- 1Department of Cardiology, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Cardiology, Peking University Third Hospital, Beijing, China
- 3Graduate School of Peking Union Medical College, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 4Department of Cardiac Catheterization Laboratory, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: High lipoprotein(a) (Lp[a]) levels are associated with increased risks of cardiovascular events in Percutaneous Coronary Intervention (PCI) patients with diabetes mellitus (DM). Peri-procedural myocardial infarction (PMI) occurs commonly during the PCI, whereas the relationship between Lp(a) and PMI remains unclear. Our study aimed to evaluate the association between Lp(a) value and the incidence of PMI in a larger-scale diabetic cohort undergoing PCI throughout 2013.
Methods: A total of 2,190 consecutive patients with DM were divided into two groups according to the median Lp(a) level of 175 mg/L: Low Lp(a) group (N = 1095) and high Lp(a) group (N = 1095). PMI was defined based on the 2018 universal definition of myocardial infarction.
Results: Patients with high Lp(a) levels exhibited higher rates of PMI compared to those with low Lp(a) levels (2.3% versus 0.8%, P = 0.006). The multivariable logistic analysis showed that PMI was independently predicted by Lp(a) as a dichotomous variable (OR 2.64, 95%CI 1.22–5.70) and as a continuous variable (OR 1.57, 95% CI 1.12–2.20). However, further investigation found that this association was only maintained in men, whose Lp(a) levels were significantly associated with the frequency of PMI, both as a dichotomous variable (OR 3.66, 95%CI 1.34–10.01) and as a continuous variable (OR 1.81, 95%CI 1.18–2.78). Lp(a) wasn’t a risk factor of PMI in women.
Conclusions: High Lp(a) levels had forceful correlations with the increased frequency of PMI in male diabetic patients undergoing PCI. Lp(a) might act as a marker of risk stratification and a therapeutic target to reduce PCI-related ischemic events.
Introduction
It is estimated that the number of patients with diabetes mellitus (DM) has doubled in the past two decades (1). DM has become one of the most common metabolic disorders in contemporary PCI practice. DM exerts the pro-thrombotic and pro-inflammatory effects in the pathogenesis of atherosclerosis and increases the risk of cardiovascular consequences (2). It’s noteworthy to identify the risk factors that predict cardiac events in diabetic patients undergoing PCI.
Peri-procedural myocardial infarction (PMI) is a procedure-related ischemic cardiac event following coronary stenting (3). There is a growing body of evidence showing the association between PMI and worse outcomes after PCI over short- and long-term periods (4–6). Previous research proposed that the distal and side-branch thromboembolism, which resulted from lipid-rich plaques rupture, gave rise to the occurrence of PMI (7). Moreover, blood lipid profiles, including high-density lipoprotein (HDL), low-density lipoprotein (LDL), and fatty acid were demonstrated to have correlations with PCI-related myocardial injury (8–10).
Lipoprotein(a) (Lp[a]) is a LDL-like lipid with additional apolipoprotein(a) attached to apolipoprotein B (11). Epidemiological studies identified Lp(a) as an atherothrombotic risk factor, and prior studies revealed the relationship between the elevation of Lp(a) and the myocardial ischemic events (12, 13). But much less is known about the impact of elevated Lp(a) on the peri-procedural coronary events among patients with DM.
Therefore, our study aimed to investigate whether high Lp(a) status was associated with an increased risk of PMI, defined by the latest 2018 fourth universal definition of myocardial infarction (UDMI) (14), among a large-scale diabetic cohort undergoing PCI. Univariable and multivariable logistic regression models and receiver-operating curve (ROC) analysis were used to investigate the predictive value of Lp(a).
Material and Methods
Study Population
The study enrolled consecutive patients with DM undergoing PCI at Fuwai Hospital (Beijing, China) between January 2013 and December 2013. Patients without available Lp(a) and cardiac biomarker data were excluded. Cardiac markers were generally measured in patients undergoing PCI, while the measurement of Lp(a) wasn’t routinely performed. Therefore, we compared the differences between patients with and without available Lp(a) data. As shown in Supplementary Table S1, most baseline characteristics and procedural features were evenly distributed between patients without available Lp(a) data and with available Lp(a) data. All patients had written informed consent. The study complied with the principles of Helsinki and was approved by the hospital review board. A total of 2,190 subjects were eligible for the present analysis. According to the median Lp(a) level of 175 mg/L, patients were divided into two parts: Low-Lp(a) group (N = 1,095) and High-Lp(a) group (N = 1,095). Baseline clinical and procedural data were extracted from medical records. Venous blood was collected from all patients after fasting overnight. Biochemical analyses were performed in the biochemistry laboratory of Fuwai Hospital. Lp(a) concentrations were measured by immunoturbidimetry (Lasay Lp(a) auto, Shima laboratories, Japan) method following the manufacturer’s instructions.
Procedure
The PCI strategies and stents were determined by treating cardiologists according to patients’ characteristics. A loading dose of 300 mg aspirin and 300 mg clopidogrel was prescribed for the patients before the procedure. After the procedure, all patients received at least 1 year of clopidogrel (75 mg/day) and lifelong aspirin (100mg/day). The Synergy between Percutaneous Coronary Intervention (PCI) with TAXUS and Cardiac Surgery (SYNTAX) score (http://www.syntaxscore.com) was calculated by treating cardiologist at Fuwai Hospital. PCI with a SYNTAX score of more than 22 were considered a high-risk PCI procedure (15). Levels of cardiac biomarkers were assayed before and after procedures. The diagnostic definition of PMI was based on the 2018 UDMI. PMI was defined as a rise of more than five times the 99th percentile upper reference limit (URL) of cardiac biomarker levels when patients showing normal baseline levels or an increase over 20% cardiac biomarker levels when patients showing elevated baseline levels but stable or declining. Additional evidence of ischemic ECG findings, imaging changes, and angiographic complications was required for the diagnosis.
Statistical Methods
Continuous characteristics were presented as mean ± SD and comparisons were made with Student’s t‐test. Categorical variables were described as frequencies with percentages and evaluated with the chi‐square test or Fisher’s exact test as appropriate. Univariate and multivariate logistic regression evaluations were performed to identify the independent predictors for PMI. Lp(a) was presented as a categorical variable [high Lp(a)] and a continuous variable (natural logarithm) in the analysis. The receiver-operating curve (ROC) was used to assess the prediction for PMI. Kaplan-Meier survival estimate was used to evaluate time-to-event data. All P values were two-sided, and a P-value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 22.0 (SPSS, Inc., Chicago, IL).
Results
Patients Characteristics
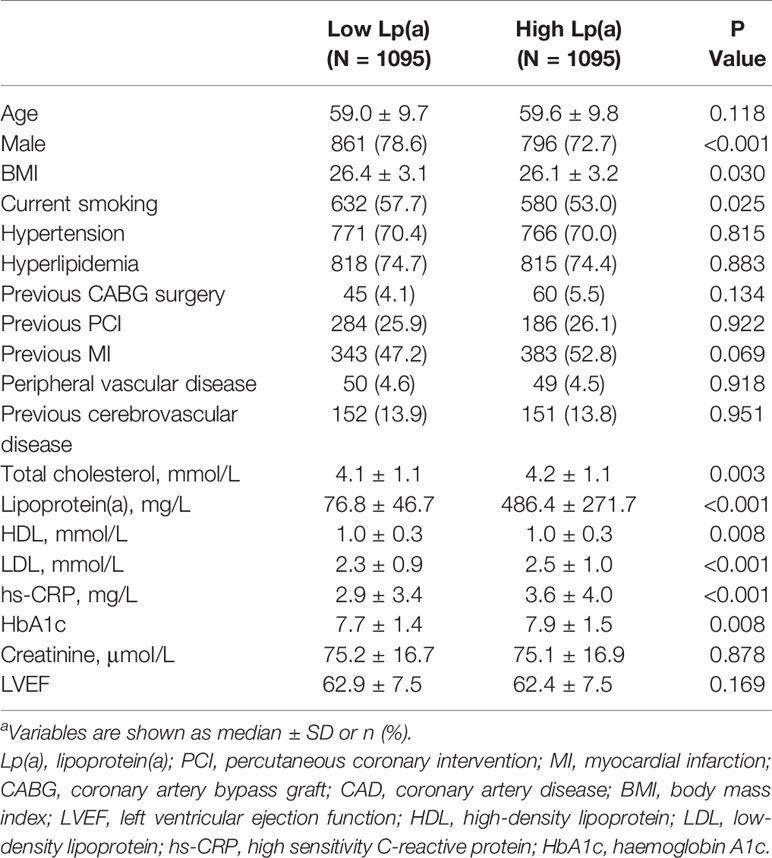
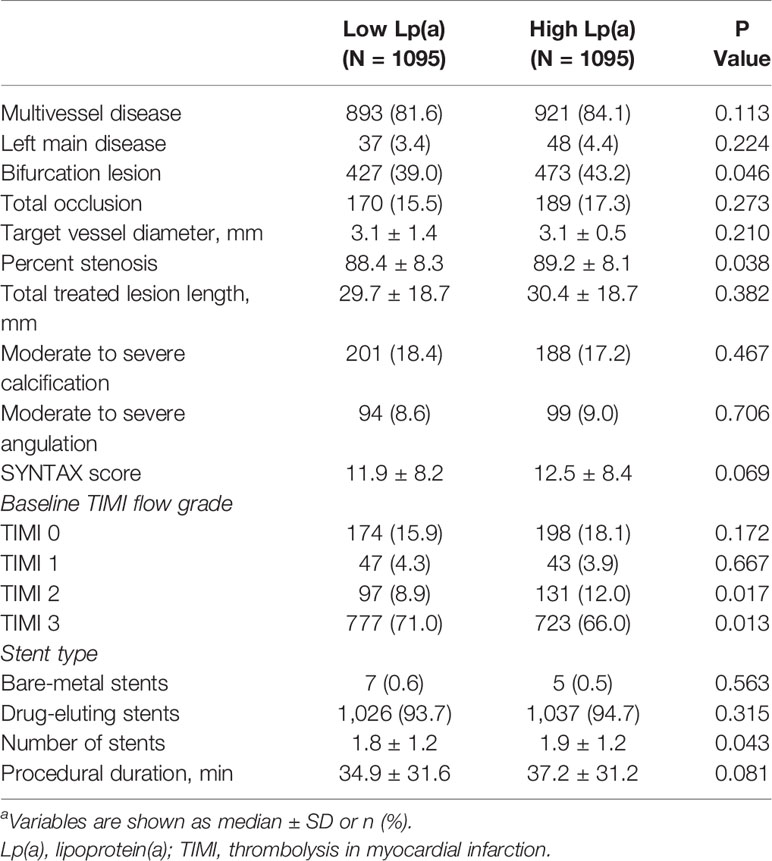
Patients were stratified into high Lp(a) group and low Lp(a) group and major clinical and angiographic variables of the study population are shown in Tables 1 and 2. 75.7% of patients were male and their mean age was 59 years old.
High Lp(a) group were more frequently female, as well as showing more complicated procedural features, compared with low Lp(a) group. As expected, significant worse metabolic profiles exhibited in the high Lp(a) group, which were composed of high LDL levels, and high Haemoglobin A1c (HbA1c) levels.
Lp(a) and PMI
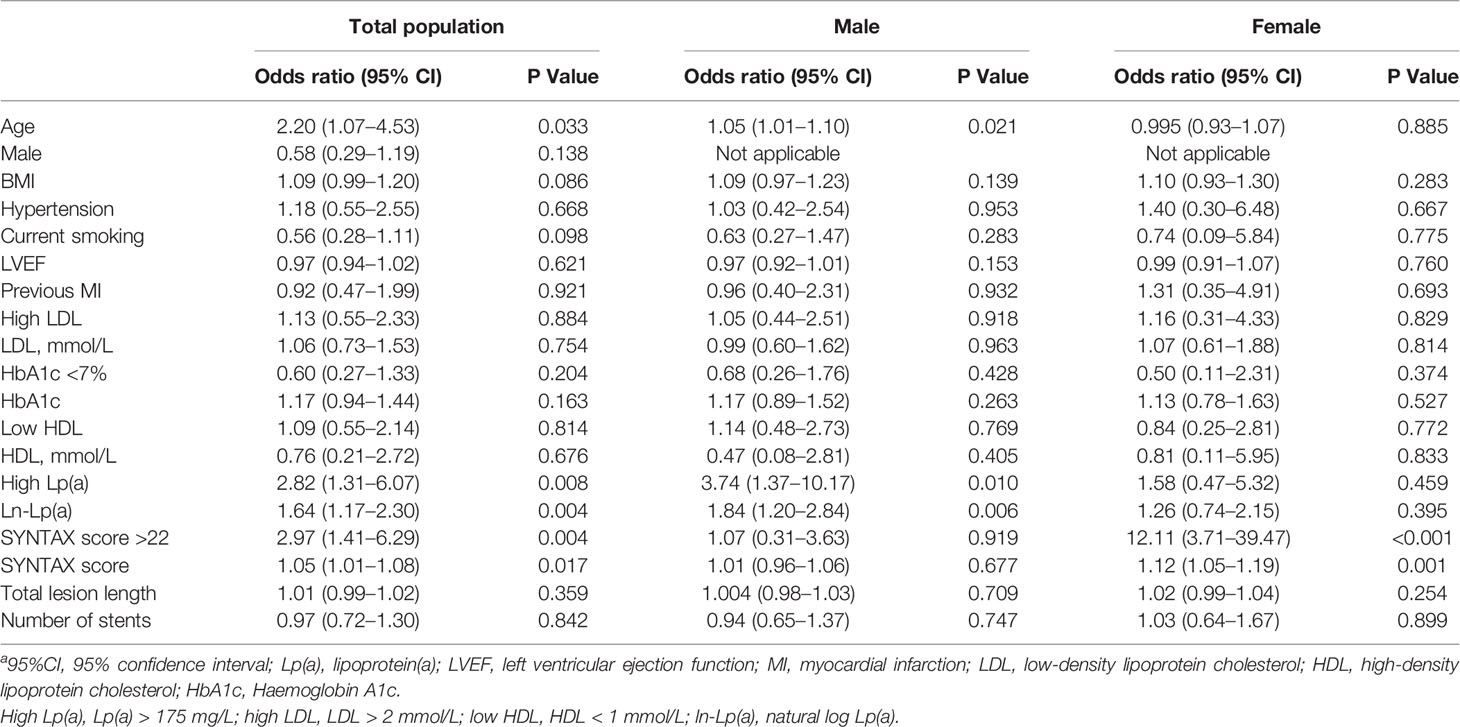
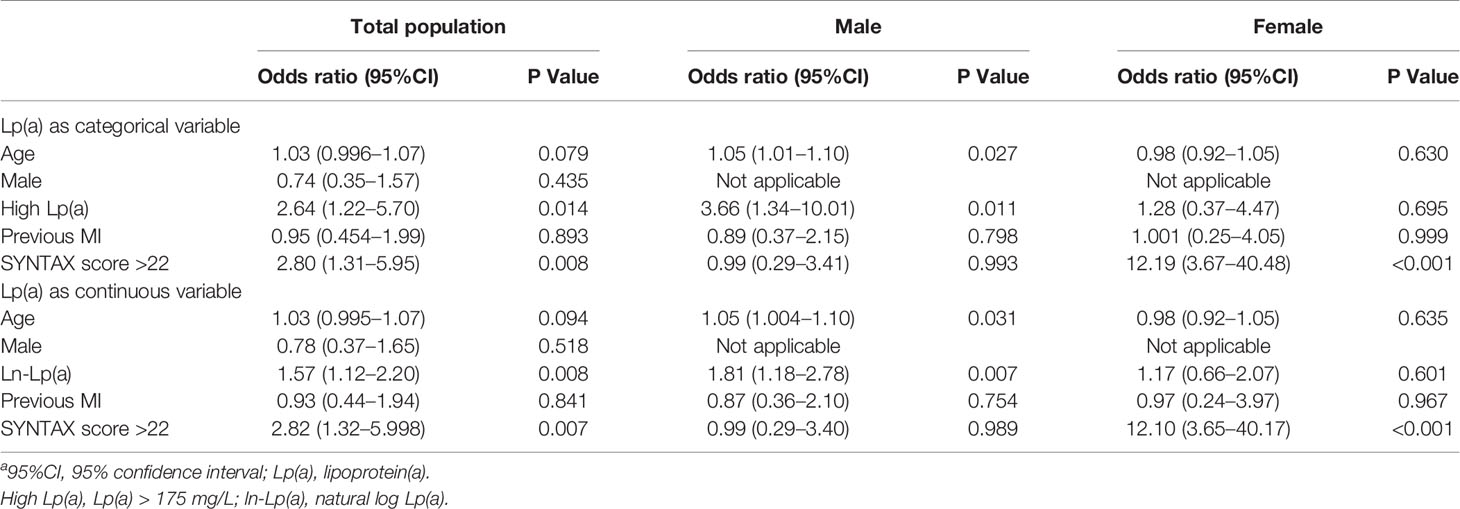
Figure 1A demonstrates the proportion of patients with PMI according to the Lp(a) levels. Patients with high Lp(a) levels exhibited an increased occurrence of PMI than those with low Lp(a) levels (2.30% versus 0.80%, P=0.006). In male patients, the incidence of PMI was considerably higher in the high Lp(a) group than the low Lp(a) group (1.60% versus 0.46%, P=0.006). In female patients, the incidences of PMI between the two groups were not significantly different (0.76% versus 0.36%, P=0.456). Table 3 provides the univariable logistic regression analysis for the prediction of PMI, which summarizes that Lp(a), as a continuous variable [log-transformed Lp(a)] (OR 1.64, 95%CI 1.17–2.30, P = 0.004) or a dichotomous variable [high Lp(a)] (OR 2.82, 95% CI 1.31–6.07, P = 0.008), was significantly associated with the increased frequency of PMI. However, after separately exploring the association between Lp(a) and PMI in males or females, we found no significant relationship of Lp(a) to PMI in females. Table 4 shows the multivariable models for the prediction of PMI. High Lp(a) levels were independently associated with increased incidence of PMI than low Lp(a) level [adjusted OR 2.64, 95% CI 1.22–5.70, P = 0.014]. Besides, the relationship between the log-transformed Lp(a) and elevated incidence of PMI was also significant (adjusted OR 1.57, 95%CI 1.12–2.20, P = 0.008). Consistently, after adjustment, Lp(a) was still the risk factor of PMI in men as a dichotomous variable (adjusted OR 3.66, 95%CI 1.34–10.01, P=0.011) or a continuous variable (adjusted OR1.81, 95%CI 1.18–2.78, P=0.007), whereas Lp(a) did not predict PMI events in women.
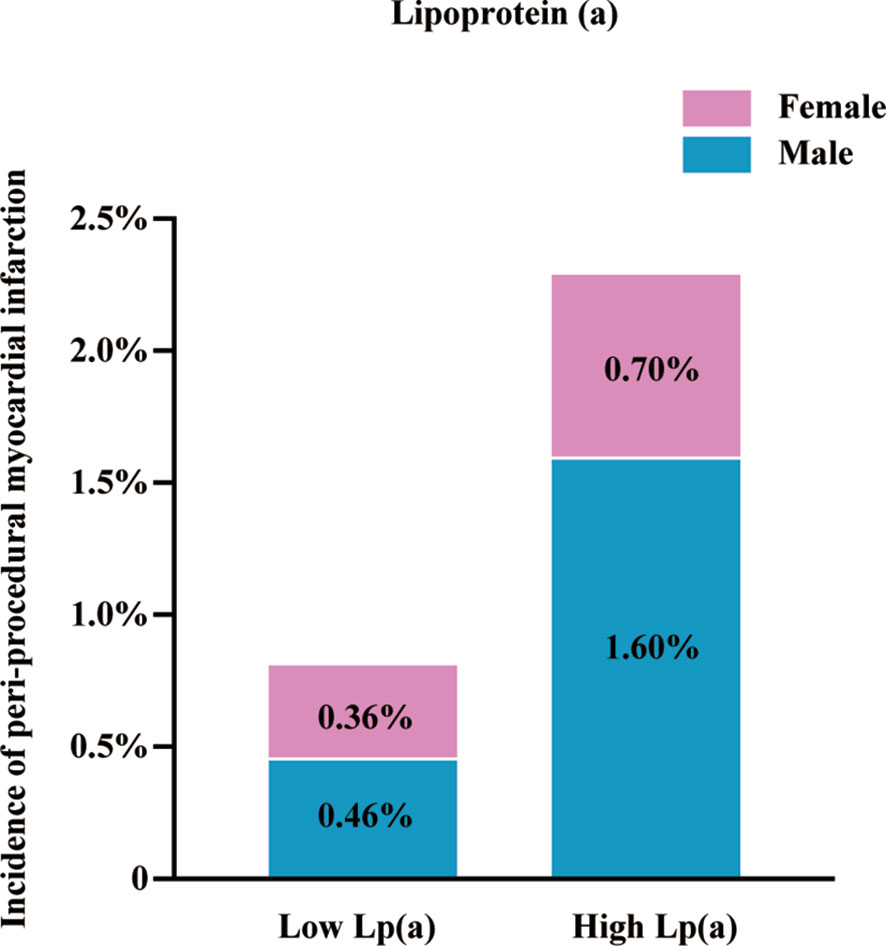
Figure 1 Incidence of peri-procedural myocardial infarction. Lp(a), lipoprotein(a); high Lp(a), Lp(a) > 175 mg/L.
To further provide an estimate that might be compared against in the future, we conducted ROC analysis in male diabetic patients. Figure 2 shows that the area under the receiver-operating curve (AUC) was 0.680 (95% CI 0.657–0.703, P <0.001) for Lp(a) to predict PMI. The optimal cut-off value was 112.4 mg/L (AUC=0.678, sensitivity 95.5%, specificity 40.1%).
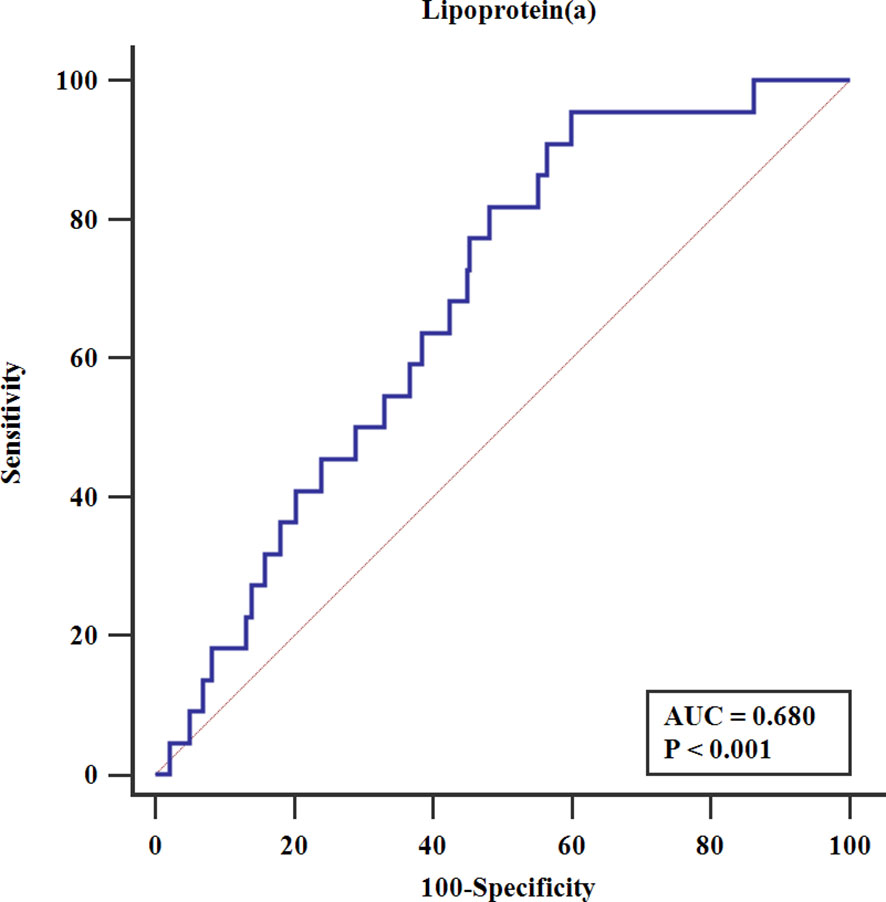
Figure 2 Receiver-operating characteristics analysis. AUC, the area under the receiver-operating curve.
Discussion
Major Findings
The major findings of our research were high Lp(a) was independently associated with the occurrence of PMI and a predictor for PMI in diabetic patients undergoing PCI, but further investigation found the association was only significant in male, and Lp(a) wasn’t a risk factor of PMI for female. Our analysis indicated that high Lp(a) might be a convenient screening marker for the assessment of PMI, and reducing Lp(a) concentrations might be one possible way to improve the outcome of PCI patients with DM.
The Clinical Implication of Lp(a) and PMI in Patients With DM
It is reported that diabetic patients had a two-fold higher risk for cardiovascular diseases than non-diabetic patients (16). However, previous studies demonstrated that intensive lowering of glucose levels didn’t reduce the risk of cardiac events (17). Thus, it is of great importance to find out the risk factor for diabetic patients to prevent adverse cardiovascular consequences.
Lipid disorders commonly occur in patients with diabetes (2). Notably, lipoprotein abnormalities advance atherosclerosis and are a strong predictor of adverse cardiovascular outcomes. Lp(a) is an inheritable lipoprotein element, which is barely affected by dietary changes and environmental alterations (18). The ability of Lp(a) to engage in cardiovascular diseases mainly depends on its pro-atherosclerotic and pro-thrombotic effect (19). Lp(a) promotes the development of atherosclerosis by advancing foam cell formation, inducing smooth muscle cell proliferation, and activating monocyte chemoattractant (20). Moreover, Lp(a) could be a marker to predict the complexity and severity of the angiographic lesions. Prior studies reported that elevated Lp(a) was significantly associated with severe coronary conditions (21, 22). Besides, Lp(a) had a plasminogen-like function, which could bind to fibrin, inhibit fibrinolysis, and promote thrombosis (20). The increased frequency of PMI in our study provided additional evidence of the association of elevated Lp(a) and post-procedure elevated risk of thrombotic cardiac events. Moreover, the two-year composite outcomes of all-cause mortality and myocardial infarction significantly increased in the PMI group than the non-PMI group (Figure 3). Reducing Lp(a) level might provide a potential way to prevent PMI and subsequent adverse consequences.
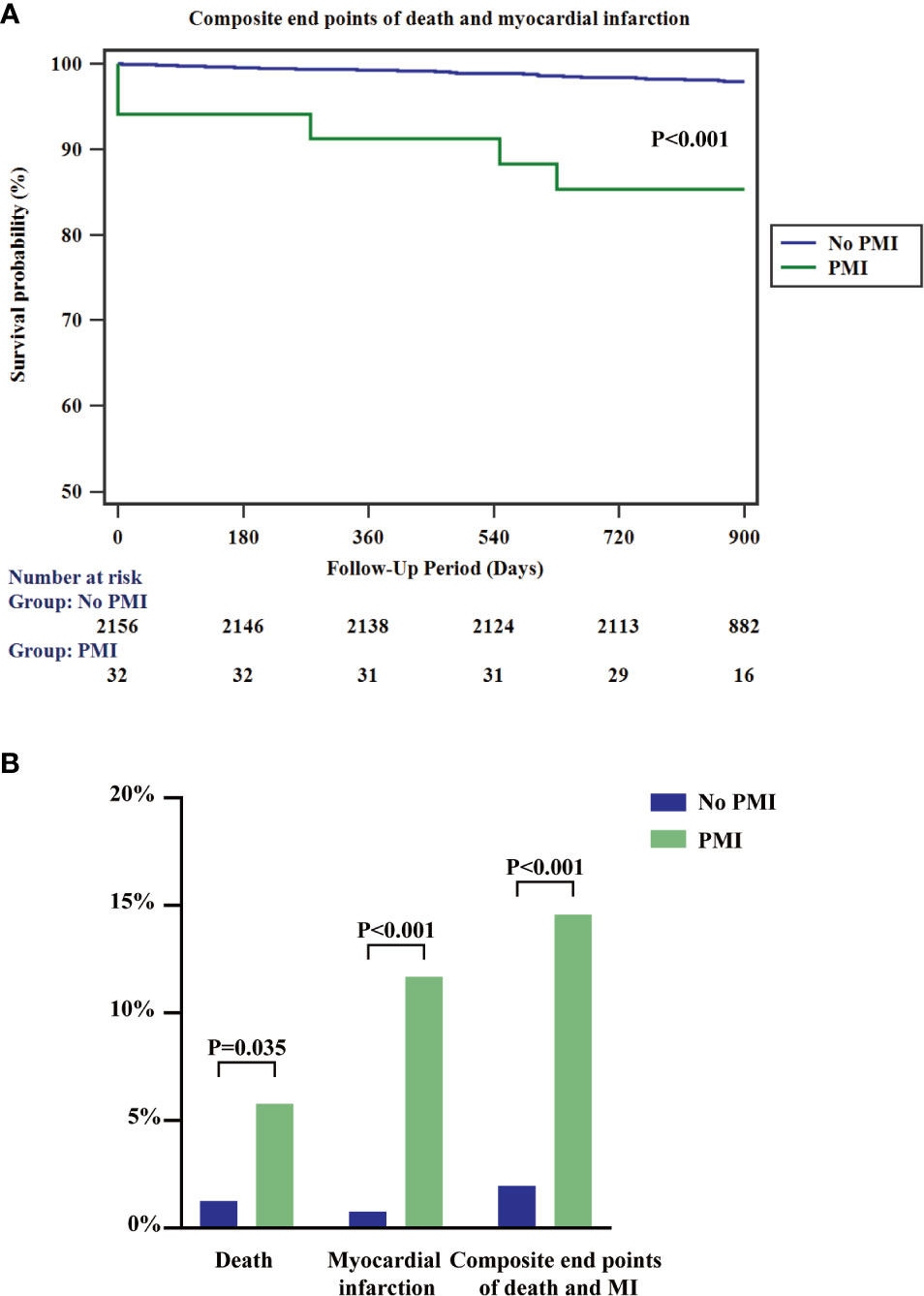
Figure 3 Kaplan-Meier survival curves for composite endpoints of all-cause mortality and myocardial infarction (A) and 2-year rates of the indicated events (B) according to the presence or absence of PMI. PMI, peri-procedural myocardial infarction; MI, myocardial infarction.
Although broadly use of statin therapy (23) markedly decreases the risk of adverse cardiovascular events by targeting LDL (24), statin shows hardly any effect on lowering Lp(a) levels. Conversely, a recent meta-analysis revealed that Lp(a) levels escalated over 10% from baseline after statin treatment (25) and Lp(a) may represent part of the residual risk of the statin therapies (26). Hence, Lp(a) plays a critical role in contemporary lipid-lowering medication administration. In a recent BiomarCaRE (Biomarkers for Cardiovascular Risk Assessment in Europe) study (27), high Lp(a) levels robustly increased the risk of cardiac events in patients with DM, indicating that the Lp(a)-lowering might greatly improve the cardiac outcomes in diabetic patients. Our study further demonstrated that the low Lp(a) population had a reduced risk of procedure-related myocardial infarction and Lp(a) might be a potential evaluation tool for risk management in diabetic patients undergoing PCI.
The most promising Lp(a)-Lowering pharmacologic treatments are hepatocyte-directed antisense oligonucleotide (ASO) and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. The latest randomized controlled trial of ASO therapy showed that Lp(a) achieved forcefully 35% to 72% reduction in a dose-dependent manner after 32 weeks (28). ODYSSEY OUTCOME (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) randomized trial demonstrated that PCSK9 inhibitor alirocumab reduced Lp(a) by a median 23.5% after 4 months (29). Currently, lipoprotein apheresis is an efficient therapy for lowering Lp(a) levels (30). Recent evidence showed a reduction of 63% for Lp(a) had been achieved by apheresis and cardiovascular events markedly reduced about 94% after treatment (31). The combination of statin and Lp(a)-lowering therapy provides us an insight into future lipid treating therapy in patients with diabetes (32).
Limitations
There are several limitations requiring careful consideration. First, this study was conducted in a single center, further multi-center validation is needed to confirm our findings. Second, the study was based on observational data, not on randomized design trials. Thus, the result of the analysis might not indicate final conclusions for the impact of Lp(a). Finally, although we adjusted the traditional risk factors, hidden unadjusted confounders may not be totally eliminated. Moreover, there was a lack of a family history record and genetic background that could allow us to further evaluate the inheritable Lp(a) influence.
Conclusions
In conclusion, Lp(a) levels were independently associated with an elevated risk of PMI during PCI in male patients with DM. Lp(a) might be a significant indicator for PCI risk stratification and a potential target for PMI treatment. Further studies should be conducted to investigate the detailed role of Lp(a) in the pathology of PMI.
Data Availability Statement
Request to the data of this study can be sent to the corresponding author, who will provide them to vetted and qualified applicants.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Fuwai Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YL: Conceptualization, methodology, investigation, formal analysis, and writing—original draft. WW: Data curation, writing—review and editing, and supervision. JS: Formal analysis, software, and validation. KZ: Project administration. BX: Resources. PL, CS, MY, JC: Investigation. Y-DT: Conceptualization, writing—review and editing, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China [81825003, 91957123]; the CAMS Innovation Fund for Medical Sciences [CIFMS 2016-I2M-1-009]; the Beijing Municipal Commission of Science and Technology (Z181100006318005); and the National Key Research and Development Program of China [2020YFC2004700].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank our medical staff from the department of cardiology for their contribution to data entry and monitoring.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.603922/full#supplementary-material
References
1. Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol (2012) 8:228–36. doi: 10.1038/nrendo.2011.183
2. Biondi-Zoccai GGL, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol (2003) 41:1071–7. doi: 10.1016/S0735-1097(03)00088-3
3. Damman P, Wallentin L, Fox KAA, Windhausen F, Hirsch A, Clayton T, et al. Long-Term Cardiovascular Mortality After Procedure-Related or Spontaneous Myocardial Infarction in Patients With Non–ST-Segment Elevation Acute Coronary Syndrome: A Collaborative Analysis of Individual Patient Data From the FRISC II, ICTUS, and RITA-3 Trials (FIR). Circulation (2012) 125:568–76. doi: 10.1161/CIRCULATIONAHA.111.061663
4. Baker NC, Lipinski MJ, Escarcega RO, Magalhaes MA, Minha S, Torguson R, et al. Definitions of Periprocedural Myocardial Infarction as Surrogates for Catheterization Laboratory Quality or Clinical Trial End Points. Am J Cardiol (2014) 113:1326–30. doi: 10.1016/j.amjcard.2014.01.408
5. Yang X, Tamez H, Lai C, Ho K, Cutlip D. Type 4a myocardial infarction: Incidence, risk factors, and long-term outcomes: Type 4a Myocardial Infarction. Cathet Cardiovasc Intervent (2017) 89:849–56. doi: 10.1002/ccd.26688
6. Bonaca MP, Wiviott SD, Braunwald E, Murphy SA, Ruff CT, Antman EM, et al. American College of Cardiology/American Heart Association/European Society of Cardiology/World Heart Federation Universal Definition of Myocardial Infarction Classification System and the Risk of Cardiovascular Death: Observations From the TRITON-TIMI 38 Trial (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis in Myocardial Infarction 38). Circulation (2012) 125:577–83. doi: 10.1161/CIRCULATIONAHA.111.041160
7. Goldstein JA. Peri-procedural myocardial infarction: Plaques and patients “at-risk.” Catheter Cardiovasc Interv (2017) 90:915–6. doi: 10.1002/ccd.27393
8. Sattler KJE, Herrmann J, Yun S, Lehmann N, Wang Z, Heusch G, et al. High high-density lipoprotein-cholesterol reduces risk and extent of percutaneous coronary intervention-related myocardial infarction and improves long-term outcome in patients undergoing elective percutaneous coronary intervention. Eur Heart J (2009) 30:1894–902. doi: 10.1093/eurheartj/ehp183
9. Wang Y, Zhang H-W, Guo Y-L, Zhu C-G, Wu N-Q, Li J-J. Free fatty acids as a marker for predicting periprocedural myocardial injury after coronary intervention. Postgrad Med J (2019) 95:18–22. doi: 10.1136/postgradmedj-2018-136137
10. Zeng R-X, Li X-L, Zhang M-Z, Guo Y-L, Zhu C-G, Guo L-H, et al. Non-HDL cholesterol is a better target for predicting periprocedural myocardial injury following percutaneous coronary intervention in type 2 diabetes. Atherosclerosis (2014) 237:536–43. doi: 10.1016/j.atherosclerosis.2014.10.030
11. Tsimikas S. A Test in Context: Lipoprotein(a). J Am Coll Cardiol (2017) 69:692–711. doi: 10.1016/j.jacc.2016.11.042
12. Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), Measured With an Assay Independent of Apolipoprotein(a) Isoform Size, and Risk of Future Cardiovascular Events Among Initially Healthy Women. JAMA (2006) 296:1363. doi: 10.1001/jama.296.11.1363
13. Chen Z, Jiang C, Qu H, Liang S, Yang J, Wu H, et al. Association of lipoprotein(a) and major adverse cardiovascular events in patients with percutaneous coronary intervention. aoms (2019) 15:1375–80. doi: 10.5114/aoms.2018.79401
14. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J (2019) 40:237–69. doi: 10.1093/eurheartj/ehy462
15. Head SJ, Farooq V, Serruys PW, Kappetein AP. The SYNTAX score and its clinical implications. Heart (2014) 100:169–77. doi: 10.1136/heartjnl-2012-302482
16. Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W. Associations of Diabetes Mellitus With Total Life Expectancy and Life Expectancy With and Without Cardiovascular Disease. Arch Intern Med (2007) 167:1145. doi: 10.1001/archinte.167.11.1145
17. Riddle MC. Effects of Intensive Glucose Lowering in the Management of Patients With Type 2 Diabetes Mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial. Circulation (2010) 122:844–6. doi: 10.1161/CIRCULATIONAHA.110.960138
18. Kronenberg F. Human Genetics and the Causal Role of Lipoprotein(a) for Various Diseases. Cardiovasc Drugs Ther (2016) 30:87–100. doi: 10.1007/s10557-016-6648-3
19. van der Valk FM, Bekkering S, Kroon J, Yeang C, Van den Bossche J, van Buul JD, et al. Oxidized Phospholipids on Lipoprotein(a) Elicit Arterial Wall Inflammation and an Inflammatory Monocyte Response in Humans. Circulation (2016) 134:611–24. doi: 10.1161/CIRCULATIONAHA.116.020838
20. Spence JD, Koschinsky M. Mechanisms of Lipoprotein(a) Pathogenicity: Prothrombotic, Proatherosclerotic, or Both? Arterioscler Thromb Vasc Biol (2012) 32:1550–1. doi: 10.1161/ATVBAHA.112.251306
21. Chieng D, Pang J, Ellis KL, Hillis GS, Watts GF, Schultz CJ. Elevated lipoprotein(a) and low-density lipoprotein cholesterol as predictors of the severity and complexity of angiographic lesions in patients with premature coronary artery disease. J Clin Lipidol (2018) 12:1019–26. doi: 10.1016/j.jacl.2018.03.090
22. Xu N, Tang X, Yao Y, Jia S, Liu Y, Zhao X, et al. Lipoprotein(a) levels are associated with coronary severity but not with outcomes in Chinese patients underwent percutaneous coronary intervention. Nutrition Metab Cardiovasc Dis (2020) 30:265–73. doi: 10.1016/j.numecd.2019.09.020
23. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation (2019) 139:e1082–143. doi: 10.1161/CIR.0000000000000625
24. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, et al. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA (2016) 316:1289. doi: 10.1001/jama.2016.13985
25. Tsimikas S, Gordts PLSM, Nora C, Yeang C, Witztum JL. Statin therapy increases lipoprotein(a) levels. Eur Heart J (2020) 41:2275–84. doi: 10.1093/eurheartj/ehz310
26. Reith C, Armitage J. Management of residual risk after statin therapy. Atherosclerosis (2016) 245:161–70. doi: 10.1016/j.atherosclerosis.2015.12.018
27. Waldeyer C, Makarova N, Zeller T, Schnabel RB, Brunner FJ, Jørgensen T, et al. Lipoprotein(a) and the risk of cardiovascular disease in the European population: results from the BiomarCaRE consortium. Eur Heart J (2017) 38:2490–8. doi: 10.1093/eurheartj/ehx166
28. Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif J-C, Baum SJ, Steinhagen-Thiessen E, et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N Engl J Med (2020) 382:244–55. doi: 10.1056/NEJMoa1905239
29. Schwartz GG, Steg PG, Szarek M, Bittner VA, Diaz R, Goodman SG, et al. Peripheral Artery Disease and Venous Thromboembolic Events After Acute Coronary Syndrome: Role of Lipoprotein(a) and Modification by Alirocumab: Prespecified Analysis of the ODYSSEY OUTCOMES Randomized Clinical Trial. Circulation (2020) 141:1608–17. doi: 10.1161/CIRCULATIONAHA.120.046524
30. Waldmann E, Parhofer KG. Apheresis for severe hypercholesterolaemia and elevated lipoprotein(a). Pathology (2019) 51:227–32. doi: 10.1016/j.pathol.2018.10.016
31. Moriarty PM, Gray JV, Gorby LK. Lipoprotein apheresis for lipoprotein(a) and cardiovascular disease. J Clin Lipidol (2019) 13:894–900. doi: 10.1016/j.jacl.2019.09.010
Keywords: lipoprotein(a), peri-procedural myocardial infarction, diabetes mellitus, percutaneous coronary intervention, coronary artery disease
Citation: Liu Y, Wang W, Song J, Zhang K, Xu B, Li P, Shao C, Yang M, Chen J and Tang Y-D (2021) Association Between Lipoprotein(a) and Peri-procedural Myocardial Infarction in Patients With Diabetes Mellitus Who Underwent Percutaneous Coronary Intervention. Front. Endocrinol. 11:603922. doi: 10.3389/fendo.2020.603922
Received: 08 September 2020; Accepted: 07 December 2020;
Published: 03 February 2021.
Edited by:
Andreas Peter, Tübingen University Hospital, GermanyReviewed by:
Sebastian Hörber, University of Tübingen, GermanyErwin Dieter Schleicher, University of Tübingen, Germany
Copyright © 2021 Liu, Wang, Song, Zhang, Xu, Li, Shao, Yang, Chen and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-Da Tang, dGFuZ3lpZGFAYmptdS5lZHUuY24=; ZHJ0YW5neWlkYUAxMjYuY29t
†These authors have contributed equally to this work
 Yupeng Liu1,2,3†
Yupeng Liu1,2,3† Kuo Zhang
Kuo Zhang Ping Li
Ping Li Yi-Da Tang
Yi-Da Tang