
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 22 March 2021
Sec. Pediatric Endocrinology
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.622901
This article is part of the Research Topic Special 2021 Frontiers in Endocrinology Collection for the 100th Anniversary of Insulin Discovery View all 12 articles
Since the 1980s, there has been a dramatic rise in the prevalence of overweight and obesity in pediatric populations, in large part driven by sedentary lifestyles and changing dietary patterns with more processed foods. In parallel with the rise in pediatric obesity in the general population, the prevalence of overweight and obesity has increased among children and adolescents with type 1 diabetes. Adiposity has been implicated in a variety of mechanisms both potentiating the risk for type 1 diabetes as well as exacerbating long-term complications, particularly cardiovascular disease. Treatment options targeting the unique needs of obese pediatric patients, both before and after diagnosis of type 1 diabetes, are limited. In this review, we discuss the history of the epidemiology of the obesity epidemic in the context of pediatric type 1 diabetes, highlight the possible role of obesity in type 1 diabetes pathogenesis and review the concept of “double diabetes”. The impact of obesity at and after diagnosis will be discussed, including noted differences in clinical and biochemical markers, lipid abnormalities, and long-term cardiovascular complications. Finally, we will review the existing literature on pharmacologic and nutritional interventions as potential treatment strategies for youth with coexisting type 1 diabetes and obesity.
Autoimmune type 1 diabetes is one of the most common chronic conditions of childhood, with the incidence increasing worldwide (1–3). Our understanding of the diverse forms of diabetes has evolved since the 1950s, with several different classification systems proposed until the most recent distinction of type 1 and type 2 diabetes in the 1990s (4). This classification focused on pathogenesis, separating diabetes resulting from absolute insulin deficiency, typically secondary to autoimmunity, or from progressive loss of insulin secretion in the setting of insulin resistance. However, continued advances have unraveled the variable genetic, immunologic, and metabolic factors that contribute to diabetes, and our current concept of type 1 diabetes in youth acknowledges significant heterogeneity of this disease. One noteworthy factor affecting this disease is the rise in childhood overweight and obesity (5). A known risk factor for insulin resistance, obesity is now a frequently recognized comorbidity in type 1 diabetes and may compound both the risk for and subsequent complications of type 1 diabetes in youth.
In this review, we highlight the epidemiological trends in obesity and new onset type 1 diabetes, including possible etiological explanations. With the rising prevalence of overweight and obesity at diagnosis of type 1 diabetes and persisting over time, we will address three important questions. First, does obesity contribute to the increased incidence of type 1 diabetes in youth and how? Second, what are the short- and long-term impacts of obesity on type 1 diabetes and its complications? And finally, can targeted treatment strategies optimize outcomes in youth with coexisting obesity and type 1 diabetes?
The obesity epidemic is one of the defining global public health issues of our time. Over the latter half of the 20th century, the prevalence of overweight and obesity in pediatric populations increased dramatically in the United States. Approximately one-third of American children are overweight or obese (6). Recent estimates among children age 2–19 years point to 17% of children with obesity [body mass index (BMI) ≥ 95th percentile] and 5.8% with extreme obesity (BMI at or above 120% of the sex-specific 95th percentile), with some leveling-off in the prevalence of obesity in younger age groups in recent years (5). Similar trends in pediatric overweight and obesity have been observed worldwide (7, 8), though the rates of obesity in the developing world continue to accelerate (9). At the heart of the obesity epidemic are sedentary lifestyles and the “westernized diet”, consisting of increased processed foods, refined sugars, and saturated fat. Metabolic derangements resulting from this diet promote weight gain and contribute to cardiovascular disease, diabetes, and cancer (10). Ultra-processed foods, both nutrient poor and calorically dense, are increasingly linked to higher risk for all-cause mortality in the United States and other countries (11, 12). Added sugars in processed foods are a significant portion of the diet of American children beginning at a young age (13), a concerning finding given the evidence for long-term health problems. Longitudinal data links obesity in childhood to adverse health outcomes in adulthood, including hypertension, fatty liver disease, dyslipidemia, and type 2 diabetes (14–16). In addition to the significant morbidity and mortality associated with adult obesity, there are enormous economic costs that are projected to continue to increase over the next decades (17).
In parallel with the obesity epidemic, the incidence of type 1 diabetes in youth has also been increasing worldwide (1–3). The multi-center SEARCH for Diabetes in Youth Study reported a 1.8% per year increase in the incidence of type 1 diabetes from 2002–2012 adjusted for age, sex, and race/ethnicity; the incidence was higher for racial/ethnic minorities at 4.2% increase per year in Hispanic youth and 2.2% increase per year in African American youth (18). In Europe, the EURODIAB study has examined trends in incidence from 1989 to 2013. Initial findings demonstrated an incidence of 3.2–4.1% per year from 1989–2003 with a subsequent leveling off; however, a follow-up investigation found a persistent and steady rise in the incidence of type 1 diabetes in more recent years of approximately 3% per year, suggesting possible cyclicity in a changing incidence rate (19, 20). No clear etiologic factor has been identified to explain this pattern. Various environmental triggers have been explored, including pathogens, nutritional changes, and obesity (21), though the increasing incidence of type 1 diabetes has continued despite some slowing in the pediatric obesity epidemic in developed countries (9).
With the increasing incidences of both type 1 diabetes and obesity, the prevalence of overweight and obesity in youth with type 1 diabetes at diagnosis has also increased. Unlike type 2 diabetes, where obesity is a known risk factor, children with new-onset type 1 diabetes in the past were not overweight at diagnosis and traditionally thought to be thin. Libman et al. was one of the first to identify the changing presentation of youth with diabetes in a study examining new onset insulin dependent diabetes in cohorts of children from 1979–1998 (22). Over a 20-year period, the prevalence of overweight and obesity at diagnosis increased threefold, from 12.6% in the first decade to 36.8% in the second decade. The prevalence of overweight and obesity at onset increased nearly five times among those with confirmed autoimmunity from the 1980s to the 1990s, suggesting that the observation was not driven by increasing cases of type 2 diabetes. Subsequent studies have similarly identified a rise in BMI at diagnosis of type 1 diabetes over time (23–25).
Following diagnosis, several groups have examined the prevalence of overweight and obesity cross-sectionally in children and adolescents with type 1 diabetes at least one year into diagnosis. In single-center reports, the estimated prevalence ranged from 25–40% of youth, with most approaching 35%, in children with a mean duration of diabetes of 5.6–8.7 years (26–29). In recent years, larger, registry-based assessments have confirmed these findings, as illustrated in Figure 1. The US-based SEARCH for Diabetes in Youth Study found that 34.7% of participants with type 1 diabetes were overweight or obese (30); another United States-based multicenter collaborative, the Type 1 Diabetes Exchange, estimated this prevalence to be 36% in their population with a mean duration of 6.8 ± 4.1 years (31). In contrast, the European-based, prospective Diabetes Patienten Verlaufsdokumentation (DPV) Registry estimated a lower prevalence of 15.3% of children being overweight/obese in their population with a mean duration of 4.7 ± 3.0 years (33). Most recently, the SWEET registry, an international consortium consisting of 26 countries, found the prevalence to be 29.6% of male patients and 34% of female patients who had a mean duration of diabetes approximately 5.5 years (32). The higher preponderance of overweight/obesity among females has been noted in other studies (26, 29–31, 33, 34), but is not a consistent finding. In the United States, racial/ethnic minorities with type 1 diabetes are more likely to be overweight/obese (30, 31) consistent with national trends in BMI by race/ethnicity among youth without diabetes (35). Importantly, the prevalence of obesity appears to be similar to youth without diabetes in most cases (23, 26, 29), though some have suggested that it may be higher among youth with type 1 diabetes (27, 34).
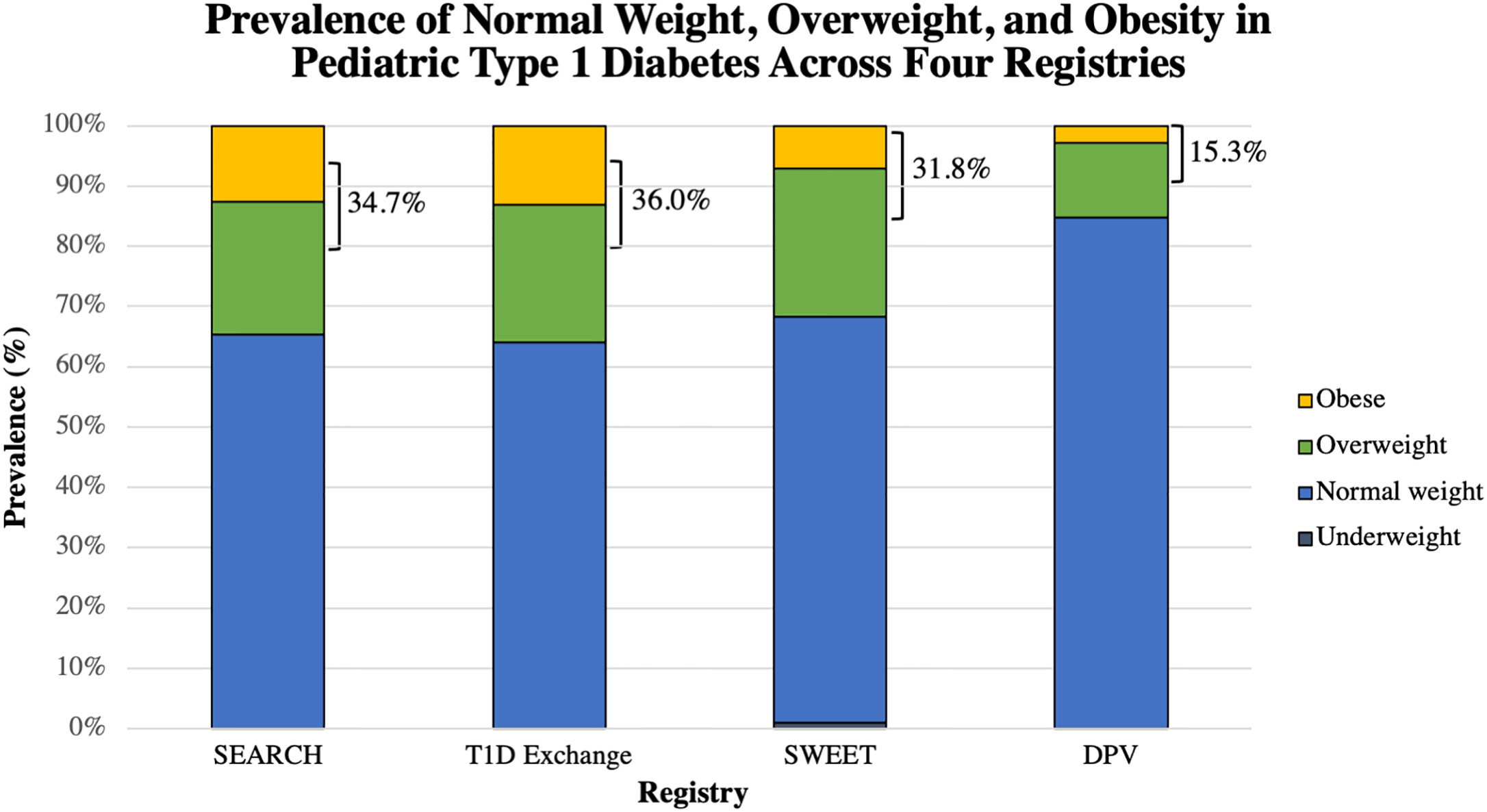
Figure 1 Graphical representation of the proportion of reported normal weight, overweight, and obese youth with type 1 diabetes in each of these four registries: SEARCH (30), T1D Exchange (31), SWEET (32), and the DPV (33). The combined percentage of overweight and obese is shown. The proportion of youth who were underweight was available only for the SWEET registry. SWEET data was reported separately for males and females; a weighted average was obtained and is reported here.
Several plausible explanations have been reported to explain the rise in obesity in youth with type 1 diabetes. Certainly, this trend mirrors the worldwide epidemic of obesity, and youth with diabetes are likely not immune to the westernized diet despite frequent nutrition counseling. Indeed, children and adolescents with type 1 diabetes generally do not meet the American Diabetes Association (ADA) and International Society for Pediatric and Adolescent Diabetes (ISPAD) dietary recommendations. More than a decade ago, our group found that adolescents with type 1 diabetes consume a higher percentage of their total calories from fat compared to peers without diabetes (36). A subsequent systematic review confirmed that children with type 1 diabetes tend to eat diets higher in saturated fat and also lower in fruits, vegetables, and whole grains, than recommended (37). These findings were echoed in more recent studies examining dietary habits in younger children (38) and adolescents (39) with type 1 diabetes. Diets with a higher percentage of total energy intake coming from fat rather than carbohydrates contribute to both weight gain and, ultimately, adverse lipid profiles (40). Carbohydrate counting, a system by which the insulin dose is calculated based upon the planned number of carbohydrates to be consumed, became widely popularized in the 2010s to increase dietary choice and flexibility for individuals with type 1 diabetes. Whether carbohydrate counting itself has contributed to weight gain is met with some controversy, with studies finding conflicting results (41). A recent study by Donzeau et al. examined carbohydrate counting versus fixed dose insulin for meals and found no change in BMI z-score in youth over one year of follow-up, though the fairly low starting mean BMI z-score (0.7 ± 0.8) of this population and possible selection bias may limit the generalizability of the findings (42).
In addition to diet, inadequate physical activity may also impact glycemia and weight in youth with type 1 diabetes. The guidelines recommend 60 min of moderate to vigorous physical activity per day (43); however, a large portion of youth with diabetes do not meet these guidelines (44). Similar to children without diabetes, various home, peer, and environmental factors may affect physical activity in youth with type 1 diabetes (45), though fear of hypoglycemia and the need for adequate supervision to monitor blood glucoses may be additional considerations (46). Regular activity has been shown to lower hemoglobin A1c, with additional benefits on weight, cardiovascular risk, and well-being (46). In the T1D Exchange cohort, youth who rated themselves as active (defined as 5–7 days/week) rather than inactive (defined as 0–1 days/week) were less likely to be overweight (OR 0.72, 95% CI 0.54–0.96) or obese (OR 0.70, 95% CI 0.49–0.99) in univariate analyses. Despite the evidence that children and adolescents with type 1 diabetes may not follow a healthy diet or get sufficient physical activity, we lack sophisticated studies examining temporal trends in relation to weight gain in this population, specifically.
An additional factor is our revised approach to treatment of type 1 diabetes, specifically with insulin therapy. The Diabetes Control and Complications Trial (DCCT) definitively identified intensive insulin therapy as the gold standard of treatment to prevent the development and progression of long-term micro- and macrovascular complications (47–49). As a result, both increased doses and frequency of dosing of insulin have become standard of care. In the 18 years following the DCCT as part of the Epidemiology of Diabetes Interventions and Complications (EDIC) trial, the prevalence of overweight increased by 47% and obesity increased seven times to 22.7% among participants (50). Prior randomization to the intensive insulin therapy group was predictive of elevated BMI. More recent studies have similarly correlated higher insulin dose and continuous subcutaneous insulin infusion with increased weight gain (28, 31, 33); in one circumstance, each unit increase in hemoglobin A1c decreased the odds of overweight by 8% (OR 0.92, 95% CI 0.87 0.97) (31), again suggesting that intensive treatment to improve glycemic control can result in weight gain. There are likely two explanations. With more insulin, the body is better able to utilize the calories from the food consumed, and in the setting of poor dietary choices and sedentary behavior, this can contribute to weight gain. Another potential adverse effect of intensive insulin therapy, hypoglycemia, may also result in excessive carbohydrate consumption to either treat or prevent low blood sugars, further adding to weight gain (51). This has improved with long-acting insulin analogues and insulin pumps, and may improve further with newer hybrid closed loop technologies, which can minimize hypoglycemia through automated basal rate adjustment (52, 53).
With the changing prevalence of obesity at onset of type 1 diabetes in youth, it has become apparent that there are youth presenting with overlapping characteristics of the different types of diabetes, including features of both autoimmunity and insulin resistance, described as “double diabetes”. Data from our group suggested a higher than expected frequency of a family history of type 2 diabetes in youth with type 1 diabetes from our Children’s Hospital of Pittsburgh Diabetes Registry, leading us to postulate that the increased insulin demands of obesity may accelerate the presentation of autoimmune type 1 diabetes (54, 55). In further support of this theory, we later observed features of insulin resistance, including obesity and acanthosis nigricans in islet autoantibody positive youth with new onset insulin-dependent diabetes (56). A similar conclusion was drawn by T. Wilkin who developed the “accelerator hypothesis”, which proposes that obesity-induced insulin resistance places increased burden on islets in genetically at-risk individuals, inducing autoimmune destruction and/or accelerating its course (57). These hypotheses have called into question the traditional classification structure of type 1 and type 2 diabetes, considering them instead to be two extremes on a spectrum of disease. Wilkin argued that the primary factor differentiating diabetes type is the tempo of progression to overt clinical disease, driven by the interplay between β-cell reserve and insulin sensitivity. This phenomenon of double diabetes, has also been described as developing after onset of diabetes. A series of case reports have described combined features of autoimmunity and insulin resistance in children, including a notable case from our group describing a 5-year-old child diagnosed with type 1 diabetes who ultimately developed obesity, acanthosis nigricans, and severe insulin resistance (58). Indeed, youth-onset type 1 diabetes is characterized by significant disease heterogeneity which may in part relate to weight. The complex relationship between environmental factors and genetic risk for type 1 or 2 diabetes likely plays a role in autoimmunity pathogenesis and presentation of clinical disease.
Various studies have investigated the role of birth weight, weight gain, and obesity in the development of islet autoimmunity, progression from single to multiple antibodies, and development of type 1 diabetes. The earliest analyses included population-based cohort and epidemiological case-control studies which correlated a diagnosis of type 1 diabetes with elevated birth weight and early childhood weight gain in infancy as compared to a referent population (59–62). However, these findings were not universal across all populations studied, as others found no supportive evidence linking weight and presentation of type 1 diabetes (63, 64). Subsequently, several prospective cohort studies from birth identified a higher rate of early weight gain and absolute BMI z-score as predictors for the development of islet autoimmunity (65–69). Notably, The Environmental Determinants of Diabetes in the Young (TEDDY) study, a multi-country cohort following children at-risk for type 1 diabetes based upon their HLA-DR-DQ genotype, has examined the associations between weight in the first few years of life with progression from single to multiple antibodies and subsequent development of type 1 diabetes. Weight z-scores at 12 and 24 months were associated with increased risk for progression to multiple antibodies (67), and a higher rate of weight gain in early childhood was associated with progression from autoimmunity to type 1 diabetes in those with the initial presenting autoantibody being directed against glutamic acid decarboxylase (69). Additionally, there may be some relationship between weight gain and earlier age at presentation of type 1 diabetes (23, 61). These studies provide some support for the accelerator hypothesis by suggesting that birth weight and excessive weight gain in early years may hasten diabetes onset, though with little evidence for an association with the onset of islet autoimmunity.
Additional investigations have examined the possible connection between BMI with the progression of type 1 diabetes after autoimmunity onset in older children and adults. Initial comparisons of autoantibody status by BMI at diagnosis were inconclusive. A cross-sectional assessment of 263 children under age 19 years at onset found no significant association between the number of antibodies and measures of adiposity, including BMI and waist circumference (70). A second study examining the TrialNet Pathway to Prevention (PTP) cohort, which follows individuals at risk for type 1 diabetes based upon family history and islet autoantibody status, also explored the relationship between BMI, BMI percentile, and insulin resistance (measured by HOMA-IR) with the progression from autoimmunity to diagnosis. Similarly, they found no association between any of these variables with the risk for developing type 1 diabetes (71). However, these studies lacked the ability to assess temporal trends. Recent, sophisticated analyses have examined the “cumulative excess BMI” (ceBMI) in the TrialNet PTP subjects, a calculated value describing the persistent elevation of BMI over time beyond the overweight threshold (BMI ≥ 25 kg/m2 in adults or ≥ 85th percentile for age and sex in children). In children, ceBMI ≥ 0 kg/m2, indicating persistent overweight/obesity over time, was associated with a higher rate of progression from a single to multiple β-cell autoantibodies in children 9 years of age or older and without high-risk HLA genotypes (72). Furthermore, ceBMI ≥ 0 kg/m2 was also associated with a 63% greater risk to develop type 1 diabetes following islet autoimmunity in children, adjusted for age, sex, and autoantibody number (p=0.0009) (73). In adults, ceBMI also increased the risk for progression to type 1 diabetes, though only in certain age and gender cohorts, namely men over 35 years of age and women younger than 35 years age, suggesting some influence from sex hormones (74).
In further support of the accelerator hypothesis, insulin resistance likely adversely affects β-cell function in youth with type 1 diabetes and may promote immune activation in at risk individuals. Among pooled European cohorts of youth with type 1 diabetes, higher BMI at diagnosis was associated with greater decline in fasting C-peptide levels among teens 10–18 years at 1 year follow-up even when adjusting for glycemia, suggesting that BMI-related insulin resistance is contributing to β -cell dysfunction in this population (75). Resulting immune activation was suggested by a study examining T-cell autoreactivities to neuronal diabetes-associated autoantigens, which are typically observed early in type 1 diabetes pathology before antibodies emerge (76). Youth with the highest quintile of BMI or elevated waist circumference at onset of insulin dependent diabetes had higher islet associated T-cell autoreactivities, suggesting that T-cell autoimmunity may be promoted by visceral adiposity-associated insulin resistance. Furthermore, all but one youth without autoantibodies had evidence of T-cell autoimmunity, suggesting some immune activation among youth who might otherwise be classified as having type 2 diabetes.
Obese youth with new onset type 1 diabetes also have altered pro-inflammatory profiles which may contribute to the acceleration of their presentation. Adipocytes secrete a variety of pro- or anti-inflammatory adipokines which correlate with insulin resistance (77). Obese children with new onset type 1 diabetes are more likely to have higher pro-inflammatory markers (leptin, visfatin, chemerin, TNF-α, CRP) and lower anti-inflammatory markers (adiponectin, omentin) compared to non-obese peers with type 1 diabetes (78). While two or more autoantibodies at diagnosis, suggesting a higher degree of autoimmunity, are associated with higher adiponectin and lower leptin levels, these relationships were negated when adjusting for BMI, suggesting that adiposity is the primary driver of abnormal adipokines (79). Together, these studies examining ceBMI, autoimmune profiles, and inflammatory markers provide reasonable evidence to hypothesize that excessive weight gain may contribute to progression of islet autoimmunity to type 1 diabetes, perhaps through insulin resistance, altered adipokines and cytokines, and β-cell stress.
Though no confirmed pathway exists linking obesity and the rising incidence of type 1 diabetes, different pathophysiological mechanisms may explain how obesity contributes to insulitis and autoimmunity, blurring the boundary with type 2 diabetes. Obesity-induced insulin resistance, insulin demand and inflammation are possible underlying mechanisms. Expanding adipose tissue through adipocyte hypertrophy leads to hypoxia and stress of the cellular endoplasmic reticulum (ER) (80). Subsequent adipocyte necrosis both activates and recruits tissue-specific macrophages, which expand in number from only 5–10% of stromal cells to 40–50%. These macrophages secrete a variety of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1 β) and chemokines (CCL1, CXCL1, and CXCL2), which further activate the immune system (81). Locally, these pro-inflammatory signals impair insulin action and promote insulin resistance, ultimately leading to reduced glucose disposal and a failure to suppress both lipolysis and hepatic glucose production. In turn, insulin resistance, hyperglycemia, elevated free fatty acids, and cytokines promote additional ER and mitochondrial stress, perpetuating this cycle.
This milieu creates low-grade, chronic inflammation which may have downstream effects for β -cells. Insulin resistance and resulting inflammation are hypothesized to increase the β-cell secretory demand, placing added stress on the β-cell and resulting in local cytokine release (IFN-γ), neo-antigen formation, and β-cell apoptosis, thus triggering an immune response and insulitis (82, 83). In response to inflammatory signals, β-cells under stress may secrete chemokines, facilitating leukocyte recruitment and contributing to islet inflammation and destruction (84). Cytokines, specifically IL-1β and IFN-γ, may also trigger β-cell apoptosis directly via upregulation of the NF-κβ transcription pathway in islets (81). Interestingly, the pattern of immune cell activation in islets may differ between type 1 and 2 diabetes; adaptive immune cells predominate in type 1 compared to innate immune cells in type 2 diabetes (83). Additional studies are needed to further clarify the immune dysregulation that may occur in type 2 diabetes to underscore any underlying similarities in pathophysiological mechanisms. In sum, insulitis is likely driven by secretion of chemokines from activated immune cells and β-cells themselves in response to pro-inflammatory stimuli (cytokines, fatty acids), furthering leukocyte inflammation and contributing to β-cell death and, ultimately, type 1 diabetes (84). At present, it is unclear if obesity may be one environmental mechanism that perpetuates insulitis caused by an underlying unknown trigger, or if obesity itself could be a primary driver of insulitis; however, the data described above currently suggests the former.
Genetic risk factors typically associated with type 2 diabetes may modulate the risk of autoimmune progression and development of clinical type 1 diabetes. The genetic risk factor most strongly associated with type 2 diabetes is TCF7L2 single nucleotide polymorphisms (SNPs). TCF7L2 SNPs are strongly associated with various aspects of type 2 diabetes, including insulin resistance, impaired insulin secretion, altered insulin processing, diminished suppression of glucagon by glucose, and increased fasting hepatic glucose release (85). These SNPs have been identified in children and adults with a single autoantibody at diagnosis of type 1 diabetes (86, 87) and seem to be inversely correlated with high-risk HLA genotypes (88). Those with these SNPs may present with a distinct phenotype characterized by less apparent islet cell damage. Oral glucose tolerance test stimulated glucose area under the curve was lower (p-0.0127) and area under the curve C-peptide was higher (p=0.008) in subjects with TCF7L2 SNPs identified at diagnosis (86). Furthermore, subjects with TCF7L2 SNPs may have altered progression from single to multiple autoantibodies, affected by both the initial antibody and the presence of overweight or obesity (89).
Taken together, obesity may augment the risk for autoimmune progression and development of type 1 diabetes when considering longitudinal assessments of excess weight. Hormonal differences in sex steroids likely play a role in potentiating this risk, given some findings in teens undergoing physiological insulin resistance of puberty (75) and differences observed in adults based upon sex and age (74). Proposed mechanisms include obesity-induced insulin resistance and inflammation increasing β-cell secretory demand, stress, and apoptosis, with autoimmune activation. Genetic factors traditionally associated with type 2 diabetes may also augment this risk. Perhaps most telling, the Diabetes Prevention Trial Type 1 Risk Score incorporates body mass index into its modern prediction model, highlighting the important, albeit complex, role of obesity in type 1 diabetes (90).
Premature CVD is a major cause of morbidity and mortality in adults with type 1 diabetes (91). Traditional risk factors for cardiovascular disease in the general population, including hypertension, dyslipidemia, and smoking, do not fully account for the increased CVD risk among those with type 1 diabetes. Insulin resistance emerged as the proposed link to explain the heightened risk for both microvascular and macrovascular complications (92). Applying a calculated measure of insulin resistance, the estimated Glucose Disposal Rate (eGDR) (93), to subjects in the longitudinal Pittsburgh Epidemiology of Diabetes Complications (EDC) study, increasing insulin resistance was associated with a higher risk of nephropathy (94), peripheral vascular disease (women only) (95), and coronary artery disease (96). This relationship in adult patients was further elucidated using data from the DCCT, where eGDR at baseline was significantly inversely associated with higher risk for nephropathy, retinopathy, cardiovascular events, and macrovascular disease (97). More recently, the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study confirmed these findings using hyperinsulinemic euglycemic clamp data comparing adult subjects with type 1 diabetes to age, BMI, and physical activity-matched controls without diabetes (98). Insulin resistance correlated with the presence and progression of coronary artery calcifications, a surrogate marker for coronary artery disease and strong predictor of adverse outcomes. The subjects with type 1 diabetes had impaired insulin-mediated free fatty acid suppression, suggesting that lipotoxicity over time may be a potential mechanistic explanation.
Youth with type 1 diabetes may also have adipose, hepatic, and peripheral insulin resistance regardless of obesity and other features of metabolic syndrome (99). Early CVD factors are present in youth with type 1 diabetes despite their shorter duration of diabetes, also believed to be related to their decreased insulin sensitivity. In hyperinsulinemic euglycemic clamps, youth with type 1 diabetes are more insulin resistant compared to matched healthy peers and have lower functional exercise capacity, a marker of cardiovascular function and predictor of mortality (100). These findings were observed in normal weight youth with no features of Metabolic Syndrome, suggesting that some degree of insulin resistance is present regardless of adiposity. Using a clamp-derived measure, insulin sensitivity is inversely correlated with more atherogenic cardiovascular risk factors, including BMI z-score, total cholesterol, low density lipoprotein-cholesterol, blood pressure, and C-reactive protein (101), as well as arterial stiffness, measured by pulse wave velocity, in a longitudinal assessment (102).
Though insulin resistance may account for much of the baseline cardiovascular risk profile in type 1 diabetes, the increased prevalence of obesity in this population likely augments this risk. Obese adults with type 1 diabetes have higher risk for micro- and macro-vascular comorbidities independent of glucose control (103). In the adult population from DCCT, the quartile with the highest weight gain in the intensively treated group also had higher BMI, blood pressure, triglycerides, total cholesterol, LDL-cholesterol, and apolipoprotein B compared to all other quartiles, indicative of a more atherogenic profile (104). These findings persisted in the EDIC follow-up of the DCCT and were associated with greater intima media thickness (105). Though the impact of obesity on cardiovascular complications is thought to be mediated by Metabolic Syndrome (e.g. hypertension, dyslipidemia), the CACTI study identified obesity as a risk factor for both the presence and progression of coronary artery calcifications independent of pre-existing metabolic abnormalities (106). How this translates to long-term cardiovascular morbidity and mortality is less clear (107). The Pittsburgh EDC study demonstrated that waist circumference, a surrogate of visceral adiposity, increases the risk for long-term mortality in type 1 diabetes (108). In the DCCT/EDIC studies, the rate of adverse cardiac events was initially similar between those who did or did not have excessive weight gain in the intensively treated group, with some divergence after 14 years, when the event rate in excessive weight gainers approached that of conventionally treated patients (109). The lack of a difference in events within 14 years of follow-up was attributed to both better management of cardiovascular risk factors (e.g. anti-hypertensive and lipid lowering medications) as well as the improved nutrition counseling this group received irrespective of overweight/obesity.
The potential compounding effect of obesity on insulin resistance and complications may appear as early as adolescence. Indeed, overweight and obese youth with type 1 diabetes are more likely to have coexisting hypertension, abnormal lipids, and elevated alanine aminotransferase compared to healthy weight peers (110, 111). In the SEARCH registry, youth with obesity and persistently elevated hemoglobin A1c over time had the highest risk for adverse cardiovascular markers and microvascular complications compared to those with either elevated hemoglobin A1c and normal weight or obesity with fairly-well controlled A1c (112). In clamp studies, our group found that obese youth with type 1 diabetes were more insulin resistant compared to non-obese peers with type 1 diabetes. Furthermore, insulin sensitivity correlated with cardiovascular risk factors, including pulse wave velocity, even when adjusting for the degree of obesity, suggesting that insulin resistance, augmented by excess weight, is the underlying factor contributing to cardiovascular complications (113). Overall, children do not meet ADA and ISPAD-determined clinical targets for glycemic control (114, 115), placing them at risk for long-term micro and macrovascular disease. Children with coexisting features of type 1 and 2 diabetes, or double diabetes, may be at heightened risk. Given the substantial changes in insulin therapy over time as a result of the DCCT with new analogs and continuous subcutaneous insulin infusion being used in children, the long-term impact on weight gain, insulin resistance, and cardiovascular health merits further study of intervention strategies.
Despite the more pronounced long-term risks of coexisting obesity and type 1 diabetes, there are limited therapeutics or guidelines to manage this condition beyond what has been traditionally available. One particular challenge for providers is the lack of a clinically meaningful definition for double diabetes which can guide treatment (116). Though glycemia is the most important predictor of outcomes, the degree of insulin resistance associated with obesity and type 1 diabetes is also important in predicting future complications, and this is not addressed by insulin titration alone. Escalating insulin doses to achieve glycemic goals may also further compound weight gain, exacerbating obesity-related complications. Adequate counseling on a healthy diet and adequate physical activity should be paramount in the care of these patients, following recommended guidelines from leading diabetes organizations (43, 46). In addition, the use of targeted nutritional therapies or pharmaceuticals for the treatment of type 2 diabetes have been proposed as adjuvant treatments for children and adults with obesity complicating type 1 diabetes; the evidence for these therapies is summarized in Table 1.
Diet is an essential component to type 1 and 2 diabetes management and is necessary to achieve optimal glycemic control (129). Both the ADA and ISPAD promote nutrition counseling and education that is tailored to the unique psychosocial and cultural needs of that family (43, 130). As a guide, children should receive approximately 45–50% of their energy from carbohydrates, 15–20% from protein, and <35% from fat (with saturated fat <10%) (130). The dietary recommendations must be commensurate with the insulin regimen, as certain approaches (e.g. sliding scales/algorithms) require fixed- macronutrient intake for each meal time. The diet for children and adolescents with type 1 diabetes is not only important for glycemic control, but also for appropriate growth and development while minimizing unnecessary weight gain. In adults with type 1 diabetes, both the Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets have been shown to promote weight loss as well as optimize glucose levels (131).
The low carbohydrate or very low carbohydrate (ketogenic) diets have gained recent popularity as part of the treatment for type 1 diabetes. They require significant carbohydrate restriction, at times to no more than 20–70 g/day. The studies examining the effectiveness of this diet are heterogeneous and often small, limiting their interpretation. Some evidence suggests there may be a beneficial reduction in hemoglobin A1c, daily insulin dose, and body weight mostly in adults, though this may be at the expense of insufficient insulin, dyslipidemia or increased hypoglycemic events (132). Use of this diet in children, especially if normal weight, is highly controversial, as carbohydrate intake and appropriate insulin dosing are critical for growth. A case series documented poor growth, significant fatigue, and adverse lipid profiles in six children with type 1 diabetes on a low carbohydrate diet (133). At present, the low carbohydrate diets are not recommended for youth with type 1 diabetes, including as treatment for obesity. The relationship between any of these diets and insulin resistance has not been studied. Similarly, studies have not examined whether weight control in children at risk for type 1 diabetes can help prevent the onset of the disease.
Metformin is considered first line therapy in both adolescents and adults with type 2 diabetes. Its mechanism is believed to be improved insulin sensitivity by inhibiting hepatic gluconeogenesis, resulting in reduced hepatic glucose output, and increasing peripheral uptake of glucose in the muscle (134). Metformin has been proposed as an adjunct therapy in both youth and adults with type 1 diabetes to address peripheral insulin resistance regardless of weight; relevant pediatric studies are summarized in Table 2. Numerous randomized controlled trials have attempted to explore the efficacy of metformin in this context. Initial studies examined adolescents with poor glycemic control, only some of whom were overweight and obese, with few finding a modest improvement in hemoglobin A1c (120, 121, 135, 136) and others finding no difference (137). These studies were small, of variable duration, and frequently used non-standard doses of metformin, limiting their interpretation. An adequately powered trial using low dose metformin (1,000 mg daily) in adolescents with sub-optimal glycemic control (hemoglobin A1c >8.5%), only approximately one-third of whom were overweight or obese, found no difference in hemoglobin A1c levels from baseline to six months (117). Two additional studies specifically examined use of metformin in overweight or obese adolescents (118, 119). A large randomized trial found that though 2,000 mg of daily metformin led to a small decline in hemoglobin A1c compared to placebo at the mid-way point, at 6 months there was no difference in hemoglobin A1c compared to placebo (119).
Despite the lack of clinical change in hemoglobin A1c over the course of these trials, many studies demonstrated a reduction in body mass index (117, 119, 121), waist circumference (117), and insulin dose per unit of body weight (117, 119–121). Indeed, the aforementioned large randomized trial of 2,000 mg metformin daily in obese adolescents demonstrated that approximately one quarter of the intervention group had at least a 25% reduction in their total daily insulin dose per unit body weight (compared to 1% in placebo group p=0.003) and at least a 10% reduction in their BMI z-score (compared to 7% in placebo group p=0.01) (119). In addition, there was a small reduction in total body fat by approximately 2 kg as determined by dual-energy x-ray absorptiometry in the metformin group compared to placebo (p<0.001).
Taken together, these findings have mirrored those in adults, which similarly have found no improvement in hemoglobin A1c, though reductions in insulin dose and body weight may occur (122, 138). The noted reductions in insulin dose were thought to imply improved insulin resistance. Two studies examined this directly using hyperinsulinemic euglycemic clamps, finding improvement in both whole-body insulin sensitivity and peripheral insulin resistance in those adolescents taking metformin (136, 139). How this actually relates to long-term cardiovascular complications is less clear, as studies are inconclusive on whether the addition of metformin impacts intermediate measures of hypertension, lipids, inflammatory markers, or carotid intima media thickness (119, 122). Through the use of advanced magnetic resonance imaging techniques, metformin may lead to some benefit in measures of aortic pulse wave velocity and wall sheer stress among youth, suggesting cardioprotective effects (123). The potential downstream benefits, particularly as pertains to the risk for micro- and macro-vascular complications, warrant further investigation.
At present, use of metformin is not recommended by the ADA for youth with type 1 diabetes (43), and though it is discussed as a consideration in the guidelines for adults with type 1 diabetes, it is not explicitly recommended (140). Real-world studies evaluating how often metformin is used within a type 1 diabetes population and which patients receive this adjunct therapy are limited. Among both the T1D Exchange and DPV registries, including close to 50,000 patients (both youth and adults), metformin was used in about 3.5% of individuals in the T1D Exchange and only 1.3% in the DPV (141). Adjuvant therapy was rare in youth under 13 years of age. An additional analysis of the DPV registry examined 525 youth with type 1 diabetes treated with metformin in addition to insulin compared to over 57,000 youth on insulin alone (142). In this observational study, the children treated with metformin tended to be slightly older and were more commonly female. Median BMI z-score of the metformin-treated youth was +1.86 (+1.33 to +2.58) compared to a median BMI z-score of +0.51 (−0.12 to +1.15) in youth on insulin alone (p<0.001). Furthermore, hemoglobin A1c was significantly higher among those on metformin, as was the presence of comorbidities, including polycystic ovary syndrome, hypertension, elevated liver enzymes, and dyslipidemia. Those youth treated with metformin required a significantly higher insulin dose per unit body weight than those on insulin alone. Over time, the metformin-treated group had modest improvements in BMI and insulin dose. The study concluded that metformin is used rarely in youth with type 1 diabetes outside of the research setting, most often in obese female adolescents, perhaps to treat concomitant polycystic ovary syndrome, and the benefits of this therapy are not fully realized.
Glucagon-like-peptide-1 (GLP-1) is an incretin hormone secreted by the gut which stimulates insulin release and inhibits glucagon secretion in a glucose-dependent manner, resulting in control of meal-induced glycemic excursions (143). GLP-1 also delays gastric emptying and acts centrally to decrease appetite (144), which can help to promote weight loss. GLP-1 receptor signaling may also support islet health by inhibiting β-cell apoptosis, promoting proliferation of β-cells, and reducing ER stress, with the net result of preserving or enhancing residual function; however, restoration of β-cell function has not been proven in human studies of islet transplantation or type 1 diabetes (145). GLP-1 is rapidly degraded by the dipeptidyl peptidase-4 enzyme (DDP-4), limiting the longevity of its effect. Both GLP-1 receptor agonists and DPP-4 inhibitors are recent advances for the treatment of type 2 diabetes, and in the case of GLP-1 agonists, for obesity in the absence of diabetes. In addition to myriad studies in adults, the GLP-1 agonist, Liraglutide, has been shown to be effective in reducing hemoglobin A1c in youth with type 2 diabetes (146) and promoting weight loss in obese adolescents with no evidence of dysglycemia (147).
While this treatment has not yet been studied in obese children with type 1 diabetes, there is some evidence supporting the use of GLP-1 agonists in adults with type 1 diabetes. A meta-analysis examined the net impact of GLP-1 agonists on glycemic control, weight, and insulin dose in seven randomized controlled trials in 206 adult subjects with type 1 diabetes also treated with insulin over study durations of 3–15 months (124). Overall, there was a net, albeit modest, improvement in hemoglobin A1c by −0.21% (95% CI −0.40, −0.02, p=0.03), without increased risk of hypoglycemia, and mean weight loss of 3.53 kg (95% CI −4.86, −2.19, p<0.05) in adults taking GLP-1 agonists compared to placebo. In the few studies that examined total daily insulin dose, specifically differentiating bolus and basal insulin, use of a GLP-1 agonist was associated with a 6% reduction in daily bolus insulin dose (95%CI, −10%, −2%, p=0.001) with no change in absolute daily insulin dose. Taken together, the authors suggest a potential benefit to adding GLP-1 to insulin therapy in adults with type 1 diabetes and offer different mechanisms by which GLP-1 may mediate these improvements. Two explanations may be improved insulin sensitivity (148) and/or reduced appetite and carbohydrate intake (149), resulting in decline in bolus insulin requirements and weight loss (124). In contrast, DPP-4 inhibitors are less promising. Two meta-analyses examined the efficacy and safety of DPP-4 inhibitors in adults with type 1 diabetes; neither found any significant improvement in hemoglobin A1c, BMI, or insulin dosage with treatment, though significant heterogeneity among studies limited the analyses (125, 126).
Altogether, adult studies have identified a slight improvement in glycemic control, weight, and bolus insulin dose among patients with type 1 diabetes on combined therapy of insulin and GLP-1 agonists, but not DPP-4 inhibitors. The therapeutic mechanisms underpinning these improvements are poorly understood based upon the available studies, though the theoretical advantage to these medications may predominantly be weight loss in the setting of overweight/obesity, which may improve insulin sensitivity. To date, no studies have examined the use of either of these therapies in youth with type 1 diabetes, and they are not currently recommended for this population.
Sodium-glucose cotransporter (SGLT) inhibitors are another fairly recent therapeutic advancement approved for the treatment of type 2 diabetes which have been proposed as an adjunct treatment for type 1 diabetes. These medications block SGLT-2 in the proximal tubule of the kidney, resulting in glycosuria and natriuresis; some medications also block SGLT-1, which can delay glucose absorption from the intestinal tract (150). In adults, SGLT inhibitors are believed to have added cardioprotective effects (151). A meta-analysis examined 10 randomized controlled trials of both SGLT-2 and combined SGLT-1/2 inhibitors, including over 5961 patients with type 1 diabetes with follow-up of 12–52 weeks (127). Across pooled studies, these medications resulted in a reduction in body weight of approximately 4% and associated reductions in hemoglobin A1c by −0.39% (95% CI −0.43 to −0.36) and fasting glucose by −1.13 mmol/L (95% CI −1.36 to −0.90). In addition, total daily insulin dose, basal insulin dose, and short acting insulin dose were all reduced by approximately 10%. Thus far in youth with type 1 diabetes, one study has examined safety and effect of SGLT-2 inhibitors, though did not comment on any effect on weight (128).
These medications essentially compensate for some food indiscretions by lowering the renal threshold, promoting the excretion of excess glucose following a meal. By reducing hyperglycemia, these drugs likely facilitate weight loss; both are proposed mechanisms for improved insulin sensitivity in adults with type 2 diabetes (152, 153). No investigations have evaluated whether the same metabolic effects are present in individuals with type 1 diabetes regardless of adiposity, and use of these medications in this population remains fairly cautionary due to the increased risk for euglycemic ketoacidosis (127, 154). Similar to the therapies described before, SLGT inhibitors are not currently recommended for adjuvant therapy in pediatric type 1 diabetes with obesity.
In all, the options to treat obese youth with type 1 diabetes remain limited. The prevention and management of obesity continues to rely upon early, consistent, and individualized dietary counseling both at diagnosis and at least annually at follow-up appointments. No therapeutic options are currently approved for this purpose, and future research is needed to understand which therapies might be safe and effective in weight reduction, optimizing glycemic control, and in preventing long-term complications.
Overweight and obesity are increasingly common in youth and adults with type 1 diabetes, perhaps a consequence of changing cultural practices surrounding diet, nutrition and exercise over the past several decades. Genetic risk and environmental factors may lead to coexisting features of type 1 and 2 diabetes, with added implications for both pathogenesis and long-term health. Data supporting these outcomes led to the concept of double diabetes. In the setting of widespread use of intensive insulin therapy, poor dietary choices and over-treatment of hypoglycemia may both contribute to weight gain. Further study is needed to understand whether use of new technologies, particularly closed loop hybrid insulin pumps and automated insulin delivery systems, may help reduce the risk of hypoglycemia and thus result in decreased weight gain by lessening the need for treatments with added food intake.
It is possible that obesity has a role in potentiating the risk for type 1 diabetes, similarly to type 2 diabetes. Obesity causes augmentation of insulin resistance, which may increase both insulin demand, stressing the islets. Though longitudinal studies have examined the role of excessive weight gain in autoimmune development and progression, future research is needed to better elucidate the mechanisms by which obesity contributes to increased stress on the insulin secretory functions and inflammation, possibly leading to or exacerbating β-cell pathology.
There is limited evidence for any beneficial effect of currently available adjuvant therapies apart from lifestyle adjustments, and these all need further investigation for both the delay of type 1 diabetes and the prevention of complications in those with double diabetes. Better measurement systems are needed to identify those with significant insulin resistance who may be at higher risk for complications that are easily adaptable for busy clinical settings. Additional investigations are needed to examine whether the use of targeted therapies for weight loss can also modulate inflammation and insulin resistance and reduce the long-term risk for cardiovascular complications. Given our current fund of knowledge, it is critical to consider how we counsel patients and families about excess weight gain for children diagnosed with type 1 diabetes to prevent double diabetes in adolescence and adulthood. Perhaps most important are practical, evidenced-based strategies to promote lay awareness of the dangers of weight gain in the type 1 diabetes population and focus efforts on obesity prevention.
All authors contributed to the conceptualization of this manuscript and identified source articles. CM drafted the manuscript. All authors contributed to the article and approved the submitted version.
The work was funded by Mentored Patient-Oriented Research, National Heart, Lung and Blood Institute, NIH 5K23HL085287-03 (Primary Investigator: IL), and UPMC Children’s Scholar Award (Primary Investigator: CM).
IL serves as Pediatric Type 2 Diabetes Expert Panel member for Novo Nordisk.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
BMI, body mass index; DPV, Diabetes Patienten Verlaufsdokumentation registry; ADA, American Diabetes Association; ISPAD, International Society for Pediatric and Adolescent Diabetes; DCCT, Diabetes Control and Complications Trial; EDIC, Epidemiology of Diabetes Interventions and Complications study; PTP, Pathway to Prevention; ceBMI, cumulative excess BMI; HOMA-IR, homeostatic model assessment of insulin resistance; ER, endoplasmic reticulum; SNPs, single nucleotide polymorphisms; CVD, cardiovascular disease; eGDR, estimated glucose disposal rate; EDC, Epidemiology of Diabetes Complications study; CACTI, Coronary Artery Calcification in Type 1 Diabetes; GLP 1, glucagon-like peptide 1; DPP4, dipeptidyl peptidase 4; SGLT, sodium glucose cotransporter.
1. Lawrence JM, Mayer-Davis EJ. What do we know about the trends in incidence of childhood-onset type 1 diabetes? Diabetologia (2019) 62(3):370–2. doi: 10.1007/s00125-018-4791-z
2. DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabetes Med (2006) 23(8):857–66. doi: 10.1111/j.1464-5491.2006.01925.x
3. Libman IM, LaPorte RE, Becker D, Dorman JS, Drash AL, Kuller L. Was there an epidemic of diabetes in nonwhite adolescents in Allegheny County, Pennsylvania? Diabetes Care (1998) 21(8):1278–81. doi: 10.2337/diacare.21.8.1278
4. Gavin JR, Alberti KGMM, Davidson MB, DeFronzo RA, Drash A, Gabbe SG, et al. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care (1997) 20(7):1183–97. doi: 10.2337/diacare.20.7.1183
5. Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988-1994 Through 2013-2014. JAMA (2016) 315(21):2292–9. doi: 10.1001/jama.2016.6361
6. Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents,and adults, 1999-2002. JAMA (2004) 291(23):2847–50. doi:10.1001/jama.291.23.2847
7. de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr (2010)92(5):1257–64. doi: 10.3945/ajcn.2010.29786
8. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesityin children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (2014) 384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8
9. NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, andobesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9million children, adolescents, and adults. Lancet (2017) 390(10113):2627–42. doi: 10.1016/S0140-6736(17)32129-3
10. Kopp W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab Syndr Obes (2019) 12:2221–36. doi: 10.2147/DMSO.S216791
11. Kim H, Hu EA, Rebholz CM. Ultra-processed food intake and mortality in the USA: results fromthe Third National Health and Nutrition Examination Survey (NHANES III, 1988-1994).Public Health Nutr (2019) 22(10):1777–85. doi: 10.1017/S1368980018003890
12. Schnabel L, Kesse-Guyot E, Alles B, Touvier M, Srour B, Hercberg S, et al. Association Between Ultraprocessed Food Consumption and Risk ofMortality Among Middle-aged Adults in France. JAMA Intern Med(2019) 179(4):490–8. doi: 10.1001/jamainternmed.2018.7289
13. Neri D, Martinez-Steele E, Monteiro CA, Levy RB. Consumption of ultra-processed foods and its association with addedsugar content in the diets of US children, NHANES 2009-2014. PediatrObes (2019) 14(12):e12563. doi: 10.1111/ijpo.12563
14. Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr (2007) 150(1):12–7. doi: 10.1016/j.jpeds.2006.08.042
15. Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic Risks and Severity of Obesity in Children and YoungAdults. N Engl J Med (2015) 373(14):1307–17. doi: 10.1056/NEJMoa1502821
16. Bendor CD, Bardugo A, Pinhas-Hamiel O, Afek A, Twig G. Cardiovascular morbidity, diabetes and cancer risk among childrenand adolescents with severe obesity. Cardiovasc Diabetol (2020) 19(1):79. doi: 10.1186/s12933-020-01052-1
17. Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in theUSA and the UK. Lancet (2011) 378(9793):815–25. doi: 10.1016/S0140-6736(11)60814-3
18. Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002-2012. N Engl J Med (2017) 376(15):1419–29. doi: 10.1056/NEJMoa1610187
19. Patterson CC, Gyurus E, Rosenbauer J, Cinek O, Neu A, Schober E, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia (2012) 55(8):2142–7. doi: 10.1007/s00125-012-2571-8
20. Patterson CC, Harjutsalo V, Rosenbauer J, Neu A, Cinek O, Skrivarhaug T, et al. Trends and cyclical variation in the incidence of childhood type 1diabetes in 26 European centres in the 25 year period 1989-2013: a multicentre prospectiveregistration study. Diabetologia (2019) 62(3):408–17. doi: 10.1007/s00125-018-4763-3
21. Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet (2016) 387(10035):2340–8. doi: 10.1016/S0140-6736(16)30507-4
22. Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ. Changing prevalence of overweight children and adolescents at onset of insulin-treated diabetes. Diabetes Care (2003) 26(10):2871–5. doi: 10.2337/diacare.26.10.2871
23. Knerr I, Wolf J, Reinehr T, Stachow R, Grabert M, Schober E, et al. The ‘accelerator hypothesis’: relationship between weight, height, body mass index and age at diagnosis in a large cohort of 9,248 German and Austrian children with type 1 diabetes mellitus. Diabetologia (2005) 48(12):2501–4. doi: 10.1007/s00125-005-0033-2
24. Kaminski BM, Klingensmith GJ, Beck RW, Tamborlane WV, Lee J, Hassan K, et al. Body mass index at the time of diagnosis of autoimmune type 1 diabetes in children. J Pediatr (2013) 162(4):736–40e1. doi: 10.1016/j.jpeds.2012.09.017
25. Flechtner-Mors M, Schwab KO, Frohlich-Reiterer EE, Kapellen TM, Meissner T, Rosenbauer J, et al. Overweight and Obesity Based on Four Reference Systems in 18,382 Paediatric Patients with Type 1 Diabetes from Germany and Austria. J Diabetes Res (2015) 2015:370753. doi: 10.1155/2015/370753
26. Ferrante E, Pitzalis G, Vania A, De Angelis P, Guidi R, Fontana L, et al. Nutritional status, obesity and metabolic control in children with type 1 diabetes mellitus. Minerva Pediatr (1999) 51(3):39–46.
27. Shenoy S, Waldron S, Cody D, Swift PG. Ethnic group differences in overweight and obese children with type 1 diabetes mellitus. Arch Dis Child (2004) 89(11):1076–7.
28. da Costa VM, de Carvalho Padilha P, de Lima GC, Ferreira AA, Luescher JL, Porto L, et al. Overweight among children and adolescent with type I diabetesmellitus: prevalence and associated factors. Diabetol Metab Syndr(2016) 8:39. doi: 10.1186/s13098-016-0154-4
29. Pinhas-Hamiel O, Levek-Motola N, Kaidar K, Boyko V, Tisch E, Mazor-Aronovitch K, et al. Prevalence of overweight, obesity and metabolic syndrome componentsin children, adolescents and young adults with type 1 diabetes mellitus. Diabetes Metab Res Rev (2015) 31(1):76–84. doi: 10.1002/dmrr.2565
30. Liu LL, Lawrence JM, Davis C, Liese AD, Pettitt DJ, Pihoker C, et al. Prevalence of overweight and obesity in youth with diabetes in USA:the SEARCH for Diabetes in Youth study. Pediatr Diabetes (2010) 11(1):4–11. doi: 10.1111/j.1399-5448-2009.00519.x
31. Minges KE, Whittemore R, Weinzimer SA, Irwin ML, Redeker NS, Grey M. Correlates of overweight and obesity in 5529 adolescents with type 1 diabetes: The T1D Exchange Clinic Registry. Diabetes Res Clin Pract (2017) 126:68–78. doi: 10.1016/j.diabres.2017.01.012
32. Maffeis C, Birkebaek NH, Konstantinova M, Schwandt A, Vazeou A, Casteels K, et al. Prevalence of underweight, overweight, and obesity in children and adolescents with type 1 diabetes: Data from the international SWEET registry. Pediatr Diabetes (2018) 19(7):1211–20. doi: 10.1111/pedi.12730
33. Frohlich-Reiterer EE, Rosenbauer J, Bechtold-Dalla Pozza S, Hofer SE, Schober E, Holl RW, et al. Predictors of increasing BMI during the course of diabetes in children and adolescents with type 1 diabetes: data from the German/Austrian DPV multicentre survey. Arch Dis Child (2014) 99(8):738–43. doi: 10.1136/archdischild-2013-304237
34. Kapellen TM, Gausche R, Dost A, Wiegand S, Flechtner-Mors M, Keller E, et al. Children and adolescents with type 1 diabetes in Germany are more overweight than healthy controls: results comparing DPV database and CrescNet database. J Pediatr Endocrinol Metab (2014) 27(3-4):209–14. doi: 10.1515/jpem-2013-0381
35. Ogden CL, Fryar CD, Hales CM, Carroll MD, Aoki Y, Freedman DS. Differences in Obesity Prevalence by Demographics and Urbanization in US Children and Adolescents, 2013-2016. JAMA (2018) 319(23):2410–8. doi: 10.1001/jama.2018.5158
36. Helgeson VS, Viccaro L, Becker D, Escobar O, Siminerio L. Diet of adolescents with and without diabetes: Trading candy for potato chips? Diabetes Care (2006) 29(5):982–7. doi: 10.2337/dc05-2197
37. Patton SR. Adherence to diet in youth with type 1 diabetes. J Am Diet Assoc (2011) 111(4):550–5. doi: 10.1016/j.jada.2011.01.016
38. Seckold R, Howley P, King BR, Bell K, Smith A, Smart CE. Dietary intake and eating patterns of young children with type 1 diabetes achieving glycemic targets. BMJ Open Diabetes Res Care (2019) 7(1):e000663. doi: 10.1136/bmjdrc-2019-000663
39. Mackey ER, O’Brecht L, Holmes CS, Jacobs M, Streisand R. Teens with Type 1 Diabetes: How Does Their Nutrition Measure Up? J Diabetes Res (2018) 2018:5094569. doi: 10.1155/2018/5094569
40. Meissner T, Wolf J, Kersting M, Frohlich-Reiterer E, Flechtner-Mors M, Salgin B, et al. Carbohydrate intake in relation to BMI, HbA1c and lipid profile in children and adolescents with type 1 diabetes. Clin Nutr (2014) 33(1):75–8. doi: 10.1016/j.clnu.2013.03.017
41. Schmidt S, Schelde B, Norgaard K. Effects of advanced carbohydrate counting in patients with type 1 diabetes: a systematic review. Diabetes Med (2014) 31(8):886–96. doi: 10.1111/dme.12446
42. Donzeau A, Bonnemaison E, Vautier V, Menut V, Houdon L, Bendelac N, et al. Effects of advanced carbohydrate counting on glucose control and quality of life in children with type 1 diabetes. Pediatr Diabetes (2020) 21(7):1240–8. doi: 10.1111/pedi.13076
43. American Diabetes Association. 13. Children and Adolescents: Standards of Medical Care in Diabetes-2021. Diabetes Care (2021) 44(Suppl 1):S180–99. doi: 10.2337/dc21-S013
44. Liese AD, Ma X, Maahs D, Trilk JL. Physical activity, sendary behaviors, physical fitness, and their relation to health outcomes in youth with type 1 and type 2 diabetes: A review of the epidemiologic literature. J Sport Health Sci (2013) 2(1):21–38. doi: 10.1016/j.jshs.2012.10.005
45. Hesketh KR, Lakshman R, van Sluijs EMF. Barriers and facilitators to young children’s physical activity and sedentary behaviour: a systematic review and synthesis of qualitative literature. Obes Rev (2017) 18(9):987–1017. doi: 10.1111/obr.12562
46. Adolfsson P, Riddell MC, Taplin CE, Davis E, Fournier PA, Annan F, Crofford O, et al. ISPAD Clinical Practice Consensu Guidelines 2018: Exercise in children and adolescents with diabetes. Pediatr Diabetes (2018) 19:205–26. doi: 10.1111/pedi.12755
47. DCCT Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med (1993) 329(14):977–86. doi: 10.1056/NEJM199309303291401
48. DCCT Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med (1995) 47(6):1703–20. doi: 10.1038/ki.1995.236
49. DCCT Research Goup. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol (1995) 75(14):894–903. doi: 10.1016/S0002-9149(99)80683-3
50. Conway B, Miller RG, Costacou T, Fried L, Kelsey S, Evans RW, et al. Temporal patterns in overweight and obesity in Type 1 diabetes. Diabetes Med (2010) 27(4):398–404. doi: 10.1111/j.1464-5491.2010.02956.x
51. Martyn-Nemeth P, Quinn L, Penckofer S, Park C, Hofer V, Burke L. Fear of hypoglycemia: Influence on glycemic variability and self-management behavior in young adults with type 1 diabetes. J Diabetes Complications (2017) 31(4):735–41. doi: 10.1016/j.jdiacomp.2016.12.015
52. Battelino T, Nimri R, Dovc K, Phillip M, Bratina N. Prevention of Hypoglycemia With Predictive Low Glucose Insulin Suspension in Children With Type 1 Diabetes: A Randomized Controlled Trial. Diabetes Care (2017) 40(6):764–70. doi: 10.2337/dc16-2584
53. Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, et al. Six-Month Randomized, Multicenter Trial of Closed-Loop Control in Type 1 Diabetes. N Engl J Med (2019) 381(18):1707–17. doi: 10.1056/NEJMoa1907863
54. Wagener DK, Sacks JM, LaPorte RE, Macgregor JM. The Pittsburgh study of insulin-dependent diabetes mellitus. Risk for diabetes among relatives of IDDM. Diabetes (1982) 31(2):136–44. doi: 10.2337/diabetes.31.2.136
55. Orchard TJ. From diagnosis and classification to complications and therapy. DCCT. Part II? Diabetes Control and Complications Trial. Diabetes Care (1994) 17(4):326–38. doi: 10.2337/diacare.17.4.326
56. Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ. Evidence for heterogeneous pathogenesis of insulin-treated diabetes in black and white children. Diabetes Care (2003) 26(10):2876–82. doi: 10.2337/diacare.26.10.2876
57. Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between Type I and Type II diabetes. Diabetologia (2001) 44(7):914–22. doi: 10.1007/s001250100548
58. Libman IM, Becker DJ. Coexistence of type 1 and type 2 diabetes mellitus: “double” diabetes? Pediatr Diabetes (2003) 4(2):110–3. doi: 10.1034/j.1399-5448.2003.00012.x
59. Johansson C, Samuelsson U, Ludvigsson J. A high weight gain early in life is associated with an increased risk of type 1 (insulin-dependent) diabetes mellitus. Diabetologia (1994) 37(1):91–4. doi: 10.1007/BF00428783
60. Hyppönen E, Kenward MG, Virtanen SM, Piitulainen A, Virta-Autio P, Tuomilehto J, et al. Infant feeding, early weight gain, and risk of type 1 diabetes. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes Care (1999) 22(12):1961–5. doi: 10.2337/diacare.22.12.1961
61. Hyppönen E, Virtanen SM, Kenward MG, Knip M, Akerblom HK. Obesity, increased linear growth, and risk of type 1 diabetes in children. Diabetes Care (2000) 23(12):1755–60. doi: 10.2337/diacare.23.12.1755
62. Stene LC, Magnus P, Lie RT, Søvik O, Joner G. Birth weight and childhood onset type 1 diabetes: population based cohort study. Bmj (2001) 322(7291):889–92. doi: 10.1136/bmj.322.7291.889
63. O’Connell MA, Donath S, Cameron FJ. Major increase in Type 1 diabetes: no support for the Accelerator Hypothesis. Diabetes Med (2007) 24(8):920–3. doi: 10.1111/j.1464-5491.2007.02203.x
64. Porter JR, Barrett TG. Braking the accelerator hypothesis? Diabetologia (2004) 47(2):352–3. doi: 10.1007/s00125-003-1291-5
65. Couper JJ, Beresford S, Hirte C, Baghurst PA, Pollard A, Tait BD, et al. Weight gain in early life predicts risk of islet autoimmunity in children with a first-degree relative with type 1 diabetes. Diabetes Care (2009) 32(1):94–9. doi: 10.2337/dc08-0821
66. Beyerlein A, Thiering E, Pflueger M, Bidlingmaier M, Stock J, Knopff A, et al. Early infant growth is associated with the risk of islet autoimmunity in genetically susceptible children. Pediatr Diabetes (2014) 15(7):534–42. doi: 10.1111/pedi.12118
67. Elding Larsson H, Vehik K, Haller MJ, Liu X, Akolkar B, Hagopian W, et al. Growth and Risk for Islet Autoimmunity and Progression to Type 1 Diabetes in Early Childhood: The Environmental Determinants of Diabetes in the Young Study. Diabetes (2016) 65(7):1988–95. doi: 10.2337/db15-1180
68. Yassouridis C, Leisch F, Winkler C, Ziegler AG, Beyerlein A. Associations of growth patterns and islet autoimmunity in children with increased risk for type 1 diabetes: a functional analysis approach. Pediatr Diabetes (2017) 18(2):103–10. doi: 10.1111/pedi.12368
69. Liu X, Vehik K, Huang Y, Elding Larsson H, Toppari J, Ziegler AG, et al. Distinct Growth Phases in Early Life Associated With the Risk of Type 1 Diabetes: The TEDDY Study. Diabetes Care (2020) 43(3):556–62. doi: 10.2337/dc19-1670
70. Cedillo M, Libman IM, Arena VC, Zhou L, Trucco M, Ize-Ludlow D, et al. Obesity, islet cell autoimmunity, and cardiovascular risk factors in youth at onset of type 1 autoimmune diabetes. J Clin Endocrinol Metab (2015) 100(1):E82–6. doi: 10.1210/jc.2014-2340
71. Meah FA, DiMeglio LA, Greenbaum CJ, Blum JS, Sosenko JM, Pugliese A, et al. The relationship between BMI and insulin resistance and progression from single to multiple autoantibody positivity and type 1 diabetes among TrialNet Pathway to Prevention participants. Diabetologia (2016) 59(6):1186–95. doi: 10.1007/s00125-016-3924-5
72. Ferrara-Cook C, Geyer SM, Evans-Molina C, Libman IM, Becker DJ, Gitelman SE, et al. Excess BMI Accelerates Islet Autoimmunity in Older Children and Adolescents. Diabetes Care (2020) 43(3):580–7. doi: 10.2337/dc19-1167
73. Ferrara CT, Geyer SM, Liu YF, Evans-Molina C, Libman IM, Besser R, et al. Excess BMI in Childhood: A Modifiable Risk Factor for Type 1 Diabetes Development? Diabetes Care (2017) 40(5):698–701. doi: 10.2337/dc16-2331
74. Ferrara CT, Geyer SM, Evans-Molina C, Libman IM, Becker DJ, Wentworth JM, et al. The Role of Age and Excess Body Mass Index in Progression to Type 1 Diabetes in At-Risk Adults. J Clin Endocrinol Metab (2017) 102(12):4596–603. doi: 10.1210/jc.2017-01490
75. Lauria A, Barker A, Schloot N, Hosszufalusi N, Ludvigsson J, Mathieu C, et al. BMI is an important driver of β-cell loss in type 1 diabetes upon diagnosis in 10 to 18-year-old children. Eur J Endocrinol (2015) 172(2):107–13. doi: 10.1530/EJE-14-0522
76. Buryk MA, Dosch HM, Libman I, Arena VC, Huang Y, Cheung RK, et al. Neuronal T-cell autoreactivity is amplified in overweight children with new-onset insulin-requiring diabetes. Diabetes Care (2015) 38(1):43–50. doi: 10.2337/dc14-1861
77. Maahs DM, Hamman RF, D’Agostino R Jr., Dolan LM, Imperatore G, Lawrence JM, et al. The association between adiponectin/leptin ratio and diabetes type: the SEARCH for Diabetes in Youth Study. J Pediatr (2009) 155(1):133–5. doi: 10.1016/j.jpeds.2008.12.048
78. Redondo MJ, Rodriguez LM, Haymond MW, Hampe CS, Smith EO, Balasubramanyam A, et al. Serum adiposity-induced biomarkers in obese and lean children with recently diagnosed autoimmune type 1 diabetes. Pediatr Diabetes (2014) 15(8):543–9. doi: 10.1111/pedi.12159
79. Hecht Baldauff N, Tfayli H, Dong W, Arena VC, Gurtunca N, Pietropaolo M, et al. Relationship of adiponectin and leptin with autoimmunity in children with new-onset type 1 diabetes: a pilot study. Pediatr Diabetes (2016) 17(4):249–56. doi: 10.1111/pedi.12267
80. Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology (2018) 155(4):407–17. doi: 10.1111/imm.13002
81. Lopes M, Kutlu B, Miani M, Bang-Berthelsen CH, Størling J, Pociot F, et al. Temporal profiling of cytokine-induced genes in pancreaticβ-cells by meta-analysis and network inference. Genomics (2014) 103(4):264–75. doi: 10.1016/j.ygeno.2013.12.007
82. Marroqui L, Dos Santos RS, Op de Beeck A, Coomans de Brachène A, Marselli L, Marchetti P, et al. Interferon-α mediates human beta cell HLA class Ioverexpression, endoplasmic reticulum stress and apoptosis, three hallmarks of early human type 1diabetes. Diabetologia (2017) 60(4):656–67. doi: 10.1007/s00125-016-4201-3
83. Brooks-Worrell BM, Palmer JP. Setting the Stage for Islet Autoimmunity in Type 2 Diabetes:Obesity-Associated Chronic Systemic Inflammation and Endoplasmic Reticulum (ER) Stress. Diabetes Care (2019) 42(12):2338–46. doi: 10.2337/dc19-0475
84. Burke SJ, Collier JJ. Transcriptional regulation of chemokine genes: a link to pancreaticislet inflammation? Biomolecules (2015)5(2):1020–34. doi:10.3390/biom5021020
85. Redondo MJ, Evans-Molina C, Steck AK, Atkinson MA, Sosenko J. The Influence of Type 2 Diabetes-Associated Factors on Type 1Diabetes. Diabetes Care (2019) 42(8):1357–64. doi: 10.2337/dc19-0102
86. Redondo MJ, Muniz J, Rodriguez LM, Iyer D, Vaziri-Sani F, Haymond MW, et al. Association of TCF7L2 variation with single islet autoantibody expression in children with type 1 diabetes. BMJ Open Diabetes Res Care (2014) 2(1):e000008. doi: 10.1136/bmjdrc-2013-000008
87. Redondo MJ, Geyer S, Steck AK, Sosenko J, Anderson M, Antinozzi P, et al. TCF7L2 Genetic Variants Contribute to Phenotypic Heterogeneity of Type 1 Diabetes. Diabetes Care (2018) 41(2):311–7. doi: 10.2337/dc17-0961
88. Redondo MJ, Grant SF, Davis A, Greenbaum C, Biobank TDE. Dissecting heterogeneity in paediatric Type 1 diabetes: association of TCF7L2 rs7903146 TT and low-risk human leukocyte antigen (HLA) genotypes. Diabetes Med (2017) 34(2):286–90. doi: 10.1111/dme.13123
89. Redondo MJ, Steck AK, Sosenko J, Anderson M, Antinozzi P, Michels A, et al. Transcription Factor 7-Like 2 (TCF7L2) Gene Polymorphism and Progression From Single to Multiple Autoantibody Positivity in Individuals at Risk for Type 1 Diabetes. Diabetes Care (2018) 41(12):2480–6. doi: 10.2337/dc18-0861
90. Sosenko JM, Skyler JS, Palmer JP, Diabetes Type T. Diabetes Prevention Trial-Type 1 Study G. The development, validation, and utility of the Diabetes Prevention Trial-Type 1 Risk Score (DPTRS). Curr Diabetes Rep (2015) 15(8):49. doi: 10.1007/s11892-015-0626-1
91. Barrett-Connor E, Orchard TJ. Insulin-dependent diabetes mellitus and ischemic heartdisease. Diabetes Care (1985) 8(Suppl1):65–70. doi: 10.2337/diacare.8.1.s65
92. Erbey JR, Kuller LH, Becker DJ, Orchard TJ. The association between a family history of type 2 diabetes andcoronary artery disease in a type 1 diabetes population. Diabetes Care (1998) 21(4):610–4. doi: 10.2337/diacare.21.4.610
93. Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1diabetes? Diabetes (2000) 49(4):626–32. doi: 10.2337/diabetes.49.4.626
94. Orchard TJ, Chang YF, Ferrell RE, Petro N, Ellis DE. Nephropathy in type 1 diabetes: a manifestation of insulinresistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh Epidemiologyof Diabetes Complication Study. Kidney Int (2002) 62(3):963–70. doi: 10.1046/j.1523-1755.2002.00507.x
95. Olson JC, Erbey JR, Forrest KY, Williams K, Becker DJ, Orchard TJ. Glycemia (or, in women, estimated glucose disposal rate) predictlower extremity arterial disease events in type 1 diabetes. Metabolism (2002) 51(2):248–54. doi: 10.1053/meta.2002.30021
96. Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care (2003) 26(5):1374–9. doi: 10.2337/diacare.26.5.1374
97. Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care (2007) 30(3):707–12. doi: 10.2337/dc06-1982
98. Schauer IE, Snell-Bergeon JK, Bergman BC, Maahs DM, Kretowski A, Eckel RH, et al. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: The CACTI study. Diabetes (2011) 60(1):306–14. doi: 10.2337/db10-0328
99. Cree-Green M, Stuppy JJ, Thurston J, Bergman BC, Coe GV, Baumgartner AD, et al. Youth With Type 1 Diabetes Have Adipose, Hepatic, and Peripheral Insulin Resistance. J Clin Endocrinol Metab (2018) 103(10):3647–57. doi: 10.1210/jc.2018-00433
100. Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab (2010) 95(2):513–21. doi: 10.1210/jc.2009-1756
101. Specht BJ, Wadwa RP, Snell-Bergeon JK, Nadeau KJ, Bishop FK, Maahs DM. Estimated insulin sensitivity and cardiovascular disease riskfactors in adolescents with and without type 1 diabetes. J Pediatr (2013) 162(2):297–301. doi: 10.1016/j.jpeds.2012.07.036
102. Shah AS, Black S, Wadwa RP, Schmiege SJ, Fino NF, Talton JW, et al. Insulin sensitivity and arterial stiffness in youth with type 1diabetes: the SEARCH CVD study. J Diabetes Complications (2015) 29(4):512–6. doi: 10.1016/j.jdiacomp.2015.02.004
103. Merger SR, Kerner W, Stadler M, Zeyfang A, Jehle P, Muller-Korbsch M, et al. Prevalence and comorbidities of double diabetes. Diabetes Res Clin Pract (2016) 119:48–56. doi: 10.1016/j.diabres.2016.06.003
104. Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control andComplications Trial. JAMA (1998) 280(2):140–6. doi: 10.1001/jama.280.2.140
105. Purnell JQ, Zinman B, Brunzell JD, Group DER. The effect of excess weight gain with intensive diabetes mellitustreatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus:results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation (2013) 127(2):180–7. doi: 10.1161/CIRCULATIONAHA.111.077487
106. Rodrigues TC, Veyna AM, Haarhues MD, Kinney GL, Rewers M, Snell-Bergeon JK. Obesity and coronary artery calcium in diabetes: the Coronary ArteryCalcification in Type 1 Diabetes (CACTI) study. Diabetes Technol Ther (2011) 13(10):991–6. doi: 10.1089/dia.2011.0046
107. Pettus JH, Zhou FL, Shepherd L, Mercaldi K, Preblick R, Hunt PR, et al. Differences between patients with type 1 diabetes with optimal andsuboptimal glycaemic control: A real-world study of more than 30 000 patients in a US electronichealth record database. Diabetes Obes Metab (2020) 22(4):622–30. doi: 10.1111/dom.13937
108. Conway B, Miller RG, Costacou T, Fried L, Kelsey S, Evans RW, et al. Adiposity and mortality in type 1 diabetes. Int J Obes (Lond) (2009) 33(7):796–805. doi: 10.1038/ijo.2009.75
109. Purnell JQ, Braffett BH, Zinman B, Gubitosi-Klug RA, Sivitz W, Bantle JP, et al. Impact of Excessive Weight Gain on Cardiovascular Outcomes in Type 1Diabetes: Results From the Diabetes Control and Complications Trial/Epidemiology of DiabetesInterventions and Complications (DCCT/EDIC) Study. Diabetes Care (2017) 40(12):1756–62. doi: 10.2337/dc16-2523
110. Redondo MJ, Foster NC, Libman IM, Mehta SN, Hathway JM, Bethin KE, et al. Prevalence of cardiovascular risk factors in youth with type 1diabetes and elevated body mass index. Acta Diabetol (2016) 53(2):271–7. doi: 10.1007/s00592-015-0785-1
111. van Vliet M, Van der Heyden JC, Diamant M, Von Rosenstiel IA, Schindhelm RK, Aanstoot HJ, et al. Overweight is highly prevalent in children with type 1 diabetes and associates with cardiometabolic risk. J Pediatr (2010) 156(6):923–9. doi: 10.1016/j.jpeds.2009.12.017
112. Kahkoska AR, Nguyen CT, Adair LA, Aiello AE, Burger KS, Buse JB, et al. Longitudinal Phenotypes of Type 1 Diabetes in Youth Based on Weight and Glycemia and Their Association With Complications. J Clin Endocrinol Metab (2019) 104(12):6003–16. doi: 10.1210/jc.2019-00734
113. Libman I, Sampene E, Arena VC, Becker D, Arslanian S. Clinical Measures to Predict In Vivo Insulin Sensitivity (IS) inYouth with Type 1 Diabetes (T1D). Diabetes (2014) 63(Suppl 1):A1–102. doi: 10.2337/db14-1-388
114. Wood JR, Miller KM, Maahs DM, Beck RW, DiMeglio LA, Libman IM, et al. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care (2013) 36(7):2035–7. doi: 10.2337/dc12-1959
115. Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, et al. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther (2019) 21(2):66–72. doi: 10.1089/dia.2018.0384
116. Kietsiriroje N, Pearson S, Campbell M, Ariens RAS, Ajjan RA. Double diabetes: A distinct high-risk group? Diabetes Obes Metab (2019) 21(12):2609–18. doi: 10.1111/dom.13848
117. Nadeau KJ, Chow K, Alam S, Lindquist K, Campbell S, McFann K, et al. Effects of low dose metformin in adolescents with type I diabetesmellitus: a randomized, double-blinded placebo-controlled study. PediatrDiabetes (2015) 16(3):196–203. doi: 10.1111/pedi.12140
118. Nwosu BU, Maranda L, Cullen K, Greenman L, Fleshman J, McShea N, et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Adjunctive Metformin Therapy in Overweight/Obese Youth with Type 1 Diabetes. PloS One (2015) 10(9):e0137525. doi: 10.1371/journal.pone.0137525
119. Libman IM, Miller KM, DiMeglio LA, Bethin KE, Katz ML, Shah A, et al. Effect of Metformin Added to Insulin on Glycemic Control AmongOverweight/Obese Adolescents With Type 1 Diabetes: A Randomized Clinical Trial. JAMA (2015) 314(21):2241–50. doi: 10.1001/jama.2015.16174
120. Hamilton J, Cummings E, Zdravkovic V, Finegood D, Daneman D. Metformin as an adjunct therapy in adolescents with type 1 diabetes and insulin resistance: a randomized controlled trial. Diabetes Care (2003) 26(1):138–43. doi: 10.2337/diacare.26.1.138
121. Urakami T, Morimoto S, Owada M, Harada K. Usefulness of the addition of metformin to insulin in pediatric patients with type 1 diabetes mellitus. Pediatr Int (2005) 47(4):430–3. doi: 10.1111/j.1442-200x.2005.02075.x
122. Petrie JR, Chaturvedi N, Ford I, Brouwers M, Greenlaw N, Tillin T, et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol (2017) 5(8):597–609. doi: 10.1016/S2213-8587(17)30194-8
123. Bjornstad P, Schafer M, Truong U, Cree-Green M, Pyle L, Baumgartner A, et al. Metformin Improves Insulin Sensitivity and Vascular Health in Youth With Type 1 Diabetes Mellitus. Circulation (2018) 138(25):2895–907. doi: 10.1161/CIRCULATIONAHA.118.035525
124. Wang W, Liu H, Xiao S, Liu S, Li X, Yu P. Effects of Insulin Plus Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RAs) in Treating Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Ther (2017) 8(4):727–38. doi: 10.1007/s13300-017-0282-3
125. Guo H, Fang C, Huang Y, Pei Y, Chen L, Hu J. The efficacy and safety of DPP4 inhibitors in patients with type 1 diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract (2016) 121:184–91. doi: 10.1016/j.diabres.2016.08.022
126. Wang Q, Long M, Qu H, Shen R, Zhang R, Xu J, et al. DPP-4 Inhibitors as Treatments for Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. J Diabetes Res (2018) 2018:5308582. doi: 10.1155/2018/5308582
127. Lu J, Tang L, Meng H, Zhao J, Liang Y. Effects of sodium-glucose cotransporter (SGLT) inhibitors in addition to insulin therapy on glucose control and safety outcomes in adults with type 1 diabetes: A meta-analysis of randomized controlled trials. Diabetes Metab Res Rev (2019) 35(7):e3169. doi: 10.1002/dmrr.3169
128. Biester T, Aschemeier B, Fath M, Frey M, Scheerer MF, Kordonouri O, et al. Effects of dapagliflozin on insulin-requirement, glucose excretion and ss-hydroxybutyrate levels are not related to baseline HbA1c in youth with type 1 diabetes. Diabetes Obes Metab (2017) 19(11):1635–9. doi: 10.1111/dom.12975
129. Mehta SN, Volkening LK, Anderson BJ, Nansel T, Weissberg-Benchell J, Wysocki T, et al. Dietary behaviors predict glycemic control in youth with type 1 diabetes. Diabetes Care (2008) 31(7):1318–20. doi: 10.2337/dc07-2435
130. Smart CE, Annan F, Higgins LA, Jelleryd E, Lopez M, Acerini CL. ISPAD Clinical Practice Consensus Guidelines 2018: Nutritional management in children and adolescents with diabetes. Pediatr Diabetes (2018) 19 Suppl 27:136–54. doi: 10.1111/pedi.12738
131. Mottalib A, Kasetty M, Mar JY, Elseaidy T, Ashrafzadeh S, Hamdy O. Weight Management in Patients with Type 1 Diabetes and Obesity. Curr Diabetes Rep (2017) 17(10):92. doi: 10.1007/s11892-017-0918-8
132. Bolla AM, Caretto A, Laurenzi A, Scavini M, Piemonti L. Low-Carb and Ketogenic Diets in Type 1 and Type 2 Diabetes. Nutrients (2019) 11(5):962. doi: 10.3390/nu11050962
133. de Bock M, Lobley K, Anderson D, Davis E, Donaghue K, Pappas M, et al. Endocrine and metabolic consequences due to restrictive carbohydrate diets in children with type 1 diabetes: An illustrative case series. Pediatr Diabetes (2018) 19(1):129–37. doi: 10.1111/pedi.12527
134. Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: anoverview. Clin Sci (Lond) (2012)122(6):253–70. doi: 10.1042/CS20110386
135. Gomez R, Mokhashi MH, Rao J, Vargas A, Compton T, McCarter R, et al. Metformin adjunctive therapy with insulin improves glycemic control in patients with type 1 diabetes mellitus: a pilot study. J Pediatr Endocrinol Metab (2002) 15(8):1147–51. doi: 10.1515/jpem.2002.15.8.1147
136. Sarnblad S, Kroon M, Aman J. Metformin as additional therapy in adolescents with poorly controlled type 1 diabetes: randomised placebo-controlled trial with aspects on insulin sensitivity. Eur J Endocrinol (2003) 149(4):323–9. doi: 10.1530/eje.0.1490323
137. Codner E, Iniguez G, Lopez P, Mujica V, Eyzaguirre FC, Asenjo S, et al. Metformin for the treatment of hyperandrogenism in adolescents with type 1 diabetes mellitus. Horm Res Paediatr (2013) 80(5):343–9. doi: 10.1159/000355513
138. Vella S, Buetow L, Royle P, Livingstone S, Colhoun HM, Petrie JR. The use of metformin in type 1 diabetes: a systematic review of efficacy. Diabetologia (2010) 53(5):809–20. doi: 10.1007/s00125-009-1636-9
139. Cree-Green M, Bergman BC, Cengiz E, Fox LA, Hannon TS, Miller K, et al. Metformin Improves Peripheral Insulin Sensitivity in Youth With Type 1 Diabetes. J Clin Endocrinol Metab (2019) 104(8):3265–78. doi: 10.1210/jc.2019-00129
140. American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2020. Diabetes Care (2020) 43(Suppl 1):S98–S110. doi: 10.2337/dc20-S009
141. Lyons SK, Hermann JM, Miller KM, Hofer SE, Foster NC, Rami-Merhar BM, et al. Use of Adjuvant Pharmacotherapy in Type 1 Diabetes: International Comparison of 49,996 Individuals in the Prospective Diabetes Follow-up and T1D Exchange Registries. Diabetes Care (2017) 40(10):e139–e40. doi: 10.2337/dc17-0403
142. Konrad K, Datz N, Engelsberger I, Grulich-Henn J, Hoertenhuber T, Knauth B, et al. Current use of metformin in addition to insulin in pediatric patients with type 1 diabetes mellitus: an analysis based on a large diabetes registry in Germany and Austria. Pediatr Diabetes (2015) 16(7):529–37. doi: 10.1111/pedi.12203
143. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev (2007) 87(4):1409–39. doi: 10.1152/physrev.00034.2006
144. Adams JM, Pei H, Sandoval DA, Seeley RJ, Chang RB, Liberles SD, et al. Liraglutide Modulates Appetite and Body Weight Through Glucagon-LikePeptide 1 Receptor-Expressing Glutamatergic Neurons. Diabetes (2018) 67(8):1538–48. doi: 10.2337/db17-1385
145. Drucker DJ. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab (2018) 27(4):740–56. doi: 10.1016/j.cmet.2018.03.001
146. Tamborlane WV, Barrientos-Perez M, Fainberg U, Frimer-Larsen H, Hafez M, Hale PM, et al. Liraglutide in Children and Adolescents with Type 2 Diabetes. N Engl J Med (2019) 381(7):637–46. doi: 10.1056/NEJMoa1903822
147. Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, et al. A Randomized, Controlled Trial of Liraglutide for Adolescents withObesity. N Engl J Med (2020) 382(22):2117–28. doi: 10.1056/NEJMoa1916038
148. Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med (1992) 326(20):1316–22. doi: 10.1056/NEJM199205143262003
149. Kuhadiya ND, Dhindsa S, Ghanim H, Mehta A, Makdissi A, Batra M, et al. Addition of Liraglutide to Insulin in Patients With Type 1 Diabetes: A Randomized Placebo-Controlled Clinical Trial of 12 Weeks. Diabetes Care (2016) 39(6):1027–35. doi: 10.2337/dc15-1136
150. Danne T, Garg S, Peters AL, Buse JB, Mathieu C, Pettus JH, et al. International Consensus on Risk Management of Diabetic Ketoacidosis in Patients With Type 1 Diabetes Treated With Sodium-Glucose Cotransporter (SGLT) Inhibitors. Diabetes Care (2019) 42(6):1147–54. doi: 10.2337/dc18-2316
151. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia (2018) 61(10):2108–17. doi: 10.1007/s00125-018-4670-7
152. Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest (2014) 124(2):509–14. doi: 10.1172/JCI70704
153. Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest (2014) 124(2):499–508. doi: 10.1172/JCI72227
Keywords: type 1 diabetes, obesity, nutrition, double diabetes, cardiovascular complications
Citation: March CA, Becker DJ and Libman IM (2021) Nutrition and Obesity in the Pathogenesis of Youth-Onset Type 1 Diabetes and Its Complications. Front. Endocrinol. 12:622901. doi: 10.3389/fendo.2021.622901
Received: 29 October 2020; Accepted: 15 February 2021;
Published: 22 March 2021.
Edited by:
Jeff M. P. Holly, University of Bristol, United KingdomReviewed by:
Andrea Vania, Sapienza University of Rome, ItalyCopyright © 2021 March, Becker and Libman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ingrid M. Libman, SW5ncmlkLmxpYm1hbkBjaHAuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.