
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 22 October 2020
Sec. Clinical Diabetes
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.541090
This article is part of the Research Topic Current Status and Emerging Health Problems Associated with Diabetes, in Asia and in Developing Countries View all 21 articles
 Chin-Hsiao Tseng1,2,3*
Chin-Hsiao Tseng1,2,3*Background: The effect of metformin on leukemia risk remains unknown.
Methods: The Taiwan’s National Health Insurance database was used to enroll 610,089 newly diagnosed type 2 diabetes patients on at least 2 anti-diabetic prescriptions during 1999–2009. We followed-up these patients until 31 December 2011, in order to determine the incidence of leukemia. We used Cox regression model (incorporated with the inverse probability of treatment-weighting using propensity scores) to estimate hazard ratios in both intention-to-treat and per-protocol analyses.
Results: We enrolled 414,783 metformin initiators and 195,306 non-metformin initiators. Among them, 598 and 372 patients developed new-onset leukemia after a median follow-up period of 5.08 years and 6.79 years, respectively. The respective incidence rates were 26.52 and 28.40 per 100,000 person-years. The hazard ratio for metformin initiators versus non-metformin initiators was 0.943 (95% confidence interval 0.828–1.074) in the intention-to-treat analysis and 0.852 (95% confidence interval 0.705–1.031) in the per-protocol analysis. Sensitivity analyses after excluding patients using the exclusion criteria (a follow-up duration < 24 and < 36 months, respectively, patients with incretin-based therapies during follow-up, and patients enrolled during 2 different periods of 1999–2003 and 2004–2009) consistently showed a neutral effect. However, metformin initiators had a significantly higher risk of leukemia in the per-protocol analyses when censoring patients at a time without regular follow-up.
Conclusion: Metformin use has an overall neutral effect on leukemia but we cannot exclude a significantly higher risk in patients who persistently use the drug.
According to a study on the global burden of cancer in 2015, the estimated number of new cases of leukemia was 606,000 and 353,000 deaths were related to leukemia, ranking it as eighth for all cancer incidences and ninth for all cancer deaths, respectively (1). Risk factors include some genetic syndromes, ionizing radiation, some environmental or occupational exposures and medications (2, 3). Diabetes patients may also suffer from a significantly higher risk of leukemia. In 2010, a Swedish study reported a significantly higher risk of leukemia in patients with type 2 diabetes mellitus (T2DM) following hospitalization (4). The estimated standardized incidence ratio compared to the general Swedish population was 1.95 (95% confidence interval: 1.72–2.17) (4). In a meta-analysis of 11 studies, the estimated odds ratio of leukemia for patients with T2DM was 1.22 (95% confidence interval: 1.03–1.44, P = 0.02) (5).
Some in vitro studies suggest that metformin may induce cell cycle arrest and apoptosis in leukemic cells (6, 7), and leukemic cell growth is activated by the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway (8, 9). Therefore, being recognized for its activating effect on the liver kinase B1/adenosine monophosphate kinase (LKB1/AMPK) resulting in the inhibition of mTOR pathway, metformin theoretically inhibits leukemic cell growth (10, 11). However, the involvement of other pathways may also provide a protective effect of metformin on leukemia because a recent in vitro study suggested that metformin may suppress the growth of leukemia cells through the downregulation of AXL receptor tyrosine kinase (12).
Some investigators envisage metformin as a new adjuvant therapeutic agent for the treatment of leukemia (8, 13). However, there is lack of evidence from human studies and several clinical trials are ongoing in order to demonstrate the therapeutic effects of metformin on leukemia in patients with or without diabetes (14). On the other hand, there is paucity of data on whether metformin is preventive for the occurrence of new-onset leukemia or not. The present study investigated the effect of metformin on the risk of leukemia in T2DM patients.
The National Health Insurance (NHI) implemented since March 1995 in Taiwan covers > 99% of the population and has contracts with 93% of medical institutions and all in-hospitals nationwide. The reimbursement database of the NHI and the methods applied in the present study were described in details in previously published papers (15, 16). This database keeps all records of diseases diagnosed, medications prescribed and procedures performed. It serves as an important base for academic research after approval by an ethics review board of the National Health Research Institutes. The approval number of the present study is 99274.
The NHI database coded the different diagnoses using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) during the study period. Diabetes was coded 250.XX and leukemia, 204–208.
Metformin initiators [metformin (+)] and non-metformin initiators [metformin (–)] were defined according to the prescriptions of anti-diabetic drugs after diagnosis during the initial 12-month period (17). Metformin (+) were patients who had been prescribed metformin during this initial 12-month period and metformin (–) were those without any metformin prescription during this period.
Figure 1 shows the procedures used to create metformin (+) and metformin (–). At first, we identified 778,300 newly diagnosed T2DM patients followed-up from 1999 to 2009 and on at least 2 anti-diabetic prescriptions in the outpatient clinics. The following patients were then excluded: patients with type 1 diabetes mellitus (n = 3,667), patients with missing data (n = 2,566) and patients with a diagnosis of any cancer prior to the start of follow-up or within 12 months of follow-up (n = 76,017). We also excluded patients aged < 25 years at the start of follow-up (n = 7,242), patients aged > 75 years at the start of follow-up (n = 62,258), and patients with a follow-up duration < 12 months (n = 16,461). As a result, we included 610,089 patients for analyses. Among them, 414,783 were metformin (+), and 195,306 were metformin (–).

Figure 1 Flowchart showing the procedures followed in creating a cohort of metformin initiators [Metformin (+)] and non-metformin initiators [Metformin (–)] from the reimbursement database of Taiwan’s National Health Insurance.
Table 1 shows the baseline characteristics in metformin (+) and metformin (–). These included demographic data [age, time elapsed since diabetes diagnosis (the time between diabetes diagnosis and the time of the first prescription of anti-diabetic drugs), sex, occupation and living region], major comorbidities (hypertension, dyslipidemia, and obesity), diabetes-related complications (nephropathy, eye disease, stroke, ischemic heart disease, and peripheral arterial disease), diagnoses that may be associated with cancer risk (chronic obstructive pulmonary disease, tobacco abuse, alcohol-related diagnoses, gallstone, history of Helicobacter pylori infection, Epstein-Barr virus-related diagnoses, hepatitis B virus infection, hepatitis C virus infection, diseases of the musculoskeletal system and connective tissue, and human immunodeficiency virus disease) and medications that are commonly used in diabetes patients that affect cancer risk (angiotensin converting enzyme inhibitor/angiotensin receptor blocker, calcium channel blocker, statin, fibrate, and aspirin). We classified the residence and occupation elsewhere in details (18). We coded diseases of musculoskeletal system and connective tissue as ICD-9-CM 710–739 and human immunodeficiency virus disease as 042. The ICD-9-CM codes for other diagnoses can be found in previously published papers (15, 16, 19).
We calculated the standardized difference for each covariate as proposed by Austin and Stuart and a value > 10% was used as an indication for potential confounding in the analyses (20).
We conducted both intention-to-treat and per-protocol analyses to emulate a target trial that compares the risk of leukemia associated to the use of metformin relative to non-metformin anti-diabetic drugs. For intention-to-treat analyses, the numerator of the incidence of leukemia was the number of newly diagnosed cases during the follow-up, and the denominator was the person-years of follow-up. We commenced follow-up at the end of the initial 12-month period for the assessment of metformin (+) and metformin (–), and ended at the diagnosis of leukemia, death or the date of the last medical record by 31 December 2011 (whichever occurred first, with no exclusion according to switching to or adding other anti-diabetic drugs thereafter).
In the per-protocol analyses, we excluded patients who were not adherent to the assigned treatment within the initial 12-month period of exposure. We then followed-up the remainder for the incidence of leukemia. Follow-up started at the end of the 12-month period and ended at the first of the following events by 31 December 2011: leukemia diagnosis, death, the last reimbursement record, or non-adherence to the assigned treatment.
We used Cox regression incorporated with the inverse probability of treatment-weighting using propensity scores (PS) to estimate hazard ratios and their 95% confidence intervals for metformin (+) versus metformin (–). This method was recommended by Austin to reduce the potential confounding effect in the different distribution in characteristics (21). We created PS using the logistic regression model from the start of follow-up plus all baseline characteristics in Table 1. The inclusion of the start of follow-up partly accounted for some unknown risk factors: such as the introduction of novel therapeutic agents or changes in treatment guidelines during the long inclusion period.
Furthermore, we examined the consistency of findings using sensitivity analyses. Firstly, we excluded the interpretation of leukemia cases followed up for < 24 and < 36 months, respectively, in the analyses (as an effect of treatment assignment) (17). Secondly, we excluded patients treated with incretin-based therapies during follow-up to avoid their potential confounding because these therapies were introduced in Taiwan only during the follow-up period. Thirdly, we conducted analyses separately with included participants during two time intervals (1999–2003 and 2004–2009), to avoid further potential effects of some unknown risk factors such as the introduction of novel therapeutic agents and changes in treatment guidelines. Finally, we censored patients at 4 months and 6 months, respectively, after the last prescription. These would have excluded patients who had not received regular refills of anti-diabetic drugs because in Taiwan, the NHI Bureau allows a prescription of not more than 3 months each time. Because metformin use can cause anemia (22–27), associated with a higher risk of leukemia (28–30), prompting the conduction of additional sensitivity analyses after excluding patients who had ever been diagnosed of anemia (ICD-9-CM 280–285).
We performed data analyses using SAS statistical software (version 9.3, SAS Institute, Cary, NC), meanwhile we considered P < 0.05 as statistically significant.
Table 1 displays the baseline characteristics of metformin (–) and metformin (+). We observed standardized difference values > 10% for age, time elapsed since diabetes diagnosis, occupation, dyslipidemia, obesity, eye disease, diseases of the musculoskeletal system, and connective tissue and statin.
The median follow-up duration for metformin (–) was 6.79 years and was 5.08 years for metformin (+) in the intention-to-treat analyses. They were 2.38 and 4.58 years, respectively, in the per-protocol analyses.
Table 2 illustrated the incidence rates of leukemia and the hazard ratios with their 95% confidence intervals comparing metformin (+) versus metformin (–) in the intention-to-treat and the per-protocol analyses. Both analyses suggested a null association between metformin use and leukemia risk. The hazard ratio in the intention-to-treat analysis was 0.943 (95% confidence interval: 0.828–1.074) and was 0.852 (95% confidence interval: 0.705–1.031) in the per-protocol analysis.
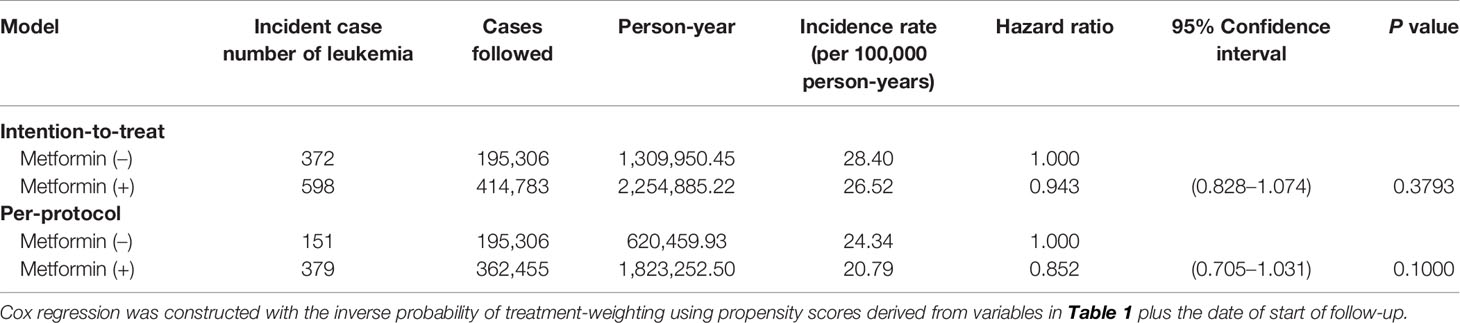
Table 2 Incidence rates of leukemia and hazard ratios comparing metformin initiators versus non-metformin initiators.
Sensitivity analyses in Table 3 supported the finding of a null association in the main analyses displayed in Table 2 (seen above). However, when censoring patients at the time of not receiving regular refills, metformin (+) had a significantly higher risk of leukemia in the per-protocol analyses (Models VI and VII). After excluding patients with a diagnosis of anemia (Model VIII), the results in the analyses did not deviate much from the main analyses shown in Table 2.
This is the first human study investigating the risk of leukemia after metformin use in T2DM patients. Unlike many previous studies that showed beneficial effects of metformin on the prevention of solid cancers (14) and some recent in vitro studies suggesting an inhibitory effect of metformin on the growth of leukemic cells (11, 12), the findings of the present study did not support a beneficial effect of metformin on leukemia in human beings (Tables 2 and 3). In the main analyses, the risk of leukemia was neutral while comparing metformin (+) versus metformin (–) in either the intention-to-treat or the per-protocol analysis (Table 2). However, from the sensitivity analyses in the per-protocol models that censored patients at the time of without regular refills, we observed a significantly increased risk among metformin (+) (Models VI and VII, Table 3). These sensitivity analyses (by including only patients with regular refills and adhering to metformin treatment within the desired follow-up person-years), implied a possible devastating effect of metformin on leukemia.
Deficiency in vitamin B12 (22, 23, 26, 27), folic acid (24), and/or iron (25) is a known potential long-term side effect of metformin treatment and deficiency in these micronutrients has been known to increase the risk of leukemia (28–30). Though not yet clarified, one of the possible explanations for a neutral or even devastating effect of metformin on leukemia is that the beneficial effects observed in in vitro studies (11, 12) could be obliterated by the deficiency in these micronutrients after long-term use of metformin.
Metformin is well known for its activation of AMPK and one of the mechanisms of preventing cancer is through its activation of AMPK resulting in the inhibition of mTOR (10, 11). A recent in vitro study suggested another potential mechanism through the downregulation of the AXL receptor tyrosine kinase (12). However, another recent in vitro and in vivo study suggested that only phenformin but not metformin could delay the development of T cell acute lymphoblastic leukemia/lymphoma (31). It is worth mentioning that some recent studies suggested that AMPK activators can also exert an opposite effect of promoting tumor cell survival through alternative pathways [involving redox regulation to maintain nicotinamide adenine dinucleotide phosphate (NADPH) and inhibit cell death] (32). Therefore, it is possible that metformin may exert anti-leukemic effects on one hand, but counteracted by its pro-survival pathways and its side effect of deficiency in micronutrients. The possible differentiation between solid tumors and leukemia in the activation of signaling pathways by AMPK on either the inhibition of mTOR or the activation of NADPH, could explain the different effects of metformin on the prevention of solid tumors and leukemia seen in previous observational studies. A recent study in a cancer center in New York compared the overall and disease-free survival rates in 924 diabetes patients on metformin with newly diagnosed solid tumors or acute myeloid leukemia. This analysis revealed a lack of metformin benefit in leukemia but a significant benefit for patients with solid tumors (33). The “two-faces” of AMPK on cancer has been an issue under vigorous discussion by some investigators recently (34–36). However, these speculations require further investigation for confirmation.
The present study has several clinical implications. Firstly, in vitro studies usually investigate a specific pathway and ignore the complex actions of and interactions with other biological pathways. Therefore, we cannot readily interpret the findings of metformin benefits on leukemic cells derived from in vitro studies (6, 7, 11, 12). Secondly, an overall neutral effect of metformin on leukemia risk (Table 2) and the potentially higher risk observed in patients in persistent use (per-protocol analyses, Models VI and VII, Table 3), calls for a cautious attention to the ongoing preclinical trials investigating the use of metformin as a therapeutic agent for leukemia (14). Thirdly, the possibility of differential responses to AMPK activators (such as metformin) between solid tumors and leukemia opens a new interesting venue for future research. Finally, though not proven, the hypothetical role of deficiency in vitamin B12, folic acid, or iron in the increased risk of leukemia speculated from the results (Models VI and VII, Table 3), calls for an attention to closely monitor levels of these micronutrients among metformin users. The timing for supplementation of these micronutrients among metformin users is an issue of clinical importance that requires vigorous research.
This study has been conducted with special attention to the potential methodological limitations commonly seen in pharmacoepidemiological studies such as selection bias, prevalent user bias, immortal time bias, and confounding by indication, as discussed previously (37).
Limitations of the study include the lack of measurement data on some potential risk factors such as hemoglobin level, serum concentrations of vitamin B12, folic acid, and iron, anthropometric factors, lifestyle, smoking, alcohol drinking, nutritional status, dietary patterns, family history, genetic markers, ionizing radiation, and environmental/occupational exposures. There are different categories of leukemia but we could not evaluate the effect of metformin on each specific category due to the lack of data. Whether the findings derived from the diabetes patients can be applied to non-diabetes ones, await additional confirmation.
In summary, this is the first observational study evaluating the effect of metformin on the risk of leukemia in humans. The findings suggest an overall neutral effect, but we cannot exclude a significantly higher risk in patients who adhered persistently to the treatment for up to five years. We recommend further studies in other populations and in non-diabetes participants in order to consolidate the findings of this study.
The datasets generated for this study will not be made publicly available because public availability of the dataset is restricted by local regulations to protect privacy. Requests to access the datasets should be directed to C-HT, Y2NrdHNoQG1zNi5oaW5ldC5uZXQ=.
The studies involving human participants were reviewed and approved by the National Health Research Institutes. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
The author confirms being the sole contributor of this work and has approved it for publication.
The study was partly supported by the Ministry of Science and Technology (MOST 107-2221-E-002-129-MY3) of Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health or National Health Research Institutes. The guarantor of this study is C-HT.
1. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol (2017) 3:524–48. doi: 10.1001/jamaoncol.2016.5688
2. Advani SH, Doval DC, Gopal R, Nair CN, Kutty PM. Therapy-related leukemia. A report of five patients and a review of the literature. Oncology (1983) 40:268–72. doi: 10.1159/000225741
3. Schüz J, Erdmann F. Environmental Exposure and Risk of Childhood Leukemia: An Overview. Arch Med Res (2016) 47:607–14. doi: 10.1016/j.arcmed.2016.11.017
4. Hemminki K, Li X, Sundquist J, Sundquist K. Risk of cancer following hospitalization for type 2 diabetes. Oncologist (2010) 15:548–55. doi: 10.1634/theoncologist.2009-0300
5. Castillo JJ, Mull N, Reagan JL, Nemr S, Mitri J. Increased incidence of non-Hodgkin lymphoma, leukemia, and myeloma in patients with diabetes mellitus type 2: a meta-analysis of observational studies. Blood (2012) 119:4845–50. doi: 10.1182/blood-2011-06-362830
6. Rodríguez-Lirio A, Pérez-Yarza G, Fernández-Suárez MR, Alonso-Tejerina E, Boyano MD, Asumendi A. Metformin Induces Cell Cycle Arrest and Apoptosis in Drug-Resistant Leukemia Cells. Leuk Res Treat (2015) 2015:516460. doi: 10.1155/2015/516460
7. Bruno S, Ledda B, Tenca C, Ravera S, Orengo AM, Mazzarello AN, et al. Metformin inhibits cell cycle progression of B-cell chronic lymphocytic leukemia cells. Oncotarget (2015) 6:22624–40. doi: 10.18632/oncotarget.4168
8. Rosilio C, Ben-Sahra I, Bost F, Peyron JF. Metformin: a metabolic disruptor and anti-diabetic drug to target human leukemia. Cancer Lett (2014) 346:188–96. doi: 10.1016/j.canlet.2014.01.006
9. Tabe Y, Tafuri A, Sekihara K, Yang H, Konopleva M. Inhibition of mTOR kinase as a therapeutic target for acute myeloid leukemia. Expert Opin Ther Targets (2017) 21:705–14. doi: 10.1080/14728222.2017.1333600
10. Green AS, Chapuis N, Maciel TT, Willems L, Lambert M, Arnoult C, et al. The LKB1/AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNA translation. Blood (2010) 116:4262–73. doi: 10.1182/blood-2010-02-269837
11. Yuan F, Cheng C, Xiao F, Liu H, Cao S, Zhou G. Inhibition of mTORC1/P70S6K pathway by Metformin synergistically sensitizes Acute Myeloid Leukemia to Ara-C. Life Sci (2020) 243:117276. doi: 10.1016/j.lfs.2020.117276
12. Saito T, Itoh M, Tohda S. Metformin suppresses the growth of leukemia cells partly through downregulation of AXL receptor tyrosine kinase. Leuk Res (2020) 94:106383. doi: 10.1016/j.leukres.2020.106383
13. Biondani G, Peyron JF. Metformin, an anti-diabetic drug to target leukemia. Front Endocrinol (Lausanne) (2018) 9:446. doi: 10.3389/fendo.2018.00446
14. Cunha Júnior AD, Pericole FV, Carvalheira JBC. Metformin and blood cancers. Clinics (Sao Paulo) (2018) 73(suppl 1):e412s. doi: 10.6061/clinics/2018/e412s
15. Tseng CH. Metformin is associated with a lower risk of colorectal cancer in Taiwanese patients with type 2 diabetes: a retrospective cohort analysis. Diabetes Metab (2017) 43:438–45. doi: 10.1016/j.diabet.2017.03.004
16. Tseng CH. Metformin and lung cancer risk in patients with type 2 diabetes mellitus. Oncotarget (2017) 8:41132–42. doi: 10.18632/oncotarget.17066
17. Tsilidis KK, Capothanassi D, Allen NE, Rizos EC, Lopez DS, van Veldhoven K, et al. Metformin does not affect cancer risk: a cohort study in the U.K. clinical practice research datalink analyzed like an intention-to-treat trial. Diabetes Care (2014) 37:2522–32. doi: 10.2337/dc14-0584
18. Tseng CH. Diabetes, metformin use, and colon cancer: A population-based cohort study in Taiwan. Eur J Endocrinol (2012) 167:409–16. doi: 10.1530/EJE-12-0369
19. Tseng CH. Diabetes, insulin use and Helicobacter pylori eradication: a retrospective cohort study. BMC Gastroenterol (2012) 12:46. doi: 10.1186/1471-230X-12-46
20. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med (2015) 34:3661–79. doi: 10.1002/sim.6607
21. Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med (2013) 32:2837–49. doi: 10.1002/sim.5705
22. Chapman LE, Darling AL, Brown JE. Association between metformin and vitamin B(12) deficiency in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab (2016) 42:316–27. doi: 10.1016/j.diabet.2016.03.008
23. Yang W, Cai X, Wu H, Ji L. Associations between metformin use and vitamin B(12) levels, anemia, and neuropathy in patients with diabetes: a meta-analysis. J Diabetes (2019) 11:729–43. doi: 10.1111/1753-0407.12900
24. Maniar K, Moideen A, Mittal A, Patil A, Chakrabarti A, Banerjee D. A story of metformin-butyrate synergism to control various pathological conditions as a consequence of gut microbiome modification: Genesis of a wonder drug? Pharmacol Res (2017) 117:103–28. doi: 10.1016/j.phrs.2016.12.003
25. Stynen B, Abd-Rabbo D, Kowarzyk J, Miller-Fleming L, Aulakh SK, Garneau P, et al. Changes of cell biochemical states are revealed in protein homomeric complex dynamics. Cell (2018) 175:1418–29. doi: 10.1016/j.cell.2018.09.050
26. Iftikhar R, Kamran SM, Qadir A, Iqbal Z, bin Usman H. Prevalence of vitamin B12 deficiency in patients of type 2 diabetes mellitus on metformin: a case control study from Pakistan. Pan Afr Med J (2013) 16:67. doi: 10.11604/pamj.2013.16.67.2800
27. Ko SH, Ko SH, Ahn YB, Song KH, Han KD, Park YM, et al. Association of vitamin B12 deficiency and metformin use in patients with type 2 diabetes. J Korean Med Sci (2014) 29:965–72. doi: 10.3346/jkms.2014.29.7.965
28. Murphy G, Dawsey SM, Engels EA, Ricker W, Parsons R, Etemadi A, et al. Cancer risk after pernicious anemia in the US elderly population. Clin Gastroenterol Hepatol (2015) 13:2282–9.e1-4. doi: 10.1016/j.cgh.2015.05.040
29. Cantarella CD, Ragusa D, Giammanco M, Tosi S. Folate deficiency as predisposing factor for childhood leukaemia: a review of the literature. Genes Nutr (2017) 12:14. doi: 10.1186/s12263-017-0560-8
30. Hung N, Shen CC, Hu YW, Hu LY, Yeh CM, Teng CJ, et al. Risk of cancer in patients with iron deficiency anemia: a nationwide population-based study. PloS One (2015) 10:e0119647. doi: 10.1371/journal.pone.0119647
31. Vara-Ciruelos D, Dandapani M, Russell FM, Grzes KM, Atrih A, Foretz M, et al. Phenformin, but not metformin, delays development of T cell acute lymphoblastic leukemia/lymphoma via cell-autonomous AMPK activation. Cell Rep (2019) 27:690–698.e4. doi: 10.1016/j.celrep.2019.03.067
32. Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature (2012) 485:661–5. doi: 10.1038/nature11066
33. Ceacareanu AC, Nimako GK, Wintrob ZAP. Missing the benefit of metformin in acute myeloid leukemia: a problem of contrast? J Res Pharm Pract (2017) 6:145–50. doi: 10.4103/jrpp.JRPP_17_37
34. Bonini MG, Gantner BN. The multifaceted activities of AMPK in tumor progression–why the “one size fits all” definition does not fit at all? IUBMB Life (2013) 65:889–96. doi: 10.1002/iub.1213
35. Hardie DG. Molecular Pathways: Is AMPK a friend or a foe in cancer? Clin Cancer Res (2015) 21:3836–40. doi: 10.1158/1078-0432.CCR-14-3300
36. Faubert B, Vincent EE, Poffenberger MC, Jones RG. The AMP-activated protein kinase (AMPK) and cancer: many faces of a metabolic regulator. Cancer Lett (2015) 356:165–70. doi: 10.1016/j.canlet.2014.01.018
Keywords: diabetes mellitus, metformin, leukemia, National Health Insurance, Taiwan
Citation: Tseng C-H (2020) Metformin Use and Leukemia Risk in Patients With Type 2 Diabetes Mellitus. Front. Endocrinol. 11:541090. doi: 10.3389/fendo.2020.541090
Received: 07 March 2020; Accepted: 30 September 2020;
Published: 22 October 2020.
Edited by:
Hiroki Mizukami, Hirosaki University, JapanReviewed by:
Sho Osonoi, Hirosaki University, JapanCopyright © 2020 Tseng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chin-Hsiao Tseng, Y2NrdHNoQG1zNi5oaW5ldC5uZXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.