
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 20 November 2020
Sec. Cancer Endocrinology
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.575799
 Yihao Liu1,2†
Yihao Liu1,2† Bin Li1†
Bin Li1† Qiuyi Zheng2†
Qiuyi Zheng2† Jia Xu1
Jia Xu1 Jie Li3
Jie Li3 Fenghua Lai2
Fenghua Lai2 Bo Lin3
Bo Lin3 Sui Peng1
Sui Peng1 Weiming Lv3*
Weiming Lv3* Haipeng Xiao2*
Haipeng Xiao2*Background: A better understanding of the current characteristics of clinical trials on thyroid cancer (TC) is important to improve trial designs and identify neglected areas of research. However, there is a lack of a thorough understanding of the clinical studies on TC. Therefore, this study aimed to present a comprehensive overview of clinical trials on TC based on the ClinicalTrials.gov database and evaluate their publication status.
Methods: We searched for TC-related clinical studies registered in the ClinicalTrials.gov database before December 2018 by using the keyword “thyroid cancer” and assessed the characteristics of the included trials. We searched the publication status of primary completed studies in PubMed and Google Scholar.
Results: A total of 450 studies were identified for analysis, including 333 (74.0%) interventional studies and 117 (26.0%) observational studies. Interventional studies about TC were commonly non-randomized (67.6%), single-arm (55.6%), single-center (76.3%), and early-phase (60.0%) trials. The major category for which studies were performed was for target drug-related therapy (53.6%). In addition, 57.0% of the primary completed interventional studies were published. The published studies were more commonly primary completed studies after 2010 and used randomization and were less commonly designed as single-arm studies and were conducted in the USA/Canada, compared to non-published studies (P < 0.05 for all). The median time from primary completion to publication was 46.5 months, and the time decreased to 36.5 months after 2010. Studies conducted in the USA/Canada [odds ratio (OR) = 9.43, P = 0.020] and multi-center studies (OR = 6.55, P = 0.021) significantly increased the potential of publication in high-impact journals.
Conclusions: High-quality, randomized phase 3 trials regarding TC are still insufficient. Therefore, more efforts are needed to improve the treatment of poor prognostic TC and timely publication.
The incidence of thyroid cancer (TC) had increased rapidly worldwide in the last few decades (1). Approximately 50% of newly diagnosed TC cases are papillary thyroid microcarcinoma (PTMC) (2), defined as papillary TC ≤1 cm in diameter, the vast majority of which are indolent in nature and do not result in death. The 2015 American Thyroid Association (ATA) guidelines recommended active surveillance as an alternative treatment for low-risk PTMC (3). Nonetheless, the potential risk and benefit of active surveillance are still unclear.
The prognosis of TC is generally excellent, owing to its natural biological behavior (4). However, the treatment of TC with a relatively poor prognosis, such as advanced or radioiodine-refractory differentiated thyroid cancer (DTC), medullary thyroid cancer (MTC), and anaplastic thyroid cancer (ATC), is a serious challenge (3). Despite advances in the understanding of cancer biology and the development of molecular-targeted drugs, there has been only a slight improvement in the outcomes for those patients. A better understanding of the current composition and characteristics of clinical trials on TC is important to improve the trial design and identify neglected areas of research.
Clinical trials, especially well-designed randomized clinical trials, have been the foundation of evidence-based medicine and the driving force in the development of medicine. In 2004, the International Committee of Medical Journal Editors (ICMJE) advocated that clinical trials should be registered in a public registry before recruiting patients to ensure transparency of the process (5, 6). The ClinicalTrials.gov database is a publicly available, worldwide, trial registry, developed and maintained by the National Library of Medicine (NLM) for the National Institutes of Health (NIH). Currently, the ClinicalTrials.gov database provides the most comprehensive information about ongoing and completed clinical studies worldwide (7, 8).
Although previous studies have evaluated a subset of oncologic-based clinical trials, none have focused exclusively on TC (8, 9). Currently, physicians still lack a thorough understanding of clinical studies on TC. Thus, given the need for better and more efficient clinical trials, it is necessary to identify new developments and to maintain the current information to guide future trial design. Moreover, registration is expected to improve transparency in the process of conducting and reporting trials. However, the characteristics associated with publication and timeliness of publication have not been studied for TC. Therefore, we aimed to present a comprehensive landscape of TC-related studies based on the ClinicalTrials.gov database and evaluate the publication status of these studies.
We searched the ClinicalTrials.gov database on December 31, 2018, using the search term “thyroid cancer.” The search date was limited from 1st January 2004 to 31st December 2018. All available results were downloaded in the form of xml files. Afterward, all the data were imported into a database to facilitate further data cleaning, classification, and management. Studies under withdrawn, unknown, terminated, and expanded access statuses were excluded. After reviewing the trial summary, studies that did not include TC were also excluded. The remaining studies were selected for further manual classification analysis. This study was approved by the Research Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University.
According to the information given on the ClinicalTrials.gov database, the following variables of the registered studies were categorized by two investigators (YL and QZ) independently: disease [only TC or multi-cancer (multi-cancer means that the study also recruit patients with other kind of cancer, but not limited to thyroid cancer)], age (only adults/adults and children), TC subtypes (DTC/MTC/ATC/advanced or radioiodine-refractory DTC/unclassified advanced or metastasis TC/unclassified TC/others), treatment used in interventional studies (targeted drugs/targeted drugs and other therapy/radiotherapy, including I131/immunotherapy/chemotherapy/others), purpose of the interventional studies (treatment/basic science/health service or preventive/supportive care/screening/diagnostic), study design of observational studies (case-only/cross-sectional/case-control/cohort), study design of interventional trials (single group/parallel/factorial/crossover/sequential), countries where the study was performed (the USA or Canada/European/Asian/others), centers (single-center or multi-center), and funder (industry/NIH/others). If an industry was listed as the lead funder, the trial was classified as being funded by the industry. When NIH was listed as the lead funder, the trial was considered as NIH-funded (10). The time to primary completion was defined as the time from the start of the study to completion of the primary endpoint. The study duration was defined as the time from the start of the study to completion of the study.
Two investigators (YL and BL) independently searched for peer-reviewed publications of studies under primary completion by using a standardized strategy. The “publications” field in the ClinicalTrials.gov database was reviewed to search for potentially matching publications. We then searched PubMed and Google Scholar by using NCT numbers in all the fields. Publication was confirmed by matching the study characteristics in the ClinicalTrials.gov database with the description in the manuscript. The earliest article reporting the primary outcome results was chosen if multiple publications were obtained from a single study. Study protocols, commentaries, interim analysis, and other non-relevant publication types were excluded. A third investigator (JX) independently reconfirmed and conducted a publication search for the studies that were found to be unpublished by the first two investigators. Differences were resolved by consensus. For each published article, the published date, study design, sample size, country, primary outcome (negative or positive), and impact factor (IF) were collected. The search for publication status was updated and finalized by April 1, 2019.
The number (percentage) for categorical variables and median [interquartile range (IQR)] for continuous variables were calculated. Fisher’s exact tests were used to compare the categorical variables, while the Mann-Whitney U tests were used to compare the continuous variables. Cox regression analysis was performed to analyze the factors influencing the time to publication. The hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for the factors. The multivariate model included each variable associated with P < 0.05 on univariate analysis. The time to publication was estimated by using the Kaplan-Meier method. As the completed studies need enough time to be published, primary studies completed after January 1, 2017, were excluded from the Cox regression analysis. Logistic regression analysis was performed to analyze the factors influencing publication in high-impact factor (≥10) journals. The odds ratios (ORs) and 95% CIs were calculated for the factors.
All statistical tests were performed using Stata/MP version 14.0 (Stata Corporation LP, College Station, TX, USA), and a two-sided P < 0.05 was considered statistically significant.
A total of 582 registered studies were identified, and 132 studies that were under the withdrawn, unknown, terminated, and expanded access statuses, and did not include TC, were excluded (Figure 1) . Finally, 450 studies were evaluated for analysis, including 333 (74.0%) interventional studies and 117 (26.0%) observational studies. The distribution of interventional and observational studies according to the registered time is summarized in Figure 2. Overall, the number of registered studies increased over the years, and the number of interventional studies increased more rapidly. More than 20 interventional studies were registered every year after 2012, and the number increased by more than 40 in the last 2 years.
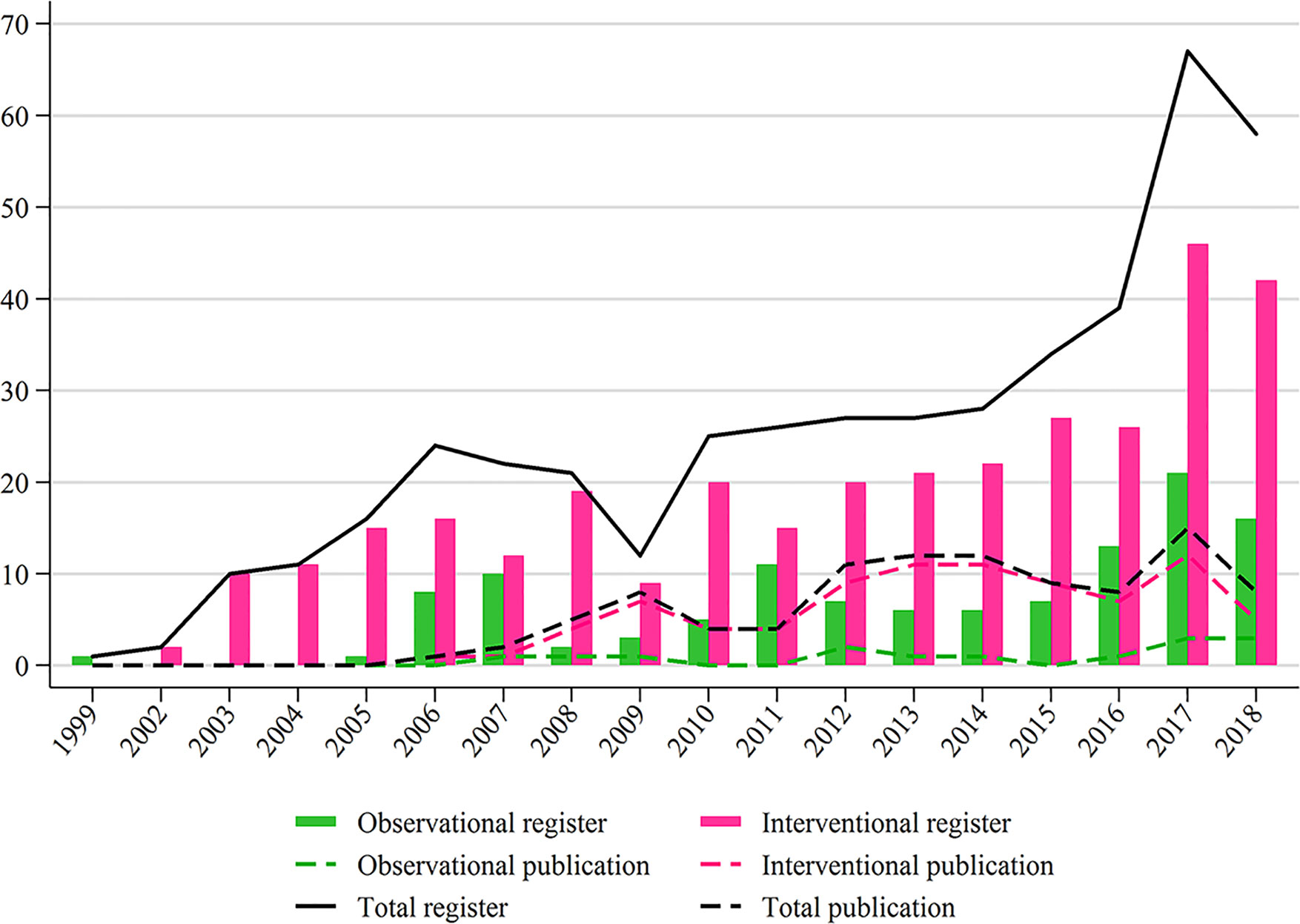
Figure 2 Study distributions of register and publication for observational and interventional studies according to the registered or published year.
The characteristics of the included interventional and observational studies are summarized in Table 1. Compared to observational studies, interventional studies included fewer children; included mostly only TC; had fewer registries after patient recruitment, a smaller sample size, more results available, and more publications; and were more often conducted in the USA/Canada, more often multi-center, and more often funded by the NIH/industry (P < 0.05). A total of 35.3% of the interventional trials focused on TC with poor prognosis, while 71% of observational studies were performed for unclassified TC. Among the interventional studies, studies for treatment and diagnosis accounted for 79 and 12%, respectively. In addition, 31 studies (9%) were registered for supportive care, health or preventive service, screening, and basic science. Among the studies for treatment, the major study category was for target drug-related therapy (53.6%). Studies for radiotherapy, chemotherapy and immune therapy accounted for 4.6, 3.8, and 4.6%, respectively.
Regarding the study design, interventional studies were mostly non-randomized (225, 67.5%), open-label (260, 78.1%), single-group (186, 55.9%), single-center (254, 76.3%), and early-phase (200, 60.0%). Only 33 phase 2/3 or 3 trials were registered, accounting for 9.9% of all interventional studies. A total of 54.7% of observational studies were cohort studies.
Among the interventional studies, 173 (52.0%) were primary completed studies, and 22 (12.8%) were still ongoing considering the long-term outcomes. The median duration for primary completion was 40.0 months (IQR: 22.0–57.2).
Only 15 registered observational studies were published. Among 149 primary completed interventional studies before January 1, 2017, 84 (57.0%) were published by April 1, 2019. Since 2012, approximately ten interventional studies were published every year (Figure 2).
The characteristics of published and unpublished interventional studies are shown in Table 2. Among the published studies, more were primary completed studies after 2010 and were randomized, while fewer were single-arm and conducted in the USA/Canada, compared to unpublished studies (P < 0.05 for all).
The median time to publication was 46.5 months (95% CI: 37.16–96.46 months), and the 1, 2, 3, and 5-year cumulative publication rates were 14.8, 28.2, 41.1, and 54.4%, respectively (Figure 3). The factors influencing the time to publication from primary completion of interventional studies is shown in Table 3. The year of primary completion, study phases, funder, study design, randomization, and country significantly influenced the time to publication on univariate Cox regression analysis. However, on multivariate Cox analysis, only the year of primary completion and country were significant factors. Primary completed studies after 2010 were more often published on time, and the median time to publication was 35.2 months (HR = 1.85, 95% CI = 1.10–3.12, P = 0.021). Asian studies had lesser time to publication, with a median of 29.0 months, compared to studies conducted in the USA/Canada (HR = 2.23, 95% CI = 1.02–4.90, P = 0.046).
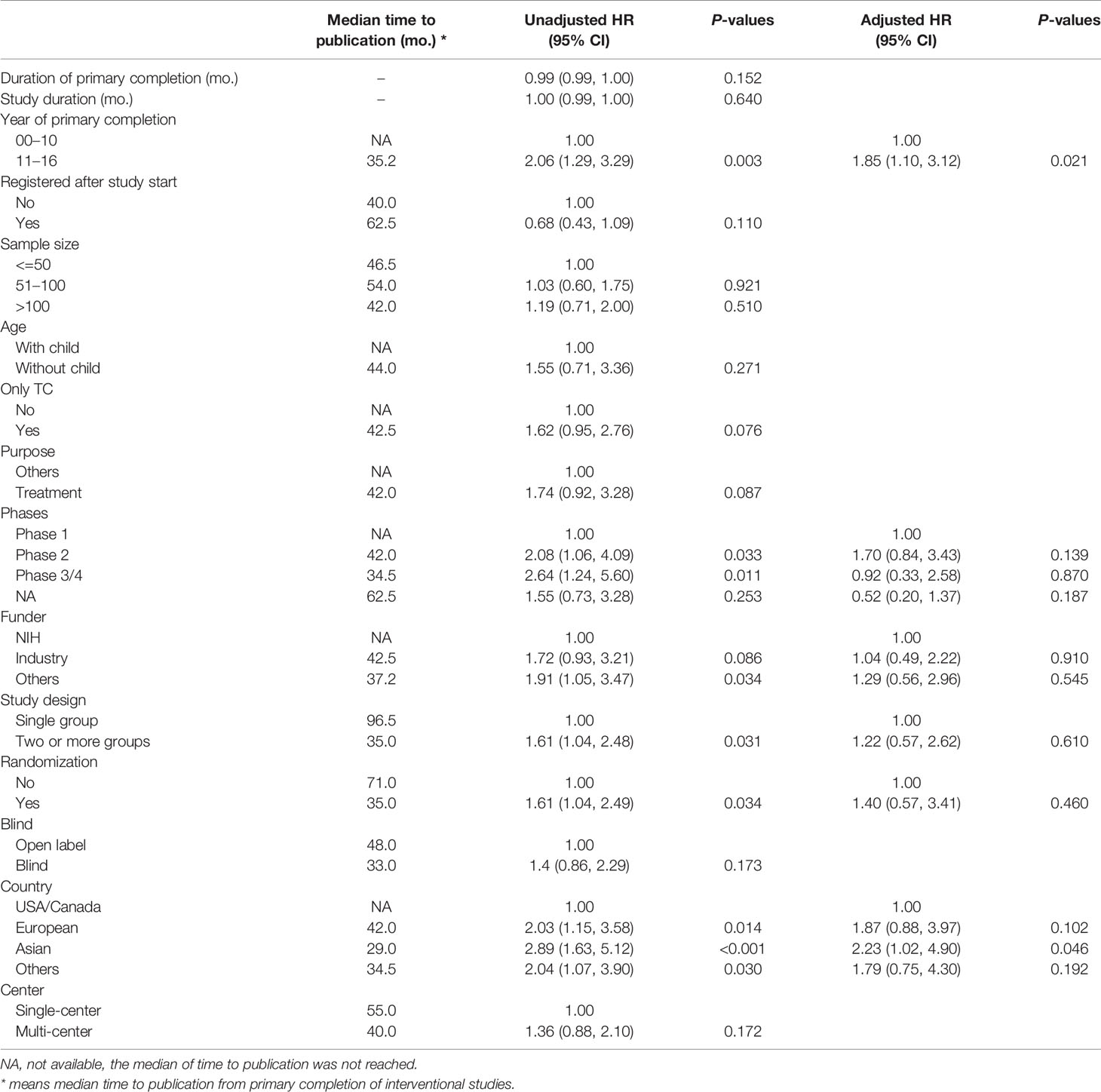
Table 3 Cox regression analysis for time to publication from primary completion of interventional studies.
Among the published interventional studies, 23 (26.7%) were published in journals with an IF ≥10. As shown in Supplementary Table S1, the studies published in high-impact journals were more often regarding treatment, multi-center studies, conducted in the USA/Canada, and funded by industry. After multivariate logistic regression analysis, compared to studies being conducted in the USA/Canada, studies performed in European (OR = 0.09, P = 0.020) and Asian (OR = 0.11, P = 0.034) countries significantly decreased the probability of being published in journals with an IF ≥10. In contrast, multi-center studies (OR = 6.55, P = 0.021) significantly increased the probability of being published in journals with an IF ≥10 (Supplementary Table S2).
To our knowledge, the current study is the most comprehensive assessment of clinical trial characteristics of studies on TC and their publication status. Our results showed that clinical trials for TC were commonly non-randomized, single-arm, single-center trials, predominantly performed as phase I or II studies. Both interventional and observational studies kept increasing in recent years. Current interventional studies were focused on targeted drug-related therapy, especially for patients with poor-prognosis TC subtypes. Only 57.0% of the primary completed interventional studies were published, and the median time to publication was 46.5 months. Encouragingly, there was an improvement in the time to publication.
Targeted therapies had been introduced and brought survival benefit to patients with different cancers over the past decades (11–13). In the current study, 59.7% of the interventional studies focused on targeted therapies for TC. Although numerous clinical studies have evaluated distinct targeted drugs, only four drugs have unequivocally shown their effectiveness in terms of improvement in the progression-free survival in randomized, placebo-controlled, phase III clinical trials: sorafenib (NCT00984282) (14) and lenvatinib (NCT01321554) (15) for radioiodine-refractory DTC and vandetanib (NCT00410761) (16) and cabozantinib (NCT00704730) (17) for MTC. Nonetheless, none of the targeted drugs have shown significant benefit in OS, except for a subgroup of patients receiving lenvatinib or cabozantinib (17, 18). Regardless of the limited treatment effect, a high rate of adverse events was observed in patients who received targeted therapy (19, 20). Immunotherapy, including immune cells and checkpoint inhibitors, may provide a new alternative option for TC with poor prognosis, as it has shown survival benefit in other oncology studies (21, 22). Currently, 12 immunotherapy trials have been registered in the ClinicalTrials.gov database and are recruiting patients.
DTC accounted for more than 90% of the new cases, and it is known to have a generally favorable prognosis, with a 5-year survival of >95% (23). It may be difficult and time-consuming to observe events such as recurrence or death; only a few pharmaceutical companies and investigators have conducted interventional studies for DTC patients and observed excellent long-term survival. The interventional studies included in the current study focused on patients with poor-prognosis TC subtypes of MTC, ATC, advanced or radioiodine-refractory DTC, as the treatment of these poor-prognosis TC subtypes remains a serious challenge. Among the studies registered in the ClinicalTrials.gov database, 35.3% of the interventional trials focused on poorer prognostic TC. Nonetheless, a worrisome and unfortunate limitation for conducting large phase III trials for MTC and ATC is the rarity of the diseases (23). Therefore, well-designed phase II randomized controlled trials for MTC and ATC may also be valuable.
Although registration in the ClinicalTrials.gov database has been associated with improved likelihood of publication, only 22.8–68% of completed studies registered in the ClinicalTrials.gov database were published (24–26). In the current study, 56.4% of the primary completed interventional studies before January 2017 were published. Typically, trials yielding negative results that do not favor or even contradict the initial hypotheses may be delayed or suppressed (27). In the current study, primary completed interventional studies after 2010 and those conducted in Asian countries were associated with improved publication likelihood. The public contribution of clinical studies may be lost when the results remain unpublished. Therefore, it is important to compel authors to publish their studies, even if they obtain negative outcomes, facilitating the publication of negative findings.
Among the published interventional studies about TC, the median time from primary completion to publication was 46.5 months. Only 52.3% of studies were published within 2 years since primary completion. Studies primary completed after 2010 and conducting in Asian had lesser time to publication. The time from primary completion to publication is quite long to some extent, and study results cannot be reported and introduced into clinical practice in time. Therefore, timely publication of trial results is a precondition for ensuring that physicians and other stakeholders make appropriate clinical decisions. Timely publication of trial results also reflects the best scientific evidence and yields maximum benefits for public health and scientific progress (24, 25). Encouragingly, the current study showed a trend of improvement in publication within 24 months compared with the studies completed before 2010. This possibly reflects the fact that investigators and sponsors attach increasing importance to timely sharing of trial results. Development in information technology may also have increased the efficiency and reduced the time needed from data collection to publication (27). Nevertheless, unremitting efforts need to continue to improve timely publication.
In the current study, an encouraging trend was that the number of both interventional and observational studies has been increasing in recent years. Similar to the studies for other oncological diseases (9, 28), clinical studies for TC are mostly small, single-institutional, and early-phase. The results of the current study revealed that early-phase studies were the most common type for TC, with phase II studies accounting for more than 42.3% of all interventional trials. Phase III randomized controlled trials are generally costly, time-consuming, and multi-center; therefore, starting and completing early-phase trials are logically easier than later-phase studies. Nonetheless, more well-designed phase III randomized controlled trials for TC are needed, as the results will play an irreplaceable role in changing clinical practice and decision-making in medicine. Another issue was that 60.1% of the interventional studies were registered after study initiation. As registration is expected to improve transparency in performing and reporting of studies, the rule that clinical trials should be registered before study initiation needs to be standardized.
This study has several limitations. The ClinicalTrials.gov does not include all clinical trials. Although many studies from other countries use the ClinicalTrials.gov database to satisfy the ICMJE registration requirements, seven other registries around the world may also be used (29). However, the ClinicalTrials.gov database still accounts for most clinical studies in the WHO portal. We relied on the ClinicalTrials.gov data provided by trial investigators or sponsors. In addition, the data sets for all trials in the database are not always complete and up to date. Moreover, we cannot exclude the possibility of errors owing to certain studies not being captured for the analysis and/or being potentially misclassified during the selection process. However, we took great care to minimize these limitations: two authors (YL and BL) crosschecked all identified studies and the trial selection steps. Furthermore, the NLM cannot verify the validity of all trial information, and some records might contain errors.
Overall, our study is the first to provide the current landscape of clinical studies about TC and indicated that high-level, randomized, phase 3 trials are still insufficient. Due to the indolent behavior of PTC and low incidence of poorer prognostic TC, it has been difficult to recruit and enroll patients in clinical trials in the past. More efforts are needed to promote the recruitment in the future. In addition, highly accurate methods are needed for the diagnosis of malignant nodules. Moreover, continued improvements in clinical trials are needed to improve the prognosis of aggressive cancers. Hence, more efforts are needed to promote the timely publication of clinical studies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YL, BL, and QZ contributed equally to this article. HX and WL supervised the study. HX, YL, and BL designed the study. YL and QZ collected the data. BL and JX did the data analysis. YL, QZ, JL, FL, and BL wrote the draft report. SP performed critical revision on the manuscript. All authors contributed to the article and approved the submitted version.
National Natural Science Foundation of China (81772850), Guangzhou Science and Technology Project (201803010057).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.575799/full#supplementary-material
1. Wiltshire JJ, Drake TM, Uttley L, Balasubramanian SP. Systematic Review of Trends in the Incidence Rates of Thyroid Cancer. Thyroid (2016) 26:1541–52. doi: 10.1089/thy.2016.0100
2. Leboulleux S, Tuttle RM, Pacini F, Schlumberger M. Papillary thyroid microcarcinoma: time to shift from surgery to active surveillance? Lancet Diabetes Endocrinol (2016) 4:933–42. doi: 10.1016/S2213-8587(16)30180-2
3. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26:1–133. doi: 10.1089/thy.2015.0020
4. Tuttle RM, Fagin JA, Minkowitz G, Wong RJ, Roman B, Patel S, et al. Natural History and Tumor Volume Kinetics of Papillary Thyroid Cancers During Active Surveillance. JAMA Otolaryngol– Head Neck Surg (2017) 143:1015–20. doi: 10.1001/jamaoto.2017.1442
5. De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med (2004) 351:1250–1. doi: 10.1056/NEJMe048225
6. Peng H, Chen L, Chen YP, Li WF, Tang LL, Lin AH, et al. The current status of clinical trials focusing on nasopharyngeal carcinoma: A comprehensive analysis of ClinicalTrials.gov database. PLoS One (2018) 13:e0196730. doi: 10.1371/journal.pone.0196730
7. Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The ClinicalTrials.gov results database–update and key issues. N Engl J Med (2011) 364:852–60. doi: 10.1056/NEJMsa1012065
8. Zwierzyna M, Davies M, Hingorani AD, Hunter J. Clinical trial design and dissemination: comprehensive analysis of clinicaltrials.gov and PubMed data since 2005. BMJ (Clin Res ed) (2018) 361:k2130. doi: 10.1136/bmj.k2130
9. Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007-2010. JAMA (2012) 307:1838–47. doi: 10.1001/jama.2012.3424
10. Anderson ML, Chiswell K, Peterson ED, Tasneem A, Topping J, Califf RM. Compliance with results reporting at ClinicalTrials.gov. N Engl J Med (2015) 372:1031–9. doi: 10.1056/NEJMsa1409364
11. Carlisle JW, Ramalingam SS. A banner year for immunotherapy and targeted therapy. Nat Rev Clin Oncol (2019) 16:79–80. doi: 10.1038/s41571-018-0138-4
12. Harbeck N, Wuerstlein R. Truly personalized therapy - an end to the era of one size fits all. Nat Rev Clin Oncol (2019) 16:77–8. doi: 10.1038/s41571-018-0165-1
13. Lordick F, Smyth EC. Two steps forward and one step back. Nat Rev Clin Oncol (2019) 16:69–70. doi: 10.1038/s41571-018-0154-4
14. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet (2014) 384:319–28. doi: 10.1016/S0140-6736(14)60421-9
15. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med (2015) 372:621–30. doi: 10.1056/NEJMoa1406470
16. Wells SA Jr., Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol (2012) 30:134–41. doi: 10.1200/JCO.2011.35.5040
17. Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol (2013) 31:3639–46. doi: 10.1200/JCO.2012.48.4659
18. Cabanillas ME, Habra MA. Lenvatinib: Role in thyroid cancer and other solid tumors. Cancer Treat Rev (2016) 42:47–55. doi: 10.1016/j.ctrv.2015.11.003
19. Ransohoff JD, Kwong BY. Cutaneous Adverse Events of Targeted Therapies for Hematolymphoid Malignancies. Clin Lymph Myeloma Leuk (2017) 17:834–51. doi: 10.1016/j.clml.2017.07.005
20. Wittayanukorn S, Qian J, Johnson BS, Hansen RA. Cardiotoxicity in targeted therapy for breast cancer: A study of the FDA adverse event reporting system (FAERS). J Oncol Pharm Pract (2017) 23:93–102. doi: 10.1177/1078155215621150
21. Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med (2011) 364:2517–26. doi: 10.1056/NEJMoa1104621
22. Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al. Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer: A meta-analysis of randomized controlled trials. Medicine (2018) 97:e11936. doi: 10.1097/MD.0000000000011936
23. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol– Head Neck Surg (2014) 140:317–22. doi: 10.1001/jamaoto.2014.1
24. Ross JS, Tse T, Zarin DA, Xu H, Zhou L, Krumholz HM. Publication of NIH funded trials registered in ClinicalTrials.gov: cross sectional analysis. BMJ (Clin Res ed) (2012) 344:d7292. doi: 10.1136/bmj.d7292
25. Chen R, Desai NR, Ross JS, Zhang W, Chau KH, Wayda B, et al. Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ (Clin Res ed) (2016) 352:i637. doi: 10.1136/bmj.i637
26. Ross JS, Mocanu M, Lampropulos JF, Tse T, Krumholz HM. Time to publication among completed clinical trials. JAMA Internal Med (2013) 173:825–8. doi: 10.1001/jamainternmed.2013.136
27. Chen YP, Liu X, Lv JW, Li WF, Zhang Y, Guo Y, et al. Publication status of contemporary oncology randomised controlled trials worldwide. Eur J Cancer (Oxford England: 1990) (2016) 66:17–25. doi: 10.1016/j.ejca.2016.06.010
28. Califf RM, Cheng SK, Tasneem A, Horton J, Chiswell K, Schulman KA, et al. Characteristics of oncology clinical trials: insights from a systematic analysis of ClinicalTrials.gov.%A Hirsch BR. JAMA Internal Med (2013) 173:972–9. doi: 10.1001/jamainternmed.2013.627
Keywords: clinical studies, thyroid cancer, targeted therapy, publication status, ClinicalTrials.gov
Citation: Liu Y, Li B, Zheng Q, Xu J, Li J, Lai F, Lin B, Peng S, Lv W and Xiao H (2020) The Current Landscape of Clinical Studies Focusing on Thyroid Cancer: A Comprehensive Analysis of Study Characteristics and Their Publication Status. Front. Endocrinol. 11:575799. doi: 10.3389/fendo.2020.575799
Received: 11 August 2020; Accepted: 21 October 2020;
Published: 20 November 2020.
Edited by:
Veronica Vella, University of Catania, ItalyReviewed by:
Dario Giuffrida, Mediterranean Institute of Oncology (IOM), ItalyCopyright © 2020 Liu, Li, Zheng, Xu, Li, Lai, Lin, Peng, Lv and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haipeng Xiao, eGlhb2hwQG1haWwuc3lzdS5lZHUuY24=; Weiming Lv, bHZ3bUBtYWlsLnN5c3UuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.