- 1Department of Endocrinology, Affiliated Zhongda Hospital, Southeast University, Nanjing, China
- 2School of Medicine, Southeast University, Nanjing, China
Background and Objectives: Irisin plays an important role in the metabolism and homeostasis of energy balance, which is involved in cognitive impairment. This study aimed to investigate the role of irisin in mild cognitive impairment (MCI) among Chinese patients with type 2 diabetes mellitus (T2DM).
Methods: We recruited 133 Chinese patients with T2DM, and divided them according to the Montreal Cognitive Assessment score. Demographic data were collected and the level of irisin in the plasma was determined. In addition, the results of neuropsychological testing were examined. The concentration of irisin in the plasma was measured using an enzyme immunoassay.
Results: A total of 59 patients were diagnosed with MCI and 74 patients were included as healthy-cognition controls. The level of irisin in the plasma (p = 0.043) and homeostasis model of assessment for insulin resistance (p = 0.032) in diabetic patients with MCI were higher than those observed in the healthy controls. A higher level of irisin in the plasma was associated with impaired overall cognition, specifically executive function. Linear regression analysis suggested that irisin (p = 0.017) and glycosylated hemoglobin (p = 0.036) were independent factors of diabetic MCI.
Conclusions: The level of irisin in the plasma correlated with cognitive impairment in T2DM patients, particularly with executive function. These results further suggest that, in addition to poor glycemic control, a high level of irisin in the plasma portends early cognitive deficits clinically in Chinese patients with T2DM.
Introduction
Individuals with type 2 diabetes mellitus (T2DM) exhibit a higher prevalence of mild cognitive impairment (MCI) in comparison with the general population (1). The risk of cognitive impairment in DM patients is 1.2 to 1.5-fold higher than that reported in non-DM individuals (2). Cognitive impairment is considered one of the chronic complications of DM (3). Several potential mechanisms promote the occurrence of cognitive impairment in T2DM, including hyperglycemic toxicity, insulin resistance, oxidative stress, accumulation of amyloid-beta peptide and tau hyper-phosphorylation (4–9).
Irisin, a novel glycosylated polypeptide hormone, plays an important role in the homeostasis and metabolism of energy balance (10). It had been reported to lead to brown-fat-like development by stimulating the expression of uncoupling protein-1 and altering that of several molecules (11, 12). Irisin induces the transformation of white adipose tissue into brown adipose tissue. Consequently, the increased thermogenesis may lead to weight loss and improve insulin sensitivity and glucose tolerance in mice (13, 14). The increase of circulating irisin is associated with endurance training induced reduction of abdominal visceral fat in old and middle-aged people (15). Many studies have found low level of irisin in individuals with T2DM compared to that in non-diabetic individuals (16–19). Continuous exposure to hyperglycemia and impaired insulin signaling are major causes of Alzheimer's disease (AD) and related to cognitive impairment, especially learning and memory loss (20, 21). Several studies reported that irisin may regulate insulin resistance and glucose homeostasis (12, 22, 23), potentially improving cognitive function. Furthermore, irisin may promote neurogenesis (24) and protect against neuronal damage caused by oxidative stress (25, 26). In addition, it was shown that irisin regulates the production of brain-derived neurotrophic factor (27, 28), which may enhance cognitive function and reduce synaptic dysfunction in AD (29). These findings suggest that irisin may improve cognitive function.
Therefore, the aim of this study was to investigate the association between the level of irisin in the plasma and cognition performance in patients with T2DM.
Materials and Methods
Patients and Study Design
We recruited 133 patients (aged 45–75 years) who were admitted to the Department of Endocrinology of the Affiliated Zhongda Hospital of Southeast University (Nanjing, China) between February 2015 and June 2017. The patients were diagnosed with T2DM for ≥3 years according to the 1999 World Health Organization criteria (30). The exclusion criteria were as follows: (1) central nervous system diseases (i.e., recent stroke, head trauma, epilepsy, Parkinson's disease, depression, or other psychological illnesses) that may cause MCI; (2) drug or alcohol abuse or dependence; (3) other major illnesses, including cancer, anemia, or thyroid dysfunction; and (4) use of potential or known cognition-impairing drugs in the previous 3 months. All patients were of Chinese Han ethnic origin and provided written informed consent prior to their participation in the study. The study was approved by the Research Ethics Committee of the Affiliated Zhongda Hospital of Southeast University, Nanjing, China.
Collection of Clinical Data
We collected the demographic and clinical characteristics of the patients, including gender, age, educational level, contact details, duration of T2DM, medical history (e.g., hypertension and fatty liver), fasting blood glucose, fasting C-peptide (FCP), glycosylated hemoglobin (HbA1c), triglyceride, total cholesterol, low-density lipoprotein, and high-density lipoprotein. We obtained physical measurements (i.e., weight, height, blood pressure, and waist and hip circumference) using a standard balance beam scale. The body mass index (BMI) is defined as the body weight (kg) divided by the square of the body height (m2).
Measurement of the Level of Irisin in the Plasma
We collected blood samples in the morning after the patients lying down for a night, and instructed the patients to avoid intense physical activity the day before. The concentration of irisin in the plasma was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Cusabio, Wuhan, China) according to the instructions provided by the manufacturer. The accuracy of this kit is comparable to that of EK-067-29 produced by Phoenix Pharmaceuticals, USA (31).
Neuropsychological Testing
Neuropsychological testing, including the Montreal Cognitive Assessment (MoCA), Mini Mental State Exam (MMSE), digit span test (DST), verbal fluency test (VFT), clock drawing test, logical memory test (LMT), auditory verbal learning test, and trail making tests A and B (TMT-A and TMT-B) were performed to assess the cognitive functions (i.e., memory, attention, executive function, psychomotor speed, and visuospatial skills). Based on the MoCA scoring system, 59 patients with MoCA scores <26 and the remaining 74 patients with MoCA scores ≥26 were classified in the MCI group and normal cognition group (control group), respectively. One point was added to the MoCA score for patients with a number of education years <12 (32).
Statistical Analysis
Statistical analyses were performed using the SPSS Version 21.0 software (IBM Corp., Armonk, NY, USA). For continuous variables, analysis of variance and Student's t-test were used to compare differences between groups at baseline. The chi-squared (χ2) test was employed for categorical variables. Spearman's correlation was used to examine the correlation between neuropsychological test scores and the level of irisin in the plasma. The relationship of cognitive performance with the level of irisin in the plasma, as well as demographic and clinical characteristics, was investigated using multiple linear regression analysis. A p < 0.05 denoted statistical significance.
Results
Demographic and Clinical Characteristics
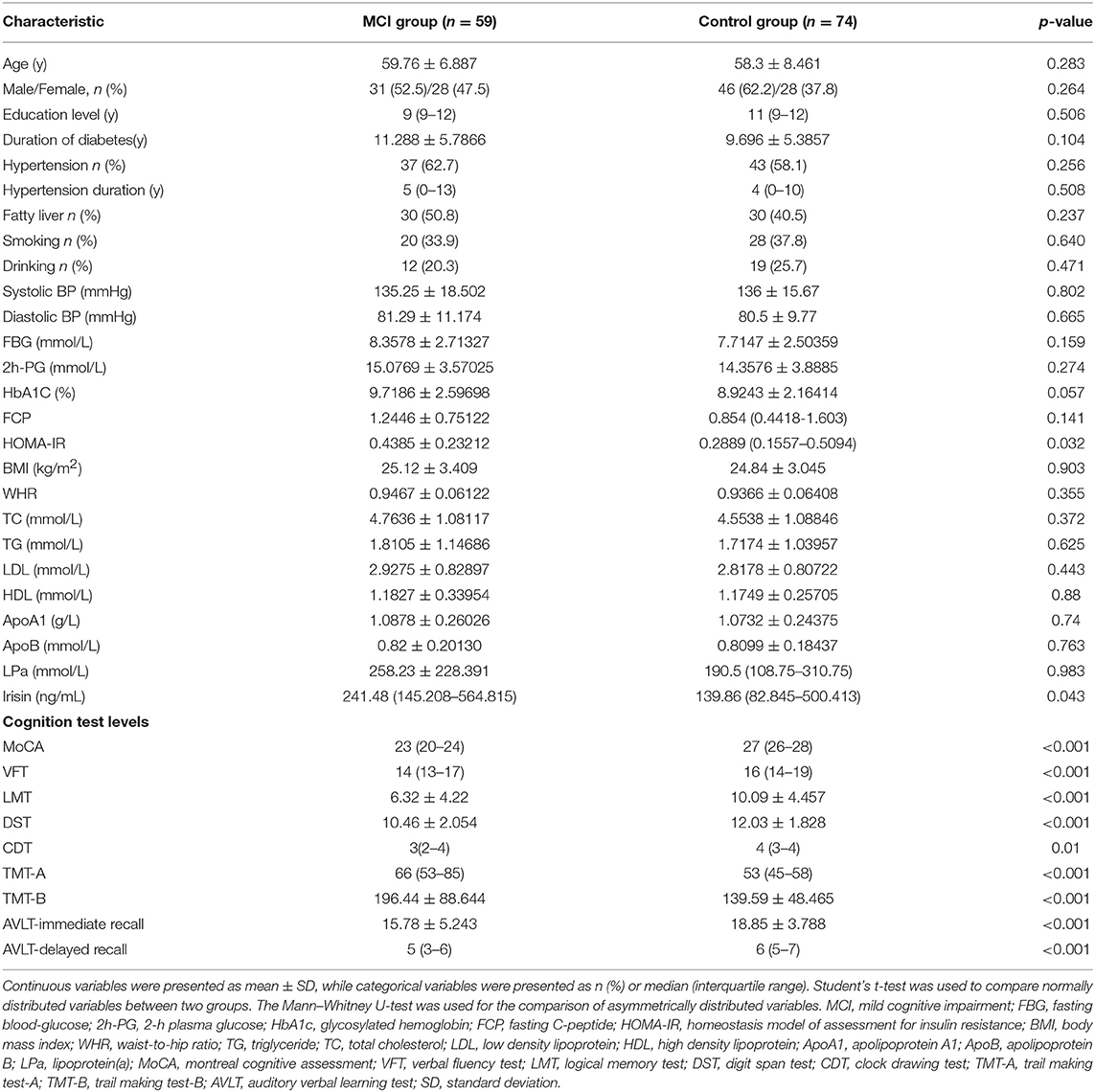
Table 1 lists the baseline characteristics and neuropsychological test scores of the patients. There were no significant differences found between the MCI and control groups in terms of age, gender, educational level, prevalence of hypertension, duration of T2DM, history of alcohol abuse or dependence, smoking history, BMI, waist-to-hip ratio, fasting blood-glucose, 2-h plasma glucose, FCP, HbA1C, total cholesterol, triglyceride, low-density lipoprotein, high-density lipoprotein, apolipoprotein A1, and apolipoprotein B (p > 0.05). The MCI group demonstrated a significantly higher level of irisin in the plasma and homeostasis model of assessment for insulin resistance (HOMA-IR) than the control group (p < 0.05). Moreover, significant differences between the two groups were also observed in the neuropsychological test scores (p < 0.01). The memory, attention, executive function, psychomotor speed, and visuospatial skills in the MCI group were significantly lower compared with those reported in the control group.
Correlation of the Level of Irisin in the Plasma With Baseline Data and Cognitive Indicators
We subsequently explored the correlation of the level of irisin in the plasma with clinical and the different neuropsychological test scores in all patients using Spearman rank correlation analysis. The level of plasma irisin was positively correlated with the BMI, FCP, HOMA-IR, and TMT-A and TMT-B scores. Of note, it was negatively correlated with the MoCA, MMSE, VFT, LMT, and DST score in total (Table 2). The level of irisin was positively associated with the FCP and the TMT-A score, whereas it was negatively associated with the MoCA, MMSE, and VFT score in the MCI group. In healthy-cognition controls, the level of irisin was only positively correlated with the FCP and TMT-A score. Considering the relationship between irisin and BMI, we performed a hierarchical analysis. The level of irisin in the plasma was only positively correlated with the TMT-A score in patients with normal weight. In overweight patients, it was significantly positively correlated with the BMI, FCP, duration of T2DM, and TMT-A and TMT-B scores. In contrast, it was negatively correlated with the MoCA, MMSE, VFT, LMT, DST, and auditory verbal learning test-delayed scores (Table 2).
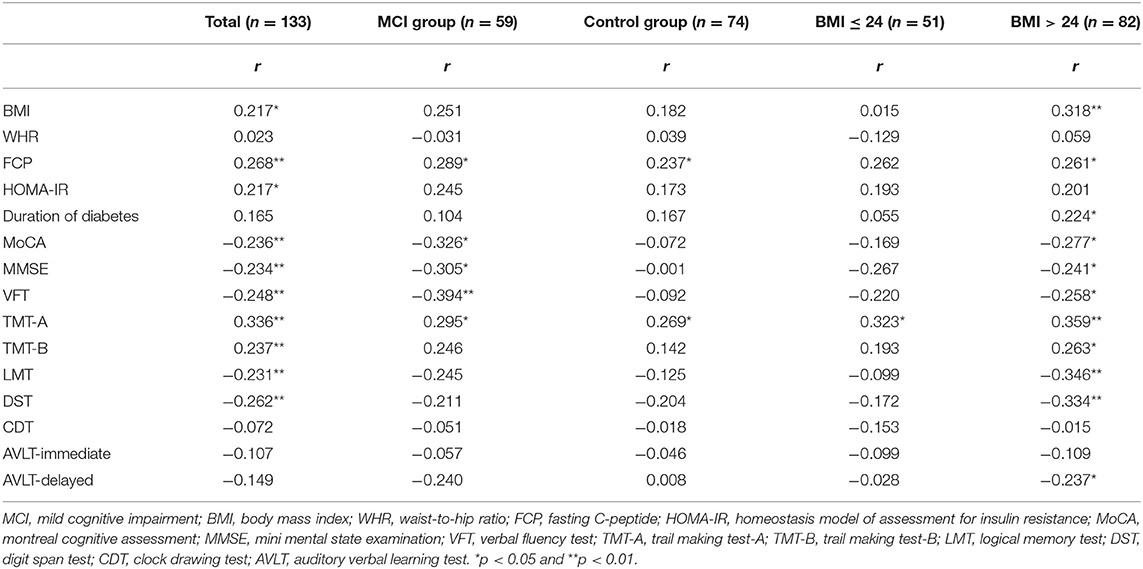
Table 2. Correlation of the level of irisin in the plasma with baseline data and cognitive indicators.
Correlation of the MoCA Score With Baseline Characteristics in All Patients
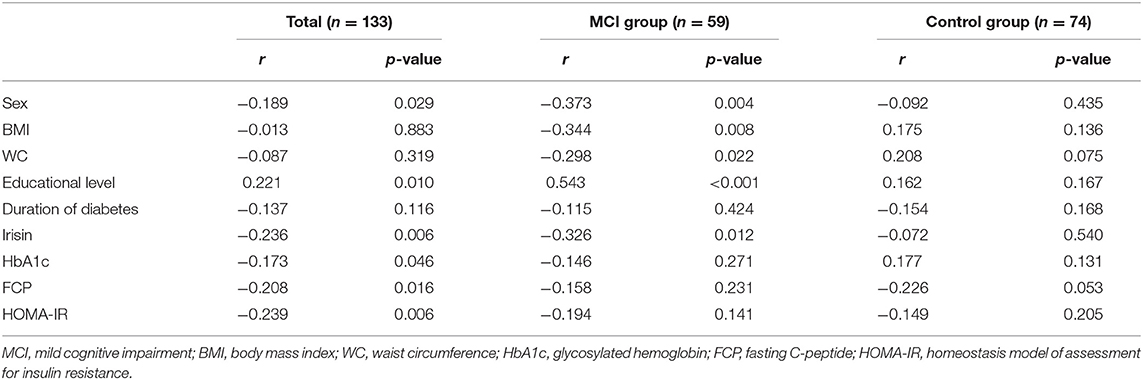
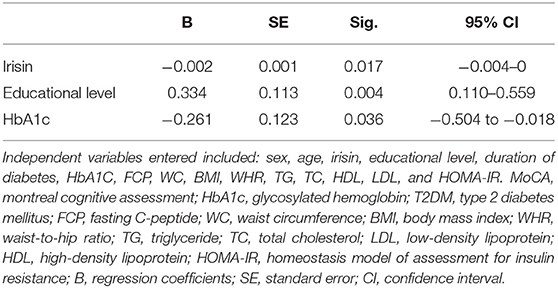
The relationship between the MoCA score and baseline characteristics was assessed through Spearman rank correlation analysis. We found that the MoCA score correlated with sex, irisin, educational level, HbA1c, FCP, and HOMA-IR (Table 3). Subsequently, we formed a multiple linear regression model to identify independent factors associated with MCI. Age, sex, irisin, educational level, duration of T2DM, HbA1C, FCP, waist circumference, BMI, waist-to-hip ratio, triglyceride, total cholesterol, high-density lipoprotein, low-density lipoprotein, and HOMA-IR were entered as independent variables in the multiple step-wise linear regression analysis, with the MoCA score as the dependent variable. The multivariable regression analysis revealed that plasma irisin, educational level, and HbA1c were associated with MCI in T2DM patients (p < 0.05) (Table 4). These three independent variables explained 17% of the variance.
Discussion
The results of the present study demonstrated that the level of irisin in the plasma of T2DM patients with MCI increased. This level was negatively associated with the MoCA, MMSE, VFT, LMT, and DST scores, whereas it was positively correlated with the TMT-A and TMT-B scores. These findings indicated worse overall cognitive, executive, and attention functions. The multivariable regression analysis indicated that a high level of plasma irisin and HbA1c may play important roles in the development of MCI in T2DM patients.
This was the first study to investigate the correlation between the level of irisin in the plasma and cognitive function in T2DM patients. Correlations were found between irisin and the MoCA, MMSE, VFT, LMT, DST, TMT-A, and TMT-B scores. Higher levels of irisin indicated poorer cognitive function in the patients. Additionally, we found that the BMI, FCP, and HOMA-IR were positively correlated with irisin, suggesting that the increased level of irisin in T2DM patients may be correlated with higher BMI, FCP, and HOMA-IR values. Previous studies suggested that the level of plasma irisin was increased in obese subjects (33–35). Furthermore, several studies showed a significant association between irisin and the HOMA-IR index (35–38), which reflects insulin resistance. All aforementioned factors have been shown to promote the development of cognitive impairment in T2DM patients (39, 40). Thus, an increased level of irisin may be a predictive factor of T2DM-associated cognitive impairment.
Based on these findings, the relationship between irisin and cognitive function is contrary to that reported by Lourenco et al. (41), which suggested that a lower level of irisin correlated with cognitive impairment. Irisin was reduced in the hippocampi and cerebrospinal fluid of AD patients compared with MCI patients or cognitively normal individuals (41). However, the level of irisin in the plasma was not significantly different in AD compared with that measured in non-demented controls. Another study suggested that irisin was positively associated with overall cognition and memory (42). Moreover, rat models showed that treatment with irisin reduced neurological deficits (25, 43) and increased hippocampal synaptic plasticity (41). The difference between our study and the others was the study population. T2DM is often accompanied by metabolic dysfunction. Similar to the increased level of insulin in insulin resistance (37, 44), the level of irisin in the plasma increased in MCI patients. In the present study, the positive association between the level of irisin in the plasma and markers of insulin resistance in T2DM patients may demonstrate an adaptive response to obesity through irisin (18). Similarly, an increased level of irisin in MCI patients may be the result of irisin resistance. This notion was supported by the negative correlation between the level of irisin and MoCA score in the MCI group rather than the control group. Considering the positive correlation between irisin and the BMI, irisin is likely to be involved in inflammatory and oxidative stress (45, 46).
In addition, in this study, the MoCA score was negatively correlated with HbA1c and positively correlated with the educational level. These findings were congruous with those reported by previous studies. Chronic exposure to hyperglycemia may damage cognitive function (47, 48). Higher education and more thinking may delay the progression of dementia (49, 50). Consequently, we encourage the elderly to participate in more intellectual activities to delay the impairment of cognitive function.
Several limitations of this study should be considered. Firstly, it was a cross-sectional study with a small sample size, which may limit the robustness of the results. Secondly, there were concerns regarding the accuracy of the irisin antibody kits (51). Further validation analyses using mass spectrometry are required to address these concerns. Thirdly, most of the hospitalized patients had uncontrolled diabetes, which lead to the selection bias in the sample. Although we have carefully considered and implemented the inclusion and exclusion criteria in order to reduce bias, it still affected the results. Moreover, we only used the MoCA for MCI diagnosis in our diabetic patients. Finally, the study did not collect data regarding the intensity of daily exercise, which may play an important role in the level of irisin and MCI.
Conclusion
In summary, this study showed that the MCI group had a higher level of irisin vs. the control group. Additionally, a higher level of irisin was associated with overall cognitive impairment, especially poorer executive function. Furthermore, irisin, HbA1c, and the educational level were identified as independent variables of MCI in all individuals, suggesting that low levels of irisin and HbA1c may be good predictors of MCI in T2DM patients. Further evidence, especially from longitudinal studies, is required to investigate the value of irisin as a predictive biomarker of MCI in T2DM patients.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee of the Affiliated Zhongda Hospital of Southeast University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SW, HL, and YY contributed to the conception and design of the study. HL conducted the study and statistical analysis, and wrote the manuscript. ST participated in the data analysis and interpretation. JH, RH, DG, JW, and KA performed the data collection. All authors read and approved the submitted version of this manuscript.
Funding
This work was partly supported by the National Natural Science Foundation of China (No. 81570732, SW; and No. 81870568, SW), and the Jiangsu Provincial Medical Youth Talent (QNRC2016819, YY).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Dolan C, Glynn R, Griffin S, Conroy C, Loftus C, Wiehe PC, et al. Brain complications of diabetes mellitus: a cross-sectional study of awareness among individuals with diabetes and the general population in Ireland. Diabet Med. (2018) 35:871–9. doi: 10.1111/dme.13639
2. Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes–systematic overview of prospective observational studies. Diabetologia. (2005) 48:2460–9. doi: 10.1007/s00125-005-0023-4
3. Mittal K, Katare DP. Shared links between type 2 diabetes mellitus and Alzheimer's disease: a review. Diabetes Metab Syndr. (2016) 10(2 Suppl. 1):S144–9. doi: 10.1016/j.dsx.2016.01.021
4. Macauley SL, Stanley M, Caesar EE, Yamada SA, Raichle ME, Perez R, et al. Hyperglycemia modulates extracellular amyloid-beta concentrations and neuronal activity in vivo. J Clin Invest. (2015) 125:2463–7. doi: 10.1172/JCI79742
5. Gaspar JM, Baptista FI, Macedo MP, Ambrosio AF. Inside the diabetic brain: role of different players involved in cognitive decline. ACS Chem Neurosci. (2016) 7:131–42. doi: 10.1021/acschemneuro.5b00240
6. Kim DJ, Yu JH, Shin MS, Shin YW, Kim MS. Hyperglycemia reduces efficiency of brain networks in subjects with type 2 diabetes. PLoS ONE. (2016) 11:e0157268. doi: 10.1371/journal.pone.0157268
7. Lemche E. (2018). Early life stress and epigenetics in late-onset Alzheimer's dementia: a systematic review. Curr. Genomics19, 522–602. doi: 10.2174/1389202919666171229145156
8. Rom S, Zuluaga-Ramirez V, Gajghate S, Seliga A, Winfield M, Heldt NA, et al. Hyperglycemia-driven neuroinflammation compromises BBB leading to memory loss in both diabetes mellitus (DM) type 1 and type 2 mouse models. Mol Neurobiol. (2019) 56:1883–96. doi: 10.1007/s12035-018-1195-5
9. Lee HJ, Seo HI, Cha HY, Yang YJ, Kwon SH, Yang SJ. Diabetes and Alzheimer's disease: mechanisms and nutritional aspects. Clin Nutr Res. (2018) 7:229–40. doi: 10.7762/cnr.2018.7.4.229
10. Grygiel-Gorniak B, Puszczewicz M. A review on irisin, a new protagonist that mediates muscle-adipose-bone-neuron connectivity. Eur Rev Med Pharmacol Sci. (2017) 21:4687–93.
11. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. (2012) 481:463–8. doi: 10.1038/nature10777
12. Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. (2014) 63:514–25. doi: 10.2337/db13-1106
13. Lee HJ, Lee JO, Kim N, Kim JK, Kim HI, Lee YW, et al. Irisin, a novel myokine, regulates glucose uptake in skeletal muscle cells via AMPK. Mol Endocrinol. (2015) 29:873–81. doi: 10.1210/me.2014-1353
14. Xin C, Liu J, Zhang J, Zhu D, Wang H, Xiong L. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int J Obes. (2016) 40, 443–451. doi: 10.1038/ijo.2015.199
15. Miyamoto-Mikami E, Sato K, Kurihara T, Hasegawa N, Fujie S, Fujita S, et al. Endurance training-induced increase in circulating irisin levels is associated with reduction of abdominal visceral fat in middle-aged and older adults. PLoS ONE. (2015) 10:e0120354. doi: 10.1371/journal.pone.0120354
16. Choi YK, Kim MK, Bae KH, Seo HA, Jeong JY, Lee WK, et al. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res Clin Pract. (2013) 100:96–101. doi: 10.1016/j.diabres.2013.01.007
17. Liu JJ, Wong MD, Toy WC, Tan CS, Liu S, Ng XW, et al. Lower circulating irisin is associated with type 2 diabetes mellitus. J Diabetes Complications. (2013) 27:365–9. doi: 10.1016/j.jdiacomp.2013.03.002
18. Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. (2013) 98:E769–78. doi: 10.1210/jc.2012-2749
19. Hu W, Wang R, Li J, Zhang J, Wang W. Association of irisin concentrations with the presence of diabetic nephropathy and retinopathy. Ann Clin Biochem. (2016) 53(Pt 1):67–74. doi: 10.1177/0004563215582072
20. Chen Z, Zhong C. Decoding Alzheimer's disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol. (2013) 108:21–43. doi: 10.1016/j.pneurobio.2013.06.004
21. Willette AA, Bendlin BB, Starks EJ, Birdsill AC, Johnson SC, Christian BT, et al. Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for Alzheimer disease. JAMA Neurol. (2015) 72:1013–20. doi: 10.1001/jamaneurol.2015.0613
22. Yang Z, Chen X, Chen Y, Zhao Q. Decreased irisin secretion contributes to muscle insulin resistance in high-fat diet mice. Int J Clin Exp Pathol. (2015) 8:6490–7.
23. Al-Daghri NM, Mohammed AK, Al-Attas OS, Amer OE, Clerici M, Alenad A, et al. SNPs in FNDC5 (irisin) are associated with obesity and modulation of glucose and lipid metabolism in Saudi subjects. Lipids Health Dis. (2016) 15:54. doi: 10.1186/s12944-016-0224-5
24. Hashemi MS, Ghaedi K, Salamian A, Karbalaie K, Emadi-Baygi M, Tanhaei S, et al. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience. (2013) 231:296–304. doi: 10.1016/j.neuroscience.2012.11.041
25. Li DJ, Li YH, Yuan HB, Qu LF, Wang P. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism. (2017) 68:31–42. doi: 10.1016/j.metabol.2016.12.003
26. Peng J, Deng X, Huang W, Yu JH, Wang JX, Wang JP, et al. Irisin protects against neuronal injury induced by oxygen-glucose deprivation in part depends on the inhibition of ROS-NLRP3 inflammatory signaling pathway. Mol Immunol. (2017) 91:185–94. doi: 10.1016/j.molimm.2017.09.014
27. Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, et al. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. (2013) 18:649–59. doi: 10.1016/j.cmet.2013.09.008
28. Belviranli M, Okudan N, Kabak B, Erdogan M, Karanfilci M. The relationship between brain-derived neurotrophic factor, irisin and cognitive skills of endurance athletes. Phys Sportsmed. (2016) 44:290–6. doi: 10.1080/00913847.2016.1196125
29. Diniz BS, Teixeira AL. Brain-derived neurotrophic factor and Alzheimer's disease: physiopathology and beyond. Neuromolecular Med. (2011) 13:217–22. doi: 10.1007/s12017-011-8154-x
30. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. (1998) 15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
31. Li M, Yang M, Zhou X, Fang X, Hu W, Zhu W, et al. Elevated circulating levels of irisin and the effect of metformin treatment in women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2015) 100:1485–93. doi: 10.1210/jc.2014-2544
32. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
33. Yilmaz H, Cakmak M, Darcin T, Inan O, Sahiner E, Demir C, et al. Circulating irisin levels reflect visceral adiposity in non-diabetic patients undergoing hemodialysis. Ren Fail. (2016) 38:914–9. doi: 10.3109/0886022X.2016.1172918
34. Sahin-Efe A, Upadhyay J, Ko BJ, Dincer F, Park KH, Migdal A, et al. Irisin and leptin concentrations in relation to obesity, and developing type 2 diabetes: a cross sectional and a prospective case-control study nested in the Normative Aging Study. Metabolism. (2018) 79:24–32. doi: 10.1016/j.metabol.2017.10.011
35. Wang W, Guo Y, Zhang X, Zheng J. Abnormal irisin level in serum and endometrium is associated with metabolic dysfunction in polycystic ovary syndrome patients. Clin Endocrinol. (2018) 89:474–80. doi: 10.1111/cen.13805
36. Park KH, Zaichenko L, Brinkoetter M, Thakkar B, Sahin-Efe A, Joung KE, et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab. (2013) 98:4899–907. doi: 10.1210/jc.2013-2373
37. Shoukry A, Shalaby SM, El-Arabi Bdeer S, Mahmoud AA, Mousa MM, Khalifa A. Circulating serum irisin levels in obesity and type 2 diabetes mellitus. IUBMB Life. (2016) 68:544–56. doi: 10.1002/iub.1511
38. Stratigou T, Dalamaga M, Antonakos G, Marinou I, Vogiatzakis E, Christodoulatos GS, et al. Hyperirisinemia is independently associated with subclinical hypothyroidism: correlations with cardiometabolic biomarkers and risk factors. Endocrine. (2018) 61:83–93. doi: 10.1007/s12020-018-1550-3
39. Umegaki H. Type 2 diabetes as a risk factor for cognitive impairment: current insights. Clin Interv Aging. (2014) 9:1011–9. doi: 10.2147/CIA.S48926
40. Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behavior Immunity. (2014) 42:10–21. doi: 10.1016/j.bbi.2014.04.001
41. Lourenco MV, Frozza RL, de Freitas GB, Zhang H, Kincheski GC, Ribeiro FC, et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer's models. Nat Med. (2019) 25:165–75. doi: 10.1038/s41591-018-0275-4
42. Kuster OC, Laptinskaya D, Fissler P, Schnack C, Zugel M, Nold V, et al. Novel blood-based biomarkers of cognition, stress, and physical or cognitive training in older adults at risk of dementia: preliminary evidence for a role of BDNF, irisin, and the kynurenine pathway. J Alzheimers Dis. (2017) 59:1097–111. doi: 10.3233/JAD-170447
43. Asadi Y, Gorjipour F, Behrouzifar S, Vakili A. Irisin peptide protects brain against ischemic injury through reducing apoptosis and enhancing BDNF in a rodent model of stroke. Neurochem Res. (2018) 43:1549–60. doi: 10.1007/s11064-018-2569-9
44. Doumatey AP, Lashley KS, Huang H, Zhou J, Chen G, Amoah A, et al. Relationships among obesity, inflammation, and insulin resistance in African Americans and West Africans. Obesity. (2010) 18:598–603. doi: 10.1038/oby.2009.322
45. Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. (2015) 3:207–15. doi: 10.1016/S2213-8587(14)70134-2
46. Reho JJ, Rahmouni K. Oxidative and inflammatory signals in obesity-associated vascular abnormalities. Clin Sci. (2017) 131:1689–700. doi: 10.1042/CS20170219
47. Munshi MN. Cognitive dysfunction in older adults with diabetes: what a clinician needs to know. Diabetes Care. (2017) 40:461–7. doi: 10.2337/dc16-1229
48. Pruzin JJ, Nelson PT, Abner EL, Arvanitakis Z. Review: relationship of type 2 diabetes to human brain pathology. Neuropathol Appl Neurobiol. (2018) 44:347–62. doi: 10.1111/nan.12476
49. Ott A, Breteler MM, van Harskamp F, Claus JJ, van der Cammen TJ, Grobbee DE, et al. Prevalence of Alzheimer's disease and vascular dementia: association with education. The Rotterdam study. BMJ. (1995) 310:970–3. doi: 10.1136/bmj.310.6985.970
50. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. (2015) 11:718–26. doi: 10.1016/j.jalz.2015.05.016
Keywords: irisin, mild cognitive impairment, executive function, diabetes mellitus, insulin resistance
Citation: Lin H, Yuan Y, Tian S, Han J, Huang R, Guo D, Wang J, An K and Wang S (2019) In Addition to Poor Glycemic Control, a High Level of Irisin in the Plasma Portends Early Cognitive Deficits Clinically in Chinese Patients With Type 2 Diabetes Mellitus. Front. Endocrinol. 10:634. doi: 10.3389/fendo.2019.00634
Received: 17 May 2019; Accepted: 30 August 2019;
Published: 13 September 2019.
Edited by:
Lei Sha, China Medical University, ChinaReviewed by:
Erwin Lemche, King's College London, United KingdomRamit Ravona-Springer, Sheba Medical Center, Israel
Copyright © 2019 Lin, Yuan, Tian, Han, Huang, Guo, Wang, An and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaohua Wang, Z3lqd3NoQDEyNi5jb20=
†These authors have contributed equally to this work as co-first authors
 Hongyan Lin
Hongyan Lin Yang Yuan
Yang Yuan Sai Tian
Sai Tian Jing Han
Jing Han Rong Huang
Rong Huang Dan Guo
Dan Guo Jiaqi Wang
Jiaqi Wang Ke An
Ke An Shaohua Wang
Shaohua Wang