- 1Central Laboratory of the Eastern Division, The First Hospital of Jilin University, Changchun, China
- 2Center for Reproductive Medicine, Center for Prenatal Diagnosis, The First Hospital of Jilin University, Changchun, China
- 3Department of Dermatology, The First Hospital of Jilin University, Changchun, China
- 4Department of Cardiology, The First Hospital of Jilin University, Changchun, China
- 5Center for Reproductive Medicine, Jilin Province People's Hospital, Changchun, China
In vitro fertilization (IVF) is an effective means to treat infertility, but the pregnancy rate is still unsatisfactory and reliable markers to predict pregnancy outcome are ill-defined. Myeloid-derived suppressor cell (MDSC) are critically involved in decisions related to the acceptance or rejection of foreign fetal antigens by the maternal immune system. However, factors that regulate peripheral blood MDSC during pre-pregnancy are poorly defined. Thus, the goal of this study was to assess the relationships among serum estradiol (E2) and endothelial growth factor (VEGF) levels, MDSC ratios, and pregnancy outcome associated with IVF. Patients undergoing IVF treatment (n = 54) were recruited from January to June 2018. Levels of E2 and VEGF were measured by ELISA, MDSC ratios among peripheral blood mononuclear cells (PBMC) were detected by flow cytometry, and the crosstalk among these parameters was analyzed. A receiver operating characteristic curve (ROC) of MDSC levels was plotted to assess this measure as an independent predictive factor for pregnancy. In addition, we analyzed the possible involvement of molecular pathways by bioinformatics. When E2 levels were <4,000 pg/ml, MDSC proportion was positively correlated with serum E2 and VEGF levels. However, when E2 levels were >4,000 pg/ml, MDSC ratio and VEGF levels were negatively correlated with E2. A ROC curve revealed that the percentage of MDSC had better sensitivity and specificity at a concentration of 8.22% (0.875 and 0.75, respectively; area under the curve (AUC) = 0.859) to predict pregnancy success, based on multiple logistic regression analysis. Furthermore, we found 12 target genes of E2 and VEGF, and also functional genes related to MDSC, indicating potential protein–protein interactions underlying these associations. In summary, we showed that E2, depending on its concentration, might play a dichotomous role in influencing the MDSC proportion by regulating VEGF. In IVF patients, an increased MDSC ratio among PBMC was highly correlated with elevated pregnancy rates, independent of the effects of E2, which might provide new insight into immune-related miscarriage and IVF failure.
Introduction
Successful pregnancy is a concerted process, involving multiple mechanisms to prevent rejection of the semi-allograft fetus, which is immunologically foreign to the mother. Although in vitro fertilization (IVF) is an effective means to treat infertility, the pregnancy rate is still unsatisfactory. Upon embryo implantation, the mother's immune system develops an immunological tolerance response to prevent miscarriage (1, 2). Dysfunctions in the maternal immune system can lead to miscarriages or severe complications during pregnancy such as preeclampsia (3) and preterm deliveries (4). Moreover, estrogen was shown to augment the secretion of inflammatory cytokines such as TNF-α, IL-6, and IFNγ in Th1 lymphocytes (5), and can also stimulate the expression of key Th17 cell transcription factors by activating STAT3 and the expression of RORα and RORγ (6). Supraphysiologic estrogen during IVF makes the body inclined to generate pro-inflammatory responses, to stabilize the immune-associated microenvironment, some anti-inflammatory immune cells can be recruited. Although some subsets of immune cells participate in maternal–fetal immune tolerance, the underlying mechanisms are still not clear (7). We postulated that E2 might affect immune tolerance during controlled ovarian stimulation (COS). Understanding such mechanisms will facilitate the development of effective therapies to improve IVF outcome.
Myeloid-derived suppressor cell (MDSC), which plays a pivotal role in suppressing innate and adaptive immune responses in the tumor microenvironment (8, 9), are defined as heterogeneous myeloid precursor cells that expand in the blood, lymphatic organs, and tumor sites (10, 11). Human MDSC is classified into two subsets, namely granulocytic MDSC (G-MDSC; CD11b+ HLA-DRlow/−CD33+CD15/CD66b+) and monocytic MDSC (M-MDSC, CD11b+ HLA-DRlow/−CD33+CD14+) (12, 13). These cells induce and maintain immune tolerance by inhibiting T cell activation and proliferation, suppressing natural killer cell cytotoxicity, inducing Foxp3 expression in CD4+CD25− T cells, and blocking macrophage-induced IL-12 (14). Several factors including hematopoietic and vascular growth factors, pro- and anti-inflammatory cytokines, and human leukocyte antigens induce the accumulation of MDSC to elicit and maintain maternal–fetal interface immune tolerance during pregnancy (14, 15). In addition to maintaining immune tolerance, MDSC can also promote angiogenesis during placental development and the remodeling of maternal uterine spiral arteries during pregnancy (15). Accordingly, recent studies showed that MDSC numbers were significantly elevated in pregnant women and decreased in the blood and endometrium during miscarriage (16, 17).
VEGF is a key regulator of angiogenesis and vascular function (18); it not only recruits MDSC to play an immunosuppressive role (19), but also inhibits the function of T cells (20). Further, MDSC can secrete VEGF, which plays an important role in reconstruction of the maternal uterine spiral artery and placental development during pregnancy (15). Although, E2 levels have been proposed to be the critical determinant of implantation (21), there is still lack of predictive marker for pregnancy outcome. In addition, factors that modulate peripheral blood MDSC during pre-pregnancy are poorly-defined. Thus, here, we investigated the relationships among serum E2 and VEGF levels, MDSC ratios, and pregnancy outcome in IVF patients and assessed possible associated molecular pathways by bioinformatics.
Materials and Methods
Patients
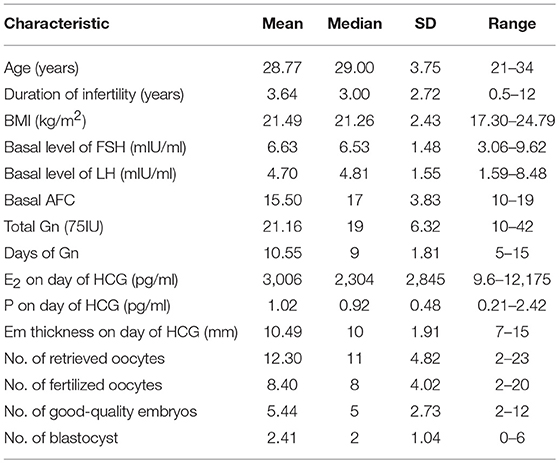
Women of an average age of 28.77 ± 3.75 years, undergoing IVF treatment (n = 54) were recruited from January to June 2018 at the Reproductive Medicine Center of Jilin Province People's Hospital. The inclusion criteria were as follows: (I) first IVF/Intracytoplasmic sperm injection (ICSI) cycle, (II) ≤35 years of age, (III) good ovarian reserve, (IV) normal hormones and karyotype, (V) without any known immunological diseases, (VI) without uterine abnormalities (fibroid, uterine septum, and uterine polyp), and (VII) without endometriosis. The exclusion criteria were as follows: (I) poor IVF/ICSI stimulation response (≤5 oocytes collected), (II) dissatisfactory appearance of endometrium at HCG day (thickness < 8 mm), (III) progesterone > 2.0 ng/ml at HCG day, (IV) poor embryo development (<2 high-quality embryos), and (V) miscarriage after pregnancy.
In vitro Fertilization and Embryo Transfer
All patients were treated with the luteal-phase GnRH-a protocol. Ovarian stimulation was induced with recombinant follicle-stimulating hormone (FSH; Gonal-F, Merck Serono, Switzerland) and human menopausal gonadotropin (HMG; menotrophins for injection, Livzon, China) after pituitary suppression was achieved with GnRH-a (Triptorelin Acetate Injection, Tiantaishan Pharmaceuticals, China). Chorionic gonadotrophin for injection (HCG, Vidrel, Merck Serono, Switzerland) was administered when the dominant follicles (two or more) reached 18 mm in diameter. Oocytes were collected 36 h after HCG administration with vaginal ultrasound-guided follicular aspiration. Mature oocytes were incubated at 37°C with 5% CO2 and then inseminated with spermatozoa 4–6 h after oocyte retrieval for IVF or ICSI according to the quality of sperm. The oocyte retrieval day was defined as day 0. Then, the embryos were scored on day 3 on a scale of 1–5 according to Scott's scoring system (6). Embryos of grades 1 and 2 were defined as high-quality embryos. The low-quality embryos were cultured to blastocysts. All surplus embryos and blastocysts were vitrified.
Intrauterine administration was performed at the estrogen-primed frozen embryo transfer (FET) cycle. The patients were administered a daily dose of 4–6 mg of estradiol valerate (Progynova; Bayer Schering Pharma, France) from menstrual cycle day 2 to prepare the endometrium and ultrasonography was performed to measure the endometrium until 8 mm, after which, the estradiol dose was adjusted, and treatment lasted for at least 10 days. Luteal phase support was performed using 90 mg of vaginal progesterone (Crinone gel 8%; Merck, Germany), administered from 3 days before the FET day. Two high-quality embryos were transferred into an adequately prepared endometrium. Serum β-hCG levels were measured from peripheral blood 2 weeks after FET. Those with positive β-hCG results underwent ultrasonography another 2 weeks later to confirm clinical pregnancy. Clinical pregnancy was defined as the presence of an intrauterine gestational sac. Luteal phase support was continued until 9 weeks of pregnancy.
E2, VEGF, and MDSC assays were performed at a random day during ovarian stimulation, but before follicular maturation, and also on the day of FET. A sample of 2 ml of peripheral blood was also collected.
Peripheral Blood Mononuclear Cell (PBMC) Isolation and Flow Cytometry
Peripheral blood was collected in sterile heparinized tubes from each patient, and PBMC, obtained by Ficoll–Hypaque gradient centrifugation, were analyzed by flow cytometry. The following anti-human fluorescence conjugated antibodies and their corresponding isotype controls used were as follows: anti-human CD11b-APC-cy7 (clone ICRF44, BD), anti-human HLA-DR-PE (clone G46-6, BD), anti-human CD14-FITC (clone 63D3, Biolegend), and anti-human CD66b-Percpcy5.5 (clone G10F5, Biolegend), FVD (BD), CD3-PE-cy7 (clone HIT3a, Biolegend), CD4-APC (clone OKT4, Biolegend), CD8-PB (clone RPA-T8, Biolegend). We used FVD to discriminate dead cells, and MDSC were classified into two subsets, G-MDSC (CD11b+ HLA-DRlow/− CD66b+) and M-MDSC (CD11b+ HLA-DRlow/CD14+). Data were analyzed with FlowJo 10.0 software package (Treestar Inc., Ashland, OR, USA).
For flow cytometric sorting, an Aria II fluorescence activated cell sorter (BD, Mountain View, CA, USA) was used. The strategy for MDSC sorting was CD11b+ HLA-DRlow/− cells from live PBMC. Depletion of MDSC was performed by harvesting the remaining PBMC after MDSC sorting.
Cells Culture
PBMC was collected from an infertile patient on menstrual cycle day 2, with basal hormone levels, and transferred to dishes with RPMI 1640 medium (Gibco, Carlsbad, USA) supplemented with 2 mM L-glutamine, 10 mM HEPES, 20 mM 2-methoxyestradiol, 150 U/ml streptomycin, 200 U/ml penicillin, and 10% fetal bovine serum. Cells (2 × 105) were cultured in 96-well plates and treated with various concentrations (0, 10, 20, 40, and 100 μM) of 17β-estradiol (Sigma-Aldrich, China). The cultures were maintained at 37°C in a 5% CO2-humidified atmosphere in 96-well plates. Medium was changed on the third day. The proportion of CD11b+HLA-DRlow/- MDSC was analyzed by flow cytometry on the sixth day.
T Cell Proliferation Assay
T cell proliferation was evaluated by carboxyfluorescein succinimidyl ester (CFSE) dilution. PBMC were labeled with CFSE (2.5 μM) (Invitrogen, Carlsbad, CA, USA) and then stimulated with anti-CD3/CD28 antibodies (2 and 1 μg/ml, separately) (eBioscience). After culturing whole PBMC or those depleted of MDSC for 3 days, T cell proliferation was determined by a flow cytometer. No stimulation served as a negative control.
ELISA
Serum samples collected from patients were kept at −80°C. Enzyme immunoassays were performed to determine the concentration of VEGF and S100A9 using ELISA kits (Beinuo Biotechnology, Shanghai, China and Biolegend, San Diego, CA, USA, separately) (n = 23), according to the manufacturer's instructions. All samples were assayed in duplicate.
Real-Time Polymerase Chain Reaction Analysis
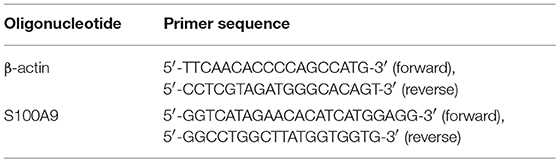
Initially, total RNA was extracted from PBMC using AxyPrep Multisource Total RNA Miniprep Kit (Axygen, Union City, CA, USA). The RNA quality was checked based on the A260/A280 ratio, and pure RNA samples were converted to cDNA using PrimeScript RT Reagent Kit with gDNA Eraser in accordance with the manufacturer's instructions (Takara Bio, Kusatsu, Japan). The polymerase chain reaction was performed using SYBR Green based on the instruction manual (Takara Bio, Kusatsu, Japan). The primer sequences are presented in Table 1 (Comate Bioscience, Changchun, China). The relative mRNA expression of different genes was quantified using the ΔΔCt method. β-actin was used as a housekeeping gene.
Hormone Assays
Serum E2 and progesterone (P) were measured by electro-chemiluminescence immunoassay (ECLIA, Cobas Roche Diagnostics, Mannheim, Germany). Intra- and inter-assay variations were <8 and 11%, respectively.
Analysis by GeneMANIA
GeneMANIA is a flexible, user-friendly web interface used for generating hypotheses regarding gene functions, analyzing gene lists, and prioritizing genes for functional assays (22). After selecting Homo sapiens from the nine optional organisms, the genes of interest were entered into the search bar and the results were collated.
GO Analysis, Pathway Analysis, and Network Construction
DAVID, a powerful tool for network biology, comprises an integrated biological knowledge base and analytic tools aimed at systematically extracting biological significance from large lists of genes or proteins (23). Potential targets were uploaded to the DAVID 6.8 server (https://david.ncifcrf.gov/home.jsp) for GO analysis, which was used to identify functionally-related genes from the list of differentially-expressed genes. Functionally related genes categorized based on biological processes, cellular components, and molecular functions were grouped by DAVID. A false discovery rate (FDR) < 5.0 and p < 0.05 were considered statistically significant. To achieve a systematic understanding of the inter-relationships among compounds, targets, and diseases, compound-target-pathway networks were constructed and analyzed in Cytoscape 3.3.
Statistical Analysis
For the development, validation, and reporting of the proposed risk score, we followed the TRIPOD (Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis) guideline (24). GraphPad Prism version 7.00 and SPSS version 21.0 software were used for statistical analysis. All data were presented as the mean ± SD. Variables between groups were analyzed using the independent-samples Student's t-test or chi-squared tests and p <0.05 was considered statistically significant. ROC curve and area under the curve (AUC) analyses were performed to compare the percentage of MDSC among PBMC between groups. Odds ratios (ORs) and 95% CIs for a better IVF outcome associated with increased MDSC were estimated based on unconditional logistic regression.
Results
Subjects
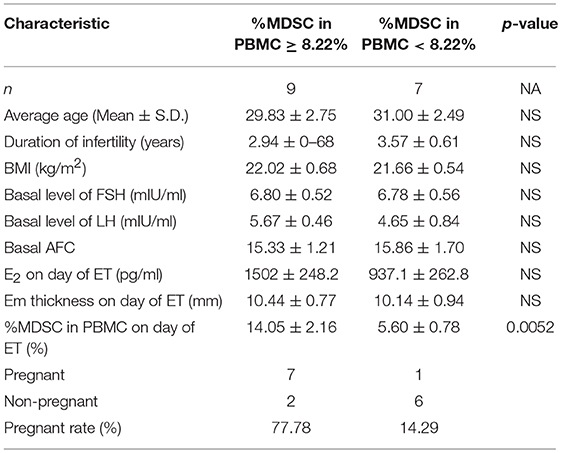
Fifty-four patients who met the inclusion criteria were enrolled and treated with COS. Clinical characteristics are shown in Table 2. During COS, four patients were excluded from the analysis due to the following exclusion criteria: poor IVF/ICSI stimulation response (n = 2) and progesterone > 2.0 ng/ml at HCG day (n = 2). We then tested serum E2, percentage of MDSC on a random day during COS (n = 50), VEGF and S100A9 levels (n = 23). The crosstalk among these factors was also analyzed. To verify that MDSC ratios are predictive of IVF outcome independently, we tested E2 and MDSC levels in estrogen-primed FET patients on the day of FET. After transferring two good-quality embryos to each patient, the remaining 16 patients were divided into two groups according to MDSC level. No significant (p > 0.05) difference was found in these characteristics (Table 3), except for the percentage of MDSC among PBMC. Finally, bioinformatic analysis was conducted for further investigation (as outlined in a following section; Figure 1).
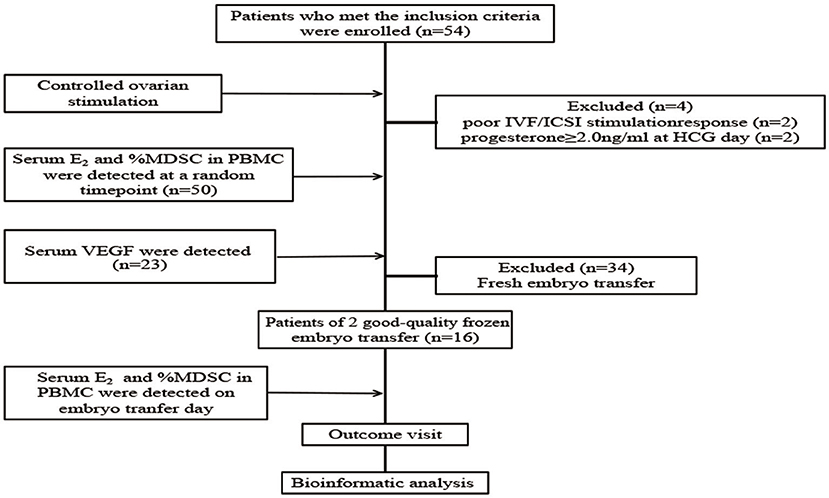
Figure 1. Flow chart of the study. Fifty-four patients were included, of which four patients were excluded from the analysis due to the exclusion criteria. Serum E2 level and percentage of MDSC among PBMC were detected in the remaining 50 patients at a random time-point during controlled ovarian stimulation, and serum VEGF level was detected (n = 23). Then, serum E2 level and percentage of MDSC in PBMC were detected on the embryo transfer day and pregnancy outcome visit were done in the patients of 2 good-quality frozen embryo transfer group (n = 16). Finally, we did bioinformatic analysis to predict the interaction between E2, VEGF and MDSC, and the probability of signal pathway.
E2, VEGF, S100A9, and MDSC Assays
To investigate the correlation between MDSC and E2 levels during COS, we analyzed these parameters in blood and serum of women who underwent IVF treatment (n = 50). MDSC subsets were identified as CD11b+HLA-DRlow/− and CD66b+ for G-MDSC or CD14+ for M-MDSC populations. As shown in Figure 2A, the percentage of MDSC among PBMC was significantly elevated in IVF patients with elevated serum E2. Further, there was a positive correlation between serum E2 levels < 4,000 pg/ml and MDSC (r2 = 0.5747, ****p < 0.0001, n = 40; Figure 2B). However, an E2 concentration > 4,000 pg/ml was inversely proportional to MDSC, indicating that hyperphysiological E2 might be detrimental to MDSC recruitment (r2 = 0.4381, *p = 0.0371, n = 10; Figure 2C). We further investigated the correlation between M-MDSC- or G-MDSC-dominant ratios and E2 (Figures 2D,E; r2 = 0.4312, ***p = 0.0007, and r2 = 0.0088, p = 0.7877, respectively, n = 40). Only M-MDSC showed a positive correlation, whereas there was no association with the G-MDSC group, when serum E2 levels were <4,000 pg/ml. Meanwhile, for E2 levels > 4,000 pg/ml, there was no obvious correlation between M-MDSC or G-MDSC and E2 (Supplemental Figures 1A,B).
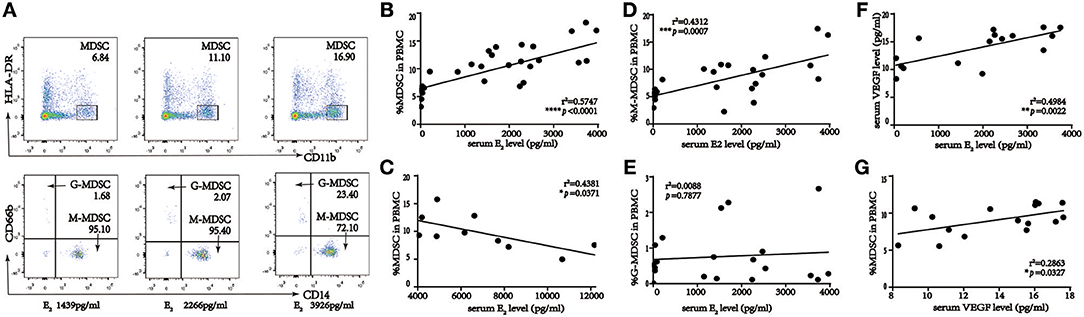
Figure 2. Percent of MDSC in the course of IVF patients, and statistical analysis of correlation among percentage of MDSC in PBMC, serum E2 and VEGF level in IVF patients. (A) Gating strategy of MDSC by flow cytometric analysis. HLA-DR−/low and CD11b+ cells were first gated from live PBMC for MDSC. The expression of cell surface markers CD14+ and CD66b+ on this population was evaluated further for M-MDSC and G-MDSC subsequently. (B) Correlation between percentage of MDSC in PBMC and serum E2 level when serum E2 level < 4,000 pg/ml (r2 = 0.5747, ****p < 0.0001, n = 40). (C) Correlation between percentage of MDSC in PBMC and serum E2 level when serum E2 level more than 4,000 pg/ml (r2 = 0.4381, *p = 0.0371, n = 10). (D) Correlation between percentage of M-MDSC in PBMC and serum E2 level when serum E2 level < 4,000 pg/ml (r2 = 0.4312, ***p = 0.0007, n = 40). (E) Correlation between percentage of G-MDSC in PBMC and serum E2 level when serum E2 level < 4,000 pg/ml (r2 = 0.0088, p = 0.7877, n = 40). (F) Correlation between serum VEGF and E2 level when serum E2 level < 4,000 pg/ml (r2 = 0.4984, **p = 0.0022, n = 16). (G) Correlation between percentage of MDSC in PBMC and serum VEGF level when serum E2 level < 4,000 pg/ml (r2 = 0.2863, *p = 0.0327, n = 16).
As E2 plays an important role in regulating VEGF in normal uterine tissues (25) and VEGF is associated with MDSC recruitment, VEGF levels were measured in the E2 < 4,000 pg/ml (n = 16) and E2 > 4,000 pg/ml (n = 7) groups separately to understand associations among E2, VEGF, and the percentage of circulating MDSC in patients receiving IVF (Figures 2F,G, Supplemental Figures 1C,D). Serum E2 levels were correlated positively and negatively with VEGF in the <4,000 and >4,000 pg/ml groups, respectively, whereas VEGF levels and MDSC ratios were positively correlated for both groups. This indicated that E2 might regulate MDSC accumulation via VEGF.
The S100 calcium-binding proteins A8 and A9 (S100A8 and S100A9), also known as migration inhibitory factor-related proteins 8 (MRP8) and 14 (MRP14), are abundantly expressed in myeloid cells (26). The function of S100A9 in myeloid cells is still controversial (27), and thus, we detected its expression both in vivo and in vitro (Supplemental Figures 1E,F). In serum, ELISA results demonstrated that there was no obvious change of S100A9 levels in 2groups. In vitro, we treated PBMC with 0–100 μM 17β-estradiol for 6 days and used qRT-PCR to confirm these results, S100A9 expression was elevated under estradiol treatment, however there was no dose-dependent tendency.
MDSC Suppress T Cell Responses in IVF Patients
The most important property of MDSC is their immunosuppressive activity (28). Therefore, we evaluated the effect of IVF-derived MDSC on T cell responses. MDSC were depleted from PBMC by flow cytometric sorting, after which the PBMC were stimulated with anti-CD3/CD28 antibodies. Compared to that with total PBMC, the results showed that the proliferation of both CD3+CD4+ and CD3+CD8+ T cells were enhanced significantly by MDSC depletion when serum E2 levels were more and less than 4,000 pg/ml, especially when more than 4,000 pg/ml (Supplemental Figure 2).
17β-Estradiol Augments MDSC in vitro
To confirm the relationship between E2 and MDSC, we treated PBMC with 0–100 μM 17β-estradiol and cell subsets were detected by a flow cytometric assay. Previous studies have reported that lymphocytes varied with E2 treatment, with CD4+ T cells elevated and CD8+ T cells decreased (29–31). In vitro culture for 6 days led to alterations in PBMC subsets, and the morphology of M-MDSC was similar to that of monocytes, whereas G-MDSC was similar to neutrophils. Accordingly, based on in vitro analysis, we also demonstrated MDSC ratios in PBMC without lymphocytes (Figure 3A, Supplemental Figure 3) and MDSC percentage in PBMC (Figure 3B). The results showed that 17β-estradiol augmented MDSC numbers in vitro. Further, statistical analysis revealed that this increase was significant among the groups. At the same time, VEGF levels in the supernatant were enhanced obviously, especially when the concentration of 17β-estradiol was 40 μM (Figure 3C).
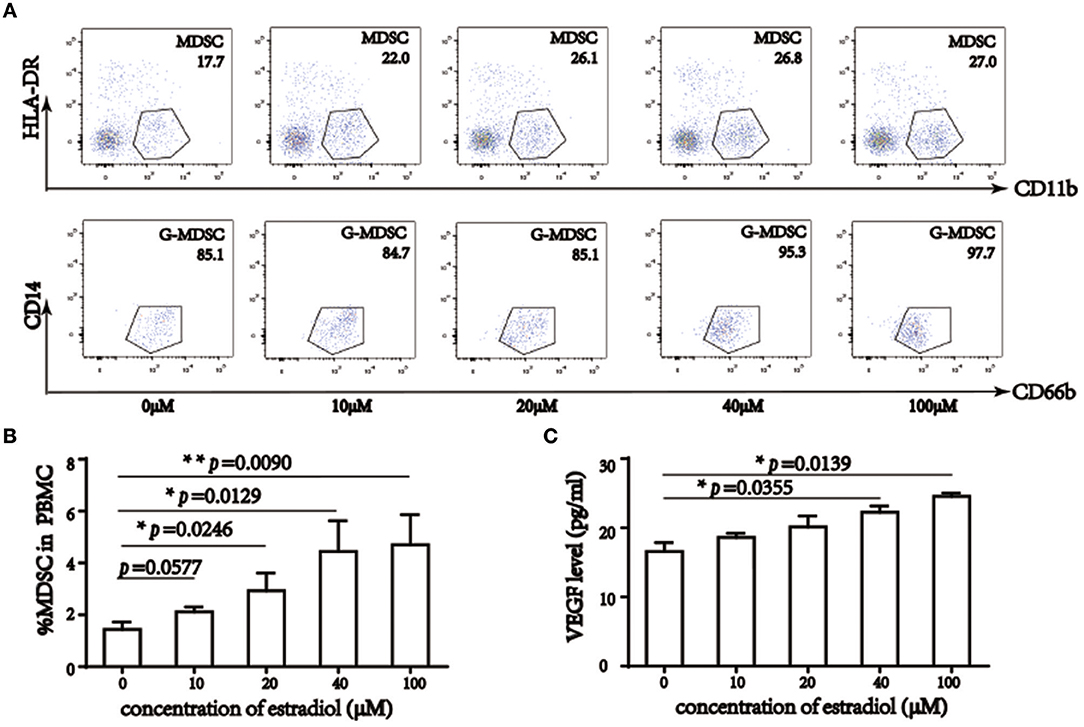
Figure 3. 17β-estradiol induced MDSC augment and elevated VEGF level in vitro. (A) Flow cytometry analysis showed that percentage of MDSC in PBMC without lymphocyte, 17β-estradiol induced MDSC augment in a dose dependent manner. (B) Qualification of MDSC in total PBMC. (C) VEGF level in liquid supernatant under 17β-estradiol treatment. *p <0.05, **p < 0.01, ***p < 0.001.
MDSC as a Predicter of IVF Outcome
We next verified that MDSC ratios were predictive of IVF outcome independently; in the estrogen-primed FET patients, after transferring two good-quality embryos to each patient, we tested E2 and MDSC levels on the day of FET. ROC analysis (AUC = 0.859) comparing the percentage of MDSC in the peripheral blood between the clinically pregnant and non-clinically pregnant groups was performed (Figure 4). The optimal cut-off value proposed by ROC analysis for MDSC was 8.22%. Study patients were further divided into two groups based on this value as follows: MDSC ≥ 8.22% group (n = 9) and MDSC < 8.22% group (n = 7). There were no significant differences between the two groups regarding baseline characteristics; however, the clinical pregnancy rate in the MDSC ≥ 8.22% group was higher than that in the <8.22% group (77.78 vs. 14.29%, p < 0.05; Table 3). An increase in MDSC (≥8.22%) in the peripheral blood was also predictive of better IVF treatment outcome (95% CI: 0.677–1.00, *p = 0.016), with a sensitivity of 87.5% and a specificity of 75.0%.
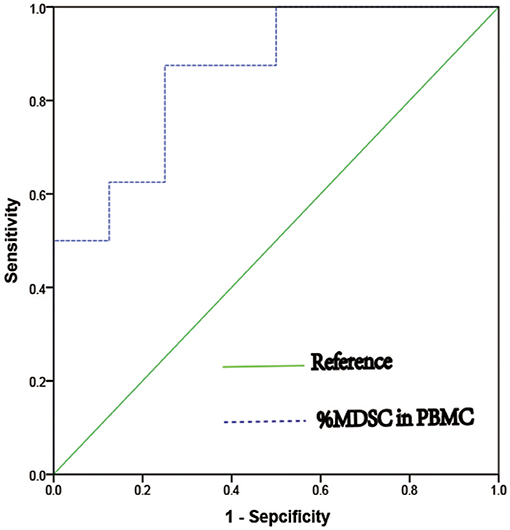
Figure 4. ROC curve of percentage of MDSC in PBMC as a predict marker of clinical pregnancy in women who undergoing IVF treatment (AUC = 0.859, 95%CI = 0.677 – 1.000, *p = 0.016).
Bioinformatic Analysis
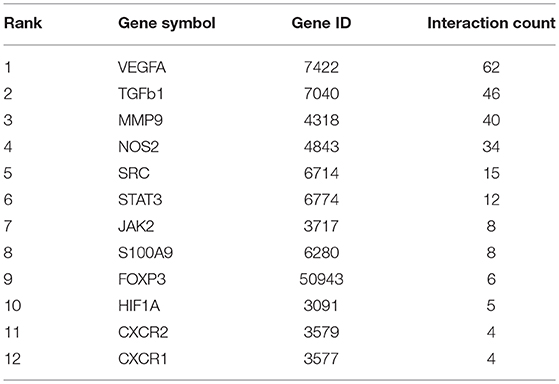
To further investigate and verify the mechanism associated with these interactions, we used bioinformatics software. CTD (The Comparative Toxicogenomics database) and the Coremine database can identify potential target proteins based on chemical–protein interaction analysis of small molecules. Both are powerful tools for computational target fishing. By utilizing CTD (http://ctdbase.org/) and COREMINE databases (http://www.coremine.com/medical/), we found 6,921 target genes related to E2 and 5,528 target genes associated with VEGF. Analysis revealed that ARG1, IDO1, and NOS2 are considered functional genes for MDSC, whereas IL-6, IL-1A, IL-2, IL-17A, and FOXP3 are cytokines associated with T cells, which can be affected by MDSC. Further, results indicated that TGF1, HIF1A, JAK2, SRC, STAT3, COX2, GM-CSF, S100A8, and S100A9 might be involved in MDSC enrichment and accumulation, and that CXCR1 and CXCR2 could affect MDSC recruitment. Among these target genes, 12 common genes were identified based on a Venn diagram (Figure 5A, Table 4), in accordance with our previous experiment on VEGF and S100A9.
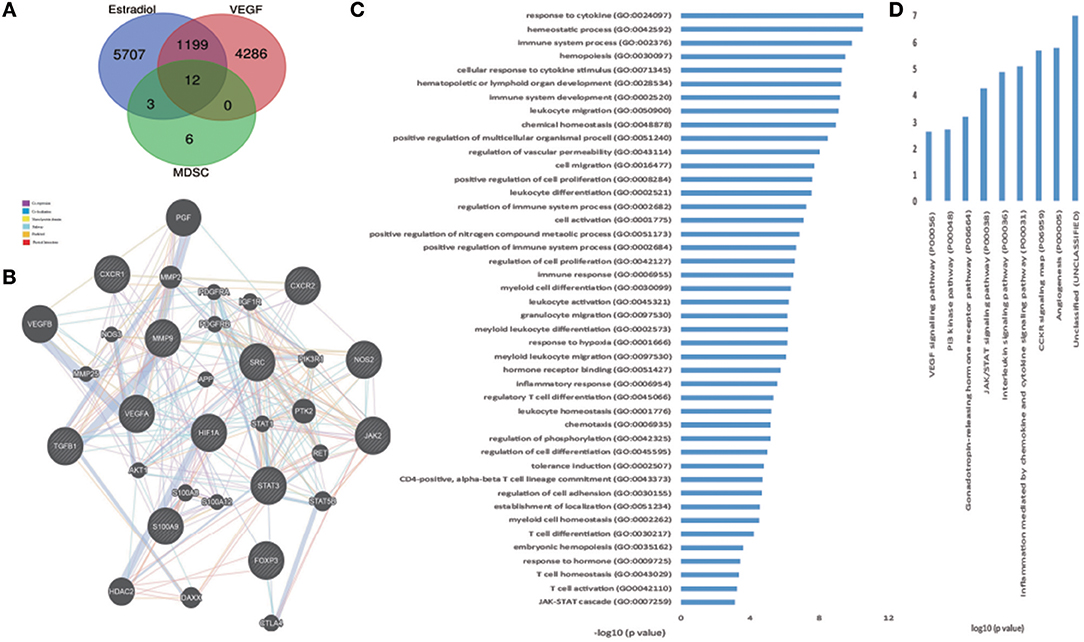
Figure 5. Hub genes of estradiol, VEGF, and MDSC, and bioinformatic analysis of the genes. (A) Venn diagrams of estradiol, VEGF, and functional genes in MDSC as hub genes. (B) Network of hub genes. Black protein nodes indicate target proteins, and different connecting colors represent different correlations. Functional association of targets was analyzed using GeneMANIA. Genes in black circles were submitted as query terms in searches. Gray circles indicate genes associated with query genes. (C) GO analysis of targets. The y-axis shows significantly enriched biological process categories of the targets, and the x-axis shows the enrichment scores of these terms (*p < 0.05). (D) Pathway analysis of targets. The y-axis shows significantly enriched pathway of the targets, and the x-axis shows the enrichment scores of these terms (*p < 0.05).
GeneMANIA revealed interacting proteins that are functionally similar to the 12 genes; 39.26% had matching co-expression characteristics, 34.02% had similar co-localization, 15.95% shared identical protein domains, 7.78% were involved in the same pathways, and 1.46% displayed similar physical interactions (Figure 5B).
To access probable signaling mechanisms, an analysis of interaction networks by DAVID showed that 93.44% of genes were enriched in biological processes such as immunological activities (GO:0002376), regulation of vascular permeability (GO:0043114), myeloid cell differentiation (GO:0030099), myeloid leukocyte migration (GO:0097529), and hormone receptor binding (GO:0051427) (Figure 5C). In addition, pathway analysis by DAVID showed that the targets were involved in angiogenesis (P00005), CCKR signaling map (P06959), JAK/STAT (P00038), and PI3K pathway (P00048) (Figure 5D). Further, based on target fishing and pathway analysis, an entire network was constructed using Cytoscape 3.0 (Figure 6).
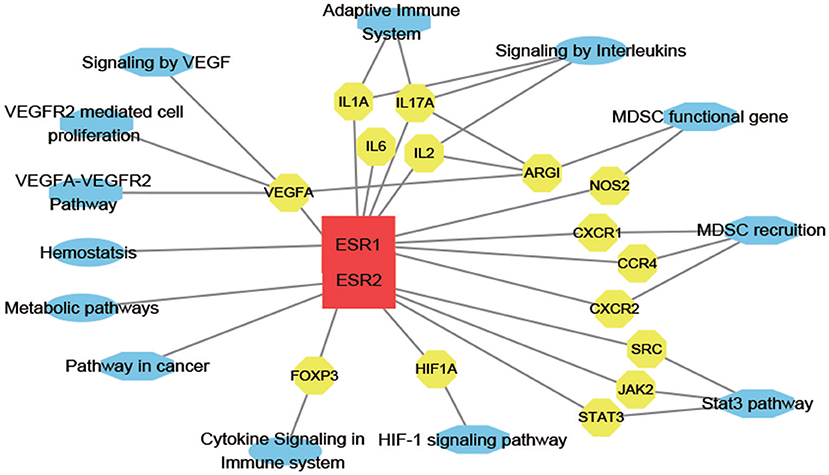
Figure 6. E2-target-pathway network. The interaction network has 30 nodes and 39 edges. The red square, yellow circle, and blue ellips, respectively, correspond to E2, target proteins and pathways.
Discussion
Failed embryo implantation is one of the major limiting factors of successful IVF. Despite advances in techniques, there is an enduring problem of recurrent implantation failure (32). Successful implantation is dependent on the quality of embryos, receptivity of the endometrium, the influence of ovarian hormones, and the balance of the immune microenvironment (32, 33). The regulatory milieu of genes encoding processes like cell adhesion, cell differentiation, cell proliferation, angiogenesis, responses to stress, and signal transduction are considered critical for successful implantation (34). In addition, the interplay between anti- and pro-inflammatory and immune responses plays an important role in the acceptance of semi-allogenic embryos, as well as the establishment of a favorable environment for endometrium receptivity (35).
Circulating E2 changes in IVF patients undergoing COS correspond to the time of stimulation and the number of oocytes. Serum E2 levels influence not only the receptivity of the endomembrane during the implantation window, but also the development and activation of immune cells; in a sense, it can predict pregnancy outcome. Further, E2 signaling accelerates the progression of ovarian cancer by mobilizing MDSC and enhancing their intrinsic immunosuppressive activity, both in vivo and in vitro (36). However, it was not clear how E2 levels affect peripheral blood MDSC during IVF and if this could modulate IVF outcome.
Our results showed that with E2 < 4,000 pg/ml, with a lower incidence of ovarian hyperstimulation (OH), the percentage of MDSC correlated positively with both serum E2 and VEGF levels; in vitro experiments corroborated these results. Thus, we postulated that E2 might drive MDSC recruitment and augment VEGF levels. Growing evidence implicates E2 as a fundamental mediator of inflammation, which also affects VEGF and MDSC functions. During pregnancy, E2 influences helper CD4+ T cell differentiation favoring humoral Th2 over cell-mediated Th1 responses to protect the infant from maternal immune responses (37, 38). E2 also regulates VEGF in normal uterine tissues (25) and VEGFR1 and VEGFR2, expressed by MDSC, are crucial for MDSC recruitment and accumulation in a mouse model (39). Further, VEGFA knockdown in tumor cells results in decreased MDSC infiltration (19), indicating an indispensable role for VEFG in MDSC regulation. In addition, VEGF is a key regulator of angiogenesis and vascular function (18) at the time of receptivity and blastocyst implantation and for stromal decidualization (40). Moreover, it has been confirmed that in the tumor microenvironment, elevated E2 levels augment and enhance the immune-suppressive functions of MDSC via the IL-6–JAK2–STAT3 pathway (36). Recently, Verma et al. showed that in miscarriage patients, lower E2 and progesterone levels can result in STAT3 downregulation and lower MDSC proportion, which leads to a breakdown of maternal–infant tolerance (41). However, it is not clear how E2 levels affect MDSC and VEGF to alter the prognosis of IVF treatment.
It has been shown that in the absence of uterine VEGF, the uterus is not receptive to implantation (42). However, recent studies have shown a decrease in total VEGF in OH pregnancies (25, 43), indicating that OH disturbs VEGF and thus vascular permeability and decidualization (44). In line with previous studies, we found that at >4,000 pg/ml, E2 concentration was negatively related to VEGF and MDSC percentage in PBMC. In tumor environment, suppressive function of MDSC has been confirmed (28). Moreover, it has been verified that the suppressive function of MDSC can be enhance by E2 treatment, and as a dose-dependent manner (36), accordance with our study. Recently, several reports have provided evidence that S100A9 can exert anti-inflammatory effects by inducing the migration of MDSC in tumor-bearing individuals and autoimmune diseases (8, 45–47). Although a previous study demonstrated that S100A8 levels are elevated during the proliferative phase of the menstrual cycle, but decreased during the secretory phase, indicating that its expression is regulated by estrogen and that it can prevent overactivated inflammatory responses for successful implantation (48). However, our result showed no obvious correlation between S100A9 and E2 levels. This is possibly because VEGF has been described to cause an accumulation of MDSC, whereas S100A9 has only been shown to regulate their migration (27).
In healthy adults, no more than 5% MDSC is present among PBMC (49). However, a recent study observed the significant expansion of MDSC in the peripheral blood of pregnant women, which suppressed the T cells response (27, 50), indicating that MDSC might play a vital role in the maintenance of maternal–fetal tolerance. To verify whether the percentage of MDSC is predictive of IVF outcome, multiple logistic regression analysis was used to establish an independent predictive factor related to clinical pregnancy in this study. We found that MDSC levels comprised an independent predictive factor of clinical pregnancy, whereas the other tested parameters had no effect on IVF outcome for this cohort. Further statistical analysis was performed based on the percentage of MDSC above and below 8.22% to examine other variables, and demonstrated that high levels of MDSC in the peripheral blood are a better predictor of IVF outcome. One study demonstrated that G-MDSC are predictive of IVF clinical pregnancy (51), contradictory to the results of the present study. Based on our preliminary experiments (49, 52), blood sample treatment could play a crucial role in flow cytometric analysis, because there is still no effective marker to distinguish G-MDSC from neutrophils (53).
Bioinformatics analysis revealed 12 genes common among E2, VEGF, and MDSC that were mostly enriched in homeostatic and immune processes and correlated with myeloid cell differentiation and migration and also associated with hormone receptor binding and cell migration, adhesion, and localization-critical processes during embryo implantation. A drug-target association network was also generated by GO and pathway analysis. These results indicated that E2 has multiple pharmacological functions including immune regulation. Although further studies are necessary to confirm and assess these precise interactions, this study provides a systematic and visual overview of possible E2-mediated molecular signaling pathways involved in mitigating IVF failure.
Gene ontology, pathway analysis, and enrichment mapping of the endomembrane from COS patients and healthy controls previously revealed the significant dysfunction of immunological and inflammatory responses upon implantation failure (34). Moreover, the activation of a pro-inflammatory immune response, which is a requisite for a receptive endometrium, could be misdiagnosed leading to corticosteroid treatment to suppress the immune response, and resulting in IVF failure (54).
Conclusion
In summary, we demonstrated that maternal–fetal interface immune tolerance is influenced by circulating E2 levels, which in turn regulates the MDSC population through VEGF and affects IVF outcomes. Further investigation of these important results will advance our understanding of the clinical application of MDSC as predictors of immune-related miscarriage and IVF failure.
Data Availability
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
Ethics Statement
The Ethics Review Boards of the First Hospital of Jilin University (No. 2018-069) and the Jilin Province People's Hospital (No.2018-Y-005) approved this research. Study participants and/or their legal guardians provided written informed consent.
Author Contributions
CH and YZ: concepted the analysis and wrote the manuscript. BP: data collection and statistical analysis of the data. XL: involved in patient's treatment and review of manuscript. HY: funding acquisition, supervision of the study concept, and review of manuscript.
Funding
The research was supported by grant from NSFC (81671592), the Science and Technology Department of Jilin Province (20180101110JC, 20190201140JC).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2019.00521/full#supplementary-material
Supplemental Figure 1. Statistical analysis of correlation among percentage of MDSC in PBMC, E2, S100A9 and VEGF level. (A) Correlation between percentage of G-MDSC in PBMC and serum E2 level when serum E2 level more than 4,000 pg/ml (n = 10). (B) Correlation between percentage of M-MDSC in PBMC and serum E2 level when serum E2 level more than 4,000 pg/ml (n = 10). (C) Correlation between serum VEGF and E2 level when serum E2 level more than 4,000 pg/ml (r2 = 0.77, **p = 0.0094, n = 7). (D) Correlation between percentage of MDSC in PBMC and serum VEGF level when serum E2 level more than 4,000 pg/ml (r2 = 0.5154, p = 0.0693, n = 7). (E) Serum S100A9 level in E2 level less and more than 4,000 pg/ml, respectively (p = 0.5821, n = 23). (F) Relative expression of S100A9 under different estradiol treatment.
Supplemental Figure 2. MDSC suppress T cell responses in IVF patients. (A) Proliferation of CD3+CD4+ T cells and CD3+CD8+ T cells in PBMC or PBMC without MDSC from peripheral blood of IVF patients. (B) Analysis of CD3+CD4+ T cells and CD3+CD8+ T cells proliferation in control, PBMC and PBMC without MDSC with different serum E2 level. (C) Statistical analysis of groups when serum E2 level less than 4,000 pg/ml. (D) Statistical analysis of groups when serum E2 level more than 4,000 pg/ml.
Supplemental Figure 3. 17β-estradiol induced MDSC augment and elevated VEGF level in vitro. Flow cytometry analysis showed that percentage of MDSC in PBMC, 17β-estradiol induced MDSC augment in a dose dependent manner.
References
1. Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat Med. (2013) 19:548–56. doi: 10.1038/nm.3160
2. Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol. (2013) 31:387–411. doi: 10.1146/annurev-immunol-032712-100003
3. Wang Y, Liu Y, Shu C, Wan J, Shan Y, Zhi X, et al. Inhibition of pregnancy-associated granulocytic myeloid-derived suppressor cell expansion and arginase-1 production in preeclampsia. J Reprod Immunol. (2018) 127:48–54. doi: 10.1016/j.jri.2018.05.002
4. Noronha LE, Antczak DF. Maternal immune responses to trophoblast: the contribution of the horse to pregnancy immunology. Am J Reprod Immunol. (2010) 64:231–44. doi: 10.1111/j.1600-0897.2010.00895.x
5. Wu R, Fujii S, Ryan NK, Van der Hoek KH, Jasper MJ, Sini I, et al. Ovarian leukocyte distribution and cytokine/chemokine mRNA expression in follicular fluid cells in women with polycystic ovary syndrome. Hum Reprod. (2007) 22:527–35. doi: 10.1093/humrep/del371
6. Daan NM, Koster MP, de Wilde MA, Dalmeijer GW, Evelein AM, Fauser BC, et al. Biomarker profiles in women with PCOS and PCOS offspring; a pilot study. PLoS ONE. (2016) 11:e0165033. doi: 10.1371/journal.pone.0165033
7. Lissauer D, Piper K, Goodyear O, Kilby MD, Moss PA. Fetal-specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J Immunol. (2012) 189:1072–80. doi: 10.4049/jimmunol.1200544
8. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. (2009) 9:162–74. doi: 10.1038/nri2506
9. Rieber N, Gille C, Köstlin N, Schäfer I, Spring B, Ost M, et al. Neutrophilic myeloid-derived suppressor cells in cord blood modulate innate and adaptive immune responses. Clin Exp Immunol. (2013) 174:45–52. doi: 10.1111/cei.12143
10. Montero AJ, Diazmontero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: a clinical perspective. J Immunother. (2015) 35:107–15. doi: 10.1097/CJI.0b013e318242169f
11. Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. (2011) 118:2254. doi: 10.1182/blood-2010-12-325753
12. Condamine T, Ramachandran I, Youn JI, Gabrilovich DI. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med. (2015) 66:97–110. doi: 10.1146/annurev-med-051013-052304
13. Serafini P. Myeloid derived suppressor cells in physiological and pathological conditions: the good, the bad, and the ugly. Immunol Res. (2013) 57:172–84. doi: 10.1007/s12026-013-8455-2
14. Kang X, Zhang X, Liu Z, Xu H, Wang T, He L, Zhao A. Granulocytic myeloid-derived suppressor cells maintain feto-maternal tolerance by inducing Foxp3 expression in CD4+CD25-T cells by activation of the TGF-β/β-catenin pathway. Mol Hum Reprod. (2016) 22:499–511. doi: 10.1093/molehr/gaw026
15. Wallace AE, Fraser R, Cartwright JE. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update. (2012) 18:458–71. doi: 10.1093/humupd/dms015
16. Fredette NC, Meyer MR, Prossnitz ER. Role of GPER in estrogen-dependent nitric oxide formation and vasodilation. J Steroid Biochem Mol Biol. (2018) 176:65–72. doi: 10.1016/j.jsbmb.2017.05.006
17. Nair RR, Sinha P, Khanna A, Singh K. Reduced myeloid-derived suppressor cells in the blood and endometrium is associated with early miscarriage. Am J Reprod Immunol. (2015) 73:479–86. doi: 10.1111/aji.12351
18. Zhang L, Xiong W, Xiong Y, Liu H, Liu Y. 17 β-estradiol promotes vascular endothelial growth factor expression via the Wnt/β-catenin pathway during the pathogenesis of endometriosis. Mol Hum Reprod. (2016) 22:526–35. doi: 10.1093/molehr/gaw025
19. Horikawa N, Abiko K, Matsumura N, Hamanishi J, Baba T, Yamaguchi K, et al. Expression of vascular endothelial growth factor in ovarian cancer inhibits tumor immunity through the accumulation of myeloid-derived suppressor cells. Clin Cancer Res. (2017) 23:587–99. doi: 10.1158/1078-0432.CCR-16-0387
20. Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. (2018) 9:978. doi: 10.3389/fimmu.2018.00978
21. Pereira N, Elias RT, Christos PJ, Petrini AC, Hancock K, Lekovich JP, et al. Supraphysiologic estradiol is an independent predictor of low birth weight in full-term singletons born after fresh embryo transfer. Hum Reprod. (2017) 32:1410–7. doi: 10.1093/humrep/dex095
22. Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. (2010) 38(Web Server issue):W214–20. doi: 10.1093/nar/gkq537
23. Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. (2009) 4:44–57. doi: 10.1038/nprot.2008.211
24. Collins GS, Reitsma JB, Altman DG, Moons KGM, Members of the Tg. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Eur Urol. (2015) 67:1142–51. doi: 10.1016/j.eururo.2014.11.025
25. Junovich G, Mayer Y, Azpiroz A, Daher S, Iglesias A, Zylverstein C, et al. Ovarian stimulation affects the levels of regulatory endometrial NK cells and angiogenic cytokine VEGF. Am J Reprod Immunol. (2011) 65:146–53. doi: 10.1111/j.1600-0897.2010.00892.x
26. Zackular JP, Chazin WJ, Skaar EP. Nutritional immunity: S100 proteins at the host-pathogen interface. J Biol Chem. (2015) 290:18991–8. doi: 10.1074/jbc.R115.645085
27. Yang J, Anholts J, Kolbe U, Stegehuis-Kamp JA, Claas FHJ, Eikmans M. Calcium-binding proteins S100A8 and S100A9: investigation of their immune regulatory effect in myeloid cells. Int J Mol Sci. (2018) 19:E1833. doi: 10.3390/ijms19071833
28. Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. (2010) 70:68–77. doi: 10.1158/0008-5472.CAN-09-2587
29. Shao MJ, Zhu YJ, Qiu YE, Hu M, He YQ. Changes in the level of immunoglobulins and CD4/CD8 ratio in young and aged mice with estradiol deficiency. Immunol Invest. (2017) 46:305–13. doi: 10.1080/08820139.2016.1267203
30. Obremski K, Wojtacha P, Podlasz P, Zmigrodzka M. The influence of experimental administration of low zearalenone doses on the expression of Th1 and Th2 cytokines and on selected subpopulations of lymphocytes in intestinal lymph nodes. Pol J Vet Sci. (2015) 18:489–97. doi: 10.1515/pjvs-2015-0064
31. Mohammad I, Starskaia I, Nagy T, Guo J, Yatkin E, Väänänen K, et al. Estrogen receptor α contributes to T cell-mediated autoimmune inflammation by promoting T cell activation and proliferation. Sci Signal. (2018) 11:eaap9415. doi: 10.1126/scisignal.aap9415.
32. Agarwal A, Safi J, Aziz N, Ly KD. Evidence-based management of infertile couples with repeated implantation failure following IVF. Curr Womens Health Rev. (2010) 6:200–18. doi: 10.2174/157340410792007073
33. Koot YE, Teklenburg G, Salker MS, Brosens JJ, Macklon NS. Molecular aspects of implantation failure. Biochim Biophys Acta. (2012) 1822:1943–50. doi: 10.1016/j.bbadis.2012.05.017
34. Pathare ADS, Zaveri K, Hinduja I. Downregulation of genes related to immune and inflammatory response in IVF implantation failure cases under controlled ovarian stimulation. Am J Reprod Immunol. (2017) 78:e12679. doi: 10.1111/aji.12679
35. Robertson SA, Moldenhauer LM. Immunological determinants of implantation success. Int J Dev Biol. (2014) 58:205–17. doi: 10.1387/ijdb.140096sr
36. Svoronos N, Perales-Puchalt A, Allegrezza MJ, Rutkowski MR, Payne KK, Tesone AJ, et al. Tumor cell-independent estrogen signaling drives disease progression through mobilization of myeloid-derived suppressor cells. Cancer Discov. (2017) 7:72–85. doi: 10.1158/2159-8290.CD-16-0502
37. Gnainsky Y, Granot I, Aldo P, Barash A, Or Y, Mor G, et al. Biopsy-induced inflammatory conditions improve endometrial receptivity: the mechanism of action. Reproduction. (2015) 149:75–85. doi: 10.1530/REP-14-0395
38. Salem ML. Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr Drug Targets Inflamm Allergy. (2004) 3:97–104. doi: 10.2174/1568010043483944
39. Huang Y, Chen X, Dikov MM, Novitskiy SV, Mosse CA, Yang L, et al. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood. (2007) 110:624–31. doi: 10.1182/blood-2007-01-065714
40. Biyiksiz PC, Filiz S, Vural B. Is sildenafil citrate affect endometrial receptivity? an immunohistochemical study. Gynecol Endocrinol. (2011) 27:767–74. doi: 10.3109/09513590.2010.540601
41. Verma P, Verma R, Nair RR, Budhwar S, Khanna A, Agrawal NR, et al. Altered crosstalk of estradiol and progesterone with Myeloid-derived suppressor cells and Th1/Th2 cytokines in early miscarriage is associated with early breakdown of maternal-fetal tolerance. Am J Reprod Immunol. (2019) 81:e13081. doi: 10.1111/aji.13081
42. Rabbani ML, Rogers PA. Role of vascular endothelial growth factor in endometrial vascular events before implantation in rats. Reproduction. (2001) 122:85–90. doi: 10.1530/rep.0.1220085
43. Danastas K, Whittington CM, Dowland SN, Combes V, Murphy CR, Lindsay LA. Ovarian hyperstimulation reduces vascular endothelial growth factor-A during uterine receptivity. Reprod Sci. (2019) 26:259–68. doi: 10.1177/1933719118768703
44. Okada H, Tsuzuki T, Shindoh H, Nishigaki A, Yasuda K, Kanzaki H. Regulation of decidualization and angiogenesis in the human endometrium: mini review. J Obstet Gynaecol Res. (2014) 40:1180–7. doi: 10.1111/jog.12392
45. Otsuka K, Terasaki F, Ikemoto M, Fujita S, Tsukada B, Katashima T, et al. Suppression of inflammation in rat autoimmune myocarditis by S100A8/A9 through modulation of the proinflammatory cytokine network. Eur J Heart Fail. (2009) 11:229–37. doi: 10.1093/eurjhf/hfn049
46. Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. (2008) 181:4666–75. doi: 10.4049/jimmunol.181.7.4666
47. Zhao F, Hoechst B, Duffy A, Gamrekelashvili J, Fioravanti S, Manns MP, et al. S100A9 a new marker for monocytic human myeloid-derived suppressor cells. Immunology. (2012) 136:176–83. doi: 10.1111/j.1365-2567.2012.03566.x
48. Nair RR, Khanna A, Singh K. Association of increased S100A8 serum protein with early pregnancy loss. Am J Reprod Immunol. (2015) 73:91–4. doi: 10.1111/aji.12318
49. Wu H, Zhen Y, Ma Z, Li H, Yu J, Xu ZG, et al. Arginase-1-dependent promotion of TH17 differentiation and disease progression by MDSCs in systemic lupus erythematosus. Sci Transl Med. (2016) 8:331ra40. doi: 10.1126/scitranslmed.aae0482
50. Pan T, Liu Y, Zhong LM, Shi MH, Duan XB, Wu K, et al. Myeloid-derived suppressor cells are essential for maintaining feto-maternal immunotolerance via STAT3 signaling in mice. J Leukoc Biol. (2016) 100:499–511. doi: 10.1189/jlb.1A1015-481RR
51. Zhu M, Huang X, Yi S, Sun H, Zhou J. High granulocytic myeloid-derived suppressor cell levels in the peripheral blood predict a better IVF treatment outcome. J Matern Fetal Neonatal Med. (2019) 32:1092–7. doi: 10.1080/14767058.2017.1400002
52. Yi H, Guo C, Yu X, Zuo D, Wang XY. Mouse CD11b+Gr-1+ myeloid cells can promote Th17 cell differentiation and experimental autoimmune encephalomyelitis. J Immunol. (2012) 189:4295–304. doi: 10.4049/jimmunol.1200086
53. Veglia F, Tyurin VA, Blasi M, De Leo A, Kossenkov AV, Donthireddy L, et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature. (2019) 569:73–8. doi: 10.1038/s41586-019-1118-2
Keywords: estradiol, VEGF, MDSC, protein–protein interactions, IVF
Citation: Hu C, Zhen Y, Pang B, Lin X and Yi H (2019) Myeloid-Derived Suppressor Cells Are Regulated by Estradiol and Are a Predictive Marker for IVF Outcome. Front. Endocrinol. 10:521. doi: 10.3389/fendo.2019.00521
Received: 28 April 2019; Accepted: 16 July 2019;
Published: 30 July 2019.
Edited by:
Elke Winterhager, University of Duisburg-Essen, GermanyReviewed by:
Rohini R. Nair, Weizmann Institute of Science, IsraelEllis Fok, The Chinese University of Hong Kong, China
Copyright © 2019 Hu, Zhen, Pang, Lin and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huanfa Yi, eWlodWFuZmFAamx1LmVkdS5jbg==
†These authors have contributed equally to this work
 Cong Hu
Cong Hu Yu Zhen3†
Yu Zhen3† Huanfa Yi
Huanfa Yi