- 1Center for Reproductive Medicine, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Department of Gynecology and Obstetrics, Tianjin Medical University General Hospital, Tianjin, China
- 3National Research Center for Assisted Reproductive Technology and Reproductive Genetics, Jinan, China
- 4Key Laboratory of Reproductive Endocrinology of Ministry of Education, Shandong University, Jinan, China
Polycystic ovary syndrome is characterized by reproductive and metabolic disturbances throughout the female lifespan. Therefore, this study aimed to determine whether genome-wide association studies (GWAS)-identified risk variants for PCOS could confer risk of metabolic syndrome (MS) or insulin resistance (IR). Fifteen independent SNPs mapping to 11 GWAS loci genotyped in a total of 2,082 Han Chinese women independent of previous GWAS and phenotype-genotype correlations were assessed. The CC group for rs12478601 in THADA was associated with decreased rate of MS after adjustment for age (23.2 vs. 27%, P = 0.042, OR = 0.81). Using a dominant model, the GG+AG group for rs2059807 in INSR was associated with increased risk of MS after adjustment for age (26.8 vs. 22.5%, P = 0.023, OR = 1.27). The GG + GT group for rs4784165 in TOX3 was found to be associated with an increased rate of IR after adjustment for age and BMI(53.3 vs. 48.5%, P = 0.027, OR = 1.27). The GG+AG group for rs2479106 in DENND1A was associated with a decreased rate of IR (48.3 vs. 53.6%, adjusted P = 0.039, OR = 0.80). After exclusion of PCOS cases with a family history of diabetes, hypertension, or dyslipidemia, the phenotype-genotype correlations between the genes INSR and TOX3 and MS or IR were still significant (P < 0.05). Three SNPs (rs13429458 in THADA, rs10818854 in DENND1A, and rs2059807 in INSR) were significantly associated with IR; however, their association was not significant after adjustment for age and BMI. This genotype-phenotype study thus provides clues that THADA, INSR, TOX3, and DENND1A play a role in PCOS possibly through a metabolic disorder-related pathway.
Introduction
Polycystic ovary syndrome (PCOS) is a common disorder estimated to affect 5–10% of women of reproductive age and 5.6% of Chinese women (1). PCOS is characterized by clustering of reproductive (oligo-/anovulation, menstrual irregularity, hirsutism, persistent acne, hyperandrogenism, infertility, and pregnancy complications) and metabolic disturbances (metabolic syndrome, type II diabetes mellitus, and cardiovascular disease) across the female lifespan (2).
Insulin resistance (IR) is universally accepted as a key pathophysiological feature of PCOS and metabolic syndrome (MS), regardless of obesity. IR is observed in approximately 50–70% of women with PCOS (3) and with more severity than that in women without PCOS. The common description of MS includes elevated waist circumference, increased triglycerides, decreased HDL-C, elevated blood pressure, and elevated fasting glucose according to the Adult Treatment Panel III criteria (4). Among women with PCOS, the prevalence of MS is reported to be 8.2% in Italy (5), 26.8% in China (1), and 43% in the USA (6). Meta-analysis of 16 studies, including both BMI and non-BMI matched studies, showed that women with PCOS had a 2-3 times higher risk of MS than women without PCOS (7).
Twin and familial studies revealed a strong evidence for the genetic basis of PCOS and IR. Increased prevalence of IR was observed in the first-degree relatives of women with PCOS (8). Candidate gene approaches identified associated genes for PCOS that are mainly involved in insulin-signaling and androgen-related pathways but lack consistent replication. Our group performed the first two genome-wide association studies (GWAS) in Han Chinese women and reported 11 susceptibility loci (15 risk variants) for PCOS (9, 10), including INSR, THADA, LHCGR, FSHR, C9orf3, DENND1A, YAP1, RAB5B, HMGA2, TOX3, and SUMO1P1. In this study, we conducted a phenotype-genotype correlation analysis to investigate the impact of these 15 SNPs on MS and IR in women with PCOS.
Methods
Subjects
The independent cohort consisted of 2,082 Han Chinese women with PCOS, recruited from the Center for Reproductive Medicine, Shandong University. PCOS was defined using the 2003 Rotterdam PCOS consensus criteria (11), which required two of the following conditions: oligo-ovulation or anovulation (OA, menstrual cycle length >35 days), clinical or biochemical hyperandrogenism (HA, Ferriman-Gallwey score ≥ 6 or circulating total testosterone ≥60 ng/dl) (9), and polycystic ovarian morphology (PCO, on ultrasound at least 12 follicles in one ovary and/or increased ovarian volume > 10 mL). Cases with congenital adrenal hyperplasia, androgen-secreting tumors, Cushing's syndrome, thyroid disease, and hyperprolactinemia were excluded. Among the 2,082 subjects, 157 had at least one parent with diabetes, 549 had at least one parent with hypertension, and 1 had at least one parent with dyslipidemia. Individuals who were taking medications such as oral contraceptives and metformin during the last 3 months were also excluded.
The study was approved by the Institutional Review Board of Center for Reproductive Medicine Shandong University and written informed consent was obtained from all participants.
Clinical and Biochemical Measurement
All subjects were assessed for family history, blood pressure, height, weight, and waist circumference. The body mass index (BMI) was calculated as weight (kg)/height (m)2. Fasting blood samples were obtained during days 2–4 of the menstrual cycle to examine circulating serum levels of hormones such as total testosterone (T). Fasting plasma glucose levels were measured using the hexokinase method, and insulin levels were measured using the electrochemiluminescence method. Homeostasis model assessment (HOMA-IR) was calculated as fasting glucose (mmol/L) × fasting insulin (mIU/L) / 22.5, and 2.69 was used as the cut-off point for IR (12). HOMA-B was calculated as 20 × fasting insulin (mIU/L) / (fasting glucose (mmol/L) - 3.5) %. Serum triglycerides (TG) and high-density lipoprotein (HDL) were detected using enzymatic methods.
MS was defined by the 2005 Adult Treatment Panel III criteria (4), which required at least three of the following criteria: waist circumference ≥80 cm, serum TG ≥1.7 mmol/l, serum HDL cholesterol <1.3 mmol/l or the use of lipid lowering medication; blood pressure ≥130/85 mmHg or the use of anti-hypertensive medication, and fasting plasma glucose ≥5.6 mmol/l.
SNP Genotyping
Genomic DNA was extracted from whole peripheral blood by a standard process using the QIAamp DNA mini kit (Qiagen). All 15 Chinese PCOS risk SNPs were genotyped using the Sequenom MassArray (Beijing, China).
Statistical Analysis
Quantitative variables of clinical characteristics of the PCOS subjects were displayed as mean ± SD. Statistical analysis was performed using SPSS22.0 for Windows (SPSS, Inc., Chicago, IL, USA) and a P < 0.05 was considered statistically significant.
Genetic models were divided into additive (+/+ vs. +/– vs. –/–), dominant (+/+ plus +/– vs. –/–), and recessive (+/+ vs. +/– plus –/–). In the genotype-phenotype analysis, an appropriate genetic model was selected considering the small numbers in the homozygous minor allele groups. Categorical variables were compared using Pearson's chi-square (χ2) test and the results were adjusted for age and BMI using logistic regression. The odds ratios (ORs) were modeled to analyze the risk variants of MS and IR in PCOS and 95% confidence intervals (95% CIs) were presented.
Results
Clinical Features
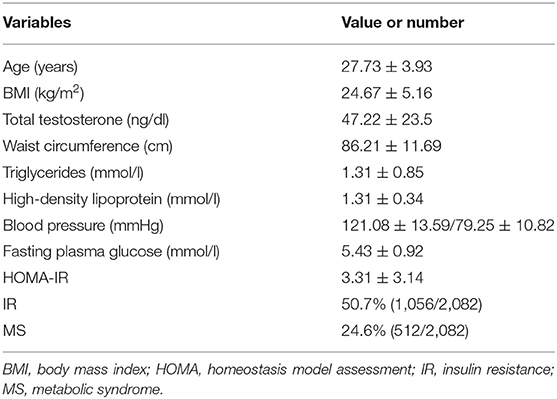
The clinical characteristics of 2,082 PCOS subjects are displayed in Table 1. The average age of these women was 27.73 years and the average BMI was 24.67 kg/m2. The mean serum level of total testosterone was 47.22 ng/dl. The prevalence of MS in women with PCOS was 24.6% and the prevalence of IR was 50.7%. The frequency of the different components of MS was as follows: elevated waist circumference (38.1%), increased triglycerides (20.8%), decreased HDL-C (56.2%), elevated blood pressure (13.9%), and elevated fasting glucose (30.0%).
MS and IR Rate in the PCOS Subgroup With or Without Hyperandrogenism (HA)
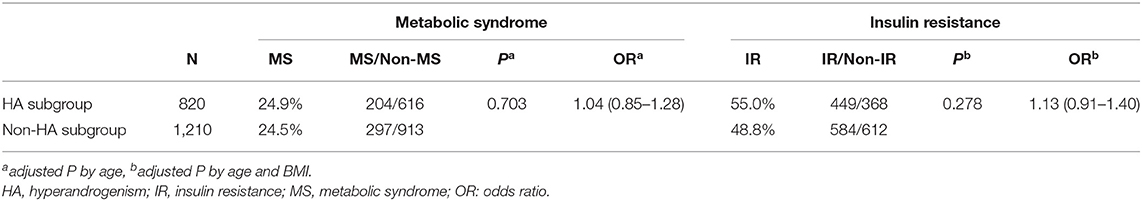
Except for 52 cases with HA status unavailable, 820 cases presented with HA (including 27 HA + OA, 36 HA + PCO and 757 HA + OA + PCO), whereas 1,210 cases presented without HA (including 1,210 OA + PCO). In PCOS women with or without the HA subgroup, the rates of MS were similar after adjustment for age (24.9 vs. 24.5%, P = 0.703 OR = 1.04, Table 2). The rate of IR in the HA group was higher than that in the non-HA group, but did not reach significant levels (55 vs. 48.8%, P = 0.278 OR = 1.13, Table 2).
Genotype-Phenotype Analysis of 15 SNPs and MS
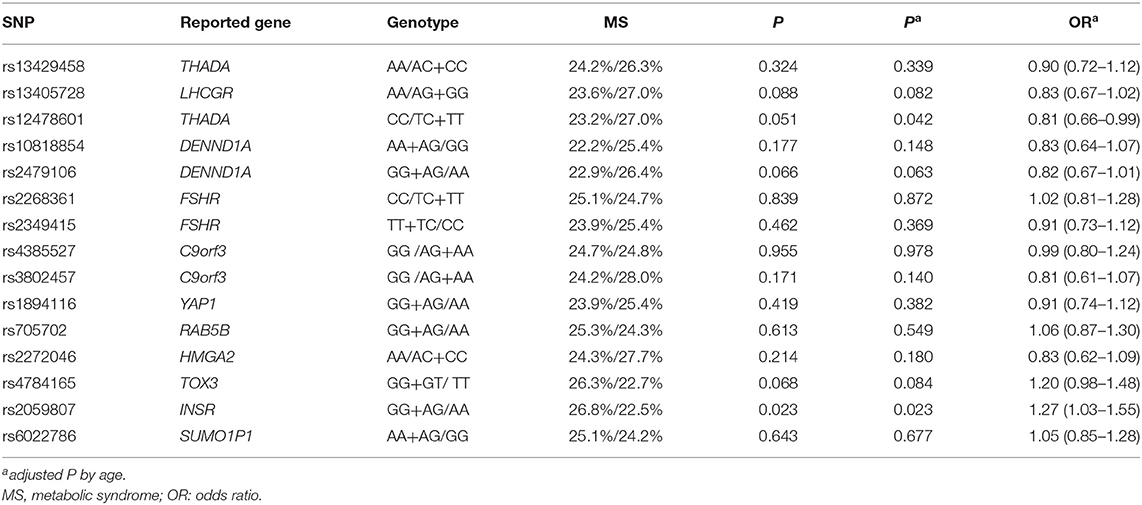
The allele frequencies of 15 SNPs are shown in Supplementary Table 1. For phenotypic and genotypic assessment, an appropriate dominant or recessive genetic model was selected considering the small numbers in the homozygous minor allele groups (Table 3), and the additive model was also performed in Supplementary Table 2. Using a recessive model, the CC group for rs12478601 in THADA was associated with decreased rate of MS (23.2 vs 27%, P = 0.051), and the association was significant after adjustment for age (P = 0.042, OR = 0.81). Using a dominant model, the rate of MS was significantly higher in the GG + AG group for rs2059807 in INSR than in the AA group (26.8 vs. 22.5%, P = 0.023, OR = 1.26, Table 3), even after adjustment for age (adjusted P = 0.023, OR = 1.27), indicating that the risk genotype of rs2059807 was robustly associated with MS in PCOS cases. After exclusion of PCOS cases with a family history of diabetes or hypertension or dyslipidemia, the phenotype-genotype correlations between the gene INSR and MS were still significant, whereas the correlation between THADA and MS was not significant (rs2059807, age-adjusted P = 0.005, OR = 1.43; rs12478601, age-adjusted P = 0.067, OR = 0.79). No association between the genotype of rs12478601, rs2059807 and waist circumference, blood pressure, TG, HDL-C were identified (Supplementary Table 3). The rate of MS in the AA group for rs2479106 in DENND1A, and the GG+GT group for rs4784165 in TOX3, were all slightly higher than that in another genotype group using dominant/recessive model (26.4 vs. 22.9%, P = 0.066; 26.3 vs. 22.7%, P = 0.068, Table 3). For rs4784165, the results were similar using additive model (Supplementary Table 2).
Genotype-Phenotype Analysis of 15 SNPs and IR
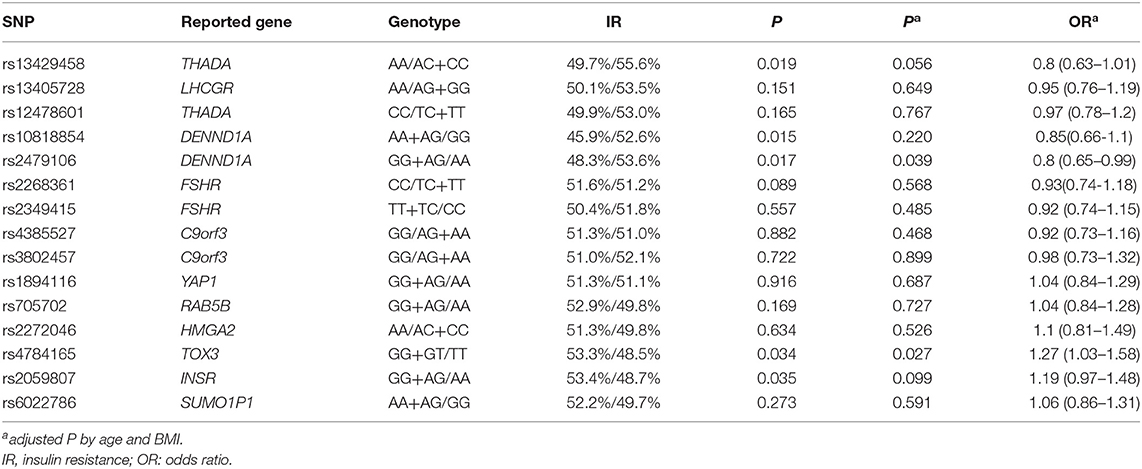
The association of HOMA-IR and the genotypes of 15 SNPs are shown in Table 4 and Supplementary Table 4. The GG + GT group for rs4784165 in TOX3 was associated with an increased rate of IR (53.3 vs. 48.5%, P = 0.027, OR = 1.27, Table 4) using a dominant model after adjustment for age and BMI. The GG+AG group for rs2479106 in DENND1A was associated with a decreased rate of IR using a dominant model (48.3 vs. 53.6%, age- and BMI-adjusted P = 0.036, OR = 0.80, Table 4). Three SNPs (rs13429458 in THADA, rs10818854 in DENND1A, and rs2059807 in INSR) were significantly associated with IR; however, the associations were not significant after adjustment for age and BMI (Table 4). Using additive model, only rs13429458 was significantly associated with IR after adjustment for age and BMI (Supplementary Table 4). After exclusion of subjects with a family history of diabetes, the phenotype-genotype correlations between gene TOX3 and IR were still significant, whereas the correlation between DENND1A and IR was not significant (rs4784165, age- and BMI-adjusted P = 0.031, OR = 1.28; rs2479106, age- and BMI-adjusted P = 0.053, OR = 0.81).
The genotype-phenotype analysis was also performed for 15 SNPs and HOMA-B, fasting insulin, and TG/HDL-C (Supplementary Table 5). Rs10818854 in DENND1A and rs2059807 in INSR were associated with HOMA-B (P < 0.05). Rs4784165 in TOX3 was associated with TG/HDL-C (P < 0.05).
Discussion
Previously our group performed the first two GWAS on women with PCOS and the results were replicated in a large number of cohorts with different ethnicities (13–19). The relationship between important clinical features of PCOS and these GWAS-identified variations have been examined in several studies. To the best of our knowledge, this is the first report on the relation of MS and PCOS-GWAS risk variations. In the present study, we performed genotype-phenotype assessment and found that PCOS susceptibility variants in THADA and INSR, identified in previous GWAS, confers risks for MS in women with PCOS, and that variants in DENND1A and TOX3 were associated with IR.
PCOS is associated with increased risk of metabolic dysfunction including MS and IR. Individuals with MS are at increased risk for serious complications in type 2 diabetes (T2D) and cardiovascular disease (CVD) (20–22). In this study, the rate of MS in women with PCOS was 24.6%, which is lower than that observed in women from the USA (43%) and higher than that in women from Italy (8.2%). The rate of MS did not differ in PCOS subgroups with or without HA. Consistent with our data, a large-scale epidemiological study in reproductive-aged Han Chinese women showed that the prevalence of MS in women with PCOS was 26.8% and did not differ among four different subtypes of PCOS based on the Rotterdam criteria (1).
THADA (thyroid adenoma associated), was initially identified in thyroid adenomas. THADA was identified as a risk locus for T2D by GWAS (23) and was replicated in Indian sib pairs (24). Gene variant in THADA was associated with pancreatic b-cell response (25). However, the association between THADA and T2D were not replicated by some studies (26, 27). In this study, rs12478601 and rs13429458 in THADA were not significantly associated with IR after adjustment for age and BMI. The relation between THADA and PCOS was firstly identified in a GWAS by our group (9) and replicated by other cohorts (13, 16). Further, its relation to PCOS was confirmed by family-based study using transmission disequilibrium test (TDT) (28). In the cross-ethnic meta-analysis of the Chinese, US and Dutch patients, the association between THADA and PCOS was confirmed across populations (29). In this study, SNP rs12478601 in THADA was associated with MS in PCOS, indicating THADA may play an important role in metabolism. The decreased risk of MS associated with CC genotype provide speculation that THADA may affect the pathogenesis of MS-PCOS and non-MS-PCOS through an independent pathway. In consistent with our study, variation in THADA was strongly associated with total cholesterol and low-density lipoprotein in Mexican Americans (30). THADA was predicted to involve in adipogenesis (31). In adipose tissues of women with PCOS carried rs12478601-C, the response to metformin with lower basal glucose was more significant (31). THADA knockout Drosophila were obese and produce less energy than controls. THADA bind the sarco/ER Ca2+ ATPase (SERCA) and regulated metabolism through calcium signaling (32).
The INSR gene is composed of 22 exons and encodes the insulin receptor, a heterotetrameric glycoprotein belonging to the tyrosine kinase receptor family. Knockout of Insr gene in mice causes extreme insulin resistance (33). Altered INSR expression causes IR and diabetes in humans and mice (34, 35). IR in individuals with PCOS is associated with decreased tyrosine autophosphorylation of the insulin receptor considering that the number and affinity of INSR is not altered (36). Single nucleotide polymorphisms in the INSR gene may introduce changes in insulin receptor function and may be associated with PCOS (37). A number of genetic association studies have been conducted in PCOS case-control cohorts, most of which have focused on a silent C/T variation in codon His 1,058; however, their results were inconsistent in different populations (38). The very important validation of the INSR gene as a PCOS risk gene was obtained in a large and well-designed case-control GWAS conducted by our group (10). The SNP rs2059807 in the INSR gene was discovered as an association signal and allele G was observed to be the risk allele for PCOS. In the present study, the risk genotype group GG+AG presented a higher rate of MS and IR than the AA group. Elouej et al. examined the association of rs2059807 and metabolic syndrome in 356 samples (male and female) from the Tunisian population and found that rs2059807 was not associated with a risk of MS (39). These conflicting results may arise due to the different criteria used to define MS and different genders.
TOX3 gene encodes a nuclear protein belonging to the high-mobility group (HMG) box family. TOX3 is a calcium-dependent neuronal transcription factor and is involved in protecting neuronal cells from cell death. TOX3 can mediate cytoprotective transcription from different promoters (either BCL-2 promoter or complement C3 promoter), depending on the presence of different components of the transcriptionally active complex (either phosphorylated CREB or CITED1). TOX3 was recently reported as a breast cancer susceptibility gene by large-scale GWAS (40, 41). TOX3 may play dual roles in cancer initiation and progression considering the decreased expression of TOX3 mRNA in breast cancers and increased expression of TOX3 mRNA in metastatic breast cancer (42, 43). TOX3 was first identified as a PCOS susceptibility gene by our previous GWAS and allele G at rs4784165 was found as the risk allele for PCOS. Recently, the association between TOX3 gene and PCOS was confirmed in another GWAS conducted by Chen Li et al. (44). However, the functional mechanism of TOX3 in PCOS pathogenesis and traits are unclear. Recently an abstract reported that Tox3 knockout rats present an obesity phenotype, male and female sterility, and a behavioral phenotype (increased anxiety). In the present study, the risk genotype group, GG+GT, presented a higher rate of IR than the TT group in women with PCOS, indicating that TOX3 may contribute to PCOS through a metabolic disorder-related pathway. Pau et al. conducted RNA sequencing for subcutaneous adipose tissue in individuals with PCOS, and found that TOX3 may be involved in inflammation (31). Sequencing of exons and the exon-intron boundary regions of the TOX3 gene did not reveal any pathogenic mutations in 200 women with PCOS (45). The expression of TOX3 was lower in the serum and granulosa cells of PCOS subjects compared with that in the control group (46). The putative role of TOX3 in the etiology of PCOS thus needs further explanation.
DENND1A gene encodes the protein DENN/MADD domain containing 1A, which is involved in endosomal membrane trafficking. DENND1A is widely expressed and its relation to PCOS was originally detected in a GWAS by our group. A second GWAS, also conducted by our group, further confirmed that DENND1A gene is a PCOS-susceptibility gene and that allele G at rs2479106 is a risk allele for PCOS. DENND1A has been validated in European cohorts of women with PCOS, but rs2479106 was not shown to be a strongly associated SNP (13, 14). The relationship between quantitative traits and risk SNP rs2479106 has been further examined to better understand its potential function. In the present study, the risk GG+AG genotype group for rs2479106 was associated with a decreased rate of IR. It is speculated that DENND1A may affect the development of IR-PCOS and non-IR PCOS through an independent pathway. Allele G at rs2479106 was associated with increased waist-to-hip ratio(WHR), Chol/HDL, and LDL levels in a European cohort (47), whereas it was associated with increased 2-h insulin levels during oral glucose tolerance tests (OGTT) in a Chinese cohort (48). Besides, allele G at rs2479106 was found to confer risk of endometrioid adenocarcinoma (49), in which insulin resistance may play an important role. Related genetic studies have provided novel biological insights for DENND1A and two principal transcripts, DENND1A variant 1 (DENND1A.V1) and DENND1A variant 2 (DENND1A.V2) have been detected. The expression levels of DENND1A.V2 protein and mRNA are increased in PCOS theca cells. Knockdown of DENND1A.V2 in PCOS theca cells resulted in decreased androgen biosynthesis. Consistently, overexpression of DENND1A.V2 in normal theca cells resulted in a PCOS phenotype including increased androgen biosynthesis (50). DENND1A.V2 thus plays a key role in hyperandrogenemia, indicating a possible biological relationship with IR.
There were some limitations in this study. First, the prevalence of MS is known to increase with age. The participants in this study were young. We thus suggest that future studies should examine the long-term risks of MS and IR in different genotype groups of women at advanced ages. Second, no multiple testing correction has been applied; hence all associations are nominally significant at 5%.
The genotype-phenotype analysis of PCOS GWAS loci is intended to shed light on the mechanism by which the risk SNPs may influence the pathogenesis of PCOS status. This genotype-phenotype study provides clues that THADA, INSR, TOX3, and DENND1A play important role in the etiology of PCOS, possibly through insulin resistance and metabolic disorder related pathways. The four variants, rs12478601, rs2059807, rs4784165, and rs2479106 are located in the intron regions of THADA, INSR, TOX3, and DENND1A genes, respectively; thus, it is unclear how these SNPs might affect the gene expression or function to influence the PCOS phenotype. These SNPs may associate with other functional causal variants in the region. A functional study of these three genes and their causal variants is thus needed to understand how they contribute to the biology of PCOS.
Conclusion
PCOS susceptibility variants in THADA and INSR are associated with metabolic syndrome and variants in TOX3 and DENND1A are associated with insulin resistance. This is the first report on the relation of metabolic syndrome (defined by 2005 Adult Treatment Panel III criteria) and PCOS-GWAS risk variations. It suggests that THADA, INSR, TOX3, and DENND1A might play a role in PCOS through a metabolic disorder related pathway.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Center for Reproductive Medicine, Shandong University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HZ and SZ designed and supported the study and revised the article. YC and ZW collected all the clinical data and blood samples. YT, JL, and SS performed the experiments. YT analyzed the data and drafted the manuscript. All authors gave their final approval for the version to be published.
Funding
This research was supported by the National Key Research and Development Program of China (2017YFC1001000, 2016YFC1000600); the National Natural Science Foundation of China (81430029, 81701410, 81622021, 31871509, 31571548, 31601199); the National Natural Science Foundation of Shandong Province (JQ201816, 2019GSF108274); and the National Institutes of Health (R01HD085527).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We especially thank all the participants in this study. The abstract of this manuscript was presented at the International Federation of Fertility Societies (IFFS) 2019 World Congress. We would like to thank Editage (www.editage.com) for English language editing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.00274/full#supplementary-material
References
1. Li R, Zhang Q, Yang D, Li S, Lu S, Wu X, et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum Reprod. (2013) 28:2562–9. doi: 10.1093/humrep/det262
2. Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. (2005) 352:1223–36. doi: 10.1056/NEJMra041536
3. Ovalle F, Azziz R. Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil Steril. (2002) 77:1095–105. doi: 10.1016/s0015-0282(02)03111-4
4. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A consensus statement from the international diabetes Federation Diabet Med. (2006) 23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x
5. Carmina E, Napoli N, Longo RA, Rini GB, Lobo RA. Metabolic syndrome in polycystic ovary syndrome (PCOS): lower prevalence in southern Italy than in the USA and the influence of criteria for the diagnosis of PCOS. Eur J Endocrinol. (2006) 154:141–5. doi: 10.1530/eje.1.02058
6. Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2005) 90:1929–35. doi: 10.1210/jc.2004-1045
7. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. (2010) 16:347–63. doi: 10.1093/humupd/dmq001
8. Yilmaz B, Vellanki P, Ata B, Yildiz BO. Diabetes mellitus and insulin resistance in mothers, fathers, sisters, and brothers of women with polycystic ovary syndrome: a systematic review and meta-analysis. Fertil Steril. (2018) 110:523–33 e514. doi: 10.1016/j.fertnstert.2018.04.024
9. Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Shi Y, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. (2011) 43:55–9. doi: 10.1038/ng.732
10. Shi Y, Zhao H, Shi Y, Cao Y, Yang D, Li Z, et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. (2012) 44:1020–5. doi: 10.1038/ng.2384
11. Rotterdam ESHRE/ASRM. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. (2004) 19:41–7. doi: 10.1016/j.fertnstert.2003.10.004
12. Xing X-Y, Wwn-ying Y, Yang ZJ. The diagnostic significance of homeostasis model assessment of insulin resistance in metabolic syndrome among subjects with different glucose tolerance. Chi J Diabetes. (2004):12:182–6.
13. Goodarzi MO, Jones MR, Li X, Chua AK, Garcia OA, Chen YD, et al. Replication of association of DENND1A and THADA variants with polycystic ovary syndrome in European cohorts. J Med Genet. (2012) 49:90–5. doi: 10.1136/jmedgenet-2011-100427
14. Welt CK, Styrkarsdottir U, Ehrmann DA, Thorleifsson G, Arason G, Gudmundsson JA, et al. Variants in DENND1A are associated with polycystic ovary syndrome in women of European ancestry. J Clin Endocrinol Metab. (2012) 97:E1342–1347. doi: 10.1210/jc.2011-3478
15. Mutharasan P, Galdones E, Penalver Bernabe B, Garcia OA, Jafari N, Shea LD, et al. Evidence for chromosome 2p16.3 polycystic ovary syndrome susceptibility locus in affected women of European ancestry. J Clin Endocrinol Metab. (2013) 98:E185–90. doi: 10.1210/jc.2012-2471
16. Brower MA, Jones MR, Rotter JI, Krauss RM, Legro RS, Azziz R, et al. Further investigation in europeans of susceptibility variants for polycystic ovary syndrome discovered in genome-wide association studies of Chinese individuals. J Clin Endocrinol Metab. (2015) 100:E182–6. doi: 10.1210/jc.2014-2689
17. Saxena R, Georgopoulos NA, Braaten TJ, Bjonnes AC, Koika V, Panidis D, et al. Han Chinese polycystic ovary syndrome risk variants in women of European ancestry: relationship to FSH levels and glucose tolerance. Hum Reprod. (2015) 30:1454–9. doi: 10.1093/humrep/dev085
18. Dallel M, Sarray S, Douma Z, Hachani F, Al-Ansari AK, Letaifa DB, et al. Differential association of DENND1A genetic variants with polycystic ovary syndrome in Tunisian but not Bahraini Arab women. Gene. (2018) 647:79–84. doi: 10.1016/j.gene.2018.01.028
19. Dapas M, Sisk R, Legro RS, Urbanek M, Dunaif A, Hayes MG. Family-based quantitative trait meta-analysis implicates rare noncoding variants in DENND1A in polycystic ovary syndrome. J Clin Endocrinol Metab. (2019). doi: 10.1210/jc.2018-02496
20. Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. (2001) 24:683–9. doi: 10.2337/diacare.24.4.683
21. Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM, San Antonio Heart S. The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care. (2003) 26:3153–9. doi: 10.2337/diacare.26.11.3153
22. Cameron AJ, Zimmet PZ, Soderberg S, Alberti KG, Sicree R, Tuomilehto J, et al. The metabolic syndrome as a predictor of incident diabetes mellitus in Mauritius. Diabet Med. (2007) 24:1460–9. doi: 10.1111/j.1464-5491.2007.02288.x
23. Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. (2008) 40:638–45. doi: 10.1038/ng.120
24. Gupta V, Vinay DG, Rafiq S, Kranthikumar MV, Janipalli CS, Giambartolomei C, et al. Association analysis of 31 common polymorphisms with type 2 diabetes and its related traits in Indian sib pairs. Diabetologia. (2012) 55:349–57. doi: 10.1007/s00125-011-2355-6
25. Simonis-Bik AM, Nijpels G, van Haeften TW, Houwing-Duistermaat JJ, Boomsma DI, Reiling E, et al. Gene variants in the novel type 2 diabetes loci CDC123/CAMK1D, THADA, ADAMTS9, BCL11A, and MTNR1B affect different aspects of pancreatic beta-cell function. Diabetes. (2010) 59:293–301. doi: 10.2337/db09-1048
26. Sanghera DK, Been L, Ortega L, Wander GS, Mehra NK, Aston CE, et al. Testing the association of novel meta-analysis-derived diabetes risk genes with type II diabetes and related metabolic traits in Asian Indian Sikhs. J Hum Genet. (2009) 54:162–8. doi: 10.1038/jhg.2009.7
27. Stancakova A, Kuulasmaa T, Paananen J, Jackson AU, Bonnycastle LL, Collins FS, et al. Association of 18 confirmed susceptibility loci for type 2 diabetes with indices of insulin release, proinsulin conversion, and insulin sensitivity in 5,327 nondiabetic Finnish men. Diabetes. (2009) 58:2129–36. doi: 10.2337/db09-0117
28. Zhao H, Xu X, Xing X, Wang J, He L, Shi Y, et al. Family-based analysis of susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Hum Reprod. (2012) 27:294–8. doi: 10.1093/humrep/der379
29. Louwers YV, Stolk L, Uitterlinden AG, Laven JS. Cross-ethnic meta-analysis of genetic variants for polycystic ovary syndrome. J Clin Endocrinol Metab. (2013) 98:E2006–2012. doi: 10.1210/jc.2013-2495
30. DeMenna J, Puppala S, Chittoor G, Schneider J, Kim JY, Shaibi GQ, et al. Association of common genetic variants with diabetes and metabolic syndrome related traits in the Arizona Insulin Resistance registry: a focus on Mexican American families in the Southwest. Hum Hered. (2014) 78:47–58. doi: 10.1159/000363411
31. Pau CT, Mosbruger T, Saxena R, Welt CK. Phenotype and tissue expression as a function of genetic risk in polycystic ovary syndrome. PLoS ONE. (2017) 12:e0168870. doi: 10.1371/journal.pone.0168870
32. Moraru A, Cakan-Akdogan G, Strassburger K, Males M, Mueller S, Jabs M, et al. THADA regulates the organismal balance between energy storage and heat production. Dev Cell. (2017) 41:450. doi: 10.1016/j.devcel.2017.05.001
33. Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, Salvatore P, et al. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet. (1996) 12:106–9. doi: 10.1038/ng0196-106
34. Foti D, Chiefari E, Fedele M, Iuliano R, Brunetti L, Paonessa F, et al. Lack of the architectural factor HMGA1 causes insulin resistance and diabetes in humans and mice. Nat Med. (2005) 11:765–73. doi: 10.1038/nm1254
35. Chiefari E, Tanyolac S, Paonessa F, Pullinger CR, Capula C, Iiritano S, et al. Functional variants of the HMGA1 gene and type 2 diabetes mellitus. JAMA. (2011) 305:903–12. doi: 10.1001/jama.2011.207
36. Li M, Youngren JF, Dunaif A, Goldfine ID, Maddux BA, Zhang BB, et al. Decreased insulin receptor (IR) autophosphorylation in fibroblasts from patients with PCOS: effects of serine kinase inhibitors and IR activators. J Clin Endocrinol Metab. (2002) 87:4088–93. doi: 10.1210/jc.2002-020363
37. Siegel S, Futterweit W, Davies TF, Concepcion ES, Greenberg DA, Villanueva R, et al. A C/T single nucleotide polymorphism at the tyrosine kinase domain of the insulin receptor gene is associated with polycystic ovary syndrome. Fertil Steril. (2002) 78:1240–3. doi: 10.1016/s0015-0282(02)04241-3
38. Diamanti-Kandarakis E, Papavassiliou AG. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med. (2006) 12:324–32. doi: 10.1016/j.molmed.2006.05.006
39. Elouej S, Rejeb I, Attaoua R, Nagara M, Sallem OK, Kamoun I, et al. Gender-specific associations of genetic variants with metabolic syndrome components in the Tunisian population. Endocr Res. (2016) 41:300–9. doi: 10.3109/07435800.2016.1141945
40. Easton DF, Pooley KA, Dunning AM, Pharoah PD, Thompson D, Ballinger DG, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. (2007) 447:1087–93. doi: 10.1038/nature05887
41. Stacey SN, Manolescu A, Sulem P, Rafnar T, Gudmundsson J, Gudjonsson SA, et al. Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet. (2007) 39:865–9. doi: 10.1038/ng2064
42. Smid M, Wang Y, Klijn JG, Sieuwerts AM, Zhang Y, Atkins D, et al. Genes associated with breast cancer metastatic to bone. J Clin Oncol. (2006) 24:2261–7. doi: 10.1200/JCO.2005.03.8802
43. Cowper-Sal lari R, Zhang X, Wright JB, Bailey SD, Cole MD, Eeckhoute J, et al. Breast cancer risk-associated SNPs modulate the affinity of chromatin for FOXA1 and alter gene expression. Nat Genet. (2012) 44:1191–8. doi: 10.1038/ng.2416
44. Chen L, Hu LM, Wang YF, Yang HY, Huang XY, Zhou W, et al. Genome-wide association study for SNPs associated with PCOS in human patients. Exp Ther Med. (2017) 14:4896–900. doi: 10.3892/etm.2017.5113
45. Cui Y, Zhao S, Zhao H, Lv Y, Yu M, Wang Y, et al. Mutational analysis of TOX3 in Chinese Han women with polycystic ovary syndrome. Reprod Biomed Online. (2014) 29:752–5. doi: 10.1016/j.rbmo.2014.08.004
46. Ning Z, Jiayi L, Jian R, Wanli X. Relationship between abnormal TOX3 gene methylation and polycystic ovarian syndrome. Eur Rev Med Pharmacol Sci. (2017) 21:2034–8.
47. Lerchbaum E, Trummer O, Giuliani A, Gruber HJ, Pieber TR, Obermayer-Pietsch B. Susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21, and 9q33.3 in a cohort of Caucasian women. Horm Metab Res. (2011) 43:743–7. doi: 10.1055/s-0031-1286279
48. Cui L, Zhao H, Zhang B, Qu Z, Liu J, Liang X, et al. Genotype-phenotype correlations of PCOS susceptibility SNPs identified by GWAS in a large cohort of Han Chinese women. Hum Reprod. (2013) 28:538–44. doi: 10.1093/humrep/des424
49. Wang Z, Li T, Zhang W, You L, Zhao Y, Xia M, et al. Variants in DENND1A and LHCGR are associated with endometrioid adenocarcinoma. Gynecol Oncol. (2012) 127:403–5. doi: 10.1016/j.ygyno.2012.08.007
Keywords: PCOS, MS, IR, GWAS, variants
Citation: Tian Y, Li J, Su S, Cao Y, Wang Z, Zhao S and Zhao H (2020) PCOS-GWAS Susceptibility Variants in THADA, INSR, TOX3, and DENND1A Are Associated With Metabolic Syndrome or Insulin Resistance in Women With PCOS. Front. Endocrinol. 11:274. doi: 10.3389/fendo.2020.00274
Received: 07 January 2020; Accepted: 14 April 2020;
Published: 30 April 2020.
Edited by:
Antonio Brunetti, University of Catanzaro, ItalyReviewed by:
Ghislain Rocheleau, Icahn School of Medicine at Mount Sinai, United StatesMohd Ashraf Ganie, Sher-I-Kashmir Institute of Medical Sciences, India
Copyright © 2020 Tian, Li, Su, Cao, Wang, Zhao and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shigang Zhao, enNnMDEwOEAxMjYuY29t; Han Zhao, aGFuemg4MEB5YWhvby5jb20=
 Ye Tian
Ye Tian Jingyu Li1,3,4
Jingyu Li1,3,4 Shizhen Su
Shizhen Su Yongzhi Cao
Yongzhi Cao Zhao Wang
Zhao Wang Shigang Zhao
Shigang Zhao Han Zhao
Han Zhao