- 1Department of Breast Surgery, The Second Hospital of Shandong University, Jinan, China
- 2Institute of Translational Medicine of Breast Disease Prevention and Treatment, Shandong University, Jinan, China
- 3School of Medicine, Shandong University, Jinan, China
- 4Breast Disease Center, Peking University First Hospital, Beijing, China
- 5Department of Breast Surgery, The First Hospital of Jilin University, Changchun, China
- 6Breast Disease Center, Peking University People's Hospital, Beijing, China
- 7Department of Breast Surgery, Affiliated Tumor Hospital of Zhengzhou University, Zhengzhou, China
- 8Breast Center, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
- 9Department of Breast Surgery, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China
- 10Department of Thyroid and Breast Surgery, The First Affiliated Hospital of Binzhou Medical University, Binzhou, China
- 11Department of Breast Surgery, Cancer Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 12Department of General Surgery, Linyi People's Hospital, Linyi, China
- 13Department of General Surgery, Beijing Chaoyang Hospital, Beijing, China
- 14Breast Center, Qingdao University Affiliated Hospital, Qingdao, China
- 15Department of Breast and Thyroid Surgery, Weifang Traditional Chinese Hospital, Weifang, China
- 16Department of Breast Surgery, The Second Affiliated Hospital of Qingdao Medical College, Qingdao Central Hospital, Qingdao, China
- 17Department of General Surgery, The Second Affiliated Hospital of Harbin Medical University, Harbin, China
- 18Department of Breast Surgery, The First Affiliated Hospital of China Medical University, Shenyang, China
- 19Department of General Surgery, Nanjing Medical University Affiliated Cancer Hospital Cancer Institute of Jiangsu Province, Nanjing, China
- 20Department of Breast Surgery, Shanxi Cancer Hospital, Taiyuan, China
- 21Suzhou Institute, Shandong University, Suzhou, China
Objective: To investigate the association between metabolic syndrome and breast cancer and to elucidate the potential mechanism underlying this association.
Patients and Methods: Based on baseline data drawn from 21 hospitals in 11 provinces of China, we performed a case–control study among 1,127 women (595 cases and 532 controls), divided into premenopausal, and postmenopausal subgroups. Student's t test, Pearson's χ2 test, and logistic regression analyses were performed to ascertain the association between breast cancer and metabolic syndrome, including all of its components. In addition, we attempted to clarify the potential role of adiponectin in this association.
Results: Among the components of metabolic syndrome, abnormal waist circumference was the component that markedly increased breast cancer risk in premenopausal women (OR 1.447, 95% CI 1.043–2.006). Metabolic syndrome with clusters of special risk factors showed an association with breast cancer risk. Among all these components of metabolic syndrome, the hypertriglyceridemic-waist (HW) phenotype significantly increased breast cancer risk (OR 1.56, 95% CI 1.02–2.39), regardless of menopausal status, rendering it a strong predictor of breast cancer. Total adiponectin levels and high-molecular-weight adiponectin were reversely associated with metabolic syndrome. In addition, total adiponectin levels among breast cancer patients were much lower than among controls (p = 0.005) only in the HW phenotype subgroup. Furthermore, the HW phenotype was associated with increased risk of estrogen receptor/progesterone receptor-positive (ER+/PR+) breast cancer, with a 95% (OR = 1.95, 95% CI:1.21–3.13) increase. However, there was no significant association between the HW phenotype and both ER+/PR– and ER–/PR– subtypes. These results suggested that low adiponectin levels may be a mechanism that explains the association between the HW phenotype and breast cancer risk.
Conclusion: Metabolic syndrome with special cluster factors is related to breast cancer risk; in particular, the HW phenotype can be regarded as a strong predictor of breast cancer. As an important factor involved in fat metabolism, adiponectin may strongly predict metabolic syndrome, especially the HW phenotype and breast cancer. Further research into this mechanism and epidemiological studies are needed. This study provides new evidence for the role of a healthy lifestyle in preventing breast cancer.
Introduction
Breast cancer is known as the most prevalent cancer among women worldwide and has been the leading cause of female cancer deaths globally (1, 2). In China, breast cancer is also the most commonly diagnosed cancer and the sixth leading cause of cancer deaths among women, with an age-standardized rate (ASR) of 22.1 cases and 5.4 cases per 100,000 women, respectively, according to data from the GLOBOCAN 2012 (2, 3). In addition to the known risk factors associated with breast cancer, such as breastfeeding and number of childbirths, the westernization of traditional lifestyles has contributed substantially to this difference and is drawing more and more attention (4–6).
China has experienced fast economic growth and urbanization since the 1980s (7). Meanwhile, a rapid lifestyle transition has occurred, including nutrition changes characterized by increased energy intake from dietary fat and red meat, which increased, respectively, from 22 to 29.8% and from 9.3 to 13.7% between 1992 and 2002, and a sedentary lifestyle (7–9). Urbanization and the shift to a Westernized lifestyle have led to a substantial increase in a series of non-communicable chronic diseases, such as diabetes, obesity, metabolic syndrome, and cancers (10, 11). Currently, metabolic syndrome (MetS), a group of medical conditions that comprises obesity along with abnormal metabolic factors, including high blood pressure (BP), impaired fasting glucose (Glu), low high-density lipoprotein (HDL), and high triglycerides (TG) (12–15), represents one of the most complex public health challenges. Metabolic syndrome is defined as the coexistence of several risk factors for cardiovascular diseases, diabetes, and certain cancers, such as endometrial cancer, prostate cancer, colorectal cancer, and breast cancer (16).
Many studies have focused on abnormal metabolic factors separately from breast cancer risk (17). However, studies considering metabolic syndrome as an entity are comparatively scarce and the results of available studies have been inconsistent. Reports from most Western countries have confirmed the association in various subgroups (18–21), yet reports to the contrary do exist (22). A similar situation is present in studies among Asian populations (23–25). In China, there are much less epidemiological data on the relationship between metabolic syndrome as an entity and breast cancer risk (26). Moreover, as one of the components of metabolic syndrome, the hypertriglyceridemic-waist (HW) phenotype is characterized by the simultaneous presence of elevated waist circumference (WC) and concentration of triglycerides, which are strong predictors of chronic diseases, such as coronary artery disease, chronic kidney disease, and abnormal glucose metabolism (27, 28). Several studies have indicated that it is visceral obesity rather than subcutaneous obesity that relate to metabolic abnormalities (29). Therefore, the HW phenotype has emerged as a stronger predictor for those chronic diseases than metabolic syndrome, for it has been validated to be one of the convenient markers of visceral obesity (28). However, the relationship between this typical phenotype and breast cancer is still unclear. The Chinese population is more likely to be viscerally obese or centrally obese in spite of generally having a low BMI (29–31). For this reason, investigating the association between the HW phenotype and breast cancer is necessary and may provide new insight into the prevention of the disease.
Figuring out the mechanisms underlying how metabolic syndrome is associated with breast cancer is particular important, as the negative impact of these risk factors could be attenuated to some extent through lifestyle intervention and conservative therapy of underlying metabolic conditions. Insulin resistance and chronic inflammation have come to be regarded as the two main mechanisms bridging metabolic syndrome and breast cancer (32, 33). Adipokines, which are involved in both insulin resistance and chronic inflammation, have also been demonstrated to contribute to the pathogenesis of abnormal metabolic factors, including obesity, diabetes, hyperlipemia, and high BP (34, 35). The dysregulation of adiponectin, the most abundant adipokine (36), does not only play a role in metabolic syndrome but also in breast cancer (37). Our previous meta-analysis and epidemiological results confirmed that a higher circulating high-molecular-weight (HMW) adiponectin (known as the active form) decreased breast cancer risk, especially in postmenopausal women (38, 39). However, most reported results were obtained from cellular and molecular experiments, and there have been few studies with intact data systematically assessing adiponectin as the molecular mechanism underlying the association between metabolic syndrome and breast cancer morbidity.
Therefore, the aim of this study was to evaluate the association between metabolic syndrome and breast cancer risk in a large-scale sample of Chinese women at large scale and to research whether adiponectin could link metabolic syndrome and breast cancer as a potential molecular mechanism, to provide new insight into the prevention of the disease.
Materials and Methods
Patients and Public Involvement
A multicenter stratified inclusion process was used to enroll the participants from 21 hospitals in 11 provinces in northern and eastern China from April 2012 to April 2013. All participants were voluntarily involved in our research, including finishing a self-designed questionnaire that was previously developed to record information through person-to-person interviews. All trained staffs were involved in the recruitment of participants and conduct of the study. Written informed consent was obtained from each participant by investigators as part of the interview. The patients' advisors have been thanked in the Acknowledgments section.
Study Participants
The inclusion criteria for cases were as follows: (1) newly diagnosed and histologically confirmed breast cancer; (2) Han ethnic group; and (3) aged 25–70 years old. For the control group, the following criteria were used: (1) negative physical examination results; (2) negative ultrasound breast scans and/or mammographic screening results; (3) matched age with cases (±3 years); (4) women who had been hospitalized or had a regular physical examination in the same hospital as matched case in the same time period; (5) no evidence of cancer or history of cancer; and (6) Han ethnic group. Furthermore, all included cases and controls should have complete data on metabolic factors, adiponectin levels by ELISA, and anthropometric measurements.
Upon applying the aforementioned criteria, there were a total 1,127 participants (595 cases and 532 controls) included in this study. According to menopausal status and excluding 5 participants with unknown menopausal status, 383 cases, and 339 controls were included in the premenopausal subgroup; 209 cases and 191 controls were included in the postmenopausal subgroup. The study protocols and procedures were approved by the Institutional Review Board at the Second Hospital of Shandong University.
Data Collection
Data were obtained through in-person interviews based on a self-designed, structured questionnaire. The questionnaire contained six sections: (1) demographic characteristics and female physiological and reproductive factors; (2) medical and family history: primarily, breast-related diseases, and family history of breast cancer; (3) lifestyle habits; (4) medication and chemical exposure history; (5) breast cancer-related knowledge; and (6) medical records. The histological and immunohistochemical diagnoses of breast cancer patients were also collected from the medical records.
Anthropometric measurement was conducted by clinicians. WC was measured to the nearest 0.1 cm, with participants wearing light clothing. High BP was defined as ≥130 mmHg systolic BP or ≥85 mmHg diastolic BP or under antihypertensive drug treatment for patients with a history of hypertension, as it was nearly the same in the four criteria for metabolic syndrome (Supplemental Table 1).
Laboratory Analyses
Total and HMW adiponectin levels were assayed from plasma using human total adiponectin and HMW adiponectin quantitative ELISA kits, respectively (SRP300, SHWAD0; RD Systems). Each sample was assayed twice and the average of the data was used. No samples were below the detection limits. All analyses were performed according to the manufacturer's recommended protocols.
Definition of Metabolic Syndrome and HW Phenotype
The criteria for metabolic syndrome have been defined by four different organizations, including the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) in the United States in 2005 (12), the International Diabetes Federation (IDF) in 2005 (12), the Chinese Diabetes Society (CDS) in 2007 (15), and the IDF and NCEP-ATPIII Joint Interim Statement published in 2009 (14) (Supplemental Table 1).
The HW phenotype was represented by the simultaneous presence of elevated WC (≥90 cm for men, ≥80 cm for women) and elevated serum triglyceride concentrations (TG concentrations ≥1.7 mmol/L) according to pre-determined cutoff points and criteria used in published work with Chinese populations (40, 41).
Statistical Analysis
We analyzed and compared the distributions of metabolic syndrome, components, and adiponectin in the case and control groups. Descriptive characteristic variables were expressed as means ± standard deviations (SD). P-values of continuous variables were determined by Student t tests and those of categorical variables by chi-squared tests. Odds ratios (ORs) and 95% confidence intervals (CIs) were obtained by single binary logistic regression analyses, and factors were related to breast cancer. Covariates considered in the adjusted model included age, number of childbirths (≤1, 2, ≥3), age at menarche (≤13, 14, ≥15 years), breastfeeding (yes, no), smoking (never, occasionally, regularly), alcohol use (never, occasionally, regularly), family history of breast cancer (yes, no), and contraceptive drug use (yes, no).
All P-values were two-sided and P < 0.05 was considered significant. All analyses were performed using IBM SPSS 21.0 statistical software (IBM Corp., Armonk, NY, USA).
Results
Participant Characteristics
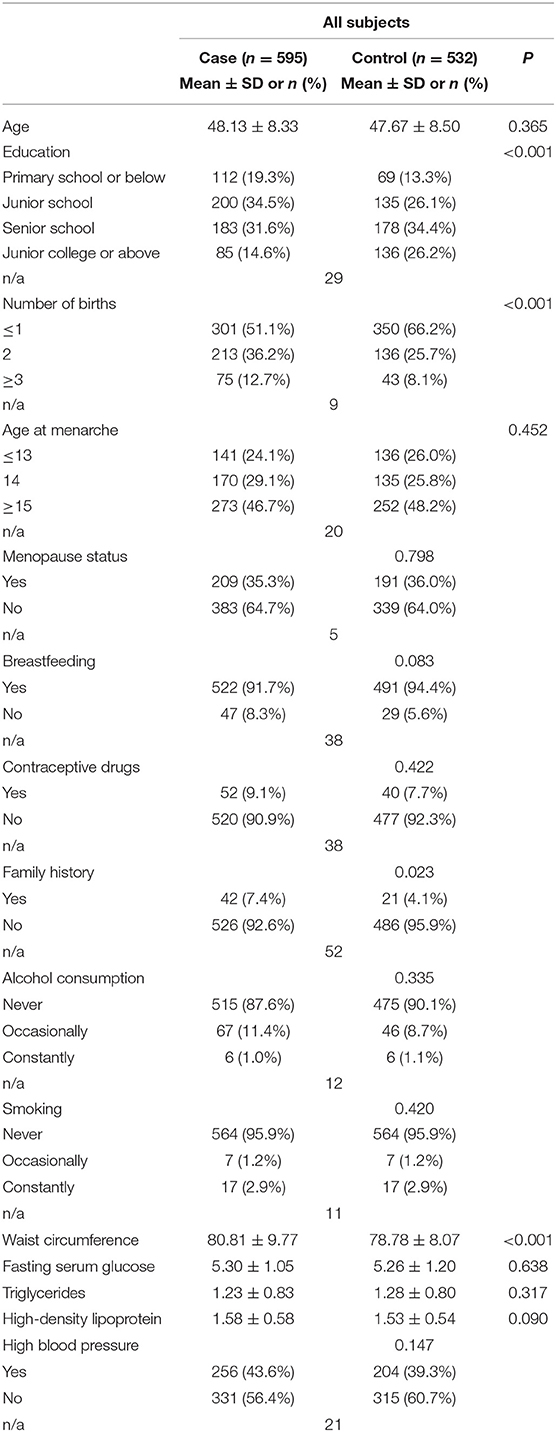
The demographic and clinical characteristics of the participants are shown in Table 1. Education level and number of births showed a significant difference between cases and controls (P < 0.001). A family history of breast cancer was more prevalent among cases than controls. Other factors, such as breastfeeding and age at menarche, showed no differences. Among metabolic components in metabolic syndrome, WC was greater among cases; other metabolic factors showed no statistical significance.
IDF Criteria Are Superior for Evaluating the Risk
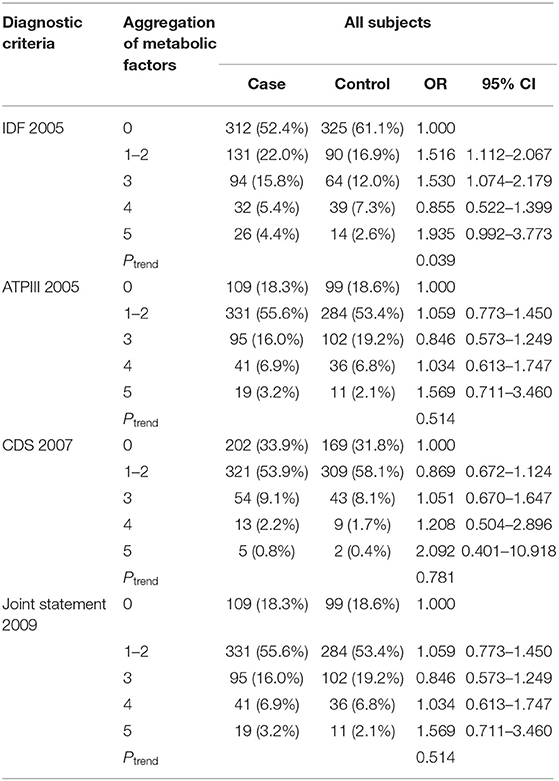
With greater understanding of metabolic syndrome, the corresponding criteria have changed. To more reasonably assess the influence of metabolic syndrome on breast cancer risk and determine the most appropriate subject of further investigation, we compared four diagnostic criteria. The results (Table 2) confirmed that the 2005 definition of the IDF yielded only positive results, with OR 1.530 (95% CI 1.074–2.197). Moreover, by IDF criteria, the result of the Ptrend indicated that breast cancer morbidity increased with the number of abnormal factors in each cluster.
As a Metabolic Symptom Component, WC Increases Breast Cancer Risk
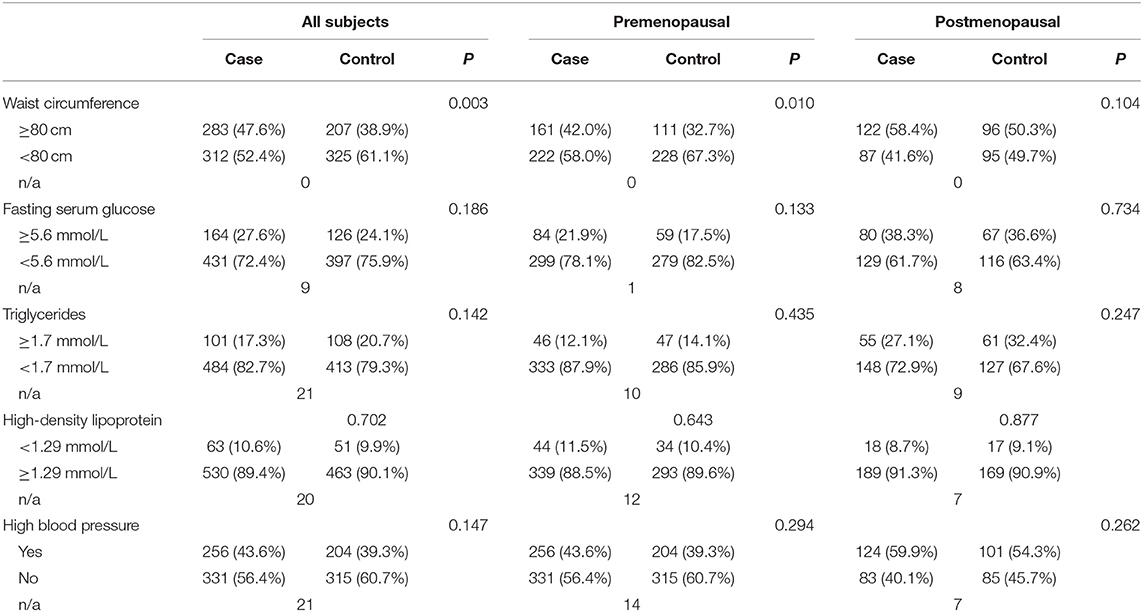
Using IDF criteria, we were able to categorize the variables and analyze the associations between breast cancer and metabolic factors according to menopausal status. By synthesizing the results (Tables 3, 4), we found that WC (an indicator of abdominal obesity) was associated with breast cancer risk in the premenopausal subgroup. Moreover, by conducting a multivariate logistic regression, a larger WC significantly increased breast cancer risk, with OR 1.447 (95% CI 1.043–2.006). However, other metabolic factors showed no association with breast cancer risk.
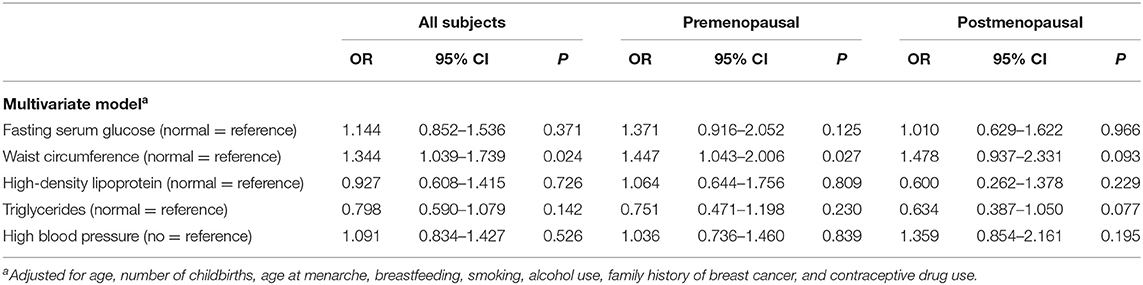
Table 4. Association between metabolic symptom components and breast cancer by multivariate logistic regression.
Cluster Mode of HW Phenotype Significantly Increases Breast Cancer Risk
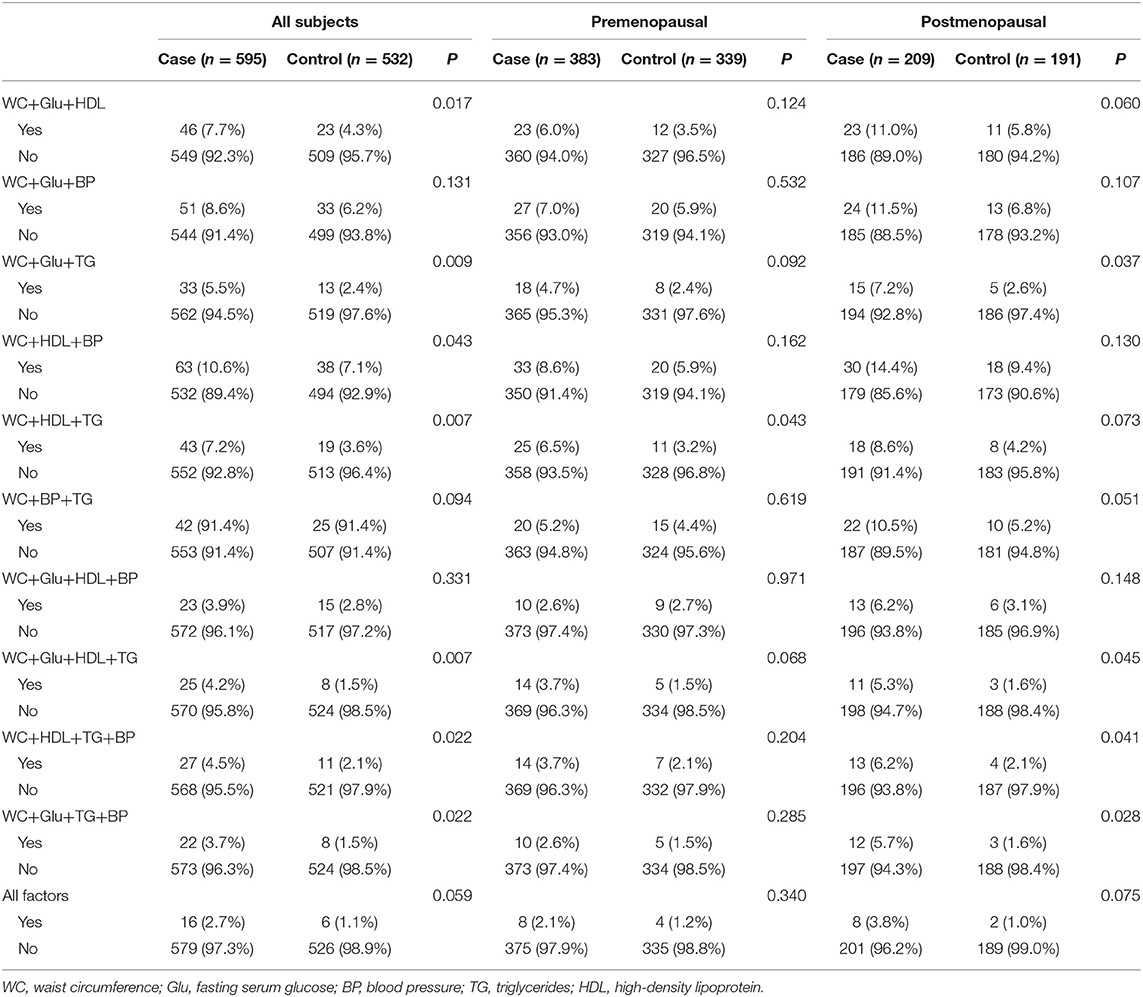
The different aggregation patterns that met the diagnosis were analyzed. As shown in Table 5, women with premenopausal metabolic syndrome who had abnormal values for WC+HDL+TG showed the highest breast cancer risk. As for the postmenopausal group, a greater number of abnormal conditions including WC+Glu+TG, WC+Glu+HDL+TG, WC+HDL+TG+BP, and WC+Glu+TG+BP increased breast cancer risk, with clusters of nearly all four risk factors. In postmenopausal women, abnormal WC+BP+TG was borderline significantly related to breast cancer risk.
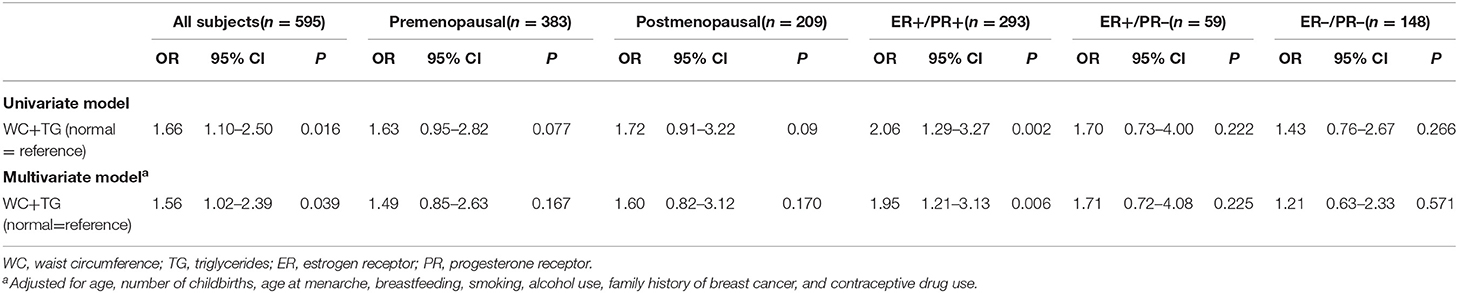
Abnormal WC+TG, known as HW phenotype, can be seen in all positive results in Table 6, in all participants and subgroups. By conducting a logistic regression, it was evident that the HW phenotype significantly increased breast cancer risk, with an OR 1.563 (95% CI 1.023–2.387), regardless of menopausal status (Table 6). Although not significant in both pre- and postmenopausal subgroups, OR values (1.492 and 1.599, respectively) predicted a link with breast cancer to some degree.
We also investigated associations between HW phenotype and breast cancer risk according to joint ER/PR status. Similar to what was found in all participant, HW phenotype was associated with ER+/PR+ breast cancer, with a 95% (OR = 1.95, 95% CI:1.21–3.13) increase in risk for women with a positive HW phenotype. However, there was no significant association between HW phenotype and both ER+/PR– and ER–/PR– subtypes.
Adiponectin Might Be the Mechanism Linking Metabolic Syndrome to Breast Cancer
As a latent mechanism, the effects of adiponectin on metabolic syndrome warrant investigation. As shown in Table 7, total adiponectin and HMW adiponectin were reversely associated with metabolic syndrome regardless of menopausal status. Similar results were obtained for total adiponectin with WC and TG, and HMW adiponectin with TG (Table 8). Differing by subgroup, total adiponectin and HMW adiponectin were associated with Glu in premenopausal women but with HDL in postmenopausal women (Table 8). Nevertheless, the HMW/total ratio was not correlated with metabolic syndrome and any of its components.
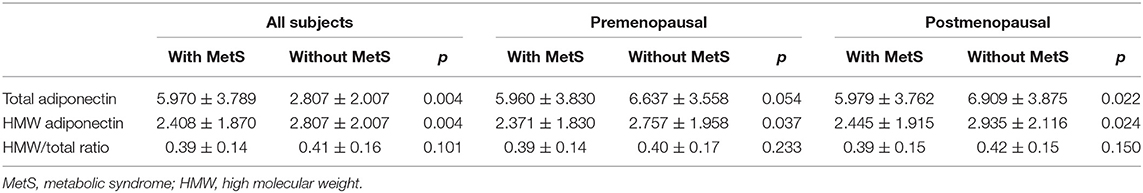
Table 7. Association between total adiponectin, HMW adiponectin, HMW/total ratio, and metabolic syndrome.
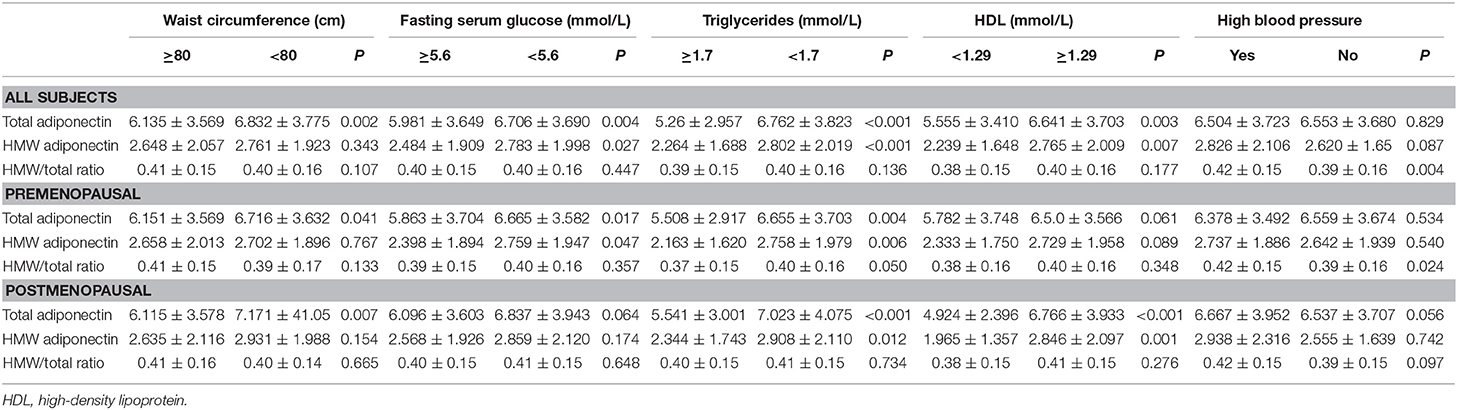
Table 8. Association between total adiponectin, HMW adiponectin, HMW/total ratio, and all components of metabolic syndrome.
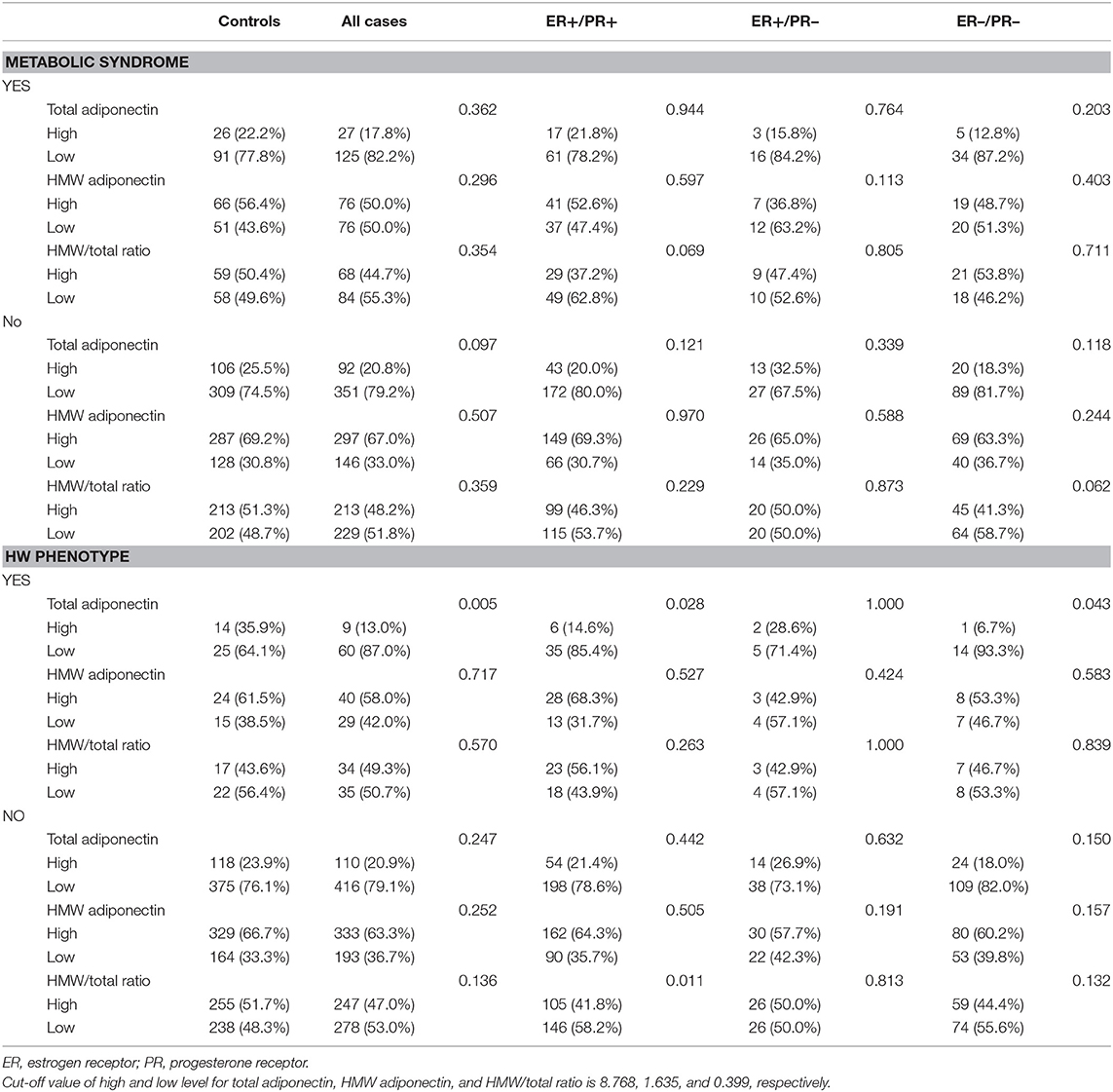
In addition, we proceeded to analyze the possible association among breast cancer, metabolic syndrome, and adiponectin. We found that such a relationship indeed existed, for there was significant difference in total adiponectin levels between breast cancer patients and the controls only in the population with the HW phenotype. As shown in Table 9, total adiponectin levels among breast cancer patients were much lower than among the controls (p = 0.005) in the HW phenotype subgroup.
To clarify the role of adiponectin in breast cancer depending on hormone receptor, we also conducted a subgroup analysis by joint ER/PR status. Similar to the findings regarding the association between the HW phenotype and breast cancer risk, there was a significant difference of total adiponectin in ER+/PR+ (p = 0.028) and ER–/PR– (p = 0.043) breast cancer compared to the controls, who were much lower in the HW phenotype subgroup. Conversely, such a difference was not found in women without the HW phenotype as well as ER+/PR– breast cancer with the HW phenotype.
Discussion
In this case–control study, we found that among components in metabolic syndrome by IDF criteria, WC (an indicator of central obesity) was strongly associated with increased premenopausal breast cancer risk. Metabolic syndrome, especially the clustering of three or four components, increases breast cancer risk, which is more common in postmenopausal women. Across various aggregation patterns, the HW phenotype showed a strong correlation with increased breast cancer risk.
Metabolic syndrome, composed of five aberrant metabolic factors, is receiving growing attention because of its close link with lifestyle (12). There are only few epidemiological studies in this area worldwide, which have yielded conflicting results. In addition to differences in study design, sample size, and ethnic groups studied, the diagnostic criteria adopted for metabolic syndrome might have contributed to the conflicting results. Therefore, we compared the four most recent metabolic syndrome definitions and found the 2005 IDF definition to be the most appropriate. This is the first time that the diagnostic criteria of the 2009 Joint Statement have been used to evaluate the influence of metabolic syndrome on breast cancer risk. Consistent with our results, a study in Korea also compared two sets of diagnostic criteria for metabolic syndrome and confirmed that the IDF criteria were superior (23). In assessing breast cancer risk, Rodriguez-Ortiz et al. (37) used IDF and ATP criteria to evaluate metabolic syndrome remission in a cohort of patients undergoing Roux-en-Y gastric bypass; the IDF criteria were confirmed as more suitable, which further verified that different metabolic syndrome criteria might yield correspondingly discrepant results.
In the present study, among components of metabolic syndrome, increased WC was the only factor related to increased premenopausal breast cancer risk. Although it is generally known that obesity is positively associated with postmenopausal breast cancer risk, this association in premenopausal women remains controversial (42–44). Central obesity, which reflects visceral fat as measured by WC or waist-to-hip ratio, could more accurately explain obesity-related health risk (45) than body mass index (BMI), which is a measure of both adipose tissue and lean mass (46). In line with our study, Nagrani et al. observed that a larger WC was associated with a threefold increased risk of breast cancer regardless of menopausal status (47). Moreover, a dose–response meta-analysis of prospective studies also confirmed that central obesity as measured by WC was associated with increased risk of premenopausal breast cancer after adjustment for BMI (48).
To thoroughly evaluate the effect of metabolic syndrome on breast cancer risk, it is important to assess the influence on breast cancer risk exerted by cluster patterns among various metabolic factors, which in the last decade has only been investigated in a study by Wang et al. (26). Correspondingly, in our study, various aggregations gave rise to different effects. Furthermore, by crossing positive associations of cluster patterns with cancer risk, abnormal WC+TG had a fundamental predictive influence on this association. Defined as HW phenotype, its involvement in coronary artery disease, insulin resistance, and hypertension has been confirmed (49–51). As WC cannot fully discriminate visceral adiposity from subcutaneous abdominal adiposity, elevated triglyceride (TG) levels have been adopted as a marker of dysfunctional visceral adipose tissue. Published data have demonstrated that the HW phenotype is a stronger predictor of certain chronic diseases than WC, BMI, as well as metabolic syndrome. As the Chinese population is likely to be viscerally obese or centrally obese in spite of generally having a low BMI, we should make efforts to improve the metabolic health of this high-risk group, and encourage their engagement in early intensive lifestyle modification, as simple weight loss might not be the optimal solution for them. Notably, to our knowledge, our study is the first to evaluate the role of the HW phenotype in breast cancer risk, even in all cancers. Verified increased inflammation in this phenotype may provide an explanation for this increased risk (52).
In terms of the mechanisms underlying the association between metabolic syndrome and breast cancer, two common mechanisms are generally accepted. One mechanism lies in insulin resistance and hyperinsulinemia, which are especially associated with abdominal obesity, and appear to be central to the development of metabolic syndrome and might contribute to dyslipidemia and altered levels of circulating estrogens (32, 33, 53). The other mechanism lies in chronic inflammation caused by the accumulation of immune cells in adipose tissue and impaired secretion of adipokines, including a variety of proinflammatory cytokines, which could be a further linking factor between breast cancer and systemic insulin resistance (32). Clearly, adipokines secreted by adipose tissue are implicated in both mechanisms. Moreover, the present results for WC and the HW phenotype point to abnormalities in lipid metabolism. Contrary to the roles of most adipokines in proinflammation and carcinogenesis (54, 55), adiponectin—the most abundant adipokine—mainly exhibits inverse properties (56). Details of the signal pathway by which adiponectin acts have been summarized in several milestone reviews (38, 43, 56). In brief, the comprehensive role of adiponectin could be embodied within two pathways: first, by directly inhibiting breast cancer cell proliferation and promoting apoptosis; second, by acting on the receptors AdipoR1/R2, binding the APPL-1 protein, and stimulating the downstream pathway leading to insulin-sensitizing and anti-inflammatory effects, in turn, directly suppressing metabolic syndrome and indirectly suppressing antineoplastic properties.
Considering the known unique cellular and molecular mechanisms and scarce epidemiological data, along with the obtained results, we attempted to assess adiponectin as a potential mechanism. We confirmed the association between increased adiponectin and decreased metabolic syndrome regardless of menopausal status and form of adiponectin. Similarly, our previous study with the same project verified that HMW adiponectin was associated with decreased breast cancer risk, especially in postmenopausal women (39). What's more, we have found that when considering the influence of metabolic abnormality on the occurrence of breast cancer, the adiponectin showed a significant association with breast cancer only in the HW phenotype population. That is, adiponectin may function as one of the potential mechanisms linking metabolic abnormality and breast cancer because of its distinctive biological behaviors in all adipokines. In this regard, the contribution of adiponectin to breast cancer occurrence and progression is still controversial. Several studies found that adiponectin acted as a negative regulator of estrogen receptor alpha negative breast cancer, while adiponectin might restrain the development of estrogen receptor alpha positive breast cancer when at relatively low concentrations (57–60). The present study also found that adiponectin was associated with estrogen receptor-positive/progestogen receptor-positive and estrogen receptor-negative/progestogen receptor-negative breast cancer with the HW phenotype. Consistent with what we found in our previous study, namely, that general obesity, as indicated by BMI, was associated with the ER+/PR+ subtype, whereas central obesity, as indicated by waist/hip ratio, was more specific for the ER–/PR– subtype (61), We revealed that HW phenotype was an independent risk factor for the ER+/PR+ subtype. Therefore, the HW phenotype might function as a stronger marker of dysfunctional visceral lipid metabolism than BMI or WHR in predicting breast cancer risk. For physiologically adiponectin governed glucose levels and lipid metabolism (62), it might mediate the cross-talk between the HW phenotype and breast cancer especially subtyped by joint of ER and PR status.
In summary, our findings from the large Chinese representative data indicated that metabolic syndrome especially the HW phenotype, can significantly increase breast cancer risk, which was closely related to the “Western/new affluence” lifestyle, characterized by high energy intake and physical inactivity. Fortunately, healthy dietary patterns and an active lifestyle may play important roles in reducing the metabolic syndrome, which could be adopted as approaches for the prevention of breast cancer (63–65). Indeed, previous studies have shown an inverse relationship between metabolic syndrome and the Mediterranean diet, and metabolic syndrome could be reversed by adherence to the Mediterranean diet, with a reduction in the prevalence of metabolic syndrome to one-third after 2 years of the diet (66–68). Moreover, multiple studies have indicated that nutritional modifications and higher physical activity could attenuate the risk of breast cancer. The Iowa Women's Health Study confirmed that high levels of physical activity reduced the risk of post-menopausal breast cancer by 14% (65). Besides, one published study estimated that more than 30% of breast cancer cases could be prevented by lifestyle modification (65, 69). Therefore, as a result of the shift to “Western/new affluence” lifestyle, the rising prevalence of metabolic syndrome as well as breast cancer could be attenuated to some extent through lifestyle intervention and conservative therapy of underlying metabolic conditions. These days, much effort is being focused on encouraging lifestyle changes in adults.
Our study had several strengths. First, we further assessed the association between breast cancer risk and metabolic syndrome with special clusters of factors, and this study provided the first confirmation that the HW phenotype increased breast cancer risk. Second, we evaluated metabolic syndrome as an entity in its association with breast cancer among Chinese women across a wide geographic region (11 provinces) and using a relatively large sample. Third, by leveraging intact data, we performed the study by strictly following the diagnostic criteria rather than neglecting or replacing components. Fourth, the diagnostic criteria for metabolic syndrome are relatively recent. The 2009 Joint Statement and 2007 CDS definitions are first used in this study to evaluate the association of metabolic syndrome with breast cancer. Concurrently, our study also has several potential limitations. First, we only analyzed the data at baseline with no follow-up conducted, which could have provided a comprehensive evaluation of metabolic syndrome and breast cancer. Second, regarding molecule subtype age, we did not obtain results of metabolic syndrome with respect to breast cancer subtype. Third, due to the observational nature of the study, the precise mechanism for the results could not be fully explained, warranting further clarification. Despite these limitations, the study is meaningful in that it is the first retrospective study regarding this issue performed with a large Chinese population.
Conclusions
To conclude, a large WC is strongly associated with increased breast cancer risk in premenopausal women. Metabolic syndrome with special cluster factors is related to breast cancer risk, and the HW phenotype significantly increases breast cancer risk. The rising prevalence of metabolic syndrome as well as breast cancer, which is attributable, at least partly, to the shift to the “Western/new affluence” lifestyle, could be attenuated to some extent through lifestyle intervention and conservative therapy of underlying metabolic conditions. As an important factor involved in fat metabolism, adiponectin, especially low adiponectin levels, may explain the association between metabolic abnormality and breast cancer to some extent. Further research into this mechanism and epidemiological studies are needed.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The study protocols and procedures were approved by the Institutional Review Board at the Second Hospital of Shandong University. Written informed consent was obtained from each participant by investigators as part of the interview.
Author Contributions
YX, WZ, and ZYu conceived and designed the experiments and wrote the paper. YX, WZ, ZYu, XD, ZF, SW, SL, FW, LY, FZ, LLi, QZ, QF, DG, SC, CG, XC, ZYa, XW, HL, HJ, HW, GL, QW, JZ, FJ, JT, FT, and CY performed the experiments. YX, WZ, and LLiu analyzed the data. SH and ZM contributed reagents, materials, analysis tools. ZYu supplied suggestion on study design as well as manuscript preparation.
Funding
This research was primarily funded by the Major Scientific and Technological Innovation Project of Shandong Province (2017CXGC1212), the National Key Research and Development Program of China (2016YFC0901304), the Seed Fund of the Second Hospital of Shandong University (S2015010015), and the Natural Science Foundation of Shandong Province (ZR2014HZ004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all subjects and patients' advisers involved in the study for their participation. We are also grateful to the Central Research Laboratory of the Second Hospital of Shandong University for their technical assistance and generous support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2019.00905/full#supplementary-material
References
1. Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. (2008) 132:1133–45. doi: 10.1002/ijc.27711
2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136:E359–86. doi: 10.1002/ijc.29210
3. Ferlay J, Shin HR, Bray F, Forman D. Cancer Incidence and Mortality Worldwide:IARC Cancer Base No. 11. (2015). Available online at: http://globocan.iarc.fr
4. Turati F, La Vecchia C. Risk factors for breast cancer in China: similarities and differences with western populations. Arch Med Sci. (2012) 8:179–82. doi: 10.5114/aoms.2012.28542
5. Shu XO, Jin F, Dai Q, Shi JR, Potter JD, Brinton LA, et al. Association of body size and fat distribution with risk of breast cancer among Chinese women. Int J Cancer. (2001) 94:449–55. doi: 10.1002/ijc.1487
6. Xu YL, Sun Q, Shan GL, Zhang J, Liao HB, Li SY, et al. A case-control study on risk factors of breast cancer in China. Arch Med Sci. (2012) 8:303–9. doi: 10.5114/aoms.2012.28558
7. Ma RC, Lin X, Jia W. Causes of type 2 diabetes in China. Lancet Diab Endocrinol. (2014) 2:980–91. doi: 10.1016/S2213-8587(14)70145-7
8. Peng X. China's demographic history and future challenges. Science. (2011) 333:581–7. doi: 10.1126/science.1209396
9. Gong P, Liang S, Carlton EJ, Jiang Q, Wu J, Wang L, et al. Urbanisation and health in China. Lancet. (2012) 379: 843–52. doi: 10.1016/S0140-6736(11)61878-3
10. Goss PE, Strasser-Weippl K, Lee-Bychkovsky BL, Fan L, Li J, Chavarri-Guerra Y, et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. (2014) 15:489–538. doi: 10.1016/S1470-2045(14)70029-4
11. Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, et al. Breast cancer in China. Lancet Oncol. (2014) 15:e279–89. doi: 10.1016/S1470-2045(13)70567-9
12. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
13. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. (2005) 112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404
14. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. (2006) 23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x
15. Joint Committee for Developing Chinese guidelines on P Treatment of Dyslipidemia in A. [Chinese guidelines on prevention and treatment of dyslipidemia in adults]. Zhonghua Xin Xue Guan Bing Za Zhi. (2007) 35:390–419. doi: 10.3760/j.issn:0253-3758.2007.05.003
16. Candi E, Tesauro M, Cardillo C, Lena AM, Schinzari F, Rodia G, et al. Metabolic profiling of visceral adipose tissue from obese subjects with or without metabolic syndrome. Biochem J. (2018) 475:1019–35. doi: 10.1042/BCJ20170604
17. Bi Y, Lu J, Wang W, Mu Y, Zhao J, Liu C, et al. Cohort profile: risk evaluation of cancers in Chinese diabetic individuals: a longitudinal (REACTION) study. J Diabetes. (2014) 6:147–57. doi: 10.1111/1753-0407.12108
18. Kabat GC, Kim M, Chlebowski RT, Khandekar J, Ko MG, McTiernan A, et al. A longitudinal study of the metabolic syndrome and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. (2009) 18:2046–53. doi: 10.1158/1055-9965.EPI-09-0235
19. Agnoli C, Berrino F, Abagnato CA, Muti P, Panico S, Crosignani P, et al. Metabolic syndrome and postmenopausal breast cancer in the ORDET cohort: a nested case-control study. Nutr Metab Cardiovasc Dis. (2010) 20:41–8. doi: 10.1016/j.numecd.2009.02.006
20. Agnoli C, Grioni S, Sieri S, Sacerdote C, Ricceri F, Tumino R, et al. Metabolic syndrome and breast cancer risk: a case-cohort study nested in a multicentre italian cohort. PLoS ONE. (2015) 10:e0128891. doi: 10.1371/journal.pone.0128891
21. Calip GS, Malone KE, Gralow JR, Stergachis A, Hubbard RA, Boudreau DM. Metabolic syndrome and outcomes following early-stage breast cancer. Breast Cancer Res Treat. (2014) 148:363–77. doi: 10.1007/s10549-014-3157-6
22. Russo A, Autelitano M, Bisanti L. Metabolic syndrome and cancer risk. Eur J Cancer. (2008) 44:293–7. doi: 10.1016/j.ejca.2007.11.005
23. Noh HM, Song YM, Park JH, Kim BK, Choi YH. Metabolic factors and breast cancer risk in Korean women. Cancer Causes Control. (2013) 24:1061–8. doi: 10.1007/s10552-013-0183-3
24. Bjørge T, Lukanova A, Jonsson H, Tretli S, Ulmer H, Manjer J, et al. Metabolic syndrome and breast cancer in the me-can (metabolic syndrome and cancer) project. Cancer Epidemiol Biomarkers Prev. (2010) 19:1737–45. doi: 10.1158/1055-9965.EPI-10-0230
25. Osaki Y, Taniguchi S, Tahara A, Okamoto M, Kishimoto T. Metabolic syndrome and incidence of liver and breast cancers in Japan. Cancer Epidemiol. (2012) 36:141–7. doi: 10.1016/j.canep.2011.03.007
26. Wang M, Cheng N, Zheng S, Wang D, Hu X, Ren X, et al. Metabolic syndrome and the risk of breast cancer among postmenopausal women in North-West China. Climacteric. (2015) 18:852–8. doi: 10.3109/13697137.2015.1071346
27. Han KJ, Lee SY, Kim NH, Chae HB, Lee TH, Jang CM, et al. Increased risk of diabetes development in subjects with the hypertriglyceridemic waist phenotype: a 4-year longitudinal study. Endocrinol Metab. (2014) 29:514–21. doi: 10.3803/EnM.2014.29.4.514
28. Haack RL, Horta BL, Gigante DP, Barros FC, Oliveira I, Silveira VM. Hypertriglyceridemic waist phenotype: effect of birthweight and weight gain in childhood at 23 years old. PLoS ONE. (2015) 10:e0134121. doi: 10.1371/journal.pone.0134121
29. Zhou C, Peng H, Yuan J, Lin X, Zha Y, Chen H. Visceral, general, abdominal adiposity and atherogenic index of plasma in relatively lean hemodialysis patients. BMC Nephrol. (2018) 19:206. doi: 10.1186/s12882-018-0996-0
30. Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr. (2007) 86:353–9. doi: 10.1093/ajcn/86.2.353
31. Zhang P, Wang R, Gao C, Jiang L, Lv X, Song Y, et al. Prevalence of central obesity among adults with normal BMI and its association with metabolic diseases in Northeast China. PLoS ONE. (2016) 11:e0160402. doi: 10.1371/journal.pone.0160402
32. Hauner D, Hauner H. Metabolic syndrome and breast cancer: is there a link? Breast Care. (2014) 9:277–81. doi: 10.1159/000365951
33. Verma S, Hussain ME. Obesity and diabetes: an update. Diabetes Metab Syndr. (2017) 11:73–9. doi: 10.1016/j.dsx.2016.06.017
34. Scheid MP, Sweeney G. The role of adiponectin signaling in metabolic syndrome and cancer. Rev Endocr Metab Disord. (2014) 15:157–67. doi: 10.1007/s11154-013-9265-5
35. Srikanthan K, Feyh A, Visweshwar H, Shapiro JI, Sodhi K. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the west virginian population. Int J Med Sci. (2016) 13:25–38. doi: 10.7150/ijms.13800
36. Simone V, D'Avenia M, Argentiero A, Felici C, Rizzo FM, De Pergola G, et al. Obesity and breast cancer: molecular interconnections and potential clinical applications. Oncologist. (2016) 21:404–17. doi: 10.1634/theoncologist.2015-0351
37. Rodriguez-Ortiz D, Reyes-Perez A, Leon P, Sanchez H, Mosti M, Aguilar-Salinas CA, et al. Assessment of two different diagnostic guidelines criteria (National Cholesterol Education Adult Treatment Panel III [ATP III] and International Diabetes Federation [IDF]) for the evaluation of metabolic syndrome remission in a longitudinal cohort of patients undergoing Roux-en-Y gastric bypass. Surgery. (2016) 159:1121–8. doi: 10.1016/j.surg.2015.11.015
38. Liu LY, Wang M, Ma ZB, Yu LX, Zhang Q, Gao DZ, et al. The role of adiponectin in breast cancer: a meta-analysis. PLoS ONE. (2013) 8:e73183. doi: 10.1371/journal.pone.0073183
39. Guo MM, Duan XN, Cui SD, Tian FG, Cao XC, Geng CZ, et al. Circulating high-molecular-weight (HMW) adiponectin level is related with breast cancer risk better than total adiponectin: a case-control study. PLoS ONE. (2015) 10:e0129246. doi: 10.1371/journal.pone.0129246
40. Ding Y, Zhang M, Wang L, Yin T, Wang N, Wu J, et al. Association of the hypertriglyceridemic waist phenotype and severity of acute pancreatitis. Lipids Health Dis. (2019) 18:93. doi: 10.1186/s12944-019-1019-2
41. Chen S, Guo X, Dong S, Yu S, Chen Y, Zhang N, et al. Association between the hypertriglyceridemic waist phenotype and hyperuricemia: a cross-sectional study. Clin Rheumatol. (2017) 36:1111–9. doi: 10.1007/s10067-017-3559-z
42. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body fatness and cancer–viewpoint of the IARC working group. N Engl J Med. (2016) 375:794–8. doi: 10.1056/NEJMsr1606602
43. Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. (2017) 356:j477. doi: 10.1136/bmj.j477
44. Chlebowski RT. Nutrition and physical activity influence on breast cancer incidence and outcome. Breast. (2013) 22 (Suppl 2: S30–S37). doi: 10.1016/j.breast.2013.07.006
45. Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. (2004) 79:379–84. doi: 10.1093/ajcn/79.3.379
46. Phillips LK, Prins JB. The link between abdominal obesity and the metabolic syndrome. Curr Hypertens Rep. (2008) 10:156–64. doi: 10.1007/s11906-008-0029-7
47. Nagrani R, Mhatre S, Rajaraman P, Soerjomataram I, Boffetta P, Gupta S, et al. Central obesity increases risk of breast cancer irrespective of menopausal and hormonal receptor status in women of South Asian Ethnicity. Eur J Cancer. (2016) 66:153–61. doi: 10.1016/j.ejca.2016.07.022
48. Chen GC, Chen SJ, Zhang R, Hidayat K, Qin JB, Zhang YS, et al. Central obesity and risks of pre- and postmenopausal breast cancer: a dose-response meta-analysis of prospective studies. Obes Rev. (2016) 17:1167–77. doi: 10.1111/obr.12443
49. Chen S, Guo X, Yu S, Yang H, Sun G, Li Z, et al. Hypertriglyceridemic waist phenotype and metabolic abnormalities in hypertensive adults: a STROBE compliant study. Medicine. (2016) 0.95:e5613. doi: 10.1097/MD.0000000000005613
50. Janghorbani M, Amini M. Utility of hypertriglyceridemic waist phenotype for predicting incident type 2 diabetes: the isfahan diabetes prevention study. J Diabetes Investig. (2016) 7:860–6. doi: 10.1111/jdi.12520
51. Arsenault BJ, Lemieux I, Després JP, Wareham NJ, Kastelein JJ, Khaw KT, et al. The hypertriglyceridemic-waist phenotype and the risk of coronary artery disease: results from the EPIC-Norfolk prospective population study. CMAJ. (2010) 182:1427–32. doi: 10.1503/cmaj.091276
52. Esmaillzadeh A, Azadbakht L. Increased levels of inflammation among women with enlarged waist and elevated triglyceride concentrations. Ann Nutr Metab. (2010) 57:77–84. doi: 10.1159/000318588
53. Stern MP. Diabetes and cardiovascular disease. The “common soil” hypothesis. Diabetes. (1995) 44:369–74. doi: 10.2337/diabetes.44.4.369
54. Harwood HJ Jr. The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology. (2012) 63:57–75. doi: 10.1016/j.neuropharm.2011.12.010
55. D'Esposito V, Liguoro D, Ambrosio MR, Collina F, Cantile M, Spinelli R, et al. Adipose microenvironment promotes triple negative breast cancer cell invasiveness and dissemination by producing CCL5. Oncotarget. (2016) 7:24495–509. doi: 10.18632/oncotarget.8336
56. Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. (2012) 33:547–94. doi: 10.1210/er.2011-1015
57. Dos Santos E, Benaitreau D, Dieudonne MN, Leneveu MC, Serazin V, Giudicelli Y, et al. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol Rep. (2008) 20:971–7. doi: 10.3892/or_00000098
58. Andò S, Gelsomino L, Panza S, Giordano C, Bonofiglio D, Barone I, et al. Obesity, leptin and breast cancer: epidemiological evidence and proposed mechanisms. Cancers. (2019) 11:62. doi: 10.3390/cancers11010062
59. Mauro L, Pellegrino M, De Amicis F, Ricchio E, Giordano F, Rizza P, et al. Evidences that estrogen receptor α interferes with adiponectin effects on breast cancer cell growth. Cell Cycle. (2014) 13:553–64. doi: 10.4161/cc.27455
60. Mauro L, Pellegrino M, Giordano F, Ricchio E, Rizza P, De Amicis F, et al. Estrogen receptor-α drives adiponectin effects on cyclin D1 expression in breast cancer cells. The FASEB J. (2015) 29:2150–60. doi: 10.1096/fj.14-262808
61. Wang F, Liu L, Cui S, Tian F, Fan Z, Geng C, et al. Distinct effects of body mass index and waist/hip ratio on risk of breast cancer by joint estrogen and progestogen receptor status: results from a case-control study in northern and eastern china and implications for chemoprevention. Oncologist. (2017) 22:1431–43. doi: 10.1634/theoncologist.2017-0148
62. Gelsomino L, Naimo GD, Catalano S, Mauro L, Andò S. The emerging role of adiponectin in female malignancies. Int J Mol Sci. (2019) 20:2127. doi: 10.3390/ijms20092127
63. Mirmiran P, Noori N, Azizi F. A prospective study of determinants of the metabolic syndrome in adults. Nutr Metab Cardiovasc Dis. (2008) 18:567–73. doi: 10.1016/j.numecd.2007.06.002
64. Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study. BMJ. (2013) 354:i3857. doi: 10.1136/bmj.i3857
65. Li YR, Ro V, Tchou JC. Obesity, Metabolic Syndrome, and Breast Cancer: From Prevention to Intervention[J]. Curr Surg Rep. (2018) 6:7. doi: 10.1007/s40137-018-0204-y
66. Bruno E, Gargano G, Villarini A, Traina A, Johansson H, Mano MP, et al. Adherence to WCRF/AICR cancer prevention recommendations and metabolic syndrome in breast cancer patients. Intc J Cancer. (2016) 38:237–44. doi: 10.1002/ijc.29689
67. Salas-Salvadó J, Fernández-Ballart J, Ros E, Martínez-González MA, Fitó M, Estruch R, et al. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: one-year results of the PREDIMED randomized trial. Arch Intern Med. (2008) 168:2449–58. doi: 10.1001/archinte.168.22.2449
68. Tortosa A, Bes-Rastrollo M, Sanchez-Villegas A, Basterra-Gortari FJ, Nuñez-Cordoba JM, Martinez-Gonzalez MA. Mediterranean diet inversely associated with the incidence of metabolic syndrome: the SUN prospective cohort. Diabetes Care. (2007) 30:2957–9. doi: 10.2337/dc07-1231
Keywords: breast cancer, metabolic syndrome, hypertriglyceridemic-waist phenotype, adiponectin, risk
Citation: Xiang Y, Zhou W, Duan X, Fan Z, Wang S, Liu S, Liu L, Wang F, Yu L, Zhou F, Huang S, Li L, Zhang Q, Fu Q, Ma Z, Gao D, Cui S, Geng C, Cao X, Yang Z, Wang X, Liang H, Jiang H, Wang H, Li G, Wang Q, Zhang J, Jin F, Tang J, Tian F, Ye C and Yu Z (2020) Metabolic Syndrome, and Particularly the Hypertriglyceridemic-Waist Phenotype, Increases Breast Cancer Risk, and Adiponectin Is a Potential Mechanism: A Case–Control Study in Chinese Women. Front. Endocrinol. 10:905. doi: 10.3389/fendo.2019.00905
Received: 21 July 2019; Accepted: 11 December 2019;
Published: 21 January 2020.
Edited by:
Eva Surmacz, Allysta Pharmaceuticals, Inc., United StatesReviewed by:
Loredana Mauro, University of Calabria, ItalyCeshi Chen, Kunming Institute of Zoology, China
Copyright © 2020 Xiang, Zhou, Duan, Fan, Wang, Liu, Liu, Wang, Yu, Zhou, Huang, Li, Zhang, Fu, Ma, Gao, Cui, Geng, Cao, Yang, Wang, Liang, Jiang, Wang, Li, Wang, Zhang, Jin, Tang, Tian, Ye and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhigang Yu, eXpnQG1lZG1haWwuY29tLmNu
†These authors have contributed equally to this work and share first authorship
 Yujuan Xiang
Yujuan Xiang Wenzhong Zhou
Wenzhong Zhou Xuening Duan4
Xuening Duan4 Zhigang Yu
Zhigang Yu