- 1Department of Endocrine Oncology, University Medical Center Utrecht, Utrecht, Netherlands
- 2Department of Medical Genetics, Wilhelmina Children's Hospital, University Medical Center Utrecht, Utrecht, Netherlands
- 3Department of Paediatrics, Wilhelmina Children's Hospital, University Medical Center Utrecht, Utrecht, Netherlands
Pituitary adenomas (PA) are amongst the most prevalent intracranial tumors, causing complications by hormonal overproduction or deficiency and tumor mass effects, with 95% of cases occurring sporadically. Associated germline mutations (AIP, MEN1, CDKN1B, PRKAR1A, SDHx) and Xq26.3 microduplications are increasingly identified, but the clinical consequences in sporadic PA remain unclear. This systematic review evaluates predictors of a genetic cause of sporadic PA and the consequences for treatment outcome. We undertook a sensitive MEDLINE/Pubmed, EMBASE, and Web of Science search with critical appraisal of identified studies. Thirty-seven studies on predictors of mutations and 10 studies on the influence on treatment outcome were included. AIP and MEN1 mutations were associated with young age of PA diagnosis. AIP mutations were also associated with gigantism and macroadenomas at time of diagnosis. Xq26.3 microduplications were associated with PA below the age of five. AIP and MEN1 mutation analysis is therefore recommended in young patients (≤30 years). AIP mutation analysis is specifically recommended for patients with PA induced gigantism and macroadenoma. Screening for Xq26.3 microduplications is advisable in children below the age of five with increased growth velocity due to PA. There is no evidence supporting mutation analysis of other genes in sporadic PA. MEN1 mutation related prolactinoma respond well to dopamine agonists while AIP mutation associated somatotroph and lactotroph adenoma are frequently resistant to medical treatment. In patients harboring an Xq26.3 microduplication treatment is challenging, although outcome is not different from other patients with PA induced gigantism. Effective use of genetic analysis may lead to early disease identification, while knowledge of the impact of germline mutations on susceptibility to various treatment modalities helps to determine therapeutic strategies, possibly lowering disease morbidity.
Introduction
Pituitary adenomas (PAs) are amongst the most frequently encountered intracranial tumors with a reported prevalence for clinically relevant PAs of 68–98 per 100,000 (1–6). Pituitary adenomas are usually benign but can lead to clinical symptoms caused by hormonal overproduction or deficiency as well as by tumor mass. The majority of cases (95%) occur sporadically (7, 8). Familial clustering can be seen in the context of an inherited syndromic condition leading to an increased risk of PAs (most frequently Multiple Endocrine Neoplasia Type 1 (MEN1)) or without other (endocrine) manifestations in case of familial isolated pituitary adenoma (FIPA).
Clinical implications of identifying germline mutations in patients with PA, in terms of treatment and prognosis, have been reported by different authors (9–12). However, to our knowledge a complete overview of literature with thorough assessment of methodological quality of studies has not been performed to date. Detection of a germline mutation enables identifying family members at risk or occult disease burden in probands. Despite the clinical need, formal guidelines defining criteria for genetic screening of patients with apparently sporadic PA are scarce. In recent years, the amount of publications concerning germline mutations in (sporadic) pituitary adenoma has increased enormously. Despite all efforts, the mechanisms underlying pituitary tumorigenesis and the role of germline mutations in PAs in a sporadic setting remain poorly understood. Still, germline mutations are often not timely identified due to de novo mutations, low penetrance of hereditary syndromic conditions, unclear family history or small family size (13–15). The reported yield of genetic screening varies enormously, presumably due to a great variety of study populations, genetic screening methods and methodological quality of studies.
To provide a useful tool for daily practice in the frequently encountered dilemma whether or not to test for the presence of germline mutations in patients with apparently sporadic PA, we aim to determine the clinical value of genetic screening in apparently sporadic PA based on a rigorous systematic review and critical appraisal of the available literature.
Methods
To assess the value of genetic testing in sporadic PA without syndromic features, we formulated two clinical questions for this review that are relevant for a physician when confronted with these patients: (1) what are predictors for the presence of a genetic cause of apparently sporadically occurring pituitary adenoma? (2) What is the impact of germline mutations on course of disease and treatment outcome of PA?
Search Strategy and Study Selection
We performed a MEDLINE/Pubmed, EMBASE, and Web of Science search in November 2018. We applied a broad search strategy using “pituitary adenoma” and “genetic analysis” with an extensive list of synonyms. The complete search string is provided in Supplementary Data Sheet 1. We included human research written in English, French, German, or Dutch without restriction for year of publication. Publications using non-original data (reviews, letters to the editor, cohort duplicates) were only used for cross referencing, case-reports up to four cases were excluded.
Studies assessing predictors of a genetic cause of PA were included if (1) it was possible to retrieve data on sporadic cases separately and (2) (likely) pathogenic germline mutations of genes associated with PA were investigated. The genes of interest include the MEN1, CDKN1B, CDKN2C, PRKAR1A, PRKACA, PRKACB, SDHx, and AIP genes and microduplications of Xq26.3. Due to insufficient evidence in literature for GPR101 allelic variants in the tumorigenesis of PA (15–21), studies on these variants were excluded from further review. Since the focus of this review is on patients with sporadically occurring PA, studies including patients with clear syndromic features suggestive for a certain genomic mutation were excluded.
Studies assessing the impact of a germline mutation on treatment outcome of PA were included if (1) results included information on treatment (type and number of treatments) and/or outcome (hormonal/disease control, tumor growth/reduction, complications) (2) information of the (sub)group of patients with a germline mutation was extractable and (3) at least five cases with a proven germline mutation were described.
After removal of duplications, two authors (MB and BN) independently screened all publications by title and abstract for possible relevance on the formulated questions. The full manuscript of all potentially eligible papers was then reviewed for in/exclusion by the same authors independently. In case of disagreement, consensus was reached by discussion, with the help of a third reviewer (RL). Reasons for exclusion at full text screening were recorded (See Supplementary Data Sheet 2). All included articles, reviews and case-reports were cross referenced for additional relevant articles.
Data Extraction
Relevant data on study population (cohort origin, number of included patients, additional selection criteria, clinical subtype of adenoma, gender distribution, and familial status) and investigated gene(s) (including method(s) of genetic analysis and investigation(s) of pathogenicity) were extracted. The prevalence of the investigated germline mutations was obtained. Age, gender, adenoma size, and functionality were considered a potential predictor. Possible predictors of germline mutations were assessed if at least five cases with a germline mutation were identified in the study population. All quantitative data describing determinants of treatment outcome of PA in patients with proven germline mutations were extracted. In order to determine the predictive value of determinants and the effect on treatment outcome, a combination of effect size, statistical significance, reproducibility (number of studies with comparable results) and methodological quality of studies were taken into consideration.
Critical Appraisal
For the systematic evaluation of risk of bias and applicability of studies on predictors of a genetic cause of PA, we adapted the Quality Assessment of Diagnostic Accuracy Studies tool (QUADAS-2) for our review purposes (22). For the evaluation of prognostic studies on the impact of germline mutation on treatment outcome, we customized the Quality In Prognosis Studies tool (QUIPS) (23). For more details, see Supplementary Data Sheets 3, 4. All included studies were appraised by two authors independently (MB and BN), in case of disagreement, consensus was reached by discussion or with the help of a third reviewer (RL). The strength of recommendations was graded using the Grading of Recommendations, Assessment, Development, and Evaluation system (24, 25).
Results
Study Selection
After removal of duplicates a total of 5,803 original records were identified. After systematic screening, a total of 37 studies on possible predictors of germline mutations and 10 studies on the impact of a germline mutation on treatment outcome were included. One record was included for answering both clinical questions (26). Cross referencing did not result in additional relevant records. For further details, see Figure 1 (Flowchart).
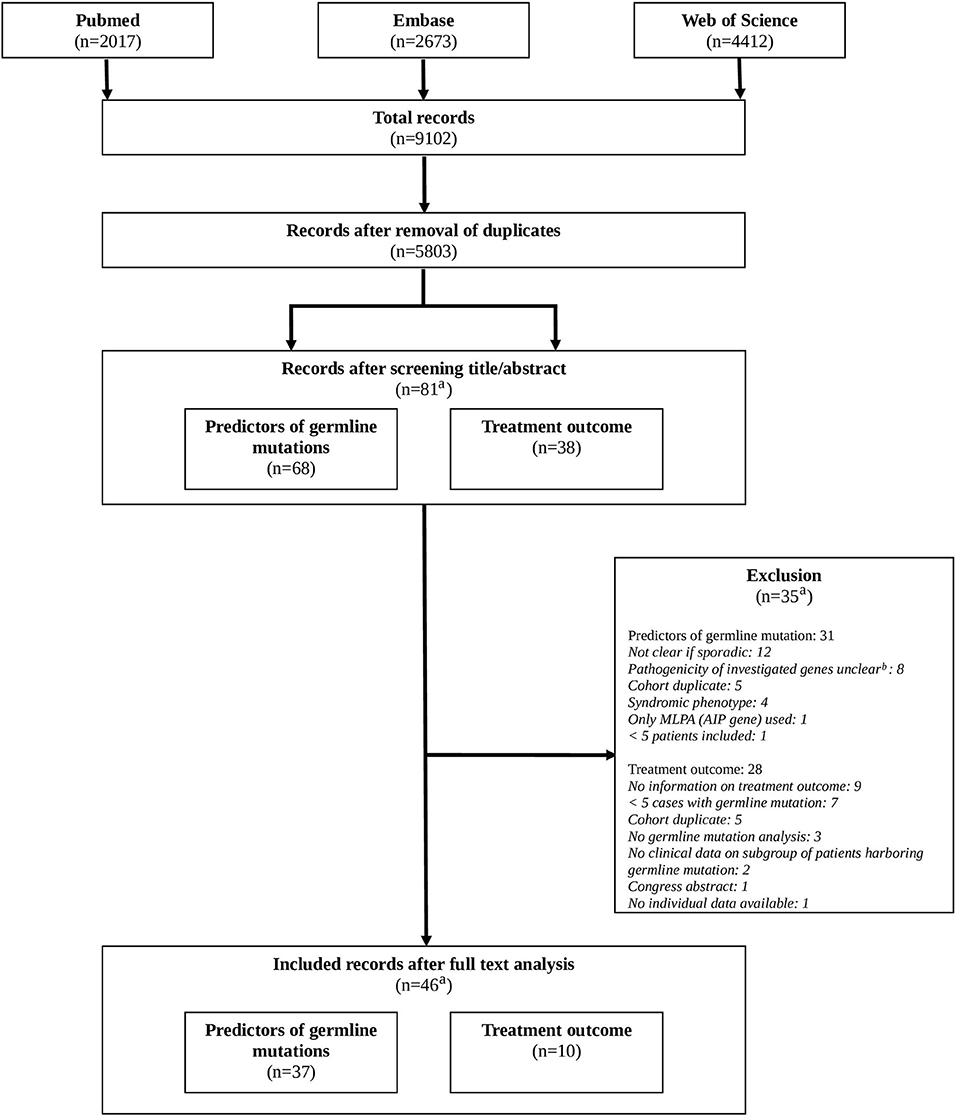
Figure 1. Flowchart. a: original records. Records can be included for both clinical questions (predictors of germline mutations, treatment outcome). b: GPR101 allelic variants, GNAI1/2/3, CABLES1, KCNQ1/2, genome wide association studies, SNP allele frequencies studies.
Predictors on Germline Mutation Status in Sporadic PA
Studies could be categorized into three separate groups: (i) patients with a somatotroph adenoma, (ii) young patients (≤30 years at diagnosis), and (iii) other groups of patients with PA.
Sporadic Somatotroph Adenoma
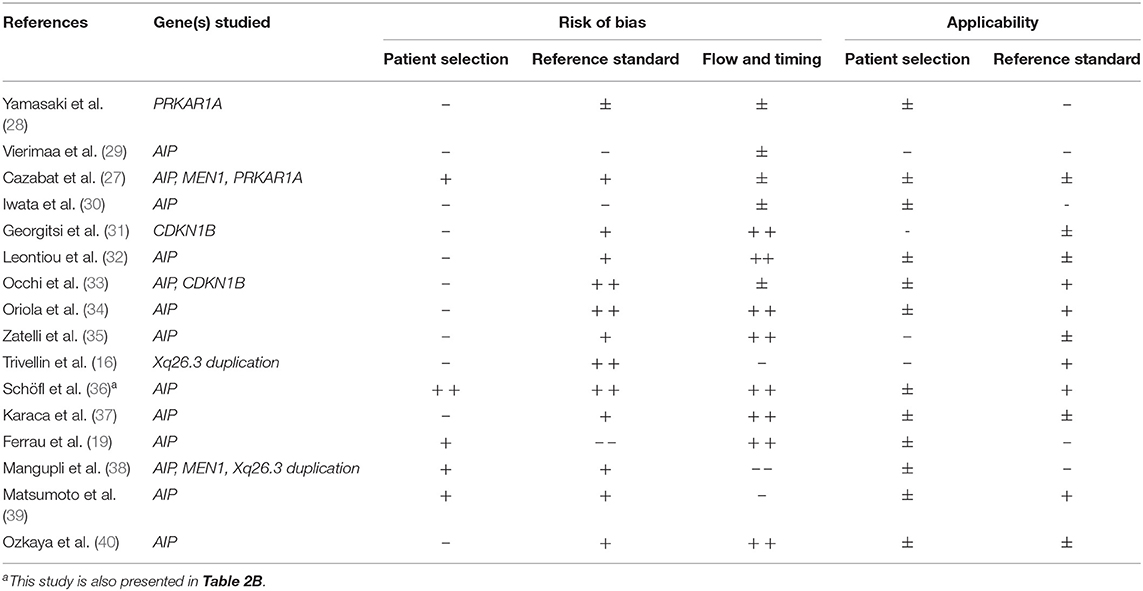
Out of 13 studies investigating the presence of an AIP gene mutation, one publication identified ≥ 5 cases with a germline mutation (27). In this study with a prevalence of an AIP mutation of 3.2%, predictors of the presence of a mutation were: younger age at diagnosis (mean age of AIP mutated patients 25 ± 10 vs. 43 ± 14 years in wildtype, P = 0.005) and gigantism (three out of five AIP mutated patients suffered from gigantism compared to 17 out of 149 patients without AIP mutation, P = 0.016). This study showed a minor risk of bias and intermediate applicability (see Tables 1A, 2A for more details).
In only two studies on Xq26.3 microduplication the data of apparently sporadically occurring PA could be extracted (16, 38). Both were at risk of bias and had a relatively low applicability for daily clinical practice. Trivellin et al. found an Xq26.3 duplication in 9 out of 38 sporadic patients with pituitary gigantism (24%). The total group of germline affected patients with gigantism (14 out of 43) had a female predominance (71 vs. 24%, P = 0.007), much earlier onset of increased growth velocity (median age 1.0 year (range 0.5–2.0) vs. 16.0 year (range 5.0–18.0), P < 0.001) and higher insulin-like growth factor (IGF-1) levels and more frequently elevated prolactin levels at diagnosis. Mangupli et al. found no cases of Xq26.3 microduplication at all.
In the five studies investigating the presence of MEN1, CDKN1B, and/or PRKAR1A mutations in sporadically occurring somatotroph adenoma, no predictors were identified (27, 28, 31, 33, 38).
The outcomes of all included studies on sporadic somatotroph adenoma are presented in Table 1A. Methodological quality assessment of studies is presented in Table 2A. For further details on study results, see Supplementary Data Sheet 5.
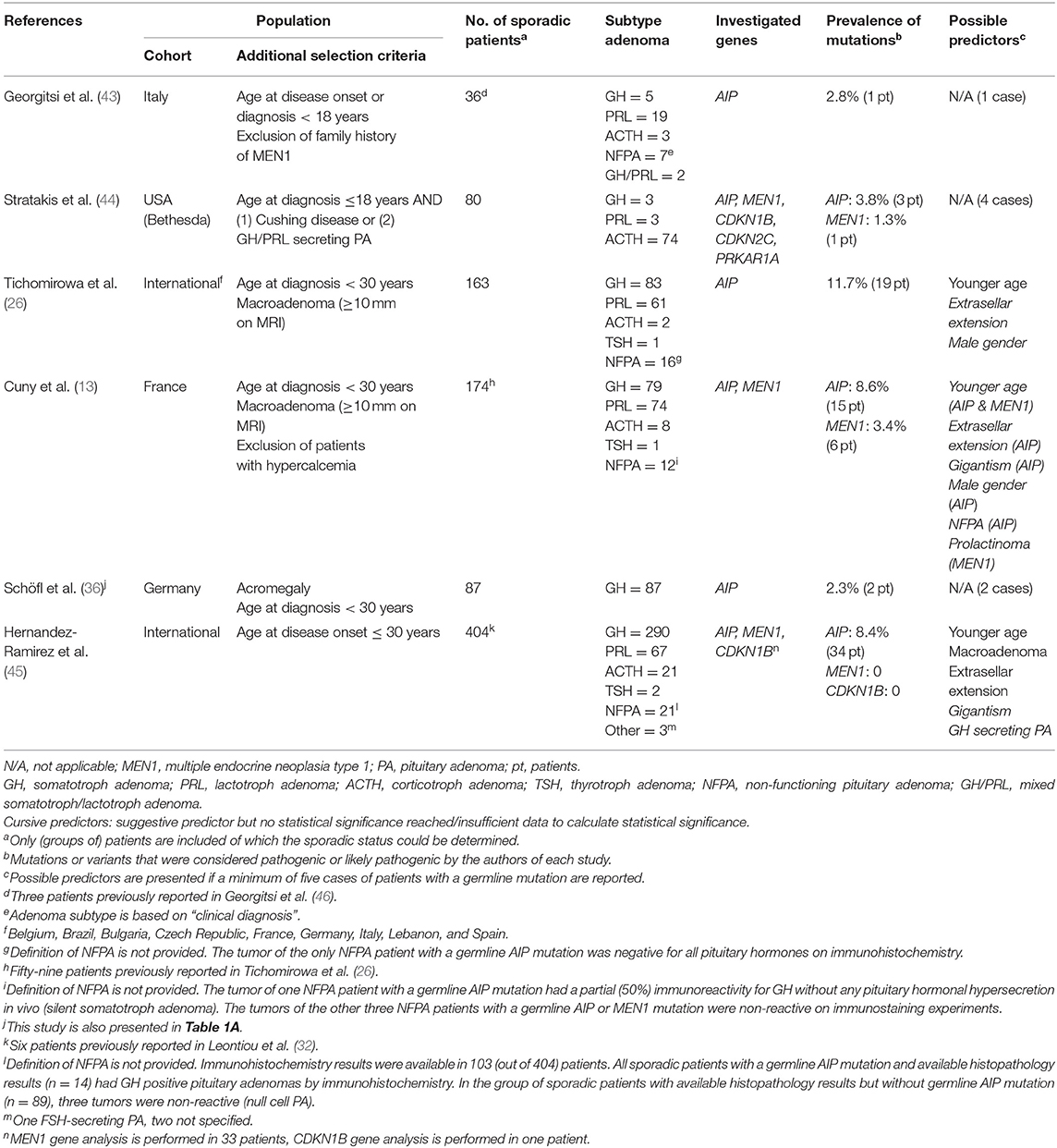
Young (≤30 Years) Patients With Sporadic PA
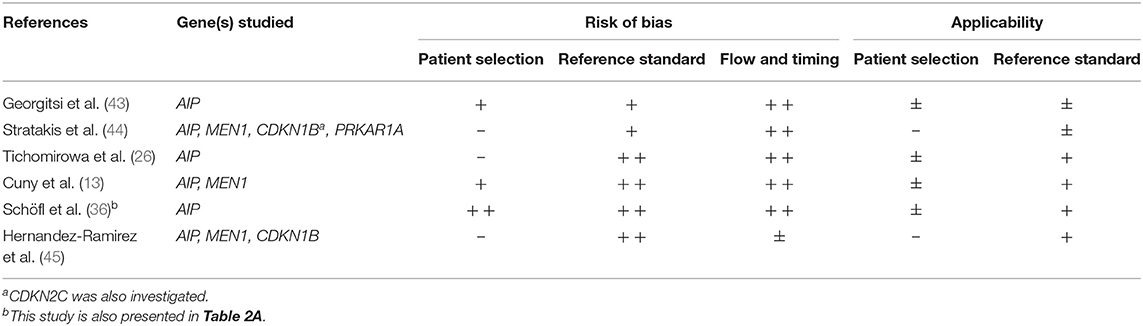
Three studies assessing the presence of an AIP mutation identified ≥5 cases with a germline mutation, reporting a mutation prevalence of 8.4, 8.6, and 11.7%, respectively (13, 26, 45). Study characteristics of all studies are displayed in Table 1B.
In all studies, the presence of an AIP mutation was related with a younger age of onset or, inversely, prevalence of AIP mutations was higher in patients with a younger age of diagnosis (≤18 years). Furthermore, the two studies only including patients with macroadenoma (≥10 mm) reported the highest frequency of AIP mutations, illustrating that macroadenoma is a predictor of this specific mutation. Extrasellar extension was a frequent feature. Thirdly, AIP mutations were more likely identified in patients suffering from gigantism. Additionally, despite a nearly equal gender distribution in study populations, male gender was overrepresented in AIP mutated patients.
Data on adenoma subtype were conflicting: although Cuny et al. reported a higher prevalence of AIP mutation in non-functioning PA, results from Hernandez-Ramirez et al. showed all AIP mutation related PA to be somatotroph adenomas. For further details on study results, see Supplementary Data Sheet 5.
The study of Cuny et al. showed only minor risk of bias and good applicability, making these results more reliable. Full quality assessment of studies can be found in Table 2B.
Regarding MEN 1 mutations, the study of Cuny et al. was at the lowest risk of bias and highest applicability (13). In this series of patients younger than 30 years (prevalence of MEN1 mutation: 3.4%), patients with a MEN1 mutation tended to be younger: 3 out of 46 (6.5%) patients ≤ 18 years harbored a germline MEN1 mutation vs. 3 out of 128 (2.3%) patients from 19 to 30 years at diagnosis. MEN1 mutations did also occur more frequently in prolactinomas (5.4%) than other PA subtypes (2%).
In the studies on the presence of the CDKN1B, CDKN2C, and PRKAR1A gene mutations no germline mutations were identified (44, 45).
Other Groups of Patients With Sporadic PA
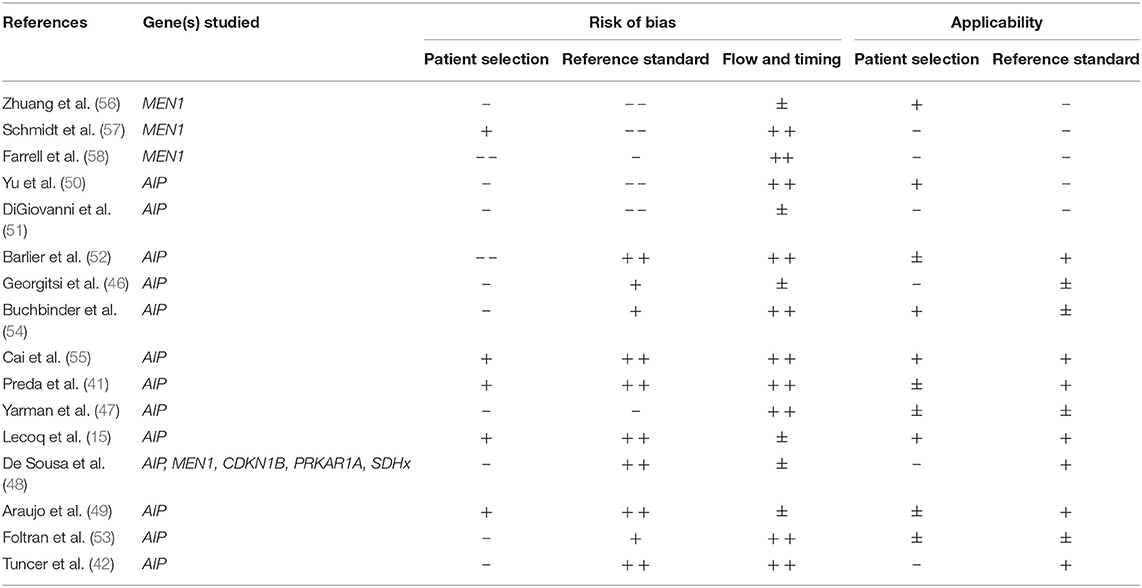
Sixteen studies applied a different set of in- and exclusion criteria than somatotroph adenoma or age at diagnosis ≤30 years, although four publications did use age criteria (41, 42, 48, 49). The reported prevalence of germline mutations within these studies is relatively low, with the exception of one study reporting a prevalence of 13.3 % (48).
The presence of AIP mutations was assessed in 13 studies. No AIP mutation was found in five of these studies (47, 50–53) and six studies described one to four cases with AIP mutation (41, 42, 46, 48, 49, 54). Lecoq et al. detected 22 cases, but unfortunately there was insufficient data reported for the identification of possible predictors of AIP status (15). In a publication of high methodological quality, Cai et al. detected six persons with AIP mutations (2.8%) in a group of 216 Han Chinese sporadic PA patients (55). The prevalence of an AIP mutation was higher in patients with a younger age at diagnosis (patients ≥ 18 years 6.3 vs. 2.5% in patients ≥ 18 years at diagnosis) and in the subgroup of somatotroph adenoma (6.3 vs. 0.7% in non-GH producing PA). In this study, male gender also appeared to be related with a higher prevalence of AIP mutations (5.3 vs. 0.8%).
Four studies on predictors for MEN1 gene mutations (48, 56–58) and one study on CDKN1B, PRKAR1A, and SDHx (48) did not reveal any mutation in the patients under study. See Table 1C for further study detail and Table 2C for all results on quality assessment.
Impact of a Germline Mutation on Treatment Outcome in PA
Ten studies reported on treatment outcome in patients with a germline mutation. In seven publications, treatment outcome was compared with a cohort of patients without germline mutation. Study characteristics are presented in Table 3.
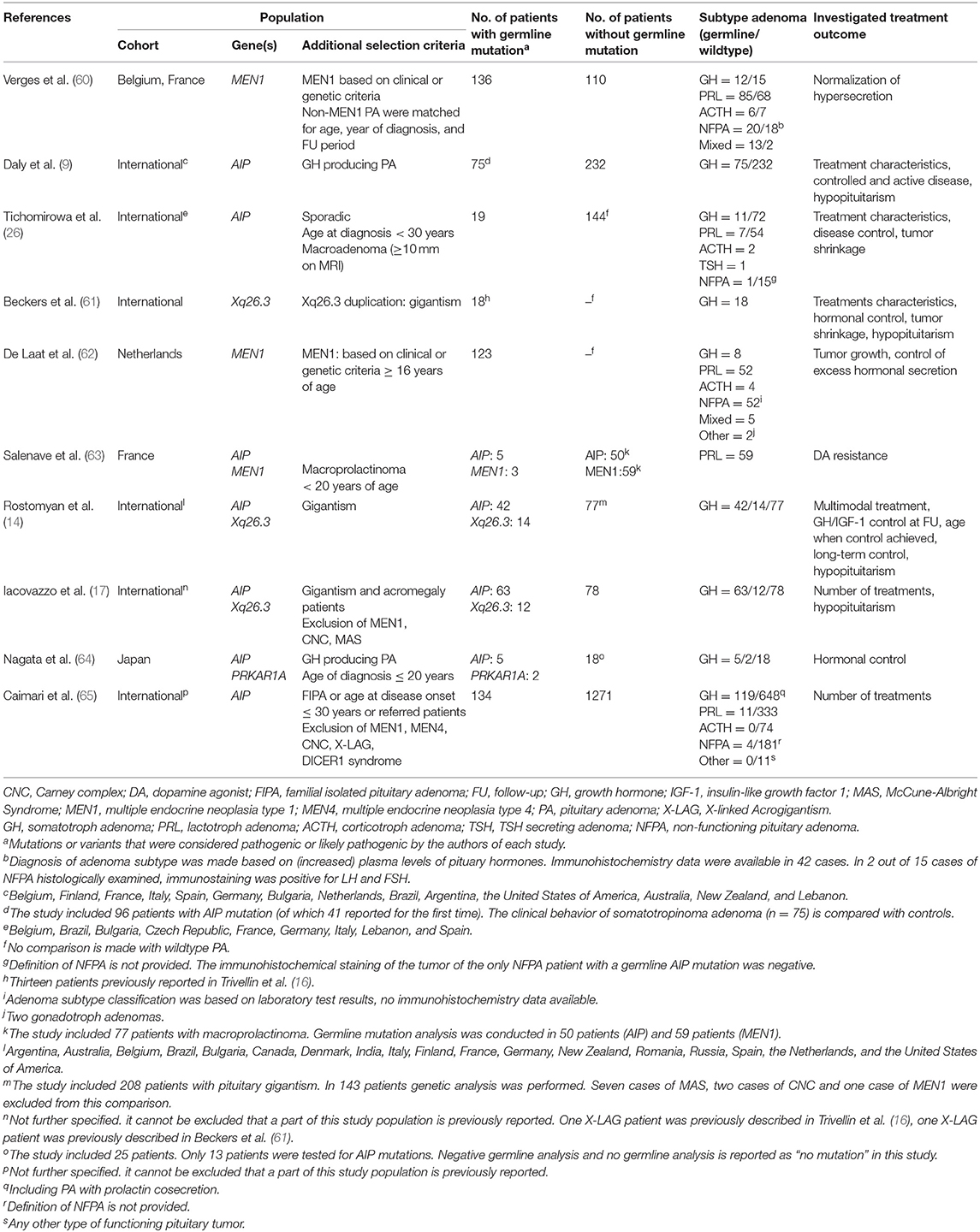
TABLE 3. Study characteristics of studies assessing the impact of a germline mutation on treatment outcome.
All seven studies on AIP mutations showed a potential risk of (patient) selection bias. The study of Daly et al. was at lowest risk of bias (9) (see Table 4 for full reporting of quality assessment). In this study 75 patients with an AIP mutation associated somatotroph adenoma were compared with 232 somatotropinomas without an AIP mutation. The proportion of patients receiving multimodal treatment was comparable (61.3 vs. 66.4%, respectively) and there was no significant difference in disease control (70.4 vs. 80.5%, respectively, P = 0.06). There were however some clear discrepancies in treatment characteristics and outcome: among patients with a higher cumulative treatment burden (≥3 distinct modalities), long-term disease control rates were significantly worse in AIP mutation associated adenoma (55.6 vs. 82.9%, P = 0.01). Furthermore, somatostatin analog (SSA)-induced GH and IGF-1 reduction and tumor size reduction was significantly less in AIP mutation associated PA. In line with these data, patients harboring an AIP mutation more often underwent a reoperation (21.9 vs. 5.5%). Although the prevalence of hypopituitarism in follow-up did not differ (AIP mutation associated 22.5 vs. controls 25.2%), patients with AIP mutation had a significantly higher number of pituitary deficiencies. Other studies on AIP mutation associated somatotropinomas showed similar results (26, 64). One study focused on AIP mutations in patients with apparently sporadically occurring PA and not familial cases (26). In this study, 4 out of 11 (36%) patients with AIP mutations underwent multiple surgical interventions, while post-operative SA therapy achieved disease control in only one out of nine patients.
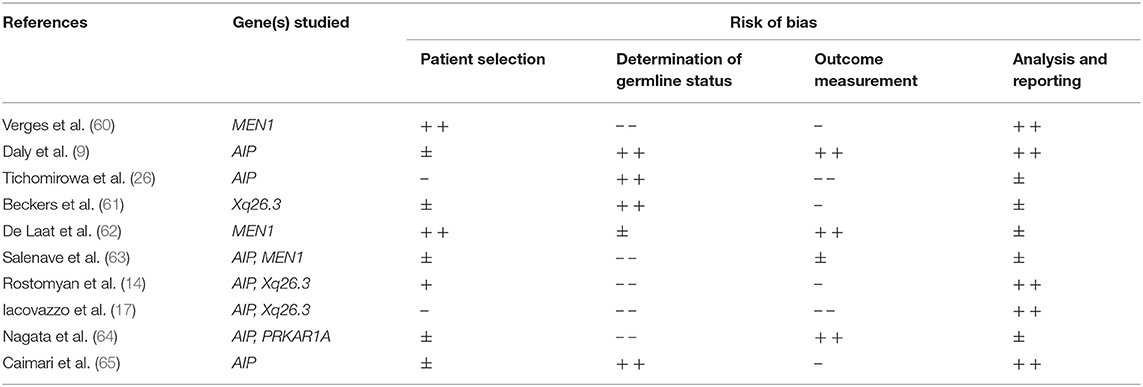
TABLE 4. Quality assessment of studies assessing the impact of a germline mutation on treatment outcome.
Two studies focused on patients with PA induced gigantism. Since these patients represent a distinct group with particularly high disease severity, these results are separately displayed. In contrast, Rostomyan et al. reported better treatment outcomes in AIP mutation associated gigantism than in patients suffering from gigantism without genetic abnormalities (14). Within an international cohort of 208 patients with pituitary gigantism, hormonal control was more frequently reached in AIP mutation associated PA. Multimodal treatment was seldom necessary in AIP mutation associated somatotropinoma gigantism (23.8 vs. 42.7% in controls, P = 0.04). Long-term control (>12 months) was reached more often in the AIP mutated patients (55.3 vs. 38.4%), but this was not statistically significant (P = 0.08). The frequency of hypopituitarism at follow-up was similar between both groups (73 vs. 66%). In another study including 153 patients with PA induced gigantism, no significant difference in number of treatments or in prevalence of hypopituitarism was found between 63 patients with AIP mutation associated gigantism and patients with gigantism but without genetic abnormalities (17).
In search for factors associated with response to dopamine agonists in macroprolactinoma, Salenave et al. found AIP mutations not to be a significant determinant. However, in this study only a small sample of AIP mutated PA (n = 4) was included (63). Failure of dopamine agonists in AIP mutation related PA has been described frequently (50% of cases) in other studies as well and multiple surgical interventions were needed regularly (9, 26). In the cohort of AIP mutations in apparently sporadically occurring PA (26), five out of seven patients (71%) underwent surgery and four out of seven patients (66.7%) had to undergo multiple surgeries, which was comparable with results from another study cohort of mainly familial AIP cases (9).
No comparative data have been published on treatment outcome in AIP mutation associated vs. wildtype non-functioning PA (NFPA). However, Daly et al. did report seven cases with AIP mutation related NFPA: six patients underwent surgery (of which one also underwent radiotherapy), long-term control of tumor size was achieved in all cases (9).
One of the largest studies on AIP mutation associated PA (134 cases) showed a trend toward a higher number of treatments in both functioning and non-functioning AIP mutation related PA (median 2 (IQR 1–3)) compared to patients without mutation (n = 1,271, median 1 (IQR 1–2)) (P = 0.055) (65). All data are shown in Supplementary Data Sheet 5.
Treatment-related outcome of PAs in MEN1 patients was described in three studies (60, 62, 63). A population based multicenter study including 123 MEN1 patients with PA by de Laat et al. was at lowest risk of bias. This study showed that prolactinomas in MEN1 patients respond well to medical treatment. Furthermore, this study showed that tumor growth was very limited over time and almost always without clinical consequences. In contrast, Verges et al. found a significant difference in normalization of pituitary hypersecretion between MEN1 and non-MEN1 functional PA (42 vs. 90%, respectively, P < 0.001). Normalization of plasma prolactin was significantly less frequent in MEN1 (44%) vs. non-MEN1 patients (90%) (P < 0.001). Salenave et al. reported the presence of a MEN1 mutation as a significant and independent predictor of dopamine agonist resistance in a regression analysis of 77 patients with prolactinoma (t = 3.052, P = 0.004). However, in this study a low number of MEN1 patients (n = 3) was included.
Treatment outcome in patients with Xq26.3 microduplications (also known as X-Linked Acrogigantism, or X-LAG) is described in three studies (14, 17, 61). Since Xq26.3 microduplications lead to an excessive growth velocity in the first years of life, X-LAG patients have a younger age at diagnosis and younger age at therapy-induced hormonal control than non-mutated counterparts (14). Due to this distinctive phenotype, it is hard to compare these results with other (sporadic) patients with PA. The proportion of patients in which disease control was reached varied due to the use of different definitions (41.7–91.7%). Multimodal treatment was necessary in the majority of cases, and hypopituitarism occurred frequently (70.6–75%). Hormonal control could almost never be achieved by medical therapy (dopamine agonists or SSA) alone (61). When comparing treatment outcome with pituitary induced gigantism without genetic abnormalities, Rostomyan et al. and Iacovazzo et al. found no differences in number of treatment modalities or prevalence of hypopituitarism between groups. The percentage of patients with long-term disease control (>12 months) did not differ significantly (X-LAG: 41.7%, controls: 38.4%), but appropriate control of GH/IGF-1 levels at last follow-up was reached more frequently in X-LAG patients (58.0 vs. 43.0%, P = 0.02) (14). For more study results, see Supplementary Data Sheet 5.
No eligible studies were found on the implications of germline mutations in PRKAR1A, CDKN1B, and SDHx.
Discussion
The prevalence of germline mutations in unselected sporadically occurring PA is low. Therefore, germline analysis is not advisable for all patients. Based on the best-available evidence, the best predictor of an AIP or MEN1 mutation appears to be a younger age at diagnosis (≤30 years). Moreover, the prevalence of an AIP mutation is significantly higher in pediatric patients in comparison to young adults (13, 26, 45).
Focusing on AIP mutations, the presence of gigantism and macroadenoma seems to be additional predictors of these mutations. The overgrowth may be attributed to the effect of GH/IGF-1 excess before full bone maturation. A male predominance in AIP affected individuals was found in a number studies (13, 26, 55). However, since it is conceivable that men are more prone to gigantism due to later growth cessation and male predominance was not observed in large families with an AIP mutation, this phenomenon might be explained by ascertainment bias (33). Both younger age at diagnosis and macroadenoma can be an expression of a more aggressive course of AIP mutation related PAs. Data on other factors such as adenoma subtype or the extent of tumor expansion are conflicting or too limited to draw clear conclusions.
MEN1 mutation analysis is recommended in young patients (≤30 years). In one study, it is even suggested that MEN1 mutations are more frequently found in prolactinomas (13). However, this is not yet confirmed in other studies.
Given the relatively high disease burden and younger age, patients suffering from pituitary related gigantism constitute a separate category. Germline Xq26.3 microduplications were strongly associated with an early increased growth velocity and female gender. Since all reported patients harboring Xq26.3 microduplication experienced a start of rapid growth already below 5 years of age, it is reasonable to perform genetic analysis for Xq26.3 microduplications especially in this subset of patients with sporadic pituitary gigantism (14, 16, 17, 61).
No cases of germline mutations in the PRKAR1A gene, SDHx genes, and CDKN1B or CDKN2C gene were reported in the included articles, which can be explained by our focus on apparently sporadically occurring PA instead of PA occurring with other syndromic manifestations. In addition, PA only very rarely occurs as manifestation of these, also rare, genetic syndromes. Therefore, genetic analysis of PRKAR1A, SDHx, and CDKN1B should only be conducted in selected cases with suggestive (syndromic) features.
AIP mutated somatotroph adenomas are more frequently resistant to SSA treatment than their non-mutated counterparts and reoperation is needed more often. Low AIP protein expression in tissue is correlated with worse response to SSA treatment (66), but since AIP downregulation may occur regardless of AIP mutations, it is still uncertain which mechanisms are involved (67). Failure of response to dopamine treatment is also described frequently in AIP mutation associated prolactinoma (9, 26). Treatment outcome seems similar when comparing study results of cohorts of sporadic and mainly familial occurring AIP mutation related PA patients, but data are too limited to draw clear conclusions (9, 26). Multimodal treatment is needed regularly but comparable with the treatment modalities in non-mutated controls, and difference in disease control did not reach statistical significance (9). There are too little reliable comparative data to determine the influence of an AIP mutation on treatment outcome in NFPA.
Best available evidence shows that MEN1 mutation associated prolactinomas respond well to medical treatment and NFPA show no to very little tumor growth in virtually all cases (62). These findings are in contrast with earlier findings (60), partially due to the population based cohort studied by de Laat et al. and the inclusion of PA diagnosed by screening (n = 66).
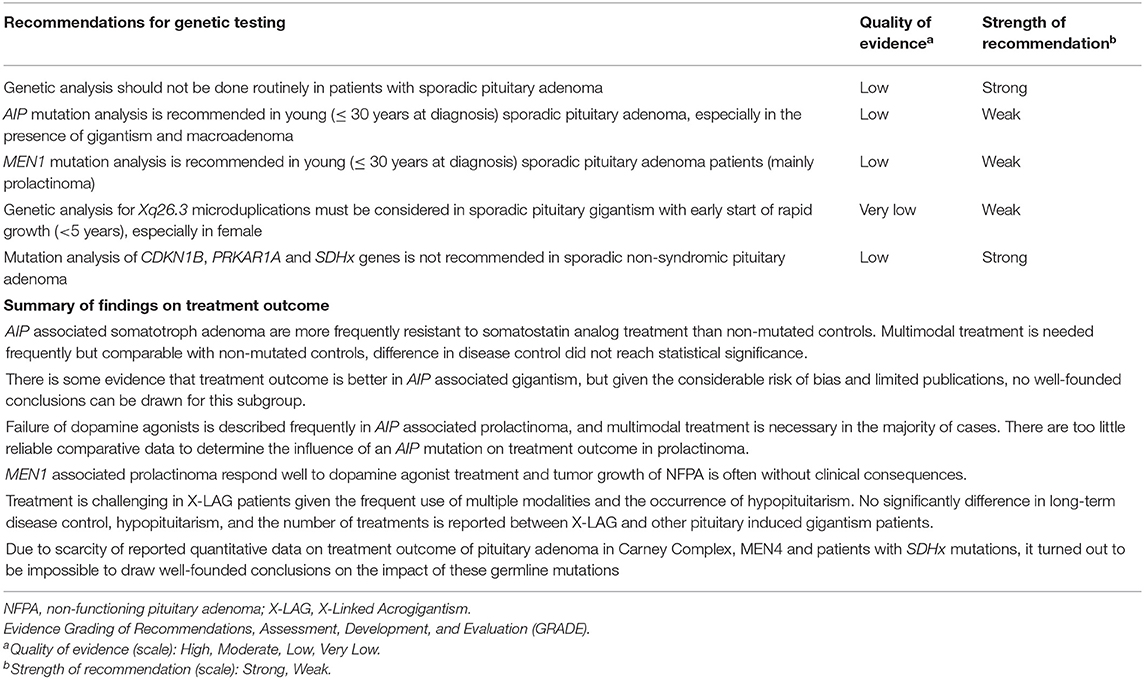
The presence of Xq26.3 microduplication is not related to a different treatment outcome compared to other cases of pituitary gigantism. Nonetheless, multiple treatment modalities are needed in most patients and complications such as hypopituitarism are frequent (14, 17, 61). Due to scarcity of reported quantitative information on treatment outcome of PA associated with mutations in PRKAR1A, CDKN1B, and SDHx, the impact of these germline mutations on therapy and outcome could not be predicted. The summary of recommendations and findings is presented in Table 5.
The majority of studies showed a considerable risk of bias, which can be partially explained by small study sizes inherent to the rarity of the disease. Most of the reported study populations were included in a non-random and non-consecutive manner and study cohorts were frequently selected from tertiary care centers, leading to potential patient selection bias. In some, mostly older studies, genetic analysis was not performed according to current quality standards. Furthermore, classification of genetic variants regarding the appropriate level of pathogenicity did not always take place according to the American College of Medical Genetics and Genomics and Association for Molecular Pathology (AMCG-AMP) guidelines (68). These genetic issues introduce a risk of detection bias. The retrospective design and lack of standardized data collection in most studies further hamper the methodological quality. Moreover, it cannot be excluded that parts of included study cohorts were reported previously, introducing a possible distortion in results. Therefore, results must be interpreted with caution before drawing conclusions and especially before being used for decision making in daily clinical practice.
Still, the aim of this review was to retrieve highly applicable best-available evidence on specific clinically relevant questions. Although we attempted to retrieve additional results, insufficient reporting of outcomes concerning our predefined topics led to exclusion of otherwise valuable records. We did exclude too small sized studies to avoid imprecise estimations. In addition, we did not perform a meta-analysis of data because of the high heterogeneity of studies to avoid unreliable outcomes. Additionally, we used the presented results on the adenoma subtype as described in the individual papers, because immunochemistry results were not always provided. This could have resulted in slightly inaccurate results in NFPA, since immunostaining can reveal clinically silent or “whispering” adenomas with some evidence of biochemical hypersecretion. Given the distinctive clinical behavior of these subtypes, a thorough investigation of adenoma subtype according to the most recent World Health Organization guidelines would have provided us with more accurate results (69, 70). However, we provided all available data on immunohistochemistry of NFPA in the results tables. Finally, the large range of publication dates introduced a challenge in the interpretation of pathogenicity of genetic variants. By adopting the author's judgement, outdated knowledge or techniques can have resulted in inaccuracy of the results. Optimally, all historic results would have to be confirmed by the current standards of DNA analysis and interpretation. Therefore, the DNA analysis techniques and interpretation of genetic variants (e.g., loss of heterozigosity studies, worldwide SNP databases, in silico analysis, functional studies) were evaluated thoroughly in our critical appraisal to put the results into the right perspective.
In general, our results support earlier findings and reviews on genetic analysis in PA (71–74). Recently, Caimari et al. developed a user-friendly risk category system to find AIP mutation associated PA using a large international cohort of 2,227 individuals. Young age of onset, familial status, GH excess, and macroadenoma were the strongest predictors (65). However, in contrast to these study results and earlier reviews, our recommendations are focused on apparently sporadically occurring PA in patients without other features of genetic syndromes. Furthermore, they come with the proper strength of recommendations as a result of the systematic literature search and critical appraisal of articles.
A number of unanswered questions and challenges for the future still remain. As a result of the rarity of diseases and/or PA as presenting manifestation, the clinical impact of a CDKN1B, PRKAR1A, and SDHx mutations on treatment outcome of PA is still uncertain. Only worldwide networks of collaborating centers sharing clinical information can help unravel this issue. Secondly, the implications of an AIP mutation in apparent unaffected family members are unknown. To our knowledge, results from systematic follow-up of unaffected AIP-positive family members are not available. Therefore, surveillance guidelines in these cases await further studies. Furthermore, the number of germline variants of uncertain significance will continue to increase in the (near) future due to the increased genetic analysis modalities, further emphasizing the need for studies of functional status combined with data on clinical outcome from large worldwide databases. Lastly, despite our efforts to produce reliable recommendations, it remains difficult to predict the benefits of our recommendations when implementing them in daily practice. For example, in a recent study by Daly et al., no germline mutations in the AIP or MEN1 gene were identified in a group of 55 PA patients, despite the use of risk criteria (75). These results show that no risk stratification system or set of screening recommendations is flawless. By external validation and further (clinical) research these tools can be optimized in the future, but will never be all comprehensive.
Based on the yet available literature on the value of genetic analysis of sporadic PA, we can conclude that effective use of genetic analysis can lead to early disease identification (with possibly beneficial treatment outcome) on the one hand, and can lower health care costs and psychological burden on the other hand if unnecessary investigations can be limited. Knowledge of the effect of germline mutations on treatment outcome helps to determine therapy strategy and possibly lowers disease morbidity. Now, large and unselected cohort studies, are needed to further guide the indications and the consequences of mutation analysis in individual patients with PA.
Author Contributions
MB, RL, and GV contributed to conception and design of the study. MB and BN contributed to data collection (selection of articles) and contributed to the critical appraisal of studies. MB contributed to data extraction and wrote the first draft of the manuscript. MB, BN, AV, RL, and GV contributed to the interpretation of data. RL and GV contributed to supervision of data collection, supervision of critical appraisal of studies, and supervison of data extraction. All authors contributed to critically reviewing the manuscript, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2019.00837/full#supplementary-material
References
1. Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, et al. The prevalence of pituitary adenomas: a systematic review. Cancer. (2004) 101:613–9. doi: 10.1002/cncr.20412
2. Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A. High prevalence of pituitary adenomas : a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab. (2006) 91:4769–75. doi: 10.1210/jc.2006-1668
3. Fontana E, Gaillard R. Epidémiologie des adénomes hypophysaires: étude dans une agglomération urbaine de Suisse. Rev Med Suisse. (2009) 5:2172–4.
4. Fernandez A, Karavitaki N, Wass JAH. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol. (2010) 72:377–82. doi: 10.1111/j.1365-2265.2009.03667.x
5. Raappana A, Koivukangas J, Ebeling T, Pirilä T. Incidence of pituitary adenomas in northern Finland in 1992-2007. J Clin Endocrinol Metab. (2010) 95:4268–75. doi: 10.1210/jc.2010-0537
6. Day PF, Loto MG, Glerean M, Picasso MFR, Lovazzano S, Giunta DH. Incidence and prevalence of clinically relevant pituitary adenomas: retrospective cohort study in a Health Management Organization in Buenos Aires, Argentina. Arch Endocrinol Metab. (2016) 60:554–61. doi: 10.1590/2359-3997000000195
7. Daly AF, Tichomirowa MA, Beckers A. The epidemiology and genetics of pituitary adenomas. Best Pract Res Clin Endocrinol Metab. (2009) 23:543–54. doi: 10.1016/j.beem.2009.05.008
8. Aflorei ED, Korbonits M. Epidemiology and etiopathogenesis of pituitary adenomas. J Neurooncol. (2014) 117:379–94. doi: 10.1007/s11060-013-1354-5
9. Daly AF, Tichomirowa MA, Petrossians P, Heliovaara E, Jaffrain-Rea M-L, Barlier A, et al. Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: An international collaborative study. J Clin Endocrinol Metab. (2010) 95:E373–83. doi: 10.1210/jc.2009-2556
10. de Pinho LKJ, Vieira Neto L, Wildemberg LEA, Moraes AB, Takiya CM, Frohman LA, et al. Familial isolated pituitary adenomas experience at a single center: clinical importance of AIP mutation screening. Arq Bras Endocrinol Metabol. (2010) 54:698–704. doi: 10.1590/S0004-27302010000800006
11. de Laat JM, van Leeuwaarde RS, Valk GD. The importance of an early and accurate MEN1 diagnosis. Front Endocrinol. (2018) 9:533. doi: 10.3389/fendo.2018.00533
12. Marques P, Palou FC, Hernández-Ramírez LC, Barry S, Iacovazzo D, Grossman A, et al. Significant phenotypic difference between clinically presenting vs. prospectively diagnosed pituitary adenoma in AIP mutation-positive kindreds. In: Marques P, editor, 100th Annual Meeting of the Endocrine Society, ENDO 2018. London; Chicago, IL: Centre for Endocrinology, William Harvey Research Institute, Queen Mary University of London (2018), p. 17–20.
13. Cuny T, Pertuit M, Sahnoun-Fathallah M, Daly A, Occhi G, Odou MF, et al. Genetic analysis in young patients with sporadic pituitary macroadenomas: Besides AIP don't forget MEN1 genetic analysis. Eur J Endocrinol. (2013) 168:533–41. doi: 10.1530/EJE-12-0763
14. Rostomyan L, Daly AF, Petrossians P, Nachev E, Lila AR, Lecoq A-L, et al. Clinical and genetic characterization of pituitary gigantism: an international collaborative study in 208 patients. Endocr Relat Cancer. (2015) 22:745–57. doi: 10.1530/ERC-15-0320
15. Lecoq A-L, Bouligand J, Hage M, Cazabat L, Salenave S, Linglart A, et al. Very low frequency of germline GPR101 genetic variation and no biallelic defects with AIP in a large cohort of patients with sporadic pituitary adenomas. Eur J Endocrinol. (2016) 174:523–30. doi: 10.1530/EJE-15-1044
16. Trivellin G, Daly AF, Faucz FR, Yuan B, Rostomyan L, Larco DO, et al. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N Engl J Med. (2014) 371:2363–74. doi: 10.1056/NEJMoa1408028
17. Iacovazzo D, Caswell R, Bunce B, Jose S, Yuan B, Hernández-Ramírez LC, et al. Germline or somatic GPR101 duplication leads to X-linked acrogigantism: a clinico-pathological and genetic study. Acta Neuropathol Commun. (2016) 4:56. doi: 10.1186/s40478-016-0328-1
18. Trivellin G, Correa RR, Batsis M, Faucz FR, Chittiboina P, Bjelobaba I, et al. Screening for GPR101 defects in pediatric pituitary corticotropinomas. Endocr Relat Cancer. (2016) 23:357–65. doi: 10.1530/ERC-16-0091
19. Ferraù F, Romeo PD, Puglisi S, Ragonese M, Torre ML, Scaroni C, et al. Analysis of GPR101 and AIP genes mutations in acromegaly: a multicentric study. Endocrine. (2016) 54:762–7. doi: 10.1007/s12020-016-0862-4
20. Pepe S, Korbonits M, Iacovazzo D. Germline and mosaic mutations causing pituitary tumours: genetic and molecular aspects. J Endocrinol. (2019) 240:R21–45. doi: 10.1530/JOE-18-0446
21. Kamenický P, Bouligand J, Chanson P. Gigantism, acromegaly, and GPR101 mutations. N Engl J Med. (2015) 372:1264–5. doi: 10.1056/NEJMc1500340
22. Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
23. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. (2013) 158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
24. Atkins D, Best D, Briss P, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. Br Med J. (2004) 328:1490. doi: 10.1136/bmj.328.7454.1490
25. Swiglo BA, Murad MH, Schünemann HJ, Kunz R, Vigersky RA, Guyatt GH, et al. A case for clarity, consistency, and helpfulness: state-of-the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J Clin Endocrinol Metab. (2008) 93:666–73. doi: 10.1210/jc.2007-1907
26. Tichomirowa MA, Barlier A, Daly AF, Jaffrain-Rea ML, Ronchi C, Yaneva M, et al. High prevalence of AIP gene mutations following focused screening in young patients with sporadic pituitary macroadenomas. Eur J Endocrinol. (2011) 165:509–15. doi: 10.1530/EJE-11-0304
27. Cazabat L, Libè R, Perlemoine K, René-Corail F, Burnichon N, Gimenez-Roqueplo A-P, et al. Germline inactivating mutations of the aryl hydrocarbon receptor-interacting protein gene in a large cohort of sporadic acromegaly: mutations are found in a subset of young patients with macroadenomas. Eur J Endocrinol. (2007) 157:1–8. doi: 10.1530/EJE-07-0181
28. Yamasaki H, Mizusawa N, Nagahiro S, Yamada S, Sano T, Itakura M, et al. GH-secreting pituitary adenomas infrequently contain inactivating mutations of PRKAR1A and LOH of 17q23-24. Clin Endocrinol. (2003) 58:464–70. doi: 10.1046/j.1365-2265.2003.01740.x
29. Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, et al. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. (2006) 312:1228–30. doi: 10.1126/science.1126100
30. Iwata T, Yamada S, Mizusawa N, Golam HMD, Sano T, Yoshimoto K. The aryl hydrocarbon receptor-interacting protein gene is rarely mutated in sporadic GH-secreting adenomas. Clin Endocrinol. (2007) 66:499–502. doi: 10.1111/j.1365-2265.2007.02758.x
31. Georgitsi M, Raitila A, Karhu A, van der Luijt RB, Aalfs CM, Sane T, et al. Germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. J Clin Endocrinol Metab. (2007) 92:3321–5. doi: 10.1210/jc.2006-2843
32. Leontiou CA, Gueorguiev M, van der Spuy J, Quinton R, Lolli F, Hassan S, et al. The role of the aryl hydrocarbon receptor-interacting protein gene in familial and sporadic pituitary adenomas. J Clin Endocrinol Metab. (2008) 93:2390–401. doi: 10.1210/jc.2007-2611
33. Occhi G, Trivellin G, Ceccato F, De Lazzari P, Giorgi G, Dematte S, et al. Prevalence of AIP mutations in a large series of sporadic Italian acromegalic patients and evaluation of CDKN1B status in acromegalic patients with multiple endocrine neoplasia. Eur J Endocrinol. (2010) 163:369–76. doi: 10.1530/EJE-10-0327
34. Oriola J, Lucas T, Halperin I, Mora M, Perales MJ, Alvarez-Escolá C, et al. Germline mutations of AIP gene in somatotropinomas resistant to somatostatin analogues. Eur J Endocrinol. (2013) 168:9–13. doi: 10.1530/EJE-12-0457
35. Zatelli MC, Torre ML, Rossi R, Ragonese M, Trimarchi F, degli Uberti E, et al. Should aip gene screening be recommended in family members of FIPA patients with R16H variant? Pituitary. (2013) 16:238–44. doi: 10.1007/s11102-012-0409-5
36. Schöfl C, Honegger J, Droste M, Grussendorf M, Finke R, Plöckinger U, et al. Frequency of AIP gene mutations in young patients with acromegaly: a registry-based study. J Clin Endocrinol Metab. (2014) 99:E2789–93. doi: 10.1210/jc.2014-2094
37. Karaca Z, Taheri S, Tanriverdi F, Unluhizarci K, Kelestimur F. Prevalence of AIP mutations in a series of Turkish acromegalic patients: are synonymous AIP mutations relevant? Pituitary. (2015) 18:831–7. doi: 10.1007/s11102-015-0659-0
38. Mangupli R, Rostomyan L, Castermans E, Caberg J-H, Camperos P, Krivoy J, et al. Combined treatment with octreotide LAR and pegvisomant in patients with pituitary gigantism: clinical evaluation and genetic screening. Pituitary. (2016) 19:507–14. doi: 10.1007/s11102-016-0732-3
39. Matsumoto R, Izawa M, Fukuoka H, Iguchi G, Odake Y, Yoshida K, et al. Genetic and clinical characteristics of Japanese patients with sporadic somatotropinoma. Endocr J. (2016) 63:953–63. doi: 10.1507/endocrj.EJ16-0075
40. Ozkaya HM, Comunoglu N, Sayitoglu M, Keskin FE, Firtina S, Khodzhaev K, et al. Germline mutations of aryl hydrocarbon receptor-interacting protein (AIP) gene and somatostatin receptor 1–5 and AIP immunostaining in patients with sporadic acromegaly with poor versus good response to somatostatin analogues. Pituitary. (2018) 21:335–46. doi: 10.1007/s11102-018-0876-4
41. Preda V, Korbonits M, Cudlip S, Karavitaki N, Grossman AB. Low rate of germline AIP mutations in patients with apparently sporadic pituitary adenomas before the age of 40: a single-centre adult cohort. Eur J Endocrinol. (2014) 171:659–66. doi: 10.1530/EJE-14-0426
42. Tuncer FN, Doganşen SC, Serbest E, Tanrikulu S, Ekici Y, Bilgiç B, et al. Screening of AIP gene variations in a cohort of Turkish patients with young-onset sporadic hormone-secreting pituitary adenomas. Genet Test Mol Biomarkers. (2018) 22:1–7. doi: 10.1089/gtmb.2018.0133
43. Georgitsi M, De Menis E, Cannavò S, Mäkinen MJ, Tuppurainen K, Pauletto P, et al. Aryl hydrocarbon receptor interacting protein (AIP) gene mutation analysis in children and adolescents with sporadic pituitary adenomas. Clin Endocrinol. (2008) 69:621–7. doi: 10.1111/j.1365-2265.2008.03266.x
44. Stratakis CA, Tichomirowa MA, Boikos S, Azevedo MF, Lodish M, Martari M, et al. The role of germline AIP, MEN1, PRKAR1A, CDKN1B and CDKN2C mutations in causing pituitary adenomas in a large cohort of children, adolescents, and patients with genetic syndromes. Clin Genet. (2010) 78:457–63. doi: 10.1111/j.1399-0004.2010.01406.x
45. Hernandez-Ramirez LC, Gabrovska P, Denes J, Stals K, Trivellin G, Tilley D, et al. Landscape of familial isolated and young-onset pituitary adenomas: prospective diagnosis in AIP mutation carriers. J Clin Endocrinol Metab. (2015) 100:E1242–54. doi: 10.1210/jc.2015-1869
46. Georgitsi M, Raitila A, Karhu A, Tuppurainen K, Makinen MJ, Vierimaa O, et al. Molecular diagnosis of pituitary adenoma predisposition caused by aryl hydrocarbon receptor-interacting protein gene mutations. Proc Natl Acad Sci USA. (2007) 104:4101–5. doi: 10.1073/pnas.0700004104
47. Yarman S, Ogret YD, Oguz FS. Do the aryl hydrocarbon receptor interacting protein variants (Q228K and Q307R) play a role in patients with familial and sporadic hormone-secreting pituitary adenomas? Genet Test Mol Biomarkers. (2015) 19:394–8. doi: 10.1089/gtmb.2014.0333
48. De Sousa SMC, McCabe MJ, Wu K, Roscioli T, Gayevskiy V, Brook K, et al. Germline variants in familial pituitary tumour syndrome genes are common in young patients and families with additional endocrine tumours. Eur J Endocrinol. (2017) May;176:635–44. doi: 10.1530/EJE-16-0944
49. Araujo PB, Kasuki L, de Azeredo Lima CH, Ogino L, Camacho AHS, Chimelli L, et al. AIP mutations in Brazilian patients with sporadic pituitary adenomas: a single-center evaluation. Endocr Connect. (2017) 6:914–25. doi: 10.1530/EC-17-0237
50. Yu R, Bonert V, Saporta I, Raffel LJ, Melmed S. Aryl hydrocarbon receptor interacting protein variants in sporadic pituitary adenomas. J Clin Endocrinol Metab. (2006) 91:5126–9. doi: 10.1210/jc.2006-1731
51. DiGiovanni R, Serra S, Ezzat S, Asa SL. AIP mutations are not identified in patients with sporadic pituitary adenomas. Endocr Pathol. (2007) 18:76–8. doi: 10.1007/s12022-007-0010-z
52. Barlier A, Vanbellinghen J-F, Daly AF, Silvy M, Jaffrain-Rea M-L, Trouillas J, et al. Mutations in the aryl hydrocarbon receptor interacting protein gene are not highly prevalent among subjects with sporadic pituitary adenomas. J Clin Endocrinol Metab. (2007) 92:1952–5. doi: 10.1210/jc.2006-2702
53. Foltran RK, Amorim PVGH, Duarte FH, Grande IPP, Freire ACTB, Frassetto FP, et al. Study of major genetic factors involved in pituitary tumorigenesis and their impact on clinical and biological characteristics of sporadic somatotropinomas and non-functioning pituitary adenomas. Brazilian J Med Biol Res. (2018) 51:e7427. doi: 10.1590/1414-431x20187427
54. Buchbinder S, Bierhaus A, Zorn M, Nawroth PP, Humpert P, Schilling T. Aryl hydrocarbon receptor interacting protein gene (AIP) mutations are rare in patients with hormone secreting or non-secreting pituitary adenomas. Exp Clin Endocrinol Diabetes. (2008) 116:625–8. doi: 10.1055/s-2008-1065366
55. Cai F, Zhang Y-D, Zhao X, Yang Y-K, Ma S-H, Dai C-X, et al. Screening for AIP gene mutations in a Han Chinese pituitary adenoma cohort followed by LOH analysis. Eur J Endocrinol. (2013) 169:867–84. doi: 10.1530/EJE-13-0442
56. Zhuang Z, Ezzat SZ, Vortmeyer AO, Weil R, Oldfield EH, Park WS, et al. Mutations of the MEN1 tumor suppressor gene in pituitary tumors. Cancer Res. (1997) 57:5446–51.
57. Schmidt MC, Henke RT, Stangl AP, Meyer-Puttlitz B, Stoffel-Wagner B, Schramm J, et al. Analysis of the MEN1 gene in sporadic pituitary adenomas. J Pathol. (1999) 188:168–73. doi: 10.1002/(SICI)1096-9896(199906)188:2<168::AID-PATH342>3.0.CO;2-4
58. Farrell WE, Simpson DJ, Bicknell J, Magnay JL, Kyrodimou E, Thakker RV, et al. Sequence analysis and transcript expression of the MEN1 gene in sporadic pituitary tumours. Br J Cancer. (1999) 80:44–50. doi: 10.1038/sj.bjc.6690319
59. Cazabat L, Bouligand J, Salenave S, Bernier M, Gaillard S, Parker F, et al. Germline AIP mutations in apparently sporadic pituitary adenomas: prevalence in a prospective single-center cohort of 443 patients. J Clin Endocrinol Metab. (2012) 97:E663–70. doi: 10.1210/jc.2011-2291
60. Verges B, Boureille F, Goudet P, Murat A, Beckers A, Sassolas G, et al. Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J Clin Endocrinol Metab. (2002) 87:457–65. doi: 10.1210/jcem.87.2.8145
61. Beckers A, Lodish MB, Trivellin G, Rostomyan L, Lee M, Faucz FR, et al. X-linked acrogigantism syndrome: clinical profile and therapeutic responses. Endocr Relat Cancer. (2015) 22:353–67. doi: 10.1530/ERC-15-0038
62. de Laat JM, Dekkers OM, Pieterman CRC, Kluijfhout WP, Hermus AR, Pereira AM, et al. Long-term natural course of pituitary tumors in patients with MEN1: Results from the dutchmen1 study group (DMSG). J Clin Endocrinol Metab. (2015) 100:3288–96. doi: 10.1210/JC.2015-2015
63. Salenave S, Ancelle D, Bahougne T, Raverot G, Kamenicky P, Bouligand J, et al. Macroprolactinomas in children and adolescents: factors associated with the response to treatment in 77 patients. J Clin Endocrinol Metab. (2015) 100:1177–86. doi: 10.1210/jc.2014-3670
64. Nagata Y, Inoshita N, Fukuhara N, Yamaguchi-Okada M, Nishioka H, Iwata T, et al. Growth hormone-producing pituitary adenomas in childhood and young adulthood: clinical features and outcomes. Pituitary. (2018) 21:1–9. doi: 10.1007/s11102-017-0836-4
65. Caimari F, Hernández-Ramírez LC, Dang MN, Gabrovska P, Iacovazzo D, Stals K, et al. Risk category system to identify pituitary adenoma patients with AIP mutations. J Med Genet. (2018) 55:254–60. doi: 10.1136/jmedgenet-2017-104957
66. Kasuki L, Vieira Neto L, Wildemberg LEA, Colli LM, de Castro M, Takiya CM, et al. AIP expression in sporadic somatotropinomas is a predictor of the response to octreotide LAR therapy independent of SSTR2 expression. Endocr Relat Cancer. (2012) 19:L25–9. doi: 10.1530/ERC-12-0020
67. Jaffrain-Rea M-L, Angelini M, Gargano D, Tichomirowa MA, Daly AF, Vanbellinghen J-F, et al. Expression of aryl hydrocarbon receptor (AHR) and AHR-interacting protein in pituitary adenomas: pathological and clinical implications. Endocr Relat Cancer. (2009) 16:1029–43. doi: 10.1677/ERC-09-0094
68. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. ACMG Standards and Guidelines Standards and guidelines for the interpretation of sequence variants : a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. (2015) 17:405–24. doi: 10.1038/gim.2015.30
69. Lloyd R, Osamura R, G K, Rosai J (eds). World Health Organization Classification of Tumours of Endocrine Organs. 4th ed. Vol. 10. Lyon: IARC Publication (2017).
70. Drummond J, Roncaroli F, Grossman AB, Korbonits M. Clinical and pathological aspects of silent pituitary adenomas. J Clin Endocrinol Metab. (2019) 104:2473–89. doi: 10.1210/jc.2018-00688
71. Jaffrain-Rea M-L, Daly AF, Angelini M, Petrossians P, Bours V, Beckers A. Genetic susceptibility in pituitary adenomas: from pathogenesis to clinical implications. Expert Rev Endocrinol Metab. (2011) 6:195–214. doi: 10.1586/eem.10.87
72. Lecoq A, Kamenický P, Guiochon-Mantel A, Chanson P. Genetic mutations in sporadic pituitary adenomas — what to screen for? Nat Rev Endocrinol. (2015) 11:43–54. doi: 10.1038/nrendo.2014.181
73. Iacovazzo D, Hernández-Ramírez L, Korbonits M. Sporadic pituitary adenomas: the role of germline mutations and recommendations for genetic screening. Expert Rev Endocrinol Metab. (2017) 12:143–53. doi: 10.1080/17446651.2017.1306439
74. Marques P, Korbonits M. Genetic aspects of pituitary adenomas. Endocrinol Metab Clin North Am. (2017) 46:335–74. doi: 10.1016/j.ecl.2017.01.004
Keywords: pituitary adenoma, germline mutation, genetic analysis, mutation, screening
Citation: van den Broek MFM, van Nesselrooij BPM, Verrijn Stuart AA, van Leeuwaarde RS and Valk GD (2019) Clinical Relevance of Genetic Analysis in Patients With Pituitary Adenomas: A Systematic Review. Front. Endocrinol. 10:837. doi: 10.3389/fendo.2019.00837
Received: 13 August 2019; Accepted: 18 November 2019;
Published: 10 December 2019.
Edited by:
Hidenori Fukuoka, Kobe University, JapanReviewed by:
Hiroshi Nishioka, Toranomon Hospital, JapanEdward Raymond Laws, Harvard Medical School, United States
Copyright © 2019 van den Broek, van Nesselrooij, Verrijn Stuart, van Leeuwaarde and Valk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gerlof D. Valk, Zy5kLnZhbGtAdW1jdXRyZWNodC5ubA==
 Medard F. M. van den Broek
Medard F. M. van den Broek Bernadette P. M. van Nesselrooij2
Bernadette P. M. van Nesselrooij2