
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Endocrinol. , 08 January 2020
Sec. Thyroid Endocrinology
Volume 10 - 2019 | https://doi.org/10.3389/fendo.2019.00884
This article is part of the Research Topic An Update on Thyroid Diseases at the 1st Italian Thyroid Preceptorship View all 8 articles
 Antonio Matrone1*
Antonio Matrone1* Maria Cristina Campopiano1
Maria Cristina Campopiano1 Alice Nervo2
Alice Nervo2 Giulia Sapuppo3
Giulia Sapuppo3 Martina Tavarelli3
Martina Tavarelli3 Simone De Leo4
Simone De Leo4Differentiated thyroid cancer (DTC) is the most frequent endocrine malignancy and represents the most rapidly increasing cancer diagnosis worldwide. In the last 20 years, this increase has been mostly due to a higher detection of small papillary thyroid cancers, with doubtful effects on patients' outcome. In fact, despite this growth, cancer-related death remained stable over the years. The growing detection of microcarcinomas associated to the indolent behavior of these cancers led to the development of strategies of active surveillance in selected centers of different countries. Moreover, toward a more personalized approach in the management of DTC patients, surgical treatments became more conservative, favoring less extensive options in patients at low risk of recurrence. The rise in lobectomy in low-risk cases and the need to avoid further therapies, with controversial impact on recurrences and cancer-related death in selected intermediate risk cases, led to reconsider the use of radioiodine treatment, too. Since clinicians aim to treat different patients with different modalities, the cornerstone of DTC follow-up (i.e., thyroglobulin, thyroglobulin autoantibodies, and neck ultrasound) should be interpreted consistently with this change of paradigm. The introduction of novel molecular target therapies (i.e., tyrosine kinase inhibitors), as well as a better understanding of the mechanisms of immune checkpoint inhibitor therapies, is radically changing the management of patients with advanced DTC, in whom no treatment option was available. The aim of this review is to analyze the most recent developments of the management of DTC, focusing on several key issues: active surveillance strategies, initial treatment, dynamic risk re-stratification, and therapeutic options in advanced DTC.
The recent advances in knowledge about differentiated thyroid cancer (DTC) showed the need of a personalized management approach. Despite the increased diagnosis of DTC, in particular small papillary thyroid cancers, cancer-related death remained stable over time. The clinical challenge is moving toward the identification of patients with indolent tumors, who can be treated and followed with a more conservative approach, as opposed to those in which aggressive therapy and intensive follow-up should be recommended. This paper discusses the key points of DTC management, from active surveillance to advanced therapy.
Active surveillance has been proposed as an alternative to immediate surgery to avoid overtreatment in unifocal intrathyroidal papillary microcarcinoma (mPTC), without metastatic lymph nodes or aggressive cytological features (1).
Many retrospective papers showed the excellent outcome of mPTC over time (2, 3) likely related to the indolent behavior of mPTC, rather than the efficacy of treatments. For the first time at Kuma Hospital in Japan, in 1993, a conservative approach for selected mPTC patients was hypothesized, and then, active surveillance was introduced in clinical practice (4).
To date, in the Japanese series, active surveillance has the same outcome of immediate surgery. After 10-year observation, only 8 and 3.8% of 1, 235 mPTC under active surveillance showed nodule enlargement or appearance of lymph node metastases, respectively (5). None of the patients who underwent surgery after disease progression showed higher rates of recurrence or mortality compared to patients who performed immediate surgery (5).
Neither clinical nor pathological features, like sex, familial history of thyroid cancer, and multifocality, were related to mPTC progression (6). Conversely, the estimated lifetime probability of mPTC progression was related to the age at diagnosis, being 60.3% for younger (25 years) and 3.5% for older patients (75 years) (7). Therefore, older mPTC patients represent the best candidates for active surveillance. However, ~40% of younger patients could be safely followed up without requiring surgical treatments during lifetimes, corroborating the feasibility of active surveillance.
Active surveillance is a likely safe and feasible strategy also in pregnancy: only 8% of pregnant women showed mPTC progression, and the following postpartum thyroidectomy was completely curative (8).
Based on the Japanese experience, active surveillance was introduced in mPTC management also in other countries, confirming the safety of this approach (9–11). It was observed that mPTC changes in volume during time, rather than in maximum diameter, represent a more sensitive tool to select those patients who could benefit from a more careful monitoring or surgical treatment. Currently, this conservative approach in clinical practice is mainly limited by the burden of cancer diagnosis, its impact on patients' everyday life, and the wide skepticism shown also by general practitioners, different specialists, and institutions (11–13). The assessment of the quality of life and the long-term results of active surveillance, also outside Japan, might improve the patients' acceptability and their compliance in future.
A risk stratification of mPTC patients according to a clinical framework (14, 15) has been proposed: clinical features (nodules with well-defined margins without evidence of extrathyroidal extension and cN0, cM0) and sociocultural and psychological evaluation (willing to accept this approach, aware of possible surgical treatment in the future, compliant to follow-up) are critical to identify the ideal candidate for active surveillance.
Several issues remain open, such as the ideal frequency of follow-up evaluations, currently recommended every 6 months for the first 2 years and yearly afterwards, the optimal timing for surgery in progressive cases, the availability of more precise radiologic tools to improve the detection of minimal extrathyroidal extension, and the identification of molecular biomarkers. To date, the role of genetic mutations (e.g., BRAF, RAS, TERT, etc.) and/or rearrangements (such as RET/PTC) are not completely understood in mPTC during active surveillance. These biomarkers could be able to distinguish the more aggressive forms of mPTC.
Accurate preoperative staging is essential to evaluate the primary tumor and the presence of lymph node metastasis to guide the extent of surgical treatment. Clinical and ultrasonographic evaluation of the neck (nUS) is the cornerstone of the initial assessment. Cross-sectional imaging (i.e., CT scan with IV contrast) might be useful in selected, locally advanced cases.
Many changes in the extent of surgery for DTC have been recently advocated. In the past (16), total thyroidectomy (TTx) was recommended for all DTC >1 cm, regardless of other pathological features, based on several studies that showed lower recurrence rates in patients treated by TTx compared to lobectomy (17–19). Currently, lobectomy is recommended for low-risk and well-differentiated unifocal thyroid cancer ≤4 cm (1, 20). In this setting, the use of nUS before surgery is essential: any suspicion of extrathyroidal extension, multifocal disease, or lymph node metastasis should lead to TTx. Lobectomy can avoid postoperative hypoparathyroidism and the need of thyroid hormone replacement (21). Conversely, in patients who perform TTx, there is no need for completion thyroidectomy, thyroglobulin (Tg) can be used as marker of recurrence, and 131I treatment can be performed, if needed.
For all DTC >4 cm, or ≤4 cm with aggressive features, TTx is usually recommended (1, 20).
In the presence of clinically evident lymph node metastasis of the central or latero-cervical compartment, an oriented therapeutic lymph node dissection is recommended (1, 22–26) because it is associated with lower recurrence (27) and disease-specific mortality rates (28, 29). Prophylactic lymph node dissection of central compartment is still debated and, to date, not routinely recommended (1, 20).
Radioiodine treatment with 131I (RAI) is not generally recommended after lobectomy (1, 20). After TTx, RAI could be used for three different purposes (30):
(1) Remnant ablation: to eliminate any thyroid tissue/cells left over the surgery, making the measurement of serum Tg more specific for persistent/recurrent disease;
(2) Adjuvant treatment: to eliminate any potential foci of thyroid cancer, which might be present after surgery and to destroy small-volume microscopic lymph node metastasis;
(3) Treatment of known disease: to treat the tumor residual disease in the case of advanced stage, both at local and distant level.
Moreover, RAI treatment allows to perform a posttherapeutic whole-body scan, with or without single-photon emission CT–CT (SPECT-CT), to evaluate the presence of radio avid local or distant metastasis, which could change the initial staging and theoretically further treatments (31).
Initial treatment of DTC consisted of TTx plus RAI for all patients until few years ago; nowadays, patients treated with RAI should be selected on the basis of the initial risk stratification (low, intermediate, and high) (1, 20, 32). Furthermore, the decision making to perform RAI should be in agreement with postoperative evaluation, too (1, 33–35). To increase thyroid-stimulating hormone (TSH) levels (36), to rise 131I uptake in thyroid cells, RAI can be performed after thyroid hormone withdrawal (THW) or administration of recombinant human TSH (rhTSH).
In high-risk patients, routine postoperative RAI with high activities is required because it improves the specific cancer survival (37). Activities up to 150 mCi are generally recommended when RAI is used as remnant ablation or adjuvant therapy. In the presence of known distant metastasis, higher activities can be used (1, 20). Furthermore, major guidelines (1, 20, 26) suggest THW preparation, while more data are needed before recommending for or against rhTSH.
In low- and intermediate-risk cases, two randomized non-inferiority trials comparing low and high activities of radioiodine, each with either THW or rhTSH, demonstrated that the ablation rate was similar in the four groups (38, 39). In addition, recurrence rates are similar in patients prepared with THW or rhTSH, both in low- (40, 41) and intermediate-risk patients (42). However, the preparation with rhTSH significantly improves quality of life (43, 44) and reduces both whole-body irradiation (45) and hospitalization time (46, 47).
Selective use of RAI is advocated for patients with intermediate risk. Aggressive variants have a worse prognosis than classic variant, and RAI increased cancer-specific survival rate (48, 49). The role of microscopic extrathyroidal extension (mETE) in deciding to perform RAI or not is debated; some studies demonstrated a risk of death (50), while others did not (51). The presence of BRAF V600E mutation is associated with increased cancer-specific mortality (52) and a higher risk of recurrence (53) compared to wild-type tumors. In intermediate-risk BRAF-positive tumors, reasonably, the associated histological features have more relevance in deciding to perform RAI, compared to the presence of mutation alone. Therefore, RAI should be performed, with low or high activities (i.e., 30–150 mCi) and after rhTSH or THW, in selected intermediate-risk cases with advanced age, aggressive histology, and higher volume of lymph node metastasis (1, 20). In other clinical scenarios (i.e., mETE, small lymph node metastasis, and intrathyroidal PTC with BRAF mutation), postoperative evaluation should guide the decision (1, 20).
In low-risk cases, including those with positive BRAF mutation and small volume metastatic lymph node involvement, RAI is not recommended anymore because the cancer-specific mortality and the persistent/recurrent disease is negligible and therefore not improved by RAI (54–56). Accordingly, based on the postoperative evaluation and in selected cases, RAI should be performed with low activity (i.e., 30–50 mCi) and after rhTSH stimulation (1, 20).
In the last few years, DTC follow-up has changed. Differently from the past, dynamic risk stratification (DRS) is widely performed at both the first postoperative and the subsequent evaluations, and it takes into account information obtained during follow-up (1).
DTC is often multifocal and bilateral and is inclined to spread to the loco-regional lymph nodes. After lobectomy, all patients should undergo basal serum Tg and TgAb measurement and periodical nUS. When patients are carefully selected to surgery, the recurrence rate is low, ranging from 1 to 7%, with no impact on overall survival (1, 57, 58). In addition, if properly treated, most of these patients remain free of disease also after recurrences.
A predefined threshold value of Tg, to recognize those patients with an incomplete response after initial treatment or with recurrence, is difficult to obtain because of the presence of the remaining lobe. For the same reason, TgAb trend loses its meaning during follow-up. However, it could be argued that a stable, non-stimulated value of Tg <30 ng/dl, in the absence of a suspicious nUS, could be predictor of an excellent response (59). On the contrary, a rising trend of Tg may predict a structural disease.
Similarly to TTx, in patients treated by lobectomy, response to treatment can be divided into four classes (20, 59):
(1) Excellent response: stable basal serum Tg levels related to the presence of a contralateral thyroid lobe and negative neck US;
(2) Biochemical incomplete response: basal serum Tg not related to the presence of a contralateral thyroid lobe, or increasing basal serum Tg levels without evidence of structural disease;
(3) Structural incomplete response: evidence of structural disease;
(4) Indeterminate response: non-specific findings on neck US and doubtful trends of Tg.
Accordingly, clinicians should tailor the intensity of treatment and follow-up.
In these patients, little evidence is available on the TSH level to be maintained during follow-up. In low-risk patients treated by lobectomy, TSH should be maintained between 0.5 and 2 mU/l, and levo-thyroxine is generally not recommended for all patients with a TSH value ≤2 mU/l (1, 20). In iodine-deficient areas, levo-thyroxine can be indicated according to patient age and comorbidities to reduce nodular hyperplasia of the remaining lobe (20).
Postoperative evaluation should be performed after 4–12 months from diagnosis and includes Tg, using high-sensitive Tg assay, TgAb measurement, and nUS (33–35). Additional imaging evaluations could be useful in selected cases.
Patients are re-evaluated over time according to the DRS and are classified into four groups, regardless of RAI (1, 20, 32, 59):
(1) Excellent response: non-stimulated Tg <0.2 ng/ml (both for TTx and TTx + RAI) and/or stimulated Tg <1 (TTx + RAI) or 2 ng/ml (TTx alone) plus undetectable TgAb and negative imaging;
(2) Indeterminate response: non-stimulated Tg 0.2–1 ng/ml (TTx + RAI) or 0.2–5 ng/ml (TTx alone) and/or stimulated Tg 1–10 ng/ml (TTx + RAI) or 2–10 ng/ml (TTx alone) and TgAb levels stable or declining, in the absence of structural or functional disease or non-specific findings on imaging studies or faint uptake in thyroid bed on RAI scanning;
(3) Biochemical incomplete response: non-stimulated Tg ≥1 ng/ml (TTx + RAI) or >5 ng/ml (TTx alone) and/or stimulated Tg ≥10 ng/ml or rising TgAb levels plus negative imaging;
(4) Structural incomplete response: structural or functional evidence of disease regardless of Tg or TgAb levels.
The risk of recurrence depends on DRS status rather than initial risk category. The impact of DRS has likely more relevance for intermediate and high-risk patients with an excellent response during follow-up (decreased risk of recurrence up to 1–2%) (60–62).
The appropriate degree and duration of TSH suppression remains to be established. Currently, the international guidelines (1, 20) suggest a degree of TSH suppression according to the risk classification after initial treatment:
(1) High risk: TSH ≤0.1 mU/l
(2) Intermediate risk: TSH 0.1–0.5 mU/l
(3) Low risk: TSH in the normal range (0.5–2 mU/l).
Despite an improved outcome in high-risk patients, currently, the use of TSH suppression is being reconsidered both in intermediate- and high-risk cases (63); no evidence of benefits has been documented in low-risk cases (55, 64). Moreover, in patients with associate comorbidities (at high risk of adverse effects by TSH suppression therapy), TSH values should be individualized, balancing risks, harms, and benefits (65).
TSH values should be titrated also after the restratification process during the follow-up:
(1) Excellent response (clinically and biochemically free of disease): TSH 0.5–2 mU/l (in patients with high-risk disease at diagnosis, TSH 0.1–0.5 mU/l during the first 5 years)
(2) Biochemical incomplete or indeterminate response: TSH 0.1–0.5 mU/l
(3) Structural incomplete response: TSH ≤0.1 mU/l.
Despite patients with DTC usually having an excellent prognosis, a small subgroup could develop distant metastasis or become radioiodine refractory (RAI-R), with a significant impact on survival rates (66). There is no fully consensus on the definition of RAI-R disease (67). According to the American Thyroid Association guidelines (1), it refers to the absence of radioiodine uptake in all or some lesions, at the first posttherapeutic whole-body scan or after previous evidence of RAI-avid disease, or in the case of progression of disease despite RAI uptake. Furthermore, also the existence of a maximum cumulative RAI dose to be administered is still debated. These patients with a structural disease should be assessed by biochemical and imaging evaluation. Tg may give an estimate of tumor burden, and Tg doubling time <1 year is associated with a poor prognosis (68). However, imaging techniques (US, MRI, and CT scan) provide the most precise information on tumor burden to evaluate tumor growth and define tumor progression according to the response evaluation criteria in solid tumors (69–71). Positron emission tomography with 2-deoxy-2-fluorine-18-fluoro-D-glucose/CT (18FDG-PET/CT) may provide additional prognostic information, since 18FDG-PET positive lesions usually have a more aggressive behavior (72, 73).
Differences among the main referral guidelines regarding the management of RAI-R DTC are reported in Table 1.
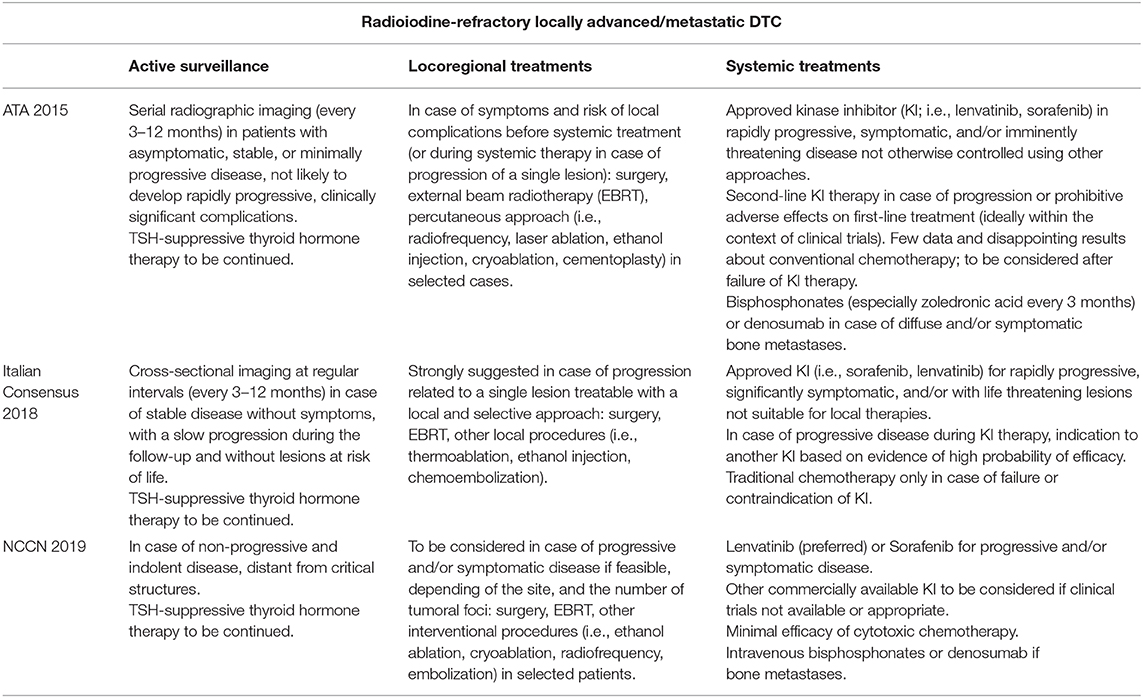
Table 1. Recommendations of the American Thyroid Association (ATA) Guidelines (2015), Italian Consensus (2018), and National Comprehensive Cancer Network (NCCN) (2019) about the management of radioiodine-refractory locally advanced/metastatic differentiated thyroid cancer (DTC) patients.
In general, in patients with oligometastatic, rapidly progressive, or symptomatic disease, a local treatment should be preferred. Surgery is the most widely used therapeutic procedure in these scenarios. Other techniques include thermal ablation (radiofrequency and cryoablation), ultrasound-guided percutaneous ethanol ablation, transarterial chemoembolization, cementoplasty, and external beam radiotherapy. Thermal ablation has been used to treat metastatic lymph nodes and distant metastasis to the bone, lung, and liver. Radiofrequency thermoablation takes advantage of the heat produced by the radiofrequency generator, while cryoablation alternates cycles of freezing and thawing to destroy tumor cells. These procedures are safe and have a high therapeutic success rate (74, 75). Ultrasound-guided percutaneous ethanol ablation has the main role for neck recurrences (76). Transarterial chemoembolization is used for small and diffuse liver metastases, placing chemotherapy and embolic agents directly into the hepatic artery and permit to treat multiple metastases in the same session treatment, when surgery and local ablative therapy have a limited role (77). In cases of osteolytic bone lesions, cementoplasty has been used to provide bone reinforcement and pain relief (78). In these cases, bisphosphonates (Zoledronic acid) and monoclonal antibodies (Denosumab) may reduce skeletal-related adverse events, such as pathological fractures, metastatic spinal cord compression, and malignant hypercalcemia (79). Finally, external beam radiotherapy was widely used in the past, but its efficacy is questionable, and it may be considered only for palliative purposes in patients with locally advanced disease.
When these local treatments are not feasible or in patients with widespread metastatic disease, a systemic therapy should be initiated. These patients had no effective therapeutic options, until few years ago, but their management has dramatically changed with the availability of tyrosine kinase inhibitors (TKIs). Sorafenib and Lenvatinib, two oral multitargeted TKIs with antiproliferative and antiangiogenetic effects, were approved by the US Food and Drug Administration and European Medicines Agency after the publication of two phase 3, randomized, double-blind, multicenter trials (80, 81). No head-to-head comparison between Sorafenib and Lenvatinib in DTC patients has been conducted so far. In the DECISION trial, Sorafenib significantly prolonged median progression-free survival in patients with advanced DTC (10.8 vs. 5.8 months with placebo) and led to an overall response rate increase (12.2 vs. 0.5%) (80). Similarly, in the SELECT trial, advanced DTC patients treated with Lenvatinib showed a significant prolongation of median progression-free survival in comparison to placebo (18.3 vs. 3.6 months), with a marked improvement in the response rate (64.8 vs. 1.5%). It must be underlined that patients treated with Sorafenib were all TKI naive; by contrast, Lenvatinib represented a second-line TKI therapy for ~25% of treated patients in the trial (81). No significant conclusions can be drawn on overall survival in TKI-treated patients, due to the crossover design of both studies which potentially misrepresents the real effect of the two TKIs. Most of TKI-treated patients experienced adverse events (AEs) in both phase III trials. The most frequent AEs reported on Sorafenib were hand–foot syndrome, diarrhea, alopecia, and rash (80). During treatment with Lenvatinib, more than half of the treated group experienced hypertension, diarrhea, fatigue, and decreased appetite (81). At least for Lenvatinib, AEs seemed to be more severe in older patients (82). In most cases, AE management was achieved by supportive care and by dose reduction or transient drug interruption. Efficacy and tolerability results of the phase III trials were confirmed in the real-life setting (83, 84), leading to an increasing awareness of the management of these patients to prevent major AEs, who are lifelong treated (85, 86). Lenvatinib discontinuation might be necessary in a limited number of cases for toxicity or marked progression. For these patients, salvage therapy with a different TKI is generally recommended, but their availability is restricted to the clinical trial setting or to the “off-label” use (1, 20, 26). Recently, immune checkpoint inhibitors have been tested in preliminary trials for the treatment of advanced thyroid cancer (87). The antitumor activity of the antiprogrammed cell death-1 monoclonal antibody Pembrolizumab has been evaluated in advanced PD-L1-positive DTC with promising results (88).
A suggested algorithm for the management of DTC from active surveillance to advanced therapies is reported in Figure 1.
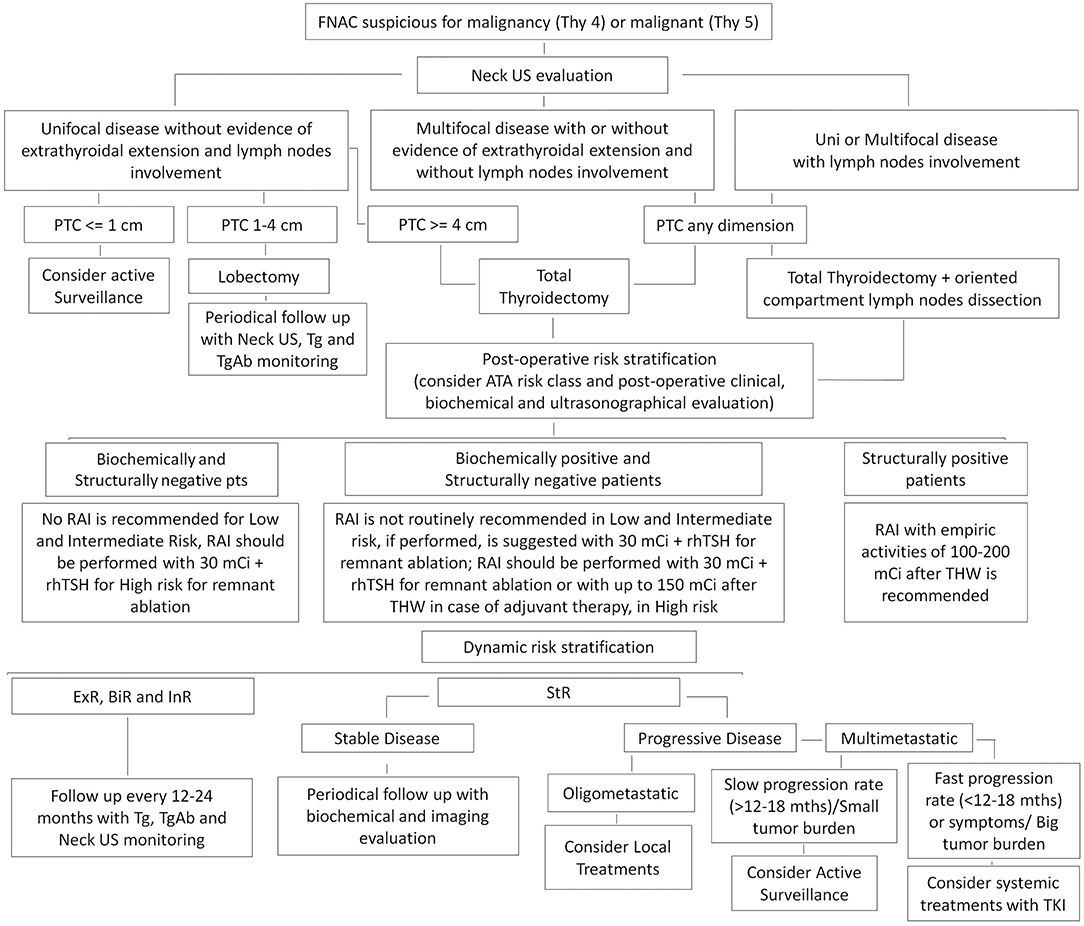
Figure 1. Suggested algorithm for the management of differentiated thyroid cancer (DTC). ExR, Excellent Response; BiR, Biochemical Incomplete Response; InR, Indeterminate Response; StR, Structural Incomplete Response.
The management of DTC is changing, and currently, a personalized approach, based on an accurate pre- and postoperative risk stratification of the patients, is required. Active surveillance and conservative surgeries are recommended for low risk, while a selective use of radioiodine treatment is advocated for the intermediate-risk patients. The introduction in the clinical practice of TKI treatments allowed to have further options in the treatment of the advanced cases.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank our colleagues Cosimo Durante, Eleonora Molinaro, and Gabriella Pellegriti for the continuing support.
1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
2. Mazzaferri EL. Management of low-risk differentiated thyroid cancer. Endocrine Pract. (2007) 13:498–512. doi: 10.4158/EP.13.5.498
3. Hay ID. Management of patients with low-risk papillary thyroid carcinoma. Endocr Pract. (2007) 13:521–33. doi: 10.4158/EP.13.5.521
4. Ito Y, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid. (2003) 13:381–7. doi: 10.1089/105072503321669875
5. Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. (2014) 24:27–34. doi: 10.1089/thy.2013.0367
6. Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. (2010) 34:28–35. doi: 10.1007/s00268-009-0303-0
7. Miyauchi A, Kudo T, Ito Y, Oda H, Sasai H, Higashiyama T, et al. Estimation of the lifetime probability of disease progression of papillary microcarcinoma of the thyroid during active surveillance. Surgery. (2018) 163:48–52. doi: 10.1016/j.surg.2017.03.028
8. Ito Y, Miyauchi A, Kudo T, Ota H, Yoshioka K, Oda H, et al. Effects of pregnancy on papillary microcarcinomas of the thyroid re-evaluated in the entire patient series at kuma hospital. Thyroid. (2016) 26:156–60. doi: 10.1089/thy.2015.0393
9. Kwon H, Oh HS, Kim M, Park S, Jeon MJ, Kim WG, et al. Active surveillance for patients with papillary thyroid microcarcinoma: a single center's experience in Korea. J Clin Endocr Metabol. (2017) 102:1917–25. doi: 10.1210/jc.2016-4026
10. Tuttle RM, Fagin JA, Minkowitz G, Wong RJ, Roman B, Patel S, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg. (2017) 143:1015–20. doi: 10.1001/jamaoto.2017.1442
11. Molinaro E, Campopiano MC, Pieruzzi L, Matrone A, Agate L, Bottici V, et al. Active surveillance in papillary thyroid microcarcinomas is feasible and safe: experience at one single Italian center. J Clin Endocrinol Metabol. (2019) dgz113. doi: 10.1210/clinem/dgz113. [Epub ahead of print].
12. Ito Y, Miyauchi A, Kudo T, Oda H, Yamamoto M, Sasai H, et al. Trends in the implementation of active surveillance for low-risk papillary thyroid microcarcinomas at kuma hospital: gradual increase and heterogeneity in the acceptance of this new management option. Thyroid. (2018) 28:488–95. doi: 10.1089/thy.2017.0448
13. Sugitani I, Ito Y, Miyauchi A, Imai T, Suzuki S. Active surveillance versus immediate surgery: questionnaire survey on the current treatment strategy for adult patients with low-risk papillary thyroid microcarcinoma in Japan. Thyroid. (2019) 29:1563–71. doi: 10.1089/thy.2019.0211
14. Brito JP, Ito Y, Miyauchi A, Tuttle RM. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid. (2016) 26:144–9. doi: 10.1089/thy.2015.0178
15. Tuttle RM, Zhang L, Shaha A. A clinical framework to facilitate selection of patients with differentiated thyroid cancer for active surveillance or less aggressive initial surgical management. Exp Rev Endocrinol Metabol. (2018) 13:77–85. doi: 10.1080/17446651.2018.1449641
16. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised american thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. (2009) 19:1167–214. doi: 10.1089/thy.2009.0110
17. Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS, et al. Extent of surgery affects survival for papillary thyroid cancer. Annals Surg. (2007) 246:375–81; discussion 81–4. doi: 10.1097/SLA.0b013e31814697d9
18. Grant CS, Hay ID, Gough IR, Bergstralh EJ, Goellner JR, McConahey WM. Local recurrence in papillary thyroid carcinoma: is extent of surgical resection important? Surgery. (1988) 104:954–62.
19. Hay ID, Grant CS, Bergstralh EJ, Thompson GB, van Heerden JA, Goellner JR. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery. (1998) 124:958–64; discussion 64–6. doi: 10.1016/S0039-6060(98)70035-2
20. Pacini F, Basolo F, Bellantone R, Boni G, Cannizzaro MA, De Palma M, et al. Italian consensus on diagnosis and treatment of differentiated thyroid cancer: joint statements of six Italian societies. J Endocrinol Invest. (2018) 41:849–76. doi: 10.1007/s40618-018-0884-2
21. Stoll SJ, Pitt SC, Liu J, Schaefer S, Sippel RS, Chen H. Thyroid hormone replacement after thyroid lobectomy. Surgery. (2009) 146:554–8; discussion 8–60. doi: 10.1016/j.surg.2009.06.026
22. Carty SE, Cooper DS, Doherty GM, Duh QY, Kloos RT, Mandel SJ, et al. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid. (2009) 19:1153–8. doi: 10.1089/thy.2009.0159
23. Perros P. Introduction to the updated guidelines on the management of thyroid cancer. Clin Med. (2007) 7:321–2. doi: 10.7861/clinmedicine.7-4-321
24. Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. (2006) 154:787–803. doi: 10.1530/eje.1.02158
25. Robbins KT, Shaha AR, Medina JE, Califano JA, Wolf GT, Ferlito A, et al. Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Head Neck Surg. (2008) 134:536–8. doi: 10.1001/archotol.134.5.536
26. Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, Callender G, et al. NCCN guidelines insights: thyroid carcinoma, Version 2.2018. J Natl Comprehens Cancer Netw. (2018) 16:1429–40. doi: 10.6004/jnccn.2018.0089
27. Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. (2006) 106:524–31. doi: 10.1002/cncr.21653
28. Sugitani I, Fujimoto Y, Yamada K, Yamamoto N. Prospective outcomes of selective lymph node dissection for papillary thyroid carcinoma based on preoperative ultrasonography. World J Surg. (2008) 32:2494–502. doi: 10.1007/s00268-008-9711-9
29. Podnos YD, Smith D, Wagman LD, Ellenhorn JD. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg. (2005) 71:731–4.
30. Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, et al. Controversies, consensus, and collaboration in the Use of. (131)I therapy in differentiated thyroid cancer: a joint statement from the american thyroid association, the european association of nuclear medicine, the society of nuclear medicine and molecular imaging, and the european thyroid association. Thyroid. (2019) 29:461–70. doi: 10.1089/thy.2018.0597
31. Avram AM. Radioiodine scintigraphy with SPECT/CT: an important diagnostic tool for thyroid cancer staging and risk stratification. J Nucl Med. (2012) 53:754–64. doi: 10.2967/jnumed.111.104133
32. Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. (2010) 20:1341–9. doi: 10.1089/thy.2010.0178
33. Matrone A, Gambale C, Piaggi P, Viola D, Giani C, Agate L, et al. Postoperative thyroglobulin and neck ultrasound in the risk restratification and decision to perform 131I ablation. J Clin Endocrinol Metabol. (2017) 102:893–902. doi: 10.1210/jc.2017-00617
34. Ibrahimpasic T, Nixon IJ, Palmer FL, Whitcher MM, Tuttle RM, Shaha A, et al. Undetectable thyroglobulin after total thyroidectomy in patients with low- and intermediate-risk papillary thyroid cancer–is there a need for radioactive iodine therapy? Surgery. (2012) 152:1096–105. doi: 10.1016/j.surg.2012.08.034
35. Mourao GF, Rosario PW, Calsolari MR. Low postoperative nonstimulated thyroglobulin as a criterion to spare radioiodine ablation. Endocr Related Cancer. (2016) 23:47–52. doi: 10.1530/ERC-15-0458
36. Edmonds CJ, Hayes S, Kermode JC, Thompson BD. Measurement of serum TSH and thyroid hormones in the management of treatment of thyroid carcinoma with radioiodine. Br J Radiol. (1977) 50:799–807. doi: 10.1259/0007-1285-50-599-799
37. Sacks W, Fung CH, Chang JT, Waxman A, Braunstein GD. The effectiveness of radioactive iodine for treatment of low-risk thyroid cancer: a systematic analysis of the peer-reviewed literature from 1966 to April 2008. Thyroid. (2010) 20:1235–45. doi: 10.1089/thy.2009.0455
38. Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. (2012) 366:1663–73. doi: 10.1056/NEJMoa1108586
39. Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. (2012) 366:1674–85. doi: 10.1056/NEJMoa1109589
40. Tuttle RM, Brokhin M, Omry G, Martorella AJ, Larson SM, Grewal RK, et al. Recombinant human TSH-assisted radioactive iodine remnant ablation achieves short-term clinical recurrence rates similar to those of traditional thyroid hormone withdrawal. J Nucl Med. (2008) 49:764–70. doi: 10.2967/jnumed.107.049072
41. Molinaro E, Giani C, Agate L, Biagini A, Pieruzzi L, Bianchi F, et al. Patients with differentiated thyroid cancer who underwent radioiodine thyroid remnant ablation with low-activity. (1)(3)(1)I after either recombinant human TSH or thyroid hormone therapy withdrawal showed the same outcome after a 10-year follow-up. J Clin Endocrinol Metabol. (2013) 98:2693–700. doi: 10.1210/jc.2012-4137
42. Castagna MG, Cevenini G, Theodoropoulou A, Maino F, Memmo S, Claudia C, et al. Post-surgical thyroid ablation with low or high radioiodine activities results in similar outcomes in intermediate risk differentiated thyroid cancer patients. Eur J Endocrinol. (2013) 169:23–9. doi: 10.1530/EJE-12-0954
43. Pacini F, Ladenson PW, Schlumberger M, Driedger A, Luster M, Kloos RT, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metabol. (2006) 91:926–32. doi: 10.1210/jc.2005-1651
44. Taieb D, Sebag F, Cherenko M, Baumstarck-Barrau K, Fortanier C, Farman-Ara B, et al. Quality of life changes and clinical outcomes in thyroid cancer patients undergoing radioiodine remnant ablation. (RRA) with recombinant human TSH. (rhTSH): a randomized controlled study. Clin Endocrinol. (2009) 71:115–23. doi: 10.1111/j.1365-2265.2008.03424.x
45. Hanscheid H, Lassmann M, Luster M, Thomas SR, Pacini F, Ceccarelli C, et al. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J Nucl Med. (2006) 47:648–54.
46. Borget I, Remy H, Chevalier J, Ricard M, Allyn M, Schlumberger M, et al. Length and cost of hospital stay of radioiodine ablation in thyroid cancer patients: comparison between preparation with thyroid hormone withdrawal and thyrogen. Eur J Nucl Med Mol Imaging. (2008) 35:1457–63. doi: 10.1007/s00259-008-0754-9
47. Molinaro E, Viola D, Passannanti P, Agate L, Lippi F, Ceccarelli C, et al. Recombinant human TSH. (rhTSH) in 2009: new perspectives in diagnosis and therapy. Q J Nucl Med Mol Imaging. (2009) 53:490–502.
48. Kazaure HS, Roman SA, Sosa JA. Aggressive variants of papillary thyroid cancer: incidence, characteristics and predictors of survival among 43,738 patients. Annals Surg Oncol. (2012) 19:1874–80. doi: 10.1245/s10434-011-2129-x
49. Regalbuto C, Malandrino P, Frasca F, Pellegriti G, Le Moli R, Vigneri R, et al. The tall cell variant of papillary thyroid carcinoma: clinical and pathological features and outcomes. J Endocrinol Invest. (2013) 36:249–54. doi: 10.3275/8515
50. Ruel E, Thomas S, Dinan M, Perkins JM, Roman SA, Sosa JA. Adjuvant radioactive iodine therapy is associated with improved survival for patients with intermediate-risk papillary thyroid cancer. J Clin Endocrinol Metabol. (2015) 100:1529–36. doi: 10.1210/jc.2014-4332
51. Nixon IJ, Ganly I, Patel S, Palmer FL, Whitcher MM, Tuttle RM, et al. The impact of microscopic extrathyroid extension on outcome in patients with clinical T1 and T2 well-differentiated thyroid cancer. Surgery. (2011) 150:1242–9. doi: 10.1016/j.surg.2011.09.007
52. Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. (2013) 309:1493–501. doi: 10.1001/jama.2013.3190
53. Tufano RP, Teixeira GV, Bishop J, Carson KA, Xing M. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: a systematic review and meta-analysis. Medicine. (2012) 91:274–86. doi: 10.1097/MD.0b013e31826a9c71
54. Schvartz C, Bonnetain F, Dabakuyo S, Gauthier M, Cueff A, Fieffe S, et al. Impact on overall survival of radioactive iodine in low-risk differentiated thyroid cancer patients. J Clin Endocrinol Metabol. (2012) 97:1526–35. doi: 10.1210/jc.2011-2512
55. Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. (2006) 16:1229–42. doi: 10.1089/thy.2006.16.1229
56. Jonklaas J, Cooper DS, Ain KB, Bigos T, Brierley JD, Haugen BR, et al. Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid. (2010) 20:1423–4. doi: 10.1089/thy.2010.0308
57. Gartland RM, Lubitz CC. Impact of extent of surgery on tumor recurrence and survival for papillary thyroid cancer patients. Annals Surg Oncol. (2018) 25:2520–5. doi: 10.1245/s10434-018-6550-2
58. Vaisman F, Momesso D, Bulzico DA, Pessoa CH, da Cruz MD, Dias F, et al. Thyroid lobectomy is associated with excellent clinical outcomes in properly selected differentiated thyroid cancer patients with primary tumors greater than 1 cm. J Thyroid Res. (2013) 2013:398194. doi: 10.1155/2013/398194
59. Momesso DP, Vaisman F, Yang SP, Bulzico DA, Corbo R, Vaisman M, et al. Dynamic risk stratification in patients with differentiated thyroid cancer treated without radioactive iodine. J Clin Endocrinol Metabol. (2016) 101:2692–700. doi: 10.1210/jc.2015-4290
60. Castagna MG, Maino F, Cipri C, Belardini V, Theodoropoulou A, Cevenini G, et al. Delayed risk stratification, to include the response to initial treatment. (surgery and radioiodine ablation), has better outcome predictivity in differentiated thyroid cancer patients. Eur J Endocrinol. (2011) 165:441–6. doi: 10.1530/EJE-11-0466
61. Han JM, Kim WB, Yim JH, Kim WG, Kim TY, Ryu JS, et al. Long-term clinical outcome of differentiated thyroid cancer patients with undetectable stimulated thyroglobulin level one year after initial treatment. Thyroid. (2012) 22:784–90. doi: 10.1089/thy.2011.0322
62. Verburg FA, Stokkel MP, Duren C, Verkooijen RB, Mader U, van Isselt JW, et al. No survival difference after successful. (131)I ablation between patients with initially low-risk and high-risk differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. (2010) 37:276–83. doi: 10.1007/s00259-009-1315-6
63. Klubo-Gwiezdzinska J, Auh S, Gershengorn M, Daley B, Bikas A, Burman K, et al. Association of thyrotropin suppression with survival outcomes in patients with intermediate- and high-risk differentiated thyroid cancer. JAMA Netw Open. (2019) 2:e187754. doi: 10.1001/jamanetworkopen.2018.7754
64. Sugitani I, Fujimoto Y. Does postoperative thyrotropin suppression therapy truly decrease recurrence in papillary thyroid carcinoma? A randomized controlled trial. J Clin Endocrinol Metabol. (2010) 95:4576–83. doi: 10.1210/jc.2010-0161
65. Biondi B, Cooper DS. Benefits of thyrotropin suppression versus the risks of adverse effects in differentiated thyroid cancer. Thyroid. (2010) 20:135–46. doi: 10.1089/thy.2009.0311
66. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metabol. (2006) 91:2892–9. doi: 10.1210/jc.2005-2838
67. Van Nostrand D. Radioiodine refractory differentiated thyroid cancer: time to update the classifications. Thyroid. (2018) 28:1083–93. doi: 10.1089/thy.2018.0048
68. Miyauchi A, Kudo T, Miya A, Kobayashi K, Ito Y, Takamura Y, et al. Prognostic impact of serum thyroglobulin doubling-time under thyrotropin suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid. (2011) 21:707–16. doi: 10.1089/thy.2010.0355
69. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline. (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
70. Sabra MM, Sherman EJ, Tuttle RM. Tumor volume doubling time of pulmonary metastases predicts overall survival and can guide the initiation of multikinase inhibitor therapy in patients with metastatic, follicular cell-derived thyroid carcinoma. Cancer. (2017) 123:2955–64. doi: 10.1002/cncr.30690
71. Sabra MM, Sherman E, Tuttle RM. Prolongation of tumour volume doubling time. (midDT) is associated with improvement in disease-specific survival in patients with rapidly progressive radioactive iodine refractory differentiated thyroid cancer selected for molecular targeted therapy. Clin Endocrinol. (2019) 90:617–22. doi: 10.1111/cen.13941
72. Deandreis D, Al Ghuzlan A, Leboulleux S, Lacroix L, Garsi JP, Talbot M, et al. Do histological, immunohistochemical, and metabolic. (radioiodine and fluorodeoxyglucose uptakes) patterns of metastatic thyroid cancer correlate with patient outcome? Endocr -Related Cancer. (2011) 18:159–69. doi: 10.1677/ERC-10-0233
73. Robbins RJ, Wan Q, Grewal RK, Reibke R, Gonen M, Strauss HW, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metabol. (2006) 91:498–505. doi: 10.1210/jc.2005-1534
74. Lee SJ, Jung SL, Kim BS, Ahn KJ, Choi HS, Lim DJ, et al. Radiofrequency ablation to treat loco-regional recurrence of well-differentiated thyroid carcinoma. Kor J Radiol. (2014) 15:817–26. doi: 10.3348/kjr.2014.15.6.817
75. Cazzato RL, Bonichon F, Buy X, Godbert Y, de Figuereido BH, Pointillart V, et al. Over ten years of single-institution experience in percutaneous image-guided treatment of bone metastases from differentiated thyroid cancer. Eur J Surg Oncol. (2015) 41:1247–55. doi: 10.1016/j.ejso.2015.06.005
76. Hay ID, Lee RA, Davidge-Pitts C, Reading CC, Charboneau JW. Long-term outcome of ultrasound-guided percutaneous ethanol ablation of selected “recurrent” neck nodal metastases in 25 patients with TNM stages III or IVA papillary thyroid carcinoma previously treated by surgery and 131I therapy. Surgery. (2013) 154:1448–54; discussion 54–5. doi: 10.1016/j.surg.2013.07.007
77. Fromigue J, De Baere T, Baudin E, Dromain C, Leboulleux S, Schlumberger M. Chemoembolization for liver metastases from medullary thyroid carcinoma. J Clin Endocrinol Metabol. (2006) 91:2496–9. doi: 10.1210/jc.2005-2401
78. Cazzato RL, Garnon J, Koch G, Shaygi B, Tsoumakidou G, Caudrelier J, et al. Current role of interventional radiology in the management of visceral and bone metastases from thyroid cancer. Gland Surg. (2018) 7:80–8. doi: 10.21037/gs.2017.12.08
79. Orita Y, Sugitani I, Takao S, Toda K, Manabe J, Miyata S. Prospective evaluation of zoledronic acid in the treatment of bone metastases from differentiated thyroid carcinoma. Ann Surg Oncol. (2015) 22:4008–13. doi: 10.1245/s10434-015-4497-0
80. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. (2014) 384:319–28. doi: 10.1016/S0140-6736(14)60421-9
81. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. (2015) 372:621–30. doi: 10.1056/NEJMoa1406470
82. Brose MS, Worden FP, Newbold KL, Guo M, Hurria A. Effect of age on the efficacy and safety of lenvatinib in radioiodine-refractory differentiated thyroid cancer in the phase III SELECT trial. J Clin Oncol. (2017) 35:2692–9. doi: 10.1200/JCO.2016.71.6472
83. Berdelou A, Borget I, Godbert Y, Nguyen T, Garcia ME, Chougnet CN, et al. Lenvatinib for the treatment of radioiodine-refractory thyroid cancer in real-life practice. Thyroid. (2017). doi: 10.1089/thy.2017.0205
84. Yu ST, Ge JN, Luo JY, Wei ZG, Sun BH, Lei ST. Treatment-related adverse effects with TKIs in patients with advanced or radioiodine refractory differentiated thyroid carcinoma: a systematic review and meta-analysis. Cancer Manage Res. (2019) 11:1525–32. doi: 10.2147/CMAR.S191499
85. Capdevila J, Newbold K, Licitra L, Popovtzer A, Moreso F, Zamorano J, et al. Optimisation of treatment with lenvatinib in radioactive iodine-refractory differentiated thyroid cancer. Cancer Treat Rev. (2018) 69:164–76. doi: 10.1016/j.ctrv.2018.06.019
86. Cabanillas ME, Takahashi S. Managing the adverse events associated with lenvatinib therapy in radioiodine-refractory differentiated thyroid cancer. Semin Oncol. (2019) 46:57–64. doi: 10.1053/j.seminoncol.2018.11.004
87. Varricchi G, Loffredo S, Marone G, Modestino L, Fallahi P, Ferrari SM, et al. The immune landscape of thyroid cancer in the context of immune checkpoint inhibition. Int J Mol Sci. (2019) 20:3934. doi: 10.3390/ijms20163934
Keywords: differentiated thyroid cancer, active surveillance, radioiodine (131I) treatment, tirosine kinase inhibitors, dynamic risk stratification
Citation: Matrone A, Campopiano MC, Nervo A, Sapuppo G, Tavarelli M and De Leo S (2020) Differentiated Thyroid Cancer, From Active Surveillance to Advanced Therapy: Toward a Personalized Medicine. Front. Endocrinol. 10:884. doi: 10.3389/fendo.2019.00884
Received: 04 October 2019; Accepted: 03 December 2019;
Published: 08 January 2020.
Edited by:
Domenico Salvatore, University of Naples Federico II, ItalyReviewed by:
Giuseppe Costante, Institut Jules Bordet, BelgiumCopyright © 2020 Matrone, Campopiano, Nervo, Sapuppo, Tavarelli and De Leo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Matrone, YW50by5tYXRyb25lQHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.