
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 19 January 2021
Sec. Reproduction
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.571705
This article is part of the Research Topic Using Technology for Healthy Lifestyle and Weight Management View all 9 articles
 Paula Gardiner1*
Paula Gardiner1* Timothy Bickmore2
Timothy Bickmore2 Leanne Yinusa-Nyahkoon3
Leanne Yinusa-Nyahkoon3 Matthew Reichert4
Matthew Reichert4 Clevanne Julce5
Clevanne Julce5 Nireesha Sidduri5
Nireesha Sidduri5 Jessica Martin-Howard5,6
Jessica Martin-Howard5,6 Elisabeth Woodhams7
Elisabeth Woodhams7 Jumana Aryan5
Jumana Aryan5 Zhe Zhang2
Zhe Zhang2 Juan Fernandez2
Juan Fernandez2 Mark Loafman8
Mark Loafman8 Jayakanth Srinivasan6,9
Jayakanth Srinivasan6,9 Howard Cabral10
Howard Cabral10 Brian W. Jack5,6
Brian W. Jack5,6Importance: Healthy nutrition and appropriate supplementation during preconception have important implications for the health of the mother and newborn. The best way to deliver preconception care to address health risks related to nutrition is unknown.
Methods: We conducted a secondary analysis of data from a randomized controlled trial designed to study the impact of conversational agent technology in 13 domains of preconception care among 528 non-pregnant African American and Black women. This analysis is restricted to those 480 women who reported at least one of the ten risks related to nutrition and dietary supplement use.
Interventions: An online conversational agent, called “Gabby”, assesses health risks and delivers 12 months of tailored dialogue for over 100 preconception health risks, including ten nutrition and supplement risks, using behavioral change techniques like shared decision making and motivational interviewing. The control group received a letter listing their preconception risks and encouraging them to talk to a health care provider.
Results: After 6 months, women using Gabby (a) reported progressing forward on the stage of change scale for, on average, 52.9% (SD, 35.1%) of nutrition and supplement risks compared to 42.9% (SD, 35.4) in the control group (IRR 1.22, 95% CI 1.03–1.45, P = 0.019); and (b) reported achieving the action and maintenance stage of change for, on average, 52.8% (SD 37.1) of the nutrition and supplement risks compared to 42.8% (SD, 37.9) in the control group (IRR 1.26, 96% CI 1.08–1.48, P = 0.004). For subjects beginning the study at the contemplation stage of change, intervention subjects reported progressing forward on the stage of change scale for 75.0% (SD, 36.3%) of their health risks compared to 52.1% (SD, 47.1%) in the control group (P = 0.006).
Conclusion: The scalability of Gabby has the potential to improve women’s nutritional health as an adjunct to clinical care or at the population health level. Further studies are needed to determine if improving nutrition and supplement risks can impact clinical outcomes including optimization of weight.
Clinical Trial Registration: ClinicalTrials.gov, identifier NCT01827215.
Healthy nutrition during preconception has significant implications for the health of the mother and newborn. Maternal nutrition and health status at the time of conception is an important determinant of embryonic and fetal growth, and emerging evidence suggests that a mother’s diet and lifestyle influences the long-term health of her infant (1). Nutritional status is affected by numerous variables including access to healthy food choices and dietary supplements (e.g., folic acid), income, community environment, lifestyle habits such as smoking and participating in physical activity, and the presence of physiological stressors (2).
A healthy diet up to three years prior to conception reduces the risk of poor pregnancy and birth outcomes, including gestational diabetes, hypertensive disorders of pregnancy, and preterm birth. Evidence-based clinical guidelines include nutrition recommendations such as daily folic acid, appropriate iron and calcium consumption, healthy food choices, a safe amount of fish, and caffeine reduction to improve birth outcomes (3–5). However, healthcare providers often lack enough time and resources to deliver individualized care to reproductive-aged women with nutritional risks during a clinical encounter (6, 7).
African American and Black women are at increased risk of poor maternal health outcomes, including risks impacted by nutrition and health status. African American and Black women are likely to seek health information predominately from informal sources, such as social media and members of their social network (8, 9). Health disparities, longstanding institutional and structural barriers, and mistrust or lack of cultural awareness during the clinical encounter may contribute to the patient-provider communication gap. Additionally, little research has been conducted assessing multi-behavioral change related to nutrition among African American and Black women of reproductive age (10–12).
Health information technology may offer a promising and cost-effective way to introduce tailored healthy nutrition and dietary habits to women. To date, many e-health interventions are resource-intensive, include limited evidence-based information, or do not address underlying motivational factors that may affect behavior change (2, 5). Although interventions providing online education and counseling through the utilization of social media and text messaging show benefits among postpartum women, these interventions are much less effective among prenatal women (13) with limited studies showing the impact on preconception women. Due to limitations in providers being able to deliver appropriate health behaviors and risk reduction education during the clinical encounter, it is appropriate to study the use of innovative health technologies to address this gap. Conversational agents, computer-generated characters that simulate face-to-face conversation and communicate key health messages, can overcome barriers to the provision of health education and counseling in a variety of clinical areas (14, 15).
“Gabby” is an animated, conversational agent developed to bridge the gap between provider and patient. Informed by focus groups from the study population, Gabby presents as an African American female character who delivers culturally relevant preconception health education for over 100 risks across 13 domains, including nutrition and dietary supplement use (14, 16). Relevant risks identified through a baseline risk assessment are addressed using tailored dialogue incorporating behavioral change techniques like motivational interviewing and shared decision making. Behaviors requiring incremental, longitudinal change are facilitated through goal setting, positive reinforcement, problem-solving, tips, and homework. Sustained engagement is monitored through self-reported behavior change over a 12 month period (15, 17).
Progress in addressing health risks is assessed using the Transtheoretical Model of health behavior change (18). According to the model, there are five “stages of change” in the behavior change process. The Gabby system helps women progress from not thinking about making any changes in specific health behaviors (“precontemplation”) to actively changing or sustaining a behavior (“action” or “maintenance”) through stage-appropriate behavior change techniques.
We previously reported the results a randomized controlled trial (RCT) of 528 African American and Black women ages 18–34 designed to evaluate the effect of using Gabby that demonstrated a significantly greater increase in preconception risks progressing forward through the stages of the Transtheoretical Model (19) at 6 and 12 months compared to a control group when all 13 domains of preconception care were analyzed (20).
In this paper, we report a secondary analysis of data from this RCT to study the impact of Gabby on a subset of 480 women who reported at least one of the ten preconception health risks in the nutrition domain. The primary outcome “progressing forward” or “achieving action or maintenance” is the self-reported stage of change on each of the ten risks in the nutrition domain at 6 and 12 months.
Participants were recruited using research websites, community programs, and referrals. Those meeting inclusion criteria (self-reported African American or Black, ages 18–34, currently not pregnant) consented and baseline data was collected. Subjects were then randomized to intervention (Gabby) or control group. The manuscript describing the primary RCT contains details of the recruitment, enrollment, consent and randomization processes (20).
Research staff collected contact information, socio-demographic characteristics including age (years), Hispanic, Latino or Spanish origin (yes/no), English as a primary language (yes/no), student status (yes/no), full or part-time employment (yes/no), household income (<$20,000, $20,000-49,999, >50,000), education (less than college, some college), marital status (single, never married, single with a partner, married, separated/divorced or widowed), how frequently they use the internet (occasionally, frequently, very frequently) and BMI (Body Mass Index) > 30.
Following consent and baseline data collection, subjects were emailed their login information and asked to complete a 15 minute-risk assessment organized into 13 domains of preconception care (20). This paper focuses on the nutrition and supplement domain. Table 1 lists the 10 nutrition and supplement risks, criteria for identification, and corresponding goal behavior.
After completing the risk assessment, subjects randomized to the Gabby intervention were asked to log on at least once every two weeks. Based on the relevant risks identified during the risk assessment, Gabby compiles a customizable list called the “My Health To-Do List” from which a woman can choose to discuss health topics that Gabby tracks over time.
Through unfolding conversational dialogue, Gabby addresses the following topics: adequate intake of fruits and vegetables, unhealthy food choices (e.g., junk food and sweets), the risk for mercury consumption when eating certain kinds of fish, omega-3 fatty acids rich food consumption, multivitamin with folic acid or folic acid supplement, calcium-rich food or supplement consumption, iron-rich food or supplement consumption, vitamin D rich food or supplement consumption, vitamin A overuse, use of herbal or weight loss supplements, and caffeine consumption. The content programmed in the Gabby system to mitigate these risks is informed by the AJOG evidence-base on nutrition and dietary supplements (5), CDC diabetes prevention curriculum (21), Five Fruit and Vegetables recommendation (22), Harvard School of Public Health’s healthy eating plate (23) and messaging based on key health behavior techniques (24–27).
The scripted dialogues Gabby delivers for healthy eating encompasses the following modules: fruits and vegetables, healthy eating plate (23), fat awareness, portion sizes, and the relationship between stress and eating. The content surrounding vitamin and mineral-rich food or supplement needs include folic acid, multivitamins, calcium, iron, vitamin D, vitamin A, herbal supplements, and omega-3 fatty acids. Gabby also provides dialogue on maintaining a healthy weight, setting goals about nutrition and supplement use, limiting caffeine intake, and references a variety of food choices that align with the range of dietary norms and preferences among subjects.
Gabby then describes why managing any risk identified is important and offers suggestions about how to take action on it. For example, Gabby will provide tips on finding low-calorie substitutions for meals, suggest healthier ways to cook meat (i.e., grilling, broiling, charring) and vegetables (i.e., steaming, boiling, microwaving), and draining fat by blotting with a paper towel. During up to 12 months of subsequent interactive dialogue, Gabby identifies the degree of progress, and provides feedback on actions taken, reassesses readiness, and recommends next steps. The system dynamically assesses the woman’s level of understanding of health information provided and repeats information when requested by the subject. Gabby uses nonverbal conversational behaviors such as hand gestures and facial expressions, while the woman responds by selecting a response from a multiple-choice menu of options updated at each turn of dialogue as depicted in Figure 1.

Figure 1 Image of Gabby, the preconception care conversational agent, with response buttons to facilitate dialogue.
Controls received a letter that listed the health risks reported on their risk assessment and encouraged women to discuss these risks with a health care professional.
At baseline, 6, and 12 months, all subjects were asked by telephone interview to report their stage of change for addressing each of the risks that were identified on the baseline risk assessment.
Self-reported stage of change data was collected and used to construct two primary outcomes: the self-reported percentage of preconception care risks from the nutrition domain that (1) progressed forward on the stage of change scale, and (2) reached the action or maintenance stage of change.
Secondary outcomes include (1) total usage of the entire system for all risks (logons and minutes), (2) the percentage of risks progressing forward and reaching action or maintenance within three subdomains within the nutrition domain, and (3) the odds of each of the individual ten nutrition risks progressing forward and reaching action or maintenance. The three subdomains constructed were: food choices, including unhealthy diet, greater than 2 servings of fish, and low omega-3 fatty acids; supplements needed for preconception care, including folic acid, iron, calcium, and vitamin D supplementation; and supplements and chemicals to use at a safe level, including safe vitamin A use, other supplement use, and caffeine use.
Subgroup analyses explored whether the relationship between nutrition risks and the primary outcome was differentially effective among subgroups of clinical and demographic variables. Variables analyzed include education, income, age, and nutrition knowledge, which was a 6-item assessment on folic acid dose, types of folic acid in diet, knowledge of supplements for bones (calcium), blood (iron), healthy fats, and number of recommend fruits and vegetables. The assessment was scored on a range of 0-6 and rescaled as a percent of correct responses.
Balance across study arms at baseline was assessed using independent sample t-tests for continuous variables and chi-squared tests for dichotomous variables. All treatment effects were reproduced using regression to control for covariates unbalanced at baseline.
Primary outcomes were assessed in two ways. First, we used intention-to-treat (ITT) analysis to detect whether the Gabby intervention exerted an effect on each of our two primary outcomes, at both 6 and 12 months, across all participants that were randomized. These analyses were conducted by transforming percentages into rates and regressing those rates on both study arms using Poisson regressions.
We then conducted an as-treated analysis by regressing the primary outcomes, at 12 months, on the number of logins to the Gabby system over those 12 months, using Poisson regression, and including all participants that logged in to the Gabby system at least once. In this analysis, we determined a priori that three potential confounding variables (education, income, and age) may correlate with progress on the stage of change and Gabby system usage, and controlled for them in the regression analysis.
For secondary outcome analyses, for each subdomain, the data was restricted only to subjects who flagged at least one risk within that subdomain, and Poisson models were used to regress the rate of progressing forward or achieving action or maintenance on both study arms. In addition, a logistic regression model was used to regress a binary measure, for each risk, of whether that risk had either progressed forward or remained at action or maintenance on study arm. Poisson and logit coefficients were used to generate odds and rate ratios with 90% and 95% confidence intervals.
A priori subgroup analyses were conducted across both study arms by using Poisson models to regress the rate of nutrition and supplement risks that progressed forward or reached action or maintenance on subgroup variables: education, income, age, and nutrition knowledge. Analyses were also conducted, with Poisson models, to determine the differential effects of Gabby across subgroups by regressing the rate of nutrition and supplement risks that progressed forward or reached action or maintenance on both study arms interacted with these subgroup variables.
An analysis was conducted to determine whether Gabby was differentially effective conditional on the stage of change from which they started at baseline. This was done by restricting the data only to risks that were reported at “precontemplation” at baseline; then by calculating the rate that progressed forward or remained at “action” or “maintenance,” and finally by regressing that rate on both study arms using a Poisson model. This procedure was repeated by restricting the data to risks reported at “contemplation” at baseline, and again by restricting to risks reported at the “preparation” and then “action” stages of change.
All statistical tests were run at alpha level a=0.05 and were performed using the R programming language, version 3.4.3.
Among the 528 subjects recruited in the full randomized trial, 480 subjects flagged at least one of the 10 risks related to nutrition and supplement use. Although randomization was not blocked on the presence of a nutrition risk, by chance there were 240 women in both the intervention and control groups who flagged a nutrition risk. Complete outcome data were obtained for 116 intervention and 146 control subjects at 6 months, and 105 intervention and 124 control subjects at 12 months – no significant differences between study arms were detected on baseline covariates (age, Hispanic origin, language, education, employment, income, marital status) amongst subjects missing 6- or 12-month data.
Table 2 shows the sociodemographic and clinical characteristics of the 480 women studied. Participants averaged 27 years old, 89 percent were either a student or worked full or part-time, and 92 percent attended at least some post-secondary school education. There were differences between study arms in the number of subjects identifying English as their primary language (P = 0.024), and the number of subjects accessing a computer primarily from home (P = 0.046) and work (P = 0.038). All subsequent ITT analyses were checked by controlling for these three unbalanced variables.
Table 3 shows the nutrition and supplement risks and the stage of change for each at baseline, 6 months, and 12 months. Of those subjects flagging a nutrition and supplement risk, 77.9% of subjects flagged the unhealthy diet risk behavior, 27.9% flagged >2 servings of fish, 25.2% flagged need omega-3 fatty acids, 62.5% flagged no folic acid supplement, 74.0% flagged no iron supplement, 62.1% flagged no calcium supplement, 50.0% flagged no vitamin D supplement, 19.2% flagged vitamin A supplement use, 61.0% flagged other supplement use, and 76.0% flagged caffeine. Both intervention and control subjects flagged an average of five out of 10 possible nutrition and supplement risks (P = 0.203). The number of risks flagged ranged from 4 at the bottom quartile to 7 at the top quartile.
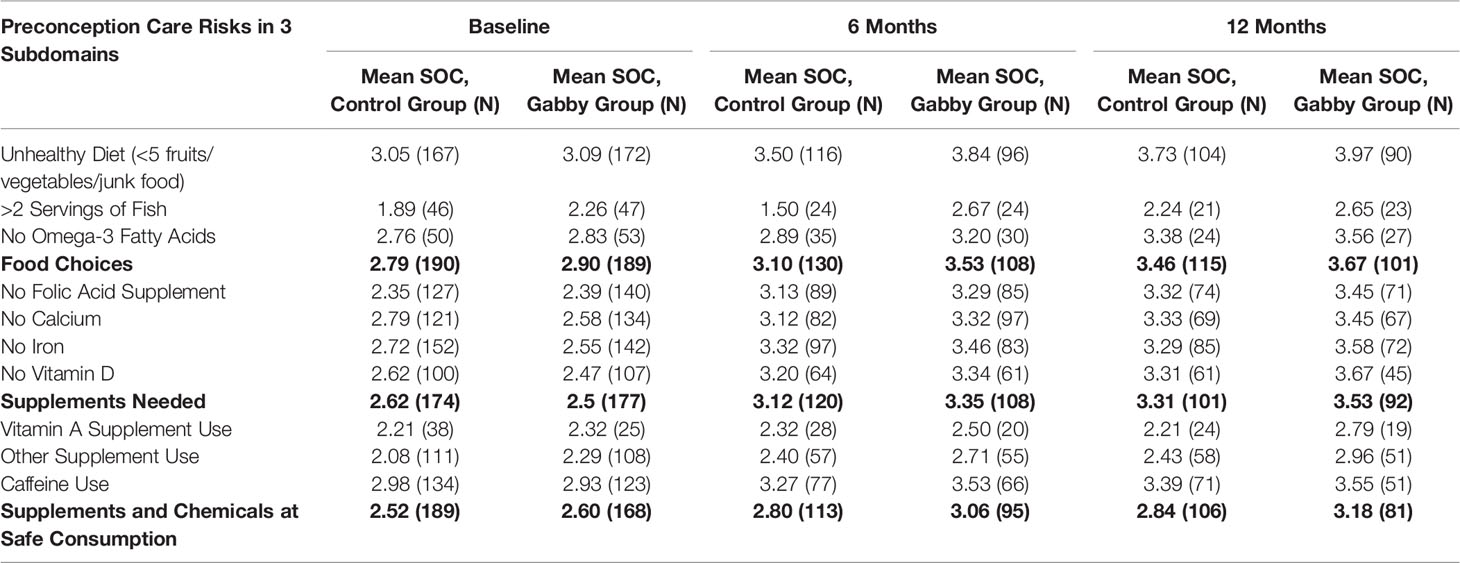
Table 3 Nutrition and supplement risks and mean stage of change (soc) values at baseline, 6 and 12 months.
Although this analysis was not powered to detect a treatment effect on nutrition and supplement risks alone, nevertheless we were able to detect an effect of Gabby at 6 months. As shown in Table 4, at 6 months, women assigned to use Gabby reported moving forward on the stage of change scale for, on average, 52.9% (SD, 35.1%) of the nutrition and supplement risks flagged compared to 42.9% (SD, 35.4%) in the control group (IRR 1.22, 95% CI 1.03–1.45; P = 0.019). Similarly, women assigned to use Gabby reported reaching the action or maintenance stage of change for, on average, 52.8% (SD, 37.1%) of the nutrition and supplement risks flagged compared to 42.8% (SD, 37.9%) in the control group (IRR 1.26, 95% CI 1.08–1.48; P = 0.004).
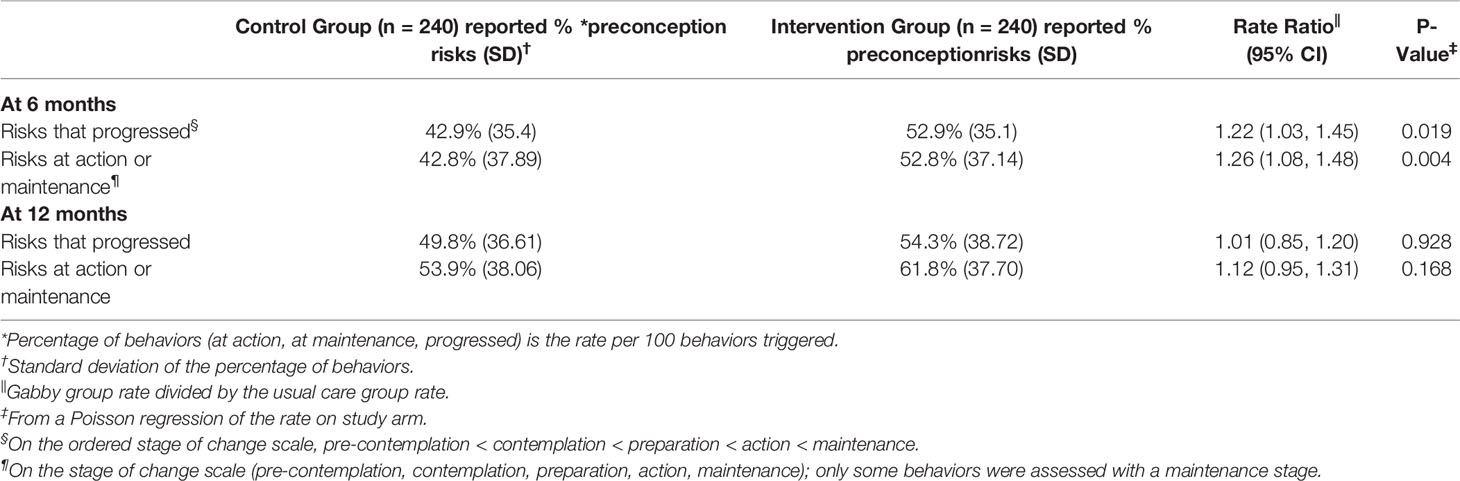
Table 4 Stage of change outcomes for diet and nutrition preconception risks at 6 and 12 months by intervention and control groups.
At 12 months, women interacting with Gabby, on average, reported making forward progress on the stage of change scale for 54.3% (SD, 38.7%) of nutrition and supplement risks compared to 49.8% (SD, 36.6%) in the control group (IRR 1.01, 95% CI 0.85–1.20; P=0.928). Similarly, women assigned to use Gabby, at 12 months, reported reaching the action or maintenance stage of change for 61.8% (SD, 37.7%) of nutrition and supplement risks compared to 53.9% (SD, 38.1%) in the control group (IRR 1.12, 95% CI 0.95–1.31; P = 0.168).
All treatment effects at 6 months remained statistically significant after controlling for primary language, which was unbalanced at baseline.
Among women using Gabby, the behaviors most often progressing forward on the stage of change scale were unhealthy diet, no folic acid supplement, and no iron supplement; these were also the behaviors most often reaching the action or maintenance stages.
Women who used Gabby more often were more effective at addressing their nutrition and supplement risks. When analyzing only those who used Gabby, each additional login was associated with an average increase of 1.01% in the reported rate of achieving the action or maintenance stage or progressing forward at 12 months (P = 0.014). This result remained significant when controlling for education, income, and age. For example, for a woman logging on to Gabby 10 times over the study period, to achieve the outcome for one additional risk, that woman would need to log on an additional 2.4 times per month, on average.
198 of the 240 women enrolled in the intervention group interacted with the entire Gabby system at least once, and the median number of logins was 6 (IQR 8) after 12 months. The median duration of a session with Gabby was 13.7 min (IQR 8.7 min). The median average interaction time (not including the risk assessment) with Gabby was 73.8 min (IQR, 134.6) per woman who used Gabby. The aforementioned data was derived from usage of the entire Gabby system (20); We are unable to report the unique usage data specific to the nutrition and supplement domain.
Figure 2 plots rate ratios with 90% and 95% confidence intervals and across three subdomains of nutrition and supplement risk behaviors and the entire domain. Rate ratios were calculated for the rate of either progressing forward or achieving action or maintenance on the stage of change scale. Because fewer subjects flagged risks within a subdomain than within the broader nutrition and supplement domain, sample sizes for within-subdomain effects are small. Nevertheless, several within-subdomain differences across study arms were of notable magnitude. Within the food choices subdomain, at 6 months, on average, subjects in the intervention group reported reaching the action or maintenance stage of change or progressing forward on the stage of change scale for 11.23% more risk behaviors than subjects in the control group (62.76% versus 49.17%, P = 0.165); at 12 months, the difference was 10.96% (73.33% versus 62.38%, P = 0.401). Similar results were found for the supplements needed subdomain (65.02% versus 58.33% at 6 months, P = 0.219).
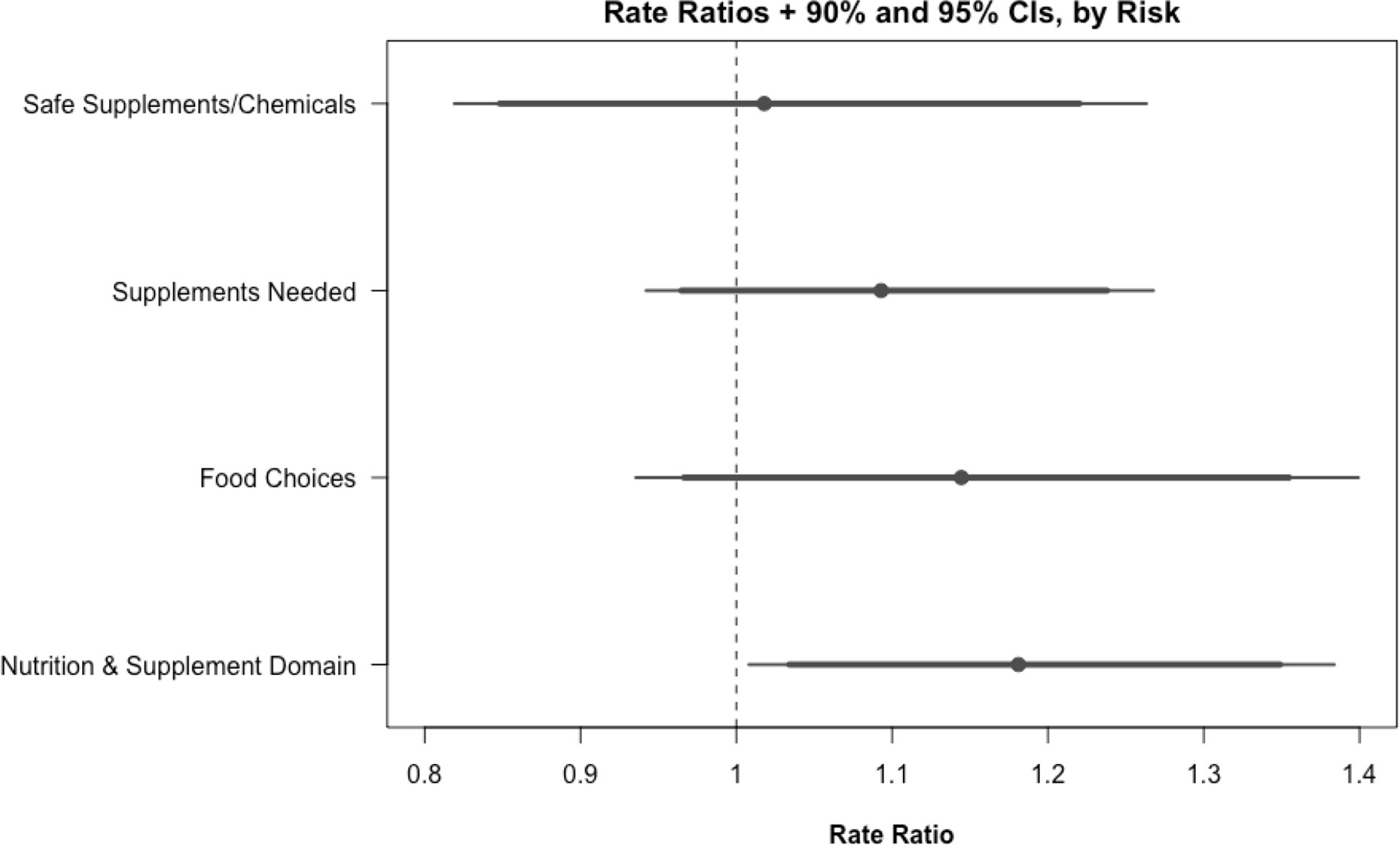
Figure 2 Rate ratios and 90%/95% CIs, by subdomain. Rate ratios and confidence intervals are calculated by regressing the rate of each risk achieving action or maintenance or making progress on the stage of change scale on each study arm using logistic regression.
Figure 3 shows the mean stage of change for the nutrition and supplement domain, subdomain, and individual risks at baseline, at 6 and 12 months for the intervention and control groups. Subjects assigned to the intervention group performed no worse than subjects assigned to the control group on any single risk behavior. For one risk behavior (i.e., having more than two servings of certain types of fish per week) subjects assigned to the intervention group were more likely to achieve action or maintenance or make forward progress than subjects in the control group (OR 5.34, CI 1.42–22.9).
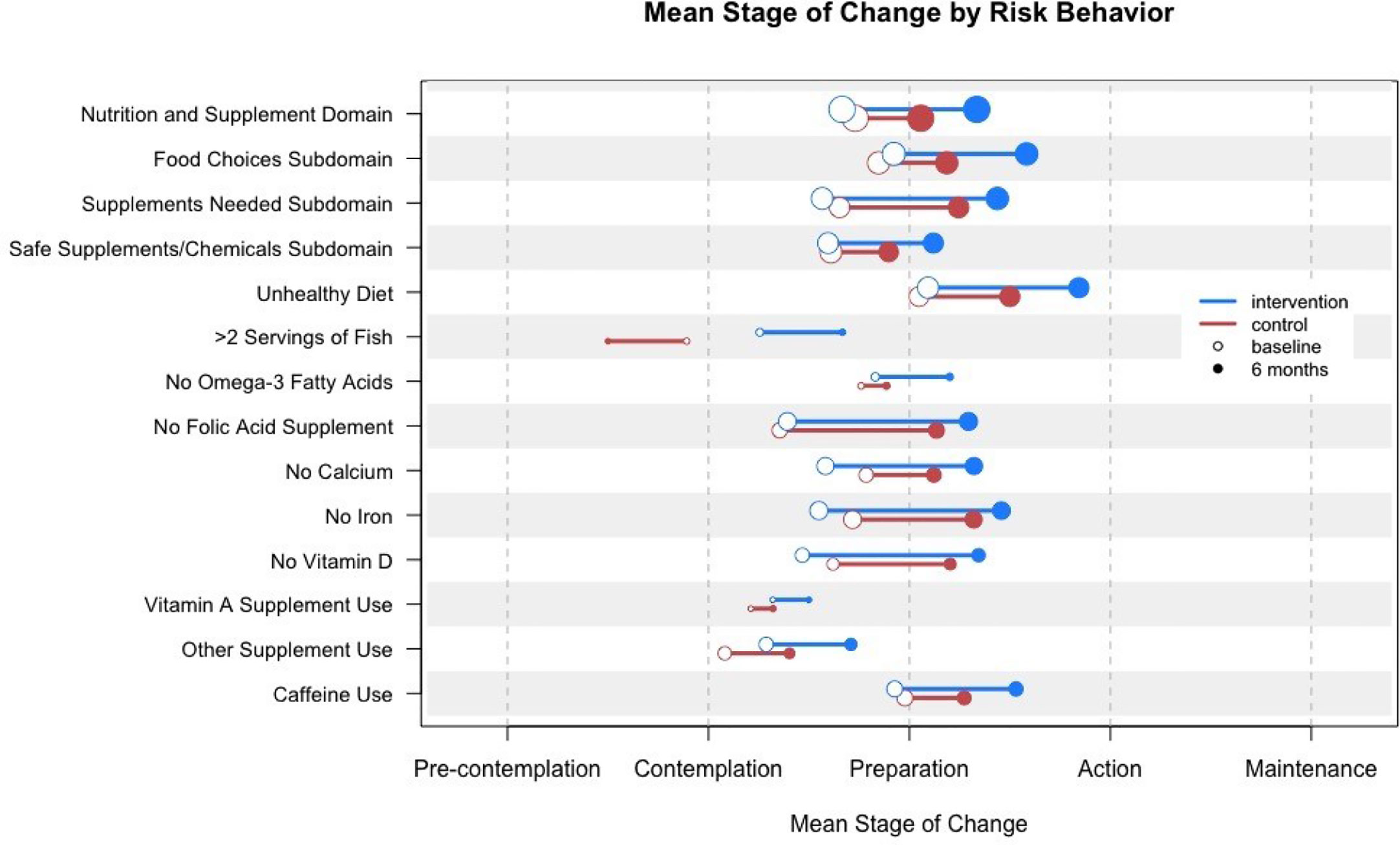
Figure 3 Mean stage of change for nutrition and supplement domain, subdomain, and individual risks at baseline and 12 months for the intervention and control groups. Circle size indicates the relative number of women in the sample who triggered the risk.
Subgroup analysis also showed that the effect of Gabby on addressing nutrition and supplement risks was larger for subjects with higher income (P = 0.029), and for subjects with lower nutrition knowledge (P = 0.065). Gabby was equally effective at addressing nutrition and supplement risks across levels of education, income, and age.
Figure 4 shows that the Gabby intervention had a significant positive effect on the primary outcomes when restricting the data only to risks beginning the study at the contemplation stage of change. For subjects at the contemplation stage at baseline, intervention subjects reported making forward progress for 75.0% (SD, 36.3%) of their health risks compared to 52.1% (SD, 47.1%) in the control group (P < 0.056). Similar results were not found for subjects beginning at the precontemplation, preparation, or action stages of change. Differences in statistical significance across subjects beginning at different stages were unlikely due to differing degrees of freedom, but effects detected for subjects beginning the study at the precontemplation, preparation, and action phases were small in magnitude and may only be detectible with larger sample sizes and increased statistical power. Results were unchanged after controlling for level of education, income, age, and nutrition knowledge.
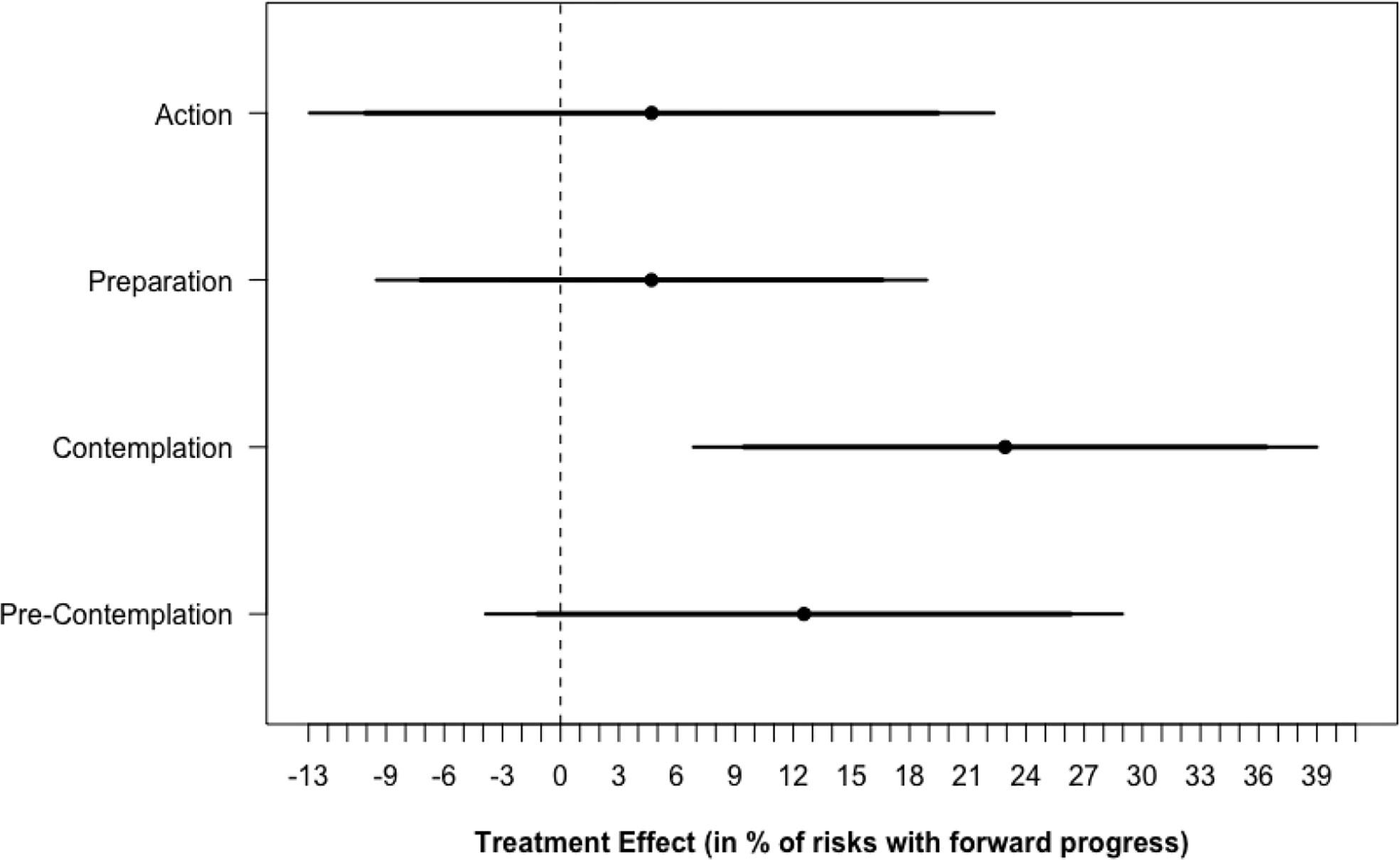
Figure 4 Treatment Effect* by risk stage of change at baseline. * Treatment effects confidence intervals are calculated by restricting the data only to subjects that began the study at each stage of change and regressing the percentage of risks making forward progress on the stage of change scale on study arm using OLS regression.
This secondary analysis shows that among women using Gabby, there was a 22% increase in the average number of preconception nutrition and supplement risks that women reported progressing forward on the stage of change scale at 6 months, and a 26% increase the number of rusks that reach the action or maintenance stages of change. The randomized trial from which these data were collected was powered to demonstrate an impact on our primary outcomes when 102 preconception risks in 13 domains of care were analyzed. The main finding of this study demonstrating the impact of the intervention in a single domain is surprising. The results are especially noteworthy given that the control group received a letter listing their nutrition and supplement risks and suggested they discuss their risks with a clinician, which is an intervention that could be potentially impactful on its own. Our data suggest that Gabby was more effective in addressing nutrition and supplement risks among women with lower nutrition knowledge but equally effective across levels of education, income, and age.
This study serves as one of the first to analyze the dietary habits and supplement use of African American and Black women of childbearing age. Interventions that aim to understand how to change underlying health behaviors that impact maternal and child health outcomes are critical in addressing poor health outcomes, particularly in health disparate populations (28). African American and Black women experience higher rates of unintended pregnancies, complications, and pregnancy-related and infant deaths due to barriers accessing health information and services (29), like gaps in nutritional knowledge and healthy food choices.
Other studies have assessed the impact of various eHealth platforms on nutrition, supplement use, and other preconception risks. Using a coaching platform, the Smarter Pregnancy study conducted in the Netherlands was designed to address the impact of an eHealth intervention on the behavior change of couples who were pregnant or considering pregnancy. After 6 months of usage, there was an increase in vegetable intake, tobacco cessation, and no alcohol consumption among study participants after receiving tips, recommendations, and recipes regarding pregnancy, BMI, and nutrition via text and email (30). Another eHealth study evaluated the SmartMoms intervention designed to manage gestational weight gain among overweight and obese pregnant women. While the in-person and remote groups experienced similar weight gain, the remote group had better adherence to the intervention than the in-person group, suggesting a benefit to mobile health interventions compared to traditional in-person interventions (31). Finally, PEARS, a smartphone application intervention that assessed diet and physical activity behavior change in overweight or obese pregnant women observed that after the intervention, the majority of participants using the application were in the maintenance stage for perceived physical activity (32).
Our data demonstrate that the more time women spent using Gabby, the more effective they were in addressing their nutrition and supplement risks. Gabby provides the opportunity for convenient counseling and advice, as our data show that the total average interaction time with Gabby was over 73 min – substantially longer than a routine annual office visit in many health care systems.
When beginning the study at the contemplation stage, subjects assigned to use Gabby reported making forward progress on 44% more risk behaviors than the control group, indicating that Gabby greatly assisted women who were already considering behavior change. However, despite our efforts to create motivational interviewing dialogue to assist women to progress from precontemplation to contemplation, we did not observe any effect of Gabby on addressing supplement and nutrition risks for this subset of women. No similar effects were detected for subjects beginning the study at the precontemplation, preparation, or action phase. While the study was not powered to detect this change, it could also be due to unsuccessful dialogue content.
This study has several limitations. As a secondary analysis of a randomized trial, the two arms were not blocked on nutrition and supplement risks during randomization. However, our analysis controlled for demographic and clinical variables that were unbalanced at baseline. Second, there were differences in the number of women reporting outcomes at 6 and 12 months. It could be that those women who did not provide outcome data at 6 months did so because they felt it to be not relevant to their current behaviors, though characteristics of women missing 6- and 12-month data were nevertheless balanced across study arms.
A second limitation to this study is that this analysis did not show that the impact on the primary outcomes was maintained at 12 months; however, the fully powered trial from which this secondary analysis is based on showed that the impact on the primary outcomes was maintained at 12 months. It is possible that the lack of maintaining behavior change is the result of an inadequate sample size.
Finally, we do not know the number of minutes that women spent specifically engaging with the nutrition and supplement domain so we cannot extrapolate whether our findings are due to interacting with the entire system or unique content.
Gabby can assist with health risks requiring long-term longitudinal behavior change such as improving nutrition and supplement use. It is an engaging system that provides tailored dialogue, including motivational interviewing and shared decision making, and monitors a woman’s progress over time – providing interaction necessary to address complex and challenging clinical problems like overweight and obesity.
A scalable health information technology tool like Gabby could undoubtedly be an important component of future health education, risk identification and mitigation. Gabby could be used as an adjunct to clinical care to overcome the barrier of clinician time restraints and could be particularly impactful as a population health tool. Gabby allows women to have evidence-based conversations about nutrition and dietary supplement use on their own time without restriction.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Individual participant data will be available, including data dictionaries. Data that will be shared: Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures). 6 and 12 months Stage of Change, System Usage, Risk Assessment, Baseline Data Collection (Demographics and Clinical Characteristics). Study Protocol, Statistical Analysis Plan, Analytic Code (R). RedCap exported data will also be available. Data will be available immediately following publication for a period of 5 years. Data will be shared with researchers who provide a methodologically sound proposal to achieve aims related to primary/secondary outcomes. Proposals should be sent toYmphY2tAYnUuZWR1. To gain access, data requestors will need to sign a data access agreement.
The studies involving human participants were reviewed and approved by the Boston University Institutional Review Board. A waiver of documentation of consent was approved and only verbal consent was obtained. As such written informed consent did not require the subjects signature for study participation in accordance with the national legislation and the institutional requirements.
BJ, PG, CJ, and NS wrote the first draft of the manuscript with input from LY-N, ML, JS, JM-H, and EW. CJ and JM-H conducted study activities. BJ, CJ, EW, and LY-N contributed to Gabby script development. JA contributed to the discussion section of this manuscript with current literature and trends. TB, ZZ, and JF created and programmed the Gabby system and added several features to the updated version. MR did the statistical analysis and wrote corresponding sections of the manuscript. HC oversaw statistical analyses and provided guidance into study-appropriate statistical measures. All authors contributed to the article and approved the submitted version.
Research reported in this publication was supported by the National Institute for Minority Health and Health Disparities grant R01MD006213.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank the following people for their assistance in carrying out this project: Karla Damus, PhD, Haley Kitasato, MPH, Lin Shi, PhD, Jingjing Ren, BEng, Abhishek Nandgaonkar, MS, Dhaval Parmar, PhD, Zack Berwaldt, Brian Penti, MD, Stephen Martin, MD, Megan Hempstead, MPH, Fatima Adigun, MPH, and Divya Mehta, MPH.
1. Stephenson J, Heslehurst N, Hall J, Schoenaker DA, Hutchinson J, Cade JE, et al. Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet (2018) 391(10132):1830–41. doi: 10.1016/S0140-6736(18)30311-8
2. Barker M, Dombrowski SU, Colbourn T, Fall CH, Kriznik NM, Lawrence WT, et al. Intervention strategies to improve nutrition and health behaviours before conception. Lancet (2018) 391(10132):1853–64. doi: 10.1016/S0140-6736(18)30313-1
3. American College of Obstetricians and Gynecologists. “Nutrition During Pregnancy.” (2018). www.acog.org/patient-resources/faqs/pregnancy/nutrition-during-pregnancy.
4. Toivonen KI, Lacroix E, Flynn M, Ronksley PE, Oinonen KA, Metcalfe A, et al. Folic acid supplementation during the preconception period: A systematic review and meta-analysis. Prev Med (2018) 114:1–17. doi: 10.1016/j.ypmed.2018.05.02
5. Gardiner PM, Nelson L, Shellhaas CS, Dunlop AL, Long R, Andrist S, et al. The clinical content of preconception care: nutrition and dietary supplements. Am J Obstet Gynecol (2008) 199(6 Suppl 2):S345–56. doi: 10.1016/j.ajog.2008.10.049
6. Tuomainen H, Cross-Bardell L, Bhoday M, Qureshi N, Kai J. Opportunities and challenges for enhancing preconception health in primary care: qualitative study with women from ethnically diverse communities. BMJ Open (2013) 3(7):e002977. doi: 10.1136/bmjopen-2013-002977
7. Mazza D, Chapman A, Michie S. Barriers to the implementation of preconception care guidelines as perceived by general practitioners: a qualitative study. BMC Health Serv Res (2013) 13(1):36. doi: 10.1186/1472-6963-13-36
8. Gollop CJ. Health information-seeking behavior and older African American women. Bull Med Libr Assoc (1997) 85(2):141–6.
9. Zimmerman MS. Assessing the Reproductive Health-Related Information-Seeking Behavior of Low-Income Women: Describing a Two-Step Information-Seeking Process. J Health Commun (2017) 23(1):72–29. doi: 10.1080/10810730.2017.1411996
10. Rosal MC, Heyden R, Mejilla R, Capelson R, Chalmers KA, Rizzo DePaoli M, et al. A Virtual World Versus Face-to-Face Intervention Format to Promote Diabetes Self-Management Among African American Women: A Pilot Randomized Clinical Trial. JMIR Res Protoc (2014) 24:e54. doi: 10.2196/resprot.3412
11. Valle CG, Deal AM, Tate DF. Preventing weight gain in African American breast cancer survivors using smart scales and activity trackers: a randomized controlled pilot study. J Cancer Surviv (2016) 11:1–. doi: 10.1007/s11764-016-0571-2
12. Bickmore TW, Schulman D, Sidner C. Automated interventions for multiple health behaviors using conversational agents. Patient Educ Couns (2013) 92:142–8. doi: 10.1016/j.pec.2013.05.011
13. Sherifali D, Nerenberg KA, Wilson S, Semeniuk K, Ali MU, Redman LM, et al. The Effectiveness of eHealth Technologies on Weight Management in Pregnant and Postpartum Women: Systematic Review and Meta-Analysis. J Med Internet Res (2017) 19(10):e337. doi: 10.2196/jmir.8006
14. Bickmore TW, Pfeifer LM, Byron D, Forsythe S, Henault L, Jack B, et al. Usability of conversational agents by patients with inadequate health literacy: evidence from two clinical trials. J Health Communication (2010) 15:197–210. doi: 10.1080/10810730.2010.499991
15. Gardiner PM, McCue KD, Negash LM, Cheng T, White LF, Yinusa-Nyahkoon, et al. Engaging women with an embodied conversational agent to deliver mindfulness and lifestyle recommendations: A feasibility randomized control trial. Patient Educ Couns (2017) 100(9):1720–9. doi: 10.1016/j.pec.2017.04.015
16. Gardiner P, Hempstead MB, Ring L, Bickmore T, Yinusa-Nyahkoon L, Tran H. Jack B. Reaching women through health information technology: the Gabby preconception care system. Am J Health Promot (2013) 27(S3):11–20. doi: 10.4278/ajhp.1200113-QUAN-1
17. Jack B, Bickmore T, Hempstead M, Yinusa-Nyahkoon L, Sadikova E, Mitchell S, et al. Reducing Preconception Risks Among African American Women with Conversational Agent Technology. J Am Board Fam Med (2015) 28(4):441–51. doi: 10.3122/jabfm.2015.04.140327
18. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot (1997) 12(1):38–48. doi: 10.4278/0890-1171-12.1.38
19. Glanz K, Rimer BK. Theory at a Glance: A Guide for Health Promotion Practice. Bethesda, MD, USA: US Department of Health and Human Services, National Cancer Institute (2005).
20. Jack BW, Bickmore T, Yinusa-Nyahkoon L, Reichert M, Julce C, Sidduri N, et al. Improving the health of young African American women in the preconception period using health information technology: a randomized controlled trial. Lancet Digital Health (2020) 2(9):E475–E85. doi: 10.1016/S2589-7500(20)30189-8
21. National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes Prevention Program. Diabetes. Diabetes Prevention Program (2008). Available at: https://www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp/Pages/default.aspx (Accessed April 1 2020).
22. World Health Organization. Promoting Fruit and Vegetable Consumption Around the World. Global Strategy on Diet. Available at: http://www.who.int/dietphysicalactivity/fruit/en/ (Accessed April 1 2020).
23. Department of Nutrition. Harvard School of Public Health. Healthy Eating Plate & Healthy Eating Pyramid. The Nutrition Source. Available at: https://www.hsph.harvard.edu/nutritionsource/healthy-eating-plate/ (Accessed April 1 2020).
24. Miller W and Rollnick S. Motivational Interviewing: Preparing People for Change. New York: Guilford Press (2012).
25. Bodenheimer T, Davis C, Holman H. Helping Patients Adopt Healthier Behaviors. Clin Diabetes (2007) 25:66–70. doi: 10.2337/diaclin.25.2.66
26. Shilts MK, Horowitz M, Townsend MS. Goal Setting as a Strategy for Dietary and Physical Activity Behavior Change: A Review of the Literature. Am J Health Promot (2004) 19:81–93. doi: 10.4278/0890-1171-19.2.81
27. Handley M, MacGregor K, Schillinger D, Sharifi C, Wong S, Bodenheimer T. Using Action Plans to Help Primary Care Patients Adopt Healthy Behaviors: A Descriptive Study. J Am Board Fam Med (2006) 19:224–31. doi: 10.3122/jabfm.19.3.224
28. Canady RB, Tiedje LB, Lauber C. Preconception Care & Pregnancy Planning: Voices of African American Women. Am J Matern Child Nurs (2008) 33(2):90–7. doi: 10.1097/01.NMC.0000313416.59118.93
29. For Women & Families, N. Black Women’s Maternal Health: A Multifaceted Approach to Addressing Persistent and Dire Health Disparities. (2018). https://www.nationalpartnership.org/our-work/resources/health-care/maternity/black-womens-maternal-health-issue-brief.pdf.
30. van Dijk MR, Oostingh EC, Koster MPH, Willemsen SP, Laven JSE, Steegers-Theunissen RPM, et al. The use of the mHealth program Smarter Pregnancy in preconception care: rationale, study design and data collection of a randomized controlled trial. BMC Pregnancy Childbirth (2017) 17:46. doi: 10.1186/s12884-017-1228-5
31. Redman LM, Gilmore LA, Breaux J, Thomas DM, Elkind-Hirsch K, Stewart T, et al. Effectiveness of SmartMoms, a Novel eHealth Intervention for Management of Gestational Weight Gain: Randomized Controlled Pilot Trial. JMIR Mhealth Uhealth (2017) 5(9):e133. doi: 10.2196/mhealth.8228
32. Ainscough KM, O’Brien EC, Lindsay KL, Kennelly MA, O'Sullivan EJ, O'Brien OA, et al. Nutrition, Behavior Change and Physical Activity Outcomes From the PEARS RCT-An mHealth-Supported, Lifestyle Intervention Among Pregnant Women With Overweight and Obesity. Front Endocrinol (Lausanne) (2020) 10:938. doi: 10.3389/fendo.2019.00938
Keywords: health information technology, health disparities, preconception care, supplement use, diet, nutrition
Citation: Gardiner P, Bickmore T, Yinusa-Nyahkoon L, Reichert M, Julce C, Sidduri N, Martin-Howard J, Woodhams E, Aryan J, Zhang Z, Fernandez J, Loafman M, Srinivasan J, Cabral H and Jack BW (2021) Using Health Information Technology to Engage African American Women on Nutrition and Supplement Use During the Preconception Period. Front. Endocrinol. 11:571705. doi: 10.3389/fendo.2020.571705
Received: 15 September 2020; Accepted: 30 November 2020;
Published: 19 January 2021.
Edited by:
Qi Chen, The University of Auckland, New ZealandCopyright © 2021 Gardiner, Bickmore, Yinusa-Nyahkoon, Reichert, Julce, Sidduri, Martin-Howard, Woodhams, Aryan, Zhang, Fernandez, Loafman, Srinivasan, Cabral and Jack. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paula Gardiner, UGF1bGEuR2FyZGluZXJAdW1hc3NtZWQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.