- 1Department of Nephrology, The 960th Hospital of the PLA Joint Logistics Support Force, Jinan, China
- 2Department of Medical Imaging, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China
- 3Department of Medical Imaging, The 960th Hospital of the PLA Joint Logistics Support Force, Jinan, China
Objectives: To investigate the serum level of osteocalcin (OC), also known as bone Gla protein, in maintenance hemodialysis (MHD) patients and its correlation with abdominal aortic calcification (AAC).
Methods: From July 2017 to February 2020, we enrolled 108 adult MHD patients. Routine fasting blood laboratory tests were performed before the start of the second hemodialysis in a week. Abdominal aortic calcification score (AACs) was assessed within 1 month. Pearson correlation and Logistic regression were used to analyze the data.
Results: The OC level was 231.56 (25.92,361.33) ng/ml, elevating significantly in this group of MHD patients. It had a positive correlation with serum phosphorus (r = 0.511, P = 0.001), intact parathyroid hormone(iPTH) (r = 0.594, P = 0.0001), fibroblast growth factor 23(FGF23) (r = 0.485, P = 0.003) and a negative correlation with age(r = -0.356, P = 0.039). Based on the AACs, patients were divided into two groups. Serum OC level were higher in patients with AACs≥5 (p=0.032). A multiple logistics regression analysis revealed that age (odds ratio [OR]1.14, P=0.005) and OC(OR=1.10, P=0.008)were risk factors for high AACs(≥5).
Conclusion: The study implicated that OC elevated significantly in this group of MHD patients.OC is positively correlated with phosphorus, iPTH, FGF23, and a negative correlation with age. OC was a risk factor for vascular calcification in this study, but this study did not classify osteocalcin as c-OC and unOC. Whether unOC is associated more directly with vascular calcification requires further study.
Introduction
Chronic kidney disease–mineral and bone disorder (CKD–MBD) is a common complication in end-stage renal disease(ESRD). CKD–MBD is characterized by abnormal mineral markers, abnormal bone metabolism, and soft tissue calcification, such as vascular calcification (VC) (1). VC is the major cause of cardiovascular disease (CVD) in ESRD, including ischemic cardiac events and subsequent vascular damage. Cardiovascular calcification contributes to approximately 50% of all deaths in hemodialysis patients (2). In contrast, MHD patients without VC always have a better prognosis with limited progression of VC over a longer period of time (3).
Abdominal aortic calcification(AAC) is highly prevalent in the hemodialysis population (4). Kauppila et al. described an AAC grading quantification method by using lateral lumbar radiography in a Framingham study subgroup (5). They showed that this method was predictive of cardiovascular events and mortality (6). Kidney Disease Improving Global Outcomes (KDIGO) in their international clinical guideline for the management of CKD-MBD suggested that lateral lumbar radiography should be used as an alternative to computerized tomography to assess VC (1).
The pathophysiologic mechanisms underlying the process of VC in ESRD have not yet been fully elucidated. VC has been associated with numerous traditional cardiovascular risk factors, including advanced age, hypertension, diabetes, and dyslipidemia, as well as with nontraditional cardiovascular risk factors, such as hyperphosphatemia, hyperparathyroidism, and excessive calcium intake. Today VC in ESRD is considered as an active, complex process, where the first, crucial step toward calcification is the transformation of vascular smooth muscle cells (VSMCs) to osteoblast phenotype, a process similar to bone formation (7).
Osteocalcin (OC), also known as bone Gla protein, a highly specific marker involved in bone formation, is the most abundant non-collagenous peptide found in the mineralized matrix of bone, concomitantly being an intriguing hormone and expanding the endocrine function of the skeleton with far-reaching extra-osseous effects. OC is produced primarily by osteoblasts in bone, it is also produced locally by VSMCs. The detection of OC in circulation raised the question whether it is involved in the process of arterial mineralization. The carboxylation pathway, vitamin K mediated, is pivotal for the transformation of OC from the undercarboxylated form (ucOC) into the fully functional carboxylated form (c-OC). CKD patients often show a subclinical vitamin K deficiency (8). Schurgers et al. evaluated inactive, dephosphorylated, uncarboxylated OC(dp-ucOC) levels in a cohort of 107 patients whose kidney function varied from CKD stages 2–5D. They reported that levels of plasma dp-ucOC increased progressively with CKD worsening (9).
Aim of the Study
This study aimed to explore the relationship between the serum OC level and other indices of VC and to determine whether serum OC level is the risk factor affecting VC in MHD patients with CKD–MBD.
Materials and Methods
The study protocol was approved by the ethics committee of 960th Hospital of the PLA Joint Logistics Support Force (protocol number: 20140214). The study conforms to the principles outlined in the Declaration of Helsinki, and written consent was obtained from all patients before the enrollment.
Study Design and Subjects
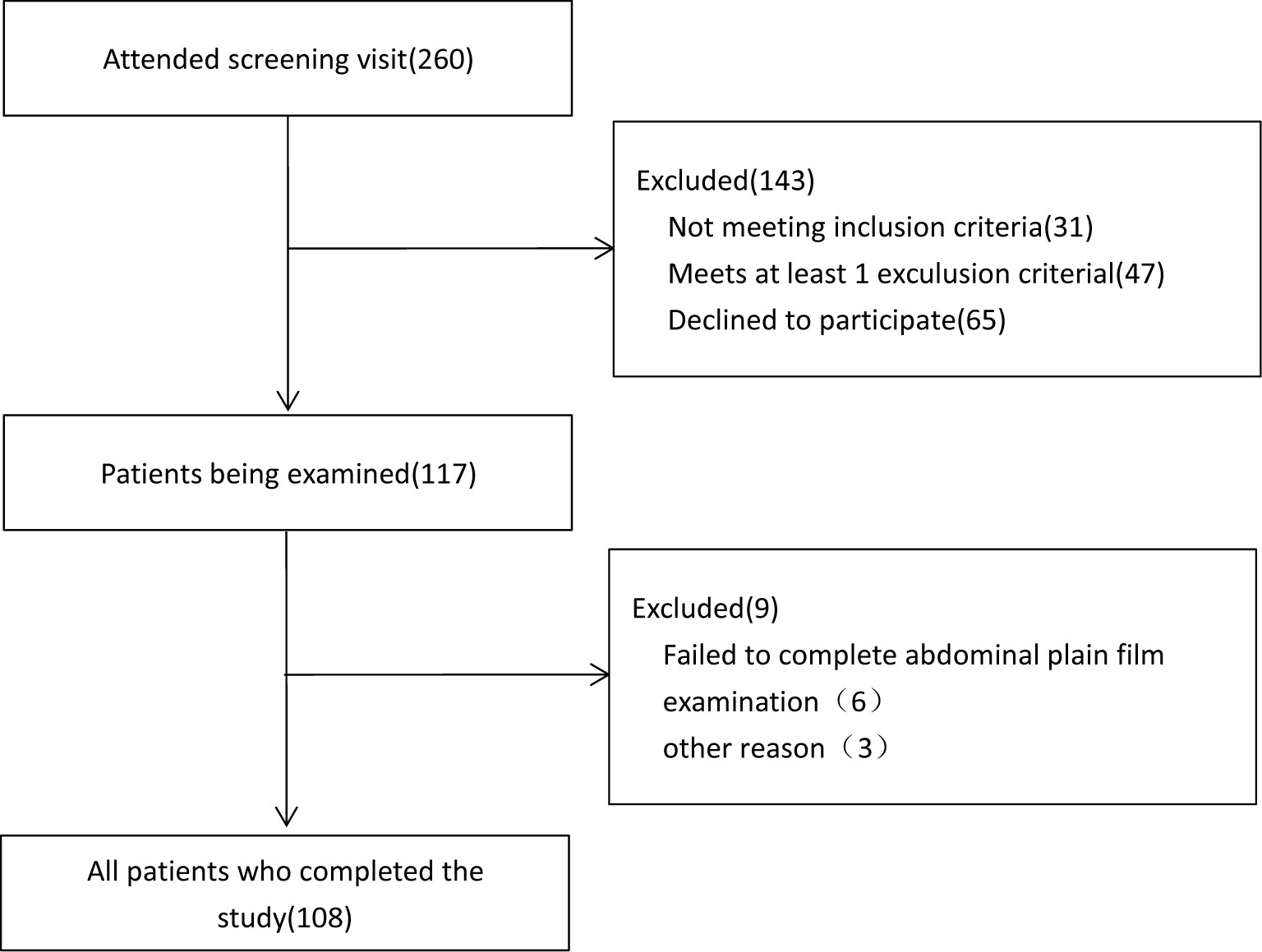
We performed an observational cross-sectional study on 108 clinically stable MHD patients, enrolled from July 2017 to February 2020, at the blood purification center of the 960th Hospital of the PLA Joint Logistics Support Force. They were screened continuously from 260 patients in our department. All of the 108 subjects completed the study (Figure 1). The study included 66 men and 42 women (age: 24–77 years), and the average age was 55.1 ± 13.6 years. The primary disease was chronic glomerulonephritis in 45 cases (41.7%), IgA nephritis and hypertensive renal damage in 12 cases each (11.1%), diabetes mellitus (DM) (13.9%) in 15 cases, polycystic kidney (5.6%) in 6 cases, and uric acid nephropathy (2.7%) in 3 cases. There were 15 cases (13.9%) with unknown causes.
The inclusion criteria were as follows: (1) age not less than 18 years, (2) dialysis vintage for more than 3 months, (3) patient or legally accepted representative willing to sign Data Release Consent Form. The exclusion criteria were as follows: (1) patients’ life expectancy <6 months. (2) patients with acute kidney injury, active inflammatory diseases, parathyroidectomy, evident malignancies, and abnormal liver function, (3) concomitant diseases that affect calcium status and soft tissue calcifications (sarcoidosis, multiple myeloma, HIV, amyloidosis),(4)patients on anticoagulants such as warfarin.
Methods
The dialysis regimen for all MHD patients was thrice a week, 4 h per session. The dialysate calcium concentration was 1.5 mmol/L, blood flow was 200–260 mL/min, and dialysate flow was 500 mL/min. The recommended protein intake ranged from 1 to 1.2 g/kg per day. The drugs dose administered for regulating calcium, phosphorus, and intact parathyroid hormone (iPTH) was as per the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder Guideline Update (10). Clinical and instrumental examinations were performed during the middle of the week, before the HD session. All blood samples were collected before the start of the second hemodialysis in a week in the morning after an overnight fast. Intact PTH were measured by electrochemiluminescence on a Cobas E601 C analyzer (Roche Diagnostics, Indianapolis, IN). Enzyme-linked immunosorbent assay (ELISA)was used for the detection of FGF23(EMD Millipore Corporation, Milford, MA, USA). Inorganic phosphorus (by Advia 1800, Siemens), and total calcium (by calorimetric method with Arsenazo, Advia 1800, Siemens) concentrations were measured in serum. Corrected calcium (mg/dL) was calculated as serum calcium (mg/dL) + 0.8 × [4 − serum albumin (g/dL)].
The complete osteocalcin (amino acid 1-49) is unstable in peripheral blood. The amino acid between 43-44 carboxyl end is easily hydrolyzed by protease, and the N-mid fragment is much more stable. In this study, the stable N-mid and intact osteocalcin in serum were detected by the kit (ECLIA Kit, Roche Diagnostics GmbH, Sandhofer strasse, Mannbeim, Germany). This result reflects the value of total osteocalcin in serum. It should be noted that this test does not distinguish between c-OC and unOC. A detailed test method can be found in the Supplementary Materials.
Abdominal aortic calcification (AAC) is assessed within 1 month of admission (11) by semiquantitative scoring of a plain lateral lumbar radiograph using previously validated 24-point aortic calcification scale (5). The scores, obtained separately for the anterior and posterill, result in a range from 0 to 6 for each vertebral level and 0 to 24 for the total AAC score (AACs). AACs was evaluated by one of the authors who was blinded to the other patient data.
Statistical analysis
Data management and analysis were performed using SPSS Statistics 17.0 and a P-value of < 0.05 was considered statistically significant. All continuous variables that follow a normal distribution were expressed as mean ( ± standard deviation), and variables without normal distribution were expressed as median (interquartile range). Categorical variables were expressed as number (percentage). Student’s t test or Mann–Whitney U test (2-tailed) were performed to determine the between-group differences. The chi-squared test was used to compare categorical variables. Pearson correlation was used to determine the correlation, bivariate relationship, and strength of association among variables. A multiple logistics regression analysis was performed to investigate the determinants of AAC.
Results
The Characteristics of OC Level
The OC level of all 108 patients was 231.56 (25.92,361.33) ng/ml. It was higher than the normal range provided by our laboratory(Table 1).
Comparison of Patients With Different AACs Levels
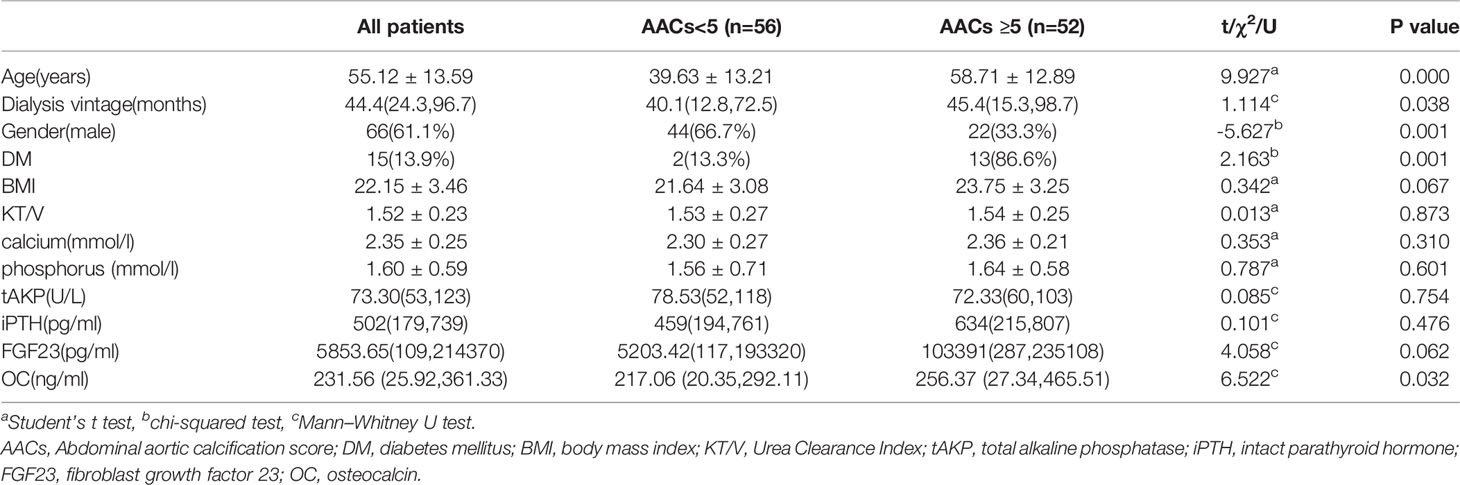
AACs scores higher than 5 were reported to have predictive value for cardiovascular disease in hemodialysis patients (12, 13). Based on this, all 108 MHD patients were divided into low AACs(AACs <5), and high AACs(AACs≥5) groups (13). The age, dialysis vintage, gender, DM and serum OC were significantly different among the groups, while there was no significant difference among the groups in BMI, KT/V, serum calcium, tAKP, iPTH and FGF23 (Table 2).
OC Level and Correlation Analysis With Laboratory Indicators
Pearson correlation analysis revealed that OC was positively correlated with serum phosphorus (r = 0.511, P = 0.001), iPTH (r = 0.594, P = 0.0001), FGF23 (r = 0.485, P = 0.003), AACs (r = 0.413, P = 0.0201), and a negative correlation with age (r = –0.356, P = 0.039). There was no correlation of OC with serum calcium (r = 0.003, P = 0.977), total alkaline phosphatase (r = 0.171, P = 0.369), and dialysis vintage (r = 0.086, P = 0.565).
Risk Factors for Vascular Calcification
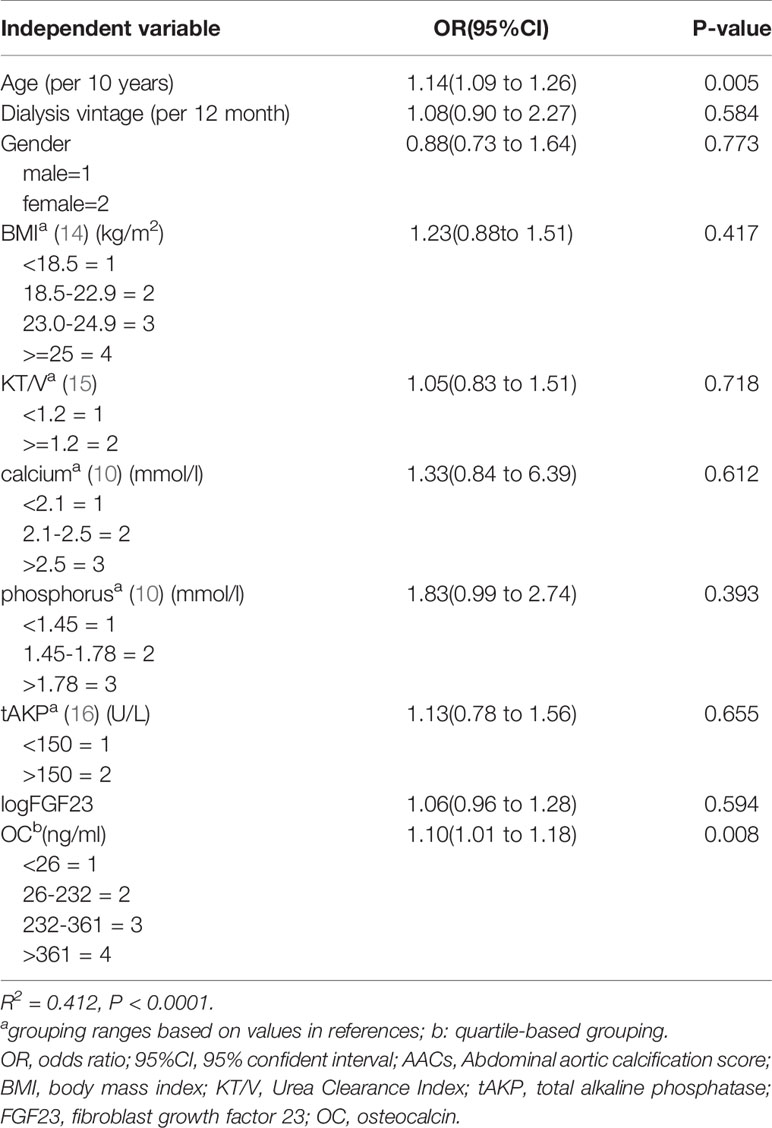
Based on the level of AACs patients were divided into 2 groups, high AACs group(AACs≥5) and low AACs group(AACs <5). In the process of logistic regression, we grouped some variables. The assignment method of grouping variables was shown in Table 3, based mainly on published literature. Considering that age and DM, OC and PTH have a high degree of multicollinearity, when we removed DM and PTH from the model, R2 remained almost unchanged, we removed DM and PTH from the variables. Finally, the multiple logistics regression analysis revealed that age (OR = 1.14, P = 0.005) and OC (OR = 1.10, P = 0.008) are risk factors for high AACs (R2 = 0.412, P<0.0001) (Table 3).
Discussion
MHD patients with CKD-MBD experience severe mixed intimal and medial vascular calcification, which contributes to the increased cardiovascular mortality and morbidity (2). VC is an active, complex process. At present, there is no good index to reflect the pathophysiological mechanism of vascular calcification, and guide clinical diagnosis and treatment. The classical markers, such as serum calcium (17), serum phosphorus (18), PTH (18), AKP (19) and FGF23 (20), are not specific and sensitive enough to reflect the pathophysiologic mechanisms underlying the process of VC (21). A review focuses on the pathophysiological mechanisms of vascular and valvular calcification in general population, list OC as one of the promoters in a table. In the body of this review, they emphasized that the circulating inactive, unOC reflect vitamin K deficiency and are markers of enhanced VC, especially in diabetic and CKD patients (22).
In vitro experiments, high OC level has been shown to stimulate VSMCs differentiation and mineralization in experimental model and OC has been found in calcified atherosclerotic plaque and calcified aortic valve (23). N-mid OC was positively associated with lower leg arterial calcification in advanced chronic kidney disease (24). In a recent study convincing evidences have been provided that OC may be implicated in the endothelial damage-related VC in patients with advanced chronic kidney disease via increasing the number of OC-positive endothelial progenitor cells (25). Giuseppe Cianciolo reported that VDR agonist therapy played a putative protective role to vascular calcification via decreased OC expression on circulating endothelial progenitor cells (26).
However, other studies in dialysis patients found no relationship between unOC and VC (27). Eiji Ishimura et al. found intact OC had no association with the presence of vascular calcification of the hand arteries of male hemodialysis patients (28). In contrast, studies in elderly men and hemodialysis patients showed that higher serum total OC levels were associated with lower AAC progression rate and lower 10-year all-cause mortality, suggesting a protective effect of OC in VC (29). Someone found an association between DM and decreased total OC and unOC levels in MHD patients, suggesting a potential protective role of OC in the bone, endocrine and vascular pathway (30).
Accurately, the level of OC in serum is influenced by many factors. Serum OC levels decreased with increasing age and increased with female sex in a general adult Danish population (31). PTH can promote osteoblasts to synthesize and secrete OC. PTH conjugates with the osteoblast PTH receptor on the cell membrane to regulate OC expression in order to increase bone formation, accelerate bone turnover, and increase the OC level in serum (32). FGF23 can upregulate OC lever via the JAK/STAT signaling pathway in vitro study (33). In patients with impaired renal function plasma OC levels are markedly elevated due to increased bone turnover and decreased renal elimination (34). Extracorporeal experiments also show that uremic serum itself promotes OC production (7).
As far as our study is concerned, OC elevated significantly in this group of MHD patients, which is consistent with previous research (35, 36). OC is positively correlated with phosphorus, iPTH, FGF23 and AACs, but the multiple logistics regression analysis revealed that only age and OC(OR=1.10, P=0.008)were risk factors for high AACs (≥5). There have been studies in dialysis patients showed increasing VC severity with age, regardless of factors such as dialysis vintage, dyslipidemia, PTH level and hypertension (21). It can be said that the effect of age on vascular calcification is well recognized. However, there are often conflicting conclusions about the relationship between other classical markers and vascular calcification (37, 38). This suggesting that the biochemical markers, vascular biomarkers and bone metabolism probably contribute to VC in a complex interconnected process. Pierre Delanaye et al. reported that a concordance between ΔPTH and Δbone biomarkers is observed in dialysis patients, but only after a long follow-up (39). For this reason, the revised 2017 KDIGO guidelines suggest that potential CKD-MBD therapies should be based on serial assessments of biomarkers, and thus on trends or variations (Δ), more than on one-single transversal result. Thus our study did not find a correlation between some classic indicators and VC, which may be related to the fact that this is only a cross-sectional study. Future research may have to focus on long-term follow-up study and multi interventional approach to prevent VC in CKD.
It’s important to note that total OC may not be a valuable measurement for risk of vascular calcification. It is reported that c-OC is the biologically active form. The hypothesis is that the functional vitamin K deficiency in hemodialysis patients with decreased c-OC is the trigger of calcification and not “unOC triggers calcification”. However, there has been difficulty in measuring ucOC and c-OC as few assays exist and it is unclear which assay system provides the most accurate measurements due to problems with comparability and heterogeneity of OC (40). Compared to healthy subjects, hemodialysis patients presented significantly reduced serum levels of active OC and increased serum levels of unOC. In a previous study which enrolled 189 hemodialysis patients together with 89 pre-dialysis CKD patients, the serum ucOC/intact OC ratio > 1.0 was observed in about 71.4% of hemodialysis patients especially those with high bone turnover (41). OC also displays a circadian rhythmicity with a nocturnal peak and thus timing of blood sampling may also contribute to variations in results (42). These factors all have an impact on the interpretation of the results.
It should be noted that serum OC levels and OC positive cells in the blood are completely different concepts and cannot be confused in the pathophysiological mechanisms of vascular calcification. Our study only discussed the correlation between serum OC levels and abdominal aortic calcification.
The current study is limited by the small number of patients, being single-center study. Vitamin K deficiency was not evaluated, The effect of external vitamin D supplementation was not assessed, either. The study was a cross-sectional observational studies, thus the cause–effect relationship cannot be concluded from the results.
In conclusion, our study shows that serum OC lever is associated with abnormal mineral parameters of CKD-MBD, OC was a risk factor for vascular calcification in this study, but this study did not classify osteocalcin as c-OC and unOC. Whether unOC is associated more directly with vascular calcification requires further study.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of 960th Hospital of the PLA Joint Logistics Support Force (protocol number: 20140214). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.620350/full#supplementary-material
References
1. KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl (2009) 113):S1–130. doi: 10.1038/ki.2009.188
2. Ruderman I, Holt SG, Hewitson TD, Smith ER, Toussaint ND. Current and Potential Therapeutic Strategies for the Management of Vascular Calcification in Patients With Chronic Kidney Disease Including Those on Dialysis. Semin Dial (2018) 31(5):487–99. doi: 10.1111/sdi.12710
3. Matsuoka M, Iseki K, Tamashiro M, Fujimoto N, Higa N, Touma T, et al. Impact of High Coronary Artery Calcification Score (CACS) on Survival in Patients on Chronic Hemodialysis. Clin Exp Nephrol (2004) 8(1):54–8. doi: 10.1007/s10157-003-0260-0
4. Biyik Z, Selcuk NY, Tonbul HZ, Anil M, Uyar M. Assessment of Abdominal Aortic Calcification At Different Stages of Chronic Kidney Disease. Int Urol Nephrol (2016) 48(12):2061–8. doi: 10.1007/s11255-016-1413-x
5. Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New Indices to Classify Location, Severity and Progression of Calcific Lesions in the Abdominal Aorta: a 25-Year Follow-Up Study. Atherosclerosis (1997) 132(2):245–50. doi: 10.1016/S0021-9150(97)00106-8
6. Wilson PW, Kauppila LI, O–Donnell CJ, Kiel DP, Hannan M, Polak JM, et al. Abdominal Aortic Calcific Deposits Are an Important Predictor of Vascular Morbidity and Mortality. Circulation (2001) 103(11):1529–34. O doi: 10.1161/01.CIR.103.11.1529
7. Ciceri P, Galassi A, Alfieri C, Messa P, Cozzolino M. Uremic Patients With Increased Vascular Calcification Score Have Serum With High Calcific Potential: Role of Vascular Smooth Muscle Cell Osteoblastic Differentiation and Apoptosis. Blood Purif (2019) 48(2):142–9. doi: 10.1159/000497229
8. Cozzolino M, Fusaro M, Ciceri P, Gasperoni L, Cianciolo G. @ the Role of Vitamin K in Vascular Calcification. Adv Chronic Kidney Dis (2019) 26(6):437–44. doi: 10.1053/j.ackd.2019.10.005
9. Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, et al. the Circulating Inactive Form of Matrix Gla Protein Is a Surrogate Marker for Vascular Calcification in Chronic Kidney Disease: a Preliminary Report. Clin J Am Soc Nephrol (2010) 5(4):568–75. doi: 10.2215/CJN.07081009
10. Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, et al. Executive Summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: What’s Changed and Why It Matters. Kidney Int (2017) 92(1):26–36. doi: 10.1016/j.kint.2017.04.006
11. Liu ZH. Vascular Calcification Burden of Chinese Patients With Chronic Kidney Disease: Methodology of a Cohort Study. BMC Nephrol (2015) 16:129. doi: 10.1186/s12882-015-0132-3
12. Chen HC, Wang WT, Hsi CN, Chou CY, Lin HJ, Huang CC, et al. Abdominal Aortic Calcification Score Can Predict Future Coronary Artery Disease in Hemodialysis Patients: a 5-Year Prospective Cohort Study. BMC Nephrol (2018) 19(1):313. doi: 10.1186/s12882-018-1124-x
13. An WS, Lee SM, Park TH, Kim SE, Kim KH, Park YJ, et al. Association Between Diastolic Dysfunction by Color Tissue Doppler Imaging and Vascular Calcification on Plain Radiographs in Dialysis Patients. Kidney Blood Press Res (2012) 35(6):619–26. doi: 10.1159/000339646
14. Li T, Liu J, an S, Dai Y, Yu Q. Body Mass Index and Mortality in Patients on Maintenance Hemodialysis: a Meta-Analysis. Int Urol Nephrol (2014) 46(3):623–31. doi: 10.1007/s11255-014-0653-x
15. National Kidney Foundation. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 Update. Am J Kidney Dis (2015) 66(5):884–930. doi: 10.1053/j.ajkd.2015.07.015
16. Chang JF, Feng YF, Peng YS, Hsu SP, Pai MF, Chen HY, et al. Combined Alkaline Phosphatase and Phosphorus Levels as a Predictor of Mortality in Maintenance Hemodialysis Patients. Med (Baltimore) (2014) 93(18):E106. doi: 10.1097/MD.0000000000000106
17. Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, et al. Effects of Phosphate Binders in Moderate CKD. J Am Soc Nephrol (2012) 23(8):1407–15. doi: 10.1681/ASN.2012030223
18. Carrillo-López N, Panizo S, Alonso-Montes C, Martínez-Arias L, Avello N, Sosa P, et al. High-Serum Phosphate and Parathyroid Hormone Distinctly Regulate Bone Loss and Vascular Calcification in Experimental Chronic Kidney Disease. Nephrol Dial Transplant (2019) 34(6):934–41. doi: 10.1093/ndt/gfy287
19. Kim DW, Hwang SY, Nam YJ, Kim D, Shin SJ, Yoon HE. @ the Combined Prognostic Significance of Alkaline Phosphatase and Vascular Calcification in Patients With End-Stage Kidney Disease. Nutr Metab Cardiovasc Dis (2020) 30(9):1476–83. doi: 10.1016/j.numecd.2020.04.029
20. Desjardins L, Liabeuf S, Renard C, Lenglet A, Lemke HD, Choukroun G, et al. FGF23 Is Independently Associated With Vascular Calcification But Not Bone Mineral Density in Patients At Various CKD Stages. Osteoporos Int (2012) 23(7):2017–25. doi: 10.1007/s00198-011-1838-0
21. Nitta K, Hanafusa N, Okazaki M, Komatsu M, Kawaguchi H, Tsuchiya K. Association Between Risk Factors Including Bone-Derived Biomarkers and Aortic Arch Calcification in Maintenance Hemodialysis Patients. Kidney Blood Press Res (2018) 43(5):1554–62. doi: 10.1159/000494441
22. Roumeliotis S, Roumeliotis A, Dounousi E, Eleftheriadis T, Liakopoulos V. Biomarkers of Vascular Calcification in Serum. Adv Clin Chem (2020) 98:91–147. doi: 10.1016/bs.acc.2020.02.004
23. Idelevich A, Rais Y, Monsonego-Ornan E. Bone Gla Protein Increases HIF-1alpha-Dependent Glucose Metabolism and Induces Cartilage and Vascular Calcification. Arterioscler Thromb Vasc Biol (2011) 31(9):E55–71. doi: 10.1161/ATVBAHA.111.230904
24. Salam S, Gallagher O, Gossiel F, Paggiosi M, Eastell R, Khwaja A. Vascular Calcification Relationship to Vascular Biomarkers and Bone Metabolism in Advanced Chronic Kidney Disease. Bone (2021) 143:115699. doi: 10.1016/j.bone.2020.115699
25. Soriano S, Carmona A, Triviño F, Rodriguez M, Alvarez-Benito M, Martín-Malo A, et al. Endothelial Damage and Vascular Calcification in Patients With Chronic Kidney Disease. Am J Physiol Renal Physiol (2014) 307(11):F1302–11. doi: 10.1152/ajprenal.00114.2014
26. Cianciolo G, La Manna G, Della Bella E, Cappuccilli ML, Angelini ML, Dormi A, et al. Effect of Vitamin D Receptor Activator Therapy on Vitamin D Receptor and Osteocalcin Expression in Circulating Endothelial Progenitor Cells of Hemodialysis Patients. Blood Purif (2013) 35(1-3):187–95. doi: 10.1159/000347102
27. Millar SA, Patel H, Anderson SI, England TJ, Sullivan SE. Osteocalcin, Vascular Calcification, and Atherosclerosis: A Systematic Review and Meta-analysis. Front Endocrinol (Lausanne) (2017) 8:183. doi: 10.3389/fendo.2017.00183
28. Ishimura E, Okuno S, Okazaki H, Norimine K, Yamakawa K, Yamakawa T, et al. Significant Association Between Bone-Specific Alkaline Phosphatase and Vascular Calcification of the Hand Arteries in Male Hemodialysis Patients. Kidney Blood Press Res (2014) 39(4):299–307. doi: 10.1159/000355807
29. Confavreux CB, Szulc P, Casey R, Boutroy S, Varennes A, Vilayphiou N, et al. Higher Serum Osteocalcin Is Associated With Lower Abdominal Aortic Calcification Progression and Longer 10-Year Survival in Elderly Men of the MINOS Cohort. J Clin Endocrinol Metab (2013) 98(3):1084–92. doi: 10.1210/jc.2012-3426
30. Fusaro M, Gallieni M, Aghi A, Rizzo MA, Iervasi G, Nickolas TL, et al. Osteocalcin (Bone GLA Protein) Levels, Vascular Calcifications, Vertebral Fractures and Mortality in Hemodialysis Patients With Diabetes Mellitus. J Nephrol (2019) 32(4):635–43. doi: 10.1007/s40620-019-00595-1
31. Diemar SS, Møllehave LT, Quardon N, Lylloff L, Thuesen BH, Linneberg A, et al. Effects of Age and Sex on Osteocalcin and Bone-Specific Alkaline Phosphatase-Reference Intervals and Confounders for Two Bone Formation Markers. Arch Osteoporos (2020) 15(1):26. doi: 10.1007/s11657-020-00715-6
32. Weng SJ, Yan DY, Gu LJ, Chen L, Xie ZJ, Wu ZY, et al. Combined treatment with vitamin K2 and PTH enhanced bone formation in ovariectomized rats and increased differentiation of osteoblast in vitro. Chem Biol Interact (2019) 300:101–10. doi: 10.1016/j.cbi.2019.01.012
33. Xu L, Zhang L, Zhang H, Yang Z, Qi L, Wang Y, et al. the Participation of Fibroblast Growth Factor 23 (FGF23) in the Progression of Osteoporosis Via JAK/STAT Pathway. J Cell Biochem (2018) 119(5):3819–28. doi: 10.1002/jcb.26332
34. Lenora J, Ivaska KK, Obrant KJ, Gerdhem P. Prediction of Bone Loss Using Biochemical Markers of Bone Turnover. Osteoporos Int (2007) 18(9):1297–305. doi: 10.1007/s00198-007-0379-z
35. Cai H, Lu R, Zhang M, Pang H, Zhu M, Zhang W, et al. Serum Soluble Klotho Level Is Associated With Abdominal Aortic Calcification in Patients on Maintenance Hemodialysis. Blood Purif (2015) 40(2):120–6. doi: 10.1159/000381937
36. Csiky B, Sági B, Peti A, Lakatos O, Prémusz V, Sulyok E. @ the Impact of Osteocalcin, Osteoprotegerin and Osteopontin on Arterial Stiffness in Chronic Renal Failure Patients on Hemodialysis. Kidney Blood Press Res (2017) 42(6):1312–21. doi: 10.1159/000486114
37. Yamada S, Giachelli CM. Vascular Calcification in CKD-MBD: Roles for Phosphate, FGF23, and Klotho. Bone (2017) 100:87–93. doi: 10.1016/j.bone.2016.11.012
38. Evrard S, Delanaye P, Kamel S, Cristol JP, Cavalier E. Vascular Calcification: From Pathophysiology to Biomarkers. Clin Chim Acta (2015) 438:401–14. doi: 10.1016/j.cca.2014.08.034
39. Delanaye P, Warling X, Moonen M, Smelten N, Jouret F, Krzesinski JM, et al. Variations of Parathyroid Hormone and Bone Biomarkers Are Concordant Only After a Long Term Follow-Up in Hemodialyzed Patients. Sci Rep (2017) 7(1):12623. doi: 10.1038/s41598-017-12808-3
40. Ducy P. @ the Role of Osteocalcin in the Endocrine Cross-Talk Between Bone Remodelling and Energy Metabolism. Diabetologia (2011) 54(6):1291–7. doi: 10.1007/s00125-011-2155-z
41. Nagata Y, Inaba M, Imanishi Y, Okazaki H, Yamada S, Mori K, et al. Increased Undercarboxylated Osteocalcin/Intact Osteocalcin Ratio in Patients Undergoing Hemodialysis. Osteoporos Int (2015) 26(3):1053–61. doi: 10.1007/s00198-014-2954-4
Keywords: osteocalcin, maintenance hemodialysis, abdominal aortic calcification, vascular calcification, chronic kidney disease-mineral and bone disorder
Citation: Jia F, Wang S, Jing Y, Zhao H, Rong P, Zhang H, Lu W, Xue Y and Sun G (2021) Osteocalcin and Abdominal Aortic Calcification in Hemodialysis Patients: An Observational Cross-Sectional Study. Front. Endocrinol. 12:620350. doi: 10.3389/fendo.2021.620350
Received: 24 November 2020; Accepted: 02 March 2021;
Published: 19 March 2021.
Edited by:
Giacomina Brunetti, University of Bari Aldo Moro, ItalyReviewed by:
Maria Fusaro, Italian National Research Council, ItalyHesham Keryakos, Minia University Hospital, Egypt
Copyright © 2021 Jia, Wang, Jing, Zhao, Rong, Zhang, Lu, Xue and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Xue, eHlhbnl4eXhAMTYzLmNvbQ==; Gang Sun, c3VuZ2FuZzIwMTdAMTI2LmNvbQ==
 Fengyu Jia
Fengyu Jia Suxia Wang1
Suxia Wang1