
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 02 March 2021
Sec. Clinical Diabetes
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.608232
This article is part of the Research TopicConditions and Results of Effective Glycemic Control in Children with Type 1 DiabetesView all 9 articles
Background: The incidence of pediatric type 1 diabetes (T1D) is increasing worldwide, and the appropriate choice of therapy regimens is important for children, especially in developing countries with inadequate resources.
Methods: We conducted a design combining meta-analysis and prospective cohort study. In meta-analysis, 14 studies involving 69,085 TID cases reported glycosylated hemoglobin (HbA1c) levels, including 48,363 multiple daily insulin injections therapy (MIT) and 20,722 continuous subcutaneous insulin infusion (CSII). In our prospective cohort study, TID cases were recruited from a tertiary children’s hospital, and randomly divided into Group MIT and Group CSII. After the 4-year follow-up, the effects of MDI (n = 112) and CSII (n = 76) therapy on glycemic control, long-term complications, as well as the growth and pubertal development were explored.
Results: Compared to CSII in TID, HbA1c levels in MDI (WMD = 0.21, 95% CI: 0.20 to 0.23) were increased significantly in meta-analysis. Among 188 clinical cases, mean age at recruitment was 7.55 (SD 2.91) years. Duration of TID was 4.23 (SD 2.61) years. 50.53% (n = 95) of them were boys. The 4-year follow-up showed that children’s HbA1c was 0.67 (95% CI −1.28, −0.05) % lower in children with CSII compared to children with MDI in multivariable regression models with adjustment for potential confounders (children’s age at follow-up, duration of TID, gender, birthweight, parity, and delivery method). CSII was associated with 2.31 kg higher in children’s weight (95% CI 0.59, 4.04) in the adjusted model. No difference was found in peripheral nerve and fundus consequences as well as the status of obesity and thin and pubertal development between CSII and MIT.
Conclusion: CSII might be associated with better glycemic control and better effect for children growth development. No higher risks of long-term complications and delayed pubertal development were observed in CSII. Our findings provided evidence for a better therapy regimen for T1D in children, nevertheless, they need to be validated by a larger sample size study.
Epidemiological and experimental studies have shown that type 1 diabetes (T1D) and its complications can affect human health. Children and adolescents are at high risk of TID. During 2010–2013, the estimated incidence of TID was 1.93 under the age of 14 in China per 100,000 person years, and this number is expected to increase continually (1, 2). TID is caused by absolutely inadequate insulin production due to the damage to the beta cells of pancreas, and requires lifelong insulin treatment. Several insulin types (rapid, short, intermediate, and long-acting) and infusion methods [multiple daily insulin injections therapy (MIT) and continuous subcutaneous insulin infusion (CSII)] can be used to treat TID.
The use of insulin pumps for CSII therapy among patients with TID increased from 0.6% to 1.3% in 1995 to 44% to 47% between 2012 and 2016 (3). In 2018, American Diabetes Association (ADA) Guidelines recommend the usage of CSII in adolescents with TID (4). However, in the field of pediatrics, the conclusion of comparison of the effects of MIT and CSII for TID were not consistent. Some studies showed that CSII therapy has more advantages in glycemic control among children (3, 5, 6), while other studies showed that CSII therapy is not more effective than MIT (7–9).
Furthermore, regarding MIT and CSII, cares should be taken as to the possible risk of complications. More importantly, for children, attentions should be paid to the impacts of different therapy regimens on growth and development. However, there is little research on this topic in China. Therefore, on the basis of literature review, first, a meta-analysis was conducted to compare MIT and CSII in achieving glycosylated hemoglobin (HbA1c) control in pediatric TID. Second, a prospective cohort study was designed to explore the effects of MIT and CSII on glycemic control, acute and chronic complications, as well as the growth and pubertal development in children.
We searched the relevant literature in four electronic bibliographic databases: PubMed, Cochrane Library, Embase and Web of Science. The published literature was searched until June, 2020. The following search terms were used in the databases:
#1 (children) OR (child) OR (childhood) or (pediatric)
#2 (Type 1 diabetes) OR (T1D)
#3 (insulin injection) OR (insulin infusion) OR (MIT)
#4 (insulin pump) OR (continuous subcutaneous insulin infusion) OR (CSII)
#5 (glycated hemoglobin) OR (GHb) OR (glycosylated hemoglobin) OR (HbA1c)
#6: #1 AND #2 AND #3 AND #4 AND #5
We selected studies which:
1. The full text of literature is available;
2. The subjects were children, not experimental animals;
3. Explicitly specified the substance that children were diagnosed as TID;
4. The data showing HbA1c concentration in both MIT and CSII therapy were provided;
5. There were no defects in research design and high literature quality.
Two assessors screened and identified the literature. Any disagreement of them was settled by discussion with a third evaluator (Figure 1). According to the Newcastle Ottawa Scale (NOS) standard (10), the quality of each literature was evaluated and total number of stars greater than or equal to six as appropriate literature (NOS ranges from 0 to 9 stars) were selected. The information of each on the 14 literature was extracted (3, 5–9, 11–18), including author name, published year, research time, region, sample size, age, gender, duration of TID, HbA1c after MDI therapy, and HbA1c after CSII therapy.
The work was approved by the Medical Ethics Committee of Nanjing Children’s Hospital. We obtained signed informed consent from all participants. All TID cases (n = 240) were recruited from a prospective cohort designed to explore the outcomes of MIT and CSII in Nanjing children’s hospital affiliated to Nanjing Medical University, a tertiary children’s hospital, from 2010 to 2015. Eligibility criteria were as follow. 1) One or more of the following criteria for TID are met: fasting plasma glucose ≥ 7.0mmol/L; 2-h plasma glucose ≥ 11.1 mmol/L following an oral glucose tolerance test (OGTT); diabetes-related symptoms and random plasma glucose ≥11.1mmol/L. 2) All children were 6 years ≤ age < 18 years, without mental disorder. 3) At least one of the three pancreatic islet autoantibodies (gluconic acid decarboxylase antibody, insulin antibody, islet cell autoantibody) is positive, or the results of insulin-C-peptide release test suggest that islet cell function is low (fasting C-peptide < 200 pmol/L). Other types of diabetes, such as type 2 diabetes and special type diabetes, were excluded.
A face-to-face questionnaire interview was conducted at study enrollment to collect demographic information. All participants were randomly divided into two groups: MIT (standard 3 + 1 mode, quick acting insulin injection before three meals combined once long-acting insulin injection) and CSII (insulin pump, basic doses of insulin continuous infusion in 24 h, large doses of insulin infusion before three meals). Of the 240 participants, 52 were excluded due to loss of follow-up. Thus, 188 participants were included in the final analysis, including 112 cases of MIT and 76 cases of CSII.
After the 4-year follow-up, laboratory tests were completed, including HbA1c, insulin, C-peptide, kidney function, liver function, blood lipid, 25(OH)D, and thyroid function. TID complications were recorded, which can be divided into acute complications of T1D (diabetic ketoacidosis or diabetic ketosis) and chronic complications (diabetic neuropathy and diabetic eye disease). Growth and pubertal development parameter following MDI and CSII for 4 years were also collected.
The fasting blood samples were collected in the morning. Blood urea nitrogen (BUN), creatinine (Cr), aspartate transaminase (AST), alanine transaminase (ALT), high-density lipoprotein (HDL), total cholesterol (TC), and triacylglycerol (TG) were determined by chemiluminescent microparticle immunoassay using the Beckman system (Beckman Coulter, American). Insulin, C-Peptide, total three iodosine adenosine (TT3), free triiodothyronine (FT3), total thyroxine (TT4), free thyroxine (FT4), thyroid-stimulating hormone (TSH), thyroid peroxidase antibody (TPOAb), and thyroglobulin antibody (TGAb) were measured by electrochemiluminescent microparticle immunoassays using the Architect system (Roche GmbH, Germany). HbA1C was measured by high performance liquid chromatography, variant II glycosylated hemoglobin detectors (Bole Company, American). Microalbuminuria (MA), IgG of urinary (IGU), and α 1microglobulin of urinary (A1M) were measured by flow cytometer (Beckman Coulter, American). A 25-hydroxyvitamin D [25(OH)D] was measured by enzymeimmunoassay (Immunodiagnostic Systems Limited, Bolden, UK).
Electromyography (Keypoint 9033A07, Dantec Company, Denmark) and eyes fundus examination (Nidek AFC-300, NIDEK Company, Japan) were performed during hospitalization follow-up.
After the 4-year follow-up, children’s weight and height were measured, with the accuracy of 0.1 kg and centimeter. Body mass Index (BMI) was calculated using the following formula: BMI = weight (kg)/height (m2). BMI categories were based on the World Health Organization classification (19), including <18.5 kg/m2, 18.5–24.99 kg/m2 and ≥25 kg/m2.
We estimated the children’s weight and height at the time of follow-up to Z-score. According to the WHO reference charts, the Z-score is determined by subtracting the median and dividing by the standard deviation using the following formula: Z-score = (XAGE - MAGE)/SDAGE. Age was the age of child, and XAGE is the actual height or weight. MAGE is the median height or weight value at this age, and SDAGE is the standard deviation at this age. Since WHO reference charts present data on height-for-age for children of all ages and weight-for-age for children aged 10 and under, we estimated the height for every child and weight for children aged 10 and under using the Z-score.
The development of breast or genitalia was recorded, and Tanner scale with a five-stage ordinal scale, described by Marshall and Tanner (20, 21), was used to assess the pubertal development of children.
Meta-analysis was processed in the software Stata 16.0. TID using CSII was set as a reference, and HbA1c levels in MDI was compared to CSII. The heterogeneity of these literature is large (P < 0.05, i2 ≥ 50%), and random effects models will be preferable to fixed effects models (22).
The individual demographic features of 188 TID cases were presented as mean ± SD for continuous variables (i.e., age, birthweight, and clinical laboratory parameter), frequency and percentages (i.e., gender, parity, delivery method, and Tanner stage) for categorical variables. Mean imputation method were imputed to replace missing values of laboratory assessments (23). T-test were used for continuous variables and chi-square test for categorical variables.
We used multiple linear regression models with HbA1c, children’s height, weight, and BMI following therapy for 4 years (continuous variables) as dependent variables and different therapy regimens (MDI and CSII) as independent variables. We used logistic regression models to estimate the associations between different therapy regimens (MDI and CSII) and odds of having diabetic ketoacidosis (DKA), abnormal electromyography and abnormal eyes fundus following therapy for 4 years. Model 1 was unadjusted; model 2 was controlled for potential confounders. First, simple linear regression was used to determine whether covariates were selected in our study. According to the standard of p < 0.1, not all covariates included met the requirements. Then, prior knowledge from the scientific literature was used to determine whether covariates were selected for statistical analysis (24). The following variables were included in the final models: children’s age at follow-up, duration of TID, gender, birthweight, parity, and delivery method.
In meta-analysis, HbA1c were reported in 14 studies involving 69,085 TID cases, including 48,363 MDI, and 20,722 CSII. Characteristics of the literatures were shown in Table 1. Compared to CSII in TID cases as a reference, HbA1c levels in MDI (WMD = 0.21, 95% CI: 0.20 to 0.23) were increased significantly (Figure 2).
We used a one-by-one elimination method for sensitivity analysis and chose a random effects model. The results showed that the pooled ES values before and after the exclusion of a study were essentially the same as the 95% confidence intervals, indicating that the original meta-analysis were reliable (Figure 3). In addition, we did not observe significant publication bias, using Begg’s test and funnel plots (Z = 0.88, P = 0.381).
In 188 clinical cases, overall individual demographic features were described in Table 2. Mean age at recruitment was 7.55 (SD 2.91) years. Mean age at follow-up was 11.75 (SD 2.53) years. Duration of TID was 4.23 (SD 2.61) years. 50.53% (n = 95) of them were boys. In addition, 112 cases used MIT and 76 used CSII. No significant differences were observed between MIT and CSII in children’s age at recruitment, children’s age at follow-up, duration of TID, gender, birthweight, parity, delivery method, and feeding method.
Table 3 showed the laboratory assessments following MDI or CSII for about 4 years. The children treated with CSII had lower concentration of HbA1c, ALT, AST, HDL, TPOAb, and A1M than those of treated with MDI (P < 0.05). There were no significant differences between the effects of MIT and CSII on acute and chronic complications, including diabetic ketoacidosis, diabetic ketosis, abnormal electromyography, and abnormal eyes fundus.
Table 4 showed the growth and pubertal development parameter following MDI and CSII therapy for about 4 years. Children treated with CSII for about 4 years showed increased weight levels and weight Z-score (P < 0.05), as well as trends of increased height levels and height Z-score in TID using MDI (P > 0.05), while there was no difference in the status of BMI and pubertal development following the two therapy regimens.
Tables 5 and 6 showed the associations between different therapy regimens with key outcomes. Specifically, children’s HbA1c was 0.67 (95% CI −1.28, −0.05) % lower in children following CSII for about 4 years compared to children following MDI in multivariable regression models with adjustment for potential confounders (children’s age at follow-up, duration of TID, gender, birthweight, parity, and delivery method). Children using CSII therapy weighed 2.31 kg heavier (95% CI 0.59, 4.04) than children in MDI group in the adjusted model. CSII did not increase the odds of long-term complications, such as diabetic ketoacidosis, diabetic ketosis, and abnormal electromyography.
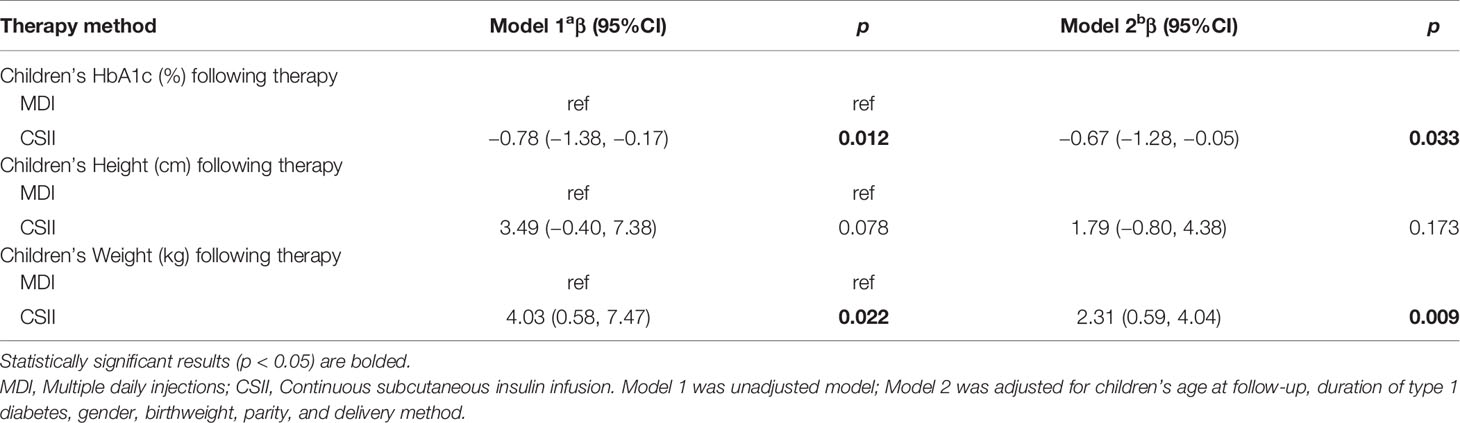
Table 5 Associations between different therapy methods with children’s HbA1c, height, weight, and BMI.
Glycemic control of TID in children with different therapy regimens has always been the focus in pediatric endocrinology. Previous observational study has proposed that CSII can effectively control glucose (3, 11, 18), but causal relationships between different therapy regimens and long-term safety outcomes remains controversial (25, 26). We completed a design combining meta-analysis and prospective cohort study, and confirmed CSII could decrease the HbA1c better than MIT in pediatric T1D. In our cohort data, no high rates of long-term complications were observed in CSII, and at the same time, there was a better effect of CSII for children growth development.
HbA1c is a key indicator for evaluating glycemic control. According to the guideline published by National Institute for Health and Care Excellence, it was recommended that children aged ≥ 12 years who cannot achieve HbA1c < 8.5% should be offered CSII therapy. Children aged < 12 years should be offered CSII therapy from diagnosis of T1D if MDI is considered inappropriate (27). Fourteen literature were included in our meta-analysis with 69,085 TID cases, of which most are observational data from clinical registry system or randomized clinical trials, and the data suggested that CSII may be better than MIT for glycemic control. The results of our cohort study confirmed the conclusion of the meta-analysis. Hence, in terms of glycemic control, our results reveal that CSII took an advantage over MIT.
Therapeutic safety was our secondary focus on the outcomes of CSII in our cohort. Previous research suggested that CSII had the risk of elevated ketoacidosis in children below 12 years, while might reduce ketoacidosis risk in adolescents (28). However, Karges B. indicated that CSII was associated with a lower rate of ketoacidosis (3), and he pointed out that different rates in the use of CSII in different studies were responsible for the discrepancy. In our cohort, the utilization rate of CSII was 40.4%, and after 4 years therapy, there was no difference in the incidence of ketoacidosis or ketosis between CSII and MIT. We also followed up nephropathy consequences and found CSII leading to lower A1M levels, a kind of urinary microalbuminuria, maybe indicating a protective effect on renal complications. In addition, no difference was found in peripheral nerve and fundus consequences between CSII and MIT.
Further, growth and pubertal development were our third focus on the outcomes of CSII in our cohort. One observation study investigated that compared to control children, children with T1D were taller and heavier at the time of the initial diagnosis throughout childhood (29), while another longitudinal study showed retardation in physical growth and delayed age at menarche in girls and full pubertal maturation in boys were in T1D children (30). In our study, the children treated with CSII for about 4 years showed significant increased weight and weight Z-score and trends of increased height levels and height Z-score, perhaps indicating a better effect of CSII for children growth development. On the other hand, there was no difference in the status of obesity and thin as well as pubertal development following the two therapy regimens.
A major advantage of this present study was that we combined meta-analysis and prospective cohort study. That is to exhibit the background part of our paper in the way of data presentation, as well as obtain the clues from the systematic evaluation and analysis of previous literature. We further verified causal relationship between insulin therapy regimens and outcomes in our prospective cohort, which avoided the limitation of single center and small sample size, and made the analysis result more comprehensive and reliable. Our cohort study focused not only on glycemic control of different therapy regimens, but also on long-term complications and growth and pubertal development, understudied outcomes, with T1D duration longer than 4 year throughout childhood. Another potential advantage was that the individual age and duration of different therapy regimens matched well, and was considered in the analyses. Thus, our findings could be important, especially for care on growth and development in children with T1D.
Nevertheless, this study had some limitations. First, the sample size of the single center cohort study is small, and our results need to be validated by a larger sample size study. In addition, studies report that factors such as family socioeconomic status (SES) and children’s nutrition are correlated with glycemic control (31–33), however, we lack some important covariables in the regression model due to the insufficient response rate of participants, so the overall stability of the model is not strong enough. HbA1c is the gold standard indirect measure of glucose control and it estimates the glycemic exposure over the last three months prior to sampling. However, glucose levels can undergo large fluctuations secondary to other factors and this glucose metric, used alone, may be insufficient to define a good control. Other parameters that we lack, such as severe hypoglycemic episodes, time in range (TIR), time below range (TBR), and time above range (TAR), could also be useful. Furthermore, during the period from T1D diagnosis to 4-year follow-up, we conducted multi-time follow-up, however, due to the high rate of lost follow-up at some time points, only the single time point of 4 years was retained as the final analysis. These individuals will be followed up for further study in the future.
Altogether, in this present study of children with T1D, compared to MIT, CSII might be associated with better glycemic control and better effects for children growth development, and no higher risks of long-term complications and delayed pubertal development were observed in CSII, nevertheless, our conclusions need to be validated by a larger sample size study.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by IRB of Children’s Hospital of Nanjing Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
The authors such as XW, XZ, and DC contributed equally to this work. They collected, analyzed data, and wrote articles. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (81803259).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Perrett KP, Jachno K, Nolan TM, Harrison LC. Association of Rotavirus Vaccination With the Incidence of Type 1 Diabetes in Children. JAMA Pediatr (2019) 173:280–2. doi: 10.1001/jamapediatrics.2018.4578
2. Weng J, Zhou Z, Guo L, Zhu D, Ji L, Luo X, et al. Incidence of type 1 diabetes in China, 2010-13: population based study. BMJ (2018) 360:j5295. doi: 10.1136/bmj.j5295
3. Karges B, Schwandt A, Heidtmann B, Kordonouri O, Binder E, Schierloh U, et al. Association of Insulin Pump Therapy vs Insulin Injection Therapy With Severe Hypoglycemia, Ketoacidosis, and Glycemic Control Among Children, Adolescents, and Young Adults With Type 1 Diabetes. Jama (2017) 318:1358–66. doi: 10.1001/jama.2017.13994
4. American Diabetes, Association, Children and Adolescents. Standards of Medical Care in Diabetes-2018. Diabetes Care (2018) 41:S126–36. doi: 10.2337/dc18-S012
5. Abaci A, Atas A, Unuvar T, Demir K, Bober E, Buyukgebiz A. A comparison of multiple daily insulin therapy with continuous subcutaneous insulin infusion therapy in adolescents with type 1 diabetes mellitus: a single-center experience from Turkey. J Pediatr Endocrinol Metab JPEM (2009) 22:539–45. doi: 10.1515/JPEM.2009.22.6.539
6. Nuboer R, Borsboom GJ, Zoethout JA, Koot HM, Bruining J. Effects of insulin pump vs. injection treatment on quality of life and impact of disease in children with type 1 diabetes mellitus in a randomized, prospective comparison. Pediatr Diabetes (2008) 9:291–6. doi: 10.1111/j.1399-5448.2008.00396.x
7. Nabhan ZM, Kreher NC, Greene DM, Eugster EA, Kronenberger W, DiMeglio LA. A randomized prospective study of insulin pump vs. insulin injection therapy in very young children with type 1 diabetes: 12-month glycemic, BMI, and neurocognitive outcomes. Pediatr Diabetes (2009) 10:202–8. doi: 10.1111/j.1399-5448.2008.00494.x
8. Skogsberg L, Fors H, Hanas R, Chaplin JE, Lindman E, Skogsberg J. Improved treatment satisfaction but no difference in metabolic control when using continuous subcutaneous insulin infusion vs. multiple daily injections in children at onset of type 1 diabetes mellitus. Pediatr Diabetes (2008) 9:472–9. doi: 10.1111/j.1399-5448.2008.00390.x
9. Weintrob N, Benzaquen H, Galatzer A, Shalitin S, Lazar L, Fayman G, et al. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens in children with type 1 diabetes: a randomized open crossover trial. Pediatrics (2003) 112:559–64. doi: 10.1542/peds.112.3.559
10. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
11. Jakisch BI, Wagner VM, Heidtmann B, Lepler R, Holterhus PM, Kapellen TM, et al. Comparison of continuous subcutaneous insulin infusion (CSII) and multiple daily injections (MDI) in paediatric Type 1 diabetes: a multicentre matched-pair cohort analysis over 3 years. Diabetic Med J Br Diabetic Assoc (2008) 25:80–5. doi: 10.1111/j.1464-5491.2007.02311.x
12. Anderson DG. Multiple daily injections in young patients using the ezy-BICC bolus insulin calculation card, compared to mixed insulin and CSII. Pediatr Diabetes (2009) 10:304–9. doi: 10.1111/j.1399-5448.2008.00484.x
13. Fendler W, Baranowska AI, Mianowska B, Szadkowska A, Mlynarski W. Three-year comparison of subcutaneous insulin pump treatment with multi-daily injections on HbA1c, its variability and hospital burden of children with type 1 diabetes. Acta Diabetol (2012) 49:363–70. doi: 10.1007/s00592-011-0332-7
14. Schreiver C, Jacoby U, Watzer B, Thomas A, Haffner D, Fischer DC. Glycaemic variability in paediatric patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI): a cross-sectional cohort study. Clin Endocrinol (2013) 79:641–7. doi: 10.1111/cen.12093
15. Bayrakdar A, Noureddine S, Farhood L, Nasrallah MP. Comparison of quality of life in a group of Lebanese type 1 diabetics on insulin pump and those on multiple daily injections. Le Journal medical libanais. Lebanese Med J (2014) 62:22–6. doi: 10.12816/0002623
16. Blackman SM, Raghinaru D, Adi S, Simmons JH, Ebner-Lyon L, Chase HP, et al. Insulin pump use in young children in the T1D Exchange clinic registry is associated with lower hemoglobin A1c levels than injection therapy. Pediatr Diabetes (2014) 15:564–72. doi: 10.1111/pedi.12121
17. Schiel R, Burgard D, Perenthaler T, Stein G, Kramer G, Steveling A. Use and Effectiveness of Continuous Subcutaneous Insulin Infusion (CSII) and Multiple Daily Insulin Injection Therapy (MIT) in Children, Adolescents and Young Adults with Type 1 Diabetes Mellitus. Exp Clin Endocrinol Diabetes Off J German Soc Endocrinol German Diabetes Assoc (2016) 124:99–104. doi: 10.1055/s-0042-101155
18. Danne T, Schwandt A, Biester T, Heidtmann B, Rami-Merhar B, Haberland H, et al. Long-term study of tubeless insulin pump therapy compared to multiple daily injections in youth with type 1 diabetes: Data from the German/Austrian DPV registry. Pediatr Diabetes (2018) 19:979–84. doi: 10.1111/pedi.12658
19. WHO. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. Vol. 894. World Health Organization technical report series (2000). p. i–xii, 1-253.
20. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child (1970) 45:13–23. doi: 10.1136/adc.45.239.13
21. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child (1969) 44:291–303. doi: 10.1136/adc.44.235.291
22. Barili F, Parolari A, Kappetein PA, Freemantle N. Statistical Primer: heterogeneity, random- or fixed-effects model analyses? Interact Cardiovasc Thoracic Surg (2018) 27:317–21. doi: 10.1093/icvts/ivy163
23. Tokodi M, Schwertner WR, Kovács A, Tősér Z, Staub L, Sárkány A, et al. Machine learning-based mortality prediction of patients undergoing cardiac resynchronization therapy: the SEMMELWEIS-CRT score. Eur Heart J (2020) 41:1747–56. doi: 10.1093/eurheartj/ehz902
24. Walter S, Tiemeier H. Variable selection: current practice in epidemiological studies. Eur J Epidemiol (2009) 24:733–6. doi: 10.1007/s10654-009-9411-2
25. Hanas R, Lindgren F, Lindblad B. A 2-yr national population study of pediatric ketoacidosis in Sweden: predisposing conditions and insulin pump use. Pediatr Diabetes (2009) 10:33–7. doi: 10.1111/j.1399-5448.2008.00441.x
26. Brorsson AL, Viklund G, Ortqvist E, Lindholm Olinder A. Does treatment with an insulin pump improve glycaemic control in children and adolescents with type 1 diabetes? A retrospective case-control study. Pediatr Diabetes (2015) 16:546–53. doi: 10.1111/pedi.12209
27. NICE. Continuous Subcutaneous Insulin Infusion for the Treatment of Diabetes Mellitus. In: Technology Appraisal Guidance [TA151]. London: NICE (2008).
28. Maahs DM, Hermann JM, Holman N, Foster NC, Kapellen TM, Allgrove J, et al. Rates of diabetic ketoacidosis: international comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the U.S., Austria, and Germany. Diabetes Care (2015) 38:1876–82. doi: 10.2337/dc15-0780
29. Hypponen E, Virtanen SM, Kenward MG, Knip M, Akerblom HK, G. Childhood Diabetes in Finland Study. Obesity, increased linear growth, and risk of type 1 diabetes in children. Diabetes Care (2000) 23:1755–60. doi: 10.2337/diacare.23.12.1755
30. Elamin A, Hussein O, Tuvemo T. Growth, puberty, and final height in children with Type 1 diabetes. J Diabetes Complications (2006) 20:252–6. doi: 10.1016/j.jdiacomp.2005.07.001
31. Hassan K, Loar R, Anderson BJ, Heptulla RA. The role of socioeconomic status, depression, quality of life, and glycemic control in type 1 diabetes mellitus. J Pediatr (2006) 149:526–31. doi: 10.1016/j.jpeds.2006.05.039
32. Noser AE, Majidi S, Finch J, Clements MA, Youngkin EM, Patton SR. Authoritarian parenting style predicts poorer glycemic control in children with new-onset type 1 diabetes. Pediatr Diabetes (2018) 19:1315–21. doi: 10.1111/pedi.12726
Keywords: type 1 diabetes, children, multiple daily insulin injections therapy, continuous subcutaneous insulin infusion, China
Citation: Wang X, Zhao X, Chen D, Zhang M and Gu W (2021) Comparison of Continuous Subcutaneous Insulin Infusion and Multiple Daily Injections in Pediatric Type 1 Diabetes: A Meta‐Analysis and Prospective Cohort Study. Front. Endocrinol. 12:608232. doi: 10.3389/fendo.2021.608232
Received: 19 September 2020; Accepted: 21 January 2021;
Published: 02 March 2021.
Edited by:
Andrea Enzo Scaramuzza, Istituti Ospitalieri di Cremona, ItalyReviewed by:
Gianvincenzo Zuccotti, University of Milan, ItalyCopyright © 2021 Wang, Zhao, Chen, Zhang and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Gu, Z3V3ZWkxNTRAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.