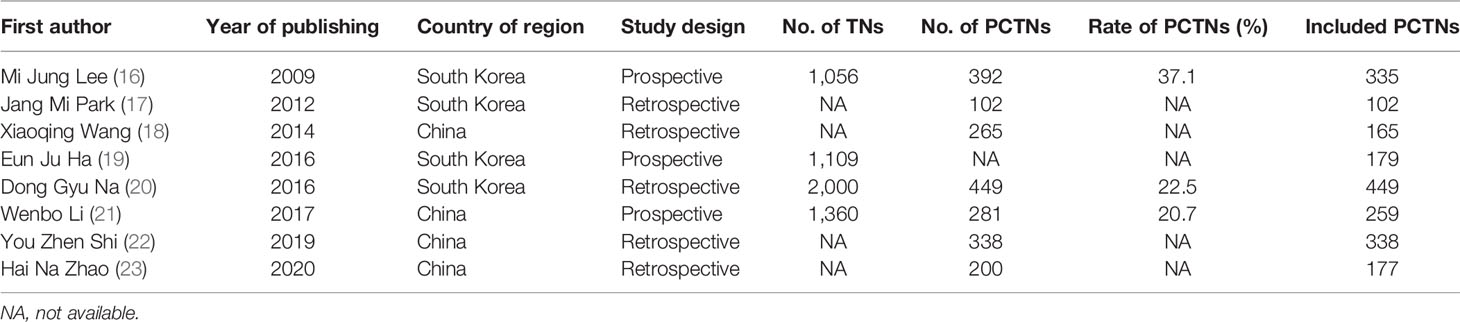
After searching the databases and deleting duplicated articles, we tab retained 56 studies for further analysis. Subsequent selection was performed by screening the titles and abstracts of all retrieved records. Comments, case reports, conference abstracts, letters, or reviews were filtered. The last round of selection was to apply strict and distinct inclusion and exclusion criteria by reviewing the full texts. Articles that met the following criteria were included in this study: (1) study on the sonographic features of PCTNs; (2) histopathologic results used as a reference standard; (3) research results available for evaluating the diagnostic value of sonographic features in PCTNs; (4) retrospective or prospective study. The exclusion criteria were as follows: (1) studies on themes other than PCTNs; (2) diagnostic classification or no specific sonographic features about PCTNs; (3) insufficient or questionable data to finish a diagnostic 2-by-2 table; (4) improper deletion of studied cases. Finally, a total of eight studies (16–23) were retained according to the selection procedure in Figure 1.
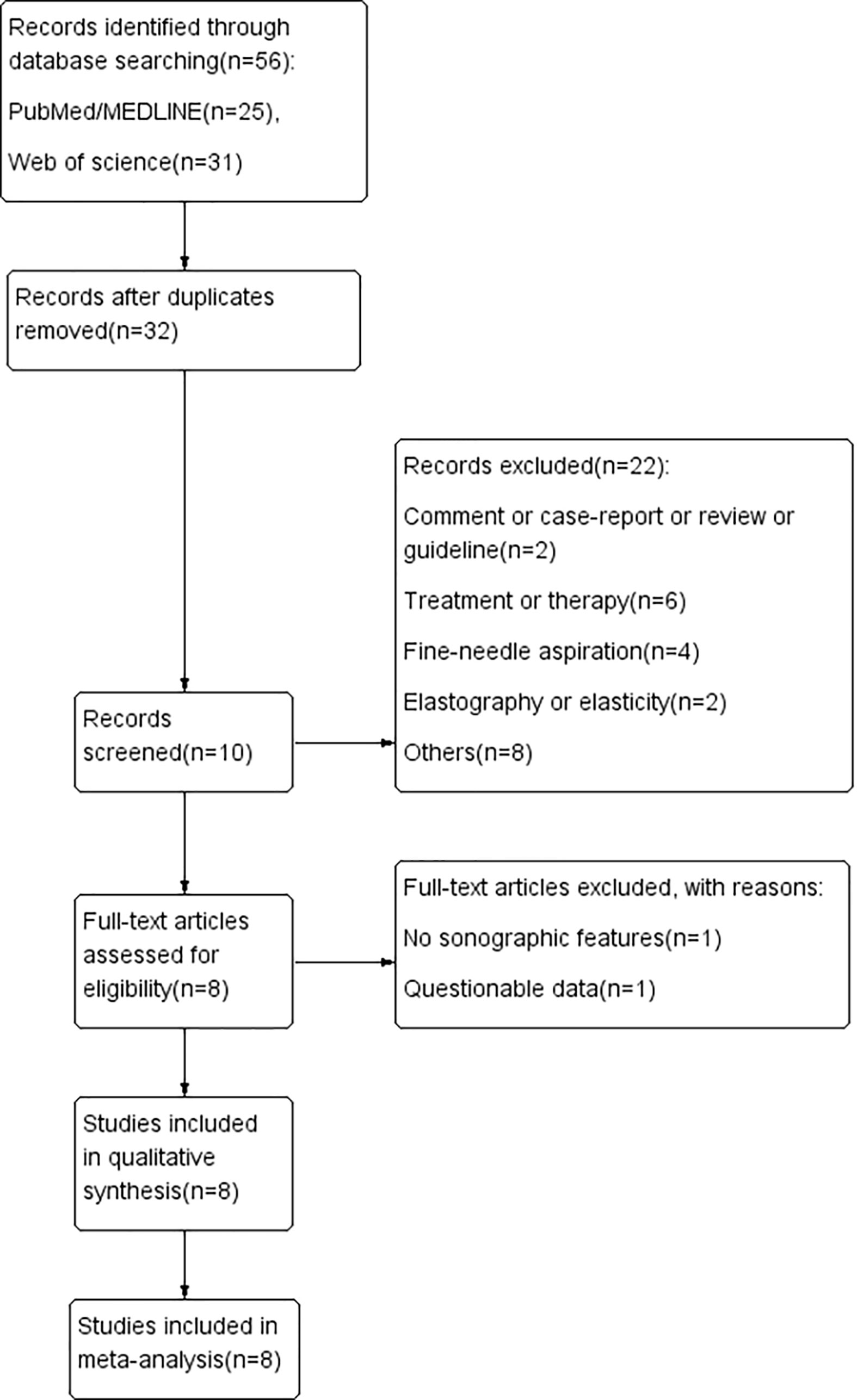
Figure 1 Flow chart of literature review process. Finally, a total of eight studies were included in our review.
Data Extraction
Two radiologists (XS and RL) individually reviewed the selected literature and extracted the data for systematic review and meta-analysis. We collected the following information from the selected articles: basic characteristics (name of first author, year of publication, country of origin, study design, number of TNs, number of included PCTNs, and scanner), sonographic performance of PCTNs, and diagnostic index of US features. According to several studies (1–5), some US features were excluded, such as vascularity. We regarded ovoid, ovoid-to-round, flat and round, and regular and parallel nodules as being wider-than-tall (anteroposterior/transverse diameter [A/T] <1) and irregular-shaped nodules were classified as taller-than-wide (A/T ≥1). Any discrepant data were discussed by XS and RL and a specialist (YX) with over 20 years of experience to reach consensus.
Quality Assessment
QUADAS-2, a recommended tool for diagnostic accuracy studies (24, 25), was used by two reviewers to evaluate the quality of the eight included studies. Another reviewer was consulted for evaluation when any disagreement occurred.
Statistical Analysis
Our first step was to find the independent risk features for thyroid malignancy. An intervention review was created in Review Manager 5.3 to calculate odds ratios (ORs), 95% confidence intervals (CIs), and p-values and to evaluate the risk bias of the included articles. The I2 inconsistency index was calculated to determine whether heterogeneity existed. If I2 ≥ 50%, the heterogeneity could not be ignored, and therefore, a random-effects model would be recommended to replace the default model. Next, independent risk features were analyzed by MetaDiSc 1.4 software to evaluate the diagnostic performance for predicting malignancy. The relationship between sensitivity and 1-specificity determines whether a threshold effect exists. When p > 0.05, the threshold effect can be ignored when analyzing the source of heterogeneity. Without a threshold effect, we would directly calculate the pooled sensitivity (Se), specificity (Sp), positive and negative likelihood ratios (LR+ and LR−), diagnostic OR (DOR), and area under the curve (AUC). A hierarchical summary receiver operating characteristic curve (HSROC) should be used to calculate AUC when a threshold exists (26–29).
Results
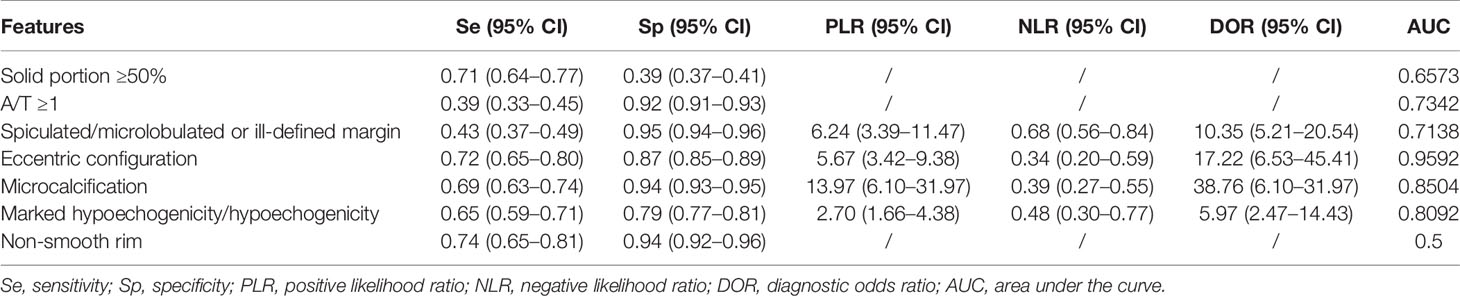
Table 1 demonstrates the basic information of the eight included studies. Half were performed in China (18, 21–23) and the other half were conducted in Korea (16, 17, 19, 20). Figure 2 shows the outcomes of the QUADAS-2 questionnaire. All included studies had a low risk of bias and were of high quality. We noted that nodules were more prone to be malignant with internal solid content ≥50%, taller-than-wide shape, and when spiculated/microlobulated or with an ill-defined margin. In terms of internal solid content of a PCTN, eccentric configuration, a non-smooth rim, marked or mild hypoechogenicity, and microcalcification were also potential malignant features for PCTNs. More details are shown in Figure 3. The overall ORs of the seven suspicious features ranged from 1.49 to 70.43. The p-values of all features were <0.01 except for nodules with a solid portion ≥50% (p = 0.03). Then, we combined RevMan 5.3 and MetaDiSc 1.4 software to evaluate the diagnostic accuracy. Figure 4 and Figure 5 show the pooled Se and Sp of diagnostic performance in the eight included studies. Except nodules with a solid portion ≥50%, the other six features revealed good specificity through a qualitative analysis. Four features (spiculated/microlobulated or ill-defined margin, eccentric configuration, microcalcification, and marked or mild hypoechogenicity) showed no threshold effect in this meta-analysis (p = 0.337, 0.285, 0.955, 0.760, respectively). Hence, we could obtain pooled diagnostic statistics from these four features. We only calculated the AUC from the HSROC for US features with an identified threshold effect. The pooled Se, Sp, LR+, LR−, DOR, 95% CIs, and AUCs are displayed in Table 2. From this table, we discovered that three features, except a non-smooth rim, of only the internal solid portion were more likely to predict the malignancy of PCTNs compared with features of the entire nodule (all AUCs >0.8). The AUC of the solid portion ≥50%, taller-than-wide shape, and spiculated/microlobulated or ill-defined margin were 0.6573, 0.7342, and 0.7138, respectively. Metaregression was conducted in MetaDiSc 1.4 to explore the source of heterogeneity. The variables were TP+FN (TP, True-positive; FN, False-negative), country of region, study design, and numbers of scanner used. We added year of publication to the metaregression of presence of microcalcification. We found that whether the study was conducted in China or South Korea was the main source of heterogeneity in terms of the presence of microcalcification (p = 0.0482, Table S1), while no other covariates could explain heterogeneity. We did not assess publication bias because our review included only eight studies, and the Cochrane Handbook recommends at least 10 studies when evaluating publication bias.

Figure 2 Outcome of QUADAS-2 for included studies. (A) Risk-of-bias summary. (B) Risk-of-bias graph. Symbols: (+), low risk of bias; (?), unclear risk of bias; (-), high risk of bias.
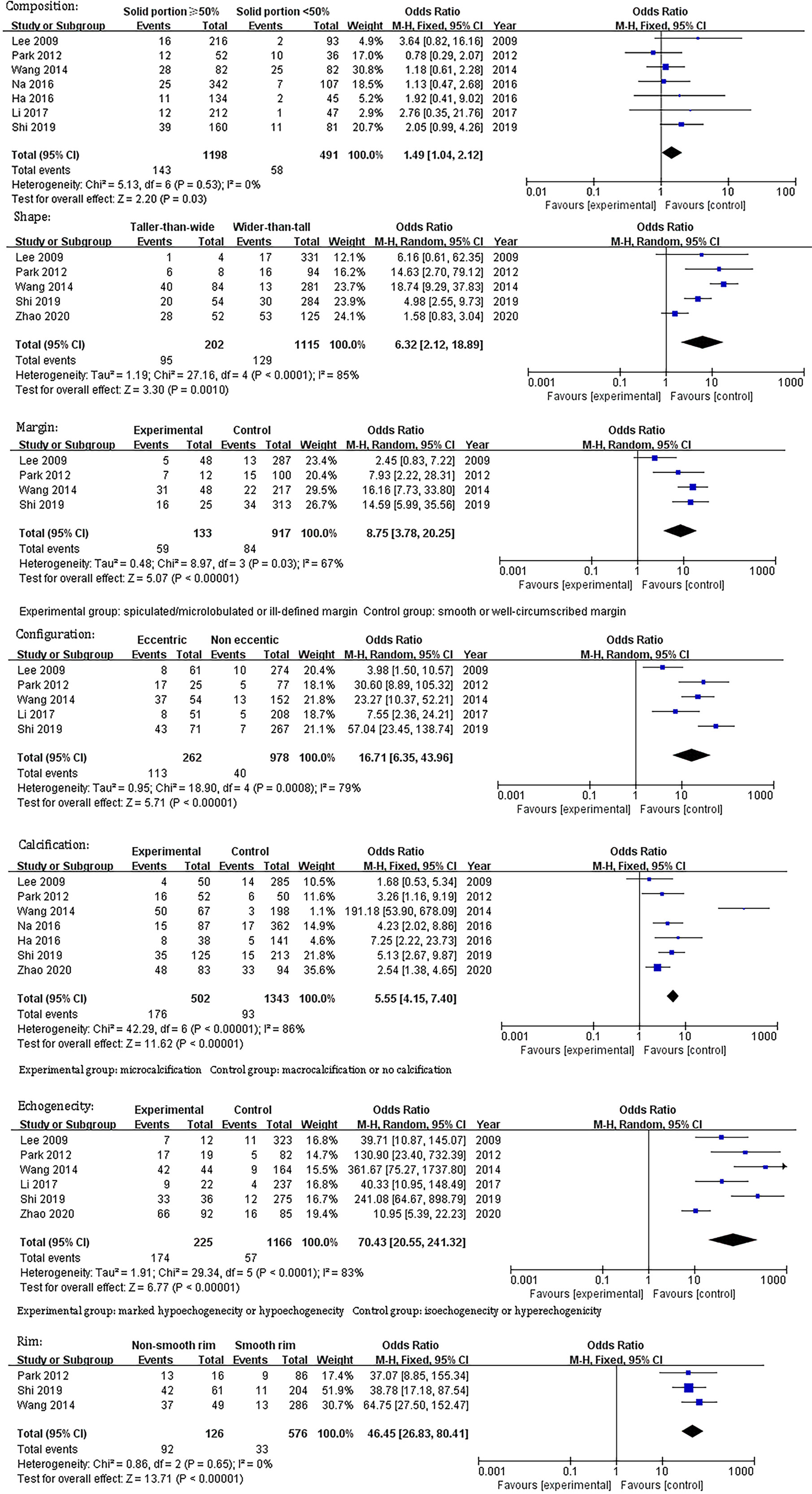
Figure 3 Odds ratio and its 95% confidence intervals of seven sonographic features of partially cystic thyroid nodules.
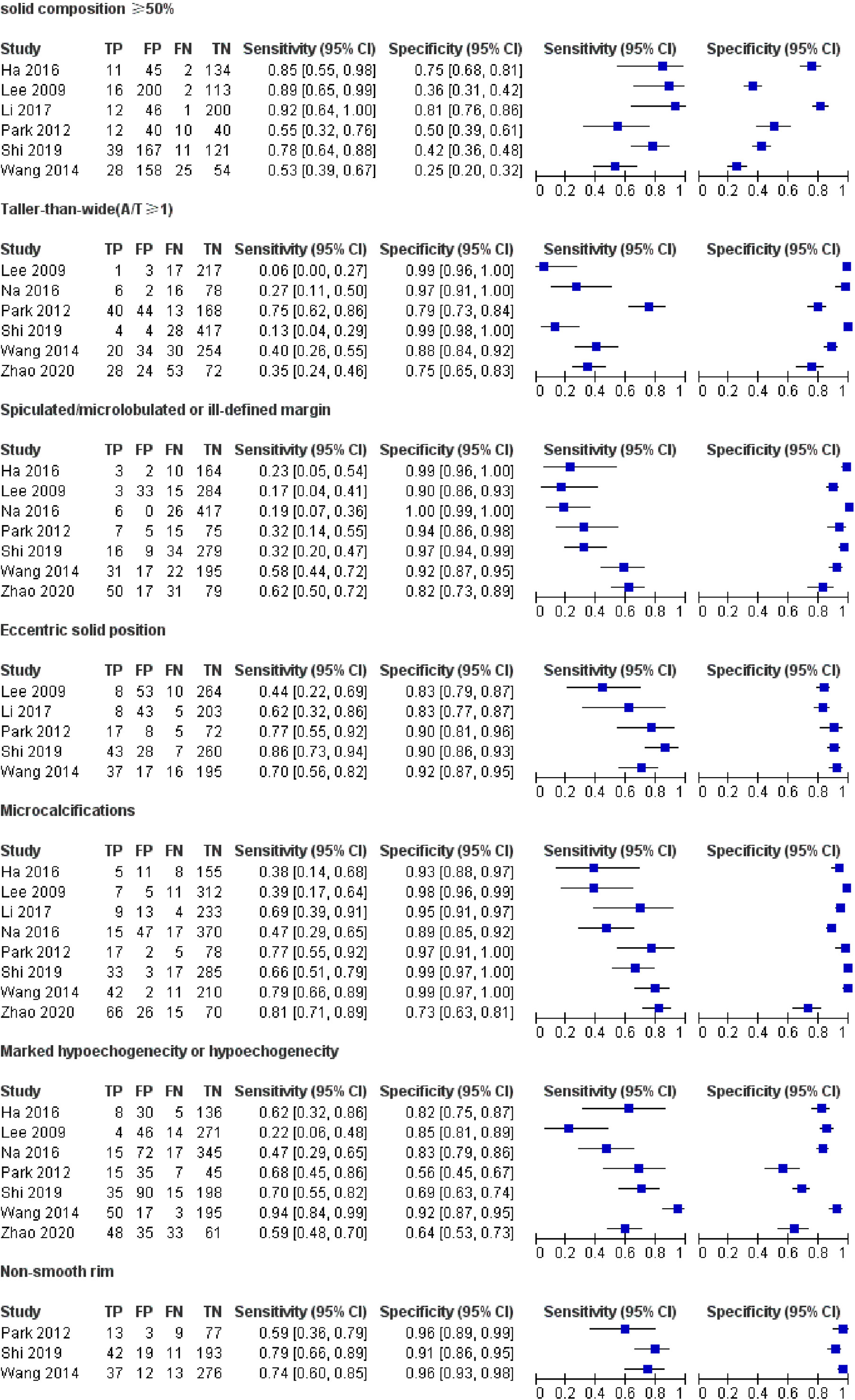
Figure 4 Forest plots of pooled sensitivity and specificity of US. Univariate analyses were performed for sensitivity and specificity, respectively. Except nodules with a solid portion ≥ 50%, the other six features revealed good specificity through a qualitative analysis.
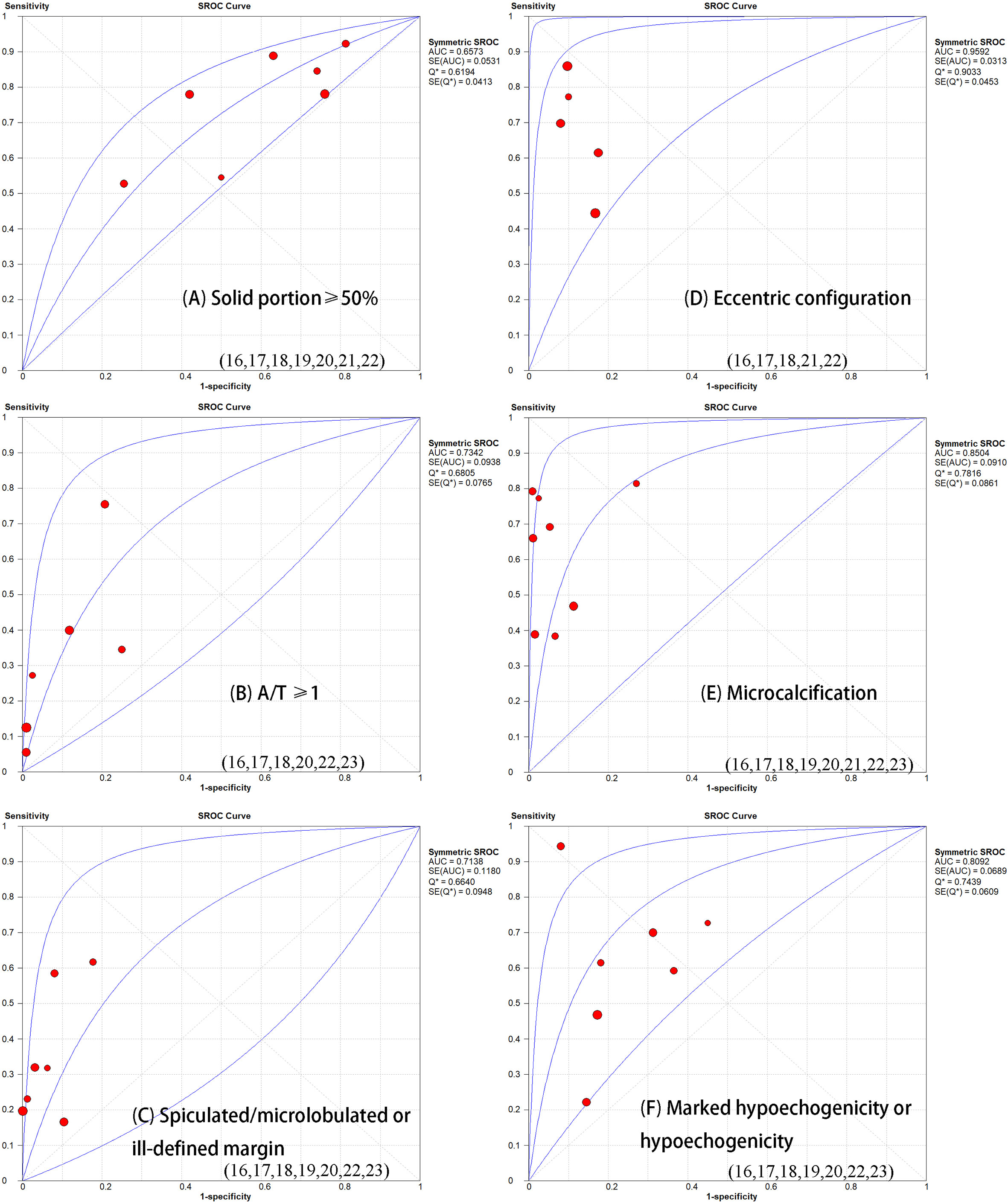
Figure 5 Summary receiver operator characteristic curve (SROC) with area under the ROC curve (AUC) of six sonographic features in diagnosing partially thyroid cancer. The size of each study is indicated by the size of the solid circles. PCTNs with an eccentric configuration are more prone to malignancy (AUC=0.09592).
Discussion
In our review, the incidence of malignant PCTNs varied from 5.0 to 45.8%. The diagnosis of malignant PCTNs is challenging, but worthy. It is of great importance to identify sonographic features that distinguish malignant PCTNs in clinical practice. Hence, we conducted this systematic review and meta-analysis to evaluate the value of US in predicting malignant PCTNs. After conducting an intervention review to determine independent risk factors for malignancy, we found PCTNs with seven US features had a higher risk of malignancy. Some of these features were in line with a previous meta-analysis regarding risky US features in all kinds of thyroid carcinoma (10). In our study, except non-smooth rim (AUC = 0.5), the AUCs of other six features were above 0.5. Notably, eccentric configuration, marked or mild hypoechogenicity, or presence of microcalcification of internal solid portion had relatively high accuracy (0.85, 0.77, 0.90, respectively) in predicting malignancy among PCTNs.
A taller-than-wide (TTW) shape, defined as an anteroposterior/transverse diameter (A/T) ratio >1, would not be reliably correlated with malignant PCTNs in our review (AUC = 0.7342). Likewise, Kim reported that a taller than wide shape did not contribute to an increased risk of malignant PCTNs. The reason may lie in the noted inter- and even intraobserver variability of taller-than-wide shape (30, 31). Hypoechogenicity showed fair diagnostic performance in our review (AUC = 0.8092). A previous study (32) that subdivided TNs based on their degree of hypoechogenicity also found that TNs with marked or moderate hypoehcogenicity had significantly higher malignant risks than mild hypoechogenicity (p < 0.001). This feature related closely with malignancy from the perspective of pathology. Kim stated that the pathogenesis of marked hypoechogenicity were associated with fibrotic regression following collapsed hemorrhagic component (31). The lack of follicular tissue arrangement may also lead to the hypoechogenicity of malignant PCTNs (33). Microcalcification of internal solid portion was significantly associated with malignancy as well (AUC = 0.8504). The degeneration of tumor cells and additional collagen produced by tumor cells could lead to psammoma bodies, a histopathological marker of microcalcification (34). They are common in any kind of papillary thyroid carcinoma regardless of the internal content. To some extent, these could explain why PCTNs with hypoechogenicity or microcalcification are prone to be malignant.
When compared to PCTNs with an eccentric configuration with a blunt angle, those with an eccentric configuration and an acute angle are more strongly associated with malignancy (p < 0.001) (18), which was also reported by Kim et al. (35). This phenomenon could be illustrated by the theory that malignant PCTNs usually develop from the wall of thyroid cysts, and the previous study has shown that the real tumor tissue is more likely to localize to the base of papillomatous lesions (36). A comment (37) reported that eccentric configuration harbors different meaning between nodules with a solid portion ≥50% and solid portion <50% (p = 0.001). Only in predominant solid nodules, an eccentric position of solid component is a significantly malignant feature. This could explain why we did not find “solid portion ≥50%” as being a high-risk factor by itself for predicting malignancy (AUC = 0.6573). Hence, we recommend integrating nodules with a solid portion ≥50% with other potential US features in future studies. And we should be alert when a PCTN presented with predominant solid and eccentric configuration simultaneously.
In addition to univariate analysis, some studies combined multiple US features to evaluate the diagnostic performance of US for PCTNs (16, 19, 20). However, because the combination of US features in these studies were different, it was impossible for us to evaluate the diagnostic accuracy of combined US features by meta-analysis. Lee et al. (16) found a high sensitivity and negative predictive value using combined US features to predict malignancy in PCTNs. Another two studies drew the same conclusion that PCTNs would have an intermediate risk of malignancy if they presented more than one suspicious US feature (38, 39). The risk of malignancy increased as more suspicious US features were detected. Although different TI-RADS were put forward to evaluate the thyroid nodule, the attention paid to PCTNs were relatively less. Therefore, we suggest that clinicians focus on the following features: eccentric configuration, presence of calcification, and marked or mild hypoechogenicity. Overall, US has the ability to diagnose malignant PCTNs if high-risk features are appropriately recognized and interpreted.
Several limitations exist in our review. Firstly, only a small number of studies were used for this research, which rendered subgroup analysis ineffective when analyzing heterogeneity. Secondly, all included studies were performed in Asia, and so there may be population and race bias. Some features are closely associated and can exist simultaneously in malignant nodules (40); however the inherent relationship between suspicious US features could not be explored and we failed to evaluate the diagnostic value of combined US features. Then, more detailed classification of specific US feature could bring new insight, but we failed to do such research: for instance, included studies (19, 20, 29) in our review did not divide the degree of hypoechogenicity when exploring associated factors for malignancy, which limited our advanced analysis. Further study could be conducted to find the relationship between degree of hypoechogenicity and malignancy. Moreover, pooled data concerning the overall diagnostic value of US for PCTNs is not available.
Conclusion
Our review selected high-quality published studies to analyze the performance of US when diagnosing malignant PCTNs. After meta-analysis, we found that several US features were highly accurate when diagnosing malignant PCTNs. With the aim of improving the diagnostic accuracy of US, we suggest combining several US features of the internal solid portion of PCTNs. More studies are needed to explore and improve the diagnostic value of US in PCTN.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
XS and RL contributed equally to this review. RL conducted the work of search and collection of literature and helped XS to write the first draft of the manuscript. XS did the work of meta-analysis. LG contributed to the discussion. YX conceived and designed this review. YX and YJ revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a grant from the Tibet Autonomous Region Science and Technology Project (XZ201901-GB-04) and the Tibet Autonomous Region Organization and Aiding Project [XZ2019ZR ZY05(Z)].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.624409/full#supplementary-material
References
1. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J Radiol (2016) 17:370–95. doi: 10.3348/kjr.2016.17.3.370
2. Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid Imaging Reporting and Data System for US Features of Nodules: A Step in Establishing Better Stratification of Cancer Risk. Radiology (2011) 260:892–9. doi: 10.1148/radiol.11110206
3. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur Thyroid J (2017) 6:225–37. doi: 10.1159/000478927
4. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26:1–133. doi: 10.1089/thy.2015.0020
5. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol (2017) 14:587–95. doi: 10.1016/j.jacr.2017.01.046
6. Salmaslioglu A, Erbil Y, Dural C, Issever H, Kapran Y, Ozarmagan S, et al. Predictive value of sonographic features in preoperative evaluation of malignant thyroid nodules in a multinodular goiter. World J Surg (2008) 32:1948–54. doi: 10.1007/s00268-008-9600-2
7. Popowicz B, Klencki M, Lewinski A, Slowinska-Klencka D. The usefulness of sonographic features in selection of thyroid nodules for biopsy in relation to the nodule’s size. Eur J Endocrinol (2009) 161:103–11. doi: 10.1530/EJE-09-0022
8. Hong YJ, Son EJ, Kim EK, Kwak JY, Hong SW, Chang HS. Positive predictive values of sonographic features of solid thyroid nodule. Clin Imag (2010) 34:127–33. doi: 10.1016/j.clinimag.2008.10.034
9. Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation - Multicenter retrospective study. Radiology (2008) 247:762–70. doi: 10.1148/radiol.2473070944
10. Remonti LR, Kramer CK, Leitao CB, Pinto LCF, Gross JL. Thyroid Ultrasound Features and Risk of Carcinoma: A Systematic Review and Meta-Analysis of Observational Studies. Thyroid (2015) 25:538–50. doi: 10.1089/thy.2014.0353
11. Bellantone R, Lombardi CP, Raffaelli M, Traini E, De Crea C, Rossi ED, et al. Management of cystic or predominantly cystic thyroid nodules: the role of ultrasound-guided fine-needle aspiration biopsy. Thyroid (2004) 14:43–7. doi: 10.1089/105072504322783830
12. La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer (2015) 136:2187–95. doi: 10.1002/ijc.29251
13. Lin Y, Li P, Shi YP, Tang XY, Ding M, He Y, et al. Sequential treatment by polidocanol and radiofrequency ablation of large benign partially cystic thyroid nodules with solid components: Efficacy and safety. Diagn Interv Imaging (2020) 101:365–72. doi: 10.1016/j.diii.2019.11.005
14. Na DG, Kim JH, Kim DS, Kim SJ. Thyroid nodules with minimal cystic changes have a low risk of malignancy. Ultrasonography (2016) 35:153–8. doi: 10.14366/usg.15070
15. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (2009) 339:b2535. doi: 10.1136/bmj.b2535
16. Lee MJ, Kim EK, Kwak JY, Kim MJ. Partially cystic thyroid nodules on ultrasound: probability of malignancy and sonographic differentiation. Thyroid (2009) 19:341–6. doi: 10.1089/thy.2008.0250
17. Park JM, Choi Y, Kwag HJ. Partially cystic thyroid nodules: ultrasound findings of malignancy. Korean J Radiol (2012) 13:530–5. doi: 10.3348/kjr.2012.13.5.530
18. Wang X, Wei X, Xu Y, Xin X, Zhang S. [Ultrasound characteristics of partially cystic thyroid nodules and their relationship with differential diagnosis of the lesions]. Zhonghua Zhong Liu Za Zhi (2014) 36(8):617–20. doi: 10.3760/cma.j.issn.0253-3766.2014.08.012
19. Ha EJ, Moon WJ, Na DG, Lee YH, Choi N, Kim SJ, et al. A Multicenter Prospective Validation Study for the Korean Thyroid Imaging Reporting and Data System in Patients with Thyroid Nodules. Korean J Radiol (2016) 17:811–21. doi: 10.3348/kjr.2016.17.5.811
20. Na DG, Baek JH, Sung JY, Kim JH, Kim JK, Choi YJ, et al. Thyroid Imaging Reporting and Data System Risk Stratification of Thyroid Nodules: Categorization Based on Solidity and Echogenicity. Thyroid (2016) 26:562–72. doi: 10.1089/thy.2015.0460
21. Li W, Zhu Q, Jiang Y, Zhang Q, Meng Z, Sun J, et al. Partially cystic thyroid nodules in ultrasound-guided fine needle aspiration: Prevalence of thyroid carcinoma and ultrasound features. Med (Baltimore) (2017) 96:e8689. doi: 10.1097/MD.0000000000008689
22. Shi YZ, Jin Y, Zheng L. Partially cystic thyroid nodules on ultrasound: The associated factors for malignancy. Clin Hemorheol Microcirc (2020) 74:373–81. doi: 10.3233/CH-190582
23. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJn, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
24. Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med (2015) 8:2–10. doi: 10.1111/jebm.12141
25. Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol (2006) 6:31. doi: 10.1186/1471-2288-6-31
26. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
27. Cronin P, Kelly AM, Altaee D, Foerster B, Petrou M, Dwamena BA. How to Perform a Systematic Review and Meta-analysis of Diagnostic Imaging Studies. Acad Radiol (2018) 25(5):573–93. doi: 10.1016/j.acra.2017.12.007
28. Lee J, Kim KW, Choi SH, Huh J, Park SH. Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers-Part II. Statistical Methods of Meta-Analysis. Korean J Radiol (2015) 16(6):1188–96. doi: 10.3348/kjr.2015.16.6.1188
29. Zhao HN, Liu JY, Lin QZ, He YS, Luo HH, Peng YL, et al. Partially cystic thyroid cancer on conventional and elastographic ultrasound: a retrospective study and a machine learning-assisted system. Ann Transl Med (2020) 8:495. doi: 10.21037/atm.2020.03.211
30. Grani G, Lamartina L, Ramundo V, Falcone R, Lomonaco C, Ciotti L, et al. Taller-Than-Wide Shape: A New Definition Improves the Specificity of TIRADS Systems. Eur Thyroid J (2020) 9(2):85–91. doi: 10.1159/000504219
31. Kim DW, Lee EJ, In HS, Kim SJ. Sonographic differentiation of partially cystic thyroid nodules: a prospective study. AJNR Am J Neuroradiol (2010) 31(10):1961–6. doi: 10.3174/ajnr.A2204
32. Lee JY, Na DG, Yoon SJ, Gwon HY, Paik W, Kim T, et al. Ultrasound malignancy risk stratification of thyroid nodules based on the degree of hypoechogenicity and echotexture. Eur Radiol (2020) 30(3):1653–63. doi: 10.1007/s00330-019-06527-8
33. Krátký J, Vítková H, Bartáková J, Telička Z, Antošová M, Límanová Z, et al. Thyroid nodules: pathophysiological insight on oncogenesis and novel diagnostic techniques. Physiol Res (2014) 63 Suppl 2(Suppl 2):S263–75. doi: 10.33549/physiolres.932818
34. Das DK. Psammoma body: a product of dystrophic calcification or of a biologically active process that aims at limiting the growth and spread of tumor? Diagn Cytopathol (2009) 37:534–41. doi: 10.1002/dc.21081
35. Kim DW, Park JS, In HS, Choo HJ, Ryu JH, Jung SJ. Ultrasound-based diagnostic classification for solid and partially cystic thyroid nodules. AJNR Am J Neuroradiol (2012) 33:1144–9. doi: 10.3174/ajnr.A2923
36. Yokozawa T, Miyauchi A, Kuma K, Sugawara M. Accurate and simple method of diagnosing thyroid nodules the modified technique of ultrasound-guided fine needle aspiration biopsy. Thyroid (1995) 5(2):141–5. doi: 10.1089/thy.1995.5.141
37. Zhang M, Fu C, Ma X, Li J, Cui K. Comment on “Partially Cystic Thyroid Nodules on Ultrasound: Probability of Malignancy and Sonographic Differentiation”. Thyroid (2016) 26:1645. doi: 10.1089/thy.2016.0389
38. Tessler FN, Middleton WD, Grant EG. Thyroid Imaging Reporting and Data System (TI-RADS): A User’s Guide. Radiology (2018) 287:29–36. doi: 10.1148/radiol.2017171240
39. Horvath E, Majlis S, Rossi R, Franco C, Niedmann JP, Castro A, et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab (2009) 94:1748–51. doi: 10.1210/jc.2008-1724
Keywords: meta-analysis, diagnostic values, sonographic features, partially cystic thyroid nodules, thyroid carcinoma
Citation: Shi X, Liu R, Gao L, Xia Y and Jiang Y (2021) Diagnostic Value of Sonographic Features in Distinguishing Malignant Partially Cystic Thyroid Nodules: A Systematic Review and Meta-Analysis. Front. Endocrinol. 12:624409. doi: 10.3389/fendo.2021.624409
Received: 31 October 2020; Accepted: 23 February 2021;
Published: 19 March 2021.
Edited by:
Valentina Drozd, International Fund “Help for patients with radiation induced thyroid cancer ‘Arnica’”, BelarusReviewed by:
Paolo Piero Limone, Hospital Mauritian Turin, ItalyTakao Ando, Nagasaki University Hospital, Japan
Copyright © 2021 Shi, Liu, Gao, Xia and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Xia, eGlheXVwdW1jaEAxMjYuY29t; Yuxin Jiang, eXV4aW5qaWFuZ3hoQDE2My5jb20=
†These authors have contributed equally to this work
 Xinlong Shi
Xinlong Shi Ruifeng Liu†
Ruifeng Liu† Luying Gao
Luying Gao