
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 25 February 2021
Sec. Obesity
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.598249
This article is part of the Research Topic Covid-19 and Obesity View all 16 articles
Background and Objective: Obesity has been reported as a risk factor for adverse outcomes in COVID-19. However, available studies presenting data on obesity prevalence in patients with COVID-19 have conflicting results. The objective of this systematic review and meta-analysis is to evaluate the prevalence of obesity in these patients and to stratify the estimates by illness severity.
Methods: We performed a literature search with the use of Medline/PubMed and Google Scholar database from December 1, 2019 to June 27, 2020 and systematically reviewed studies reporting the number of obese patients with real-time reverse transcriptase polymerase chain reaction (rRT-PCR)-confirmed SARS-CoV-2 infection.
Results: Nineteen studies were identified. The pooled obesity prevalence rates were 0.32 (95% CI: 0.24–0.41) in hospitalized patients, 0.41 (95% CI: 0.36–0.45) in patients admitted to intensive care unit, 0.43 (95% CI: 0.36–0.51) in patients needing invasive mechanic ventilation (IMV), and 0.33 (95% CI: 0.26–0.41) in those who died. Obesity was associated with a higher risk for hospitalization [Odds ratio (OR): 1.3, 95% CI: 1.00–1.69; I2 52%, p = 0.05], ICU admission (OR: 1.51, 95% CI: 1.16–1.97; I2 72%, p = 0.002), and IMV requirement (OR: 1.77, 95% CI: 1.34–2.35; I2 0%, p < 0.001). The increase in risk of death did not reach statistical significance (OR: 1.28, 95% CI: 0.76–2.16, p = 0.35) which might be due to obesity survival paradox and/or unidentified factors.
Conclusions: Our data indicate that obese subjects may be at higher risk for serious illness if infected and obesity may play a role in the progression of COVID-19.
Coronavirus disease 19 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly spread and become a global pandemic, with more than 13 million confirmed cases resulting in over 585,000 confirmed deaths as of July 17, 2020 (1). Clinical manifestations of COVID-19 vary in a broad spectrum, ranging from asymptomatic or mild infection to life-threatening acute respiratory distress syndrome and multiorgan failure. Older age and the presence of comorbidities including hypertension (HT), type 2 diabetes (T2DM), and cardiovascular disease (CVD) seems to be associated with a more severe course of COVID-19 (2–4).
Obesity represents a major and urgent global health problem (5). It tends to increase with increasing age and is a known risk factor for the abovementioned comorbidities identified as predisposing factors for adverse outcomes in COVID-19 (5). Although there are several reports evaluating the burden of obesity on the clinical course of COVID-19, it has not been fully documented whether people living with obesity have a higher risk of getting COVID-19. Most of the earlier studies on COVID-19 did not provide information about body mass index (BMI) or other measures of adiposity of the patients (2–4, 6–8). Other studies which present data on obesity prevalence in patients with COVID-19 have conflicting results, reporting similar, lower, or higher rates of obesity compared to general population (9–12). The aim of this systematic review and meta-analysis was, therefore, to evaluate the prevalence of obesity in patients with COVID-19 and to stratify the estimates by illness severity.
We report this systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (13). The review protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42020199145).
The following medical subject titles, key words, and their combinations were used to search on Medline/PubMed and Google Scholar database for retrospective cohorts, cross-sectional and longitudinal studies including gray literature as pre-prints, conference papers, and reports which were accessed online between December 1, 2019 and June 27, 2020: 2019 nCoV, SARS-CoV-2, COVID-19, coronavirus disease 2019, obesity, body mass index, BMI, clinical features, risk factors. Reference lists of relevant articles were also screened to capture other potentially eligible studies. The literature search was concluded by June 27, 2020 and only reports written in English language were assessed.
The primary outcome measure was to evaluate the overall prevalence of obesity in COVID-19 infection and stratify the estimates by disease severity and geographic region. Studies reporting the number of obese patients with real-time reverse transcriptase polymerase chain reaction (rRT-PCR)-confirmed SARS-CoV-2 infection at least in two of the following groups were included in the meta-analysis: all cases, hospitalized patients, patients admitted to an intensive care unit (ICU), patients needing invasive mechanical ventilation (IMV), and patients who died. Duplicate publications, reviews, perspectives and letters not presenting original data, studies lacking information on BMI or obesity were excluded, as well as studies including any intervention, pediatric population, patients with any specific condition (e.g., malnutrition, pregnancy), or fewer than 50 patients. Among studies involving the same patient groups, the largest cohort was selected. Studies that applied a BMI cut-off value other than ≥30 kg/m2 for obesity were also excluded; however, studies defining obesity as BMI ≥28 kg/m2 in Asian Pacific populations were eligible. To avoid selection bias, two reviewers (NH and ND) identified and selected articles that met the inclusion criteria. The full text of each study was assessed independently by these reviewers and any disagreement among them was discussed and resolved by consensus or discussion with a third reviewer (BY).
Information on first author, publication year, country, study design, time and duration of follow-up, total sample size, gender composition, obesity criteria, numbers reported for obesity at different outcome levels were extracted from all included studies. The methodological quality of studies was evaluated through the National Heart, Lung, and Blood Institute Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (14). Data extraction and quality assessment was conducted independently by two reviewers (NH and NE). Disagreements were resolved by joint discussion or by the third reviewer (BY).
All statistical analyses were done through metaprop and metabin commands in meta package version 4.13 in R software ver. 3.6.3. Meta-analysis of obesity prevalence was performed through the DerSimonian-Laird, random-effects method, to account for a high heterogeneity with 95% confidence interval. The Freeman–Tukey double arcsine transformation was applied for prevalences to normalize and stabilize the variance of the sampling distribution. Higgins’ I² was used to assess heterogeneity with significance set at >75%.
Initial search strategy identified a total of 3,883 records, of which 2,470 remained after the removal of duplicates. After assessment of titles and abstracts of these records, 72 articles were found to be potentially relevant. Full texts of these articles were reviewed and finally, 19 studies (9–12, 15–29) that met the inclusion criteria were included in this systematic review and meta-analysis. A PRISMA study flow diagram of this search and selection process is shown in Figure 1.
The main characteristics of the included studies are summarized in Table 1. The results of the risk of bias assessment of individual studies are shown in Table 2. All included studies were observational cohort studies. Their sample size ranged from 50 to 51,633, describing a total of 68,214 patients having confirmed COVID-19. The research time period of the included studies ranged from January 11 to May 18, 2020. Out of 19 studies, eight were conducted in the U.S., two in Mexico, six in Europe, two in China, and one in Kuwait respectively.
Five studies with 58,419 patients provided data for the combined analysis (inpatient + outpatient), 15 studies with 7,758 patients reported on hospitalization, 17 studies with 10,391 patients reported on ICU admission, and 9 studies with 5,107 patients reported on IMV requirement.
Anthropometric measurements were performed at hospital admission in only two studies (16, 22). In the rest, data from previous medical records were used to define obesity. Out of 19 studies, five had missing BMI data of the patients (9, 11, 20, 21, 29). The proportion of patients without BMI data ranged from 4.5 to 45.3% in these studies.
The pooled obesity prevalence rate in all cases (admitted + non-admitted) with COVID-19 was 0.34 [95% confidence interval (CI): 0.22–0.46] as presented in Figure 2A. The pooled obesity prevalence rate was 0.35 (95% CI: 0.22–0.49) in non-admitted patients (Figure 2B). There was significant heterogeneity among studies. I2 estimates were 100 and 95%, respectively (p < 0.01 for both).
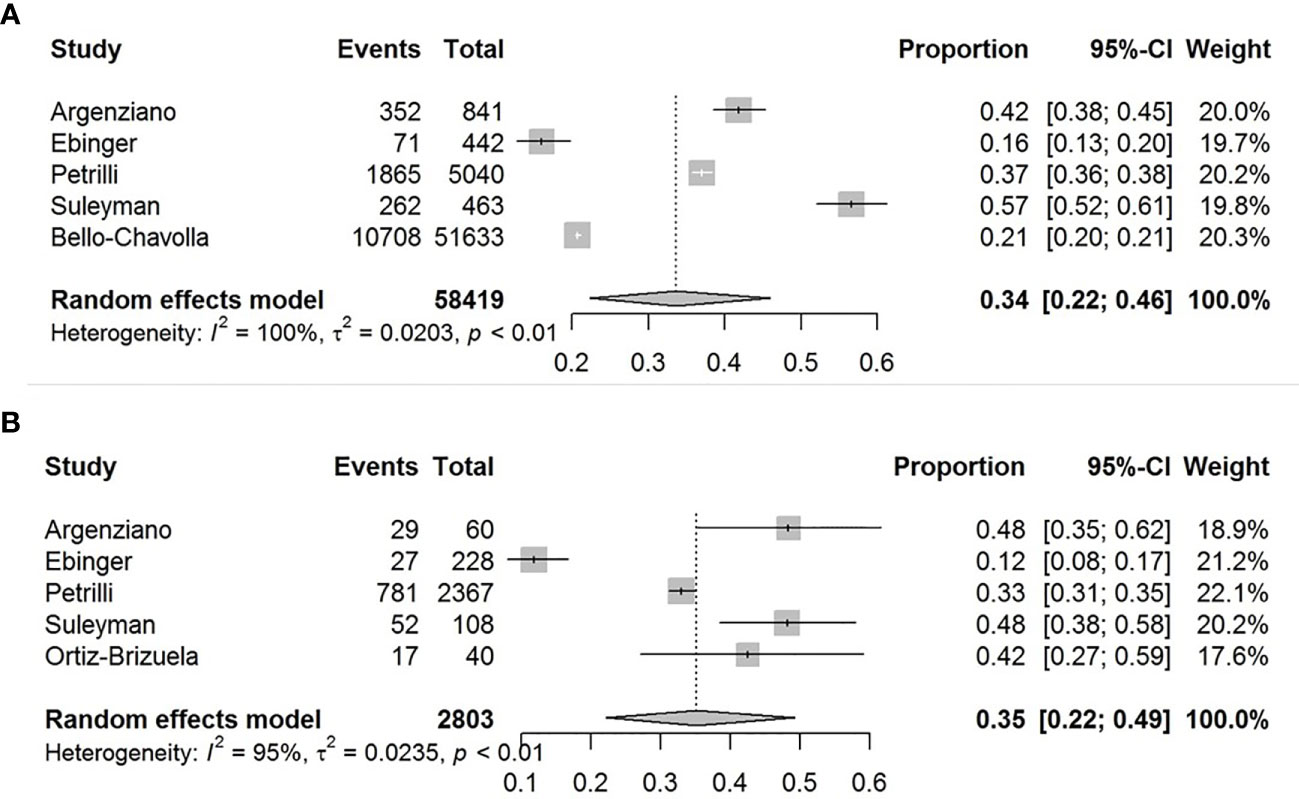
Figure 2 Forest-plots showing the prevalence rates of obesity in all cases (A) and in non-admitted patients with COVID-19 (B).
The pooled obesity prevalence rates were 0.32 (95% CI: 0.24–0.41) in hospitalized patients (Figure 3), 0.30 (95% CI: 0.21–0.39) in patients admitted to non-critical care wards (Figure 4), 0.41 (95% CI: 0.36–0.45) in patients admitted to an ICU (Figure 5), 0.43 (95% CI: 0.36–0.51) in patients with IMV requirement (Figure 6), and 0.33 (95% CI: 0.26–0.41) in those who died (Figure 7) respectively. Studies from the U.S., Europe, and Asia were included in these analyses. All rates were significantly higher than the 13.2% worldwide prevalence of obesity (30). I2 estimates were 98, 98, 92, 94, and 94%, indicating significant heterogeneity among studies (all p < 0.01).
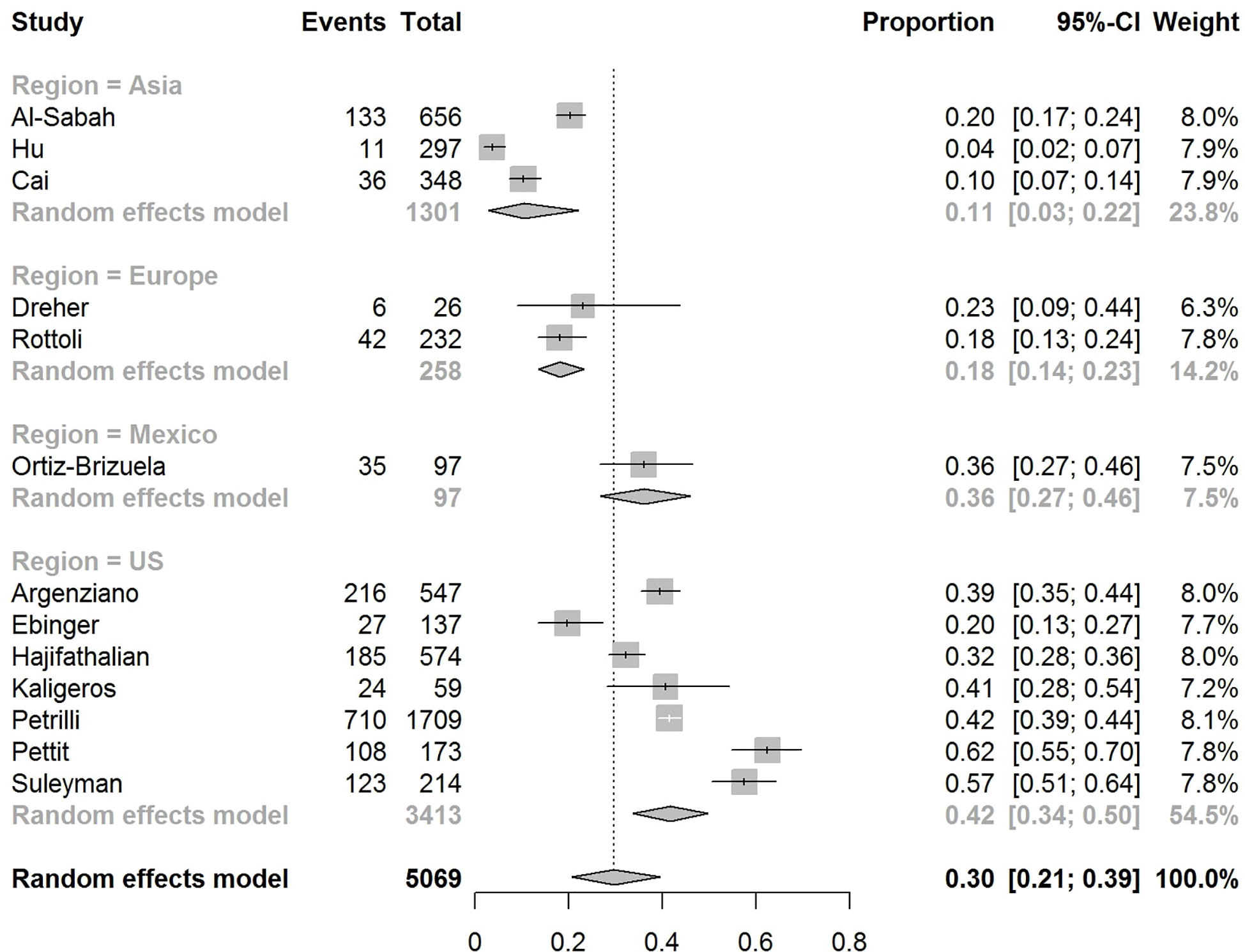
Figure 4 Forest-plot showing the prevalence rate of obesity in patients with COVID-19 admitted to non-critical care wards.
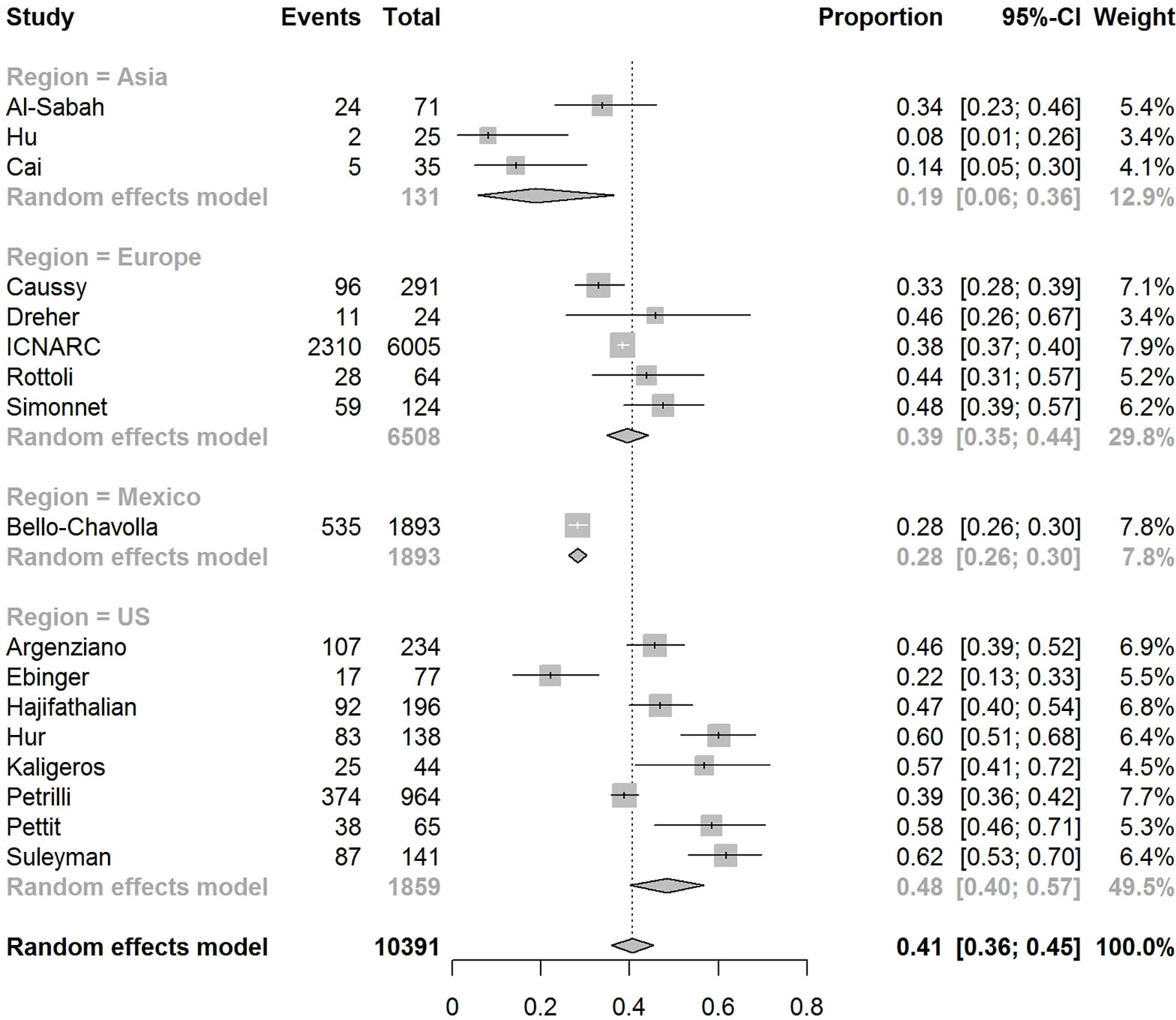
Figure 5 Forest-plot showing the prevalence rate of obesity in patients with CVOID-19 admitted to intensive care unit (ICU).
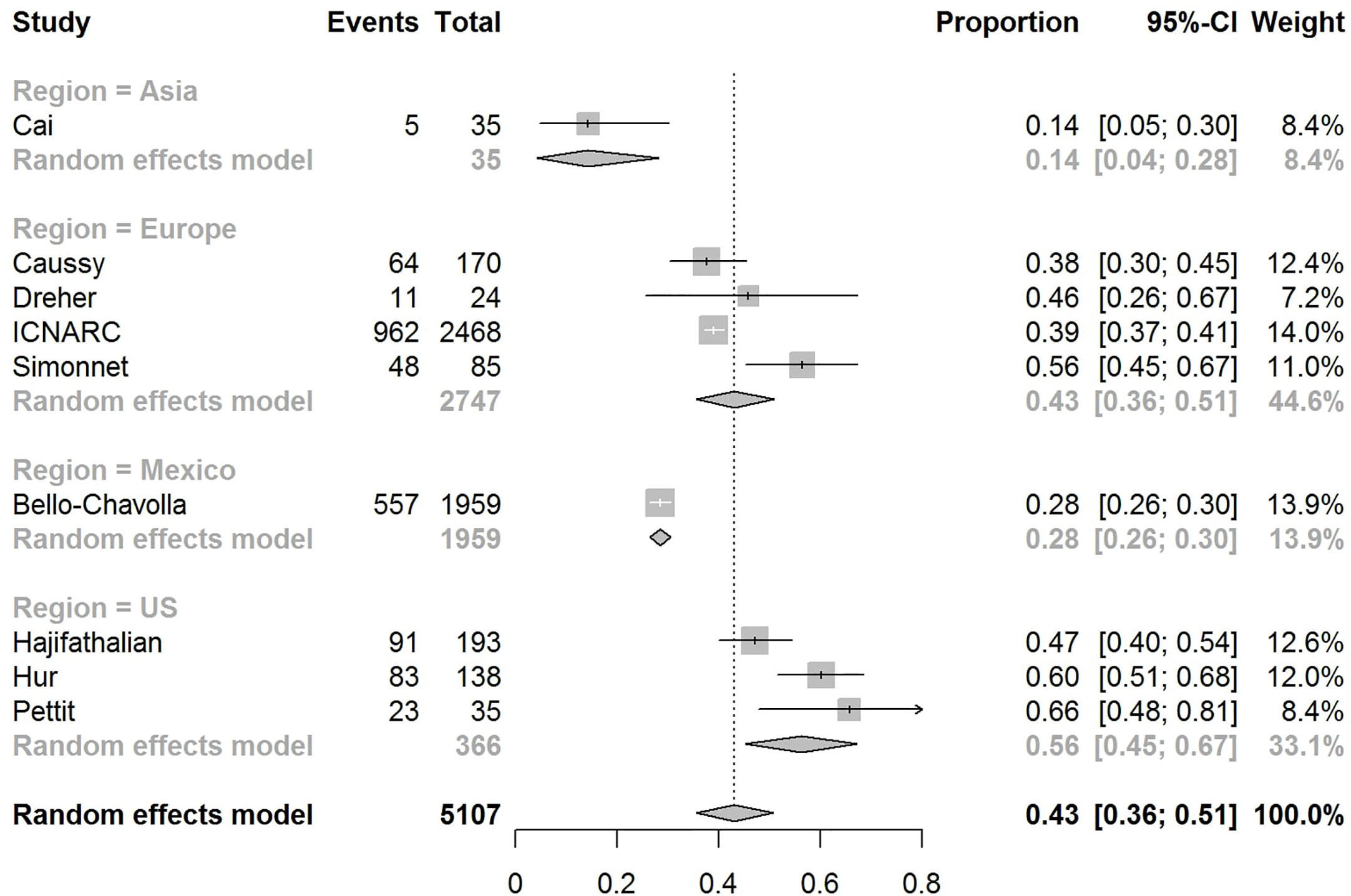
Figure 6 Forest-plot showing the prevalence rate of obesity in patients with COVID-19 needing invasive mechanical ventilation (IMV).
Subgroup analysis according to the geographic region were as follows:
In the U.S., the prevalence rates of obesity were 0.45 (95% CI: 0.38–0.52) in hospitalized patients (Figure 3), 0.42 (95% CI: 0.34–0.50) in patients admitted to non-critical care wards (Figure 4), 0.48 (95% CI: 0.40–0.57) in patients admitted to ICU (Figure 5), 0.56 (95% CI: 0.45–0.67) in patients with IMV requirement (Figure 6), and 0.40 (95% CI: 0.11–0.73) in those who died (Figure 7) respectively. Obesity rate was higher only in patients who required IMV compared to the background general population prevalence of obesity in the U.S., which is 42.4% (31).
In Europe, the prevalence rates of obesity were 0.23 (95% CI: 0.15–0.31) in hospitalized patients (Figure 3), 0.18 (95% CI: 0.14–0.23) in patients admitted to non-critical care wards (Figure 4), 0.39 (95% CI: 0.35–0.44) in patients admitted to ICU (Figure 5), 0.43 (95% CI: 0.36–0.51) in patients with IMV requirement (Figure 6), and 0.37 (95% CI: 0.32–0.42) in those who died (Figure 7) respectively. Prevalence rates of obesity in patients admitted to ICU, in patients needing IMV, and in those who died were significantly higher than the obesity prevalence of the countries where the included studies were conducted, which are 27.8% in UK, 22.3% in Germany, 21.6% in France, and 19.9% in Italy (32).
In Asia, the prevalence rates of obesity were 0.11 (95% CI: 0.03–0.23) in hospitalized patients (Figure 3), 0.11 (95% CI: 0.03–0.22) in patients admitted to non-critical care wards (Figure 4), 0.19 (95% CI: 0.06–0.36) in patients admitted to ICU (Figure 5), 0.14 (95% CI: 0.04–0.28) in patients with IMV requirement (Figure 6), and 0.33 (95% CI: 0.00–0.94) in those who died (Figure 7) respectively. Among the included studies, one was conducted in Kuwait (approximately 48% of included patients were Indian), two in China. Compared to the general obesity prevalences, which are 6.2% in China, 3.9% in India, and 37.9% in Kuwait (32), these rates are not significantly increased. However, the wide confidence intervals should be considered while interpreting these results.
Our pooled analyses showed that COVID-19 patients with obesity had a borderline higher risk for hospitalization [Odds ratio (OR):1.3, 95% CI: 1.00–1.69, p = 0.05; I2 52%, pheterogeneity = 0.08] (Figure 8). Obesity was related to significantly higher risk for ICU admission (OR:1.51, 95% CI: 1.16–1.97, p = 0.002; I2 72%, pheterogeneity < 0.01) (Figure 9), and IMV requirement (OR:1.77, 95% CI: 1.34–2.35, p < 0.001; I2 0%, pheterogeneity = 0.64) (Figure 10). However, obesity was not associated with increased risk for death in patients with COVID-19 (OR:1.28, 95% CI: 0.76–2.16, p = 0.35; I2 80%, pheterogeneity < 0.01) (Figure 11).
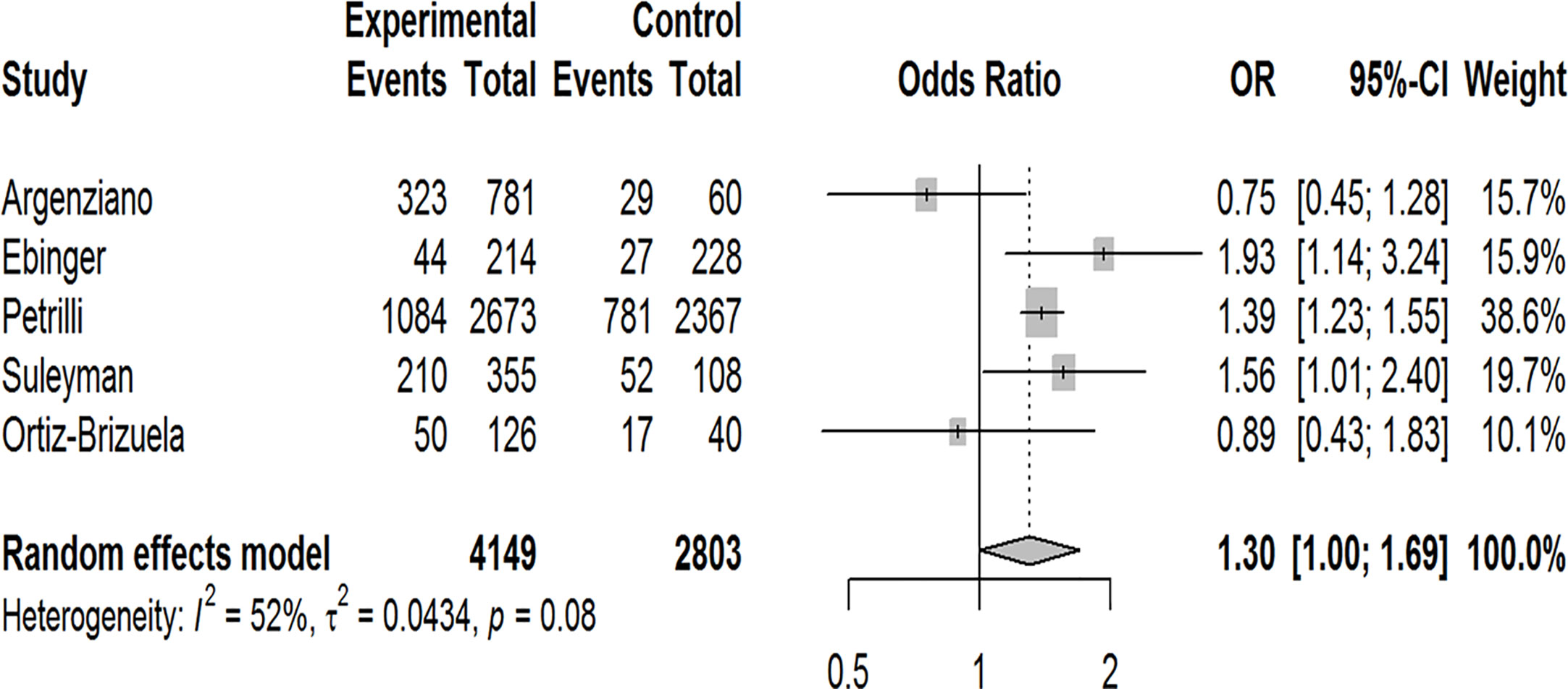
Figure 8 Forest-plot showing the association between obesity and hospitalization in patients with COVID-19.
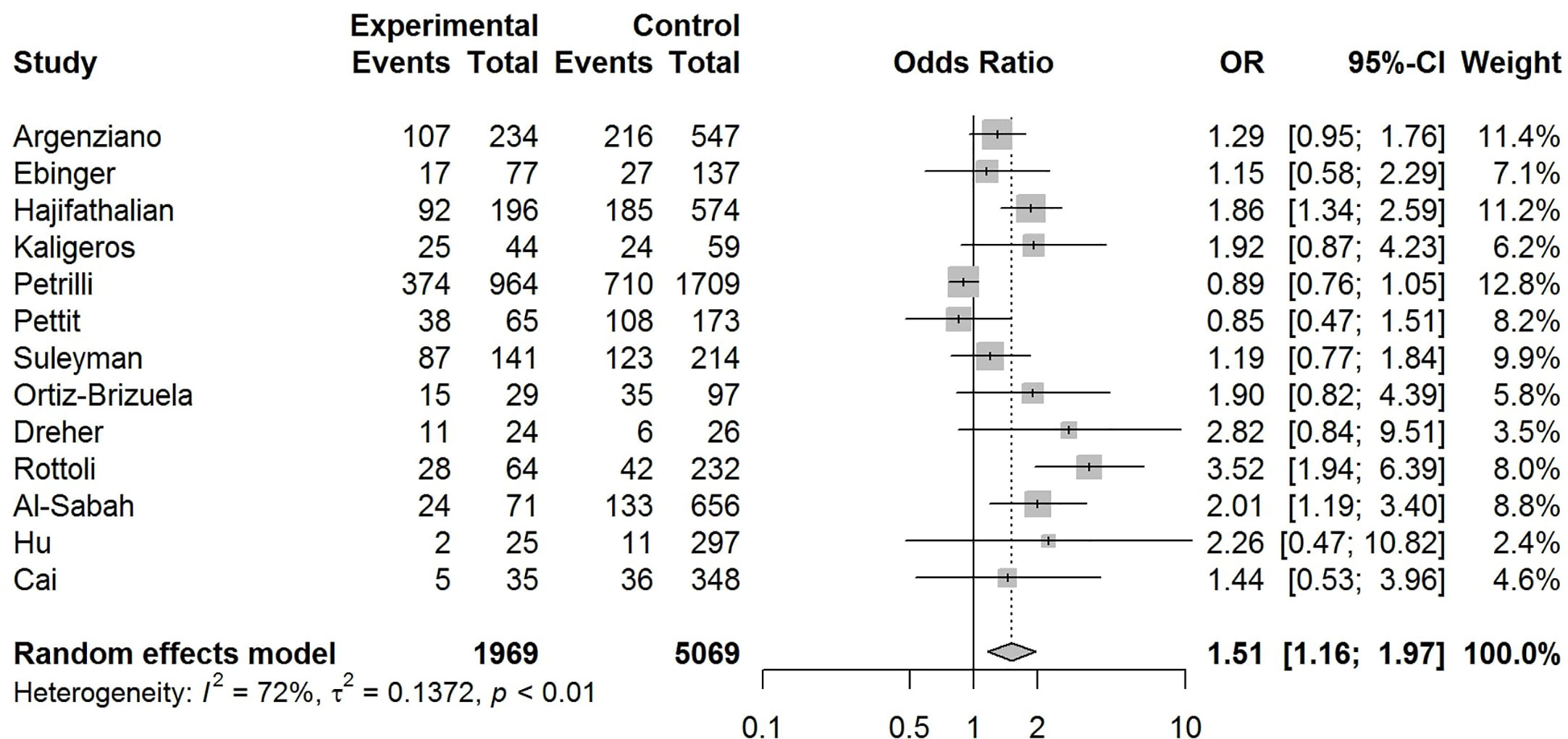
Figure 9 Forest-plot showing the association between obesity and admission to intensive care unit (ICU) in patients with COVID-19.
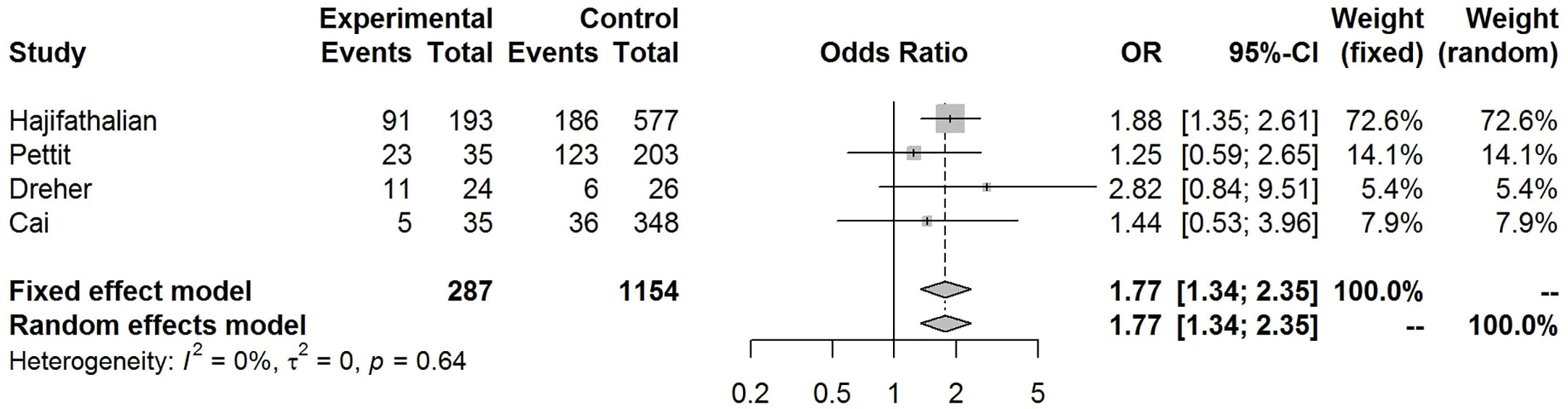
Figure 10 Forest-plot showing the association between obesity and invasive mechanical ventilation (IMV) requirement in patients with COVID-19.
The funnel-plot analysis for the association between obesity and risk of admission to ICU showed an asymmetrical shape (Figure 12), indicating the possibility of publication bias. Funnel-plot analysis was not performed for obesity and risk of hospitalization, IMV, and death because the number of included studies were less than 10.
In this systematic review and meta-analysis, we found that the pooled obesity prevalence rates were higher in patients with COVID-19 who were hospitalized, admitted to an ICU, or in need for IMV. Our pooled analysis revealed 1.3 times increased risk for hospitalization, 1.5 times increased risk for ICU admission, and approximately 1.8 times increased risk for IMV requirement among patients with obesity compared to patients without obesity.
In the fast-moving field of COVID-19, other meta-analyses have also assessed the relationship between this disorder and obesity. Using various inclusion criteria and definitions for the outcome variables, most of these meta-analyses indicated that in patients with COVID-19, obesity is significantly related to increased risk of severe disease and composite poor outcomes with reported ORs varying from 1.39 to 2.35 (33–41). A few meta-analyses presenting separate analyses for different outcomes reported increased risk of hospitalization with ORs varying from 1.4 to 2.13 (34, 42, 43), ICU admission with ORs varying from 1.21 to 1.74 (34, 43, 44), and IMV requirement with ORs varying from 1.66 to 2.29 (34, 36, 42–44) in obese patients with COVID-19. Our findings are in line with these reports in that we found 1.3 times higher risk for hospitalization, 1.51 times higher risk for ICU admission, and 1.77 times higher risk for IMV in obese patients with COVID-19 suggesting that obesity increases the severity of this disorder.
Prevalence of obesity varies across geographic region and obesity prevalence observed in hospitalized patients or in ICUs may depend on the local prevalence of obesity. Thus, we performed subgroup analysis by geographic region and found that in European countries, obesity was more common in patients with COVID-19 who needed ICU admission, IMV, or in those who died compared to background population. On the other hand, obesity rates only in COVID-19 patients requiring IMV exceeded the background general population prevalence of obesity in the U.S. In addition to the differences of obesity in the general population, the inconsistencies between Europe and the U.S. regarding the measured outcomes may be related with missing data, confounding factors, and variations in reporting methods and management protocols.
In our study, pooled prevalence rates of obesity seem to increase progressively with increasing disease severity, being highest in patients with IMV requirement. However, pooled obesity prevalence rate in patients who died was lower than that of those admitted to ICU or those with IMV requirement. Similar results were obtained in subgroup analysis by geographic region in the U.S. and Europe. Accordingly, our results showed that risk of death due to COVID-19 was not significantly increased in patients with obesity (OR:1.28, 95% CI: 0.76–2.16, p = 0.35). Consistent with our results, another meta-analysis including 34 studies from 9 different countries reported that obesity was significantly related to the increased risk of IMV during ICU admission, but not associated with excess mortality (OR: 1.15, 95% CI: 0.98–1.34) (36). These observations may be in agreement with obesity survival paradox. That is, despite the increased risk of pneumonia, pneumonia mortality might be lower in overweight and moderate obese individuals compared to those with normal BMI, as shown in a previous meta-analysis (45). Several hypothetical explanations have been proposed for this inverse association between obesity and risk of mortality, including clinicians’ lower threshold for ICU admission of obese patients, confounding, reverse causality, secretion of immunomodulatory substances by adipocytes (e.g., leptin, interleukin-10, and soluble TNF-α receptor) that may attenuate the inflammatory response, and the increased metabolic reserve provided by excess fat stores and lean body mass in obese patients that may counteract the increased catabolic stress and improve survival during critical illness (46). However, during the 2009 H1N1 pandemic, severe obesity (BMI ≥40 kg/m2) was identified as an independent risk factor for admission to ICU and death (47). Similarly, obesity has been reported to be associated with increased mortality due to COVID-19 in some of the very recently published meta-analyses with ORs varying from 1.37 to 3.68 (34, 35, 42, 43, 48) challenging the obesity survival paradox in COVID-19 in contrast to our results. The differences in the study populations and the discrepancies in the cut off values for BMI to define obesity among the included studies as well as the differences between healthcare systems, testing strategies, and indications for hospitalization, ICU admission, or IMV might explain these inconsistencies.
In the present study, we found that the pooled prevalence of obesity in all cases (admitted + non-admitted) with COVID-19 was 34% (22–46%). Among five studies that included both inpatient and outpatient confirmed COVID-19 cases, four were conducted in the U.S (9–12). and one in Mexico (19). Compared with the obesity prevalence in the general population of the U.S., this result suggests that obesity may not be associated with a higher test positivity for COVID-19. This contradicts the result of a study conducted in the United Kingdom (UK) (49). In this study, Yates et al., used UK Biobank data, in which 882 of 2,494 tests were positive for COVID-19. Although limited by possible selection bias, authors reported that both BMI and waist circumference were associated with testing positive for COVID-19 in a dose-response fashion. After adjustment for possible confounders, the OR for overweight, obese, and severe obese subjects was 1.31, 1.55, and 1.57, respectively, compared to those with healthy weight (49).
Overall, our data indicate that obese subjects may be at higher risk for serious illness if infected and obesity may play a role in the progression of COVID-19. Several mechanisms have been proposed about the association between obesity and poor COVID-19 outcomes (Figure 13). Higher expression of angiotensin-converting enzyme 2 (ACE-2; an important functional receptor for SARS-CoV-2 invasion) in adipose tissue may lead to prolonged viral shedding and exposure in patients with obesity, increasing the susceptibility to SARS-CoV-2 infection and the risk of disease aggravation (35). Obesity driven chronic inflammation, aberrant cytokine activation, decreased adiponectin and increased leptin secretions, and dysfunction of innate and adaptive immunity may contribute to worse clinical outcomes in patients with COVID-19 (50–52). Obesity is also associated with hypercoagulability and increased risk of thrombosis (53), which seems to be one of the important factors leading to a more severe clinical course in infected patients. Moreover, obesity, particularly when it is severe, is associated with significant changes in pulmonary mechanics and respiratory muscle performance, which predispose patients to develop respiratory failure in the case of lung infection (54). Although these are plausible mechanisms, future studies are needed to prove that they are actually linked to COVID-19 outcomes.
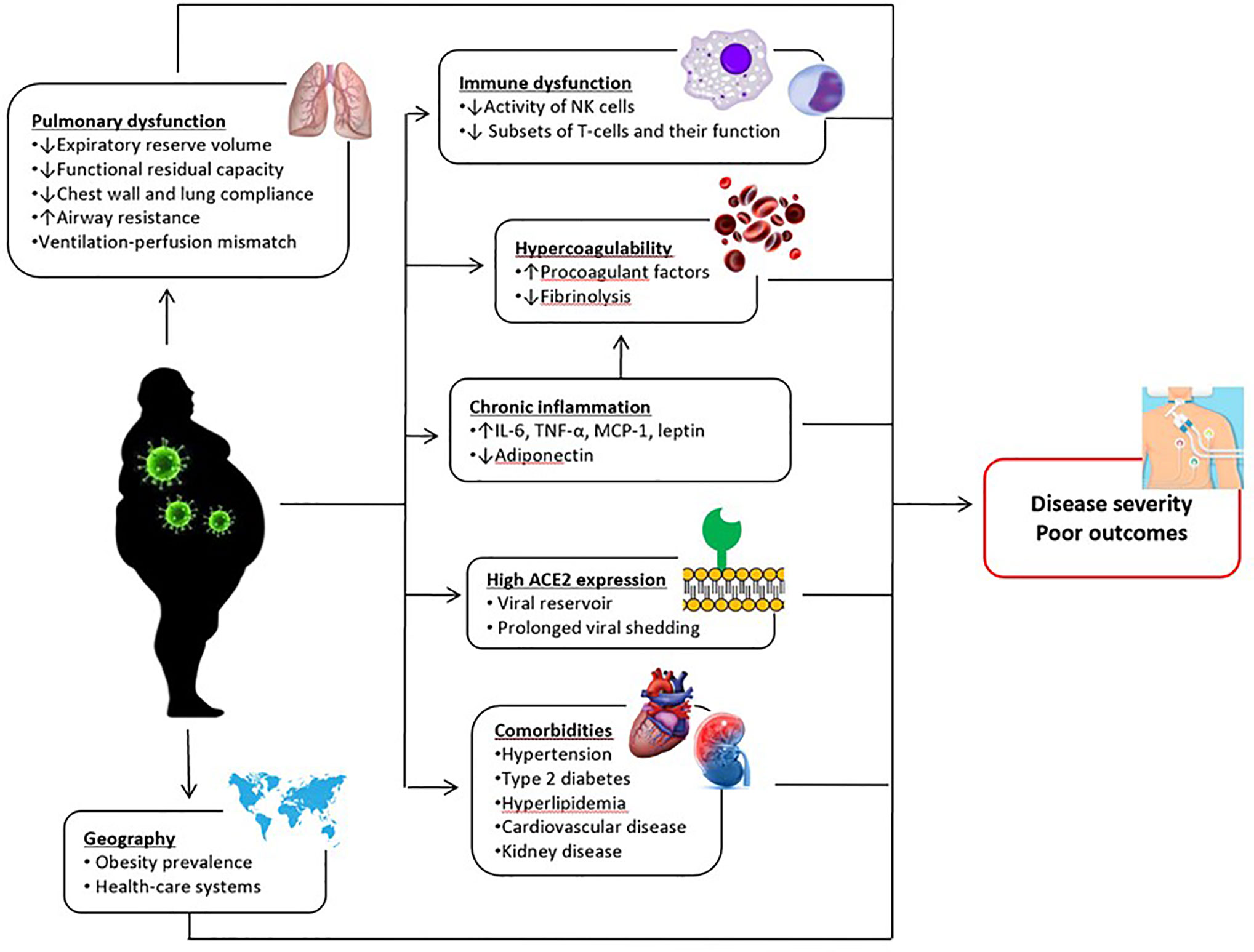
Figure 13 Postulated mechanisms underlying the relationship between obesity and poor COVID-19 outcomes. IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein-1; NK, natural killer; TNF-α, tumor necrosis factor alpha.
This study, with its large sample size and inclusion of studies from different regions worldwide, presents further evidence about the relationship between obesity and COVID-19 outcomes. However, several limitations exist in this work. There was a significant heterogeneity among studies, probably due to the differences in sample sizes and baseline characteristics of the patients. Most included studies were retrospective and analyzed data of patients who were hospitalized, leading to a selection bias towards those with a more severe disease. Sampling and testing strategies, indications for hospitalization or admission to ICU, or indications for IMV were not adequately defined in most reports. Moreover, studies included in this analysis were short-term observational studies; outcomes could be different with a longer time of observation. Anthropometric measurements were not performed in most studies; using data from previous medical records may have led to incorrect BMI assessment and categorization. Besides, a substantial proportion of patients had missing BMI data in some studies. Furthermore, confounding factors including age, sex, ethnicity, deprivation, and comorbidities were not addressed in some reports. Given that obesity is related to these factors, it is difficult to interpret the potential role of obesity as an independent risk factor for poor COVID-19 outcomes. It should also be kept in mind that variations in treatment protocols over time across studies may have affected reported outcomes. Lastly, due to the small number of studies in subgroup analyses, strong conclusions could not be drawn on geographic variation of observations regarding the association between obesity and outcomes of COVID-19.
Despite its limitations, available data and results of our study consistently suggest that people living with obesity are at increased risk of poor COVID-19 outcomes. Thus, measurement of anthropometric parameters should be routinely performed in patients tested positive for COVID-19 as a part of risk assessment. Obese patients with COVID-19 should be followed and treated as a higher risk population. Testing priority, close monitoring, and earlier intensive treatment may be considered in these patients to avoid unfavorable clinical outcomes.
Special attention to obesity is also important in the aspect of disease prevention. Restriction on leaving home for several weeks was introduced in many countries as a measure to reduce rapid transmission of COVID-19. This may result in physical inactivity and, in long term, increase the susceptibility of people to develop obesity (55). Eventually, this may increase the number of individuals who will likely have a more severe course when infected with COVID-19. Thus, people should be encouraged to increase physical activity and gain healthy eating habits during pandemic.
In conclusion, our systematic review and meta-analysis indicated that the prevalence of obesity is higher in patients with severe COVID-19 and obesity is associated with increased risk for hospitalization, ICU admission, and IMV. However, abovementioned limitations of the included studies should be kept in mind while interpreting the results. Prospective cohort studies with a large sample size and addressing all potential confounding factors including age, sex, ethnicity, deprivation, and comorbidities are needed to clarify the independent role of obesity on the risk of COVID-19 and its clinical course. The pathogenesis of COVID-19 in patients with obesity should also be investigated to identify the causal mechanisms and interfere with prophylactic and therapeutic measures.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
BY supervised the project. BY and NE conceptualized the meta-analysis protocol. NH, NE, and BY screened the literature search results and assessed for the eligibility criteria. EK quantitatively synthesized the results of involved studies. NH and NE produced original form of the manuscript. BY reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
The authors received no financial support for the research and authorship of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. World Health Organization. Coronavirus disease (COVID-19) Situation Report – 179. (2020). https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200717-covid-19-sitrep-179.pdf?sfvrsn=2f1599fa_2.
2. Guan W-j, Liang W-h, Zhao Y, Liang H-r, Chen Z-s, Li Y-m, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: A Nationwide Analysis. Eur Respir J (2020) 55(5):2000547. doi: 10.1183/13993003.01227-2020
3. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med (2020) 46(5):846–8. doi: 10.1007/s00134-020-05991-x
4. Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol (2020) 109(5):531–8. doi: 10.1007/s00392-020-01626-9
5. Dai H, Alsalhe TA, Chalghaf N, Riccò M, Bragazzi NL, Wu J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: An analysis of the Global Burden of Disease Study. PloS Med (2020) 17(7):e1003198. doi: 10.1371/journal.pmed.1003198
6. Guan W-j, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x, et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med (2020) 382(18):1708–20. doi: 10.1056/NEJMoa2002032
7. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA (2020) 323(16):1574–81. doi: 10.1001/jama.2020.5394
8. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (2020) 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
9. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ (2020) 369:m1966. doi: 10.1136/bmj.m1966
10. Ebinger JE, Achamallah N, Ji H, Claggett BL, Sun N, Botting P, et al. Pre-Existing Characteristics Associated with Covid-19 Illness Severity. PLoS One (2020). 15:e0236240. doi: 10.1371/journal.pone.0236240
11. Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR, Barr RG, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ (2020) 369:m1996. doi: 10.1136/bmj.m1996
12. Suleyman G, Fadel RA, Malette KM, Hammond C, Abdulla H, Entz A, et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan detroit. JAMA Network Open (2020) 3(6):e2012270–e. doi: 10.1001/jamanetworkopen.2020.12270
13. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
14. National Institute of Health, National Heart Lung, and Blood Institute. Study quality assessment tools. (2018). https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
15. Hajifathalian K, Kumar S, Newberry C, Shah S, Fortune B, Krisko T, et al. Obesity is associated with worse outcomes in COVID-19: Analysis of Early Data From New York City. Obes (Silver Spring) (2020) 28(9):1606–12. doi: 10.1002/oby.22923
16. Hur K, Price CP, Gray EL, Gulati RK, Maksimoski M, Racette SD, et al. Factors Associated With Intubation and Prolonged Intubation in Hospitalized Patients With COVID-19. Otolaryngol Head Neck Surg (2020) 163(1):170–8. doi: 10.1177/0194599820929640
17. Kalligeros M, Shehadeh F, Mylona EK, Benitez G, Beckwith CG, Chan PA, et al. Association of Obesity with Disease Severity among Patients with COVID-19. Obes (Silver Spring) (2020) 28:1200–4. doi: 10.1002/oby.22859
18. Pettit NN, MacKenzie EL, Ridgway J, Pursell K, Ash D, Patel B, et al. Obesity is Associated with Increased Risk for Mortality Among Hospitalized Patients with COVID-19. Obesity Silver Spring (2020) 28(10):1806–10. doi: 10.1002/oby.22941
19. Bello-Chavolla OY, Bahena-Lopez JP, Antonio-Villa NE, Vargas-Vázquez A, González-Díaz A, Márquez-Salinas A, et al. Predicting mortality due to SARS-CoV-2: A mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab (2020) 105:dgaa346. doi: 10.1210/clinem/dgaa346
20. Ortiz-Brizuela E, Villanueva-Reza M, González-Lara MF, Tamez-Torres KM, Román-Montes CM, Díaz-Mejía BA, et al. Clinical and epidemiological characteristics of patients diagnosed with covid-19 in a tertiary care center in mexico city: a prospective cohort study. Rev Invest Clin (2020) 72(3):165–77. doi: 10.24875/RIC.20000211
21. Al-Sabah SK, Al-Haddad M, Al Youha S, Jamal MH, AlMazeedi S. COVID-19: Impact of Obesity and Diabetes in Disease Severity. medRxiv (2020) 10:e12414. doi: 10.1101/2020.05.24.20111724
22. Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, et al. Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China. Diabetes Care (2020) 43(7):1392–8. doi: 10.2337/dc20-0576
23. Hu L, Chen S, Fu Y, Gao Z, Long H, J-m W, et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin Infect Dis (2020) 71(16):2089–98. doi: 10.1093/cid/ciaa539
24. Caussy C, Wallet F, Laville M, Disse E. Obesity is Associated with Severe Forms of COVID-19. Obes (Silver Spring) (2020) 28(7):1175. doi: 10.1002/oby.22842
25. Dreher M, Kersten A, Bickenbach J, Balfanz P, Hartmann B, Cornelissen C, et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Deutsches Ärzteblatt Int (2020) 117(10):271. doi: 10.3238/arztebl.2020.0271
26. Giacomelli A, Ridolfo AL, Milazzo L, Oreni L, Bernacchia D, Siano M, et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res (2020) 158:104931. doi: 10.1016/j.phrs.2020.104931
27. Rottoli M, Bernante P, Belvedere A, Tonetti T, Garelli S, Giannella M, et al. Obesity Is One of the Strongest Risk Factor for Respiratory Failure and Death in COVID-19 Patients: A Retrospective Multicentric Cohort Study. Lancet Diabetes Endocrinol (2020) 3578779. doi: 10.2139/ssrn.3578779https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3578779. in press.
28. Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obes (Silver Spring) (2020) 28(7):1195–9. doi: 10.1002/oby.22831
29. ICNARC report on COVID-19 in critical care. Available at: https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports (Accessed 24 April 2020).
30. World Health Organization. Global Health Observatory data repository. Prevalence Obes Among Adults BMI≥30 Age-standardized Estimates. https://www.who.int/data/gho/data/indicators/indicator-details/GHO/prevalence-of-obesity-among-adults-bmi-=-30-(age-standardized-estimate)-(-).
31. Centers for disease control and prevention. Adult Obesity Facts. Disponible (2020). https://wwwcdcgov/obesity/data/adulthtml.
32. World Health Organization. Global Health Observatory data repository. Prevalence Obes Among Adults BMI≥30 Age-standardized Estimates By WHO Region. https://apps.who.int/gho/data/view.main.REGION2480A?lang=en.
33. Malik P, Patel U, Patel K, Martin M, Shah C, Mehta D, et al. Obesity a predictor of outcomes of COVID-19 hospitalized patients-A systematic Review and Meta-Analysis. J Med Virol (2021) 93(2):1188–93. doi: 10.1002/jmv.26555
34. Huang Y, Yao L, Huang Y-M, Min W, Wei L, Yi S, et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism (2020) 113:154378. doi: 10.1016/j.metabol.2020.154378
35. Pranata R, Lim MA, Yonas E, Vania R, Lukito AA, Siswanto BB, et al. Body mass index and outcome in patients with COVID-19: A dose–response meta-analysis. Diabetes Metab (2020) 101178. doi: 10.1016/j.diabet.2020.07.005
36. Zhou Y, Yang Q, Chi J, Dong B, Lv W, Shen L, et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int J Infect Dis (2020) 99:47–56. doi: 10.1016/j.ijid.2020.07.029
37. Sharma A, Garg A, Rout A, Lavie CJ. Association of obesity with more critical illness in COVID-19. Mayo Clin Proc (2020) 95(9):2040–2. doi: 10.1016/j.mayocp.2020.06.046
38. Soeroto AY, Soetedjo NN, Purwiga A, Santoso P, Kulsum ID, Suryadinata H, et al. Effect of increased BMI and obesity on the outcome of COVID-19 adult patients: A systematic review and meta-analysis. Diabetes Metab Syndrome (2020) 14(6):1897–904. doi: 10.1101/2020.06.28.20142240
39. Seidu S, Gillies C, Zaccardi F, Kunutsor SK, Hartmann-Boyce J, Yates T, et al. The impact of obesity on severe disease and mortality in people with SARS-CoV-2: A systematic review and meta-analysis. Endocrinol Diabetes Metab (2020) 4(1):e00176. doi: 10.1002/edm2.176
40. Du Y, Lv Y, Zha W, Zhou N, Hong X. Association of Body mass index (BMI) with Critical COVID-19 and in-hospital Mortality: a dose-response meta-analysis. Metabolism (2020) 117:154373. doi: 10.1016/j.metabol.2020.154373
41. Yang J, Hu J, Zhu C. Obesity aggravates COVID-19: a systematic review and meta-analysis. J Med Virol (2020). doi: 10.1002/jmv.26677
42. Hussain A, Mahawar K, Xia Z, Yang W, Shamsi E-H. Obesity and mortality of COVID-19. Meta-analysis Obes Res Clin Practice (2020) 14(4):295–300. doi: 10.1016/j.orcp.2020.07.002
43. Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes Rev (2020) 21(11):e13128. doi: 10.1111/obr.13128
44. Földi M, Farkas N, Kiss S, Zádori N, Váncsa S, Szakó L, et al. Obesity is a risk factor for developing critical condition in COVID-19 patients: A systematic review and meta-analysis. Obes Rev (2020) 21(10):e13095. doi: 10.1111/obr.13095
45. Nie W, Zhang Y, Jee SH, Jung KJ, Li B, Xiu Q. Obesity survival paradox in pneumonia: a meta-analysis. BMC Med (2014) 12(1):61. doi: 10.1186/1741-7015-12-61
46. Schetz M, De Jong A, Deane AM, Druml W, Hemelaar P, Pelosi P, et al. Obesity in the critically ill: a narrative review. Intensive Care Med (2019) 45(6):757–69. doi: 10.1007/s00134-019-05594-1
47. Fezeu L, Julia C, Henegar A, Bitu J, Hu FB, Grobbee DE, et al. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obes Rev (2011) 12(8):653–9. doi: 10.1111/j.1467-789X.2011.00864.x
48. Noor FM, Islam MM. Prevalence and Associated Risk Factors of Mortality Among COVID-19 Patients: A Meta-Analysis. J Community Health (2020), 45(6):1270–1282. doi: 10.1007/s10900-020-00920-x
49. Yates T, Razieh C, Zaccardi F, Davies MJ, Khunti K. Obesity and risk of COVID-19: analysis of UK biobank. Prim Care Diabetes (2020) 14(5):566–7. doi: 10.1101/2020.07.10.20150003
50. Ryan PM, Caplice NM. Is Adipose Tissue a Reservoir for Viral Spread, Immune Activation, and Cytokine Amplification in Coronavirus Disease 2019? Obesity (2020) Silver Spring (2020) 28(7):1191–4. doi: 10.1002/oby.22843
51. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol (2011) 11(2):85–97. doi: 10.1038/nri2921
52. Malavazos AE, Corsi Romanelli MM, Bandera F, Iacobellis G. Targeting the adipose tissue in COVID-19. Obesity Silver Spring (2020) 28(7):1178–9. doi: 10.1002/oby.22844
53. Samad F, Ruf W. Inflammation, obesity, and thrombosis. Blood J Am Soc Hematol (2013) 122(20):3415–22. doi: 10.1182/blood-2013-05-427708
54. Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med (2018) 12(9):755–67.(1). doi: 10.1080/17476348.2018.1506331
55. Public Health England. Excess weight and COVID-19: insights from new evidence. (2020). https://www.gov.uk/government/publications/excess-weight-and-covid-19-insights-from-new-evidence.
Keywords: obesity, body mass index, COVID-19, SARS-CoV-2, prognosis
Citation: Helvaci N, Eyupoglu ND, Karabulut E and Yildiz BO (2021) Prevalence of Obesity and Its Impact on Outcome in Patients With COVID-19: A Systematic Review and Meta-Analysis. Front. Endocrinol. 12:598249. doi: 10.3389/fendo.2021.598249
Received: 24 August 2020; Accepted: 19 January 2021;
Published: 25 February 2021.
Edited by:
Katherine Samaras, St Vincent’s Hospital Sydney, AustraliaReviewed by:
Catherine Itsiopoulos, Murdoch University, AustraliaCopyright © 2021 Helvaci, Eyupoglu, Karabulut and Yildiz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bulent Okan Yildiz, eWlsZGl6Ym9AeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.