
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 31 March 2021
Sec. Bone Research
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.599339
Objective: Nonalcoholic fatty liver disease (NAFLD) and sarcopenia, which are common in elderly men, are known as risk factors of fracture. However, few studies have examined the association with fracture in these patients. Therefore, we aimed to investigate the association between NAFLD with or without sarcopenia and 10-year fracture probability in Korean men aged ≥50 years.
Materials and Methods: Data of 2,525 individuals from the 2010–2011 Korea National Health and Nutrition Examination Survey were analyzed. NAFLD was defined using the fatty liver index (FLI) and comprehensive NAFLD score (CNS), and liver fibrosis using the fibrosis 4 calculator. Sarcopenia was defined as the lowest quintile for sex-specific sarcopenia index cutoff; values. The Fracture Risk Assessment (FRAX) tool was used to predict the 10-year probability of major osteoporotic and hip fractures.
Results: Compared to the no NAFLD group, the 10-year major osteoporotic fracture probability was significantly associated with the FLI-defined (β = 0.16, P = 0.002) and CNS-defined (β = 0.20, P < 0.001) NAFLD groups with liver fibrosis. Similarly, the 10-year hip fracture probability was significantly associated with the FLI- and CNS-defined NAFLD with liver fibrosis groups compared to the group without NAFLD (FLI-defined group, β = 0.04, P = 0.046; CNS-defined group, β = 0.05, P = 0.048). Furthermore, in the group with sarcopenia, the 10-year major osteoporotic fracture probability was significantly associated with the FLI- and CNS-defined NAFLD with liver fibrosis groups compared to the group without NAFLD (FLI-defined group, β = 0.29, P = 0.003; CNS-defined group, β = 0.38, P < 0.001).
Conclusions: NAFLD with liver fibrosis is significantly associated with a higher 10-year major osteoporotic and hip fracture probability in Korean men aged ≥50 years, and this positive association was more profound in patients with sarcopenia. Therefore, screening middle-aged to elderly men who have NAFLD combined with liver fibrosis and sarcopenia may help prevent fractures.
Fractures not only reduce the quality of life, but also increase medical and health care cost, thereby imposing a significant financial and socioeconomic burden (1). Therefore, it is important to predict and prevent fractures. Currently, the Fracture Risk Assessment (FRAX), which is a country-specific instrument developed by the World Health Organization (WHO), is the most widely used fracture prediction tool for individuals aged > 40 years (2). In FRAX, the 10-year probability of both major osteoporotic and hip fractures can be calculated by inputting ten clinical risk factors for fracture (2). Based on FRAX, 37.7% of menopausal women and 12.7% of men aged over 50 years in South Korea are at high risk of osteoporotic fracture (3). Another study also found that South Korean women and men have an average of 59.5% and 23.8% risk of osteoporotic fractures, respectively, in their lifetime (4).
Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases. It initially manifests as mild steatosis or nonalcoholic steatohepatitis, but can progress to liver fibrosis, end-stage liver disease with cirrhosis, and hepatocellular carcinoma (5). The incidence of NAFLD is constantly increasing, and it is associated with liver-related morbidity and mortality (1). NAFLD is considered a hepatic manifestation of metabolic syndrome. It is associated with various metabolic abnormalities and may be associated with an increased risk of fractures (6). However, evidence on the association between NAFLD and bone mineral density (BMD) are conflicting (7). Most studies investigating this association with BMD have targeted mild liver steatosis (8), and few studies have evaluated patients with advanced NAFLD, such as nonalcoholic steatohepatitis (9). Furthermore, there is no direct analysis of the association between NAFLD and fracture using the FRAX tool.
The prevalence of NAFLD is twice as high in men than in women (10), and one study also reported that NAFLD and sarcopenia often co-occur in elderly patients (11). Sarcopenia is associated with decreased bone density and loss of muscle mass, thus making it a risk factor for osteoporotic fracture and a predisposing factor for falls (12). A systematic review also reported that sarcopenia was independently associated with NAFLD and, possibly, advanced fibrosis (13). Therefore, we focused on the effect of NAFLD with liver fibrosis and sarcopenia on the 10-year fracture probability evaluated using the FRAX tool in middle-aged to elderly Korean men. One study that evaluated the 10-year fracture probability using the FRAX tool showed that such probability was increased in middle-aged Korean women with metabolic syndrome (14). However, to the best our knowledge, the 10-year fracture probability evaluated using FRAX tool has not been investigated in patients with NAFLD, liver fibrosis, and sarcopenia. In this study, we hypothesized that NAFLD is significantly associated with a higher 10-year fracture probability, and this association are more profound in patients with sarcopenia. Therefore, we aimed to investigate the association between 10-year fracture risk and NAFLD with or without sarcopenia in Korean men aged ≥50 years, using data from the Korea National Health and Nutrition Examination Survey (KNHANES).
This cross-sectional study used data from the 2010–2011 KNHANES. The KNHANES is a nationwide population-based survey conducted by the Korean Ministry of Health and Welfare and the Division of Chronic Disease Surveillance of the Korean Centers for Disease Control and Prevention.
Among the 17,476 subjects of the 2010–2011 KNHANES, 2,915 men aged ≥50 years were initially evaluated. Of them, we excluded 191 patients with physician-diagnosed hepatitis B, hepatitis C, liver cirrhosis, hepatocellular carcinoma, and rheumatoid arthritis, which is risk factor for fracture and patients who were high-risk drinkers (> 210 g per week for men and > 140 g per week for women). From the remaining 2,724 patients, those with missing data on factors needed for calculating the fatty liver index (FLI), comprehensive NAFLD score (CNS), fibrosis 4 calculator (FIB-4), and FRAX were further excluded. Finally, 2,525 subjects were included in the final analysis (Figure 1).
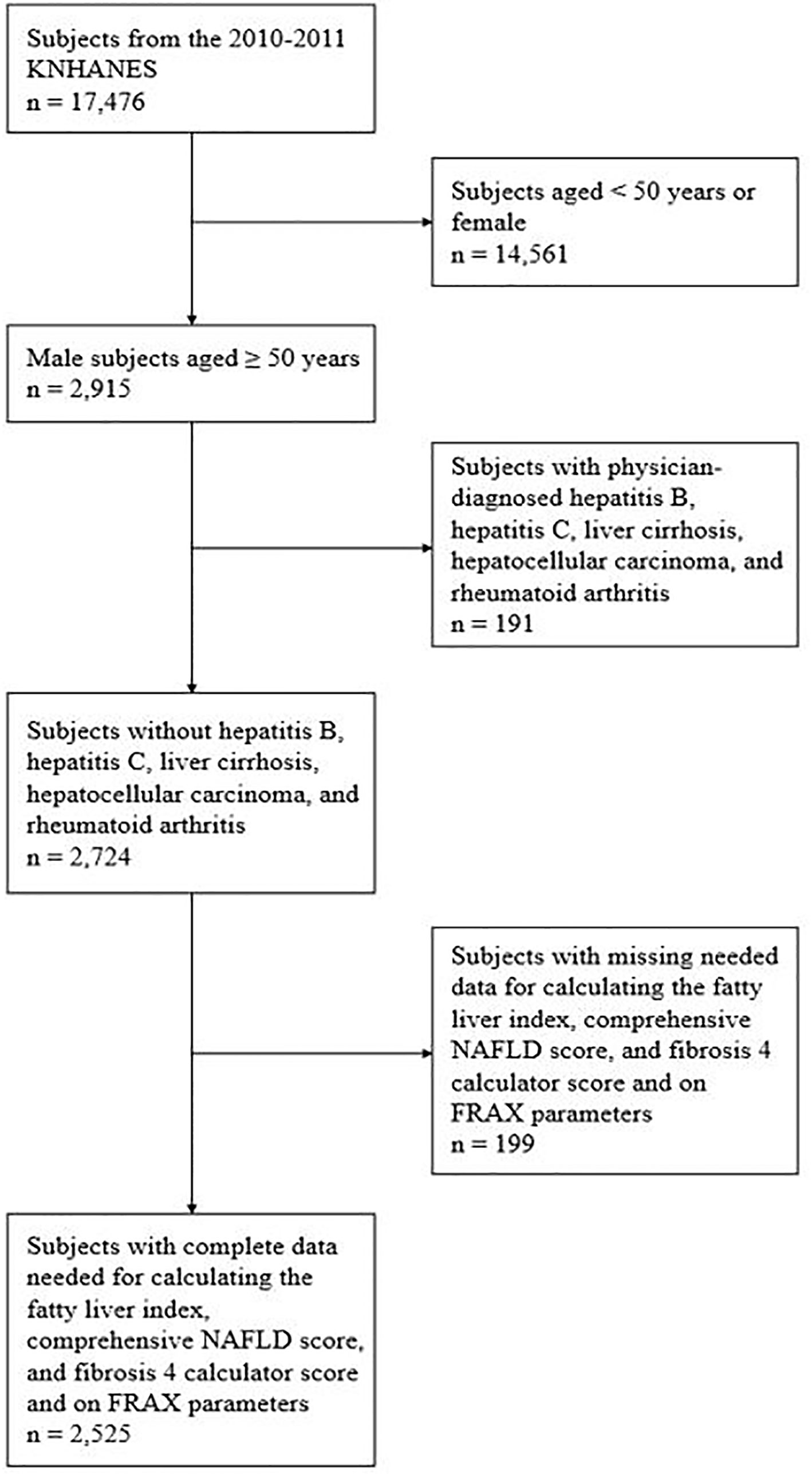
Figure 1 Subject inclusion flow chart. KNHANES, Korea National Health and Nutrition Examination Survey; NAFLD, nonalcoholic fatty liver disease; FRAX, fracture risk assessment.
All participants provided written informed consent before participation in the study, and the KNHANES was conducted following ethical approval by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (No. 2010-02CON-21-C, 2011-02CON-06C). The protocol of the current study was approved by the Institutional Review Board of Yonsei University, Seoul, Korea (No. 4-2020-0744).
Subjects were interviewed by trained staff using standardized health questionnaires, which included questions on demographic information, medical history of osteoporosis diagnosis or fracture, parental history of fracture, history of smoking, and alcohol intake. In our study, the variables used in the FRAX tool to predict the 10-year probability of major osteoporotic fracture (spine, forearm, hip, or shoulder fractures) and hip fracture (2) were considered when adjusting for the risk factors. The variables included age, sex, previous history of fracture, parental history of hip fracture, prevalence of rheumatoid arthritis, secondary osteoporosis, use of steroids, current smoking, drinking more than 3 units per day, body weight, height, and bone mineral density of the femoral neck (2). Anthropometric measurements and laboratory tests were also conducted.
NAFLD was defined using FLI and CNS, and liver fibrosis using FIB-4. The FLI model was calculated using four variables: body mass index (BMI), waist circumference, serum triglycerides (TG), and serum γ-glutamyl transferase (15). The CNS model was calculated using the parameters of age, BMI, waist circumference, prevalence of diabetes, alcohol intake, exercise, serum fasting glucose, serum TG, serum high density lipoprotein-cholesterol, serum uric acid, serum aspartate aminotransferase, and serum alanine aminotransferase (16). A FLI of ≥60 and a CNS of ≥40 were considered indicative of NAFLD (15, 16). The FIB-4 score was calculated using the parameters of age, plasma platelet count, serum aspartate aminotransferase, and serum alanine aminotransferase, with liver fibrosis defined as scores above 1.3 (17). According to the presence of NAFLD and liver fibrosis, it was classified into three groups; no NAFLD group, NAFLD without liver fibrosis group, and NAFLD with liver fibrosis group.
Sarcopenia was determined using the sarcopenia index (sarcopenia index = total appendicular skeletal muscle mass (kg)/BMI (kg/m2)), which involved measurement of the appendicular skeletal muscle mass via dual-energy x-ray absorptiometry (DEXA, QDR 4500A; Hologic, Bedford, MA). Sarcopenia was defined as the lowest quintile for sex-specific sarcopenia index cutoff values (< 0.807 for men) based on a modified recommendation from the Foundation for the National Institutes of Health (18). BMD of the femoral neck was also examined using DEXA. BMI was calculated by dividing weight (kg) with the square of height (m2). Smoking was defined as current smoking. Monthly alcohol intake was defined as drinking more than once a month during the past year.
The characteristics of the subjects classified according to the presence of NAFLD and liver fibrosis were expressed as the means and standard deviations for continuous variables and as numbers and percentages for categorical variables. A one-way analysis of variance was used to compare the continuous variables and a chi-squared test for categorical variables. For post hoc analysis, the Bonferroni method was used.
Multivariate linear regression analysis was performed to analyze the association between NAFLD with liver fibrosis and sarcopenia and the 10-year major osteoporotic and hip fracture probability. The regression coefficients (β) and 95% confidence intervals (CIs) were estimated. We adjusted for multiple variables that showed significant associations in the univariate analysis and those based on clinical relevance. After calculating the crude β (model 1), model 2 was adjusted for age, osteoporosis diagnosis, fracture history, parental history of fracture, BMI, BMD of the femoral neck, current smoking, and alcohol intake. All variables in the linear regression analysis were examined for multi-collinearity, and only those variables with a variance inflation factor of < 5 were used. All statistical analyses were performed using IBM SPSS version 25 (Armonk, NY, USA). All P values were two-tailed, and a P value of < 0.05 was considered statistically significant.
A total of 2,525 subjects were included in this study (Figure 1). Table 1 shows the demographic characteristics of the subjects according to NAFLD and liver fibrosis. The prevalence rates of FLI- and CNS-defined NAFLD were 20% (n = 512) and 46% (n = 1158), respectively. The prevalence rate for NAFLD accompanied by FIB-4–defined liver fibrosis was 11% (n = 279) in patients with FLI-defined NAFLD and 24% (n = 614) in patients with CNS-defined NAFLD.
In the analysis of the three groups of NAFLD defined by FLI and CNS, age was the youngest and the 10-year hip fracture probability was the lowest in the NAFLD without liver fibrosis group (P < 0.001 and P = 0.001, respectively). Meanwhile, the BMI, BMD of the femoral neck, prevalence of sarcopenia, and alcohol intake were the lowest in the group without NAFLD (all P < 0.001). The 10-year major osteoporotic fracture probability was also the lowest in the group without NAFLD (P = 0.042 for FLI-defined NAFLD; P < 0.001 for CNS-defined NAFLD).
Table 2 shows the β and 95% CIs for the 10-year major osteoporotic fracture probability according to FLI- and CNS-defined NAFLD with FIB-4–defined liver fibrosis in the overall cohorts. The model was adjusted for age, osteoporosis diagnosis, previous and parental history of fracture, BMD of the femoral neck, BMI, current smoking, and alcohol intake.
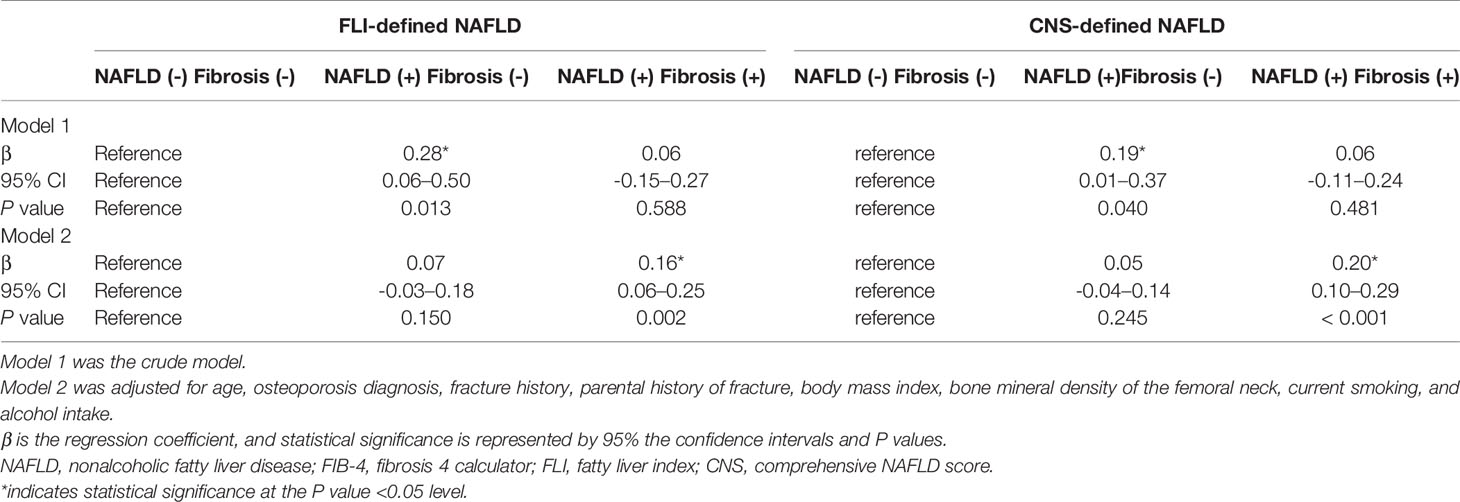
Table 2 Association between NAFLD with FIB-4–defined liver fibrosis and 10-year major osteoporotic fracture probability in Korean men aged ≥50 years.
The 10-year major osteoporotic fracture probability was higher in the FLI- and CNS-defined NAFLD without liver fibrosis groups than that in the group without NAFLD, although the difference was not significant. However, compared to the no NAFLD group, the 10-year major osteoporotic fracture probability showed significant positive associations with the FLI-defined (β = 0.16, P = 0.002) and CNS-defined (β = 0.20, P < 0.001) NAFLD groups with liver fibrosis.
For the 10-year hip fracture probability, compared with the group without NAFLD, the FLI- and CNS-defined NAFLD with liver fibrosis groups showed significant positive associations between the fracture probability and NAFLD with liver fibrosis (Table 3, FLI-defined group, β = 0.04, P = 0.046; CNS-defined group, β = 0.05, P = 0.048).
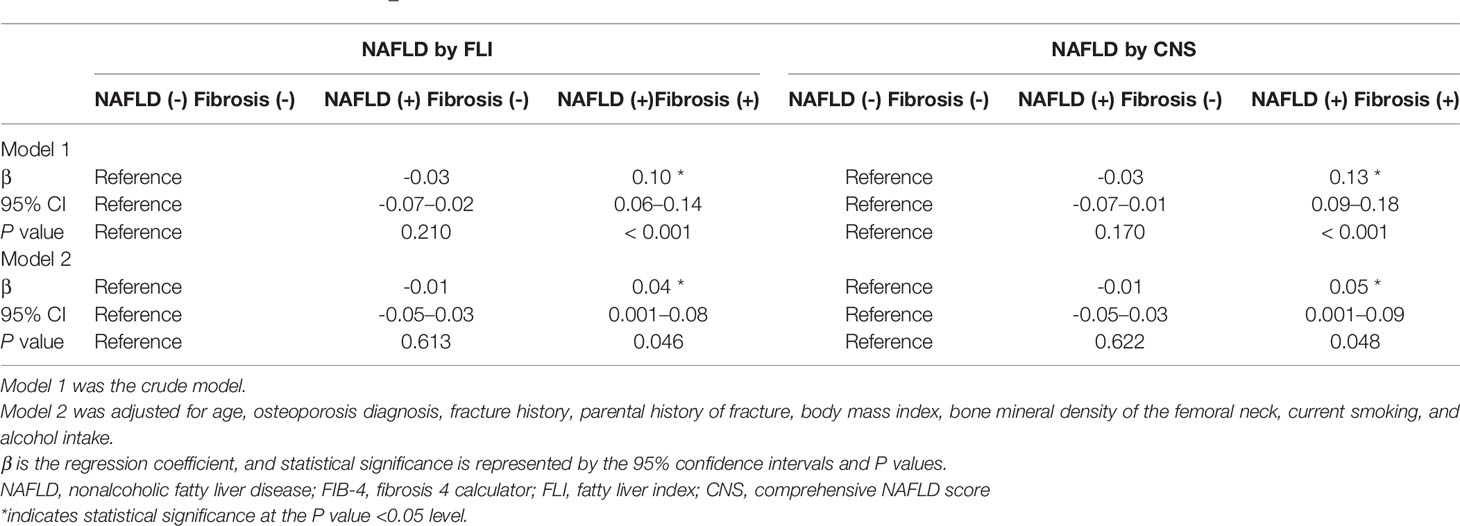
Table 3 Association of NAFLD with FIB-4–defined liver fibrosis and 10-year hip fracture probability in Korean men aged ≥50 years.
Table 4 shows the β and 95% CIs for the 10-year major osteoporotic fracture probability according to FLI- and CNS-defined NAFLD with FIB-defined liver fibrosis in 413 men with sarcopenia aged ≥50 years. This model was also adjusted for age, osteoporosis diagnosis, previous or parental history of fracture, BMD of the femoral neck, BMI, current smoking, and alcohol intake.
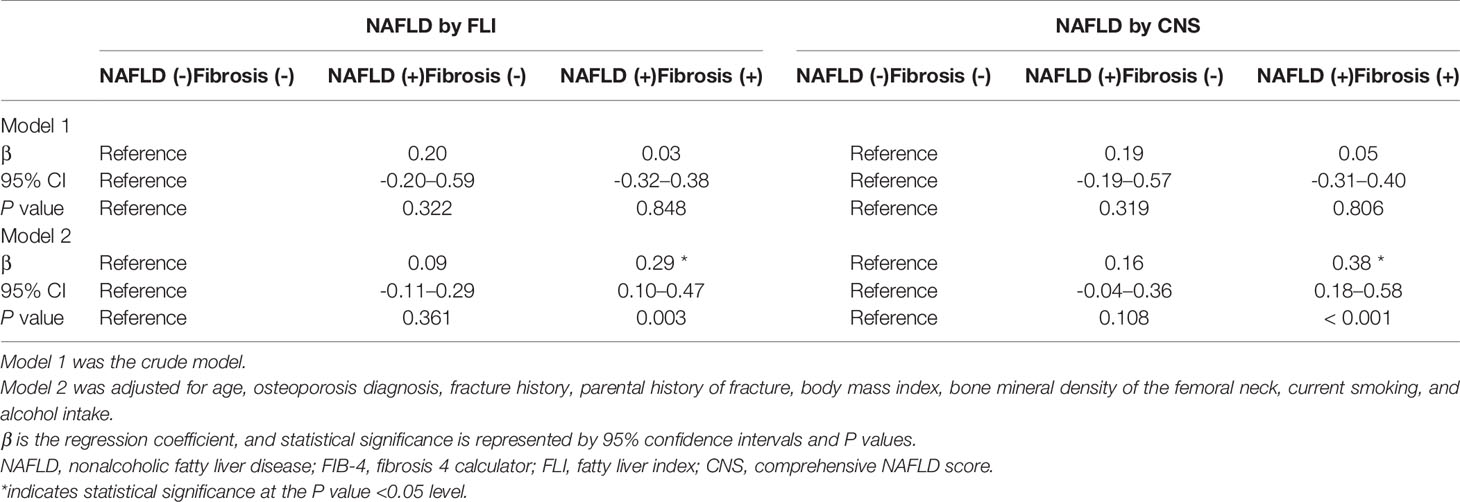
Table 4 Association of NAFLD with FIB-4 defined liver fibrosis and 10-year major osteoporotic fracture probability in Korean men with sarcopenia aged ≥50 years.
Compared with the group without NAFLD, the 10-year major osteoporotic fracture probability was significantly higher in the FLI-defined (β = 0.29, P = 0.003) and CNS-defined (β = 0.38, P < 0.001) NAFLD with liver fibrosis groups.
This cross-sectional study of data from the 2010–2011 KNHANES showed that NAFLD with liver fibrosis and sarcopenia were significantly associated with the 10-year major osteoporotic probability in Korean men aged ≥50 years. Therefore, we suggested that developing preventive and pharmacological approaches for the management of NAFLD with liver fibrosis and sarcopenia are needed as a preventive strategy for fracture (6).
Known risk factors for osteoporosis and fracture, such as poor nutrition, excess alcohol intake, hypogonadism, and corticosteroid use, are commonly found in patients with chronic liver disease (8). In addition, chronic liver disease in adults (e.g., cirrhosis and advanced liver disease) is a risk factor itself for osteoporosis and increased bone fragility (19). Several studies reported a 10-56% reduction in BMD in adult patients with liver fibrosis or viral liver cirrhosis (20, 21). These results suggest that advanced liver fibrosis, which has progressed in the absence of hepatic decompensation, has a negative effect on bone mass and quality (22). However, there is no research on the direct association between NAFLD with liver fibrosis and fracture probability. Further, the pathophysiological mechanisms by which NAFLD is associated with fractures have not been well established (6).
Our results showed a higher risk of fracture in the group with NAFLD with liver fibrosis, which could be explained by the following mechanisms. Insulin-like growth factor-1 is an important driver of bone formation from mesenchymal stem cells and promotes osteoblast survival and differentiation (23). It is produced primarily in the liver and can lead to impaired production during liver disease (24), likely resulting in a lower bone turnover (25). In addition, excess fat accumulation in the liver causes low-grade, chronic inflammation associated with the development of bone loss and osteoporosis and a higher likelihood of fractures (26). Increased osteoclast activity is mediated by osteoclastogenic proinflammatory cytokines. For example, interleukin 1 and tumor necrosis factor (TNF) are involved in hepatic inflammation and fibrosis (8). In particular, TNF-α can reduce trabecular and cortical bone formation by inhibiting osteoblast differentiation and collagen secretion and by inducing osteoblast apoptosis (27). This cytokine can also promote trabecular and cortical bone resorption by inducing the expression of the receptor activator of nuclear factor kappa B ligand, which inhibits osteoclast activation and osteoblast apoptosis (28).
Sarcopenia increases the risk of falls and fractures by reducing mobile function and increasing frailty and imbalance (10). A systematic review reported a high prevalence of sarcopenia in men with fragility fractures and that sarcopenia was a risk factor for fractures when accompanied by low BMD in men (29). In our study, the association of NAFLD with liver fibrosis and 10-year major osteoporotic fracture probability increased in the group with sarcopenia, indicating the synergic effect of NAFLD with liver fibrosis and sarcopenia on fracture. Although the exact mechanism is unknown, one study of elderly patients reported that NAFLD and sarcopenia share common pathophysiological mechanisms that may increase the risk of fracture, including insulin resistance and chronic low-grade inflammation (13).
Our study had some limitations. First, because this was a cross-sectional study, causal inference cannot be drawn. Further larger longitudinal prospective studies with multiple comparative groups are needed to establish precise interrelationships. Second, among the variables included in the FRAX tool (2), the history of steroid uses and diseases that could cause secondary osteoporosis was not included in the analysis. Therefore, the fracture risk may have been underestimated. In addition, there are other risk factors of fracture (e.g., physical activity, calcium/vitamin D intake) that are not included in the FRAX tool. Therefore, further research including additional confounding factors is needed (30). Third, the gold standard for NAFLD diagnosis is liver biopsy. However, NAFLD and liver fibrosis were diagnosed in our study using FLI, CNS, and FIB-4. It should be noted, however, that liver biopsy is not routinely used in epidemiological studies or actual practice due to its invasiveness and potential for complication (31). In addition, the FLI has relatively high accuracy for Koreans, with an area under receiver operating characteristic curve (AUROC) of 0.86 (16). The CNS is a prediction model developed using data from 15,676 Koreans diagnosed via ultrasound. Further, its validation in 66,868 independent examination cohorts yielded a relatively high AUROC value of 0.8-0.9 (16). FIB-4 has also been validated to be a relatively accurate method for diagnosing NAFLD in various races (17). Therefore, the above three diagnostic tools can be considered reliable and easily accessible for the diagnosis of NAFLD and liver fibrosis.
Despite these limitations, this study is valuable because to our best knowledge, this is the first to investigate the association between NAFLD with liver fibrosis and sarcopenia and the 10-year fracture probability. It also provides evidence on the potential of NAFLD with more advanced liver fibrosis and sarcopenia as a therapeutic target for reducing the risk of fractures. The potential impact and precise pathophysiological mechanisms by which NAFLD with liver fibrosis and sarcopenia is associated with the development of fracture warrants further study (32).
In conclusion, this quantitative cross-sectional analysis of data from the 2010–2011 KNHANES revealed that the 10-year major osteoporotic and hip fracture probability was significantly associated with FLI- and CNS-defined NAFLD with liver fibrosis. Notably, this association was more profound in the subjects with sarcopenia. These findings indicate that screening middle-aged to elderly men who have NAFLD combined with liver fibrosis and sarcopenia for the prediction of further fracture may be a potential preventive strategy against future fractures (32).
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
All participants provided written informed consent before participation in the study, and the KNHANES was conducted following ethical approval by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (No. 2010-02CON-21-C, 2011-02CON-06C). This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Review Board of Yonsei University, Seoul, Korea (Approval No. 4-2020-0744).
HL, DL, and CK: contributed to the study design. HL: performed data collection, interpretation, and analysis. HL and CK participated in the writing and modification of the article. DL and CK: performed critical review. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
1. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int (2006) 17(12):1726–33. doi: 10.1007/s00198-006-0172-4
2. Kanis JA, McCloskey EV, Johansson H, Strom O, Borgstrom F, Oden A. Case finding for the management of osteoporosis with FRAX – assessment and intervention thresholds for the UK (2008). Available at: www.sheffield.ac.uk/FRAX (Accessed August 26, 2020).
3. Kim JW, Jeon YJ, Baek DH, Kim TN, Chang JS. Percentage of the population at high risk of osteoporotic fracture in South Korea: analysis of the 2010 Fifth Korean National Health and Nutrition Examination survey data. Osteoporos Int (2014) 25(4):1313–9. doi: 10.1007/s00198-013-2595-z
4. Park C, Ha YC, Jang S, Yoon HK, Lee YK. The incidence and residual lifetime risk of osteoporosis-related fractures in Korea. J Bone Miner Metab (2011) 29(6):744–51. doi: 10.1007/s00774-011-0279-3
5. Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology (2002) 123:134–40. doi: 10.1053/gast.2002.34168
6. Li M, Xu Y, Xu M, Ma L, Wang T, Liu Y, et al. Association between nonalcoholic fatty liver disease (NAFLD) and osteoporotic fracture in middle-aged and elderly Chinese. J Clin Endocrinol Metab (2012) 97(6):2033–8. doi: 10.1210/jc.2011-3010
7. Lee SH, Yun JM, Kim SH, Seo YG, Min H, Chung E, et al. Association between bone mineral density and nonalcoholic fatty liver disease in Korean adults. J Endocrinol Invest (2016) 39(11):1329–36. doi: 10.1007/s40618-016-0528-3
8. Collier J. Bone disorders in chronic liver disease. Hepatology (2007) 46(4):1271–8. doi: 10.1002/hep.21852
9. Purnak T, Beyazit Y, Ozaslan E, Efe C, Hayretci M. The evaluation of bone mineral density in patients with nonalcoholic fatty liver disease. Wien Klin Wochenschr (2012) 124:526–31. doi: 10.1007/s00508-012-0211-4
10. Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. Prevalence of primary non-alcoholic fatty liver disease in a population-based study and its association with biochemical and anthropometric measures. Liver Int (2006) 26:856–63. doi: 10.1111/j.1478-3231.2006.01311.x
11. Wijarnpreecha K, Panjawatanan P, Thongprayoon C, Jaruvongvanich V, Ungprasert P. Sarcopenia and risk of nonalcoholic fatty liver disease: A meta-analysis. Saudi J Gastroenterol (2018) 24(1):12–7. doi: 10.4103/sjg.SJG_237_17
12. Hida T, Harada A, Imagama S, Ishiguro N. Managing sarcopenia and its related-fractures to improve quality of life in geriatric populations. Aging Dis (2013) 5(4):226–37. doi: 10.14336/AD.2014.0500226
13. Tovo CV, Fernandes SA, Buss C, de Mattos AA. Sarcopenia and non-alcoholic fatty liver disease: Is there a relationship? A systematic review. World J Hepatol (2017) 9(6):326–32. doi: 10.4254/wjh.v9.i6.326
14. Lee HJ, Hwang SY, Kim SC, Joo JK, Suh DS, Kim KH. Relationship Between Metabolic Syndrome and Bone Fracture Risk in Mid-Aged Korean Women Using FRAX Scoring System. Metab Syndr Relat Disord (2020) 18(4):219–24. doi: 10.1089/met.2019.0060
15. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol (2006) 6:33. doi: 10.1186/1471-230X-6-33
16. Lee YH, Bang H, Park YM, Bae JC, Lee BW, Kang ES, et al. Nonlaboratory-based self-assessment screening score for non-alcoholic fatty liver disease: development, validation and comparison with other scores. PloS One (2014) 9:e107584. doi: 10.1371/journal.pone.0107584
17. Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology (2007) 46:32–6. doi: 10.1002/hep.21669
18. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci (2014) 69:547–58. doi: 10.1093/gerona/glu010
19. Leslie WD, Bernstein CN, Leboff MS. AGA technical review on osteoporosis in hepatic disorders. Gastroenterology (2003) 125:941–66. doi: 10.1016/S0016-5085(03)01062-X
20. Bonkovsky HL, Hawkins M, Steinberg K, Hersh T, Galambos JT, Henderson JM, et al. Prevalence and prediction of osteopenia in chronic liver disease. Hepatology (1990) 12:273–80. doi: 10.1002/hep.1840120214
21. Crawford BA, Kam C, Donaghy AJ, McCaughan GW. The heterogeneity of bone disease in cirrhosis: a multivariate analysis. Osteoporos Int (2003) 14:987–94. doi: 10.1007/s00198-003-1495-z
22. Lo Re V3, Lynn K, Stumm ER, Long J, Nezamzadeh MS, Baker JF, et al. Structural Bone Deficits in HIV/HCV-Coinfected, HCV-Monoinfected, and HIV-Monoinfected Women. J Infect Dis (2015) 212(6):924–33. doi: 10.1093/infdis/jiv147
23. Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, et al. Targeted overexpression of insulin-like growth factor 1 to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology (2000) 141:2674–82. doi: 10.1210/endo.141.7.7585
24. Moller S, Juul A, Becker U, Flyvbjerg A, Skakkebaek NE, Henriksen JH. Concentrations, release, and disposal of insulin-like growth factor(IGF)-binding proteins(IGFBP), IGF-1, and growth hormone in different vascular beds in patients with cirrhosis. J Clin Endocrinol Metab (1995) 80:1148–57. doi: 10.1210/jcem.80.4.7536200
25. Bedimo R, Cutrell J, Zhang S, Drechsler H, Gao A, Brown G, et al. Mechanisms of bone disease in HIV and hepatitis C virus: impact of bone turnover, tenofovir exposure, sex steroids and severity of liver disease. AIDS (2016) 30(4):601–8. doi: 10.1097/QAD.0000000000000952
26. Ding C, Parameswaran V, Udayan R, Burgess J, Jones G. Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults: a longitudinal study. J Clin Endocrinol Metab (2008) 93:1952–8. doi: 10.1210/jc.2007-2325
27. Gilbert L, He X, Farmer P, Rubin J, Drissi H, van Wijnen AJ, et al. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem (2002) 277:2695–701. doi: 10.1074/jbc.M106339200
28. Gilbert L, He X, Farmer P, Boden S, Kozlowski M, Rubin J, et al. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology (2000) 141:3956–64. doi: 10.1210/endo.141.11.7739
29. Wong RMY, Wong H, Zhang N, Chow SKH, Chau WW, Wang J, et al. The relationship between sarcopenia and fragility fracture-a systematic review. Osteoporos Int (2019) 30(3):541–53. doi: 10.1007/s00198-018-04828-0
30. Reid IR. Effects of vitamin D supplements on bone density. J Endocrinol Invest (2015) 38:91–4. doi: 10.1007/s40618-014-0127-0
31. Wang Y, Wen G, Zhou R, Zhong W, Lu S, Hu C, et al. Association of Nonalcoholic Fatty Liver Disease With Osteoporotic Fractures: A Cross-Sectional Retrospective Study of Chinese Individuals. Front Endocrinol (2018) 9:408. doi: 10.3389/fendo.2018.00408
Keywords: nonalcoholic fatty liver disease, liver fibrosis, sarcopenia, 10-year fracture probability, fracture risk assessment tool
Citation: Lee HJ, Lee DC and Kim CO (2021) Association Between 10-Year Fracture Probability and Nonalcoholic Fatty Liver Disease With or Without Sarcopenia in Korean Men: A Nationwide Population-Based Cross-Sectional Study. Front. Endocrinol. 12:599339. doi: 10.3389/fendo.2021.599339
Received: 27 August 2020; Accepted: 16 March 2021;
Published: 31 March 2021.
Edited by:
Elaine Dennison, MRC Lifecourse Epidemiology Unit (MRC), United KingdomReviewed by:
Subhashis Pal, Emory University, United StatesCopyright © 2021 Lee, Lee and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Duk Chul Lee, ZmFpdGhAeXVocy5hYw==; Choon Ok Kim, ZGVsaXZlcnk5OEB5dWhzLmFj
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.