
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Endocrinol., 14 May 2020
Sec. Neuroendocrine Science
Volume 11 - 2020 | https://doi.org/10.3389/fendo.2020.00236
This article is part of the Research TopicRecent Progress and Perspectives in Neurosteroid ResearchView all 20 articles
A correction has been applied to this article in:
Corrigendum: Allopregnanolone, the Neuromodulator Turned Therapeutic Agent: Thank You, Next?
Allopregnanolone, today best known as brexanolone and marketed as Zulresso™ for the treatment of postpartum depression is part of only two recently Food and Drug Administration (FDA)-approved fast-acting antidepressants, with esketamine nasal spray, an NMDA receptor antagonist used in treatment-resistant depression being the other (1).
The trajectory, lasting 80 years, that brought allopregnanolone from its discovery (2) in 1938 in the adrenal glands, to understanding its fast non-genomic mechanism in potentiating membrane neurotransmitter receptors, including GABAA receptors (3, 4), underlying its role in acute and chronic stress (5–7), discovering its powerful non-sedative pharmacological effects as anxiolytic and antidepressant agent in animal models and humans (8–10), to the design of the first clinical trials for postpartum depression (11), and finally to the shelves of the clinics in 2019, is regarded as one of the best examples of translational drug development in neuropsychopharmacology (12, 13).
This article redraws the most significant milestones in allopregnanolone discoveries and evaluates future perspective for a new generation of neurosteroid-based treatments in neuropsychiatry. The role of allopregnanolone as a potential biomarker for mood disorders and its pharmacological mechanism in improving behavioral deficits will be discussed.
Following its discovery in the adrenal glands (2), Baulieu's laboratory observed (1981) that allopregnanolone can be produced in brain in a manner unrelated to peripheral renovation rates (14). This finding led to coin the term “neurosteroid” to define a chemically identical steroid specifically produced by the brain as opposed to “neuroactive steroids,” coined by Paul and Purdy (15), which defines steroids produced peripherally that reach and act in the brain. However, it took 25 years to demonstrate that allopregnanolone and its biosynthetic enzymes, 5α-reductase type I (5α-RI) and 3α-hydroxysteroid dehydrogenase (3α-HSD), are expressed in glutamatergic neurons in cortex, hippocampus and basolateral amygdala, and in long-projecting GABAergic neurons in reticulus thalamic nucleus, striatum, central amygdala, and cerebellum but not in glial cells of rodent and human brain (16–20). In 1986, Paul's laboratory observed allopregnanolone is a potent positive allosteric modulator of GABA's action at synaptic and extrasynaptic GABAA receptors (3, 4, 21). Costa and Guidotti' laboratories later cloned and described the function of 18 kDa translocator (TSPO), involved in gating cholesterol entry into the inner mitochondrial membranes, where cholesterol is converted to pregnenolone, the precursors of all neurosteroids (22, 23). Acute stress in rodents fast induces allopregnanolone biosynthesis underlying its role in stress response and demonstrating allopregnanolone present in brain is synthesized independently from peripheral glands (24). However, prolonged stress in rodent models of behavioral dysfunction correlated with downregulated allopregnanolone biosynthesis in corticolimbic circuitry regulating fear responses, anxiety-like, and depression-like phenotypes (7, 25, 26). The first evidence suggesting that allopregnanolone is involved in the etiopathology of depression originated by studies in rodents and depressed patients (27–29). Evidence showed that treatment with the SSRI, fluoxetine normalized the stress—induced decrease of allopregnanolone in rodent brain as well as its lower levels observed in CSF/serum of patients with depression (25, 28, 30). This finding was corroborated by observing SSRIs act as selective brain steroidogenic stimulants (SBSSs), increasing selectively allopregnanolone in a manner independent from SSRI mechanisms, underlying a novel mechanism of classical antidepressants (7, 29, 31). Endogenously-produced allopregnanolone in corticolimbic neurons modulates the fine-tuning of GABAA receptors for GABAmimetic, GABAA receptor agonists and positive allosteric modulators. This function underlies allopregnanolone's neurophysiological role. This finding also suggested that allopregnanolone, by this mechanism, may regulate emotional behavior in corticolimbic circuitry (32). Indeed, decreased allopregnanolone biosynthesis in these neurons occurred in association with behavioral dysfunction that are reminiscent of deficits observed in the spectrum of mood disorders (17, 19, 20). Independent laboratories, meanwhile, discovered that allopregnanolone enhances tonic inhibition in δ-containing GABAA receptors, that pregnancy reduces GABAA γ and δ-containing subunits, and that two membrane binding sites on GABAA receptors mediate activation and potentiation of neurosteroid signaling (21, 33–35). Preclinical studies of stress-induced allopregnanolone biosynthesis downregulation contributed to the discovery of several neurosteroidogenic targets through which, agents that increase allopregnanolone biosynthesis, are beneficial in improving behavioral deficits (36–39).
Collectively, these and many more observations in the field by many talented neurosteroid scientists, led to clinical trials that demonstrated the efficacy of intravenous allopregnanolone in postpartum depression (40, 41). Given the remarkable pharmacological efficacy of this novel therapeutic, on March 19th, 2019, the FDA approved intravenous allopregnanolone (i.e., brexanolone) as the first specific treatment for postpartum depression (Figure 1). Clinical studies are currently evaluating the pharmacological efficacy of an orally-active allopregnanolone called SAGE 217 for the treatment of major depressive disorders (47). A new era of fast-acting, short-course, long-lasting, neurosteroid-based treatments is born.
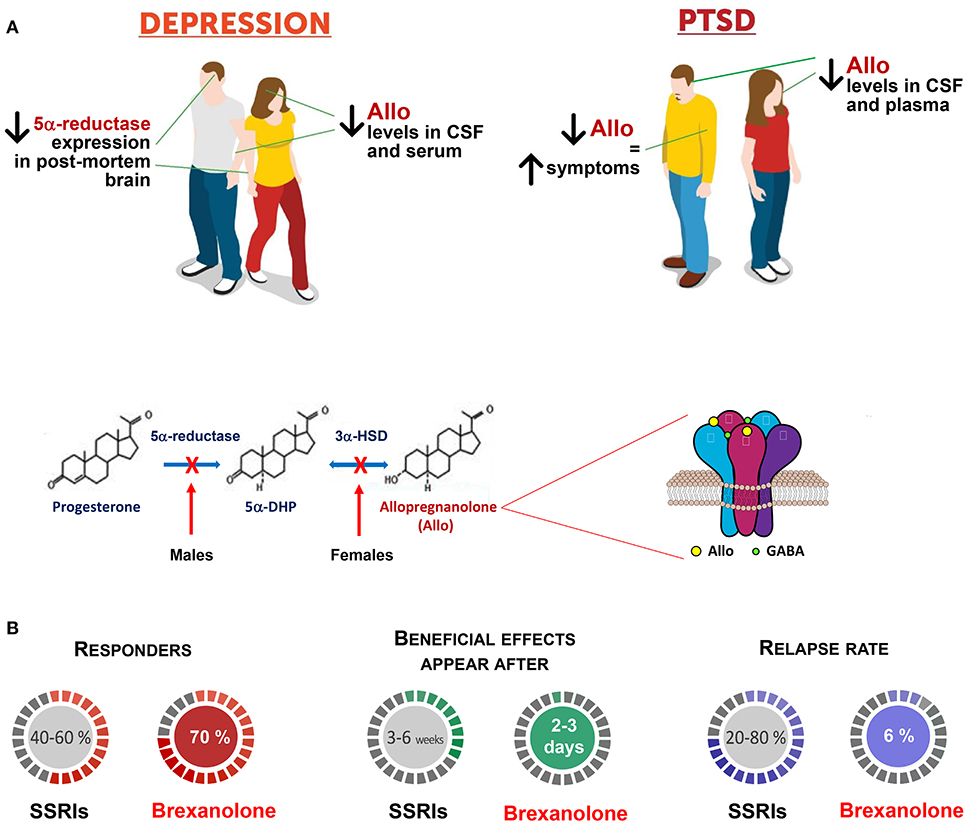
Figure 1. Brexanolone is superior to traditional antidepressants in the treatment of mood disorders. (A) Patients with mood disorders, including major unipolar depression and PTSD, exhibit serum, plasma, CSF, and brain reduction of allopregnanolone levels and/or biosynthesis, which includes the enzymes, 5α-reductase type I (5α-RI), and 3α-hydroxysteroid dehydrogenase (3α-HSD) [(18, 28, 30); reviewed in (10, 42)]. In women with PTSD, progesterone, and the immediate allopregnanolone precursor, 5α-dihydroprogesterone (5α-DHP) levels fail to change and their ratios with allopregnanolone and pregnanolone (allopregnanolone's equipotent GABAergic isomer), concentration in the CSF and plasma points to a possible deficit at the 3α-HSD enzyme expression/function levels (43). Likewise, in PTSD male patients, the CSF allopregnanolone concentrations are decreased for an apparent deficit in 5α-RI expression/function, which negatively correlates with PTSD and depression symptoms (43–45). Thus, the concentration and the ratio of allopregnanolone with its parental neuroactive steroids can suggest deficits in their enzymatic pathway, which may unveil biomarkers of sex hormone-related mood disorders. Allopregnanolone's mechanism of action includes activation of mainly extrasynaptically-expressed GABAA receptors. GABAA receptor offers two residues for neurosteroid action; one is located between α and β subunits, and the second is a cavity on α subunits (34). The efficacy of neurosteroids at GABAA receptors is greatly enhanced by the αβδ-containing GABAA receptor subtype, which is characteristic of tonic inhibition mediated by extrasynaptic receptors (21). Allopregnanolone plays a pivotal neurophysiological role by modulating the fine-tuning and strength of GABAA receptors (32). By this mechanism, allopregnanolone appears to regulate emotional behavior and the pharmacological response of GABAA receptor. Altered GABAA receptor subunit composition has been observed in several pathophysiological conditions, including across the menstrual cycle, changes in hormonal shape during pregnancy, as well as during protracted stress (46). Stress, specifically, results in a GABAA receptor composition with increased sensitivity for neurosteroids and neurosteroid-like molecules (e.g., synthetic allopregnanolone analogs) [(29); reviewed in (42)]. These observations are in support of treatments that stimulate allopregnanolone biosynthesis for the therapeutic management of stress-induced psychiatric disorders, for which traditional anxiolytics or antidepressants are ineffective. (B) Brexanolone, a β-cyclodextrin-based parenterally administered soluble formulation of allopregnanolone is marketed as Zulresso™ and it is the first and only specific treatment for postpartum depression. Brexanolone is one of only two recently FDA-approved fast-acting antidepressants. In clinical trials, women with postpartum depression treated with brexanolone improved their symptoms compared with placebo in 2.5 days. Symptoms were measured before and after treatment. Follow-up studies showed that women receiving the treatment maintained the therapeutic gains for at least 30 days (41). Side effects include risk of sedation or loss of consciousness during treatment. For these reasons women who undergo treatment will be monitored by a healthcare professional in a healthcare setting. Other side effects may include sleepiness, dry mouth, flushing of the skin or face. A clinical trial using the orally-active allopregnanolone analog, SAGE 217 has recently failed for non-compliance issues that were noted with about 10% of patients presenting no blood drug levels. However, statistical significance was achieved at days 3, 8, 12, and 15 in patients with measurable drug concentration levels of SAGE-217. Hence, these allopregnanolone derivatives are highly promising in the treatment of mood disorders, from postpartum depression to major depression and, probably, in PTSD, which, as mentioned above, is characterized by low allopregnanolone levels (43, 45). Another approach is to use neurosteroidogenic drugs (38). These agents may selectively elevate allopregnanolone levels by stimulating enzyme activity/expression levels where a deficit emerges thereby improving mood symptoms avoiding a global expression of allopregnanolone levels.
Allopregnanolone, a positive allosteric modulator of GABA's action at GABAA receptors (3, 4, 15, 48), is deficient in mood disorders (28, 30, 43, 45). Allopregnanolone and progesterone change significantly in pregnancy and after parturition (33, 49). The increase in plasma progesterone throughout pregnancy triggers upregulation of allopregnanolone levels, which reaches the highest blood concentrations during the third trimester (49, 50). Following childbirth, these neurohormones abruptly decrease (51, 52). Among the hypotheses linking allopregnanolone decrease and post-partum depression, the suggestion that allopregnanolone drops quicker and to lower levels than in mothers who fail to develop post-partum depression is particularly intriguing. This effect may be resulting from abnormal neurosteroid enzyme expression. Mechanistically, GABAA receptor function may fail to adapt to the rapid allopregnanolone level decline during the weeks following parturition (53). Studies conducted in estrous cycle in rats demonstrated that the drastic decrease of progesterone concentrations during diestrus is associated with overexpression of extrasynaptic α4β1δ-containing GABAA receptors in periaqueductal gray, which mediates anxiolytic and mood regulating effects of allopregnanolone in this estrous phase (54, 55). The expression of specific subunits of the GABAA receptor is coordinated with fluctuations in neurosteroid concentrations during menstrual/estrous cycle, pregnancy, and perinatally function (21, 33, 56). Pharmacological treatments, including finasteride and oral contraceptives, that inhibit 5α-RI, which results in a blood and brain allopregnanolone decrease also affect subunit expression of GABAA receptor and are associated with mood symptoms and suicide and are part of postfinasteride syndrome (57, 58). Post-finasteride syndrome, in addition to depression, anxiety and cognitive deficits also induces sexually-related side effects, such as loss of libido, erectile dysfunction, decreased arousal and difficulty in achieving an orgasm that persist despite drug withdrawal (58). Evidence suggests during pregnancy and across the estrous cycle a switch of extrasynaptic δ with synaptic γ2 subunits may be operative (33, 56). Rapid and dynamic changes among synaptic and extrasynaptic GABAA receptor conformation in areas that regulate cognitive functions and emotions, including the hippocampus have been reported (59).
Altogether, stressful condition, hormonal changes, pharmacological treatment (e.g., finasteride, oral contraceptives) may coordinately change GABAA receptor expression resulting in alterations in receptor function underlying mood disorders. They may alter GABAA receptor pharmacology in response to anxiolytics (42). Conversely, allopregnanolone, its analogs, and neurosteroidogenic agents may offer a therapeutic advantage for disorders that arise by these deficits.
To contrast the rapid post-partum depletion of allopregnanolone and the rise of mood deficits, directly supplementing synthetic neuroactive steroids or their analogs, may offer a quick strategy in treating post-partum depression and other mood disorders linked with the drastic drop in endogenous allopregnanolone (53, 60). Following this concept, brexanolone, a β-cyclodextrin-based parenterally-administered soluble formulation of allopregnanolone, was developed and FDA-approved for treating post-partum depression. In an open-label study, a single brexanolone IV administration showed rapid and long-lasting antidepressant effects in severe post-partum depression (40). Safety and efficacy was further confirmed in two double-blind, randomized clinical trials (41). Brexanolone presumably acts by reinstating normal allopregnanolone levels, and thereby tuning GABAergic neurotransmission function, promptly improved symptom severity in with post-partum depression patients (Figure 1). However, it still remains to be clarified the precise treatment targets, including levels of endogenous allopregnanolone, verify altered biosynthetic enzyme expression/function, and GABAA receptor assembly modifications pre, during, and post-brexanolone treatment. The elevation of brain derived neurotropic factor (BDNF) is also conceivable among allopregnanolone's mechanisms (61). Each of these factors may be critical for understanding why—and for whom—brexanolone is best indicated to improve mood symptoms. First, deficient allopregnanolone levels may be critical for predicting who may benefit from varying doses of direct neurosteroid replacement (via brexanolone or other allopregnanolone analogs). Second, baseline allopregnanolone concentrations are crucial to select the most effective brexanolone dose and avoid unwanted side-effects, including excessive sedation (62). Third, by directly affecting both the HPA and HPG axes, allopregnanolone may alter expression of key biosynthetic enzymes (e.g., 5α-RI and 3α-HSD) involved in neurosteroid synthesis. Indeed, the HPA axis is modulated by GABAergic neuron activation within the hypothalamus (63). Allopregnanolone potently inhibits HPA axis activity and repress stress elevation of ACTH and corticosterone (64, 65). This finding suggests that allopregnanolone administration may alter HPA axis responsiveness by affecting gonadal steroid concentrations (e.g., estradiol) with documented roles in maintaining expression/function of neurosteroidogenic enzymes (e.g., 3α-HSD) and sustainably change endogenous neurosteroids production (66).
Collectively, these reports suggest that more studies are needed to verify the diverse mechanisms involved in brexanolone treatment.
While converging evidence suggests a neurosteroid biosynthesis deficit involvement in the underlying neurobiology of mood disorders, the yet unanswered question is whether allopregnanolone biosynthesis (allopregnanolone levels and expression of rate-limiting biosynthetic enzymes) provide a reliable biomarker to prevent mood disorder, predict occurrence, diagnose, and indicate treatment selection. Another valid option suggests analyzing neurosteroid biosynthesis relative to GABAA receptor subunit dynamic changes. GABAA receptor expression and neurosteroid biosynthesis in post-partum depression and in general in mood disorders remains underinvestigated. Furthermore, analysis of neurosteroids that positively modulate GABAA receptors (allopregnanolone and pregnanolone), and of their sulfates (e.g., pregnanolone sulfate), that inhibit NMDA-mediated tonic neurotransmission, which results in neuroprotection and cognitive improvement (67), has been poorly investigated. Establishing predictive biomarkers of treatment response will enable follow-up analysis of neuroactive steroid biosynthesis and GABAA receptor composition that will help predict whether brexanolone pharmacological effects are associated with permanent neurobiological improvements or, alternatively, whether GABAergic functional deficits may anticipate relapses following drug discontinuation. Assessing a biomarker axis, indicating the dynamic changes of several inter-related neurobiological deficits will facilitate a more thorough diagnosis of mood disorders as well as predict which patients will likely respond to treatment. This will increase efficacy and limit occurrence of side-effects.
In neuropsychopharmacology establishing reliable biomarkers and efficient treatments is urgently needed. Currently, patients show large non-response and relapse-rate to traditional antidepressants and significant side-effects.
Eighty years of neurosteroid research originated from many talented neuroscientists around the world guided investigations that from the discovery of allopregnanolone led to its approval as a fast-acting agent to treat post-partum depression. One of the most significant achievements still remaining to be accomplished in neuropsychopharmacology and, in general in psychiatry, is the assessment of valid biomarkers to predict, diagnose, select, and treat patients more efficiently, avoiding drug non–responders and side-effects. Neurosteroidogenic targets have been recently suggested that may result in new drug development (38, 68). The opportunity of increasing allopregnanolone levels and improving deficits with functional foods (69) is an emerging novel approach to treat mood disorders in a more natural way without exposing pregnant women to drugs.
The author confirms being the sole contributor of this work and has approved it for publication.
This work was supported by the US Department of Defense Grant W81XWH-15-1-0521 to GP.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Krystal JH, Abdallah CG, Duman RS. Ketamine: a paradigm shift for depression research and treatment. Neuron. (2019) 101:774–8. doi: 10.1016/j.neuron.2019.02.005
2. Beall D, Reichstein T. Isolation of progesterone and allopregnanolone from the adrenal. Nature. (1938) 142:479. doi: 10.1038/142479b0
3. Majewska MD, Harrison NL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. (1986) 232:1004–7. doi: 10.1126/science.2422758
4. Puia G, Santi MR, Vicini S, Seeburg PH, Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. (1990) 4:759–65. doi: 10.1016/0896-6273(90)90202-Q
5. Purdy RH, Morrow AL, Moore PH Jr, Paul SM. Stress-induced elevations of gammaaminobutyric acid type A receptor-active steroids in the rat brain. PNAS. (1991) 88:4553–7. doi: 10.1073/pnas.88.10.4553
6. Matsumoto K, Puia G, Dong E, Pinna G. GABAA receptor neurotransmission dysfunction in a mouse model of social isolation-induced stress. Stress. (2007) 10:3–12. doi: 10.1080/10253890701200997
7. Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. PNAS. (2003) 100:2035–40. doi: 10.1073/pnas.0337642100
8. Crawley JN, Glowa JR, Majewska MD, Paul SM. Anxiolytic activity of an endogenous adrenal steroid. Brain Res. (1986) 398:382–5. doi: 10.1016/0006-8993(86)91500-3
9. Girdler SS, Straneva PA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatr. (2001) 49:788–97. doi: 10.1016/S0006-3223(00)01044-1
10. Zorumski CF, Paul SM, Mennerick S. Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. (2013) 37:109–22. doi: 10.1016/j.neubiorev.2012.10.005
11. Meltzer-Brody SE, Kanes SJ. Allopregnanolone in postpartum depression: Role in pathophysiology and treatment. Neurobiol Stress. (2020) 19:22. doi: 10.1016/j.ynstr.2020.100212
12. Walton N, Maguire J. Allopregnanolone-based treatments for postpartum depression: why/how do they work? Neurobiol Stress. (2019) 11:100198. doi: 10.1016/j.ynstr.2019.100198
13. Paul SM, Pinna G, Guidotti A. “NEUROSTEROIDS: from molecular pathophysiology to therapeutics - a brief historical perspective. Neurobiol Stress. (2020) 378:112309. doi: 10.1016/j.ynstr.2020.100215
14. Corpéchot C, Robel P, Baulieu EE. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. PNAS. (1981) 78:4704–7. doi: 10.1073/pnas.78.8.4704
15. Paul SM, Purdy RH. Neuroactive steroids. FASEB J. (1992) 6:2311–22. doi: 10.1096/fasebj.6.6.1347506
16. Agis-Balboa RC, Pinna G, Costa E, Guidotti A. Location and expression of brain enzymes catalyzing neurosteroid biosynthesis. PNAS. (2006) 103:14602–7. doi: 10.1073/pnas.0606544103
17. Agís-Balboa RC, Pinna G, Costa E, Guidotti A. Downregulation of 5αreductase type I mRNA expression in cortico-limbic glutamatergic neurons in socially-isolated mice. PNAS. (2007) 104:18736–41. doi: 10.1073/pnas.0709419104
18. Agis-Balboa RC, Guidotti A, Pinna G. Allopregnanolone biosynthesis is downregulated in the prefrontal cortex/Brodmann's area 9 (BA9) of depressed patients. Psychopharmacology. (2014) 231:3569–80. doi: 10.1007/s00213-014-3567-5
19. Pinna G, Agis-Balboa R, Pibiri F, Nelson M, Guidotti A, Costa E. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochem Res. (2008) 33:1990–2007. doi: 10.1007/s11064-008-9718-5
20. Pibiri F, Nelson M, Costa E, Guidotti A, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. PNAS. (2008) 105:5567–72. doi: 10.1073/pnas.0801853105
21. Maguire J, Mody I. GABAAR plasticity during pregnancy: relevance to postpartum depression. Neuron. (2008) 59:207–13. doi: 10.1016/j.neuron.2008.06.019
22. Sprengel R, Werner P, Guidotti A, Krueger KE. Molecular cloning and expression of cDNA encoding a peripheral-type benzodiazepine receptor. J Biol Chem. (1989) 264:20415–21.
23. Papadopoulos V, Baraldi M, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor. Trends Pharmacol Sci. (2006) 27:402–9. doi: 10.1016/j.tips.2006.06.005
24. Purdy RH, Moore PH Jr, Morrow AL, Paul SM. Neurosteroids and GABAA receptor function. Adv Biochem Psychopharmacol. (1992) 47:87–92.
25. Matsumoto K, Uzunova V, Pinna G, Guidotti A, Costa E. Permissive role of brain allopregnanolone content in the regulation of pentobarbital-induced righting reflex loss. Neuropharmacology. (1999) 38:955–63. doi: 10.1016/S0028-3908(99)00018-0
26. Guidotti A, Dong E, Matsumoto K, Pinna G, Rasmusson AM, Costa E. The socially-isolated -dihydroprogesterone in psychiatric disorders. Brain Res Rev. (2001) 37:110–5. doi: 10.1016/S0165-0173(01)00129-1
27. Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. PNAS. (1996) 93:12599–604. doi: 10.1073/pnas.93.22.12599
28. Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. PNAS. (1998) 95:3239–44. doi: 10.1073/pnas.95.6.3239
29. Pinna G, Costa E, Guidotti. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses inactive on 5-HT reuptake. Psychopharmacology. (2006) 186:362–72. doi: 10.1007/s00213-005-0213-2
30. Romeo E, Strohle A, Spalletta G, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatr. (1998) 155:910–3. doi: 10.1176/ajp.155.7.910
31. Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically facilitate pentobarbital sedation by increasing neurosteroids. Proc Natl Acad Sci USA. (2004) 101:6222–5. doi: 10.1073/pnas.0401479101
32. Pinna G, Uzunova V, Matsumoto K, Puia G, Mienville JM, Costa E, et al. Brain allopregnanolone regulates the potency of the GABAA receptor agonist muscimol. Neuropharmacology. (2000) 39:440–8. doi: 10.1016/S0028-3908(99)00149-5
33. Concas A, Mostallino MC, Porcu P, Biggio G. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. PNAS. (1998) 95:13284–9. doi: 10.1073/pnas.95.22.13284
34. Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. (2006) 444:486–9. doi: 10.1038/nature05324
35. Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. (2012) 73:23–34. doi: 10.1016/j.neuron.2011.12.012
36. Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. (2010) 9:971–88. doi: 10.1038/nrd3295
37. Rupprecht R, Rammes G, Kucher K. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. (2009) 325:490–3. doi: 10.1126/science.1175055
38. Raber J, Arzy S, Bertolus JB, Depue B, Haas HE, Hofmann SG, et al. Current understanding of fear learning and memory in humans and animal models and the value of a linguistic approach for analyzing fear learning and memory in humans. Neurosci Biobehav Rev. (2019) 105:136–77. doi: 10.1016/j.neubiorev.2019.03.015
39. Locci A, Pinna G. Stimulation of PPAR-α by N-palmitoylethanolamine engages allopregnanolone biosynthesis to modulate emotional behavior. Biol Psychiatr. (2019) 85:1036–45. doi: 10.1016/j.biopsych.2019.02.006
40. Kanes S, Colquhoun H, Gunduz-Bruce H, Epperson CN, Rubinow D, Paul S, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. (2017) 390:480–9. doi: 10.1016/S0140-6736(17)31264-3
41. Meltzer-Brody S, Colquhoun H, Kanes S. Brexanolone injection in postpartum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. (2018) 392:1058–70. doi: 10.1016/S0140-6736(18)31551-4
42. Locci A, Pinna G. Neurosteroid biosynthesis downregulation and changes in GABAA receptor subunit composition: A biomarker axis in stress-induced cognitive and emotional impairment. Br J Pharmacol. (2017) 174:3226–41. doi: 10.1111/bph.13843
43. Rasmusson AM, Pinna G, Krystal J, Guidotti A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatr. (2006) 60:704–13. doi: 10.1016/j.biopsych.2006.03.026
44. Pineles SL, Nillni YI, Pinna G, Irvine J, Webb A, Arditte Hall KA, et al. PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology. (2018) 93:133–41. doi: 10.1016/j.psyneuen.2018.04.024
45. Rasmusson AM, Nillni G, Anderson M, Pinna G. Relationships between cerebrospinal fluid GABAergic neurosteroid levels and symptom severity in men with PTSD. Psychoneuroendocrinology. (2018) 102:95–104. doi: 10.1016/j.psyneuen.2018.11.027
46. Pinna G, Agis Balboa RC, Zhubi A, Matsumoto K, Grayson DR, Costa E et al. Imidazenil and diazepam increase locomotor activity in mice exposed to protracted social isolation. Proc Natl Acad Sci USA. (2006) 103:4275–80.
47. Gunduz-Bruce H, Silber C, Paul SM, Kanes SJ. Trial of SAGE-217 in patients with major depressive disorder. N Engl J Med. (2019) 381:903–11. doi: 10.1056/NEJMoa1815981
48. Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. (2005) 6:565–75.
49. Luisi S, Petraglia F, Luisi M, Genazzani AR. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. (2000) 85:2429–33. doi: 10.1210/jcem.85.7.6675
50. McEvoy K, Payne JL, Osborne LM. Neuroactive steroids and perinatal depression: a review of recent literature. Curr Psychiatry Rep. (2018) 20:78. doi: 10.1007/s11920-018-0937-4
51. Osborne LM, Betz JF, Payne JL. The role of allopregnanolone in pregnancy in predicting postpartum anxiety symptoms. Front Psychol. (2019) 10:1033. doi: 10.3389/fpsyg.2019.01033
52. Payne JL, Maguire J. Pathophysiological mechanisms implicated in postpartum depression. Front Neuroendocrinol. (2019) 52:165–80. doi: 10.1016/j.yfrne.2018.12.001
53. Mody I. GABAA R modulator for postpartum depression. Cell. (2019) 176:1. doi: 10.1016/j.cell.2018.12.016
54. Griffiths JL, Lovick TA. GABAergic neurones in the rat periaqueductal grey matter express alpha4, beta1 and delta GABAA receptor subunits: plasticity of expression during the estrous cycle. Neuroscience. (2005) 136:457–66. doi: 10.1016/j.neuroscience.2005.08.013
55. Lovick TA. Plasticity of GABAA receptor subunit expression during the oestrous cycle of the rat: implications for premenstrual syndrome in women. Exp Physiol. (2006) 91:655–60. doi: 10.1113/expphysiol.2005.032342
56. Maguire JL, Stell BM, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. (2005) 8:797–804. doi: 10.1038/nn1469
57. Porcu P, Mostallino MC, Concas A. Long-term administration with levonorgestrel decreases allopregnanolone levels and anxiety-like behavior. Pharmacol Biochem Behav. (2012) 102:366–72. doi: 10.1016/j.pbb.2012.05.011
58. Diviccaro S, Melcangi RC, Giatti S. Post-finasteride syndrome: an emerging clinical problem. Neurobiol Stress. (2020) 12:100209. doi: 10.1016/j.ynstr.2019.100209
59. Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. (2008) 9:331–43. doi: 10.1038/nrn2370
60. Pinna G. Targeting neurosteroidogenesis as therapy for PTSD. Front Pharmacol. (2014) 4:166. doi: 10.3389/fphar.2013.00166
61. Nin MS, Martinez LA, Pibiri F, Nelson M, Pinna G. Neurosteroids reduce social isolation-induced behavioral deficits: a proposed link with neurosteroid-mediated upregulation of BDNF expression. Front Endocrinol (Lausanne). (2011) 2:73. doi: 10.3389/fendo.2011.00073
62. Leader LD, O'Connell M, VandenBerg A. Brexanolone for postpartum depression: clinical evidence and practical considerations. Pharmacotherapy. (2019) 39:1105–12. doi: 10.1002/phar.2331
63. Boudaba C, Szabo K, Tasker JG. Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J Neurosci. (1996) 16:7151–60. doi: 10.1523/JNEUROSCI.16-22-07151.1996
64. Brunton PJ, McKay AJ, Russell JA. Central opioid inhibition of neuroendocrine stress responses in pregnancy in the rat is induced by the neurosteroid allopregnanolone. J Neurosci. (2009) 29:6449–60. doi: 10.1523/JNEUROSCI.0708-09.2009
65. Biggio G, Pisu MG, Biggio F, Serra M. Allopregnanolone modulation of HPA axis function in the adult rat. Psychopharmacology (Berl). (2014) 231:3437–44. doi: 10.1007/s00213-014-3521-6
66. Mitev YA, Darwish M, Wolf SS, Holsboer F, Almeida OF, Patchev VK. Gender differences in the regulation of 3 alpha-hydroxysteroid dehydrogenase in rat brain and sensitivity to neurosteroid-mediated stress protection. Neuroscience. (2003) 120:541–9. doi: 10.1016/S0306-4522(03)00287-2
67. Vyklicky V, Borovska J, Krausova B, Balik A, Korinek M, Borovska J, et al. Preferential inhibition of tonically over phasically activated NMDA receptors by pregnane derivatives. J Neurosci. (2016) 36:2161–75. doi: 10.1523/JNEUROSCI.3181-15.2016
68. Pinna G. Animal models of PTSD: the socially isolated mouse and the biomarker role of allopregnanolone. Front Behav Neurosci. (2019) 13:114. doi: 10.3389/fnbeh.2019.00114
Keywords: brexanolone, allopregnanolone (3α,5α-THP), postpartum depression, fast-acting antidepressant, GABAA receptor, 5α-reduced steroids, 5α-reductase, 3α-HSD
Citation: Pinna G (2020) Allopregnanolone, the Neuromodulator Turned Therapeutic Agent: Thank You, Next? Front. Endocrinol. 11:236. doi: 10.3389/fendo.2020.00236
Received: 19 February 2020; Accepted: 31 March 2020;
Published: 14 May 2020.
Edited by:
Hubert Vaudry, Université de Rouen, FranceReviewed by:
Jamie Maguire, Tufts University School of Medicine, United StatesCopyright © 2020 Pinna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Graziano Pinna, Z3Bpbm5hQHVpYy5lZHU=; Z3Jhemlhbm9fcGlubmFAeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.