
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 15 October 2019
Sec. Reproduction
Volume 10 - 2019 | https://doi.org/10.3389/fendo.2019.00655
This article is part of the Research Topic Fertility Preservation in Asia View all 11 articles
 Seido Takae1*
Seido Takae1* Jung Ryeol Lee2
Jung Ryeol Lee2 Nalini Mahajan3
Nalini Mahajan3 Budi Wiweko4
Budi Wiweko4 Nares Sukcharoen5
Nares Sukcharoen5 Virgilio Novero6,7
Virgilio Novero6,7 Antoinette Catherine Anazodo8,9,10
Antoinette Catherine Anazodo8,9,10 Debra Gook11
Debra Gook11 Chii-Ruey Tzeng12
Chii-Ruey Tzeng12 Alexander Kenneth Doo13
Alexander Kenneth Doo13 Wen Li14
Wen Li14 Chau Thi Minh Le15
Chau Thi Minh Le15 Wen Di16
Wen Di16 Ri-Cheng Chian17
Ri-Cheng Chian17 Seok Hyun Kim18
Seok Hyun Kim18 Nao Suzuki1
Nao Suzuki1Background: At present, fertility is one of the main concerns of young cancer patients. Following this trend, “fertility preservation (FP)” has been established and has become a new field of reproductive medicine. However, FP for child and adolescent (C-A) cancer patients is still developing, even in advanced countries. The aim of the present study was to assess the barriers to FP for C-A patients by investigating the current status of FP for C-A patients in Asian countries, which just have started FP activities.
Method: A questionnaire survey of founding members of the Asian Society for Fertility Preservation (ASFP) was conducted in November 2018.
Main findings: Of the 14 countries, 11 country representatives replied to this survey. FP for C-A patients is still developing in Asian countries, even in Australia, Japan, and Korea, which have organizations or academic societies specialized for FP. In all countries that replied to the present survey, the patients can receive embryo cryopreservation (EC), oocyte cryopreservation (OC), and sperm cryopreservation (SC) as FP. Compared with ovarian tissue cryopreservation (OTC), testicular tissue cryopreservation (TTC) is an uncommon FP treatment because of its still extremely experimental status (7 of 11 countries provide it). Most Asian countries can provide FP for C-A patients in terms of medical technology, but most have factors inhibiting to promote FP for C-A patients, due to lack of sufficient experience and an established system promoting FP for C-A patients. “Don't know how to provide FP treatment for C-A” is a major barrier. Also, low recognition in society and among medical staff is still a particularly major issue. There is also a problem with cooperative frameworks with pediatric departments. To achieve high-quality FP for C-A patients, a multidisciplinary approach is vital, but, according to the present study, few paramedical staff can participate in FP for C-A patients in Asia. Only Australia and Korea provide FP information by video and specific resources.
Conclusion: The present study demonstrated the developing status of FP for C-A patients in Asian countries. More intensive consideration and discussion are needed to provide FP in Asian societies based on the local cultural and religious needs of patients.
Based on the Global Burden of Disease study, cancer incidence rate continues to increase in the world including Asian countries (1). Also, incidence of childhood cancer is increasing (2). Development of cancer therapy has resulted in increasing numbers of cancer survivors. In particular, more than 70% of child cancer patients will be cancer survivors (3). Unfortunately, one in 10 cancer patients experience fertility due to impairment in ovarian or testis function, as a result of the gonadotoxic treatments (chemotherapy and radiation therapy) as cancer therapy. Recently, several reviews of fertility preservation (FP) which based on assured clinical study have indicated the risk of infertility associated with specific diseases and therapies among different age groups. Especially, high-dose Alkylating agent represented by Cyclophosphamide may cause serious damage to gonads (4, 5). In addition, cancer itself and cancer treatment could cause the sexual dysfunction due to physical and psychological problems for cancer survivors (both men and women) including survivors of childhood cancer. To begin with, couple infertility and sexual dysfunction are highly prevalent in general population. Therefore, cancer and cancer treatment have possibilities getting worse this contemporary condition (6–8).
For adult patients with cancer, fertility preservation treatments have been established to improve quality of life for cancer survivors. In 2006, the “Oncofertility consortium” and “FertiPROTEKT,” which are representative associations to promote FP for young cancer patients, were established (9). The “International Society for Fertility Preservation (ISFP)” was established in 2009 as the first academic society specialized in FP treatments. Additionally, the “Japan Society for Fertility Preservation (JSFP)” and the “Fertility Preservation Society of India (FPSI),” and the “Asian Society for Fertility Preservation (ASFP)” were founded in 2012 and 2014, and 2015, respectively. Also, Australasian Oncofertility Consortium started 2015. As a consequence of efforts or actions to promote FP by these organizations, FP is now becoming a new field of reproductive medicine.
Based on the latest guideline that was updated by the American Society of Clinical Oncology (ASCO), only oocyte and embryo cryopreservation is endorsed as an “established method” for fertility preservation for female patients who face a threat to their own fertility due to cancer treatment (10). Meanwhile, ovarian tissue cryopreservation (OTC) is still an “experimental method” according to this guideline, although many experts believe that OTC fulfills the criteria for an “established method” (11, 12). The indications for OTC are specifically FP for child and adolescent patients and adult patients who do not have enough time to receive another fertility preservation treatment (5, 10, 11). Based on the literature, around 1,000 cases per year of oocyte cryopreservation (OC) for serious medical reasons and until now, more than 4,500 cases of OTC are performed in Europe (13), and more than 1,000 cases of OC and 200 cases of OTC are performed in Japan as FP (2006-2016, unpublished data). For male cancer patients, sperm cryopreservation before receiving chemotherapy is strongly recommended as the sole effective FP treatment. Hormonal therapy is not recommended as FP treatment for men. Testicular tissue cryopreservation (TTC) with later re-implantation is considered a highly experimental method (10). To determine the FP procedure for child and adolescent (C-A) patients, sexual maturity as we say “puberty” is one of important factors. It is menarche for female and spermarche for male. Generally, OC is the FP procedure which method is the most likely to result in subsequent pregnancy, but this is only for post-menarchal females (those who have begun to menstruate) since it would require developing follicles. Therefore, for pre-pubertal females, OTC is the only FP option. As a FP options for males, sperm cryopreservation is the most established option and should be offered to all peri- and post-pubertal male adolescents with a fertility-threatening situation. Although the age at which to offer sperm cryopreservation is unclear, an adequate semen specimen can be obtained in adolescents as young as 11 years of age. For pre-pubertal boys with lack of mature sperm, TTC is solely option as FP treatment (14, 15). At present, there are only two live birth cases from transplanted ovarian tissues that were cryopreserved before menarche, and there are no live birth cases from patients who underwent TTC (15, 16). In addition, there are few reports of OC for C-A patients. Even OC for late teenagers is still challenging because it needs ovarian stimulation with multiple hormonal injections and follicle monitoring using ultrasound, with subsequent oocyte retrieval under sedation or anesthesia (these procedures need a transvaginal approach) (15, 17). For these reasons, FP for C-A patients is still uncommon compared with FP for adult patients, even in advanced countries performing FP although they have OTC and TTC cases for infant (18, 19). The aim of the present study was to assess the barriers to FP for C-A patients by investigating the current status of FP for C-A patients in Asian countries whom are members of the Asian Society of Fertility Preservation (ASFP).
On November 2018, a survey was sent to country representatives of ASFP (Australia, China, Hong Kong, India, Indonesia, Japan, Korea, Philippines, Taiwan, Thailand, Vietnam, Pakistan, Singapore, Turkey) to collect information about the current status of FP services for child patients and the barriers that inhibit promoting this treatment. The participating countries gross national income per capita is very different (five high income countries, three upper-middle income countries, five lower income countries, and one with no data). The survey was approved by the institutional review board of our institution with revisions in keeping with the Declaration of Helsinki. The final version was sent by email to 14 contacts of the ASFP.
Potential survey participants were identified from existing members of the ASFP and international experts in the field. Potential participants received an email with an invitation to participate in the survey. Following the initial email, each participant received two reminders, one on November 1, 2018 and one on November 15, 2018, in order to maximize the number of responses.
Surveys were excluded from the analysis if participants failed to provide contact or identification information, if the survey was left blank, or if duplicate responses were submitted.
Survey participants were asked a total of 12 questions about the following areas: organization to promote FP treatment, patient access to medical professionals, current status of FP for adult and child patients, barriers that inhibit promotion of FP for C-A patients, and systems for providing information about FP for child patients. Three questions were dichotomous scaled questions (yes/no) with space for providing open-ended comments. Three questions were multiple-choice format, where only one answer could be selected. Four questions were multiple response questions, where participants could select one or more answers. One question was for free descriptive answer, and one was defining the priority order.
Survey responses were exported to Microsoft Excel. The dichotomous and multiple response questions were coded with numerical values to facilitate statistical analysis.
The present study was approved by the IRB of St. Marianna University (approval No. 4191, UMIN000035723). This survey is questionnaire survey targeted to medical professionals (representatives of society). On the explanation of this survey, we had written about consent to participate this survey at the front of questionnaires. We told them to reply when they could agree with participating this survey as participants.
From the 14 countries, 11 country representatives replied to the survey. Of the 11 countries, five had organizations or academic societies to promote FP, and three countries (Australia, Japan, and Korea) had organizations or academic societies that are specialized for FP in the true sense, whereas two (China and Indonesia) had a committee or branch society of a large academic society in the area of reproductive medicine or maternal-child health medicine. Two countries (Hong Kong and Philippines) are planning to establish organizations or academic societies specialized for FP. Although most countries do not have aid funds or insurance for FP, only Australia has a registration system for FP which requires individual patient consent and partial financial assistance or insurance system (Medicare) covering extensive FP treatment [embryo cryopreservation (EC), OC, consultation, ovarian transposition, sperm cryopreservation (SC)]. Also, Korea has partial funds for FP treatment (EC only).
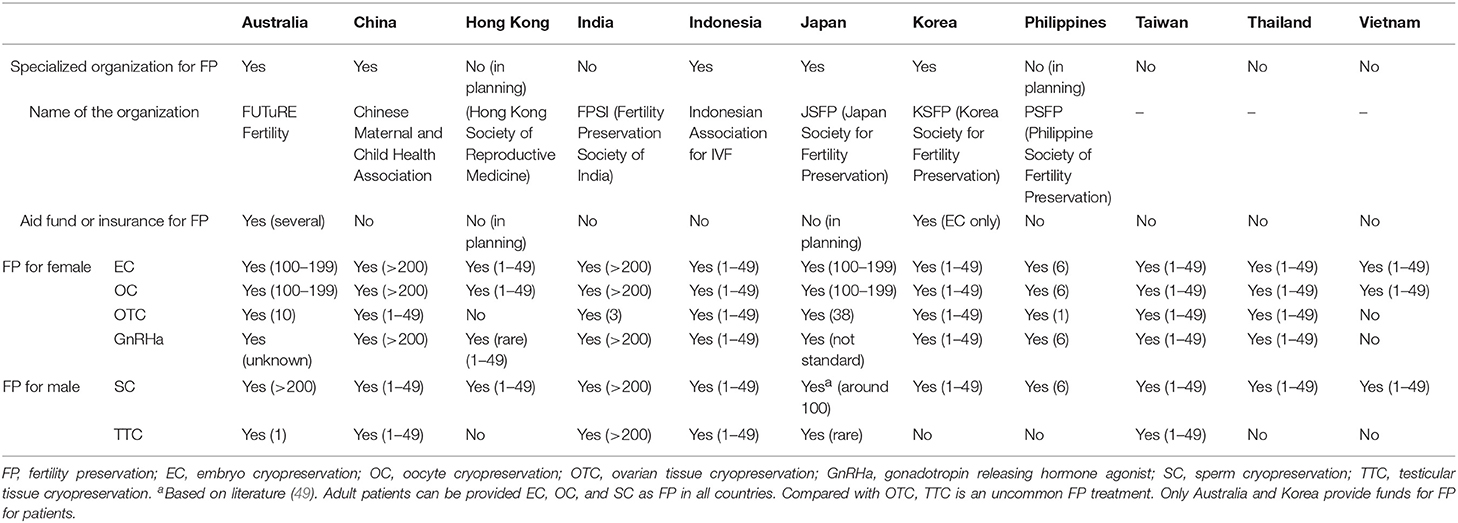
Table 1. Organizations to promote FP, patient access to medical professionals, and current status of FP for adult patients in Asian countries.
In all countries that replied to the survey, the patients can receive EC, OC, and SC as FP. Compared with OTC, TTC is uncommon FP treatment because of its still extremely experimental status. Therefore, even Australia, which is an advanced country for FP, has only one institution that has ethics approval for TTC although TESE can be done in post-pubertal patients in a number of centers if required.
All of Asian countries have experience of FP for C-A patients. However, in most countries, the opportunities for FP for C-A patients are limited compared with FP for adult patients, because all participants (except for Indonesia) chose “not so often” regarding opportunities for FP for C-A patients. The main reasons were “not enough information for physicians, oncologists, patients and family” and “lack of public awareness.” Also, the numbers of facilities that can provide FP treatment for C-A patients are limited. Especially, in Australia, the facilities that can do OTC and TTC are strictly consolidated.
To investigate the barriers that inhibit promotion of FP for C-A patients, multiple-choice questionnaires were prepared (Table 3). Although there was variation in ranking, 9 of 11 participants identified “b: Low recognition among medical staff” as one of the major issues. Also, “f: Information is insufficient,” “a: Low recognition in society,” and “g: There is a problem with the cooperative system with the pediatrics department” were major reasons for inhibiting the promotion of FP for C-A patients (Table 3). Three of the 11 selected “e: There is technology, but we don't know how to provide it” and “j: Economically impossible.” Only one participant from Thailand chose “k: It is not necessary because the adoption system is popular.” As other comments, participants from Australia mentioned “weakness of evidence for FP for C-A patients.” To improve the level of FP awareness, 3 of 11 participants (India, Japan, Korea) are providing opportunities for lecture presentations, oral presentations at scientific conferences, and education for parents or patients.
To improve FP treatment for C-A patients, the kinds of specialists that provided FP for C-A patients were investigated, and 10 of 11 participants replied. In half of the countries (5 of 10), only a medical doctor could provide FP treatment for C-A patients. On the other hand, in four of five countries, nurses and/or psychologists could collaborate with the medical team in FP treatment for C-A patients. Although, patient navigators as independent position and child life specialists are not involved in FP for C-A patients, in Australia, nurses and psychologist are involved as patient navigators aiming to assist decision-making and psychological support. In addition, peer supporters including cancer survivors are not involved in FP treatment for individual cases (Table 4). However, as described below, patient consumer organization and Consumer Charter are collaborating with FP organization to develop FP in Australia (20). Also, JSFP have peer supporter group to promote FP.
All of the participants selected “Oral explanation” for informed assent, and “article” is used for informed assent as supplementary material (China, Japan, Philippines, Vietnam). To improve the quality of informed assent, Korea has animations about FP treatment, including sexual education. Only Australia has an “online or printed resource” and a “video a peer supporter has done” as “other” means (Table 5).
Improvement of the survival rate following childhood cancer has led to an increased focus on the late effects of cancer treatment (3, 21) and “fertility” is a prime concern for both female and male cancer survivors (3, 22) which can result in psychological distress (23). Although Asia consists of 48 countries that have various backgrounds in terms of culture, economic status, religion, and status of medical care, FP is becoming increasingly common as medical care. In particular, countries that participate in the ASFP and have specialized organizations for FP can provide contemporary FP treatment. Indeed, Australia is one of the advanced countries in the FP area, which has already established its own registration system and partial public funding for patients receiving FP treatment. Japan is also one of advanced country which has guideline of FP treatment collaborate JSFP with JSCO (Japan Society of Clinical Oncology) (24). JSFP may start a registration system for FP treatment within 1 year to understand the present status of FP in Japan based on national survey for FP (25, 26). However, FP for C-A patients is not as common as FP for adult cancer patients (27). The present study data have shown that the numbers of hospital or institutions that can provide FP for C-A patients are much fewer than for adult patients. The reason for lower number of FP in C-A patients are multi-factorial (28).
Barriers to promoting FP treatment for C-A patients may be divided into “medical factors” and others. For female C-A patients, OC and OTC are options as FP treatments, with SC and TTC for male C-A patients. In general, the selection of FP treatments depends on the patient's pubertal status. For post-pubertal female patients, EC with OC is one of the options for FP treatment (15). Although OC has been the standard FP treatment for young or unmarried female patients since 2013 as per ASCO (5), it is uncertain whether will be acceptable OC for teenagers. In fact, reports of OC for post-pubertal female patients as FP are very few, and its status is challenging, as mentioned above. Some reports and clinical data already demonstrated that OC is a practical technology for children (17), but there are issues to be resolved before pediatric fertility preservation programs can be universally available (ovarian stimulation, transvaginal procedure, sedation) (14). Furthermore, concern about delays in therapy is one of the greatest barriers to offering OC for patients (15), especially C-A patients who often require the urgent initiation of treatment due to hematological or systemic disease. In addition, OC for pre-pubertal female patients is also challenging. Although there is a report of a pre-pubertal OC patient (29), in general, only OC as a combined procedure (oocyte retrieved from ovarian cortex which extracted OTC) is available for pre-pubertal female patients (30, 31). Based on the literature, OC as a combined procedure can be available to around 40% of under 15-year-old child patients (minimum 3.5 months) (30). However, the effectiveness of the combined procedure is still very limited (32), and it has been demonstrated that the percentage of degenerated oocytes was significantly higher in girls than in adult patients (33).
OTC is the only FP treatment for pre-pubertal females and for post-pubertal patients who are unable to delay the initiation of chemotherapy, although its status is still experimental. It has been completed in patients of all ages and has been demonstrated to be safe and effective, with a low complication rate with minimal delay (15) allowing cancer treatment to commence very soon after laparoscopic surgery for OTC (34). In promoting OTC for C-A patients, the primary disease is one of the major issues. For C-A patients, leukemia is a representative primary disease. Although there is a live birth case with leukemia who received Ovarian tissue transplantation (OTT) after treatment (35), OTT following OTC in leukemia patients is challenging and requires further investigation to avoid re-introducing minimum residual disease (MRD) (10, 36). According to the European Society for Blood and Marrow Transplantation (EBMT), both pre-pubertal and post-pubertal OTC from patients with leukemia can be considered, in view of future developments, for in vitro maturation and subsequent in vitro fertilization (37). Already, as future developments, an artificial ovary and multiple-step primordial follicle culture system has demonstrated encouraging results (38). Currently, OTC has been becoming an established treatment in some countries (10); there have already been more than 130 live birth cases (11). In general, the hospital or institution that provides OTC treatment for adult patients can perform OTC for C-A patients, because both are technically the same procedure. Indeed, based on the present study, most countries that can provide OTC for adult patients replied that “it is possible to do OTC for C-A patients.” However, there are few countries that can provide OTC for C-A patients at the same level as for adult patients (although the actual numbers of OTC cases for C-A patients are unknown), due to several child-specific barriers.
For post-pubertal male patients, SC with patient assent and parent or guardian consent is an actual established method for FP (10, 34). Although the minimum age for SC is unclear (15), the success rate of SC has been reported to be up to 64.5% for adolescents aged 11–14 years (15, 39). At least Tanner stage 3 pubertal development is needed for successful SC (15, 39, 40). In general, ejaculated sperm is collected by masturbation, but penile vibratory or electro ejaculation under general anesthesia is used for patients who cannot perform masturbation (15). Also, surgical sperm extraction called “ONCO-TESE” (TESE: Testicular sperm extraction) is one of the effective procedures for patients who show cancer-induced azoospermia with a testicular tumor or lymphoma (41–43). Based on the literature, patients who underwent “ONCO-TESE” can be started on chemotherapy the same day as sperm retrieval (43). For pre-pubertal male patients, TTC is the sole treatment for FP, even though its status is still extremely experimental (10). Until now, there have been no retrievals of mature sperm or achievement of pregnancy using this treatment (15). These current situations are congruent with the findings of the present study. In conclusion, based on present survey, almost of female child cancer patients can receive OTC (except Hong Kong and Vietnam), and female adolescent patients can receive OC in Asian countries which participate this survey, although OC is uncertain whether will be acceptable for teenagers. And all adolescent male patients can receive sperm cryopreservation, also almost child male patients can receive sperm cryopreservation according to their sexual maturation (except China, Thailand, Vietnam). However, TTC for male child and adolescent cancer patients is still uncommon procedure as described above in Asia. As a limitation, age restriction was still unclear on this survey (almost participants did not clearly state). There are some possibilities that these differences to select the procedure of FP is ascribed to the developing and economical status of country.
According to the present study, there are several factors based on “medical aspects” and “social aspects” that impede the progress of FP for C-A patients. Importantly, “How to provide FP treatment for C-A” is a major issue, more so than “medical technology” as a medical factor. When we provide FP treatment for C-A patients, there are some difficulties in explaining FP treatment and obtaining informed assent/consent from children/parents. For discussion about FP with C-A patients, “Knowledge about FP (guidelines, costs, facilities and specialist, informed assent/consent process),” “low referrals,” “low priority,” “Sense of comfort for health care professionals (they feel embarrassed to discuss FP),” “Patient factors (prognosis, cost, age, feel discomfort),” “Parent factors (contradictory opinions, feel discomfort),” and “Educational resources for patients and families” (44, 45) are issues (28). Also, “provider bias” is identified as a potential barrier. Providers feel difficulties giving information about FP to patients who have low potential for fertility and/or cure, and who have a lower socioeconomic status. Furthermore, if the hospital does not have the capability to perform experimental FP treatments, it is difficult to discuss FP with patients (15, 46). These situations are among the reasons for “low referrals.” Until now, we had only five studies about decision-making for C-A patients, and all of them were performed in Western European countries (47). Therefore, we should perform surveys in Asian countries based on the different and varied cultures, including many different religions. In addition, for investigating this survey accurately, we need to consider economic status (GDP: gross domestic product) of countries, developing status of fertility treatment (especially ART: assisted reproductive technology), cost issue, distance between centers which provide FP treatment currently. And as a social aspect, difference of sanitary system is one of important factor. In Japan, the government had stated the policy for supporting young cancer patients to promote FP in 2018. Also, leading society for cancer treatment in Australia and Japan had published the guidance for FP. To promote the FP, academic societies are established in each Asian country. These societies hold opportunities of scientific meeting and symposium for advertising, dissemination in the territory.
The present study demonstrated the variety of frameworks for FP treatment among countries and the need to implement consistent oncofertility models of care in Asian countries (28). In most countries, pediatricians and pediatric oncologist/hematologist can participate in FP, but participation of pediatric surgeons is still not common. Based on the reports investigating the safety of OTC for pediatric patients by pediatric surgeons, there are no cases of delay, and they concluded that OCT is safe procedure (18). They considered port placement according to the size of the patient's body. We strongly agree with them that collaboration with pediatric surgeons is needed for OTC. The participation of paramedical staff (multidisciplinary approach) is also vital to improve FP treatment (28). According to the present study, nursing staff, social workers, and psychologists participated in FP in a few countries. Based on the national guidelines of FP for C-A patients in Sweden, involvement of a psychologist and/or counselor to give information about FP is recommended as part of a multidisciplinary approach (48). Not only medical staffs, but peer supporter and cancer survivor are important for developing FP treatment. In Australia, The FUTuRE Fertility Research Group led a collaborative consultation process with the Australasian Oncofertility Consumer group and oncofertility specialists to explore consumers' experiences of oncofertility care (20). The importance of resources (brochures and videos) for decision-making has also been emphasized (48). Although only Australia and Korea can provide video information about FP for C-A patients, most countries provide information by oral explanations. Unfortunately, there are no Asian countries in which child-life specialists and patient navigators can participate in FP treatment, likely because there are still very few child-life specialists and patient navigators in Asian countries. On the other hand, some child-life specialists are already participating in FP in the USA. As a future task, establishment of system to follow-up the reproductive issue of C-A patients after cancer treatment. In almost of Asian countries don't have system and network to follow-up C-A patients focused on reproductive issues, although some countries have guideline of long-term follow-up C-A patients.
As limitations, we investigated current status of FP for C-A patients in Asian countries, however it is difficult to compare them simply. Because they have various backgrounds of priority, culture, religion, and economical situation among them. Also, our survey had covered mainly developed countries in Asia. To assess the current status more accurately, we need to investigate remaining 34 of Asian countries which didn't participate this study.
The present study demonstrated the developing status of FP for C-A patients in Asian countries. The problem that needs to be resolved is how to establish a system providing FP for C-A patients while being part of the research strategy to improve the current FP options. Asian countries hold a high value on family and so it is important that we develop an oncofertility model of care which will support the implementation of local, national and international guidelines and include healthcare providers and patients. In addition, greater consideration and more discussion needs to occur about “How to apply FP to our own society” are needed based on the various cultures and religions in the region.
The datasets generated for this study are available on request to the corresponding author.
ST drafted the manuscript. NSuz and AA revised manuscript. ST and NSuz designed the research and contributed to the critical discussion. JL, NM, BW, NSuk, VN, AA, DG, C-RT, AD, CL, WL, WD, R-CC, and SK contributed to collecting and analyzing data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
FP, fertility preservation; C-A, child and adolescent; ISFP, International Society for Fertility Preservation; ASFP, Asian Society for Fertility Preservation; JSFP, Japan Society for Fertility Preservation; FPSI, Fertility Preservation Society of India; ASCO, American Society of Clinical Oncology; JSCO, Japan Society of Clinical Oncology; EC, embryo cryopreservation; OC, oocyte cryopreservation; OTC, ovarian tissue cryopreservation; OTT, ovarian tissue transplantation; GnRHa, gonadotropin releasing hormone agonist; SC, sperm cryopreservation; TTC, testicular tissue cryopreservation; GDP, gross domestic product; ART, assisted reproductive technology.
1. Global Burden of Disease Cancer C, Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, et al. Global, Regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. (2018) 4:1553–68. doi: 10.1001/jamaoncol.2018.2706
2. Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F, et al. International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol. (2017) 18:719–31. doi: 10.1016/S1470-2045(17)30186-9
3. Wallace WH, Smith AG, Kelsey TW, Edgar AE, Anderson RA. Fertility preservation for girls and young women with cancer: population-based validation of criteria for ovarian tissue cryopreservation. Lancet Oncol. (2014) 15:1129–36. doi: 10.1016/S1470-2045(14)70334-1
4. Poorvu PD, Frazier AL, Feraco AM, Manley PE, Ginsburg ES, Laufer MR, et al. Cancer treatment-related infertility: a critical review of the evidence. JNCI Cancer Spectr. (2019) 3:1–14. doi: 10.1093/jncics/pkz008
5. Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. (2013) 31:2500–10. doi: 10.1200/JCO.2013.49.2678
6. Lotti F, Maggi M. Sexual dysfunction and male infertility. Nat Rev Urol. (2018) 15:287–307. doi: 10.1038/nrurol.2018.20
7. Valpey R, Kucherer S, Nguyen J. Sexual dysfunction in female cancer survivors: a narrative review. Gen Hosp Psychiatry. (2019). doi: 10.1016/j.genhosppsych.2019.04.003. [Epub ahead of print].
8. Frederick NN, Recklitis CJ, Blackmon JE, Bober S. Sexual dysfunction in young adult survivors of childhood cancer. Pediatr Blood Cancer. (2016) 63:1622–8. doi: 10.1002/pbc.26041
9. von Wolff M, Andersen CY, Woodruff TK, Nawroth F. FertiPROTEKT, oncofertility consortium and the danish fertility-preservation networks - what can we learn from their experiences? Clin Med Insights Reprod Health. (2019) 13:1179558119845865. doi: 10.1177/1179558119845865
10. Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 36:1994–2001. doi: 10.1200/JCO.2018.78.1914
11. Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med. (2017) 377:1657–65. doi: 10.1056/NEJMra1614676
12. von Wolff M, Sanger N, Liebenthron J. Is ovarian tissue cryopreservation and transplantation still experimental? it is a matter of female age and type of cancer. J Clin Oncol. 36:3340–1. doi: 10.1200/JCO.18.00425
13. Shenfield F, de Mouzon J, Scaravelli G, Kupka M, Ferraretti AP, Prados FJ, et al. Oocyte and ovariantisse cryopreservation in European countries: statutory background, practice, storage and use. Human Reproduction Open. (2017) 2017:hox003. doi: 10.1093/hropen/hox003
14. Moravek MB, Appiah LC, Anazodo A, Burns KC, Gomez-Lobo V, Hoefgen HR, et al. Development of a pediatric fertility preservation program: a report from the pediatric initiative network of the oncofertility consortium. J Adolesc Health. (2019) 64:563–73. doi: 10.1016/j.jadohealth.2018.10.297
15. Burns KC, Hoefgen H, Strine A, Dasgupta R. Fertility preservation options in pediatric and adolescent patients with cancer. Cancer. (2018) 124:1867–76. doi: 10.1002/cncr.31255
16. Demeestere I, Simon P, Dedeken L, Moffa F, Tsepelidis S, Brachet C, et al. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod. (2015) 30:2107–9. doi: 10.1093/humrep/dev128
17. Lavery SA, Islam R, Hunt J, Carby A, Anderson RA. The medical and ethical challenges of fertility preservation in teenage girls: a case series of sickle cell anaemia patients prior to bone marrow transplant. Hum Reprod. (2016) 31:1501–7. doi: 10.1093/humrep/dew084
18. Rowell EE, Corkum KS, Lautz TB, Laronda MM, Walz AL, Madonna MB, et al. Laparoscopic unilateral oophorectomy for ovarian tissue cryopreservation in children. J Pediatr Surg. (2019) 54:543–9. doi: 10.1016/j.jpedsurg.2018.06.005
19. Corkum KS, Lautz TB, Johnson EK, Reimann MB, Walz AL, Lockart BA, et al. Testicular wedge biopsy for fertility preservation in children at significant risk for azoospermia after gonadotoxic therapy. J Pediatr Surg. (2019) 54:1901–5. doi: 10.1016/j.jpedsurg.2019.01.055
20. Anazodo AC, Gerstl B, Stern CJ, McLachlan RI, Agresta F, Jayasinghe Y, et al. Utilizing the experience of consumers in consultation to develop the Australasian oncofertility consortium charter. J Adolesc Young Adult Oncol. (2016) 5:232–9. doi: 10.1089/jayao.2015.0056
21. Wallace WH, Thompson L, Anderson RA, Guideline Development G. Long term follow-up of survivors of childhood cancer: summary of updated SIGN guidance. BMJ. (2013) 346:f1190. doi: 10.1136/bmj.f1190
22. Peate M, Meiser B, Hickey M, Friedlander M. The fertility-related concerns, needs and preferences of younger women with breast cancer: a systematic review. Breast Cancer Res Tr. (2009) 116:215–23. doi: 10.1007/s10549-009-0401-6
23. Logan S, Perz J, Ussher JM, Peate M, Anazodo A. Systematic review of fertility-related psychological distress in cancer patients: informing on an improved model of care. Psycho Oncol. (2019) 28:22–30. doi: 10.1002/pon.4927
24. Suzuki N. Clinical practice guidelines for fertility preservation in pediatric, adolescent, and young adults with cancer. Int J Clin Oncol. (2019) 24:20–7. doi: 10.1007/s10147-018-1269-4
25. Furui T, Takai Y, Kimura F, Kitajima M, Nakatsuka M, Morishige KI, et al. Problems of reproductive function in survivors of childhood-and adolescent and young adult-onset cancer revealed in a part of a national survey of Japan. Reprod Med Biol. (2018) 18:105–10. doi: 10.1002/rmb2.12255
26. Furui T, Takai Y, Kimura F, Kitajima M, Nakatsuka M, Morishige K, et al. Fertility preservation in adolescent and young adult cancer patients: from a part of a national survey on oncofertility in Japan. Reprod Med Biol. (2018) 18:12256. doi: 10.1002/rmb2.12256
27. McDougall RJ, Gillam L, Delany C, Jayasinghe Y. Ethics of fertility preservation for prepubertal children: should clinicians offer procedures where efficacy is largely unproven? J Med Ethics. (2018) 44:27–31. doi: 10.1136/medethics-2016-104042
28. Anazodo A, Laws P, Logan S, Saunders C, Travaglia J, Gerstl B, et al. How can we improve oncofertility care for patients? A systematic scoping review of current international practice and models of care. Hum Reprod Update. (2019) 25:159–79. doi: 10.1093/humupd/dmy038
29. Reichman DE, Davis OK, Zaninovic N, Rosenwaks Z, Goldschlag DE. Fertility preservation using controlled ovarian hyperstimulation and oocyte cryopreservation in a premenarcheal female with myelodysplastic syndrome. Fertil Steril. (2012) 98:1225–8. doi: 10.1016/j.fertnstert.2012.07.1056
30. Poirot C, Brugieres L, Yakouben K, Prades-Borio M, Marzouk F, de Lambert G, et al. Ovarian tissue cryopreservation for fertility preservation in 418 girls and adolescents up to 15 years of age facing highly gonadotoxic treatment. Twenty years of experience at a single center. Acta Obstet Gynecol Scand. (2019) 98:630–7. doi: 10.1111/aogs.13616
31. Abir R, Ben-Aharon I, Garor R, Yaniv I, Ash S, Stemmer SM, et al. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Hum Reprod. (2016) 31:750–62. doi: 10.1093/humrep/dew007
32. Kedem A, Yerushalmi GM, Brengauz M, Raanani H, Orvieto R, Hourvitz A, et al. Outcome of immature oocytes collection of 119 cancer patients during ovarian tissue harvesting for fertility preservation. J Assist Reprod Genet. (2018) 35:851–6. doi: 10.1007/s10815-018-1153-1
33. Fasano G, Dechene J, Antonacci R, Biramane J, Vannin AS, Van Langendonckt A, et al. Outcomes of immature oocytes collected from ovarian tissue for cryopreservation in adult and prepubertal patients. Reprod Biomed Online. (2017) 34:575–82. doi: 10.1016/j.rbmo.2017.03.007
34. Anderson RA, Mitchell RT, Kelsey TW, Spears N, Telfer EE, Wallace WH. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diab Endocrinol. (2015) 3:556–67. doi: 10.1016/S2213-8587(15)00039-X
35. Shapira M, Raanani H, Barshack I, Amariglio N, Derech-Haim S, Marciano MN, et al. First delivery in a leukemia survivor after transplantation of cryopreserved ovarian tissue, evaluated for leukemia cells contamination. Fertil Steril. (2018) 109:48–53. doi: 10.1016/j.fertnstert.2017.09.001
36. Dolmans MM, Luyckx V, Donnez J, Andersen CY, Greve T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril. (2013) 99:1514–22. doi: 10.1016/j.fertnstert.2013.03.027
37. Balduzzi A, Dalle JH, Jahnukainen K, von Wolff M, Lucchini G, Ifversen M, et al. Fertility preservation issues in pediatric hematopoietic stem cell transplantation: practical approaches from the consensus of the Pediatric Diseases Working Party of the EBMT and the International BFM Study Group. Bone Marrow Transplant. (2017) 52:1406–15. doi: 10.1038/bmt.2017.147
38. Anderson RA, Wallace WHB, Telfer EE. Ovarian tissue cryopreservation for fertility preservation: clinical and research perspectives. Hum Reprod Open. (2017) 2017:hox001. doi: 10.1093/hropen/hox001
39. DiNofia AM, Wang X, Yannekis G, Ogle S, Hobbie WL, Carlson CA, et al. Analysis of semen parameters in a young cohort of cancer patients. Pediatr Blood Cancer. (2017) 64:381–6. doi: 10.1002/pbc.26221
40. Picton HM, Wyns C, Anderson RA, Goossens E, Jahnukainen K, Kliesch S, et al. A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod. (2015) 30:2463–75. doi: 10.1093/humrep/dev190
41. Schrader M, Muller M, Sofikitis N, Straub B, Krause H, Miller K. “Onco-tese”: testicular sperm extraction in azoospermic cancer patients before chemotherapy-new guidelines? Urology. (2003) 61:421–5. doi: 10.1016/s0090-4295(02)02264-1
42. Guo DP, Hwang K. Optimizing fertility preservation with microscopic onco-testicular sperm extraction. Fertil Steril. (2018) 109:625–6. doi: 10.1016/j.fertnstert.2018.02.010
43. Berookhim BM, Mulhall JP. Outcomes of operative sperm retrieval strategies for fertility preservation among males scheduled to undergo cancer treatment. Fertil Steril. (2014) 101:805–11. doi: 10.1016/j.fertnstert.2013.11.122
44. Vindrola-Padros C, Dyer KE, Cyrus J, Lubker IM. Healthcare professionals' views on discussing fertility preservation with young cancer patients: a mixed method systematic review of the literature. Psycho-Oncol. (2016) 26:4–14. doi: 10.1002/pon.4092
45. Frederick NN, Campbell K, Kenney LB, Moss K, Speckhart A, Bober SL. Barriers and facilitators to sexual and reproductive health communication between pediatric oncology clinicians and adolescent and young adult patients: the clinician perspective. Pediatr Blood Cancer. (2018) 65:e27087. doi: 10.1002/pbc.27087
46. Gupta AA, Donen RM, Sung L, Boydell KM, Lo KC, Stephens D, et al. Testicular biopsy for fertility preservation in prepubertal boys with cancer: identifying preferences for procedure and reactions to disclosure practices. J Urol. (2016) 196:219–24. doi: 10.1016/j.juro.2016.02.2967
47. Li N, Jayasinghe Y, Kemertzis MA, Moore P, Peate M. Fertility preservation in pediatric and adolescent oncology patients: the decision-making process of parents. J Adolesc Young Adult Oncol. (2017) 6:213–22. doi: 10.1089/jayao.2016.0061
48. Rodriguez-Wallberg KA, Borgstrom B, Petersen C, Thurin-Kjellberg A, Morse H, Giwercman A, et al. National guidelines and multilingual age-adapted patient brochures and videos as decision aids for fertility preservation (FP) of children and teenagers with cancer-a multidisciplinary effort to improve children's information and access to FP in Sweden. Acta Obstet Gynecol Scand. (2019) 98:679–80. doi: 10.1111/aogs.13588
Keywords: fertility preservation, child cancer patients, ovarian tissue cryopreservation, oncofertility, Asia
Citation: Takae S, Lee JR, Mahajan N, Wiweko B, Sukcharoen N, Novero V, Anazodo AC, Gook D, Tzeng C-R, Doo AK, Li W, Le CTM, Di W, Chian R-C, Kim SH and Suzuki N (2019) Fertility Preservation for Child and Adolescent Cancer Patients in Asian Countries. Front. Endocrinol. 10:655. doi: 10.3389/fendo.2019.00655
Received: 28 May 2019; Accepted: 09 September 2019;
Published: 15 October 2019.
Edited by:
Tom Kelsey, University of St. Andrews, United KingdomReviewed by:
Francesco Lotti, University of Florence, ItalyCopyright © 2019 Takae, Lee, Mahajan, Wiweko, Sukcharoen, Novero, Anazodo, Gook, Tzeng, Doo, Li, Le, Di, Chian, Kim and Suzuki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seido Takae, czJ0YWthZUBtYXJpYW5uYS11LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.