
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 25 March 2021
Sec. Thyroid Endocrinology
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.591015
This article is part of the Research Topic Risk-benefit Considerations and Staging of Differentiated Thyroid Cancer View all 16 articles
 Xingchen Li1†
Xingchen Li1† Yuansheng Duan1†
Yuansheng Duan1† Dandan Liu1†
Dandan Liu1† Hongwei Liu2†
Hongwei Liu2† Mengqian Zhou1
Mengqian Zhou1 Kai Yue1
Kai Yue1 Yanjie Shuai1
Yanjie Shuai1 Yu Wang1
Yu Wang1 Chenyan Ji1
Chenyan Ji1 Chao Jing1*
Chao Jing1* Yansheng Wu1*
Yansheng Wu1* Xudong Wang1*
Xudong Wang1*The Delphian lymph node (DLN), also known as the prelaryngeal node, is one component of the central lymph node. The DLN has been well studied in laryngeal cancer, although its significance in papillary thyroid cancer (PTC) remains unclear. We retrospectively analyzed 936 patients with PTC who underwent thyroidectomy by a single surgeon in Tianjin Cancer Hospital from 2017 to 2019. Moreover, 250 PTC patients who underwent thyroidectomy by another surgeon in Tianjin Cancer Hospital from January 2019 to April 2019 were used as a validation cohort. Among the 936 patients with PTC, 581 patients (62.1%) had DLNs, of which 177 samples with metastasis (177/581, 30.5%) were verified. DLN metastasis was significantly correlated with sex, age, tumor size, bilateral cancer, multifocality, extrathyroidal extension, lymphovascular invasion and central and lateral neck lymph node metastasis. Multivariate analysis revealed that independent risk factors for DLN metastasis included age, gender, tumor size, extrathyroid extension, lymphovascular invasion and central lymph node metastasis, which determined the nomogram. In particular, tumor size was proven to be one of the most predominant single predictors. The diagnostic model had an area under the curve (AUC) of 0.829 (95% confidence interval, 0.804–0.854). The internal and external validations of the nomogram were 0.819 and 0.745, respectively. Our results demonstrate that DLN metastasis appears to be a critical parameter for predicting metastatic disease of the central compartments. Furthermore, this study provides a precise criterion for assessing DLN metastasis and has great clinical significance for treating PTC.
Thyroid neoplasms are the fifth most prevalent malignancy in women and account for 3% of all human malignancies (1). Papillary thyroid carcinoma (PTC) is the most common histopathological subtype, accounting for approximately 90% of all thyroid carcinomas (2). Although the 20-year overall survival rate of PTC reaches up to 90% and its disease-specific survival rate is approximately 97% (3), metastasis to regional cervical lymph nodes occurs early and frequently, and the rate of lymph node metastasis is as high as 90% in PTC (4). The central neck nodes are the most common sites of nodal metastasis in PTC patients (5, 6). Emerging evidence has suggested that cervical lymph node metastasis acts as an unfavorable factor that contributes to locoregional recurrence (7–9).
The DLN, also known as the prelaryngeal or precricoid lymph node, usually consists of a single node or a group of lymph nodes and receives lymphatic drainage from the larynx and thyroid (10, 11). The Delphian, pretreacheal, and paratracheal lymph node groups compose the central neck lymph nodes. Increasing evidence indicates that DLN metastasis is an effective predictor of regional lymph node disease and recurrence in many malignant head and neck cancers, including PTC (12–16). Currently, the role of DLN metastasis in PTC has gained substantial attention (17–19). Reports have indicated that DLN metastasis is an aggressive disease and predicts a high risk of recurrence in PTC (14, 20). At the moment, it is controversial whether clinically lymph node-negative (cN0) PTC patients should undergo prophylactic central lymph node dissection. The DLN, one component of central neck node clusters, may be an available preoperative indicator to help surgeons to make individualized treatment plans. Hence, a high-efficiency DLN metastasis evaluation system will be of clinical significance.
Thus far, DLN has been well studied in laryngeal cancer while its role in thyroid cancer remains unknown. The aim of this study was to assess the incidence and risk factors of DLN metastasis, and to develop a clinicopathologic characters-based diagnostic model to help surgeons predict preoperative DLN metastasis.
We reviewed 936 patients who were diagnosed pathologically with PTC. All the patients underwent thyroidectomy by a single surgeon at the Head and Neck Surgery Department of Tianjin Cancer Hospital from June 2017 to January 2019. The precricoid region was deliberately excised and labeled as DLNs (Figure 1) and then diagnosed by histopathology. Overall, 250 patients who underwent thyroidectomy by another surgeon at Tianjin Cancer Hospital from January 2019 to April 2019 were used as a validation cohort to evaluate the performance of the diagnostic model. Of the 250 patients with PTC, 127 (50.8%) patients were found to have DLNs. DLN metastasis was observed in 28 (22.4%) patients. The mean number of metastatic DLNs was 1.89. The clinicopathologic information was gathered, and the protocol in this study was approved by the Tianjin Cancer Hospital Ethics Committee.
All the patients underwent thyroidectomy according to the Chinese practice guidelines in thyroid cancer (21, 22). For bilateral PTC patients, Total thyroidectomy was performed. For unilateral PTC patients, total thyroidectomy or lobectomy plus isthmusectomy were performed. When unilateral PTC patients met one of following conditions: tumor size >4 cm, multifocal in one lobe, extrathyroid invasion or distant metastasis, which were diagnosed by the use of frozen section analysis and preoperative examination, total thyroidectomy plus isthmusectomy may be considered, according to the guidelines of Chinese Thyroid Association. Meanwhile, pretracheal nodes, ipsolateral or bilateral paratracheal nodes, and peripheral fatty tissue were routinely dissected. The DLNs were removed once they were identified by the surgeon. Ipsilateral prophylactic central node dissection (pCND) was performed in cN0 PTC patients. When any of the cervical lymph nodes were verified as suspicious through preoperative ultrasonography, palpation or intraoperative inspection, a contralateral CND was performed. If lateral neck lymph node (Level II-V) was suspected with metastasis by preoperative ultrasound examination or affirmed with metastasis by fine needle aspiration, lateral node dissection (LND) would be performed.
The characteristics of patients were displayed as the mean ± SD or frequencies with percentages. The correlation between DLN metastasis and various clinical factors was analyzed by univariable analysis using Student’s t-test (for continuous variables) or the chi-squared test (for categorical variables). Logistic regression was used to identify the factors that were independently associated with DLN metastasis. The tolerance for all the potential predictors was > 0.1 and the variance inflation factor (VIF) was < 10. A multiple-variable logistic regression model, including all the variables with P values less than 0.05 in multivariate analysis, was used for translation into a nomogram diagnostic model. The model performance was assessed using discrimination and calibration (23). A nomogram was created to calculate individual probabilities (24). We evaluated the performance of the diagnosis model in the validation cohort and determined the means and 95% confidence intervals of the AUROC. The analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R-software version 3.5.1 (R foundation for statistical computing, Vienna, Austria. URL http://www.R-project.org).
Of all the 936 patients, DLNs were detected in 581 (62.07%, 581/936) patients, and the median numbers of DLNs were 2.1 (range, 1–10). In the group with identified DLNs, 177 (30.46%, 177/581) patients were confirmed to be DLN positive. The percentage of central lymph node metastasis in PTC patients was 59.40% (Table 1).
The univariate analysis revealed that metastatic disease to the DLN was associated with male sex (52.5% vs. 22.2%, p<0.001), younger age at diagnosis (38.27 ± 11.997 vs. 43.86 ± 10.752, p<0.001), larger tumor (13.79 ± 8.8659 vs. 9.38 ± 4.3466, p<0.001), bilateral (39.0% vs. 24.9%, p<0.001), multifocality (46.3% vs. 30.7%, p<0.001), extrathyroid extension (ETE) (87.0% vs. 73.3%, p<0.001), lymphovascular invasion (10.7% vs. 1.5%, p<0.001), central neck node metastases (43.2% vs. 7.9%, p<0.001) and lateral neck node metastases (58.9% vs. 25.0%, p=0.022) (Table 2), whereas the correlations between DLN metastasis and thyroiditis (p=0.636) or tumor location (p=0.774) were not statistically significant.
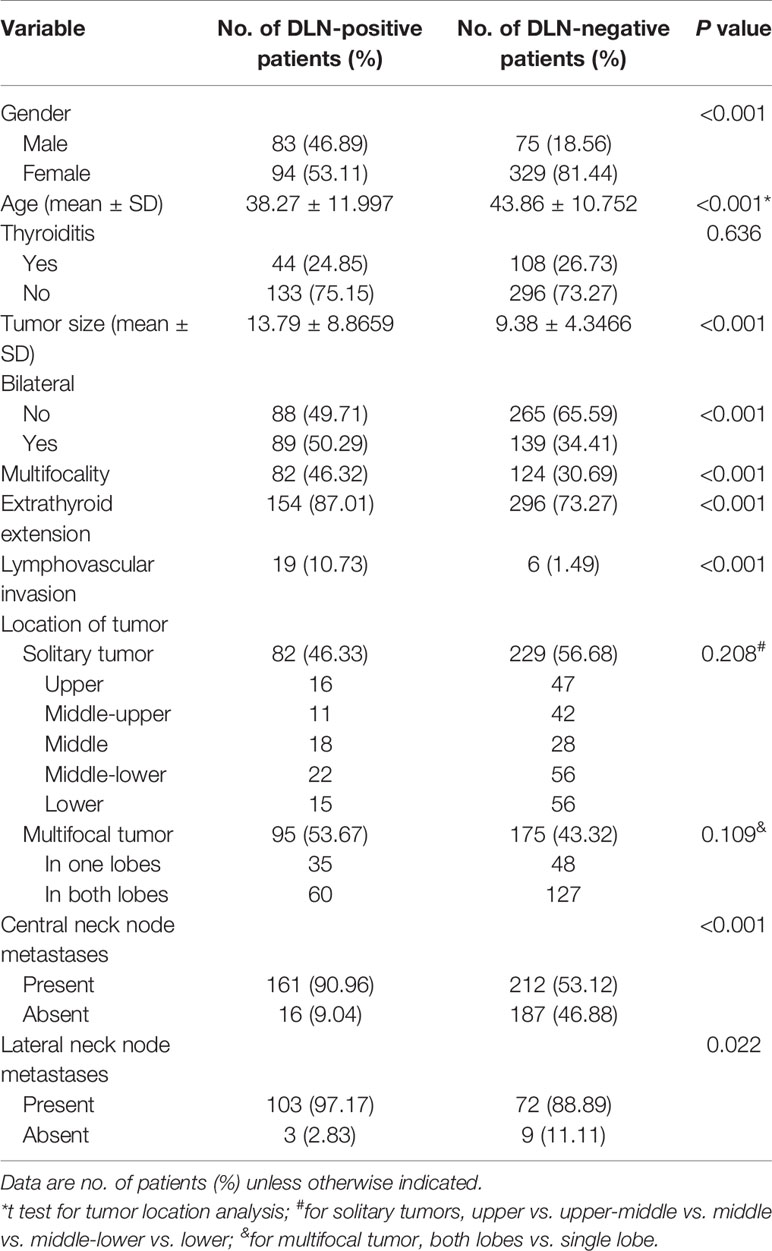
Table 2 Comparison of clinicopathological characteristics between the patients with and without DLN metastasis.
All the variables with P values less than 0.05 in univariate analysis were selected for multivariate logistic analysis. As shown in Figure 2, gender (male, p<0.0001), age (younger age, p=0.0039), tumor size (>10mm, p<0.0001), ETE (p=0.0081), lymphovascular invasion (p=0.0071) and central neck node metastasis (p<0.0001) were independent risk factors of DLN metastasis. However, lateral neck lymph node metastasis was not included in the multivariate logistic analysis due to the limited number of cases. For central compartment metastasis, DLN status had a sensitivity, specificity, positive and negative predictive values of 43%, 92%, 91% and 47%, respectively. And for lateral lymph node metastasis, DLN metastasis had a respective sensitivity, specificity, positive and negative predictive values of 59%, 75%, 97% and 11% (Table 3).
A multivariate logistic regression model was adopted to establish the diagnostic model for DLN metastasis. Gender, age, tumor size, ETE, lymphovascular invasion and central neck node metastasis were included as the clinicopathologic features. For internal validation, the study population (n=556) was randomly assigned to training data sets (66%) or test data sets (33%). The diagnostic model was established with the training data while the test data were used to assess model performance with AUC. The area under the ROC curve (AUC) of the training data and testing data was 0.829 (95% CI, 0.804–0.854) and 0.819 (95% CI, 0.764–0.870), respectively (Figures 3 and 4). For external validation, 250 PTC patients who underwent thyroidectomy by another surgeon were used to evaluate the performance of the diagnostic model. The area under the ROC curve was 0.745. Calibration plots recalibrated the diagnostic models to predict risk of DLN metastasis. Calibration of the diagnosis model was satisfactory (Figure 5).
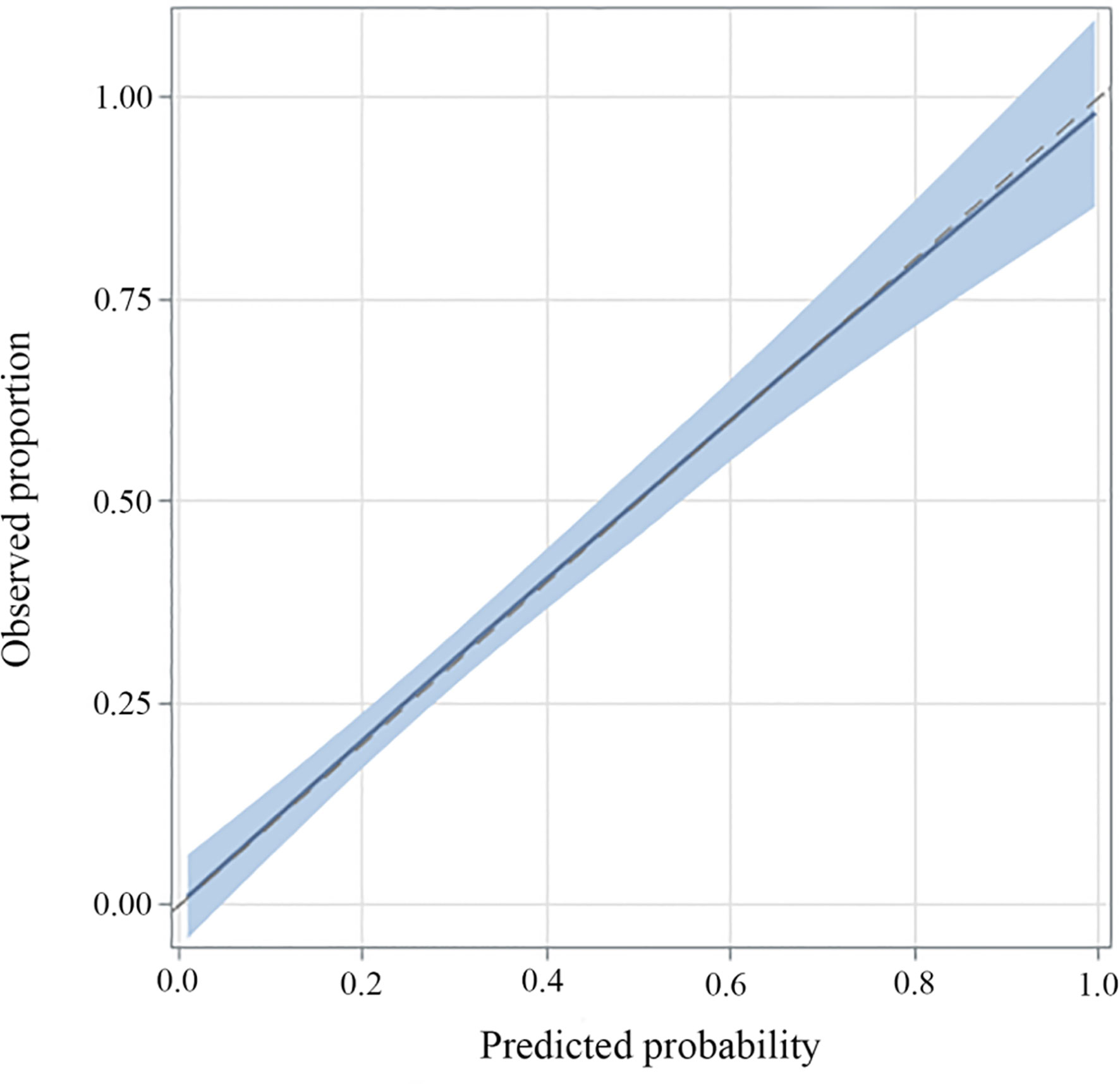
Figure 5 Calibration plots of recalibrated prognostic models to predict risk of DLN metastasis. In the case of perfect calibration, all the groups of predicted probabilities fit close to the blue diagonal line, corresponding to an intercept of 0 and a slope of 1 for the calibration plot. The vertical lines in grouped observations represent 95% confidence intervals.
A nomogram was developed to calculate the degree of individual risk in order to improve clinical diagnosis. The nomogram based on these significant variables was established (Figure 6). Among the six significant features, tumor size was one predominant predictor of DLN metastasis in the nomogram. When all these criteria were satisfied, the specificity was infinite close to 100%. For example, a 45-year-old man was diagnosed initially with PTC. His tumor size was 3 mm, and central lymph node metastasis on preoperative ultrasonography was positive. ETE was confirmed by intraoperative frozen biopsy, although there was no lymphovascular invasion. The DLNs were excised, and then, histopathological examination showed that the DLN had no metastasis. The probability of DLN metastasis assessed by nomogram was approximately 30%.
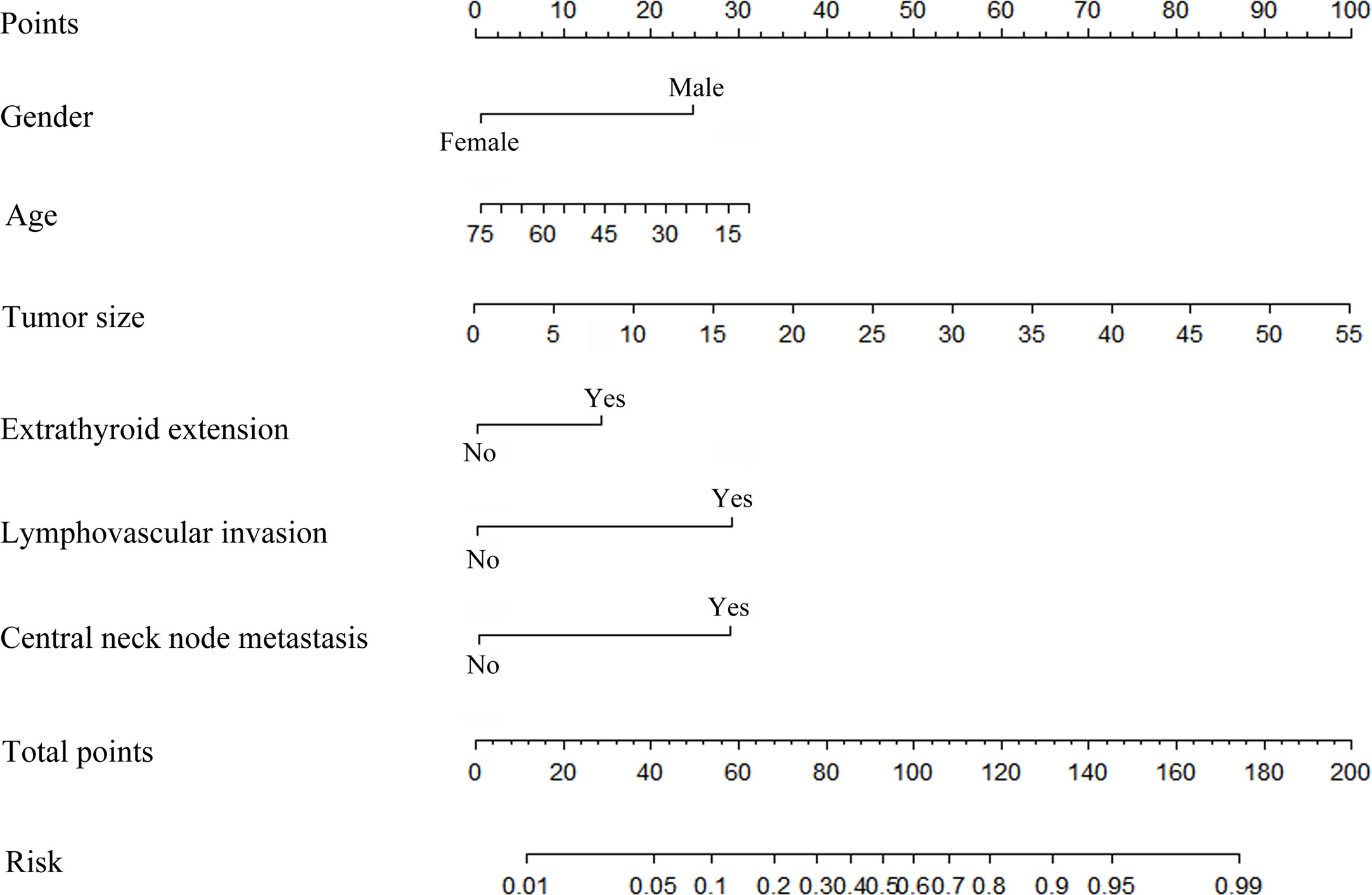
Figure 6 Risk factor-based nomogram for predicting DLN metastasis. Predictor points (“Points” scale; top) correspond to each variable.
Delphian was first used as an eponym for the prelaryngeal node in thyroid disease by Raymond B. Randall, and it is hypothesized that metastasis to this lymph node predicts a poor prognosis from cancer (13, 20). Previous literature has reported that DLN positivity is a measurable parameter to predict extensive lymph node metastasis, recurrence and poor overall survival in laryngeal and hypopharyngeal cancer (25, 26). As for PTC, recent reports detected the DLN in 23% to 75% of PTC patients, and the DLN positivity rate was 8% to 25% (12, 13, 15, 16, 27–30). Metastasis to the DLN is associated with several clinicopathological characteristics of PTC patients, including age, gender, tumor size, tumor location, multifocality, ETE, lymphovascular invasion and central and lateral neck node metastasis (12–14, 16, 27, 28, 30). In our series, male PTC patients were more likely to have positive DLNs. In addition, the rate of positive DLNs in younger patients was higher than that in elderly patients, suggesting that age was negatively correlated with DLN metastasis. Crucially, several adverse prognostic factors in PTC, including ETE, increased volume (number and size) of primary tumor, lymphovascular invasion and central and lateral node metastasis were verified to be positively related to DLN metastasis. Multivariable analyses revealed that gender, age, tumor size, ETE, lymphovascular invasion and central neck node metastasis were independent risk factors.
The DLN receives afferent lymph flow from the larynx and thyroid gland, which then flows towards the central and lateral neck lymph nodes (12). Studies have reported that patients with DLN metastasis are five to eight times more likely to have central compartment disease and 3.5 to 4 times more likely to have lateral neck lymph node metastasis (12–14). DLN positivity is predictive of further central and lateral lymph node metastasis (20). Hence, once metastatic disease to the DLN is identified, the surgeon should pay greater attention to the central and lateral neck compartment. We confirmed that the metastatic rate to the central lymph node and lateral neck lymph node in DLN-positive patients was 161 of 177 (90.96%) and 103/106 (97.17%), respectively. There has been debate over many years regarding whether prophylactic central node dissection should be performed in clinically N0 (cN0) PTC patients, and the prognostic significance of metastasis in this node group is controversial. Although NCCN clinical practice guideline in thyroid carcinoma and the American Thyroid Association guidelines no longer recommend prophylactic central compartment clearance in all cN0 PTC patients (31, 32), the Chinese Thyroid Association guidelines (21, 22) still recommend prophylactic central neck dissection, because the occult neck lymph node metastasis rate in cN0 PTC is still up to 72% (14), which could be the seeds of recurrence. Therefore, the central compartment should still be critically evaluated in all patients with cN0 PTC. The DLN, as one component of Level VI, is relatively sensitive for estimating Level VI metastatic disease. Joseph D. et al. (13) investigated 103 patients with thyroid cancer, and 21.4% of the patients were DLN positive. In that analysis, DLN involvement was associated with greater nodal disease (9.8 vs. 1.6 nodes), larger tumor size (19.4 mm vs. 11.1 mm) and younger age (41 vs. 47 years). Importantly, these anthors confirmed that DLN positivity remarkably predicted further disease in the neck lymph nodes. DLN-positive patients were approximately 8 times, 4 times and 60 times more likely to have central node disease, lateral node disease, and any neck nodal disease respectively. Our results were similar to the data mentioned above. Additionally, in our study, the diameter of the tumor was more than 5mm in 96.05% (170/177) of DLN-positive PTC and more than 10 mm in 66.67% (118/177) of DLN-positive PTC patients. Moreover, our results showed that DLN involvement was predictive of further disease in the central lymph node (sensitivity: 41%, specificity: 92%, positive predictive value (PPV): 91% and negative predictive value (NPV): 60%) and moderately predictive of further disease in the lateral neck compartment (sensitivity: 59%, specificity: 75%, PPV: 97%, NPV: 11%). Collectively, evaluating the status of the DLN is beneficial for the selection of lymph node management. Based on these data, once the status of DLN is sensitively evaluated, we can better predict the criticality of lymph node involvement.
In our study, conclusive evidence of DLN metastasis was obtained by a nomogram that was developed according to all adverse factors. These factors greatly contribute to a high risk of disease metastasis and recurrence (31). Ideally, a more accurate measurement before the operation and evaluation of the frozen sections of samples collected during the operation is essential for diagnosing DLN and Level VI nodal metastases. The nomogram proposed in this study, incorporating independent risk factors, provides a useful tool for surgeons to improve metastatic disease prediction and decision-making. To the best of our knowledge, this is the first retrospective study in PTC patients to search for clinicopathologic risk factors and further develop a diagnostic model for DLN metastasis. However, further studies should be conducted to validate the potential application value of nomograms in PTC patients with suspicious or confirmed DLN metastasis. Admittedly, our study was inherently limited by the retrospective single-center design with a probable selection bias. Moreover, lateral lymph node dissection was performed only when there was evidence of metastasis, which resulted in insufficient enrollment of negative cases. Hence, we excluded lateral lymph node metastasis from the multivariable analyses and the significance of lateral neck node metastasis might be underestimated.
In conclusion, our findings demonstrate that metastatic PTC to the DLN is associated with a number of clinicopathologic characteristics. As the DLN could be a clinical sensitive predictor of further neck lymph nodes, particularly the central compartment, we developed a diagnostic model that includs all adverse factors. If there is strong suspicion of metastatic DLN disease in cN0 PTC, the remainder of the central compartment should be evaluated for further nodal metastasis, and the appropriate dissection should be performed.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the Medical Ethics Committee of the Tianjin Medical University Cancer Institute & Hospital (bc2020046), and written informed consent was obtained from each patient.
CJ and YSD conceived and designed the study. XCL, DDL, and HWL collected the clinical information and performed most of the statistical analyses. MQZ, KY, YJS, and YW contributed to the data analysis and interpretation. XDW and YSW provided clinical samples and information. The manuscript was written by XCL and revised by CJ and YSD. CYJ contributed to the revision of the manuscript. CJ, XDW, and YSW supervised the research. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This manuscript has been released as a pre-print at https://www.researchsquare.com/article/rs-42289/v1, (33).
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin (2018) 68(1):7–30. doi: 10.3322/caac.21442
2. Mao Y, Xing M. Recent incidences and differential trends of thyroid cancer in the USA. Endocr Relat Cancer (2016) 23(4):313–22. doi: 10.1530/ERC-15-0445
3. Ito Y, Miyauchi A, Kihara M, Fukushima M, Higashiyama T, Miya A. Overall Survival of Papillary Thyroid Carcinoma Patients: A Single-Institution Long-Term Follow-Up of 5897 Patients. World J Surg (2018) 42(3):615–22. doi: 10.1007/s00268-018-4479-z
4. Wang LY, Ganly I. Nodal metastases in thyroid cancer: prognostic implications and management. Future Oncol (2016) 12(7):981–94. doi: 10.2217/fon.16.10
5. So YK, Kim MJ, Kim S, Son YI. Lateral lymph node metastasis in papillary thyroid carcinoma: A systematic review and meta-analysis for prevalence, risk factors, and location. Int J Surg (2018) 50:94–103. doi: 10.1016/j.ijsu.2017.12.029
6. Sturgeon C, Yang A, Elaraj D. Surgical Management of Lymph Node Compartments in Papillary Thyroid Cancer. Surg Oncol Clin N Am (2016) 25(1):17–40. doi: 10.1016/j.soc.2015.08.013
7. Chow SM, Law SC, Chan JK, Au SK, Yau S, Lau WH. Papillary microcarcinoma of the thyroid-Prognostic significance of lymph node metastasis and multifocality. Cancer (2003) 98(1):31–40. doi: 10.1002/cncr.11442
8. Adam MA, Pura J, Goffredo P, Dinan MA, Reed SD, Scheri RP, et al. Presence and Number of Lymph Node Metastases Are Associated With Compromised Survival for Patients Younger Than Age 45 Years With Papillary Thyroid Cancer. J Clin Oncol (2015) 33(21):2370–5. doi: 10.1200/JCO.2014.59.8391
9. Tran Cao HS, Johnston LE, Chang DC, Bouvet M. A critical analysis of the American Joint Committee on Cancer (AJCC) staging system for differentiated thyroid carcinoma in young patients on the basis of the Surveillance, Epidemiology, and End Results (SEER) registry. Surgery (2012) 152(2):145–51. doi: 10.1016/j.surg.2012.02.015
10. Olsen KD, DeSanto LW, Pearson BW. Positive Delphian lymph node: clinical significance in laryngeal cancer. Laryngoscope (1987) 97(9):1033–7. doi: 10.1288/00005537-198709000-00007
11. Thaler ER, Montone K, Tucker J, Weinstein GS. Delphian lymph node in laryngeal carcinoma: a whole organ study. Laryngoscope (1997) 107(3):332–4. doi: 10.1097/00005537-199703000-00010
12. Isaacs JD, Lundgren CI, Sidhu SB, Sywak MS, Edhouse PJ, Delbridge LW. The Delphian lymph node in thyroid cancer. Ann Surg (2008) 247(3):477–82. doi: 10.1097/SLA.0b013e31815efdc4
13. Iyer NG, Kumar A, Nixon IJ, Patel SG, Ganly I, Tuttle RM, et al. Incidence and significance of Delphian node metastasis in papillary thyroid cancer. Ann Surg (2011) 253(5):988–91. doi: 10.1097/SLA.0b013e31821219ca
14. Zheng G, Zhang H, Hao S, Liu C, Xu J, Ning J, et al. Patterns and clinical significance of cervical lymph node metastasis in papillary thyroid cancer patients with Delphian lymph node metastasis. Oncotarget (2017) 8(34):57089–98. doi: 10.18632/oncotarget.19047
15. saacs JD, McMullen TP, Sidhu SB, Sywak MS, Robinson BG, Delbridge LW. Predictive value of the Delphian and level VI nodes in papillary thyroid cancer. ANZ J Surg (2010) 80(11):834–8. doi: 10.1111/j.1445-2197.2010.05334.x
16. Tan Z, Ge MH, Zheng CM, Wang QL, Nie XL, Jiang LH. The significance of Delphian lymph node in papillary thyroid cancer. Asia Pac J Clin Oncol (2017) 13(5):e389–e93. doi: 10.1111/ajco.12480
17. Roh JL, Kim JM, Park CI. Central lymph node metastasis of unilateral papillary thyroid carcinoma: patterns and factors predictive of nodal metastasis, morbidity, and recurrence. Ann Surg Oncol (2011) 18(8):2245–50. doi: 10.1245/s10434-011-1600-z
18. Roh JL, Kim JM, Park CI. Central cervical nodal metastasis from papillary thyroid microcarcinoma: pattern and factors predictive of nodal metastasis. Ann Surg Oncol (2008) 15(9):2482–6. doi: 10.1245/s10434-008-0044-6
19. Roh JL, Kim JM, Park CI. Lateral cervical lymph node metastases from papillary thyroid carcinoma: pattern of nodal metastases and optimal strategy for neck dissection. Ann Surg Oncol (2008) 15(4):1177–82. doi: 10.1245/s10434-008-9813-5
20. Huang J, Sun W, Zhang H, Zhang P, Wang Z, Dong W, et al. Use of Delphian lymph node metastasis to predict central and lateral involvement in papillary thyroid carcinoma: A systematic review and meta-analysis. Clin Endocrinol (Oxf) (2019) 91(1):170–8. doi: 10.1111/cen.13965
21. Ming Gao MG, Ji Q, Xu Z, Lu H. Ruochuan Cheng, Haixia Guan. Chinese consensus on diagnosis and treatment of thyroid micropapillary carcinoma. Chin J Clin Oncol (2016) 43(10):405–11.
22. Association CM. Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Chin J Clin Oncol (2012) 39(17):1249–72.
23. Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, et al. Discrimination and Calibration of Clinical Prediction Models: Users’ Guides to the Medical Literature. JAMA (2017) 318(14):1377–84. doi: 10.1001/jama.2017.12126. 2656816.
24. Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation (2010) 121(14):1630–6. doi: 10.1161/CIRCULATIONAHA.109.925214
25. Ferlito A, Shaha AR, Rinaldo A. Prognostic value of Delphian lymph node metastasis from laryngeal and hypopharyngeal cancer. Acta Otolaryngol (2002) 122(4):456–7. doi: 10.1080/00016480260000201
26. Wierzbicka M, Leszczynska M, Mlodkowska A, Szyfter W, Bartochowska A. The impact of prelaryngeal node metastases on early glottic cancer treatment results. Eur Arch Otorhinolaryngol (2012) 269(1):193–9. doi: 10.1007/s00405-011-1747-z
27. Kim WW, Yang SI, Kim JH, Choi YS, Park YH, Kwon SK. Experience and analysis of Delphian lymph node metastasis in patients with papillary thyroid carcinoma. World J Surg Oncol (2012) 10:226. doi: 10.1186/1477-7819-10-226
28. Lee YC, Shin SY, Kwon KH, Eun YG. Incidence and clinical characteristics of prelaryngeal lymph node metastasis in papillary thyroid cancer. Eur Arch Otorhinolaryngol (2013) 270(9):2547–50. doi: 10.1007/s00405-013-2471-7
29. Oh EM, Chung YS, Lee YD. Clinical significance of Delphian lymph node metastasis in papillary thyroid carcinoma. World J Surg (2013) 37(11):2594–9. doi: 10.1007/s00268-013-2157-8
30. Chai YJ, Kim SJ, Choi JY, Koo do H, Lee KE, Youn YK. Papillary thyroid carcinoma located in the isthmus or upper third is associated with Delphian lymph node metastasis. World J Surg (2014) 38(6):1306–11. doi: 10.1007/s00268-013-2406-x
31. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
32. National Comprehensive Cancer Network I. 2019 Practice Guidelines in Oncology- Thyroid Carcinoma v.2. National Comprehensive Cancer Network (2019). Available at: www.nccn.org/professionals/physician_gls/PDF/thyroid.pdf.
Keywords: risk factor, nomogram, diagnostic model, papillary thyroid cancer, Delphian lymph node
Citation: Li X, Duan Y, Liu D, Liu H, Zhou M, Yue K, Shuai Y, Wang Y, Ji C, Jing C, Wu Y and Wang X (2021) Diagnostic Model Incorporating Clinicopathological Characteristics of Delphian Lymph Node Metastasis Risk Profiles in Papillary Thyroid Cancer. Front. Endocrinol. 12:591015. doi: 10.3389/fendo.2021.591015
Received: 03 August 2020; Accepted: 01 February 2021;
Published: 25 March 2021.
Edited by:
Christoph Reiners, University Hospital Würzburg, GermanyReviewed by:
Giorgio Napolitano, University of Studies G. d’Annunzio Chieti and Pescara, ItalyCopyright © 2021 Li, Duan, Liu, Liu, Zhou, Yue, Shuai, Wang, Ji, Jing, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xudong Wang, V1hELjExMzNAMTYzLmNvbQ==; Yansheng Wu, eWFuc2hlbmcxOTgxQDE2My5jb20=; Chao Jing, U3VwZXJEd2VsbEBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.