
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 17 September 2020
Sec. Reproduction
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.572384
Polycystic ovary syndrome (PCOS) is reported to be associated with certain trace elements. However, previous data are inconsistent and potentially biased due to small sample sizes. The potential utility of trace element levels for screening of PCOS remains to be established. The aim of this meta-analysis was to investigate the potential relationships between PCOS and serum levels of zinc (Zn), copper (Cu), magnesium (Mg), iron (Fe) and ferritin. We carried out a literature search of PubMed, EMBASE, and Web of Science for relevant cross-sectional/case-control studies published prior to October 2019. Random-effect models were used to estimate the overall standard mean differences (SMDs) between PCOS and healthy control subjects. The screening value of potential microelement biomarkers for PCOS was assessed using the receiver operating characteristic (ROC) curve. Twenty-one studies featuring 2,173 women with PCOS and 1,897 healthy women were selected for analysis. Our results showed that Cu and ferritin levels were significantly higher in women with PCOS than healthy controls, with SMDs of 0.52 [95% confidence interval (CI): 0.38–0.67, I2 = 47.6%] and 1.05 (95% CI: 0.25–1.86, I2 = 97.0%), respectively. The serum ferritin concentration was distinguished as a potential biomarker for PCOS based on the high area under ROC curve value of 0.71 (95% CI: 0.57–0.86). Although we did not identify a statistical association between serum Zn concentration and PCOS overall, the concentration of Zn in PCOS women with insulin resistance (IR) was lower than that in healthy women (SMD = −0.89, 95% CI: −1.73 to −0.06). Furthermore, the concentrations of Mg (SMD = 0.31, 95% CI: −0.32–0.94, I2 = 95.4%) and Fe (SMD = −0.59, 95% CI: −1.29–0.12, I2 = 97.2%) were not statistically significant between the PCOS and control groups. We generated hypothetical pathways for associations among serum Cu, ferritin and PCOS. The serum concentrations of both Cu and ferritin were significantly higher in women with PCOS, and ferritin was identified as a potential early indicator for PCOS screening. Further studies are essential to determine the specific underlying mechanisms.
Polycystic ovary syndrome (PCOS) is a multifactorial and polygenic disorder of the endocrine system characterized by anovulation, hyperandrogenism, and polycystic ovarian morphology (1). According to different diagnostic criteria, the global prevalence of PCOS ranges from 4 to 21% (2) Women with PCOS have significant reproductive effects, including increased risk of infertility, miscarriage, and pregnancy-related complications (3), along with metabolic disorders, such as obesity (4), insulin resistance (IR) (5), and type 2 diabetes mellitus (6). The causes of PCOS are currently unclear and no effective biomarkers for early PCOS screening have been identified to date (7). Recently, alterations in trace element levels in PCOS have attracted considerable research attention (8).
Trace elements, such as zinc (Zn), copper (Cu) and magnesium (Mg), are essential for normal cellular functions, and play major roles in metabolic pathways involving of enzymes, hormones, and vitamins (9). Considerable evidence suggests that abnormal levels of trace elements are associated with metabolic syndrome (10) and PCOS is characteristically accompanied by metabolic dysfunction. However, epidemiological findings on the associations between trace elements and PCOS are inconsistent. For example, Revathi et al. (11) showed that serum levels of Cu and Zn were higher while Mg levels were lower in PCOS patients than the control group. In contrast, Li et al. (12) reported no significant differences in the levels of serum Zn, Mg, and iron (Fe) between PCOS and healthy control groups in a Chinese cohort. A meta-analysis conducted by Spritzer and co-workers in 2015 did not offer a robust conclusion, since only four related articles were included that used the same unit of measurement for specific trace elements (8). In view of the increased related epidemiological evidence in recent years (11, 13, 14), meta analysis data need to be urgently updated. Standardized mean difference (SMD) is a practical meta-analysis statistical method to overcome the inconsistencies in measurement units among different studies (15).
Here, we conducted a meta-analysis of existing publications until 2019 to establish accurate and reliable associations of serum levels of Zn, Fe, Mg, Cu, and ferritin with PCOS and further evaluated the utility of these trace element levels for PCOS screening.
We electronically searched PubMed, Embase, and Web of Science using a logical combination of key words. The search terms used were (“polycystic ovary syndrome” OR “poly cystic ovarian syndrome” OR “polycystic ovary disease” OR “polycystic ovaries” OR “polycystic” OR “pcos” OR “stein Leventhal syndrome” OR “SLS”) and (“zinc” or “copper” or “magnesium” or “iron” or “microelement” or “macro elements” or “trace elements”). All articles identified between inception and 26th October 2019 were screened. We also screened the reference lists of these publications for additional references. Conference abstracts were carefully read and screened for unpublished “insignificant results.” We additionally attempted to contact the corresponding authors to request the full text or original data.
Studies were included in this meta-analysis if they were confirmed to meet the following inclusion criteria. First, reports needed to be observational studies that included PCOS patients and non-PCOS controls. Moreover, only confirmed PCOS diagnoses were acceptable. Second, studies needed to contain specific data relating to the serum concentrations of Zn, Fe, ferritin, Cu, or Mg, Finally, studies needed to involve humans. Publications were excluded if they were: (1) commentaries, reviews, or conference abstracts, (2) repetitive studies, (3) clinical interventions, (4) animal studies, (5) lacking a control group, and (6) not in English. Additionally, publications reporting data on plasma levels but not serum levels of microelements were excluded.
Based on these factors, the identified titles and abstracts were first independently reviewed by YJ and HX; only relevant publications were selected for full screening and analysis.
Two independent reviewers (YJ and JM) extracted a range of data, including the date of publication, first author, study population, study design, sample size, the characteristics of PCOS patients, and the methods used to measure microelements in the serum. We recorded the different concentration units (ng/mL, μg/mL, μg/dL, mg/dL, μg/L, and mmol/L) and the methods used to describe data [mean ± standard deviation (SD), median & interquartile range, median, and range]. All data were rechecked by LR.
We assessed the quality of the included studies in accordance with the Newcastle-Ottawa Scale (NOS) (16). Two reviewers independently scored the NOS grade from three aspects: selection, comparability, and exposure. Any discrepancies between the two reviewers were resolved by reaching a consensus, or by involving a third reviewer (BY).
All data relating to the concentration of specific trace elements are represented by mean ± standard deviation (SD). We used sample size, median, and range or interquartile range, to estimate the mean and SD using a method that was described previously (17). Since the existing literature is not consistent with regard to units, we used the pooled standardized mean difference (SMD) to determine the associations between PCOS and serum concentrations of microelements. SMD was determined as mean difference divided by standard deviation derived from both groups estimated using Cohen's method (15). The “meta” package in R software was used to estimate effect sizes. Heterogeneity in the studies was tested using Cochran's Q two-sided test of homogeneity (18). The I2 statistic was a crucial factor when determining the model that should be used to pool the effect size (if I2 < 50%, we used a fixed model, otherwise, we used a random model). Begg's Funnel plots (in cases where the number of included studies was >9) and Egger's regression test were used to test publication bias. The overall strength of evidence was assessed using GRADE criteria (https://gradepro.org/). Sensitivity analyses were performed to test the robustness of the pooled SMD by excluding the study with the largest effect size. Subgroup analyses were conducted according to different diagnostic criteria and sub-classifications of PCOS (e.g., PCOS with obesity, or PCOS with insulin resistance). The receiver operating characteristic (ROC) curve was employed to evaluate the screening value of specific trace elements for PCOS. All analyses were performed using R software. A two-sided P ≤ 0.05 was considered to be statistically significant.
Our database screening identified 1,921 articles. By removing duplications, and by screening abstracts, we were able to select 33 articles for full-text assessment. In addition, one additional record was identified in the reference list of one of the 33 articles. According to the inclusion and exclusion criteria, there were 21 publications included in our final meta-analysis (11–14, 19–35) (Figure 1). All of these studies featured a cross-sectional design and included individual data from 2,173 women with PCOS and 1,897 healthy controls. The baseline characteristics, such as author, year, country, microelement, units, study design, number of PCOS/control subjects, and specific criteria for PCOS diagnosis included in the studies are shown in Table 1. Three articles separately reported data relating to obese or non-obese PCOS women, (19, 24, 28) while two articles separately reported data relating to IR or NIR PCOS women (14, 30). The overall quality of these articles was relatively high (NOS score ≥6). The specific details are shown in Supplementary Table 1.
Overall, 10 articles focused on the association between zinc concentration and PCOS (11–13, 23, 26, 29, 30, 33–35). We did not find a statistical association between serum zinc concentration and PCOS. The SMD between PCOS and healthy controls ranged from −2.34 (95% CI: −2.76 to −1.92) to 0.76 (95% CI 0.25–1.26) (Figure 2A). Using the random-effects model, pooled SMD was −0.31 (95% CI −0.74–0.12), and I2 = 95%. Although the Funnel plot presented obvious asymmetry (see Supplementary Figure 2A), publication bias was not statistically significant (Begger test: P = 0.175, Egger test: P = 0.211). After excluding the results of Shahrokhi et al. (13) (SMD = −2.34), which reported the maximum effect, the pooled SMD remained statistically insignificant (SMD = −0.10, 95% CI: −0.39–0.20) (Supplementary Figure 2B). We further excluded the results of Shahrokhi and Sharif, since they did not use the Rotterdam criteria to diagnose PCOS. The pooled SMD remained insignificant (SMD = −0.09, 95% CI: −0.42–0.24, I2 = 90%). Subgroup analysis showed that the serum Zn concentration in PCOS women with IR was significantly lower than that of healthy women (SMD = −0.89, 95% CI: −1.71 to −0.06). However, there was no significant difference in serum Zn concentration when compared between healthy controls and PCOS women without IR (SMD = −0.25, 95% CI: −0.67–0.16) (see Figure 3A).
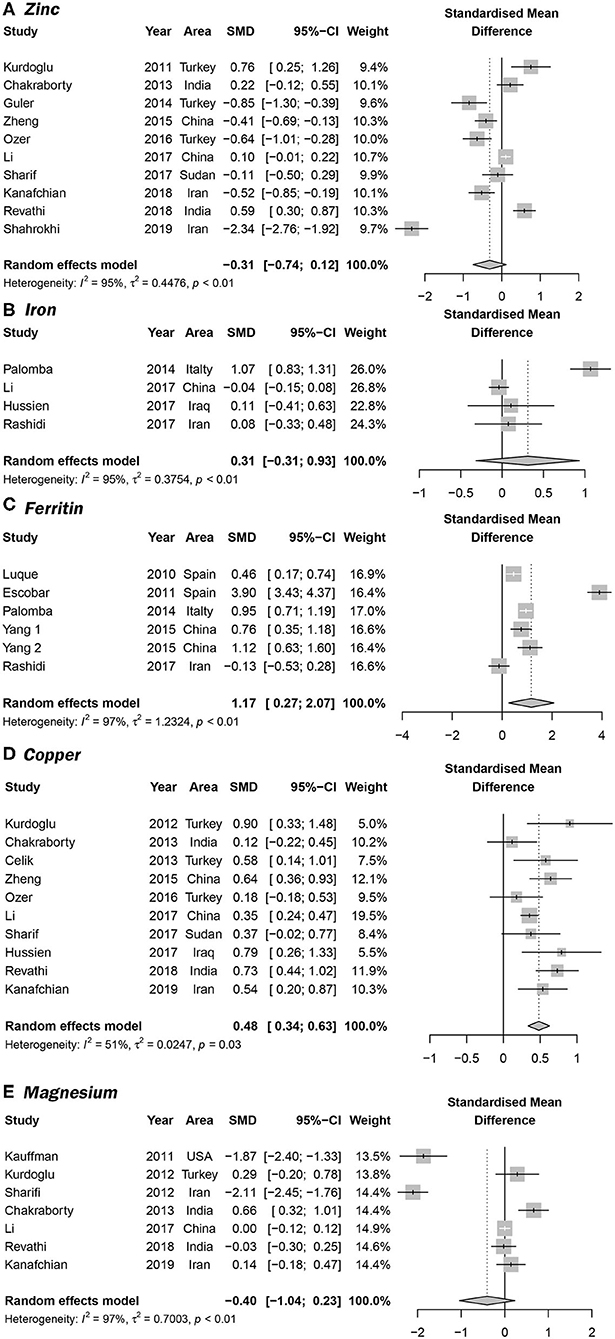
Figure 2. Forrest plots showing serum trace element concentrations in women with PCOS and healthy controls. (A–E) represent the association between PCOS and the serum concentrations of serum Zn, Fe, ferritin, Cu, and Fe, respectively.
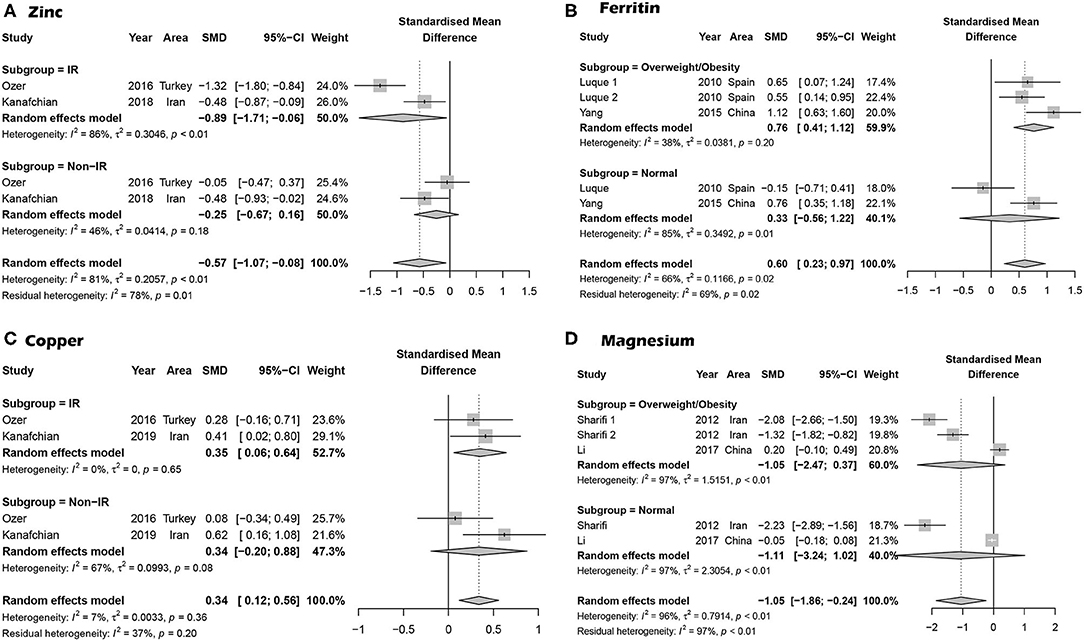
Figure 3. Subgroup analysis based on PCOS with trace elements. (A) Subgroup analysis for the association between serum Zn concentration and PCOS based on IR and PCOS. (B) Subgroup analysis for the association between serum ferritin concentration and PCOS based on overweight/obese PCOS cases. (C) Subgroup analysis for PCOS cases with insulin resistance. (D) Subgroup analysis for PCOS patients who were overweight/obese.
Four studies focused on serum Fe concentrations in patients with PCOS (12, 27, 31, 32), while 5 studies focused on ferritin concentrations (19, 20, 27, 28, 32). The serum concentration of Fe in PCOS patients was not significantly different from that in healthy controls (SMD = 0.31, 95% CI: −0.31–0.93, I2 = 95%) (Figure 2B). There was no significant publication bias with regards to these publications (Begger test: P = 0.734; Egger test: P = 0.601). The SMD of serum ferritin concentration between PCOS and healthy controls ranged from −0.13 (95% CI: −0.53–0.28) to 3.90 (95% CI: 3.43–4.37) (Figure 2C). The pooled SMD was 1.17 (95% CI: 0.27–2.07, I2 = 97.0%), indicating that the serum concentration of ferritin in PCOS patients was higher than that of healthy women. This association was confirmed with moderate evidence (Supplementary Table 6). Begg's (P = 0.734) and Egger tests (P = 0.601) revealed no significant publication bias. After excluding the results of Escobar et al. (20), pooled SMD was also < 0 (SMD = 0.63, 95% CI: 0.24–1.03) (Supplementary Figure 3). We further excluded the results of Luque and Escobar, since they did not use the Rotterdam criteria to diagnose PCOS. The pooled SMD remained significant (SMD = 0.68, 95% CI: 0.16–1.19, I2 = 87%). Subgroup analysis further showed that among overweight/obese women with PCOS, the serum concentration of ferritin was greater than that of healthy women (SMD = 0.76, 95% CI: 0.41–1.12); there was no such increase in serum concentrations of ferritin in women with PCOS who were within the normal weight range (SMD = 0.33, 95% CI: −0.56–1.22) (Figure 3B). Data obtained using the ROC curve suggest that the serum ferritin concentration could be effectively used to distinguish between PCOS and healthy controls to some extent (Figure 4A; area under the curve (AUC) = 0.71, 95% CI: 0.57–0.86).
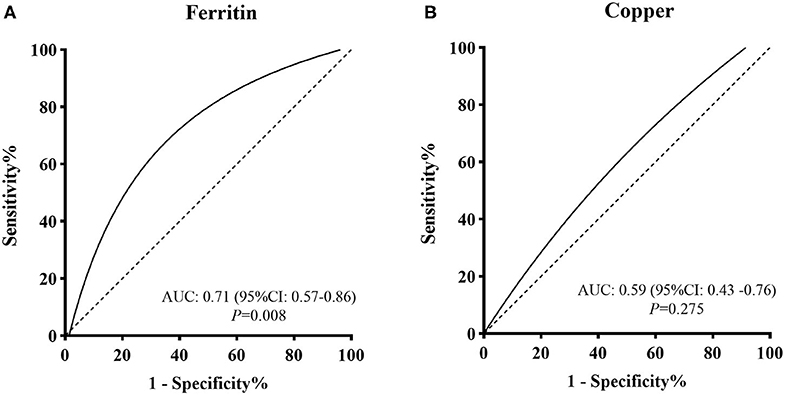
Figure 4. ROC curve evaluations for screening value of (A) serum ferritin concentration and (B) serum Cu concentration for PCOS.
We identified 10 articles that focused on the serum concentration of Cu in women with PCOS (11, 12, 14, 23, 25, 29–31, 33, 35). The SMD for serum Cu concentration between PCOS and healthy controls ranged from 0.12 (95% CI: −0.22–0.45) to 0.90 (95% CI: 0.33–1.48) (Figure 2D). Using a random effects model, the pooled SMD was 0.48 (95% CI: 0.34–0.63) and I2 = 51%; this indicated that the serum concentration of Cu in PCOS patients was higher than that of healthy women. This association was verified by moderate evidence (Supplementary Table 6). We observed no significant publication bias (Supplementary Figure 4, Begger test: P = 0.348, Egger test: P = 0.080). We further excluded the results of Celik, Sharif and Hussien since they did not use the Rotterdam criteria to diagnose PCOS. The pooled SMD remained significant (SMD = 0.47, 95% CI: 0.29–0.64, I2 = 63%). Subgroup analysis showed that among PCOS women with IR, the serum concentration of Cu was greater than that of healthy controls (SMD = 0.35, 95% CI: 0.06–0.64); the SMD was not significant when we analyzed PCOS patients without IR (SMD = 0.34, 95% CI: −0.02–0.88) (see Figure 3C). Nevertheless, data from ROC curve analysis indicated that the predictive value of serum Cu was not statistically significant (Figure 4B; AUC = 0.59, 95% CI: 0.43–0.76; P = 0.275).
We identified 7 articles that focused on the serum concentration of Mg in women with PCOS (11, 12, 14, 21, 23, 24, 35). All the included studies used the Rotterdam criteria for PCOS diagnosis. No significant differences in the serum concentration of Mg were evident between PCOS patients and healthy controls (SMD = −0.40, 95% CI: −1.04–0.23). Further analysis revealed high levels of heterogeneity among the 7 articles (I2 = 97%); the SMD ranged from −2.11 (95% CI: −2.45 to −1.76) to 0.66 (95% CI: 0.32–1.01) (Figure 2E). Begg's test (P = 0.260) and Egger test (P = 0.320) showed no significant publication bias. After excluding the studies of Sharifi (33) and Kauffman (21), which reported relatively extreme results, the pooled SMD was −0.07 (95% CI: −0.02–0.17) (Supplementary Figure 5). Subgroup analysis further showed that the serum concentration of serum Mg was not significantly different between PCOS patients and healthy controls, irrespective of whether or not PCOS patients were obese (overweight/obese: SMD = −1.05, 95% CI: −2.47–0.37; normal weight: SMD = −1.11, 95% CI: −3.24–1.02) (Figure 3D).
Knowledge of the potential associations of trace elements with PCOS occurrence and development should provide effective new strategies to prevent, screen and treat PCOS, which has public health significance. Here, we identified 21 specific articles on the associations between PCOS and serum concentrations of Zn, Mg, Cu, Fe, and ferritin. The results showed that PCOS patients had significantly higher serum concentrations of Cu and ferritin than healthy controls. However, no significant differences were observed with regard to the levels of Zn, Fe, and Mg between the PCOS and control groups. Our report provides not only an update on meta-analysis data but also preliminary evidence of the screening value of serum ferritin concentration for PCOS.
Our results showed that serum Cu and ferritin are associated with PCOS. Cu is an essential trace element in the human body and required as a cofactor for a range of enzymes in critical metabolic pathways, including cytochrome oxidase, superoxide dismutase, ascorbic acid oxidase, and tyrosinase (36). Recent studies have shown that Cu interacts with key neuropeptides in the hypothalamic-pituitary-gonadal axis, notably, gonadotropin-releasing hormone (GnRH) and neurokinin B, and promotes anovulatory menstruation (37). Excessive levels of Cu induce oxidative stress via Fenton and redox reactions, resulting in increased production of reactive oxygen species (ROS) (38). A previous study showed significantly higher levels of oxidative stress parameters, including total antioxidant and oxidant status and oxidative stress index, in PCOS patients than healthy controls (39), indicating a role of oxidation in the pathogenesis of the disease. ROS can alter the steroidogenesis process in the ovary, leading to increased androgen levels, disturbance in follicular development, and infertility (40). Moreover, IR is reported to be linked with oxidative stress, which may mediate PCOS occurrence through facilitating secretion of excessive levels of androgens from ovaries and adrenal glands (41).
Ferritin, the cellular storage protein for iron, serves as a biomarker for estimating the levels of iron stored in the body. Several factors potentially contribute to elevation of serum ferritin levels in women with PCOS, including the iron-sparing effect caused by prolonged menstrual cycle and hyperinsulinism (42). Meanwhile, higher insulin may facilitate intestinal absorption and deposition of iron in tissue, with IR leading to higher levels of ferritin (42). Our results also showed an association of obese/overweight PCOS subjects with higher serum ferritin but not those with normal BMI, indicating a critical role of overweight/obesity. This finding was consistent with that of Hitha et al. (43), which showed a significant positive relationship between ferritin and metabolic parameters in obese subjects. Although serum Fe showed a similar increasing trend among PCOS subjects, the data were not statistically significant, suggesting that the serum Fe level may be a less sensitive parameter than ferritin. Based on the collective findings, a hypothetical pathway was drawn to describe the potential associations among Cu, ferritin and PCOS (Figure 5).
The complex effects of trace elements on body functions may partly explain the inconsistency of epidemiological results. Zn acts as a stabilizer and cofactor for many enzymes and is an essential element for hormonal function (44). In addition, Zn is a regulator of islet function and glucose homeostasis and combines with insulin hexamers to promote the stability and binding ability of insulin receptors (45, 46). Although no association with PCOS has been established, Zn supplementation is reported to ameliorate insulin sensitivity, improve glucose homeostasis, and alleviate insulin resistance (47–49). Mg is involved in over 300 enzyme systems and has been identified as a necessary nutrient for energy production and synthesis of nucleic acids. Considerable evidence suggests that IR can be improved in women with PCOS following Mg supplementation (50, 51), but the specific mechanisms are still unclear.
To explore the causal correlations between trace elements and PCOS, many randomized controlled trials (RCTs) have been performed to establish whether trace element supplements have beneficial effects on PCOS treatment (52, 53). The group of Afshar (53) showed that Mg and Zn co-supplementation decreased serum high-sensitivity C-reactive protein and increased plasma total antioxidant capacity levels. However, inconsistent results have been obtained from different studies (52). Notably, increases in Cu and ferritin were difficult to adjust through simple supplements. To our knowledge, no RCTs have focused on the significance these elements in PCOS. Identification of practical biomarkers to screen for PCOS among childbearing women remains an urgent medical requirement. PCOS is a clinical outcome of long-term changes in the endocrine system (54). We assume that subtle alterations do not raise clinical concerns, including changes in trace elements, which may slowly cause PCOS. Here, we reported the screening value of serum ferritin for PCOS for the first time, which requires further verification. Our findings were similar to the results of Spritzer et al. (8). Our study provides more robust evidence since a larger number of studies were included, some of which were published in recent years. Additionally, we addressed two problems reported by the group of Spritzer. SMD was employed to overcome the challenge of heterogeneity of measurement units used among different studies (15). The method of Wan (17) was used to process non-normal data. Despite the possibility of introducing greater heterogeneity, comparison of data from different sources could provide valuable information.
A number of limitations in our study should be acknowledged. First, heterogeneity existed among the original articles due to differences in participant backgrounds and methods used to detect trace elements. Second, SMD was used to estimate the difference, which simply reflected the variation trends of trace elements among PCOS but not the actual levels, that would impact clinical application. Third, the cross-sectional or case-control designs of original articles would limit causal inference. We could not conclude whether the changes in trace elements induce PCOS or exert a converse effect. Fourth, the sample sizes of studies focusing on trace element analysis were small, potentially resulting in bias of results. Fifth, due to data limitations, the main confounding variables were not adjusted for and we could not analyze the possible confounding effect of obesity or IR on all associations through sub-group analysis. These factors could have influenced our final comparative analyses. Furthermore, only literature published in English was included. Although a number of researchers propose that the language of publication has little effect on the pooled effect estimates (55), the possibility of publication bias cannot be overlooked. Potential unpublished data may additionally contribute to publication bias.
In conclusion, serum concentrations of Cu and ferritin are significantly higher in subjects with PCOS. Moreover, ferritin may serve as an early indicator of PCOS screening. Further studies are required to investigate the significance of other elements, including Mg, Zn and Fe, in PCOS and the specific mechanisms involved.
All the original data were presented in the main text and Supplemental Materials. Any other questions can contact the corresponding author: Ran Liu, cmFubGl1QHNldS5lZHUuY24=.
JY, XH, and JM: literature search, screening, and data extraction. XH and JY: data analysis and results visualization. JY, XH, YB, and RL: manuscription draft and modification. RL and YB: fund acquirement. All authors reviewed the final version of the manuscript and approve it for publication.
This work was supported by grants from the National Natural Science Foundation of China (Reference numbers: 81872579, 81273123), the Major Science and Technology Program for Water Pollution Control and Treatment (2014ZX07405002), the Graduate Student Scientific Practice Innovation Projects in Jiangsu province (KYCX19_0123), and the Fundamental Research Fund of Central Public Welfare Research Institutions in 2019 (innovation team project for new approaches and applications on substitution toxicology for environmental hormone substance).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank International Science Editing Company for English language editing.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.572384/full#supplementary-material
PCOS, Polycystic ovary syndrome; IR, insulin resistance; ROS, reactive oxygen species; Zn, zinc; Cu, copper; Mg, magnesium; Fe, iron; SMD, standard mean difference.
1. Krishnan A, Muthusami S. Hormonal alterations in PCOS and its influence on bone metabolism. J Endocrinol. (2017) 232:R99–113. doi: 10.1530/JOE-16-0405
2. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. (2016) 106:6–15. doi: 10.1016/j.fertnstert.2016.05.003
3. Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update. (2015) 21:575–92. doi: 10.1093/humupd/dmv029
4. Naderpoor N, Shorakae S, Joham A, Boyle J, de Courten B, Teede HJ. Obesity and polycystic ovary syndrome. Minerva Endocrinol. (2015) 40:37–51.
5. Chen C, Jing G, Li Z, Juan S, Bin C, Jie H. Insulin resistance and polycystic ovary syndrome in a Chinese population. Endocrine Pract. (2017). doi: 10.4158/EP171849.OR. [Epub ahead of print].
6. Ollila MM, West S, Keinanen-Kiukaaniemi S, Jokelainen J, Auvinen J, Puukka K, et al. Overweight and obese but not normal weight women with PCOS are at increased risk of Type 2 diabetes mellitus-a prospective population-based cohort study. Hum Rep. (2017) 32:423–31. doi: 10.1093/humrep/dex030
7. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. (2018) 110:364–79. doi: 10.1016/j.fertnstert.2018.05.004
8. Spritzer PM, Lecke SB, Fabris VC, Ziegelmann PK, Amaral L. Blood trace element concentrations in polycystic ovary syndrome: systematic review and meta-analysis. Biol Trace Element Res. (2017) 175:254–62. doi: 10.1007/s12011-016-0774-4
9. Fraga CG. Relevance, essentiality and toxicity of trace elements in human health. Mol Aspects Med. (2005) 26:235–44. doi: 10.1016/j.mam.2005.07.013
10. Shi Y, Zou Y, Shen Z, Xiong Y, Zhang W, Liu C, et al. Trace elements, PPARs, and metabolic syndrome. Int J Mol Sci. (2020) 21:2612. doi: 10.3390/ijms21072612
11. Revathi R, Julius A, Singaravelu S. Correlation of serum copper, zinc, magnesium with insulin resistance in Pcos female of reproductive age group. Int J Pharm Res. (2018) 10:789–92. doi: 10.5958/0976-5506.2019.01225.7
12. Li MY, Tang YY, Lin CL, Huang QY, Lei DQ, Hu YL. Serum macroelement and microelement concentrations in patients with polycystic ovary syndrome: a cross-sectional study. Biol Trace Element Res. (2017) 176:73–80. doi: 10.1007/s12011-016-0782-4
13. Shahrokhi SA, Naeini AA. The association between dietary antioxidants, oxidative stress markers, abdominal obesity and poly-cystic ovary syndrome: a case control study. J Obstetr Gynaecol. (2019) 40:77–82. doi: 10.1080/01443615.2019.1603215
14. Kanafchian M, Esmaeilzadeh S, Mahjoub S, Rahsepar M, Ghasemi M. Status of serum copper, magnesium, and total antioxidant capacity in patients with polycystic ovary syndrome. Biol Trace Element Res. (2019) 193:1117. doi: 10.1007/s12011-019-01705-7
15. Takeshima N, Sozu T, Tajika A, Ogawa Y, Hayasaka Y, Furukawa TA. Which is more generalizable, powerful and interpretable in meta-analyses, mean difference or standardized mean difference? BMC Med Res Methodol. (2014) 14:30. doi: 10.1186/1471-2288-14-30
16. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
17. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
18. Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med. (1998) 17:841–56. doi: 10.1002/(SICI)1097-0258(19980430)17:8<841::AID-SIM781>3.0.CO;2-D
19. Luque-Ramírez M, Alpañés M, Escobar-Morreale HF. The determinants of insulin sensitivity, β-cell function, and glucose tolerance are different in patients with polycystic ovary syndrome than in women who do not have hyperandrogenism. Fertil Steril. (2010) 94:2214–21. doi: 10.1016/j.fertnstert.2009.11.049
20. Escobar-Morreale HF, Luque-Ramírez M. Role of androgen-mediated enhancement of erythropoiesis in the increased body iron stores of patients with polycystic ovary syndrome. Fertil Steril. (2011) 95:1730–5.e1. doi: 10.1016/j.fertnstert.2011.01.038
21. Kauffman RP, Tullar PE, Nipp RD, Castracane VD. Serum magnesium concentrations and metabolic variables in polycystic ovary syndrome. Acta Obstetric Gynecol Scand. (2011) 90:452–8. doi: 10.1111/j.1600-0412.2010.01067.x
22. Luque-Ramirez M, Alvarez-Blasco F, Alpanes M, Escobar-Morreale HF. Role of decreased circulating hepcidin concentrations in the iron excess of women with the polycystic ovary syndrome. J Clin Endocrinol Metab. (2011) 96:846–52. doi: 10.1210/jc.2010-2211
23. Kurdoglu Z, Kurdoglu M, Demir H, Sahin HG. Serum trace elements and heavy metals in polycystic ovary syndrome. Hum Exp Toxicol. (2012) 31:452–6. doi: 10.1177/0960327111424299
24. Sharifi F, Mazloomi S, Hajihosseini R, Mazloomzadeh S. Serum magnesium concentrations in polycystic ovary syndrome and its association with insulin resistance. Gynecol Endocrinol. (2012) 28:7–11. doi: 10.3109/09513590.2011.579663
25. Celik C, Bastu E, Abali R, Alpsoy S, Guzel EC, Aydemir B, et al. The relationship between copper, homocysteine and early vascular disease in lean women with polycystic ovary syndrome. Gynecol Endocrinol. (2013) 29:488–91. doi: 10.3109/09513590.2013.774361
26. Guler I, Himmetoglu O, Turp A, Erdem A, Erdem M, Onan MA, et al. Zinc and homocysteine levels in polycystic ovarian syndrome patients with insulin resistance. Biol Trace Element Res. (2014) 158:297–304. doi: 10.1007/s12011-014-9941-7
27. Palomba S, Falbo A, Chiossi G, Orio F, Tolino A, Colao A, et al. Low-grade chronic inflammation in pregnant women with polycystic ovary syndrome: a prospective controlled clinical study. J Clin Endocrinol Metabol. (2014) 99:2942–51. doi: 10.1210/jc.2014-1214
28. Yang JH, Chou CH, Yang WS, Ho HN, Yang YS, Chen MJ. Iron stores and obesity are negatively associated with ovarian volume and anti-Müllerian hormone levels in women with polycystic ovary syndrome. Taiwanese J Obstetr Gynecol. (2015) 54:686–92. doi: 10.1016/j.tjog.2014.11.025
29. Zheng G, Wang L, Guo Z, Sun L, Wang L, Wang C, et al. Association of serum heavy metals and trace element concentrations with reproductive hormone levels and polycystic ovary syndrome in a Chinese population. Biol Trace Element Res. (2015) 167:1–10. doi: 10.1007/s12011-015-0294-7
30. Ozer A, Bakacak M, Kiran H, Ercan O, Kostu B, Kanat-Pektas M, et al. Increased oxidative stress is associated with insulin resistance and infertility in polycystic ovary syndrome. Ginekologia Polska. (2016) 87:733–8. doi: 10.5603/GP.2016.0079
31. Hussien KA, Al-Salih RMH, Ali SA. Oxidative stress and some related trace elements in women with unexplained infertility. J Glob Pharma Technol. (2017) 9:362–71.
32. Rashidi BH, Shams S, Shariat M, Jaliseh HK, Mohebi M, Haghollahi F. Evaluation of serum hepcidin and iron levels in patients with PCOS: a case-control study. J Endocrinol Invest. (2017) 40:779–84. doi: 10.1007/s40618-017-0632-z
33. Sharif ME, Adam I, Ahmed MA, Rayis DA, Hamdan HZ. Serum level of zinc and copper in sudanese women with polycystic ovarian syndrome. Biol Trace Element Res. (2017) 180:23–7. doi: 10.1007/s12011-017-1000-8
34. Kanafchian M, Mahjoub S, Esmaeilzadeh S, Rahsepar M, Mosapour A. Status of serum selenium and zinc in patients with the polycystic ovary syndrome with and without insulin resistance. Middle East Fertil Soc J. (2018) 23:241–5. doi: 10.1016/j.mefs.2017.11.003
35. Chakraborty P, Ghosh S, Goswami SK, Kabir SN, Chakravarty B, Jana K. Altered trace mineral milieu might play an aetiological role in the pathogenesis of polycystic ovary syndrome. Biol Trace Element Res. (2013) 152:9–15. doi: 10.1007/s12011-012-9592-5
36. Lorincz MT. Wilson disease and related copper disorders. Handb Clin Neurol. (2018) 147:279–292. doi: 10.1016/B978-0-444-63233-3.00018-X
37. Peacey L, Elphick MR, Jones CE. Roles of copper in neurokinin B and gonadotropin-releasing hormone structure and function and the endocrinology of reproduction. Gen Comp Endocrinol. (2020) 287:113342. doi: 10.1016/j.ygcen.2019.113342
38. Pereira TCB, Campos MM, Bogo MR. Copper toxicology, oxidative stress and inflammation using zebrafish as experimental model. J Appl Toxicol. (2016) 36:876–85. doi: 10.1002/jat.3303
39. Sak S, Uyanikoglu H, Incebiyik A, Incebiyik H, Hilali NG, Sabuncu T, et al. Associations of serum fetuin-A and oxidative stress parameters with polycystic ovary syndrome. Clin Exp Reprod Med. (2018) 45:116–21. doi: 10.5653/cerm.2018.45.3.116
40. Sulaiman MA, Al-Farsi YM, Al-Khaduri MM, Saleh J, Waly MI. Polycystic ovarian syndrome is linked to increased oxidative stress in Omani women. Int J Womens Health. (2018) 10:763–71. doi: 10.2147/IJWH.S166461
41. Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A. Insulin resistance: review of the underlying molecular mechanisms. J Cell Physiol. (2019) 234:8152–61. doi: 10.1002/jcp.27603
42. Escobar-Morreale HF. Iron metabolism and the polycystic ovary syndrome. Trends Endocrinol Metab. (2012) 23:509–15. doi: 10.1016/j.tem.2012.04.003
43. Hitha H, Gowda D, Mirajkar A. Serum ferritin level as an early indicator of metabolic dysregulation in young obese adults - a cross-sectional study. Can J Physiol Pharmacol. (2018) 96:1255–60. doi: 10.1139/cjpp-2018-0433
44. Yaman M, Kaya G, Yekeler H. Distribution of trace metal concentrations in paired cancerous and non-cancerous human stomach tissues. World J Gastroenterol. (2007) 13:612–8. doi: 10.3748/wjg.v13.i4.612
45. Cruz KJC, de Oliveira ARS, Morais JBS, Severo JS, Mendes PMV, de Sousa Melo SR, et al. Zinc and insulin resistance: biochemical and molecular aspects. Biol Trace Element Res. (2018) 186:407–12. doi: 10.1007/s12011-018-1308-z
46. Wijesekara N, Chimienti F, Wheeler MB. Zinc, a regulator of islet function and glucose homeostasis. Diabetes Obes Metab. (2009) 11(Suppl. 4):202–14. doi: 10.1111/j.1463-1326.2009.01110.x
47. Thoen RU, Barther NN, Schemitt E, Bona S, Fernandes S, Coral G, et al. Zinc supplementation reduces diet-induced obesity and improves insulin sensitivity in rats. Appl Physiol Nutr Metab. (2019) 44:580–6. doi: 10.1139/apnm-2018-0519
48. Taylor CG. Zinc, the pancreas, and diabetes: insights from rodent studies and future directions. Biometals. (2005) 18:305–12. doi: 10.1007/s10534-005-3686-x
49. Chausmer AB. Zinc, insulin and diabetes. J Am Coll Nutr. (1998) 17:109–15. doi: 10.1080/07315724.1998.10718735
50. Muneyyirci-Delale O, Kaplan J, Joulak I, Yang L, Von Gizycki H, Nacharaju VL. Serum free fatty acid levels in PCOS patients treated with glucophage, magnesium oxide and spironolactone. Gynecol Endocrinol. (2013) 29:474–7. doi: 10.3109/09513590.2013.769515
51. Jamilian M, Sabzevar NK, Asemi Z. The effect of magnesium and vitamin E Co-supplementation on glycemic control and markers of cardio-metabolic risk in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Hormone Metab Res. (2019) 51:100–5. doi: 10.1055/a-0749-6431
52. Jamilian M, Foroozanfard F, Bahmani F, Talaee R, Monavari M, Asemi Z. Effects of zinc supplementation on endocrine outcomes in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Element Res. (2016) 170:271–8. doi: 10.1007/s12011-015-0480-7
53. Afshar Ebrahimi F, Foroozanfard F, Aghadavod E, Bahmani F, Asemi Z. The effects of magnesium and zinc co-supplementation on biomarkers of inflammation and oxidative stress, and gene expression related to inflammation in polycystic ovary syndrome: a randomized controlled clinical trial. Biol Trace Element Res. (2018) 184:300–7. doi: 10.1007/s12011-017-1198-5
54. Sattar N. Review: PCOS, insulin resistance and long-term risks for diabetes and vascular disease. Br J Diabetes Vasc Dis. (2009) 9:15–8. doi: 10.1177/1474651408101369
Keywords: PCOS, trace elements, meta-analysis, zinc (Zn), copper (Cu), magnesium (Mg), iron (Fe)
Citation: Yin J, Hong X, Ma J, Bu Y and Liu R (2020) Serum Trace Elements in Patients With Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front. Endocrinol. 11:572384. doi: 10.3389/fendo.2020.572384
Received: 14 June 2020; Accepted: 18 August 2020;
Published: 17 September 2020.
Edited by:
Yang Xu, Peking University First Hospital, ChinaReviewed by:
William Atiomo, University of Nottingham, United KingdomCopyright © 2020 Yin, Hong, Ma, Bu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ran Liu, cmFubGl1QHNldS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.