- Department of Assisted Reproduction, Shanghai Ninth People's Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Background: Endometriosis is one of the most challenging diseases for doctors helping infertile women conceive, which has become a common method to help maternal endometriosis-associated infertility. Women with advanced endometriosis possess a higher risk of several adverse outcomes both during pregnancy and at the time of delivery. Whether endometriosis gives rise to a higher occurrence of congenital abnormalities in infants via in vitro fertilization and frozen–thawed embryo transfer (IVF-ET) remains unknown.
Methods: Data collected on 22,865 women undergoing IVF using a freeze-all strategy from 2007 to 2017 were analyzed to estimate the rate of congenital malformations. We used an adjusted OR to compare the fertility outcomes of women with advanced endometriosis to the control group.
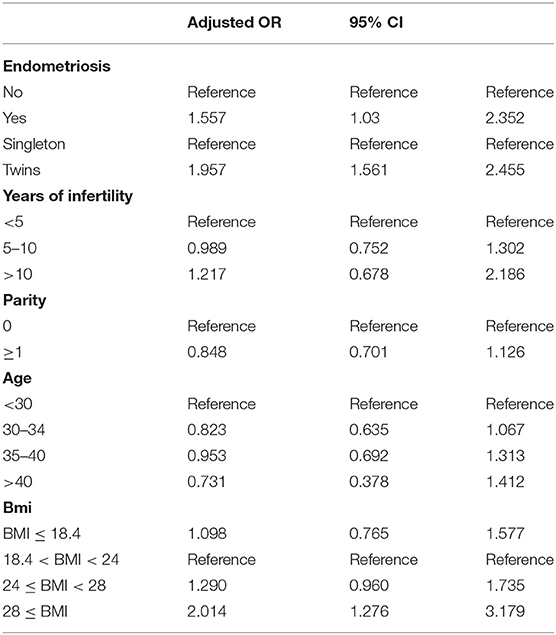
Results: We studied 1,495 infants born from women with advanced endometriosis and 27,105 infants born from endometriosis-free women. There was a 1.557-fold risk that the infants with advanced maternal endometriosis would develop a congenital malformation (adjusted OR: 1.557, 95% CI: 1.03–2.35). Compared with singletons, twins were 1.957 times more likely to experience an adverse outcome (OR: 1.957, 95% CI: 1.561–2.455). When analyzing specific categories of birth defects, the proportion of circulatory system defects was higher than the other categories of birth defects in total (0.56%), followed by musculoskeletal system defects (0.15%).
Conclusions: Maternal advanced endometriosis might increase the risk of congenital malformations for infants born after IVF-ET. The organ system most frequently affected by congenital malformations was the cardiovascular system, followed by the musculoskeletal system.
Introduction
The disease in women, endometriosis, is identified by endometrial glands, also known as stroma in sites besides the uterine cavity. Normally, the lesion is located in the ovaries or the peritoneum. Doctors have to face immense challenges while helping infertile women conceive due to endometriosis. It affects over 150 million women worldwide and is also estimated to affect 7–12% of women of reproductive age; there is a much higher incidence in infertile women. In total, 25% of patients undergoing assisted reproductive technology (ART) are affected and 20–40% of these patients show ovarian endometriosis (1, 2).
Several etiologies, such as oxidative stress (3), inflammatory factors, cytokines (4), genetic etiology (5, 6), immunity (7), and hormone role (8), have been reported as catalysts for endometriosis.
Though it is known that the prevalence peaks of the disease occur in the reproductive age, endometriosis leads to complications in both pregnancy and conception. Women with endometriosis have failed to draw the attention on the eminent personalities toward the health of children born to them until recently. Recent studies have revealed that pregnancy complications are more common in women inflicted with endometriosis, leading to miscarriage, and ectopic pregnancy at times (9). Moreover, it has been substantially proven from the start that premature births are more prevalent in women with endometriosis and pose a major health hazard (10–13), despite the existence of conflicting results (14, 15). Women with advanced endometriosis lead to enhanced risks with adverse results in pregnant women and the time of delivery. Besides, advanced endometriosis has given rise to increased risk of neonatal deaths and unfavorable obstetrics in women (16).
Now, one of the most preferred methods to help maternal endometriosis-associated infertility is in vitro fertilization (IVF). The natural cycles that are inflicted by the presumably disturbed functions due to endometriosis can be bypassed with the use of IVF and frozen–thawed embryo transfer (IVF-ET). However, there is pretty little knowledge and information regarding the higher incidence of congenital abnormalities in infants through IVF-ET to women with advanced endometriosis.
The purpose of this study is to compare the risk of congenital malformations for infants born after IVF-ET in women with or without endometriosis.
Materials and Methods
Population Study and Design
The laparotomy or laparoscopy enabled the diagnosis of patients afflicted with endometriosis, while all were treated surgically. The procedure of endometriosis was conducted based on the guidelines of the revised American Society for Reproductive Medicine (ASRM) classification (17). The data in this study were gathered from the database of ART in the Department of Assisted Reproduction of the Shanghai Ninth People's Hospital, under the aegis of the Jiaotong University School of Medicine, Shanghai, China, for the time period between January 2007 and December 2017. The treatment and/or birth taking place in ART are documented and updated in this database. These details of the births and treatments in ART and the resultant birth through ART are required by the Technical Standard for Human Assisted Reproduction under the Chinese Ministry of Health (CMOH). The parental medical condition (pre-existing and gestational) and the neonatal medical data were also recorded for each participant. The information regarding the obstetric and perinatal outcomes and the hospital medical records were provided by women within 3 months after delivery. This study involved the population that conceived using the benefits of ART, whether live or otherwise, with a gestational age of minimum 23 weeks or with weight on birth of not <500 g.
Follow-up and Definitions
The data in this study were obtained from an IVF database that included all records for IVF treatments and outcomes in patients who have undertaken IVF treatment in the Shanghai Ninth People's Hospital affiliated to JiaoTong University School of Medicine (a large hospital-based tertiary care reproductive center in Shanghai, China) since 2007. In this study, the clinical outcomes of IVF are presented via a patient-anchored approach with all embryo transfer cycles attached to the patient undergoing IVF treatment.
Endometriosis in the advanced form was diagnosed through laparotomy or laparoscopy and following the new guidelines on classification of endometriosis (17) staged as III–IV. The definition of a live birth was given as a birth that displayed any signs of life irrespective of the duration of gestation, based on the definition provided by the World Health Organization. The 10th Revision (ICD-10) of the International Classification of Diseases specified the definition and codes for the birth defects.
Statistical Analysis
The SPSS 24.0 software (SPSS, Inc.) was used in the statistical analyses. The data were represented with ±SD where they showed normal distributions or as medians (ranges) when they exhibited abnormal distributions. Qualitative data were displayed in percentages. t-tests were used to analyze the variations via continuous parametric data, while the rate comparisons between groups were performed through the χ2 test or the exact test by Fisher. Chi-square test was used to compare birth characteristics in births after assisted conception and in the total population (including all births in Shanghai). Fisher exact test was applied to assess the significance of the difference in these groups when the expected number in any of the cells was less than five. Quantification of the risk factors leading to congenital malformations between the groups' binary logistic regression was carried out. The adjusted odds ratio (OR) along with a 95 confidence interval (CI) illustrated the influence of the risk factors on congenital malformation.
Results
Pregnancy and Delivery Outcomes
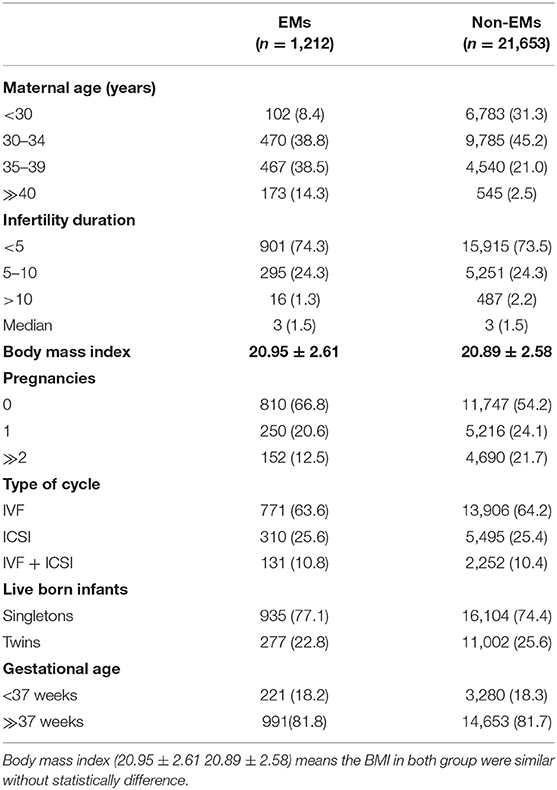
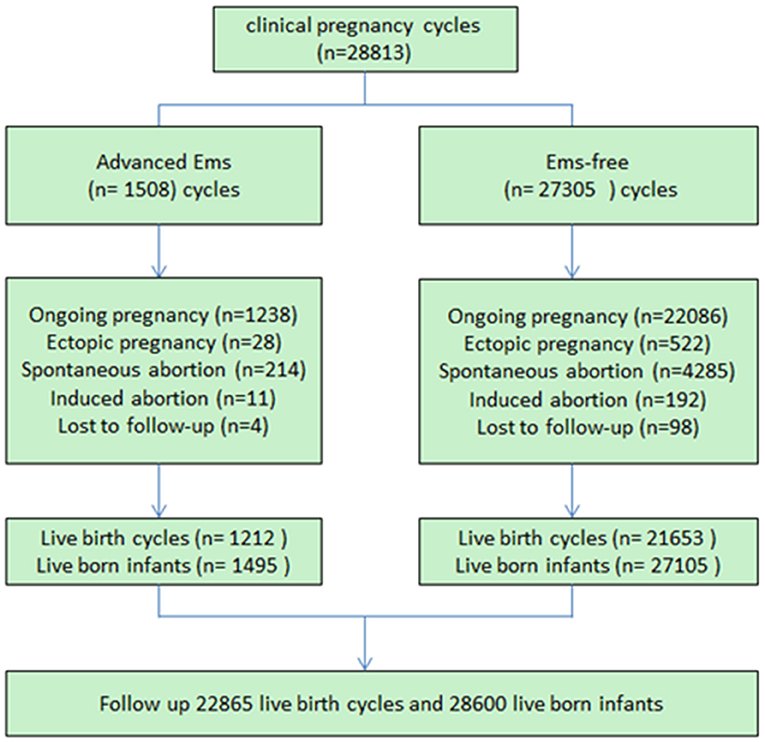
In total, 1,495 live infants were born in the endometriosis (EMs) group and 27,105 infants were born in the control group. The baseline maternal attributes across the two groups are listed in Table 1. More pregnancies were experienced by the women in the control group as compared with the women in the EMs group (P < 0.01). Besides, the singleton births proportion was more in the EMs group than in the control group (77.1 vs. 74.4%, P < 0.01). Other maternal attributes such as infertility duration, body mass index, gestational age, and type of cycles, were similar across both the groups.
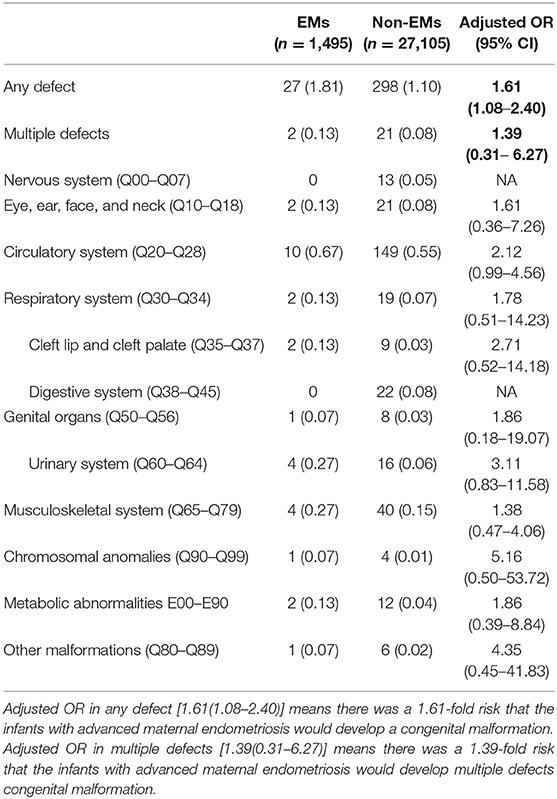
Table 2 presents the complete breakdown of the various organ systems and their detected malformations. Around 1.81% of infants born in the EMs group had defects at birth, while the ratio was 1.10% for the control group infants. Multiple birth defect proportions of about 0.13 and 0.08%, respectively, were observed. Circulatory system defects had a higher incidence of defects at birth (0.56%), followed by musculoskeletal system defects (0.15%), than the total birth defects in other categories, revealed by an analysis of the extent of birth defects in specific categories.
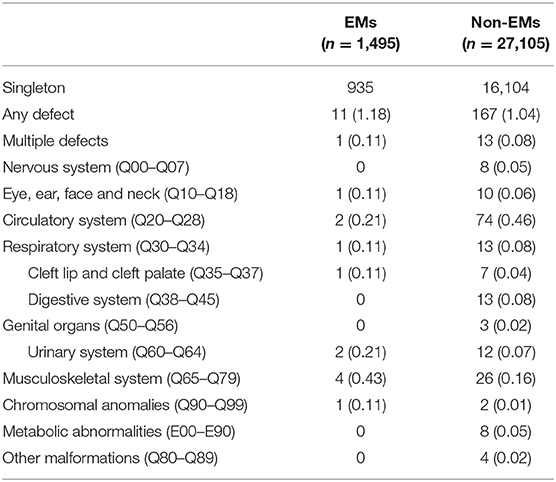
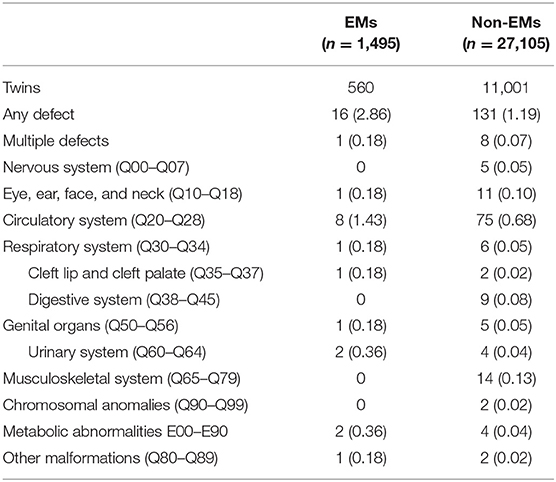
Table 3 presents the proportion of birth defects stratified by singletons. Table 4 presents the proportion of birth defects stratified by twins.
Logistical analyses exploring the effect of advanced endometriosis on birth defects via IVF treatment, adjusting for maternal age, duration of infertility, pregnancies, BMI, and parity, are listed in Table 5. An increase in the statistical probability of a congenital malformation for EMs and multiple births in the controlled models was illustrated by the results. EMs were found to be associated more likely with increased afflictions of congenital malformation (adjusted OR: 1.557, 95% CI: 1.03–2.35). Figure 1 presents the flowchat of the study.
Discussion
This study explores the risk of congenital malformations among children born after IVF treatment in women with advanced endometriosis that analyzes different organ systems of the live-born infants.
As some of the confounding variables were different between the two groups, adjusted odds ratios by logistic regression were executed to determine the factors that affected the risk of congenital malformations in offspring. In general, an association among maternal endometriosis and congenital malformations was observed. Multiple pregnancies proved to be a serious risk factor for congenital malformation in consistency with the earlier studies (18, 19).
In women with EMs (22.8%), the occurrence of multiple pregnancies was seen to be lower than that in the control group (25.6%, P < 0.01). Moreover, the primiparas in the EMs group were substantially more compared with the control group. These divergences could be explained by the lower implantation rate in the EMs than in the control group. Infertility in endometriosis-afflicted women is known to be influenced by numerous factors such as pelvic adhesions, defective implantation, ovulatory dysfunction, disturbed folliculogenesis (20–25), abnormal expression of proteins, higher FSH levels, and lower anti-Müllerian hormone levels (26). Furthermore, patients with endometriosis revealed an increase in cytokine and inflammatory factor secretion, inducing changes in paracrine-, endocrine-, and inflammation-altered peritoneal environment, distorted pelvic anatomy (27), abnormal uterine transport, and altered hormonal and additional unknown mechanisms (28, 29).
The “junctional zone,” which is another term for the functional and structural abnormalities in the inner myometrium, was observed from the MRI scans and biopsies of women with endometriosis (30–32). The defective transformation of the spiral arteries may be caused by endometriosis, thereby affecting placentation (4). This defective transformation has been documented for contributing to a greater risk of preeclampsia, intrauterine growth restriction, placental abruption, and preterm labor (33). This may also cause the occurrence of birth defects in endometriosis patients.
In our study, the logistic regression showed that the incidence of congenital malformation of twins in both EMs group and the control group were higher than that of singleton pregnancies (adjusted OR = 1.96, 95% CI: 1.56–2.45, P < 0.01), which was same as the results of the reference (34, 35). The twins are prone to congenital malformations than the singletons by four times, as has been revealed by certain studies earlier (19). Twinning itself could be the reason for these congenital abnormalities, from vascular connection among the twins or by compression deformation due to uterine crowding. Developmental disruptions are also likely to occur, in the process of twinning, thereby leading to enhanced susceptibility to environmental agents (36). With the increase in the usage of fertility drugs, along with the advancements in assisted reproductive technology, the rate of twinning has also seen an increasing trend, consequently leading to an increased incidence of congenital defects. The factors contributing to the substantially increased risk of birth defects in multiple births have been discussed elaborately in numerous previous studies, which included insufficient nutrition supply, crowded uterine conditions, and common genetic background (18, 37, 38).
In IVF-induced pregnancies, the incidence of birth defects was 1.13% within 7 days of delivery and was found to be in concurrence with the 1.11–1.58% observed in the earlier studies, as reported in a Chinese population-based study (39).
Very few previous studies have discussed the maternal factors' influence on congenital malformation. Only two studies previously focused on the boys having anomalies with their genitals among boys delivered by women having endometriosis (without IVF). Possible linkage with specific factors from the maternal angle and the genital anomaly cryptorchidism (undescended testis) was investigated by a case–control study by Mavrogenis in 2014. The investigators in that study determined that the risk of cryptorchidism among sons born to mothers with endometriosis was double (40). Nevertheless, a register-based Danish study in 2017 revealed the lack of any substantial evidence for the greater incidence of endometriosis-afflicted women giving birth to boys with genital anomalies (41).
Stratifications for organ systems in singleton and twin pregnancies are illustrated in Tables 2–4. The cardiovascular system was found to be the most frequently affected organ system by congenital malformations in our cohort study (0.56%), followed by the musculoskeletal system (0.15%). Among the two, the highest adjusted OR values appear in the chromosomal anomalies (adjusted OR: 5.16, 95% CI: 0.50–3.72; P = 0.17), urinary system (adjusted OR: 3.11, 95% CI: 0.83–11.58; P = 0.09), and cleft lip/palate (adjusted OR: 2.71, 95% CI: 0.52–14.18; P = 0.24). Unfortunately, these three results are not significantly different. This may be due to the fact that the sizes of the positive samples in separated systems are too small.
According to the logistic regression, when the age, duration of infertility, parity, BMI, and number of fetus was controlled, it showed that advanced endometriosis has a 1.557-fold risk that the infants with advanced maternal endometriosis would develop a congenital malformation (adjusted OR: 1.557, 95% CI: 1.03–2.35). Compared with singletons, twins were 1.957 times more likely to experience an adverse outcome (OR: 1.957, 95% CI: 1.561–2.455). Furthermore, we compared patients with different BMIs (18.4 < BMI < 24) and it showed that when BMI exceeds 28, there was a 2.014-fold risk that the infants would develop a congenital malformation. Years of infertility and parity (the number of times a female has given birth) have no significant effect to the outcome of the pregnancy. We have set several groups according to age and ran the logistic regression again. There was no statistically significant difference in the incidence of birth defects between age groups. One explanation was that the infant defects rate in the first age group (<30) of maternal EMs was higher than other groups (5/95), which was accidental.
It is pertinent to note that this study was conducted on more than 20,000 live-born infants treated with IVF, which, to date, is the largest number reported by any study on this topic. However, there are some limitations in the current study: (1) The data lacked the complete details of all birth defects, as the rate of malfunctions of congenital nature was calculated using live newborns and pregnancies that were terminated, rather than all those also associated with stillbirths and miscarriages. Hence, the actual rate of congenital malformation could be higher than what our data imply. (2) Albeit the size of the sample in the control group is relatively big, the analytical power to detect the abnormalities in rare incidence measures was limited, like in the case of congenital malformations by different organ systems. (3) Finally, we failed to take into account some significant variables, like screenings of prenatal fetal abnormalities, environmental exposures, family history of birth defects, parental smoking, and maternal smoking. (4) The weakness of a retrospective study for detection of anomalies, particularly without a standardized dysmorphology assessment.
In conclusion, it can be surmised that advanced maternal endometriosis increases the prevalence of congenital malformations in newborns through inducement of IVF. The cardiovascular system was the organ system that seemed to be most frequently influenced by congenital malformations, followed by the musculoskeletal system. The incidence of congenital malformation of twin pregnancies in both the EMs group and the control group was higher than that of singleton pregnancies. These results may provide evidence for endometriosis etiology and treatment research in the future.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
This study was approved by the Ethics Committee (Institutional Review Board) of Shanghai Ninth People's Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All participants provided written informed consent.
Author Contributions
ZL contributed to data collection and drafted the article. MY and MM were responsible for the analysis of data. YW and YK participated in the ultimate interpretation of the study data and in revisions to the article. All authors have read and approved the final manuscript.
Funding
This study was supported by the National Nature Science Foundation of China (Grant Nos. 31770989, 981701523, and 81801527), the Shanghai Ninth People's Hospital Foundation of China (Grant No. JYLJ030), and the Interdisciplinary Program of Shanghai Jiao Tong University (Grant No. YG2019QNA14).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully acknowledge all staff of the Department of Assisted Reproduction in Shanghai Ninth People's Hospital for their support and cooperation.
References
1. Benaglia L, Candotti G, Papaleo E, Pagliardini L, Leonardi M, Reschini M, et al. Pregnancy outcome in women with endometriosis achieving pregnancy with IVF. Hum Reprod. (2016) 31:2730–6. doi: 10.1093/humrep/dew210
2. Keay SD, Cahill DJ. Different aetiological mechanisms for unexplained and endometriosis-associated infertility cannot be inferred from unstimulated IVF cycles using HCG to induce ovulation. Hum Reprod. (2002) 17:1926–7. doi: 10.1093/humrep/17.7.1926
3. Asghari S, Valizadeh A, Aghebati-Maleki L, Nouri M, Yousefi M. Endometriosis: perspective, lights, and shadows of etiology. Biomed Pharmacother. (2018) 106:163–74. doi: 10.1016/j.biopha.2018.06.109
4. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. (2012) 98:511–9. doi: 10.1016/j.fertnstert.2012.06.029
5. Fung JN, Mortlock S, Girling JE, Holdsworth-Carson SJ, Teh WT, Zhu Z, et al. Genetic regulation of disease risk and endometrial gene expression highlights potential target genes for endometriosis and polycystic ovarian syndrome. Sci Rep. (2018) 8:11424. doi: 10.1038/s41598-018-29462-y
6. Christofolini DM, Mafra FA, Catto MC, Bianco B, Barbosa CP. New candidate genes associated to endometriosis. Gynecol Endocrinol. (2018) 35:1–4. doi: 10.1080/09513590.2018.1499090
7. Zhang T, De Carolis C, Man GCW, Wang CC. The link between immunity, autoimmunity and endometriosis: a literature update. Autoimmun Rev. (2018) 17:945–55. doi: 10.1016/j.autrev.2018.03.017
8. Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril. (2012) 98:572–9. doi: 10.1016/j.fertnstert.2012.06.044
9. Aris A. A 12-year cohort study on adverse pregnancy outcomes in Eastern Townships of Canada: impact of endometriosis. Gynecol Endocrinol. (2014) 30:34–7. doi: 10.3109/09513590.2013.848425
10. Carassou-Maillan A, Pouly JL, Mulliez A, Dejou-Bouillet L, Gremeau AS, Brugnon F, et al. Adverse pregnancy outcomes after Assisted Reproduction Technology in women with endometriosis. Gynecol Obstet Fertil. (2014) 42:210–5. doi: 10.1016/j.gyobfe.2014.01.012
11. Tzur T, Weintraub AY, Arias Gutman O, Baumfeld Y, Soriano D, Mastrolia SA, et al. Pregnancy outcomes in women with endometriosis. Minerva Ginecol. (2018) 70:144–9. doi: 10.23736/S0026-4784.17.04123-5
12. Jacques M, Freour T, Barriere P, Ploteau S. Adverse pregnancy and neo-natal outcomes after assisted reproductive treatment in patients with pelvic endometriosis: a case-control study. Reprod Biomed Online. (2016) 32:626–34. doi: 10.1016/j.rbmo.2016.03.005
13. Scala C, Leone Roberti Maggiore U, Racca A, Barra F, Vellone VG, Venturini PL, et al. Influence of adenomyosis on pregnancy and perinatal outcomes in women with endometriosis. Ultrasound Obstet Gynecol. (2018) 52:666–71. doi: 10.1002/uog.18989
14. Benaglia L, Bermejo A, Somigliana E, Scarduelli C, Ragni G, Fedele L, et al. Pregnancy outcome in women with endometriomas achieving pregnancy through IVF. Hum Reprod. (2012) 27:1663–7. doi: 10.1093/humrep/des054
15. Mekaru K, Masamoto H, Sugiyama H, Asato K, Heshiki C, Kinjyo T, et al. Endometriosis and pregnancy outcome: are pregnancies complicated by endometriosis a high-risk group? Eur J Obstet Gynecol Reprod Biol. (2014) 172:36–9. doi: 10.1016/j.ejogrb.2013.10.024
16. Berlac JF, Hartwell D, Skovlund CW, Langhoff-Roos J, Lidegaard Ø. Endometriosis increases the risk of obstetrical and neonatal complications. Acta Obstet Gynecol Scand. (2017) 96:751–60. doi: 10.1111/aogs.13111
17. Revised American Fertility Society classification of endometriosis: 1985. Fertil Steril. (1985) 43:351–2. doi: 10.1016/S0015-0282(16)48430-X
18. Sunday-Adeoye I, Okonta PI, Egwuatu VE. Congenital malformations in singleton and twin births in rural Nigeria. Niger Postgrad Med J. (2007) 14:277–80.
19. Glinianaia SV, Rankin J, Wright C. Congenital anomalies in twins: a register-based study. Hum Reprod. (2008) 23:1306–11. doi: 10.1093/humrep/den104
20. Pellicer A, Oliveira N, Ruiz A, Remohí J, Simón C. Exploring the mechanism(s) of endometriosis-related infertility: an analysis of embryo development and implantation in assisted reproduction. Hum Reprod. (1995) 10(Suppl 2):91–7. doi: 10.1093/humrep/10.suppl_2.91
21. Navarro J, Garrido N, Remohi J, Pellicer A. How does endometriosis affect infertility? Obstet Gynecol Clin North Am. (2003) 30:181–92. doi: 10.1016/S0889-8545(02)00060-8
22. Pouly JL, Canis M, Velemir L, Brugnon F, Rabischong B, Botchorichvili R, et al. Endometriosis related infertility. J Gynecol Obstet Biol Reprod. (2007) 36:151–61. doi: 10.1016/j.jgyn.2006.12.009
23. Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. Pathogenic mechanisms in endometriosis-associated infertility. Fertil Steril. (2008) 90:247–57. doi: 10.1016/j.fertnstert.2008.02.093
24. Barri PN, Coroleu B, Tur R, Barri-Soldevila PN, Rodríguez I. Endometriosis-associated infertility: surgery and IVF, a comprehensive therapeutic approach. Reprod Biomed Online. (2010) 21:179–85. doi: 10.1016/j.rbmo.2010.04.026
25. Doody MC, Gibbons WE, Buttram VC Jr. Linear regression analysis of ultrasound follicular growth series: evidence for an abnormality of follicular growth in endometriosis patients. Fertil Steril. (1988) 49:47–51. doi: 10.1016/S0015-0282(16)59646-0
26. Prieto L, Quesada JF, Cambero O, Pacheco A, Pellicer A, Codoceo R, et al. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertil Steril. (2012) 98:126–30. doi: 10.1016/j.fertnstert.2012.03.052
27. Vicente-Muñoz S, Morcillo I, Puchades-Carrasco L, Payá V, Pellicer A, Pineda-Lucena A. Nuclear magnetic resonance metabolomic profiling of urine provides a noninvasive alternative to the identification of biomarkers associated with endometriosis. Fertil Steril. (2015) 104:1202–9. doi: 10.1016/j.fertnstert.2015.07.1149
28. Hickman TN. Impact of endometriosis on implantation. Data from the Wilford Hall Medical Center IVF-ET Program. J Reprod Med. (2002) 47:801–8.
29. Rodriguez-Purata J, Coroleu B, Tur R, Carrasco B, Rodriguez I, Barri PN. Endometriosis and IVF: are agonists really better? Analysis of 1180 cycles with the propensity score matching. Gynecol Endocrinol. (2013) 29:859–62. doi: 10.3109/09513590.2013.808327
30. Maubon A, Faury A, Kapella M, Pouquet M, Piver P. Uterine junctional zone at magnetic resonance imaging: a predictor of in vitro fertilization implantation failure. J Obstet Gynaecol Res. (2010) 36:611–8. doi: 10.1111/j.1447-0756.2010.01189.x
31. Levy G, Dehaene A, Laurent N, Lernout M, Collinet P, Lucot JP, et al. An update on adenomyosis. Diagn Interv Imaging. (2013) 94:3–25. doi: 10.1016/j.diii.2012.10.012
32. Novellas S, Chassang M, Delotte J, Toullalan O, Chevallier A, Bouaziz J, et al. MRI characteristics of the uterine junctional zone: from normal to the diagnosis of adenomyosis. Am J Roentgenol. (2011) 196:1206–13. doi: 10.2214/AJR.10.4877
33. Brosens I, Pijnenborg R, Benagiano G. Defective myometrial spiral artery remodelling as a cause of major obstetrical syndromes in endometriosis and adenomyosis. Placenta. (2013) 34:100–5. doi: 10.1016/j.placenta.2012.11.017
34. Bahtiyar MO, Dulay AT, Weeks BP, Friedman AH, Copel JA. Prevalence of congenital heart defects in monochorionic/diamniotic twin gestations: a systematic literature review. J Ultrasound Med. (2007) 26:1491–8. doi: 10.7863/jum.2007.26.11.1491
35. Sperling L, Kiil C, Larsen LU, Brocks V, Wojdemann KR, Qvist I, et al. Detection of chromosomal abnormalities, congenital abnormalities and transfusion syndrome in twins. Ultrasound Obstet Gynecol. (2007) 29:517–26. doi: 10.1002/uog.3918
36. Yang MJ, Tzeng CH, Tseng JY, Huang CY. Determination of twin zygosity using a commercially available STR analysis of 15 unlinked loci and the gender-determining marker amelogenin–a preliminary report. Hum Reprod. (2006) 21:2175–9. doi: 10.1093/humrep/del133
37. Hajdú J, Beke A, Marton T, Hruby E, Pete B, Papp Z. Congenital heart malformations is twin pregnancies. Orv Hetil. (2005) 146:355–60.
38. Mitchell SE, Reidy K, Da Silva Costa F, Palma-Dias R, Cade TJ, Umstad MP. Congenital malformations associated with a single umbilical artery in twin pregnancies. Twin Res Hum Genet. (2015) 18:595–600. doi: 10.1017/thg.2015.59
39. Yan J, Huang G, Sun Y, Zhao X, Chen S, Zou S, et al. Birth defects after assisted reproductive technologies in China: analysis of 15,405 offspring in seven centers (2004 to 2008). Fertil Steril. (2011) 95:458–60. doi: 10.1016/j.fertnstert.2010.08.024
40. Mavrogenis S, Urban R, Czeizel AE, Acs N. Possible association of maternal factors with the higher risk of isolated true undescended testis: a population-based case-control study. Congenit Anom. (2014) 54:178–83. doi: 10.1111/cga.12061
Keywords: endometriosis, IVF, congenital, malformation, frozen–thawed embryo transfer
Citation: Liang Z, Yin M, Ma M, Wang Y and Kuang Y (2019) Effect of Maternal Advanced Endometriosis on Risk of Congenital Malformations for Infants Born After in vitro Fertilization and Frozen–Thawed Embryo Transfer: Analysis of 28,600 Newborns. Front. Endocrinol. 10:763. doi: 10.3389/fendo.2019.00763
Received: 21 July 2019; Accepted: 21 October 2019;
Published: 12 November 2019.
Edited by:
Elke Winterhager, University of Duisburg-Essen, GermanyReviewed by:
Linli Hu, Zhengzhou University, ChinaHimanshu Arora, University of Miami, United States
Copyright © 2019 Liang, Yin, Ma, Wang and Kuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Wang, sammy20080228@icloud.com; Yanping Kuang, kuangyp1506@sh9hospital.org
 Zhou Liang
Zhou Liang Mingru Yin
Mingru Yin Yun Wang
Yun Wang