
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 19 August 2020
Sec. Clinical Diabetes
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.00553
This article is part of the Research Topic Achieving Effective Management and Treatment of Diabetes Mellitus in Future Primary Care View all 11 articles
Many patients with type 1 diabetes (T1D) do not achieve the glycemic target goal with insulin treatment. In this study, we aimed to evaluate the efficacy and safety of add-on to insulin therapy in patients with T1D. We conducted direct and indirect network meta-analyses using Bayesian models and ranked hypoglycemic agents via mixed treatment comparison, using data from the CENTRAL, MEDLINE, EMBASE, and Science Citation Index Expanded databases. Randomized controlled trials (RCTs) involving patients with T1D treated with insulin and add-on metformin or sodium-glucose cotransporter inhibitors or glucagon-like peptide-1 receptor agonists from January 1970 to September 2019 were included in this study. Twenty-three RCTs with 5,151 subjects were divided into the following groups: insulin alone, insulin+metformin, insulin+canagliflozin, insulin+dapagliflozin, insulin+empagliflozin, insulin+sotagliflozin, insulin+liraglutide, and insulin+exenatide. HbA1c level in the insulin+sotagliflozin group was significantly lower than that in the insulin alone group (mean difference: −0.43, 95% credible interval: −0.62 to −0.23). Total daily insulin dose in the insulin+sotagliflozin group was significantly lower than that in the insulin alone group. Compared with that in the insulin alone group, body weight in the groups treated with insulin+add-on canagliflozin, sotagliflozin, and exenatide was significantly decreased by 4.5, 2.8, and 5.1 kg, respectively. Hypoglycemic episodes did not differ among the groups. In patients with T1D, insulin+sotagliflozin decreased the HbA1c level, daily insulin dose, and body weight without hypoglycemia compared with insulin monotherapy. Insulin+canagliflozin or insulin+exenatide was effective in reducing body weight compared with insulin alone. In conclusion, sotagliflozin treatment decreased not only the HbA1c levels and insulin dose but also the body weight without causing hypoglycemia in patients with T1D. Treatment with canagliflozin and exenatide effectively reduced body weight in patients with T1D. However, ketoacidosis associated with the use of SGLT inhibitors should be considered in these patients. Thus, our results suggest that sotagliflozin has a high probability of being ranked first as an adjunctive therapy to insulin in patients with T1D.
The incidence of type 1 diabetes (T1D) is continuously increasing. According to a report from the International Diabetes Federation (IDF), T1D currently affects 29 million adults worldwide (1). The IDF reported that the number of children and adolescents with T1D in 2017 was 1,106,500. Moreover, 132,600 patients are newly diagnosed with T1D every year. According to the T1D Exchange Registry data, in more than 70% of patients with T1D, glycated hemoglobin (HbA1c) levels lower than 7% was not achieved (2).
Compared with the treatment of type 2 diabetes (T2D) using various novel medications, that of T1D mostly depends on insulin. Several drugs have been investigated as an adjunct therapy for T1D, but the US FDA approved only pramlintide, which mimics a β-cell hormone that is co-secreted with insulin in the postprandial period, in 2005 (3). However, the effects of pramlintide on HbA1c level and weight changes are mild and unsatisfactory (4).
Metformin is the most studied oral antidiabetic drug used as an adjunct for T1D treatment. It suppresses hepatic gluconeogenesis and increases glucose uptake by muscles via the amplification of glucose transporter 4 (5). Treatment of T1D with metformin reportedly reduces insulin requirement and decreases body mass index (BMI), although the HbA1c level was similar to that of the placebo treatment (6, 7). Dipeptidyl peptidase-4 (DPP-4) inhibitors prolong the half-life of endogenous glucagon-like peptide-1 (GLP-1), which stimulates glucose-dependent insulin secretion and inhibits glucagon release. Although the effect of DPP-4 inhibitors in chronic T1D has not been elucidated, a study reported more than 20 IU reduction in daily insulin dose in newly diagnosed patients with T1D (4). GLP-1 receptor agonists (GLP-1RA), such as liraglutide and exenatide, are also reported to reduce insulin dose and decrease body weight in patients with T1D. However, their effect on HbA1c was not consistent among studies (8–15). Sodium-glucose cotransporter-2 (SGLT-2) inhibitors, which reduce glucose reabsorption in the proximal tubules of the kidney, are one of the most attractive drugs for T2D owing to their beneficial effects on cardiovascular and renal functions (16, 17). Compared with that of insulin administration alone, administration of SGLT-2 inhibitors, such as dapagliflozin, empagliflozin, and canagliflozin, decreased HbA1c level, body weight, and insulin requirement in patients with T1D (18–23). Recently, researchers have focused on SGLT-1/2 co-inhibitors because they can simultaneously inhibit the absorption of sugars in the kidneys and intestine (24). Garg et al. showed that sotagliflozin, an SGLT-1/2 co-inhibitor, decreased HbA1c level in patients with T1D (1).
Several trials with GLP-1 RA, SGLT-2 inhibitors, or SGLT-1/2 co-inhibitors as adjuncts to insulin therapy for T1D have been conducted. However, no conclusive suggestion has been made because of the following reasons: the glucose-lowering efficacy of these agents was not satisfactory; the trials had a small sample size; and most importantly, these agents were only compared with insulin and not with other agents. Therefore, the aim of our study was to identify the most efficient drug as an add-on to insulin therapy in patients with T1D through a network meta-analysis.
The results are presented in accordance with the guidelines of Preferred Reporting Items for Systematic Reviews and Network Meta-Analyses statement (NMA Checklist) (25). All analyses were conducted using previously published studies; therefore, ethical approval and patient consent were not required.
We performed a comprehensive search of the following databases, from the time of the inception of each database until September 2019: MEDLINE (via PubMed), EMBASE, CINAHL, Web of Science, and the Cochrane Central Register of Controlled Trials in the Cochrane Library. The following terms were used to identify RCTs: Metformin or Sodium-Glucose Transport Proteins or Sodium-Glucose Transporter 2 or Sodium-Glucose Transporter 2 Inhibitors or SGLT1/2 inhibitor or Exenatide or Liraglutide and Diabetes Mellitus, Type 1. Detailed search terms are provided in Supplementary Information 1.
The following studies were included in the review: RCTs, reviews, observational studies, and clinical trials. The search was limited to human studies but was not restricted to any particular language or publication date. Reference lists from all available review articles and RCTs were searched manually (Figure 1).
The abstracts and full texts obtained were independently checked by two researchers (YJK and SDH). Any disagreements were resolved through discussions and consultations with another researcher. The inclusion criteria for studies used in the analysis were as follows: (1) randomized controlled studies; (2) studies referring to at least two of the following eligible antidiabetic medications: placebo, metformin, canagliflozin, dapagliflozin, empagliflozin, sotagliflozin, liraglutide, and exenatide; and (3) studies that reported one or more of the primary or secondary outcomes (Figure 2). Treatments with direct comparisons are linked with a line, whose thickness corresponds to the number of trials evaluating that comparison. For example, when insulin is used as a reference, the line comparing insulin and metformin is the boldest indicating that these two interventions are most evaluated, while being solid line (rather than dotted) indicates a direct evaluation. Conversely, dotted lines indicate indirect connections expressed using direct comparison and indirect comparison due to lack of head-to-head study. For example, line comparing insulin sotagliflozin and insulin dapagliflozin indicates no direct study; the indirect connection in the network was, therefore, was calculated. Trials that recruited patients with T2D or latent autoimmune diabetes were excluded.
Two researchers (YJK and SDH) independently assessed the risk of bias of each trial using the Cochrane Collaboration's Risk of Bias tool (26). The risk of bias was assessed during the generation of random sequence, concealment of allocation, blinding of participants and personnel, blinding of outcome assessment, analysis of incomplete outcome data, selective reporting, and in other areas. All these judgments were categorized as “yes” (low risk of bias) or “unclear” or “no” (high risk of bias) (Supplementary Figures 1, 2) (26, 27).
We assessed the overall evidence quality for the primary outcomes using an adapted Grading of Recommendations Assessment, Development, and Evaluation approach (28). The evidence quality for a specific outcome was based on performance vs. limitations of the study design, inconsistency of results, indirectness of evidence, imprecision of results, and publication bias of all studies measuring a particular outcome. The overall evidence quality for the outcome was determined by combining assessments from all domains (Supplementary Figure 3) (29).
We aimed to determine the efficacy of eligible medications on changes in HbA1c level (mean ± standard deviation [from the baseline to endpoint]) as the primary outcome. The occurrence of hypoglycemia, reduction in insulin daily dose, and change in body weight were determined as secondary outcomes. Moreover, the potential for adverse outcomes associated with these medications, including diabetic ketoacidosis, heart failure, stroke, diarrhea, pancreatitis, renal event, urinary tract infection, and genital infection, were investigated.
Bayesian network meta-analysis was performed to compare the efficacy of eight types of diabetes treatments in terms of HbA1c and weight reduction outcomes and adverse outcomes in patients with type 1 diabetes. Direct and indirect network meta-analyses were performed using Bayesian models, and the different agents were ranked by mixed treatment comparison (GeMTC) and using Stata version 13 (StataCorp) (30–32). The relative ranking probability of each treatment was estimated, and the treatment hierarchy of competing interventions was obtained using rankograms, surface under the cumulative ranking curves, and mean ranks. The network meta-analysis was performed on studies evaluating multiple treatments, which allows the estimation of pooled effects within each treatment (33). For multi-arm trials, correlations among the treatment effects among arms were included in the investigations. Studies with j+1 treatment arms were based on comparison of the treatment effects with the reference treatment effects through multivariate normal distribution, whereas treatment-as-usual studies were based on the homogeneity among study variances across treatments (34, 35). Inconsistency tests, homogeneity analysis, and sensitivity analysis were performed using the node analysis method in R software (The R Foundation for Statistical Computing c/o Institute for Statistics and Mathematics, Vienna, Austria). The results of inconsistency tests were assessed according to the Bayesian p-value, where the results with p < 0.5 were considered an evidence for the existence of significant inconsistency (36, 37). An I2 test was performed (I2 > 50% indicated significant heterogeneity) to assess homogeneity. Furthermore, a sensitivity analysis was conducted by comparing the differences between fixed-effect and random-effect models. The clinical outcome indicators were evaluated using the mean difference or odds ratio (OR) with a 95% credible interval (CrI) (mean difference for continuous outcomes and OR for binary outcomes) (34, 38). When a loop connected three treatments, it was possible to evaluate the inconsistency between direct and indirect evidence (39). We also used the node-splitting method to calculate the inconsistency of the model, which separated evidence for a particular comparison into direct and indirect evidence (37). Subsequently, the agreement between direct and indirect evidence was evaluated, and its Bayesian p-value was obtained. Sensitivity analyses were carried out using the same methods, after the omission of data obtained from specific studies (studies with a small number of patients and events in a specific treatment arm and studies with a large population that may dominate the data of specific treatment arms) (40).
In total, 23,267 records were initially retrieved from the electronic database search; of these, 12,361 duplicate records were removed. Among the remaining records, 10,829 were excluded based on a review of either the title or abstract and 77 records were retrieved for full-text review. Among these studies, 54 were excluded based on the following criteria: contained wrong dose of medication (n = 7), duplicated data (n = 9), contained patients with T2D (n = 17), review articles (n = 7), contained patients with liver cirrhosis and on dialysis (n = 4), contained adults with latent autoimmune diabetes (n = 5), editorial comment (n = 3), and failed to extract subject event (n = 2) (Figure 1).
Finally, 23 trials reporting outcomes for 5,151 patients (2,610 women and 2,541 men) were included in the analysis (Table 1). The average study duration was 30.8 ± 14.5 weeks. The trials were conducted in the following countries: the United States (1, 9, 11, 18, 23, 48, 51, 52) Denmark (12, 13, 42, 44), Canada (21, 41), Italy (46, 49), Austria (20), Belgium (14), Chile (47), France (45), Germany (50), India (10), and United Kingdom (1 each) (43). The number of patients per study ranged from 12 to 1,402, and the mean follow-up period was 17.01 (range, 11.5–38.0) years (Table 1).
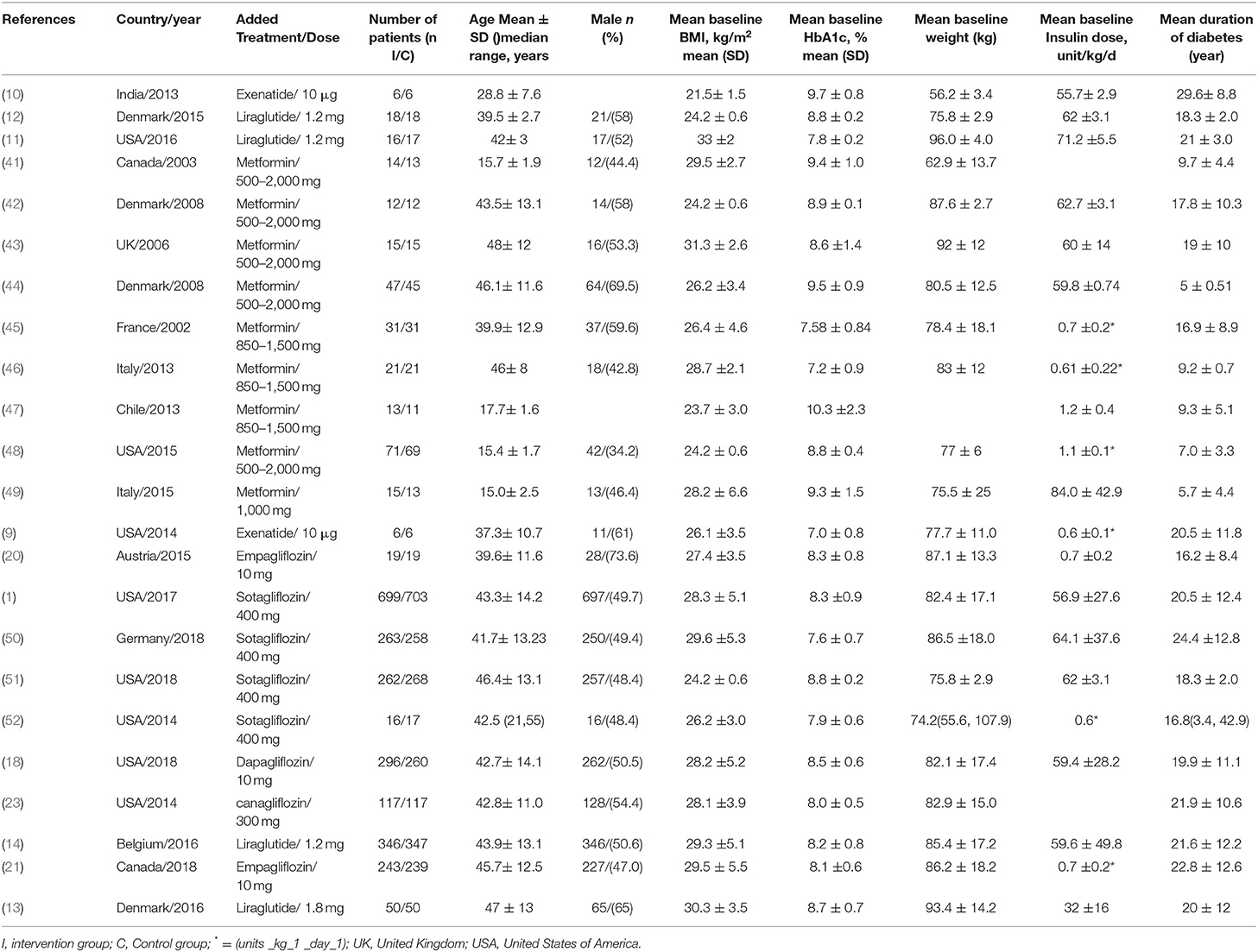
Table 1. Important characteristics of the included studies and proportions of patients with using type 1 treatment.
Although all included studies were described as randomized, a few studies provided specific details of either the method of randomization or concealment of allocation. For all the included studies, blinding had been done adequately.
Data obtained from all the 23 studies (n = 5,151) were subjected to the network analysis. The primary endpoint was a change in HbA1c level. Compared with the insulin alone treatment as the reference, sotagliflozin treatment significantly reduced the HbA1c level (MD: −0.43, 95% CrI: −0.62 to −0.23) (Figure 3A). However, canagliflozin (−0.28, 95% CrI:−0.65 to 0.11), dapagliflozin (−0.37, 95% CrI: −0.75 to 0.01), empagliflozin (−0.15, 95% CrI: −0.43 to 0.13), metformin (−0.12, 95% CrI: −0.28 to 0.03), liraglutide (−0.20, 95% CrI: −0.41 to 0.03), and exenatide (−0.42, 95% CrI: −0.88 to 0.06) showed no significant changes in HbA1c compared with insulin alone. Among the studies with sotagliflozin, a study by Sands et al. had a noticeably short study treatment duration (29 days) (52). The sensitivity analysis was performed after excluding this study and showed that sotagliflozin therapy reduced HbA1c level significantly (Supplementary Figure 4 and Supplementary Tables 2, 3).
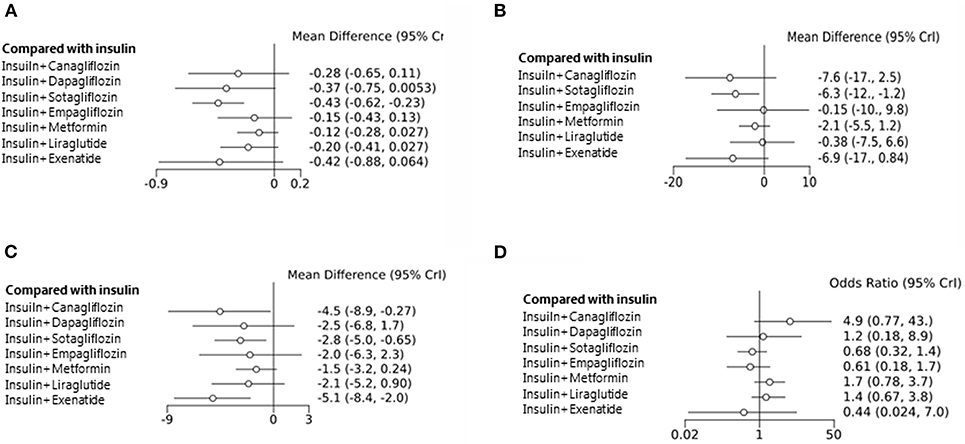
Figure 3. Mean change in HbA1c level from the baseline (A). Mean change in daily insulin dose from the baseline (B). Mean change in body weight from the baseline (C). Hypoglycemic events (D) associated with different types of treatment compared with the placebos used as the reference.
We further analyzed the total insulin daily dose (TIDD), weight change, and adverse effects as the secondary endpoints. Among the eight studied agents, sotagliflozin decreased the TIDD compared with insulin alone, whereas the other drugs showed no change in the TIDD (MD: −6.3 IU, 95% CrI: −12 to −1.20) (Figure 3B). A decrease in body weight from the baseline was observed after treatment with canagliflozin (−4.5 kg, 95% CrI: −8.90 to −0.27), sotagliflozin (−2.8, 95% CrI: −5.0 to −0.65), and exenatide (−5.1, 95% CrI: −8.4 to −2.0) (Figure 3C). However, the frequency of hypoglycemia was not significantly different among the intervention groups (Figure 3D).
Diabetic ketoacidosis (DKA) is one of the most serious adverse effects observed in patients with type 1 diabetes. In the present study, DKA was more frequently observed with canagliflozin (OR = 18.0, 95% CrI: 1.5 to 6.7e+0.2) and sotagliflozin (OR = 6.9, 95% CrI: 2.0 to 29.0) treatments.
Other adverse events, such as myocardial infarction, heart failure, stroke, hospitalization, peripheral artery disease, diarrhea, pancreatitis, renal event, and urinary tract infection or genital infection, did not differ among the medication groups. These side effects were similar to those of only sotagliflozin subgroup, which showed the best effect. Compared to placebo, the event of acidosis, defined as lactic acidosis, metabolic acidosis, renal tubular acidosis, and uremic acidosis, in the sotagliflozin group was approximately 2.82 times higher (Odds ratio: 2.82, 95% CI: 1.87 to 4.26). Using meta-analysis, diabetic ketoacidosis (DKA) was also observed in patients, more frequently with sotagliflozin (OR = 5.91, 95% CI: 2.45 to 14.2) treatment group (Supplementary Figure 5).
In terms of changes in the HbA1c level as the primary outcome, network meta-analysis can statistically rank the outcomes by measuring their probability. Figure 4A shows several probabilities in Rank 1, indicating that the interventions will be ranked first in the network flow. The highest probability of insulin exenatide was 0.369 but was not statistically significant. However, the next probability, insulin sotagliflozin, was statistically significant in the network meta-analysis, and the probability of being first in the rankogram was 0.277, which was the highest reduction in the HbA1c level among the treatments (Figure 4A). Model fit was assessed by comparing deviance information criterion and residual deviance. Deviance information criterion (DIC) measures the deviance, estimated by the posterior mean of minus twice the log-likelihood plus the effective number of parameters in the model. The DIC measures the model fit that penalizes model complexity—lower DIC values suggest a more parsimonious model. The DIC and residual deviance for HbA1c were 90.0 and 46.6, respectively (Figure 4B). The model that analyzed HbA1c showed a small DIC number of <150, therefore, the change was minimized, and the model could be selected as an appropriate model.
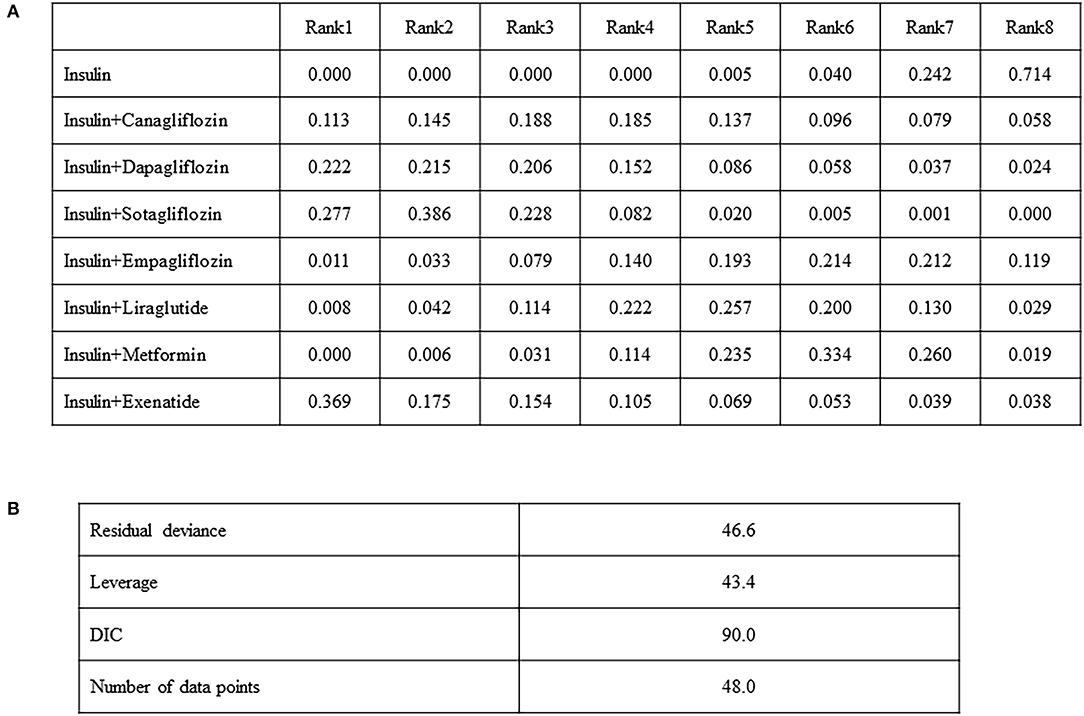
Figure 4. Comparison of the included diabetes treatments for HbA1c, odds ratio (95% CI). Each cell indicates the effect of the column-defining intervention relative to the row-defining intervention (A), model fit statistics (B).
The rank probabilities of mean change in body weight from the baseline for add-on drugs were in the following order: exenatide (0.482), canagliflozin (0.290), and sotagliflozin (0.169) (Supplementary Figure 6A). Model fit statistic of DIC of any weight reduction was 63.2 and the residual deviance was 33.8 (Supplementary Figure 7A).
In the present study, we evaluated the effect and safety of adding oral hypoglycemic agents (metformin, SGLT2 inhibitor, or SGLT1/2 co-inhibitor) or injectable GLP-1 RAs to insulin therapy in patients with T1D. Among these agents, sotagliflozin add-on therapy was found to be the most effective in reducing HbA1c levels. Treatment with canagliflozin, sotagliflozin, and exenatide decreased the body weight (a secondary outcome) by 4.5, 2.8, and 5.1 kg, respectively. The TIDD was significantly decreased in the sotagliflozin treatment group (6.3 IU/day) compared with that in the insulin monotherapy group. Hypoglycemic episodes and other adverse events did not differ between the groups. These data suggest that sotagliflozin and short-acting GLP-1RA and SGLT inhibitor add-on therapies could have beneficial effects in lowering the HbA1c level, insulin dose, and body weight in patients with T1D undergoing insulin treatment.
In previous clinical trials, early intensive glucose control in patients with T1D was reported to reduce all-cause mortality and prevent or delay late microvascular and macrovascular complications of diabetes (53). Therefore, insulin therapy is essential for T1D, but weight gain is a major concern. According to an analysis of physician electronic health records in the United States, 47.8% of people with T1D were found to be obese (54). Obesity is associated with insulin resistance and increased cardiovascular complications. Patients with T1D with more than two complications have significantly higher BMI than those with less than one complication (55).
Another important issue in the management of T1D is glycemic variability. A recent study demonstrated that variability in the HbA1c level was significantly and additively associated with mortality in participants (>13 years old) with T1D (56). Potential underlying mechanisms are unclear, but the variability in HbA1c could result in a poor response to insulin therapy or hypoglycemia. In a study on 1,706 adolescents with T1D, HbA1c variability significantly increased the risk of retinopathy, albuminuria, and cardiac autonomic neuropathy (57). Oxidative stress and systemic inflammation induced by inflammatory cytokines have been hypothesized as a potential mechanism underlying the association between glycemic variability and increased risk of diabetic complications (58, 59).
Taken together, intensive insulin treatment is essential for preventing diabetic complications caused by hyperglycemia, but it is associated with adverse effects, such as weight gain, hypoglycemia, and hyperglycemia, which cause low compliance leading to glycemic variability. In addition, the risk of hyperglycemia or hypoglycemia decreases the quality of life of patients with T1D (60).
Therefore, considerable effort has been made for better glycemic control without hypoglycemia using new antidiabetic medications. SGLT2 inhibitors target the proximal tubular SGLT2 transport protein, which is responsible for ~90% of renal glucose reabsorption (16). Glucosuria caused by SGLT2 inhibition can result in a caloric loss of 250–300 kcal/day and, consequently, a weight loss of 2–3 kg. Given their insulin-independent mechanism, SGLT2 inhibitors have been used in several studies on T1D. In the Empagliflozin as Adjunctive to Insulin Therapy-2 and−3 studies, empagliflozin add-on to insulin improved glycemic control and weight change without increasing hypoglycemia in patients with T1D (21). However, adjudicated DKA occurred more frequently with 10 mg (4.3%) and 25 mg empagliflozin (3.3%). In the DEPICT-1 study, dapagliflozin treatment significantly reduced HbA1c by 0.42–0.45%, body weight by 2.96–3.72%, and TIDD by 8.8–13.2% after 24 weeks (19). DKA rates were higher after dapagliflozin treatment than after placebo treatment. Canagliflozin treatment also showed a similar efficacy in HbA1c reduction and body weight control, but the incidence of DKA requiring hospitalization was significantly increased with canagliflozin treatment compared with placebo treatment (22).
Sotagliflozin is a novel dual inhibitor of SGLT1 and SGLT2 that can reduce glucose absorption in the proximal intestine. SGLT1 inhibition was shown to increase the delivery of glucose to the distal small intestine and augment GLP-1 release (16, 61). In a phase III RCT of sotagliflozin administered in combination with insulin to 1,402 adults with T1D, 24 weeks of treatment with sotagliflozin decreased the HbA1c level by 0.46%, body weight by 2.98 kg, and insulin dose by 2.8 U/day (1). However, sotagliflozin treatment was associated with a higher rate of ketoacidosis (3.0%) than placebo (0.6%).
The present study data revealed that sotagliflozin add-on to insulin improved glycemic control and decreased weight. These positive effects may be related to an improvement in glucose variability. Although the clinical role of SGLT1 inhibition at therapeutic doses is unlikely, the beneficial effects of sotagliflozin on glycemic improvement and weight loss in the present study suggest that a more marked inhibition of SGLT1 should be explored.
Canagliflozin, which also inhibits SGLT1, was also effective in body weight reduction in patients with T1D. A study showed that canagliflozin inhibited intestinal glucose absorption at a concentration 10-times the IC50 of SGLT1 in the intestinal lumen (62). A recent randomized trial in patients with type 2 diabetes showed that pre-meal administration of canagliflozin increased the plasma GLP-1 levels (63). These findings suggest that canagliflozin has a positive role in weight reduction in patients with T1D.
A brief report on cardiovascular effects of exenatide add-on therapy in 69 metformin-treated patients with T2D showed a significant reduction in total body fat mass, trunk fat mass, and waist circumference compared with the insulin glargine therapy. According this study, treatment with exenatide for 1 year reduced body weight (6%), waist circumference (5%), and total body (11%) and truncal fat mass (13%) (64).
A recent prospective, randomized study investigated exenatide or glargine add-on therapy in 37 overweight or obese patients with T2D, who were inadequately treated with metformin. After 16 weeks, the exenatide treatment group had lower body weight (−4.5 kg), BMI (−1.6 kg/m2), body fat mass, and percent fat mass (except for gynoid fat) than the insulin glargine group. Weight loss by exenatide was mainly owing to reduced body fat content rather than lean tissue (65).
A study on metformin-treated patients with T2D showed that 1 year treatment with exenatide reduced the total body fat mass by 6% and the waist circumference by 5% compared with the insulin glargine-treated patients (37). In another recent prospective, randomized study in overweight or obese patients with T1D, 16 weeks of treatment with exenatide significantly decreased the body weight by 4.5 kg and BMI by 1.6 kg/m2 compared with insulin glargine treatment (38). Moreover, exenatide resulted in weight loss mainly by reducing body fat but not lean tissue mass. The findings of our study suggest that sotagliflozin has potential benefits of HbA1c reduction and weight loss, whereas canagliflozin and exenatide have a potential benefit of weight loss in patients with T1D.
Our study had several strengths. A traditional meta-analysis can compare only two groups based on one intervention, which is a limitation. However, our network meta-analysis is a complement method to the groups, interventions, or conflict interests that are difficult to be directly compared with each other. In this study, we performed an indirect analysis that can explain the accuracy of model by 20,000 repetitive learning in computer and rank among interventions by comparing several groups at the same time. This is pivotal to guideline development for T1D since, in the absence of head-to-head evidence, guideline development groups will rely more strongly on expert opinion. Hence, they may make comparisons that do not adequately account for potential biases in study designs, intervention characteristics, and study populations. Indirect comparisons connect treatments via a common control or comparator (e.g., a placebo like insulin or a standard of care) thus having a comparative effect between treatments that have not been compared head-to-head in randomized controlled trials. Another benefit of this analysis is that it comprises a simultaneous analysis of all potential treatment options and makes full use of the available evidence within a single analysis. Doing so, provides a more concise assessment of the clinical landscape and enables better decision-making. This analysis can be helpful in selecting add-on drugs for a specific condition. Secondly, this is the first network meta-analysis involving the SGLT1/2 inhibitor, sotagliflozin. Finally, we exclusively included well-designed RCTs. Therefore, less accurate studies were excluded, and the results were less biased to increase reliability.
Our study also had some limitations. Some of the trials included had a relatively small number of participants, and they were conducted for a short duration. Thus, the assessment of long-term outcomes such as cardiovascular events and renal complications was not performed. Another limitation is that the sotagliflozin study included HbA1c reduction results with an insufficient drug duration, which may weaken the findings. However, the sensitivity analysis proved that sotagliflozin therapy reduced HbA1c level significantly in patients with T1D.
In conclusion, sotagliflozin treatment decreased not only the HbA1c levels and insulin dose but also the body weight without causing hypoglycemia in patients with T1D. Treatment with canagliflozin and exenatide was effective in body weight reduction in patients with T1D. However, when we performed a meta-analysis using only four studies, including sotagliflozin, the sotagliflozin group had an increased risk of acidosis and diabetic ketoacidosis compared to the placebo. Therefore, adverse effects associated with SGLT inhibitors should be considered in these patients.
In March 2019, the US FDA rejected the use of sotagliflozin as an adjunct to insulin for the treatment of T1D. The decision followed a split vote in January 2019 by the FDA's Endocrinologic and Metabolic Drugs Advisory Committee, during which panel members expressed concerns over an increased risk of ketoacidosis with the drugs used for T1D. However, after 1 month, the European Commission approved sotagliflozin for prescription in the European Union for certain overweight patients with T1D. According to the results of our network meta-analysis, we suggest that sotagliflozin has a high probability of being ranked first as an adjunctive therapy to insulin in patients with T1D. However, to avoid ketoacidosis and other adverse events, risk mitigation strategies, such as continuation of insulin and discontinuation of SGLT inhibitors on sick days, should be strictly implemented when these drugs are introduced for patients with T1D (66).
All datasets presented in this study are included in the article/Supplementary Material.
YK, SH, and SL: conceptualization, methodology and data acquisition, data analysis and interpretation, statistical analysis, writing—original draft preparation, writing—review and editing, and funding acquisition. All authors contributed to the article and approved the submitted version.
This network meta-analysis was supported by grants from the Basic Science Research Program through the Bio & Medical Technology Development Program of the National Research Foundation (NRF) and funded by the Korean government (MSIT) (SDH: NRF-2019M3E5D1A02069619). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (SDH: 2020R1F1A1073560).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.00553/full#supplementary-material
1. Garg SK, Henry RR, Banks P, Buse JB, Davies MJ, Fulcher GR, et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med. (2017) 377:2337–48. doi: 10.1056/NEJMoa1708337
2. Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care. (2015) 38:971–8. doi: 10.2337/dc15-0078
3. Chapman I, Parker B, Doran S, Feinle-Bisset C, Wishart J, Strobel S, et al. Effect of pramlintide on satiety and food intake in obese subjects and subjects with type 2 diabetes. Diabetologia. (2005) 48:838–48. doi: 10.1007/s00125-005-1732-4
4. Frandsen CS, Dejgaard TF, Madsbad S, Holst JJ. Non-insulin pharmacological therapies for treating type 1 diabetes. Expert Opin Pharmacother. (2018) 19:947–60. doi: 10.1080/14656566.2018.1483339
5. Wiernsperger NF, Bailey CJ. The antihyperglycaemic effect of metformin: therapeutic and cellular mechanisms. Drugs. (1999) 58(Suppl. 1):31–82. doi: 10.2165/00003495-199958001-00009
6. Al Khalifah RA, Alnhdi A, Alghar H, Alanazi M, Florez ID. The effect of adding metformin to insulin therapy for type 1 diabetes mellitus children: a systematic review and meta-analysis. Pediatr Diabetes. (2017) 18:664–73. doi: 10.1111/pedi.12493
7. Liu C, Wu D, Zheng X, Li P, Li L. Efficacy and safety of metformin for patients with type 1 diabetes mellitus: a meta-analysis. Diabetes Technol Ther. (2015) 17:142–8. doi: 10.1089/dia.2014.0190
8. Wang W, Liu H, Xiao S, Liu S, Li X, Yu P. Effects of insulin plus glucagon-like peptide-1 receptor agonists (GLP-1RAs) in treating type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Ther. (2017) 8:727–38. doi: 10.1007/s13300-017-0282-3
9. Sarkar G, Alattar M, Brown RJ, Quon MJ, Harlan DM, Rother KI. Exenatide treatment for 6 months improves insulin sensitivity in adults with type 1 diabetes. Diabetes Care. (2014) 37:666–70. doi: 10.2337/dc13-1473
10. Kumar KVSH, Shaikh A, Prusty P. Addition of exenatide or sitagliptin to insulin in new onset type 1 diabetes: a randomized, open label study. Diabetes Res Clin Pract. (2013) 100:e55–8. doi: 10.1016/j.diabres.2013.01.020
11. Kuhadiya ND, Dhindsa S, Ghanim H, Mehta A, Makdissi A, Batra M, et al. Addition of liraglutide to insulin in patients with type 1 diabetes: a randomized placebo-controlled clinical trial of 12 weeks. Diabetes Care. (2016) 39:1027–35. doi: 10.2337/dc15-1136
12. Frandsen CS, Dejgaard TF, Holst JJ, Andersen HU, Thorsteinsson B, Madsbad S. Twelve-week treatment with liraglutide as add-on to insulin in normal-weight patients with poorly controlled type 1 diabetes: a randomized, placebo-controlled, double-blind parallel study. Diabetes Care. (2015) 38:2250–7. doi: 10.2337/dc15-1037
13. Dejgaard TF, Frandsen CS, Hansen TS, Almdal T, Urhammer S, Pedersen-Bjergaard U, et al. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. (2016) 4:221–32. doi: 10.1016/S2213-8587(15)00436-2
14. Mathieu C, Zinman B, Hemmingsson JU, Woo V, Colman P, Christiansen E, et al. Efficacy and safety of liraglutide added to insulin treatment in type 1 diabetes: the ADJUNCT ONE treat-to-target randomized trial. Diabetes Care. (2016) 39:1702–10. doi: 10.2337/dc16-0691
15. Ahrén B, Hirsch IB, Pieber TR, Mathieu C, Gómez-Peralta F, Hansen TK, et al. Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the ADJAUNCT TWO randomized trial. Diabetes Care. (2016) 39:1693–701. doi: 10.2337/dc16-0690
16. Wright EM, Loo DDF, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. (2011) 91:733–94. doi: 10.1152/physrev.00055.2009
17. Kluger AY, Tecson KM, Barbin CM, Lee AY, Lerma EV, Rosol ZP, et al. Cardiorenal outcomes in the CANVAS, DECLARE-TIMI 58, and EMPA-REG OUTCOME trials: a systematic review. Rev Cardiovasc Med. (2018) 19:41–9. doi: 10.31083/j.rcm.2018.02.907
18. Dandona P, Mathieu C, Phillip M, Hansen L, Tschöpe D, Thorén F, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT-1 52-week study. Diabetes Care. (2018) 41:2552–9. doi: 10.2337/dc18-1087
19. Dandona P, Mathieu C, Phillip M, Hansen L, Griffen SC, Tschöpe D, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. (2017) 5:864–76. doi: 10.1016/S2213-8587(17)30308-X
20. Pieber TR, Famulla S, Eilbracht J, Cescutti J, Soleymanlou N, Johansen OE, et al. Empagliflozin as adjunct to insulin in patients with type 1 diabetes: a 4-week, randomized, placebo-controlled trial (EASE-1). Diabetes Obes Metab. (2015) 17:928–35. doi: 10.1111/dom.12494
21. Rosenstock J, Marquard J, Laffel LM, Neubacher D, Kaspers S, Cherney DZ, et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care. (2018) 41:2560–9. doi: 10.2337/dc18-1749
22. Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care. (2015) 38:2258–65. doi: 10.2337/dc15-1730
23. Rodbard HW, Peters AL, Slee A, Cao A, Traina SB, Alba M. The effect of canagliflozin, a sodium glucose cotransporter 2 inhibitor, on glycemic end points assessed by continuous glucose monitoring and patient-reported outcomes among people with type 1 diabetes. Diabetes Care. (2017) 40:171–80. doi: 10.2337/dc16-1353
24. Musso G, Gambino R, Cassader M, Paschetta E. Efficacy and safety of dual SGLT 1/2 inhibitor sotagliflozin in type 1 diabetes: meta-analysis of randomised controlled trials. BMJ. (2019) 365:l1328. doi: 10.1136/bmj.l1328
25. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
26. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
27. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. (2004) 328:1490. doi: 10.1136/bmj.328.7454.1490
28. Furlan AD, Pennick V, Bombardier C, van Tulder M, Editorial Board CBRG. 2009 updated method guidelines for systematic reviews in the cochrane back review group. Spine. (2009) 34:1929–41. doi: 10.1097/BRS.0b013e3181b1c99f
29. Bae JM, Choi YY, Park CO, Chung KY, Lee KH. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. (2013) 132:110–7. doi: 10.1016/j.jaci.2013.02.044
30. Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS ONE. (2013) 8:e76654. doi: 10.1371/journal.pone.0076654
31. Liu Y, Wang W, Zhang A-B, Bai X, Zhang S. Epley and Semont maneuvers for posterior canal benign paroxysmal positional vertigo: a network meta-analysis. Laryngoscope. (2016) 126:951–5. doi: 10.1002/lary.25688
32. van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. (2016) 7:80–93. doi: 10.1002/jrsm.1167
33. Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. (2005) 331:897–900. doi: 10.1136/bmj.331.7521.897
34. Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. (2004) 23:3105–24. doi: 10.1002/sim.1875
35. Higgins JP, Whitehead A. Borrowing strength from external trials in a meta-analysis. Stat Med. (1996) 15:2733–49.
36. Du S, Ye J, Chen H, Li X, Lin Q. Interventions for Treating 3- or 4-part proximal humeral fractures in elderly patient: a network meta-analysis of randomized controlled trials. Int J Surg. (2017) 48:240–6. doi: 10.1016/j.ijsu.2017.09.002
37. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. (2010) 29:932–44. doi: 10.1002/sim.3767
38. Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. (2011) 64:163–71. doi: 10.1016/j.jclinepi.2010.03.016
39. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. (1997) 50:683–91. doi: 10.1016/S0895-4356(97)00049-8
40. Wu H-Y, Huang J-W, Lin H-J, Liao W-C, Peng Y-S, Hung K-Y, et al. Comparative effectiveness of renin-angiotensin system blockers and other antihypertensive drugs in patients with diabetes: systematic review and Bayesian network meta-analysis. BMJ. (2013) 347:f6008. doi: 10.1136/bmj.f6008
41. Hamilton J, Cummings E, Zdravkovic V, Finegood D, Daneman D. Metformin as an adjunct therapy in adolescents with type 1 diabetes and insulin resistance: a randomized controlled trial. Diabetes Care. (2003) 26:138–43. doi: 10.2337/diacare.26.1.138
42. Jacobsen IB, Henriksen JE, Beck-Nielsen H. The effect of metformin in overweight patients with type 1 diabetes and poor metabolic control. Basic Clin Pharmacol Toxicol. (2009) 105:145–9. doi: 10.1111/j.1742-7843.2009.00380.x
43. Khan ASA, McLoughney CR, Ahmed AB. The effect of metformin on blood glucose control in overweight patients with type 1 diabetes. Diabet Med. (2006) 23:1079–84. doi: 10.1111/j.1464-5491.2006.01966.x
44. Lund SS, Tarnow L, Astrup AS, Hovind P, Jacobsen PK, Alibegovic AC, et al. Effect of adjunct metformin treatment in patients with type-1 diabetes and persistent inadequate glycaemic control. A randomized study. PLoS ONE. (2008) 3:e3363. doi: 10.1371/journal.pone.0003363
45. Meyer L, Bohme P, Delbachian I, Lehert P, Cugnardey N, Drouin P, et al. The benefits of metformin therapy during continuous subcutaneous insulin infusion treatment of type 1 diabetic patients. Diabetes Care. (2002) 25:2153–8. doi: 10.2337/diacare.25.12.2153
46. Pitocco D, Zaccardi F, Tarzia P, Milo M, Scavone G, Rizzo P, et al. Metformin improves endothelial function in type 1 diabetic subjects: a pilot, placebo-controlled randomized study. Diabetes Obes Metab. (2013) 15:427–31. doi: 10.1111/dom.12041
47. Codner E, Iñíguez G, López P, Mujica V, Eyzaguirre FC, Asenjo S, et al. Metformin for the treatment of hyperandrogenism in adolescents with type 1 diabetes mellitus. Horm Res Paediatr. (2013) 80:343–9. doi: 10.1159/000355513
48. Libman IM, Miller KM, DiMeglio LA, Bethin KE, Katz ML, Shah A, et al. Effect of Metformin added to insulin on glycemic control among overweight/obese adolescents with type 1 diabetes: a randomized clinical trial. JAMA. (2015) 314:2241–50. doi: 10.1001/jama.2015.16174
49. Nwosu BU, Maranda L, Cullen K, Greenman L, Fleshman J, McShea N, et al. A randomized, double-blind, placebo-controlled trial of adjunctive metformin therapy in overweight/obese youth with type 1 diabetes. PLoS ONE. (2015) 10:e0137525. doi: 10.1371/journal.pone.0137525
50. Danne T, Cariou B, Banks P, Brandle M, Brath H, Franek E, et al. HbA(1c) and hypoglycemia reductions at 24 and 52 weeks with sotagliflozin in combination with insulin in adults with type 1 diabetes: the European inTandem2 study. Diabetes Care. (2018) 41:1981–90. doi: 10.2337/dc18-0342
51. Buse JB, Garg SK, Rosenstock J, Bailey TS, Banks P, Bode BW, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the north American in Tandem1 study. Diabetes Care. (2018) 41:1970–80. doi: 10.2337/dc18-0343
52. Sands AT, Zambrowicz BP, Rosenstock J, Lapuerta P, Bode BW, Garg SK, et al. Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, as adjunct therapy to insulin in type 1 diabetes. Diabetes Care. (2015) 38:1181–8. doi: 10.2337/dc14-2806
53. Nathan DM, Group DER. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. (2014) 37:9–16. doi: 10.2337/dc13-2112
54. Bae JP, Lage MJ, Mo D, Nelson DR, Hoogwerf BJ. Obesity and glycemic control in patients with diabetes mellitus: analysis of physician electronic health records in the US from 2009-2011. J Diabetes Complicat. (2016) 30:212–20. doi: 10.1016/j.jdiacomp.2015.11.016
55. Rydén A, Sörstadius E, Bergenheim K, Romanovschi A, Thorén F, Witt EA, et al. The humanistic burden of type 1 diabetes mellitus in Europe: examining health outcomes and the role of complications. PLoS ONE. (2016) 11:e0164977. doi: 10.1371/journal.pone.0164977
56. Wightman SS, Sainsbury CAR, Jones GC. Visit-to-visit HbA1c variability and systolic blood pressure (SBP) variability are significantly and additively associated with mortality in individuals with type 1 diabetes: an observational study. Diabetes Obes Metab. (2018) 20:1014–7. doi: 10.1111/dom.13193
57. Virk SA, Donaghue KC, Cho YH, Benitez-Aguirre P, Hing S, Pryke A, et al. Association between HbA1c variability and risk of microvascular complications in adolescents with type 1 diabetes. J Clin Endocrinol Metab. (2016) 101:3257–63. doi: 10.1210/jc.2015-3604
58. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. (2010) 107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545
59. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. (2002) 287:2563–9. doi: 10.1001/jama.287.19.2563
60. Ceriello A, Monnier L, Owens D. Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol. (2019) 7:221–30. doi: 10.1016/S2213-8587(18)30136-0
61. Zambrowicz B, Freiman J, Brown PM, Frazier KS, Turnage A, Bronner J, et al. LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo-controlled trial. Clin Pharmacol Ther. (2012) 92:158–69. doi: 10.1038/clpt.2012.58
62. Dominguez Rieg JA, Rieg T. What does sodium-glucose co-transporter 1 inhibition add: Prospects for dual inhibition. Diabetes Obes Metab. (2019) 21(Suppl. 2):43–52. doi: 10.1111/dom.13630
63. Takebayashi K, Hara K, Terasawa T, Naruse R, Suetsugu M, Tsuchiya T, et al. Effect of canagliflozin on circulating active GLP-1 levels in patients with type 2 diabetes: a randomized trial. Endocr J. (2017) 64:923–31. doi: 10.1507/endocrj.EJ17-0065
64. Bunck MC, Diamant M, Eliasson B, Cornér A, Shaginian RM, Heine RJ, et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care. (2010) 33:1734–7. doi: 10.2337/dc09-2361
65. Yin T-T, Bi Y, Li P, Shen S-M, Wang W-M, Jiang C, et al. Effects of exenatide versus insulin glargine on body composition in overweight and obese T2DM patients: a randomized controlled trial. Nutr Metab. (2018) 15:67. doi: 10.1186/s12986-018-0295-6
Keywords: SGLT inhibitor, GLP-1 receptor agonist, type 1 diabetes, add on to insulin therapy, body weight, glycemic level
Citation: Kim YJ, Hwang SD and Lim S (2020) Effects of Sodium-Glucose Cotransporter Inhibitor/Glucagon-Like Peptide-1 Receptor Agonist Add-On to Insulin Therapy on Glucose Homeostasis and Body Weight in Patients With Type 1 Diabetes: A Network Meta-Analysis. Front. Endocrinol. 11:553. doi: 10.3389/fendo.2020.00553
Received: 22 March 2020; Accepted: 06 July 2020;
Published: 19 August 2020.
Edited by:
Indah Suci Widyahening, University of Indonesia, IndonesiaCopyright © 2020 Kim, Hwang and Lim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soo Lim, bGltc29vQHNudS5hYy5rcg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.