
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 26 October 2020
Sec. Thyroid Endocrinology
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.583565
This article is part of the Research Topic Mechanisms and Novel Therapies in Graves’ Orbitopathy: Current Update View all 21 articles
Background: While orbital decompression can alleviate optic nerve compression and prevent further vision loss in dysthyroid optic neuropathy (DON), it cannot relieve inflammatory symptoms. Very high doses of intravenous glucocorticoids (GCs) are the first-line therapy for DON; however, the effective rate is only 40% and might be much lower in patients who fail high-dose GC pulse therapy and progressed to DON. The results of two case series studies indicated that rituximab treatment had a much better curative effect compared to very high doses of intravenous GCs, but some patients required urgent orbital decompression after rituximab injection because rituximab might lead to the release of cytokines, aggravated intraorbital edema, and further vision loss.
Methods: We retrospectively studied the therapeutic process of two Grave’s ophthalmopathy (GO) patients complicated with DON who failed high-dose GC pulse therapy and underwent orbital decompression. Both patients received single-dose (500 mg) rituximab treatment.
Results: During more than 2 years of follow-up, rituximab treatment exhibited significant improvement in inflammatory symptoms, as manifested by a substantial decrease in Clinical Activity Score (CAS); meanwhile, the vision of both patients improved significantly and their diplopia was relieved.
Conclusions: The results of this study were consistent with those of two previous case series studies indicating the significant and lasting effect of rituximab treatment on DON, especially for patients with GC resistance or recurrence after GC therapy. Orbital decompression before rituximab treatment might reduce the incidence of rapid vision loss and urgent orbital decompression surgery caused by aggravated orbital edema after rituximab injection; however, the necessity for preventive decompression surgery requires further study.
Grave’s disease (GD) is an autoimmune disease involving the thyroid, skin, and orbit, with an incidence in the adult population of 1–2% (1). Grave’s ophthalmopathy (GO) is the most frequent extrathyroidal manifestation of GD, occurring in up to 50% of GD patients throughout the course of the disease (2). GO is generally self-limiting, with the signs and symptoms improving naturally or following thyrotoxicosis control, smoking cessation, and local treatment (3). However, for some patients, the signs and symptoms persist or aggravate gradually, which require specific treatment besides smoking cessation and anti-thyroid drug administration (4).
The pathogenesis of GO is incompletely understood, although immunological cross-reactivity between the thyroid and orbital antigens may play a key role, and disorders of inflammatory cytokines, thyrotropin receptor autoantibodies, and immunoglobulins targeting the insulin-like growth factor 1 receptor may be correlated with GO (2, 5). Hence, the current internal medicine treatments for GO mostly target immunological disorders, especially for moderate-to-severe and sight-threatening GO.
High-dose intravenous glucocorticoid (GC) pulse treatment is the first-line treatment for moderate-to-severe and active GO, with a 4.5 g cumulative dose of methylprednisolone divided into 12 weekly intravenous injections recommended. A higher dose is acceptable for severe forms; however, the cumulative doses should not exceed 8.0 g (4, 6). The response rate of high-dose intravenous GCs pulse treatment is 70–80% (4, 6, 7); however, in clinical practice, many patients responding well to intravenous GCs may re-develop active disease after therapy completion. For patients with a recurrence or poor response to intravenous GCs, shared decision-making with patients to select an appropriate second-line treatment is recommended (6). A second course of intravenous GCs, orbital radiotherapy, combination use of cyclosporine and oral GCs, and rituximab are the most common second-line treatments (6).
As an alternative second-line treatment for GO, rituximab has been proven to be effective and safe in patients with GO who fail high-dose intravenous GC pulse treatment and shows potential to become a first-line treatment (6). For GO patients progressing to DON, non-control studies have also shown that rituximab was effective in relieving DON; however, a small number of patients in the studies required urgent orbital decompression surgery after receiving rituximab injection as rituximab may have aggravated intraorbital edema (8–10). This finding is consistent with the recommendation in the 2016 European Group of Graves’ Orbitopathy Guidelines for the Management of GO that rituximab should not be used in patients with impending DON (6). Thus, the use of rituximab in patients with GO complicated with DON requires further clinical studies.
This study retrospectively analyzed the therapeutic processes of two GO patients who failed high-dose intravenous GC pulse treatment and progressed to DON. Although a very high dose of intravenous GCs is the first-line treatment for DON, patient 1 was not sensitive to GCs and the cumulative doses of GCs exceeded 8 g. Two patients underwent orbital decompression surgery first to avoid further vision loss. However, there was no significant improvement, especially the clinical activity score (CAS) and diplopia. Moreover, the recovery of vision was not ideal. Thus, rituximab treatment was initiated. During 2 years of follow-up after rituximab treatment, both patients achieved stable and significant remission.
Both patients were initially diagnosed with moderate-to-severe and active GO and received high-dose intravenous GC pulse treatment. The patients also received basic treatments including selenium supplementation and artificial tears. Orbital decompression surgery and sequential rituximab were initiated when the patients suffered from DON and resisted to GCs or the total methylprednisolone dose exceeded 8g. All patients received a single dose of 500 mg rituximab as a slow intravenous infusion, with 5 mg of dexamethasone to prevent allergic reactions.
Case 1: A 54-year-old man (non-smoker) was diagnosed with GD and GO in December 2017 at a local hospital, where he received methimazole treatment only. Three months later, he was transferred to Tongji Hospital, Wuhan, China, for further treatment due to rapidly declining vision (OD 0.3, OS 0.08), eyeball movement disorder, and inability to close his eyelid. The patient was then diagnosed with DON, with a CAS of 7/7. Two days after transfer to this hospital, we initiated high-dose intravenous GC pulse treatment (0.5 g methylprednisolone seven times) and performed bilateral orbital decompression and eyelid margin suture surgery as ophthalmologic examination showed persisting DON with no significant improvement in vision. The orbital decompression surgery was balanced decompression of the inner and outer orbital walls combined with lipectomy, part of the bone in both medial and lateral orbital walls and about 3ml of adipose tissue were removed in the surgery. After the above treatment, the patient’s CAS decreased to 5/7, and ophthalmologic examination showed normal intraocular tension; however, the eyeball movement disorder remained and his vision did not improve (OD 0.1, OS 0.1). The patient then left the hospital and received oral GC treatment at home (methylprednisolone 40 mg/d for 1 week and 36 mg/d for another week). Two weeks later, the patient returned to the hospital. Ophthalmologic examination showed a CAS of 6/7; therefore, we administered an eighth intravenous GC treatment (0.5 g methylprednisolone). His vision improved 7 days after the intravenous GC treatment (OD 0.5, OS 0.6), but the CAS was still 6/7, and the eyeball movement disorder and inability to close the eyelids did not improve. Finally, we decided to administer rituximab treatment (a single dose of 500 mg) and oral GC treatment simultaneously.
Case 2: A 58-year-old man (smoker, quit smoking 10 years ago) was diagnosed with hyperthyroidism and moderate-to-severe GO in March 2017 at the local hospital, where he received methimazole and intravenous GC treatment (11 weekly intravenous injections of 0.5 g methylprednisolone), without significant improvement. The patient was then transferred to Tongji Hospital, Wuhan, China. Ophthalmologic examination showed eyeball movement disorder; both eyes had 1.0 vision, and his CAS was 5/7. We administered intravenous GC treatment (12 weekly intravenous injections of 0.25 g methylprednisolone) combined with right orbital radiotherapy (2 Gy daily for 10 days). After the above treatment, his vision was normal (OD 1.2, OS 1.2), the intraocular tension was also normal, and the CAS decreased to 2/7. However, the disease relapsed approximately 2 months after completion of intravenous GC therapy, which manifested as severe exophthalmos, diplopia, vision decrease (OD 0.1, OS 1.0), and inability to close his eyelid. The CAS increased to 7/7. The patient was then diagnosed with DON and right orbital decompression surgery was performed. The surgical type and methods were similar to those described for patient 1. After orbital decompression surgery, his vision improved slightly (OD 0.4, OS 1.0); however, the CAS and diplopia did not improve. Therefore, we decided to administer rituximab treatment (a single dose of 500 mg) and oral GC treatment.
The treatment process of patient 1 and patient 2 was summarized in Figure 1. Table 1 showed the baseline data of patients before rituximab treatment, Table 2 showed in detail the patients undergoing orbital decompression surgery and rituximab treatment, and Table 3 showed the changes in patients' vision before rituximab treatment.
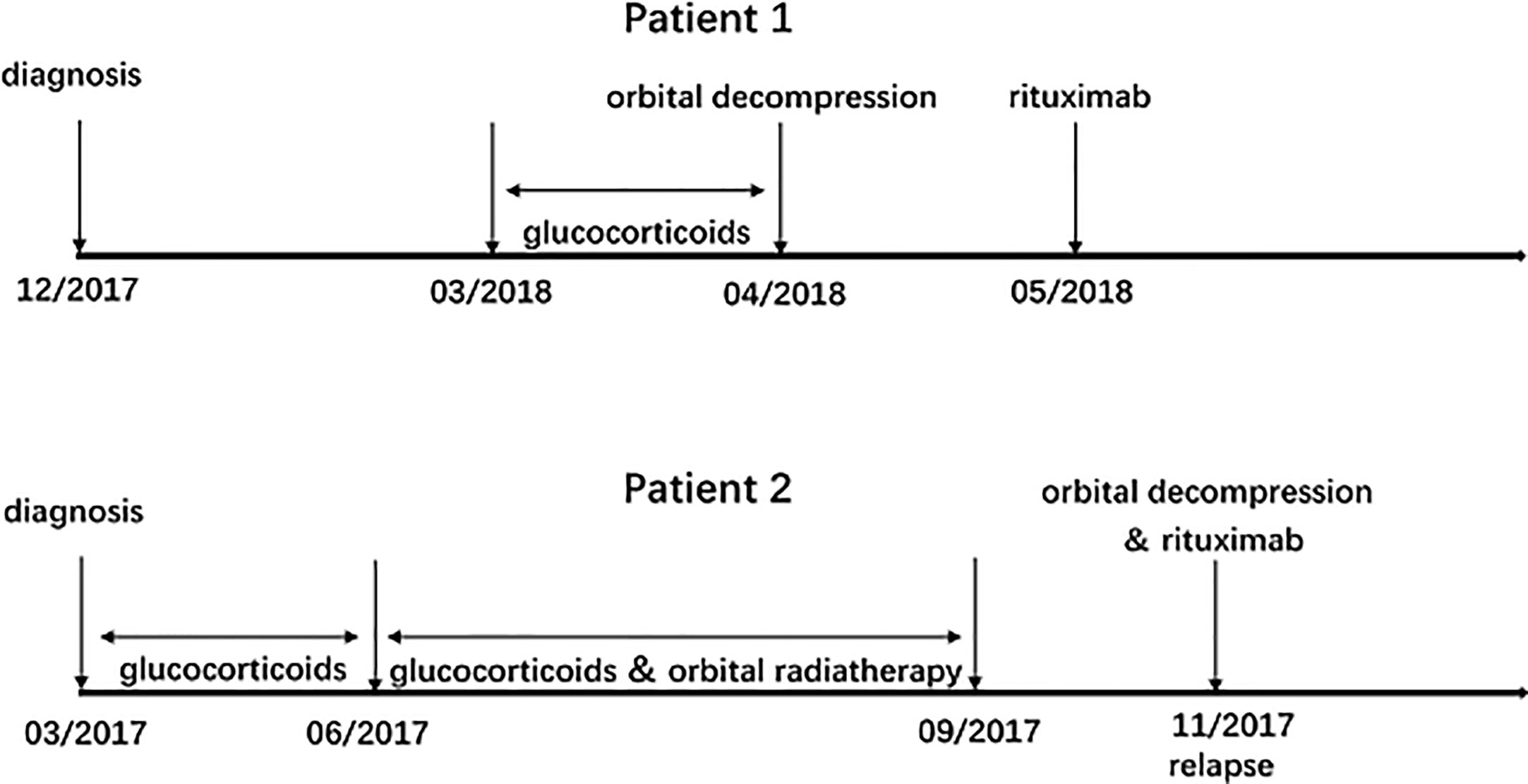
Figure 1 Treatment process: corticosteroids, orbital decompression, and rituximab for patient 1 and patient 2.
As shown in Table 4 and Figure 2, rituximab treatment was administered after orbital decompression and showed significant improvement in inflammatory symptoms, as manifested by a substantial decrease in CAS. Meanwhile, the vision of both patients improved significantly, and their diplopia was relieved after rituximab treatment.
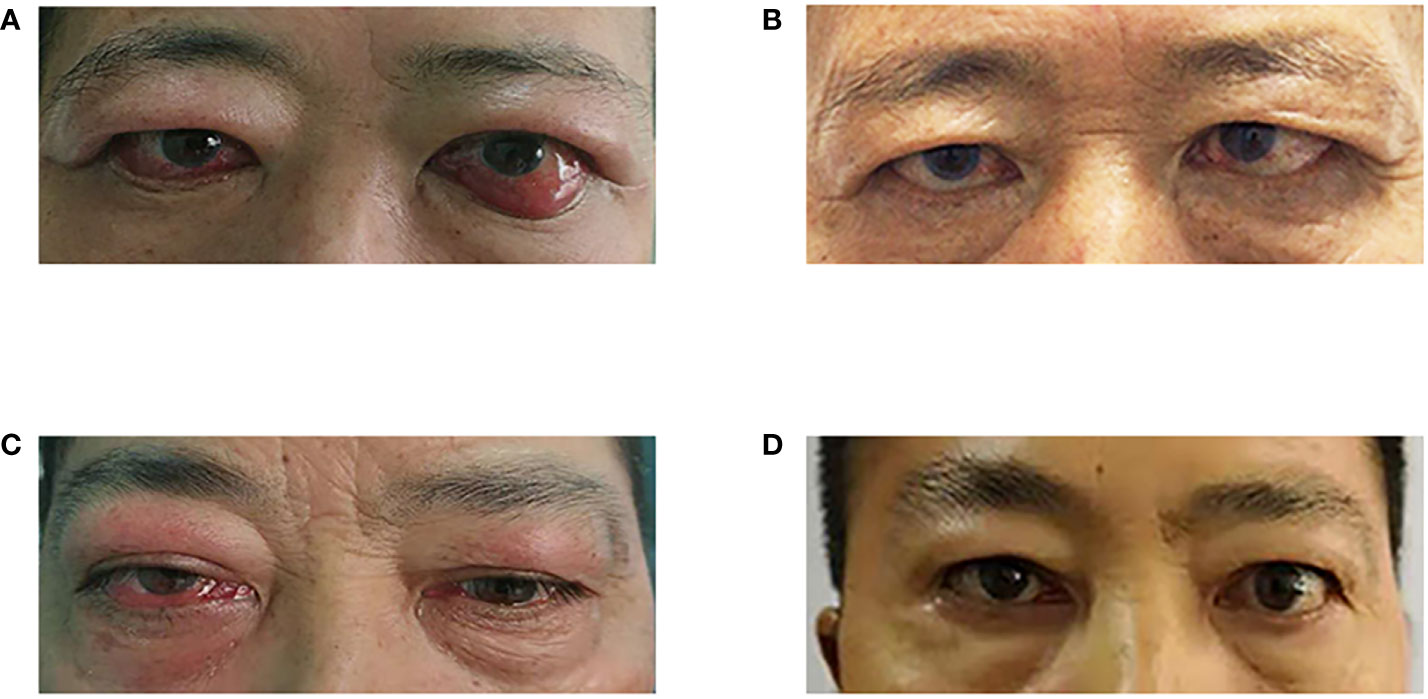
Figure 2 Changes in the appearance of the two patients' eyes. (A) Before rituximab treatment (patient 1); (B) 24 months after rituximab treatment; (C) Before rituximab treatment (patient 2); (D) 26 months after rituximab treatment (patient 2).
In patient 1, the CAS decreased from 5/7 to 2/7 (OD) and from 6/7 to 3/7 (OS) 5 months after rituximab treatment, with a final CAS of both eyes of 2/7 at the last follow-up to 24 months after rituximab treatment. His vision had improved from 0.5 to 1.0 OD and from 0.6 to 0.8 (OS) at the last follow-up. The severity of diplopia decreased but persisted until the last follow-up. Slight improvements in intraocular pressure and proptosis were also observed.
In patient 2, similar to patient 1, inflammatory signs and vision improved significantly after rituximab treatment, while intraocular pressure and proptosis improved slightly. Specifically, the CAS decreased from 7/7 to 2/7 (OD) 6 months after rituximab treatment, with a final CAS of 0/7 (OD) at the last follow-up. The patient’s vision had improved from 0.4 to 1.0 OD and from 1.0 to 1.2 (OS) at 26 months after rituximab treatment. In addition, diplopia was alleviated at 6 months and completely relieved at 26 months after rituximab treatment.
Rituximab is a mouse anti-human monoclonal antibody that directly targets CD20, a B-lymphocyte-specific antigen (11). It was initially developed and used in patients with CD20-expressing lymphoid malignancies, including chronic lymphocytic leukemia and B-cell non-Hodgkin lymphoma. Rituximab induces cell apoptosis by binding to the CD20 membrane antigen in both normal and malignant B cells (12). In addition, it significantly depletes lymphocytes not only in the blood but also in the target tissue, and the effect remained significant 2 months after rituximab infusion (13). Based on the pharmacological mechanism of rituximab, clinical trials on autoimmune diseases have indicated the potential use of rituximab as a candidate treatment for autoimmune diseases (14).
In 2006, two case reports indicated that corticosteroid-resistant GO might be significantly relieved after treatment with rituximab (15, 16). Following the two successful treatment cases, several non-controlled studies on the effects of rituximab in GO have been published, with most showing that rituximab can be used in active moderate-to-severe GO, especially when intravenous methylprednisolone therapy fails (5). In one study, Salvi et al. reported good therapeutic effects after rituximab treatment and improved activity and severity of GO in 98 and 91% of 43 GO patients who failed to respond to corticosteroids, respectively (17). In recent years, the results of two randomized clinical trials of rituximab as a first-line treatment in moderate-to-severe and active GO were published. In one study, Salvi et al. reported that GO was more significantly inactivated after 500 or 2,000 mg (administered twice over 2 weeks) rituximab injection (100 vs. 69% after 7.5 g intravenous methylprednisolone) at 24 weeks (18). However, another study by Stan et al. did not observe differences in either short- or long-term (24- or 52-week) outcomes between rituximab and placebo; meanwhile, two patients developed DON after rituximab. One possible explanation for the occurrence of DON is that the release of cytokines induced by rituximab treatment resulted in aggravated intraorbital edema, which transformed subclinical DON to DON (5, 19). The contradictory results of the two clinical trials suggest the need for more evidence before rituximab can be implemented as a first-line treatment for GO. However, the 2016 European Group of Graves’ Orbitopathy Guidelines for the Management of Graves’ orbitopathy recommended that, although it is still difficult to say which second-line option is more effective because of the limited evidence regarding differences in efficiency difference, treatment has relatively more evidence suggesting that rituximab is a good option for active moderate-to-severe GO when high-dose intravenous GC pulse treatment fails (6).
In GO patients progressing to DON, very high doses of intravenous GCs (e.g. 500–1,000 mg of methylprednisolone for 3 consecutive days) is the first-line therapy; however, the effective rate is only 40%, and many patients require urgent decompression surgery when the response is poor (6). In the formulation of the patients’ treatment plans in the present report, we considered that very high doses of intravenous GCs might be ineffective in patient 1 as he showed resistance GCs, which manifested as non-remission of the disease after seven intravenous GC treatments. Meanwhile, very high doses of intravenous GCs might have led to serious side effects in patient 2 as the cumulative doses of GCs exceeded 8 g. To relieve compression of the optic nerve and avoid further loss of vision, we performed orbital decompression surgery first instead of administering very high doses of intravenous GCs. However, as it was predictable before the surgery that the patients’ condition, especially the inflammatory symptoms and eye movement disorder, did not significantly improve as orbital decompression could only relieve orbital pressure and optic nerve compression; thus, further drug treatment was urgently required.
No evidence-based medical recommendations exist regarding the further treatment of GO patients complicated with DON who fail high-dose GC pulse therapy and require orbital decompression. We finally decided to administer rituximab based on the results of several non-control studies that indicated a better curative effect for rituximab treatment than for first-line therapy in GO patients with DON (8–10). Among these studies, Chong et al. successfully treated four patients who failed to respond to GCs and developed DON. In these patients, the CAS decreased significantly 2 months after rituximab treatment and remained so in all patients. The vision also improved bilaterally in all four patients. One of the four patients received decompression surgery and fractionated orbital irradiation 2 months before rituximab infusion, and another patient received urgent decompression surgery 12 days after the first rituximab infusion due to continued DON (8). Two other studies by Salvi et al. and Mitchell et al. also reported the successful treatment of five patients with DON, with significantly improved disease activity and vision in all five patients, similar to Chong’s study. One of the five patients received urgent decompression surgery after the first rituximab infusion (9, 10). The longest interval between rituximab infusion and orbital decompression surgery was 2 months. Gess et al. administered rituximab to a patient with GO with DON who had failed very high-dose intravenous GCs. The patient improved initially but subsequently worsened 2 months later and underwent orbital decompression surgery (13).
The common characteristics of both patients in this study were significant enlarged extraocular muscle and orbital fat, which led to optic nerve compression and progression of DON. Previous studies revealed that the enlargement of extraocular muscle and orbital fat was associated with hyaluronic acid (HA) accumulation in the muscles and connective tissues and adipogenesis (20, 21). B cells play an important role in HA accumulation and adipogenesis. Briefly, B cells can present self-antigen and activate T cells through the complement receptor 2 (CR2, CD35), then activate helper T cells, recognize TSHR, ligate TSHR and TRAb together, and enhance hyaluronic acid (HA) production and adipogenesis (21, 22). Therefore, rituximab inhibits the activation of T cells by B-cell depletion and blockade of antigen presentation, which may be the main reason why rituximab is more effective compared with GC.
In conclusion, the treatment processes of these two patients indicated that rituximab might be safe and persistently effective for GO patients complicated with DON who fail high-dose GC pulse therapy and require orbital decompression. This finding is consistent with those of two previous non-control studies suggesting that rituximab treatment may have a better curative effect than very high doses of intravenous GCs. However, rituximab treatment may lead to the release of cytokines, aggravated intraorbital edema, and rapid vision loss. Orbital decompression before rituximab injection might mitigate this side effect of rituximab; thus, the patients in this study might have obtained additional benefits from orbital decompression, although the surgeries were not performed preventively. The necessity for prophylactic orbital decompression surgery before rituximab injection requires additional evidence.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Commission of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
BZ: Data curation, Project administration, Formal analysis, Writing—original draft, Writing—review and editing. YL: Data curation, Writing—review and editing. WX: Data curation, Writing—review and editing. BP: Data curation, Writing—review and editing. GY: Conceptualization, Data curation, Project administration, Formal analysis, Investigation, Methodology, Project administration, Validation, Writing—review and editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
CAS, Clinical Activity Score; DON, dysthyroid optic neuropathy; GC, glucocorticoid; GD, Grave’s disease; GO, Grave’s ophthalmopathy.
1. Weetman AP. Graves’ disease. N Engl J Med (2000) 343(17):1236–48. doi: 10.1056/NEJM200010263431707
2. Minakaran N, Ezra DG. Rituximab for thyroid-associated ophthalmopathy. Cochrane Database Syst Rev (2013) (5):CD009226. doi: 10.1002/14651858.CD009226.pub2
3. Dolman PJ. Evaluating Graves’ orbitopathy. Best Pract Res Clin Endocrinol Metab (2012) 26(3):229–48. doi: 10.1016/j.beem.2011.11.007
4. Zang S, Ponto KA, Kahaly GJ. Clinical review: Intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab (2011) 96(2):320–32. doi: 10.1210/jc.2010-1962
5. Campi I, Vannucchi G, Salvi M. THERAPY OF ENDOCRINE DISEASE: Endocrine dilemma: management of Graves’ orbitopathy. Eur J Endocrinol (2016) 175(3):R117–33. doi: 10.1530/EJE-15-1164
6. Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, et al. The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy Guidelines for the Management of Graves’ Orbitopathy. Eur Thyroid J (2016) 5(1):9–26. doi: 10.1159/000443828
7. Stiebel-Kalish H, Robenshtok E, Hasanreisoglu M, Ezrachi D, Shimon I, Leibovici L. Treatment modalities for Graves’ ophthalmopathy: systematic review and metaanalysis. J Clin Endocrinol Metab (2009) 94(8):2708–16. doi: 10.1210/jc.2009-0376
8. Khanna D, Chong KK, Afifiyan NF, Hwang CJ, Lee DK, Garneau HC, et al. Rituximab treatment of patients with severe, corticosteroid-resistant thyroid-associated ophthalmopathy. Ophthalmology (2010) 117(1):133–9.e2. doi: 10.1016/j.ophtha.2009.05.029
9. Salvi M, Vannucchi G, Campi I, Curro N, Simonetta S, Covelli D, et al. Rituximab treatment in a patient with severe thyroid-associated ophthalmopathy: effects on orbital lymphocytic infiltrates. Clin Immunol (2009) 131(2):360–5. doi: 10.1016/j.clim.2008.12.005
10. Mitchell AL, Gan EH, Morris M, Johnson K, Neoh C, Dickinson AJ, et al. The effect of B cell depletion therapy on anti-TSH receptor antibodies and clinical outcome in glucocorticoid-refractory Graves’ orbitopathy. Clin Endocrinol (Oxf) (2013) 79(3):437–42. doi: 10.1111/cen.12141
11. Engel P, Gomez-Puerta JA, Ramos-Casals M, Lozano F, Bosch X. Therapeutic targeting of B cells for rheumatic autoimmune diseases. Pharmacol Rev (2011) 63(1):127–56. doi: 10.1124/pr.109.002006
12. Salles G, Barrett M, Foa R, Maurer J, O’Brien S, Valente N, et al. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv Ther (2017) 34(10):2232–73. doi: 10.1007/s12325-017-0612-x
13. Gess AJ, Silkiss RZ. Orbital B-Lymphocyte Depletion in a Treatment Failure of Rituximab for Thyroid Eye Disease. Ophthal Plast Reconstr Surg (2014) 30(1):e11–3. doi: 10.1097/IOP.0b013e31828956a8
14. Anolik JH, Barnard J, Owen T, Zheng B, Kemshetti S, Looney RJ, et al. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum (2007) 56(9):3044–56. doi: 10.1002/art.22810
15. El FD, Nielsen CH, Hasselbalch HC, Hegedus L. Treatment-resistant severe, active Graves’ ophthalmopathy successfully treated with B lymphocyte depletion. Thyroid (2006) 16(7):709–10. doi: 10.1089/thy.2006.16.709
16. Salvi M, Vannucchi G, Campi I, Rossi S, Bonara P, Sbrozzi F, et al. Efficacy of rituximab treatment for thyroid-associated ophthalmopathy as a result of intraorbital B-cell depletion in one patient unresponsive to steroid immunosuppression. Eur J Endocrinol (2006) 154(4):511–7. doi: 10.1530/eje.1.02119
17. Salvi M, Vannucchi G, Beck-Peccoz P. Potential utility of rituximab for Graves’ orbitopathy. J Clin Endocrinol Metab (2013) 98(11):4291–9. doi: 10.1210/jc.2013-1804
18. Salvi M, Vannucchi G, Curro N, Campi I, Covelli D, Dazzi D, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab (2015) 100(2):422–31. doi: 10.1210/jc.2014-3014
19. Stan MN, Garrity JA, Carranza LB, Prabin T, Bradley EA, Bahn RS. Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab (2015) 100(2):432–41. doi: 10.1210/jc.2014-2572
20. Blandford AD, Zhang D, Chundury RV, Perry JD. Dysthyroid optic neuropathy: update on pathogenesis, diagnosis, and management. Expert Rev Ophthal (2017) 12(2):111–21. doi: 10.1080/17469899.2017.1276444
21. Bahn RS. Current Insights into the Pathogenesis of Graves’ Ophthalmopathy. Horm Metab Res (2015) 47:773–8. doi: 10.1055/s-0035-1555762
Keywords: Grave’s disease, Grave’s ophthalmopathy, Dysthyroid optic neuropathy, orbital decompression, rituximab
Citation: Zhang B, Li Y, Xu W, Peng B and Yuan G (2020) Use of Rituximab After Orbital Decompression Surgery in Two Grave’s Ophthalmopathy Patients Progressing to Optic Neuropathy. Front. Endocrinol. 11:583565. doi: 10.3389/fendo.2020.583565
Received: 22 July 2020; Accepted: 05 October 2020;
Published: 26 October 2020.
Edited by:
Huifang Zhou, Shanghai Jiao Tong University, ChinaReviewed by:
Mario Salvi, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, ItalyCopyright © 2020 Zhang, Li, Xu, Peng and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Yuan, eXVhbmdhbmc4OEBob3RtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.