
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 19 August 2021
Sec. Clinical Diabetes
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.610893
This article is part of the Research TopicAchieving Effective Management and Treatment of Diabetes Mellitus in Future Primary CareView all 11 articles
Vitamin D deficiency could play an important role in the pathogenesis of type 2 diabetes mellitus (T2DM) as it may alter several crucial processes in the development of diabetes and its complications, such as pancreatic insulin secretion, peripheral insulin resistance, persistence of systemic „sterile” inflammation and immune activation. Vitamin D may also have an antioxidant effect through the inhibition of free radicals generation. The reported study was designed with eligible consecutively recruited patients with T2DM on standard metformin therapy (n=130), randomized in 1:1 ratio, considered to have undergone Vitamin D supplementation according to the guidelines proposed by the Endocrine Society, or to have continued with metformin only. The potential benefit was monitored through the influence on glycemia level, glycated haemoglobin (HbA1c), insulin resistance index (calculated as homeostatic model assessment; HOMA-IR), Castelli Risk Index I and Tryglicerides/Thiobarbituric acid-reactive substances (TG/TBARS) Index in a 6-month follow up period. Our study indicates that oral daily doses of vitamin D improve HbA1c levels over the 3-month and 6-month period, followed by a significant decrease in advanced oxidation protein products levels over the 3-month period when higher vitamin D doses are given. The effect of vitamin D on HOMA-IR index, malondialdehyde levels and TG/TBARS index was not statistically significant. Further investigation should consider defining the doses of vitamin D in patients with T2DM which may attenuate the oxidative stress risk, the risk of metabolic syndrome and the risk of related cardiovascular events.
During the past years there has been a growing interest in extra-skeletal effects of vitamin D since it has been discovered that vitamin D receptors are expressed not only in tissues related to bone metabolism, but also in many other tissues, such as brain, prostate, breasts, immune cells etc. (1, 2). This has linked vitamin D to many chronic diseases, such as cancer, heart disease, metabolic syndrome, prediabetes, diabetes, inflammatory and autoimmune diseases (3, 4).
Patients with type 2 diabetes mellitus (T2DM) usually have a lack of vitamin D but it is still unknown whether this is a coincidence or whether low levels of vitamin D may contribute to the disease appearance. Recent studies have suggested that vitamin D deficiency could play an important role in T2DM pathogenesis through altering several crucial processes in the development of diabetes and its complications: pancreatic insulin secretion, peripheral insulin resistance, down-regulation of the insulin receptor gene, systemic „sterile” inflammation and immune activation (3, 5). Besides hyperinsulinemia and hyperglycaemia, T2DM is a condition characterized by an increased formation of free radicals and a decreased antioxidant capacity. Some experimental studies have shown that vitamin D may have an antioxidant effect followed by the inhibition of free radicals generation, consequent lipid peroxidation and oxidative modification of other biomolecules (6). Due to the abovementioned effects, vitamin D supplementation has been proposed as a possible therapeutic tool for T2DM to optimize glycaemic control and to prevent the occurrence of complications (7).
Despite the promising data from observational studies, vitamin D supplementation trials have yielded inconsistent results. Some of them have reported a significant reduction of fasting blood glucose (FBG) and glycated haemoglobin (HbA1c) while others have shown no statistically significant improvement in vitamin D group vs. placebo (7–10). These results should be interpreted with caution because most of the trials included small heterogeneous groups of patients in different stages of disease with suboptimal doses of vitamin D, various ways of administration, lifestyle habits, dietary habits, age, ethnicity, season of the year, location and climate conditions (5, 7).
Moreover, little or no research has determined any long-term effect of vitamin D supplementation in T2DM patients with co-administration of the standard pharmacological regimen, by using daily doses of vitamin D according to the available guidelines (11). Therefore, the study reported was aimed at determining whether vitamin D supplementation in T2DM patients, who underwent standard metformin therapy, may have a benefit through the influence on insulin resistance, Castelli risk index I and oxidative stress markers, in a 6-month follow up randomized controlled study.
A prospective, randomized, controlled open-label study was conducted in a summer-winter period (overall trial start date 02.04.2018; overall trial end date 15.12.2018.) in Primary Health Care Center Podgorica, Montenegro. The study was conducted according to the Declaration of Helsinki and Good clinical Practice guidelines. Ethical approval was obtained by the Ethical Committee of Primary Health Care Center in Podgorica (Ethical Committee of Primary Health Care Center in Podgorica, ID number 05/01-E.K.-5989/1) and all participants provided a written informed consent. Study was also registered on ISRCTN registry platform (02.12.2019. ID number ISRCTN25609316). In the region, Mediterranean climate prevails, at latitude 42°, where regular diet is characterized by Mediterranean eating habits, which may reflect a basal vitamin D level.
A sample size was determined according to the key variable HbA1c of previous study (12) considering α = 0.05 and statistical power 1-β =0.8. The calculated sample size was 49 patients per group. Out of 560 pre-screened T2DM patients, 150 met the eligibility criteria. Allowing the drop-out rate of 30%, a total of 130 participants (65 in each group) were enrolled, ≥ 30 years of age, diagnosed with T2DM according to American Diabetes Association (ADA) 2011 criteria (13), who had good metabolic control (HbA1c ≤ 7%), who were treated with metformin and were given lifestyle advice.
The exclusion criteria were as follows: the use of vitamin D supplements and any diabetes pharmacotherapy other than metformin 6 months prior the study; the use of drugs that affect the metabolism of vitamin D; the presence of severe anemia; a chronic liver or kidney failure; alcoholism, pregnancy; malabsorption; urolithiasis; hypercalcemia; body mass index (BMI) ≥ 40 kg/m2 and the presence of acute or chronic inflammatory conditions.
The primary outcome of the study was change in insulin resistance and glycemic control measured by the homeostasis model of assessments (HOMA-IR) and glycemic parameters (FBG, HbA1c). The oxidative stress parametres [malondialdehyde (MDA) and advanced oxidation protein products (AOPP)] and inflammation markers [C-reactive protein (CRP)] were considered secondary outcomes. Furthermore, alteration of vitamin D levels over time, blood pressure, lipid profile, BMI, calcium total (Ca), calcium ionized (Ca ++) and atherogenic risk were also considered as secondary outcomes.
The eligible participants recruited consecutively were randomly assigned to two groups in 1:1 ratio. The Serum level of 25(OH)D (marker of vitamin D status) was measured at baseline in all participants. Half of the patients (n=65) were randomly prescribed vitamin D3 therapy and continued their prescribed metformin therapy for 6 months, while the other group (n=65) continued their prescribed metformin therapy only. The dosing was carried out according to the Endocrine Society’s clinical practice guidelines as for the vitamin D baseline levels (10). The supplements were given in the form of Vigantol oil drops (Merck KGaA, 0.5 mg/ml, one drop equals 500 IU). The participants in the first group who were vitamin D deficient [defined as serum levels of 25(OH)D ≤ 50 nmol/L] were asked to take 50 000 IU of vitamin D3 weekly (equal to 15 drops of vitamin D supplement-Vigantol oil daily) during the first 3 months and 14 000 IU weekly (4 drops daily) for the next 3 months. The participants in the same group whose 25(OH)D levels were > 50 nmol/L were asked to take 14 000 IU weekly (4 drops daily) until the end of the study. The study protocol is shown in Figure 1. At the screening visit, data about participant’s level of physical activity, sun exposure and average dietary intake of vitamin D of three consecutive days were also reported. Every participant received a table with a list of food containing vitamin D and was advised not to alter the usual dietary amounts of vitamin D. All participants also agreed not to change physical activity and sun exposure patterns while enrolled. The compliance was carefully controlled by the medical staff. The Patients whose diabetes therapy has changed during the study period were excluded. The report involved clinical and laboratory analyses of data through the 3- and the 6-month assessment period. All supplements were provided to the patients by the authors themselves through the medical staff. Supplement adherence was assessed at the end of every month by returning the empty bottles of Vigantol oil. In the Metformin + Vitamin D group, a total of 16 patients were excluded (Six with assigned regimen of 50 000 IU+14 000 IU, and 10 with assigned regimen of 14 000 IU): 1 patient was diagnosed with pancreatic tumor, 1 developed urticaria-like skin lesions after taking first dose of supplement and reaction was classified as moderate, 5 patients did not take the supplement regularly and 9 patients changed diabetes medication. In the Metformin group all 65 patients completed the study successfully.
All participants were subjected to anthropometric measurements and laboratory tests at baseline, 3 and 6 months of intervention. Body height in centimeters was measured without shoes using a wall-mounted stadiometer. Body weight was measured in wearing light clothes and without shoes on electronic (digital reading) scales previously calibrated with a possible error of ± 100g. BMI was calculated as kg/m2. Waist circumference (WC) was measured while standing and with clothes on using the non-stretchable tape positioned parallel to the floor over the umbilicus. Systolic (SBP) and diastolic blood pressure (DBP) were measured in a seated position after 10 minutes of rest with an electronic blood pressure monitoring device (Microlife’s BP A150-30 AFIB). Two measurements were done one minute apart and the average value was used.
Samples were drawn from venous blood early in the morning after 12 hours of overnight fasting. Serum levels of FBG, creatinine, total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides (TG) and Ca were measured using standard enzymatic procedures (Roche Cobas 6000 c 501, Mannheim, Germany). Ca ++ was determined using the ion-selective method on an electrolyte analyzer [Roche Diagnostics AVL 9180 Electrolyte Analyzer (AVL 9180, Roche, Japan)].
Serum CRP level was determined by immunoturbidimetric method (Roche Cobas 6000 c 501, Mannheim, Germany).
For HbA1c, blood was used with K2EDTA anticoagulants and it was determined immunoturbidimetrically (Roche Cobas 6000 c 501, Mannheim, Germany).
Fasting insulin (FI) and 25(OH)D serum levels were measured by electrochemiluminescence using commercial Roche tests on the Cobas 6000/e601 automated analyzer (Roche Diagnostics, Mannheim, Germany). This is a competitive electrochemiluminescence assay which employs a vitamin D binding protein as capture protein, for the quantitative determination of total 25-OH vitamin D in human serum and plasma. According to the manufacturer’s instruction measuring range is 3 to 70 ng/mL (7.5 - 175.0 nmol/L), functional sensitivity 4.01 ng/mL (10.0 nmol/L) (CV 18.5%), within-run precision: < 15 ng/mL: SD ≤ 1 ng/mL > 15 ng/mL: ≤ 6.5%, intermediate precision: < 15 ng/mL: SD ≤ 1.7 ng/mL > 15 ng/mL: ≤ 11.5%.
Lipid peroxidation was measured in plasma and expressed as the Thiobarbituric acid-reactive substances (TBARS) biomarker malondyadehide (MDA). The mean recovery was 90% (SD ± 2%), the CV was 4% (14). The TG/TBARS index was used to monitor the ratio between the potentially oxidizable substrates-polyunsaturated fatty acids of circulating triglycerides and their oxidized counterparts-lipid peroxides.
Advanced oxidation protein product level (AOPP) was measured in serum spectrophotometrically, expressed as chloramine-T equivalents. Before the analysis, plasma samples were diluted in 1:10 ratio (15). In order to avoid turbidity interference with lipids and drugs, plasma samples were previously treated with 2 mol/L magnesium chloride (MgCl2) and 4% phosphortungstate dissolved in 0.19 mol/L sodium hydroxide (NaOH), centrifuged at 1000g for 20 minutes, and supernatant was used for AOPP analyses (ubaci referencu).
Castelli risk index I was determined through the TC/HDL cholesterol ratio, reflecting cholesterol-related atherogenic risk, characteristic of the metabolic syndrome (16), recently studied as the potential surrogate marker related to the insufficiency of vitamin D and metabolic syndrome (17).
HOMA-IR was calculated by the formula HOMA−IR=FBG(mmol/L)×FI(μIU/L)/22.5 (18).
All normally distributed data were presented as mean ± SD. All data not normally distributed were presented as median and interquartile range. A Student’s t test was used to compare the continuous variables between the Metformin + Vitamin D and Metformin group, when the distribution was normal, and Mann-Whitney test when the data have not been distributed normally. Skewed continuous variables were natural log transformed before the analysis. Two way ANOVA for repeated measures was used to monitor parameters over time. Linear regression analysis was used to assess the mean difference between Metformin + Vitamin D and Metformin group after 3 and 6 months (the mean difference is reported as β). All effects were adjusted for gender, baseline age, BMI, FI and HbA1c. The significant p value was set as 0.05 (two-sided). All statistical analyses were performed using R software (version 3.4.3) (R Foundation for Statistical Computing, Vienna, Austria) (19).
Out of 560 pre-screened patients 130 consented to participate in the study. Table 1 shows baseline demographic characteristics of the study population. Clinical and biochemical parameters were balanced between the groups.
For vitamin D concentration, repeated measures two-way ANOVA revealed significant interaction (F=75.349, p<0.001), significant effect of group (F=37.976, p<0.001), and significant effect of time (F=115.201, p<0.001) (Table 3). In Metformin + Vitamin D group 25(OH)D levels increased significantly during intervention period (p<0.001). In Metformin group 25(OH)D levels significantly increased during the first 3-month period and then significantly decreased (p<0.001 for all) (Table 2). After 3 and after 6 months of supplementation, 25(OH)D levels differed significantly between groups (p<0.001) adjusted for participants’ age, gender and baseline levels of BMI, FI and HbA1c (Table 4).
For HbA1c, there are significant effect of group (F=9.782, p=0.037), and significant effect of time (F=9.782, p<0.001) (Table 3).
In Metformin + Vitamin D group, HbA1c levels decreased significantly after 3 months of supplementation (p=0.011) and increased significantly between 3 and 6 month of supplementation (p<0.001). Significant difference in HbA1c levels between groups was seen after 3 (p=0.031) and after 6 months (p=0.017) (Figure 2).
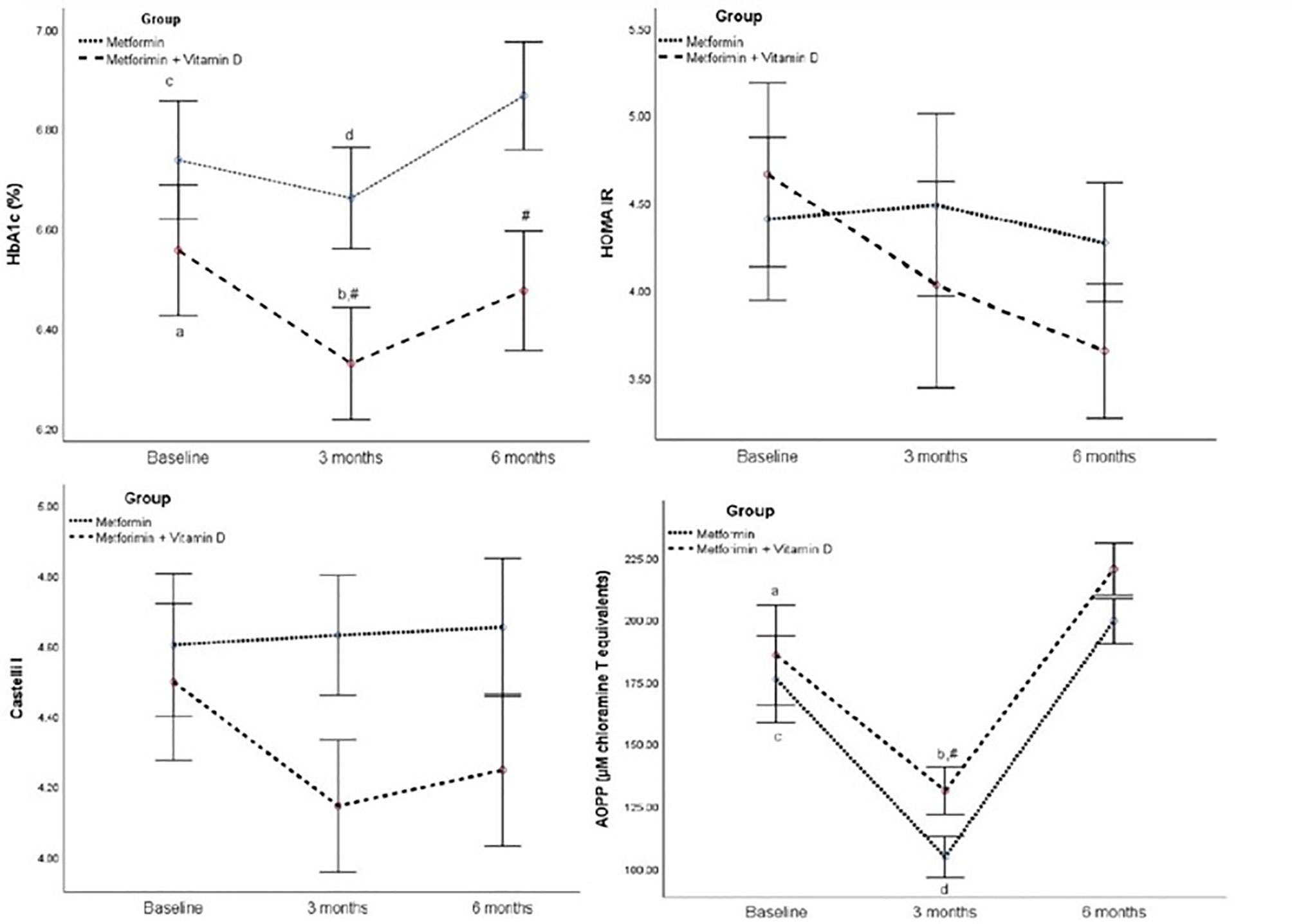
Figure 2 HbA1c, HOMA IR, Castelli I, and AOPP in the follow-up of 6 months between Metformin and Metformin + Vitamin D group. Data are presented mean ± SE, In two way ANOVA for repeated measures: In the Metformin + Vitamin D group: p<0.05 a vs 3 months, b vs 6 months, In the Metformin: p<0.05 c vs 3 months, d vs 6 months, # between group at specific time point.
For FBG, SBP, MDA, TC, Ca, and Ca ++ repeated measures two-way ANOVA revealed significant effect of time (p=0.020, p=0.046, p<0.001, p=0.008, p<0.001, and p<0.001) (Table 3), but there was no significant difference between groups during the intervention period.
For BMI, repeated measures two-way ANOVA revealed no significant interaction (F=2.175, p=0.129), significant effect of group (F=4.489, p=0.039), and significant effect of time (F=4.926, p=0.015) (Table 3). For DBP, repeated measures two-way ANOVA revealed no significant interaction (F=0.528, p=0.567), significant effect of group (F=4.501, p=0.039), and significant effect of time (F=3.989, p=0.029) (Table 3).
For AOPP, repeated measures two-way ANOVA revealed no significant interaction (F=0.098, p=0.907), significant effect of group (F=4.214, p=0.047), and significant effect of time (F=90.323, p<0.001) (Table 3). Significant difference between groups was seen only in 3rd month of intervention time (Table 4).
Regarding mean change, regression analysis significant differences between groups were seen in Vitamin D after 3 months (B 42.25 (95%CI 35.43 - 49.98), p<0.001), and after 6 months (B 38.76 (95%CI 31.72 – 45.80), p<0.001). Also, it is demonstrated a significant mean difference of 0.20 mmol/L in HbA1c after 3 months (p=0.050), of 0.24 mmol/L in HbA1c after 6 months (p=0.045).
In the present study we have found that the recommended doses of vitamin D significantly decreased the level of HbA1c after 3 as well as after 6 months of vitamin D supplementation in patients with T2DM treated with metformin, compared to the metformin group. The most recent meta-analysis carried out by Hu at al. indicated that vitamin D supplementation had beneficial effects on HbA1c, insulin resistance and FI in the subgroup of subjects with short-term follow-up intervention, but had no effect among those with more than 3 months of intervention (20). Similar results were obtained in the systematic review by Nigil Haroon et al. (21). There are many causes which could support these results. First of all, it should not be ignored that the 6-month period is the time of three seasonal changes, in our case, from summer to winter, when the climate conditions, the amount of clouds and a smaller extent of UV exposure may have a significant influence on the endogenous production of vitamin D. Furthermore, the diet and daily habits may differ significantly in winter, which may contribute to the worsening of the metabolic control. Additionally, the patient’s perception of treatment and motivation may have a significant influence on the treatment efficacy. To our knowledge, this is the first study with individual dosing plan for patients regarding 25(OH)D levels according to the Endocrine Society guidelines (11). The daily doses proposed were 7142 IU (50 000 IU weekly) for vitamin D deficient patients and 2000 IU for patients who had 25(OH)D levels > 50 nmol/L. This dose of 2000 IU was not sufficient to provide 25(OH)D levels beyond 100 nmol/L, which is believed to be crucial for extra-skeletal effects (22). The same fact could explain why we did not maintain a significant effect of vitamin D on FI and HOMA-IR levels over the six-month period. Talaei et al. showed that the effect of vitamin D on insulin resistance was significant only when the vitamin D concentration was between 100 and 150 nmol/L (22). Since the definition of the optimal vitamin D level remains controversial, vitamin D deficiency would not be the inclusion and standard judgment criterion (23, 24). However, it is possible that vitamin D has beneficial effects only in vitamin D deficient patients especially in those with poor glycaemic control (25, 26). Yet, Krul-Poel et al. found that vitamin D supplementation was not sufficient to improve glycaemic control in patients with well controlled T2DM, but they used intermittent high-dose of vitamin D. They may have an impact on different outcomes compared to daily doses (27, 28).
A meta-analysis that included 17 randomized controlled trials evaluated the effect of vitamin D supplementation on lipid profile in patients with T2DM. Results revealed that vitamin D supplementation had positive effects in vitamin D deficient patients who had received vitamin D intervention for ≤ 12 weeks. Our results also showed that vitamin D had beneficial effect on HDL cholesterol after 3 months of supplementation when there was higher prevalence of vitamin D deficiency in the Vitamin D+Metformin group and higher vitamin D doses were given (29).
The proposed mechanisms of vitamin D beneficial effects may occur on different levels. By acting as an immunomodulator, vitamin D can regulate innate and adaptive immunity, particularly dendritic cell maturation, macrophage differentiation and T-cell proliferation, while decreasing inflammatory response, inflammatory cytokine secretion and apoptosis of beta cells. On the cellular level, it can down-regulate inflammatory and redox sensitive nuclear transcription factor kappa beta (NFκB) (30–33). Vitamin D stimulates insulin secretion by calcium flux-dependent mechanisms (34).
Although we observed decrease in CRP levels in Metformin + Vitamin D group this decrease did not reach statistical significance. The lack of significant difference between groups in our study could be explained by a small sample size.
We also observed significant increase in Ca and Ca ++ levels in both groups during the study period (p<0.0001). Still, there was no significant difference between groups in Ca and Ca++ levels. Seasonal changes in sunlight and dietary habits in spite of given recommendations could be the reason for this result.
Oxidative stress is strongly associated with T2DM and plays a key role in insulin peripheral sensitivity and insulin secretory response (6, 35). It is well documented that hyperglycaemia contributes mostly to the generation of the reactive oxygen species (ROS), through glucose autoxidation and consequent non-enzymatic glycation of proteins in T2DM (36, 37). Recent evidence has suggested that Vitamin D can decrease oxidative stress. Meta-analysis performed by Sepidarkish et al. has shown that vitamin D supplementation significantly reduced levels of MDA, a primary biomarker of lipid peroxidation (38, 39). In the same meta-analysis the positive effect on MDA was observed only in subgroups with bi-weekly administration of vitamin D doses that are between 100 000 and 200 000 IU per month. Doses less than 100 000 IU and more than 200 000 IU per month were under significant effect. Different dosing and different way of administration could be the reason for not having more pronounced effects of vitamin D on MDA levels during our study. Similar results were reported by Eftekhari et al. (40). It is well documented that vitamin D possess the antioxidant potential in vivo and in vitro, serving as a lipophilic substance, as a membrane and lipoprotein free radical scavenger and antioxidant (41–43). It seems that vitamin D treatment was not a promising approach in improving the AOPP level, most probably because of its lipophilic properties (Table 2). Beneficial effect was seen only after 3 month period when higher doses of vitamin D were given. After 3rd month AOPP had the same trend of rising in both groups, which can be attributed to the interaction of drugs present in circulation (most probably metformin) with method, since all samples were made at once, under the same conditions.
Our study had some limitations. Firstly, it was a randomized but not a placebo controlled trial. Levels of 25(OH)D were used as vitamin D status markers, but vitamin D has several forms with different levels of activity (1). Insulin resistance was assessed using HOMA IR instead of hyperinsulinemic-euglycemic clamps, which is proposed as a gold standard (44). Serum 25(OH)D was measured by an electrochemiluminescence immunoassay, not a gold standard method-liquid chromatography mass spectrometry (45). The strengths of our study include its randomized, prospective design; the use of vitamin D status dependent on oral daily doses according to ES guidelines; the study was mediumd and long-term follow-up randomized.
Our study has indicated that oral daily doses of vitamin D proposed by ES guidelines reduce the levels of HbA1c over a 3-month and over a 6-month period. Its effect on metabolic control, through the improvement on HOMA-IR, and oxidative stress measured through AOPP improvment might have a promising effect if vitamin D could be maintained in optimal dose. Further investigation would reconsider vitamin D doses in patients with T2DM, which may attenuate the oxidative stress risk, the risk of metabolic syndrome and the related cardiovascular events.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethical Committee of Primary Health Care Center in Podgorica (ID number 05/01-E.K.-5989/1). The patients/participants provided their written informed consent to participate in this study.
MC contributed in the conception of the work, participated in the study design, and wrote the manuscript. RK contributed in the conception of the work, participated in the study design, and critically revised the manuscript. AK and GK contributed in the conception of the work, performed biochemistry analyses, and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
The work was supported by the Project TR31060 (Ministry of education, science and technological development).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Holick MF. The D-Lightful Vitamin D for Health. J Med Biochem (2013) 32:1–58 doi: 10.2478/v10011-012-0025-y
3. Martin T, Campbell RK. Vitamin D and Diabetes. Diabetes Spectr (2011) 24(2):113–8. doi: 10.2337/diaspect.24.2.113
4. Holick MF, Chen TC. Vitamin D Deficiency: A Worldwide Problem With Health Consequences. Am J Clin Nutr (2008) 87(4):1080S–6S. doi: 10.1093/ajcn/87.4.1080S
5. Cojić MM. The Role of Vitamin D in Treating Patients With Type 2 Diabetes Mellitus. Acta Med Median (2019) 58:116–24. doi: 10.5633/amm.2019.0116
6. Saif-Elnasr M, Ibrahim IM, Alkady MM. Role of Vitamin D on Glycemic Control and Oxidative Stress in Type 2 Diabetes Mellitus. J Res Med Sci (2017) 22:22. doi: 10.4103/1735-1995.200278
7. Mirhosseini N, Vatanparast H, Mazidi M, Kimball SM. The Effect of Improved Serum 25-Hydroxyvitamin D Status on Glycemic Control in Diabetic Patients: A Meta-Analysis. J Clin Endocrinol Metab (2017) 102:3097–110. doi: 10.1210/jc.2017-01024
8. Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The Role of Vitamin D and Calcium in Type 2 Diabetes: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab (2007) 92:2017–29. doi: 10.1210/jc.2007-0298
9. George PS, Pearson ER, Withm MD. Effect of Vitamin D Supplementation on Glycaemic Control and Insulin Resistance: A Systematic Review and Meta-Analysis. Diabetes Med (2012) 29:e142–50. doi: 10.1111/j.1464-5491.2012.03672.x
10. Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, et al. Effect of Vitamin D3 Supplementation on Improving Glucose Homeostasis and Preventing Diabetes: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab (2014) 99:3551–60. doi: 10.1210/jc.2014-2136
11. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2011) 96:1911–30. doi: 10.1210/jc.2011-0385
12. Nasri H, Behradmanesh S, Maghsoudi AR, Ahmadi A, Nasri P, Rafieian-Kopaei M. Efficacy of Supplementary Vitamin D on Improvement of Glycemic Parameters in Patients With Type 2 Diabetes Mellitus; A Randomized Double Blind Clinical Trial. J Renal Inj Prev (2013) 3:31–4. doi: 10.12861/jrip.2014.10
13. American Diabetes Association. Standards of Medical Care in Diabetes-2011. Diabetes Care (2011) 34:S11–61. doi: 10.2337/dc11-S011
14. Jean CD, Maryse T, Marie JF. Plasma Malondialdehyde Levels During Myocardial Infarction. Clin Chim Acta (1983) 129:319–22. doi: 10.1016/0009-8981(83)90035-9
15. Witko-Sarsat V, Friedlander M, Nguyen Khoa T, Capeillère-Blandin C, Nguyen AT, Canteloup S, et al. Advanced Oxidation Protein Products as Novel Mediators of Inflammation and Monocyte Activation in Chronic Renal Failure. J Immunol (1998) 161:2524–32.
16. Vargas HO, Vargas Nunes SO, Barbosa DS, Vargas MM, Cestari A, Dodd S, et al. Castelli Risk Indexes 1 and 2 Are Higher in Major Depression But Other Characteristics of the Metabolic Syndrome Are Not Specific to Mood Disorders. Life Sci (2014) 102:65–71. doi: 10.1016/j.lfs.2014.02.033
17. Barbalho SM, Tofano RJ, de Campos AL, Rodrigues AS, Quesada K, Bechara MD, et al. Association Between Vitamin D Status and Metabolic Syndrome Risk Factors. Diabetes Metab Syndrome: Clin Res Rev (2018) 12:501–7. doi: 10.1016/j.dsx.2018.03.011
18. Klisic A, Kavaric N, Jovanovic M, Soldatovic I, Gligorovic-Barhanovic N, Kotur-Stevuljevic J. Bioavailable Testosterone Is Independently Associated With Fatty Liver Index in Postmenopausal Women. Arch Med Sci (2017) 13:1188–96. doi: 10.5114/aoms.2017.68972
19. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing (2014). Available at: http://www.R-project.org/ (Accessed Accessed: 1st September 2019).
20. Hu Z, Chen J, Sun X, Wang L, Wang A. Efficacy of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Meta-Analysis of Interventional Studies. Med (Baltimore) (2019) 98:e14970. doi: 10.1097/MD.0000000000014970
21. Nigil Haroon N, Anton A, John J, Mittl M. Effect of Vitamin D Supplementation on Glycemic Control in Patients With Type 2 Diabetes: A Systematic Review of Interventional Studies. J Diabetes Metab Disord (2015) 14:3. doi: 10.1186/s40200-015-0130-9
22. Talaei A, Mohamedi M, Adgi Z. The Effect of Vitamin D on Insulin Resistance in Patients With Type 2 Diabetes. Diabetol Metab Syndr (2013) 5:8. doi: 10.1186/1758-5996-5-8
23. Vogeser M, Seger C. Vitamin D–Challenges in Diagnosing and Monitoring of Hypovitaminosis D. J Med Biochem (2012) 31:316–25. doi: 10.2478/v10011-012-0023-0
24. Binkley N, Ramamurthy R, Krueger D. Low Vitamin D Status: Definition, Prevalence, Consequences, and Correction. Endocrinol Metab Clin North Am (2010) 39:287–301. doi: 10.1016/j.ecl.2010.02.008
25. Krul-Poel YH, Ter Wee MM, Lips P, Simsek S. Management of Endocrine Disease: The Effect of Vitamin D Supplementation on Glycaemic Control in Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Eur J Endocrinol (2017) 176:R1–14. doi: 10.1530/EJE-16-0391
26. Soric MM, Renner ET, Smith SR. Effect of Daily Vitamin D Supplementation on HbA1c in Patients With Uncontrolled Type 2 Diabetes Mellitus: A Pilot Study. J Diabetes (2012) 4:104–5. doi: 10.1111/j.1753-0407.2011.00164.x
27. Krul-Poel YH, Westra S, ten Boekel E, ter Wee MM, van Schoor NM, van Wijland H, et al. Effect of Vitamin D Supplementation on Glycemic Control in Patients With Type 2 Diabetes (SUNNY Trial): A Randomized Placebo-Controlled Trial. Diabetes Care (2015) 38:1420–6. doi: 10.2337/dc15-0323
28. Dalle Carbonare L, Valenti MT, Del Forno F, Caneva E, Pietrobelli A. Vitamin D: Daily vs. Monthly Use in Children and Elderly-What Is Going on? Nutrients (2017) 9:652. doi: 10.3390/nu9070652
29. Jafari T, Fallah AA, Barani A. Effects of Vitamin D on Serum Lipid Profile in Patients With Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Clin Nutr (2016) 35:1259–68. doi: 10.1016/j.clnu.2016.03.001
30. Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-Dihydroxyvitamin D3 Receptor in the Immune System. Arc Biochem Biophys (2000) 374:334–8. doi: 10.1006/abbi.1999.1605
31. Dong X, Lutz W, Schroeder TM, Bachman LA, Westendorf JJ, Kumar R, et al. Regulation of relB in Dendritic Cells by Means of Modulated Association of Vitamin D Receptor and Histone Deacetylase 3 With the Promoter. Proc Nati Acad Sci USA (2005) 102:16007–12. doi: 10.1073/pnas.0506516102
32. Chagas CEA, Borges MC, Martini LA, Rogero MM. Focus on Vitamin D, Inflammation and Type 2 Diabetes. Nutrients (2012) 4:52–67. doi: 10.3390/nu4010052
33. Van Etten E, Mathieu C. Immunoregulation by 1,25-Dihydroxyvitamin D3: Basic Concepts. J Ster Biochem Mol Biol (2005) 97:93–101. doi: 10.1016/j.jsbmb.2005.06.002
34. Beaulieu C, Kestekian R, Havrankova J, Gascon-Barre M. Calcium Is Essential in Normalizing Intolerance to Glucose That Accompanies Vitamin D Depletion In Vivo. Diabetes (1993) 42:35–43. doi: 10.2337/diabetes.42.1.35
35. Kocić R, Pavlović D, Kocić G, Pesić M. Susceptibility to Oxidative Stress, Insulin Resistance, and Insulin Secretory Response in the Development of Diabetes From Obesity. Vojnosanit Pregl (2007) 64:391–7. doi: 10.2298/VSP0706391K
36. Kocic G, Sokolovic D, Jevtovic T, Veljkovic A, Kocic R, Nikolc G, et al. Hyperglycemia, Oxidative and Nitrosative Stress Affect Antiviral, Inflammatory and Apoptotic Signaling of Cultured Thymocytes. Redox Rep (2010) 15:179–84. doi: 10.1179/174329210X12650506623564
37. Rains JL, Jain SK. Oxidative Stress, Insulin Signaling, and Diabetes. Free Radic Biol Med (2011) 50:567–75. doi: 10.1016/j.freeradbiomed.2010.12.006
38. Sepidarkish M, Farsi F, Akbari-Fakhrabadi M, Namazi N, Almasi-Hashiani A, Hagiagha AM, et al. The Effect of Vitamin D Supplementation on Oxidative Stress Parameters: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmacol Res (2019) 139:141–52. doi: 10.1016/j.phrs.2018.11.011
39. Klisic A, Isakovic A, Kocic G, Kavaric N, Jovanovic M, Zvrko E, et al. Relationship Between Oxidative Stress, Inflammation and Dyslipidemia With Fatty Liver Index in Patients With Type 2 Diabetes Mellitus. Exp Clin Endocrinol Diabetes (2018) 126:371–8. doi: 10.1055/s-0043-118667
40. Eftekhari MH, Akbarzadeh M, Dabbaghmanesh MH, Hassanzadeh J. The Effect of Calcitriol on Lipid Profile and Oxidative Stress in Hyperlipidemic Patients With Type 2 Diabetes Mellitus. ARYA Atheroscler (2014) 10(2):82–8.
41. Hamden K, Carreau S, Jamoussi K, Miladi S, Lajmi S, Aloulou D, et al. 1Alpha, 25 Dihydroxyvitamin D3: Therapeutic and Preventive Effects Against Oxidative Stress, Hepatic, Pancreatic and Renal Injury in Alloxan-Induced Diabetes in Rats. J Nutri Sci Vitaminol (2009) 55:215–22. doi: 10.3177/jnsv.55.215
42. Wiseman H. Vitamin D Is a Membrane Antioxidant. Ability to Inhibit Iron-Dependent Lipid Peroxidation in Liposomes Compared to Cholesterol, Ergosterol and Tamoxifen and Relevance to Anticancer Action. FEBS Lett (1993) 326:285–8. doi: 10.1016/0014-5793(93)81809-E
43. Cetinkalp S, Delen Y, Karadeniz M, Yüce G, Yilmaz C. The Effect of 1alpha, 25(OH)2D3 Vitamin Over Oxidative Stress and Biochemical Parameters in Rats Where Type 1 Diabetes Is Formed by Streptozotocin. J Diabetes Complicat (2009) 23:401–8. doi: 10.1016/j.jdiacomp.2008.09.005
44. Tam CS, Xie W, Johnson WD, Cefalu WT, Redman LM, Ravussin E. Defining Insulin Resistance From Hyperinsulinemic-Euglycemic Clamps. Diabetes Care (2012) 35:1605–10. doi: 10.2337/dc11-2339
Keywords: vitamin D supplementation, HbA1c, insulin resistance, oxidative stress, type 2 diabetes
Citation: Cojic M, Kocic R, Klisic A and Kocic G (2021) The Effects of Vitamin D Supplementation on Metabolic and Oxidative Stress Markers in Patients With Type 2 Diabetes: A 6-Month Follow Up Randomized Controlled Study. Front. Endocrinol. 12:610893. doi: 10.3389/fendo.2021.610893
Received: 27 September 2020; Accepted: 30 July 2021;
Published: 19 August 2021.
Edited by:
Boon-How Chew, Putra Malaysia University, MalaysiaReviewed by:
Justyna Kuliczkowska-Płaksej, Wroclaw Medical University, PolandCopyright © 2021 Cojic, Kocic, Klisic and Kocic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Milena Cojic, bWlsZW5hcm92Y2FuaW5AeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.