
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 24 July 2019
Sec. Thyroid Endocrinology
Volume 10 - 2019 | https://doi.org/10.3389/fendo.2019.00477
This article is part of the Research Topic Combination Therapy for Hypothyroidism View all 17 articles
Background: The standard of care in management of hypothyroidism is treatment with levothyroxine (L-T4). Sometimes patients are dissatisfied with L-T4 and the combination of levo-triiodothyronine (L-T3) with L-T4 is considered.
Methods: We performed a systematic review and meta-analysis of blinded randomized controlled trials (RCTs), reporting how often hypothyroid patients prefer combination L-T3/L-T4 treatment to L-T4 alone. We also explored for explanatory factors for combination therapy preference in sensitivity analyses examining trial, patient, and disease characteristics. Potential dose-response relationships were explored using meta-regression analyses. We searched 9 electronic databases (from inception until February, 2019), supplemented with a hand-search. Two reviewers independently screened abstracts and citations and reviewed full-text papers, with consensus achieved on the included studies. Two reviewers independently critically appraised the quality of included studies and abstracted the data. Random effects meta-analyses were reported for the percentage of patients preferring combination L-T3/T-T4 therapy over L-T4 alone. A binomial distribution of choices (i.e., preference of combination therapy or no preference for combination therapy) was assumed.
Results: We included 7 blinded RCTs including 348 hypothyroid individuals in the primary meta-analysis. The pooled prevalence rate for preference of combination therapy over L-T4 was 46.2% (95% confidence interval 40.2%, 52.4%) (p = 0.231 for the difference from chance). There was no significant statistical heterogeneity among study results (Q = 7.32, degrees of freedom = 6, p = 0.293, I2 = 18.0%). In sensitivity analyses, combination treatment preference was explained in part by treatment effects on TSH concentration, mood and symptoms, but not quality of life nor body weight. In a secondary dose-response meta-regression analyses, a statistically significant association of treatment preference was identified for total daily L-T3 dose, but not L-T3:L-T4 dose ratio.
Conclusions: In conclusion, in RCTs in which patients and investigators were blinded to treatment allocation, approximately half of participants reported preferring combination L-T3 and L-T4 therapy compared to L-T4 alone; this finding was not distinguishable from chance. An observed potential positive L-T3 dose effect on treatment preference deserves further study, with careful consideration of thyroid biochemical indices and patient reported outcomes.
Internationally, levothyroxine (L-T4) treatment is the established first choice as a standard of care in the management of hypothyroidism (1–7). However, some patients are dissatisfied with L-T4 standard care treatment (8). Factors contributing to dissatisfaction with thyroid hormone treatment include persistent hypothyroid symptoms, such as excess weight, fatigue, mood problems, or memory/cognitive concerns (8). In the clinical context of persistent symptoms after achieving a normalized thyroid stimulating hormone (TSH) concentration on L-T4, after excluding or managing other potential causes of symptoms, patients and clinicians sometimes consider utilizing alternative thyroid hormone preparations, such as combination therapy using L-T4 and levo-triiodothyronine (L-T3). The rationale for this approach would be normalizing potentially low tissue T3 levels, which are not readily measurable. We conducted a systematic review and meta-analysis, examining how often hypothyroid patients prefer L-T3/L-T4 combination therapy over L-T4 monotherapy. In order to minimize the risk of bias, we restricted our review to blinded randomized controlled trials (RCTs). We also explored for explanatory factors relating to patient preferences.
Our systematic review was registered (PROSPERO CRD42019123920). We included blinded RCTs, examining how often hypothyroid adult patients prefer combination L-T3/L-T4 therapy, compared to the standard of care of L-T4 monotherapy. Trial settings were restricted to be ambulatory outpatient clinics (i.e., not hospitalized patients) and participants were required to be aged ≥ 18 years of age, with hypothyroidism of any etiology. Trials were required to report some level of blinding, including blinding of study participants. All studies were required to have measured thyroid function in study participants using a thyroid stimulating hormone (TSH) measurement. Studies focusing on desiccated thyroid hormone were excluded. Due to limited resources for translation, only English language studies were included. For overlapping or duplicate studies reporting the same primary outcome, the largest study was included. As we expected strong reader interest in factors explaining patient preferences, we separately abstracted data from secondary explanatory analyses (such as deiodinase polymorphism status), which would typically be published in subsequent publications from the original studies. The explanatory data was not included in the meta-analysis of combination therapy preference rate.
An experienced library information specialist (RF) executed a comprehensive search strategy from inception to February 2019 in the following databases: MEDLINE, Ovid MEDLINE Epub Ahead of Print and In-Process and Other Non-Indexed Citations, Embase Classic + Embase, “Cochrane Central Register of Controlled Trials,” “Cochrane Database of Systematic Reviews,” Emcare, and PsycInfo all from the OvidSP platform; Web of Science from the Clarivate Analytic, and ClincalTrials.gov. We limited our search to adults (age ≥ 18 years) and the following types of studies: randomized controlled trials, systematic reviews, and meta-analyses. There was no language restriction on the search. Where available, both controlled vocabulary terms (“exploded” where applicable), and text words were used to identify as many relevant results as possible (Supplementary Data). We supplemented the electronic search by cross-referencing included papers, relevant sections of clinical practice guidelines, relevant systematic and narrative reviews, as well as reviewing the personal files of one of the authors who had participated in development of a hypothyroidism clinical practice guideline (AMS).
Two investigators (AA and AMS) independently, in duplicate, screened citations from the electronic search, reviewed full-text papers for inclusion, critically appraised the quality included studies, and abstracted the data. Consensus was achieved for inclusion of papers and abstracted data by discussion of reviewers; a third reviewer/clinical content expert (SE) was consulted in the event of any discrepancies that could not otherwise be resolved by reviewer discussion. We contacted the corresponding authors of original studies if there were questions relating to potential eligibility or results of studies or the results. The risk of bias of included trials was evaluated using the most current Risk of Bias evaluation tool developed by the Cochrane Collaboration (ROB 2.0) (9). The systematic review was reported according to PRISMA standards (10).
Descriptive data were summarized as numbers and percentages for categorical data and means or medians and standard deviations or ranges for continuous data. We performed random effects meta-analyses, estimating the percentage (with 95% confidence intervals, CI) of patients preferring combination L-T4 with L-T3 (any dose) over L-T4 monotherapy (Comprehensive Meta-Analysis software, version 2.0). A binomial distribution for preferring (or not preferring) combination therapy was assumed, such that a significant preference in combination therapy (beyond chance) would be defined by the lower limit of the 95% CI exceeding 50%. Individuals who preferred L-T4 or those who had no treatment preference, were judged to not prefer combination therapy. For the primary meta-analysis, treatment preference was evaluated only in individuals who had been exposed to L-T3 in a random fashion during the trial (i.e., individuals not receiving L-T3 at any point in the trial or those assigned L-T3 in non-random fashion, were not included). We evaluated for statistical heterogeneity in the meta-analysis using a Cochrane's Q (chi-squared) test (11) and an I2 estimate (12). We planned to evaluate for potential publication bias using a funnel plot (13), assuming at least 10 studies were included in the meta-analysis (for meaningful interpretation). We planned to explore for sources of heterogeneity of combination therapy treatment preference by performing the following sensitivity analyses (examining for difference in treatment effects among studies according to study characteristics): (a) study characteristics (study quality, study drug treatment duration [3 months or less, or longer than 3 months]), (b) participant characteristics (mean or median age <50 years or ≥ 50 years], sex [inclusion of any men], molecular characteristics, treatment (frequency of daily treatment dosing), other treatment effects (differential changes between groups on TSH concentration, quality of life, mood, psychological outcomes, or body weight), and disease characteristics (etiology of hypothyroidism, i.e., inclusion of any patients who had thyroidectomy or radioactive iodine treatment as an etiology for hypothyroidism). Fixed effects univariate meta-regression was performed to investigate for any dose-effect on thyroid hormone treatment preference, relating to study- and study subgroup- specific combination therapy dose, specifically: L-T3 total daily dose (ug) and L-T3:L-T4 ratio of daily dose. These variables were calculated for the hypothetical scenario of an individual receiving a baseline L-T4 dose of 100 ug daily, in order to account for differences in calculation of combination therapy dose among trials (i.e., dose ratios or fixed dose substitutions). We utilized the L-T3:L-T4 ratio (and not vice versa), to enable inclusion of data from individuals receiving a dosage of 0 ug of L-T3 (in the case of individuals randomized to an L-T4 arm, in a parallel design trial). Thus, in the case of parallel design randomized controlled trials, the L-T4 arm data (not exposed to combination therapy) was not included in the primary analysis of prevalence of combination therapy preference but would be eligible for inclusion in the secondary meta-regression dose analysis, where the outcome was study drug treatment preference (i.e., placebo compared to pre-trial L-T4 use in the case of parallel design trials or combination therapy compared to intra-trial L-T4 use for cross-over trials). We defined statistical significance of all analyses at an alpha level of 0.05; however, in examining for heterogeneity using Cochrane's Q test, we set that alpha level at 0.10 (11).
As detailed in our study flow diagram (Figure 1), we retrieved a total of 4,192 citations from our electronic searches, ultimately yielding 2,436 unique citations after removing any duplicates. References from the hand search were all included in the electronic database searches, so the hand search yielded no additional relevant papers. We reviewed 62 full-text papers for eligibility and there were 7 trials included in the systematic review and meta-analysis (14–20). Relevant data on secondary subgroup analyses relating to molecular data were reported in two additional publications for the trial of Appelhof et al. [original publication (14), secondary publications (21, 22)] as well as Nygaard et al. [original publication (18), secondary publication (23)]. The reviewed full-text papers excluded from this review, and the reasons for exclusion are shown in the Supplementary Table 1. We excluded some RCTs comparing combination therapy compared to levothyroxine for the following reasons: (a) no data on patient preference—Clyde et al. (24), Kaminski et al. (25), Saravanan et al. (26), Sawka et al. (27), Siegmund et al. (28), Valizadeh et al. (29), (b) no blinding—Fadayev et al. (30), and (c) no TSH measurement (so safety and appropriateness of thyroid hormone dosing could not be established)—Smith et al. (31).
A summary of the details of the RCTs included in both the systematic review and meta-analysis is shown in Table 1. Of the included studies, five were conducted in Europe (14–18), one was conducted in the United States (19), and one was conducted in Australia (20). The number of participants randomized ranged from 13 to 141 hypothyroid patients (14–20). The majority of participants in the studies were female, with two studies recruiting only females (16, 17). Furthermore, the mean age of participants was younger than 50 years in all of the studies (14–20). The etiology of hypothyroidism was autoimmune primary hypothyroidism in the majority of participants in 5/7 studies (14, 17–20). None of the studies included patients with secondary hypothyroidism due to hypothalamic/pituitary disease. Of the 6 studies reporting on recruitment setting (14–16, 18–20), 5 reported recruiting participants in ambulatory Endocrinology clinics (15, 16, 18–20), and one reported recruiting participants from primary care practices (14). One study used a parallel design (14), whereas the other 6 studies utilized a cross-over design (15–20). Pre-trial L-T4 dose was required to be stable for at least 2 months in one study (20), 3 months in 2 studies (15, 19), 6 months in 2 studies (14, 18), and 1 year in one study (17); there was no reported requirement for duration of pre-trial stability of levothyroxine dosage in one study of surgically-treated patients who had Graves disease (16). The study treatment periods ranged from 5 to 15 weeks (14–20). The details of combination therapy are shown in Table 1; only one study (14) reported twice daily administration of L-T3. A summary of the risk of bias of included trials is shown in Table 2.
In a random effects meta-analysis, the pooled prevalence rate for preference of combination therapy over L-T4 was 46.2% (95% CI 40.2%, 52.4%) (p = 0.231 for the difference from chance, using data from 7 trials including 348 hypothyroid individuals) (Figure 2). There was no significant statistical heterogeneity among study results (Q = 7.32, degrees of freedom [df ] = 6, p = 0.293, I2 = 18.0%). A funnel plot investigating for publication bias was not performed due to an insufficient number of studies for meaningful interpretation (i.e., fewer than 10 trials in the meta-analysis).
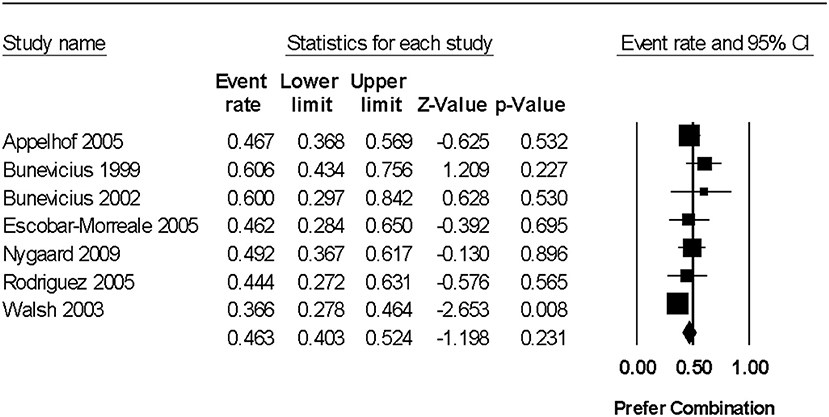
Figure 2. Forest plot from a random effects meta-analysis examining prevalence of preference of combination levo-triodothyronine (L-T3) and levothyroxine (L-T4) therapy over L-T4 alone. 95% CI, 95% confidence interval. For the studies reporting the number of individuals who had no preference (and thus were assumed to favor standard care), the rates were as follows: Bunevicius et al. (15)−33.3% (11/33), Bunevicius et al. (16)−20.0% (2/10), Nygaard et al. (18)−35.6% (21/59), Rodriguez et al. (19)−29.6% (8/27), and Walsh et al. (20)−17.8% (18/101). It is not known if the individuals with no preference were indifferent or indecisive (i.e., unable to make a decision).
In spite of the lack of statistically significant heterogeneity in our primary meta-analysis, we proceeded with planned sensitivity analyses, to explore for any potential differences in treatment benefits according to patient, study, and disease characteristics. We were not able to examine the impact of study duration as all of the included trials (14–20) were ≤ 3 months in duration; furthermore, we were not able to examine any potential impact of age, as the mean age of study participants was relatively young (<50 years of age) in all trials (14–20). In terms of study quality, there was no significant difference in combination therapy preference between 4 studies in which there were some concerns about selection bias (randomization/concealment of allocation) (15, 16, 18, 20) compared to 3 studies which were considered at low risk of bias for that variable (14, 17, 19) (between study heterogeneity Q = 0.027, df = 1, p = 0.871). There was also no significant difference between 5 studies that included some men (14, 15, 18–20), compared to two that included only women (16, 17) (Q = 0.196, df = 1, p = 0.658). Furthermore, there were no significant difference between 5 trials that included individuals who had a thyroidectomy or radioactive iodine treatment (15–17, 19, 20) compared to 2 trials that included only individuals with autoimmune primary hypothyroidism (14, 18) (Q = 0.083, df = 1, p = 0.773). In examining the effect of frequency of dosing of combination therapy, there was no significant difference between one study that utilized twice daily dosing (14) compared to the 6 other studies that utilized once daily dosing or did not report on dosing (assuming once daily dose) (15–20) (Q = 0, df = 1, p = 0.998). In examining whether trials with end of trial TSH differences between treatment groups were associated with differences in treatment preferences, a trend for a possible marginal association was observed. Specifically the preference rate for combination therapy was 38.6% (95% CI 30.5%, 47.4%) in the 2 studies where the TSH was significantly higher in the combination therapy group compared to the L-T4 group (17, 20), 46.7% (95% CI 36.8%, 56.9%) in one trial where TSH was reduced in the combination therapy group compared to the L-T4 group (14) and 51.9% (95% CI 43.2%, 60.4%) in the 4 trials where end of trial TSH was not significantly different between treatment groups (15, 16, 18, 19) (between group difference for categories of TSH differences, Q = 4.504, df = 2, p = 0.105). In summary, study quality (reflected by randomization/concealment of allocation method), inclusion of males, inclusion of individuals who had a thyroidectomy or radioactive iodine treatment, and frequency of combination therapy daily dosing, did not explain combination therapy preference; however a possible marginal relationship between end of study TSH differences and treatment preference was observed.
In order to investigate any relationship between dose and treatment preference over L-T4 monotherapy taken during or before the trial, the respective L-T3 and L-T4 total daily dosage, and the ratio of these two doses, was calculated for a hypothetical baseline L-T4 dosage of 100 ug/day, according to each respective trial protocol (Table 2). Respective fixed effects meta-regression analyses were performed (Figure 3). Data from the L-T4 monotherapy arm in the parallel design randomized trial of Appelhof et al. (14) was included in these analyses, assuming that the L-T4 baseline dosage was continued and the L-T3 dosage was 0 ug/day. Data from 7 trials of 396 participants [incorporating 3 subgroups from the trial by Appelhof et al. (14)] were used in the meta-regression analyses. There was a statistically significant positive association between total daily L-T3 dosage (which ranged from 0 to 20 ug/day) and treatment preference (slope regression model 0.043, 95% CI 0.007, 0.078, p = 0.020) (Figure 3A). However, there was no significant association of treatment preference with the L-T4:L-T3 ratio of 1:0 to 4:1) (slope 0.124, −0.489, 0.738, p = 0.691) (Figure 3B).
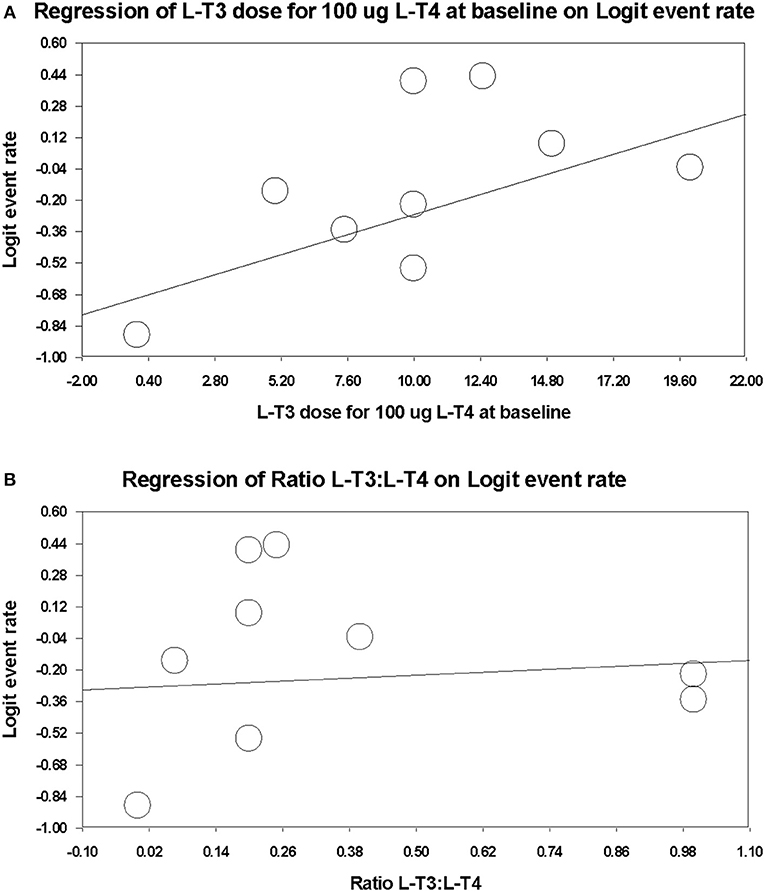
Figure 3. Meta-regression plots examining for any dose-response relationship between combination therapy dose and treatment preference over L-T4 monotherapy (L-T4 monotherapy during or before trial). (A) Total daily L-T3 dosage (ug) on combination therapy. (B) Ratio of L-T3 divided by L-T4 dosage (ug) on combination therapy.
We performed several sensitivity analyses of combination therapy preferences, where we grouped trials according to changes in other specific outcomes. Specifically, grouping studies that demonstrated differences between treatment groups for validated measures of quality of life, changes in body weight, mood, and symptoms. We found no significant difference in rate of preference for combination therapy in comparing one trial reporting improved quality of life with combination therapy (18) to 3 trials where there was no significant treatment group difference in any quality of life measure (14, 17, 20) (Q = 1.644, df = 2, p = 0.439). Furthermore, there was no significant difference in treatment preference rate in comparing one study in which body weight was statistically significantly reduced in the combination therapy group (14) to 5 other trials reporting no significant body weight difference (Q = 0.637, df = 1, p = 0.723). However, a marginally higher rate of preference for combination therapy (53.2%, 95% CI 42.9%, 63.2%) was observed in two trials reporting significant improvement in mood and symptoms (respectively) with combination treatment (15, 18), compared to 5 other trials where there was no significant difference between treatment groups in either measure (43.1%, 95% CI 37.1%, 49.2%) (14, 16–20) (between group difference Q = 2.762, df = 1, p = 0.097). In summary, the minority of trials reporting improvement in mood and symptoms, tended to report higher rates of combination therapy preference.
Although none of the primary reports of the trials in this systematic review included molecular biomarker data, given the importance of potential relationship between molecular characteristics of patients and treatment preference, reports of secondary publications from included trials were descriptively summarized. The methodologic quality of respective secondary analysis papers (21–23) was considered consistent with that of the original trials, so is not reported separately. In secondary analyses of original randomized trial data (14), Appelhof et al. compared rates of preference for combination therapy, according to genetic polymorphism status of type 2 deiodinase enzymes for Thr92Ala and ORFa-Gly3Asp (also known as rs12885300) (21). The prevalence rate of the Thr92Ala polymorphism among 141 trial participants was as follows: 74 (52%) heterozygous, 20 (14%) homozygous, and 47 (33%) wild type (21). The number of individuals and percentage with the ORFa-Gly3Asp polymorphism was: 52 (37%) heterozygous, 19 (13%) homozygous, and 70 (50%) wild type (21). Among the 92 patients who received combination therapy, no significant differences in rates of combination therapy preference were observed according to Thr92Ala polymorphism genotype (53% heterozygous, 39% homozygous, 41% wild type) nor ORFa-Gly3Asp genotype (49% heterozygous, 43% homozygous, and 46% wild type) (21). The authors concluded there was no association between D2 polymorphisms and well-being or subjective preference for combination treatment over L-T4 monotherapy (21). In another secondary analysis of the same original trial by Appelhof et al. (14), Van der Deure et al. (22) examined polymorphisms in OATP1C1 gene, encoding a protein capable of thyroid hormone transport into the brain. Genotyping was successfully executed in140/141 patients in the original trial (22). The prevalence of OATP1C1-intron3C>T, OATP1C1-Thr143 and OATP1C1-3035T alleles were 46, 3, and 43%, respectively (22). Among 92 trial patients who received combination therapy, there was no significant difference in combination therapy preference according to genotype status: nOATP1C1-intron 3C (p = 0.68); OATP1C1-pro143Thr (p = 0.22), or OATP1C1-C3035T (p = 0.95 for respective chi-squared tests). However, in a secondary analysis of the trial from Nygaard et al. (18), Carlé et al. reported that in a subgroup of 45 patients from the 59 participants completing the original trial (18), the presence of the combination of 2 polymorphisms (rs225014 encoding the DIO2 enzyme and rs12885300 encoding the MCT10 transporter) was associated with a higher rate of preference for combination treatment: 63% if one polymorphism present, 100% if both polymorphisms present, and 42% if wild type (p = 0.009) (23).
In conclusion, in this systematic review and meta-analysis of relatively short-term blinded RCTs, approximately 46% of adult hypothyroid patients preferred combination therapy with L-T3 and L-T4 and L-T3 over L-T4 monotherapy; yet these findings were not distinguishable from chance. Some differences between this study with prior guideline narrative summaries of combination therapy preference rates (1, 32), is our strict inclusion criteria relating to blinding (to minimize the risk of bias), exclusion data from any add-on non-randomized treatment arms (also to minimize the risk of bias), and the a priori definition of statistical significance relative to chance in our meta-analysis. The fundamental clinical assumption of our analyses was that patients either prefer combination therapy to the standard of care or not, so patients who prefer L-T4 monotherapy or those who have no preference, would be grouped together as they would be treated with the same standard of care of L-T4 monotherapy. We found no patient demographic, disease, or study characteristics associated with variability in combination therapy preference. Studies reporting improvements in symptoms and mood (15, 18), tended to report higher rates of preference rates for combination therapy. The types of physical and emotional symptoms that were reported on questionnaires to be improved in these studies (15, 18) included: feeling cold (15), blurred vision (15), nausea (15), fatigue (15), depression/sadness (15, 18), anger (15), confusion (15), fearfulness (15), irritability (15), anxiety (18), and general health (18). However, the majority of studies did not report any significant difference in quality of life (using validated quality of life questionnaires) nor body weight with combination therapy and patient preference did not vary with these measures. Significant weight loss was reported only in a high dose combination therapy arm (5:1 L-T4 to L-T3 dose ratio) in one trial, where the TSH was suppressed with combination therapy (14). There was some preference variability of marginal statistical significance associated with end of trial TSH difference between study groups; specifically, for trials reporting an end of trial combination therapy group TSH that was either significantly lower or higher than the L-T4 monotherapy arm, tended to be associated with diminished patient preference for combination therapy. A positive association of L-T3 total daily dosage and treatment preference was observed in an exploratory univariate meta-regression analysis, where the dosages of L-T3 varied from zero to 20 ug/day. We were not able to make any firm conclusion on any potential relationship between patient molecular characteristics and combination therapy due to paucity of data. The secondary analyses summarized in this review should be interpreted as hypothesis generating and further confirmatory research is needed.
It is important to acknowledge that in the clinical practice setting, patient preference rates for combination therapy may differ from those observed in blinded randomized trials, particularly if patients may have some negative pre-established perceptions of L-T4 therapy (nocebo effect) and positive expectations with combination therapy (particularly L-T3). In clinical practice, experiences of patients treated with combination therapy may be highly variable, including the reasons preferring combination therapy (or not), and the degree of benefits on symptoms, well-being, and functional ability. However, the potential therapeutic benefits are likely be enhanced in a supportive, encouraging clinical care environment where patients' symptoms and concerns are acknowledged and their views incorporated in medical decision-making. Of note, intense monitoring for treatment benefits (e.g., using detailed questionnaires) and adverse effects is expected to be more rigorous in a research trial setting compared to clinical practice. The experience of clinicians prescribing and adjusting the dose of combination therapy may be also different in clinical practice compared to the clinical trial setting (e.g., specialized clinical trial centers with investigators experienced in use of combination therapy). In an effort to address physician expertise, authors from the European Thyroid Association have recommended that only specialists accredited in Endocrinology or Internal Medicine should be the ones prescribing combination therapy (32). Yet even among endocrinologists, experience prescribing combination therapy may be variable. Some practical potential challenges in clinically utilizing combination therapy using commercially-available existing short-acting L-T3 preparations may include: complexity of administration of two different thyroid hormone preparations (often more than once a day for higher total daily doses), increased medication expense (the extent of which varies globally), and greater complexity/expense in medication monitoring (e.g., inclusion of T3 measurements in bloodwork, potentially including peak levels). Saravanan et al. have reported that in hypothyroid patients receiving combination therapy, where the baseline L-T4 dose is reduced by 50 ug and replaced with 10 ug of L-T3, peak blood free T3 levels rise 42% (4 h after dose administration) (33). As such, use of higher dose L-T3 in combination treatment may necessitate measurement of peak T3 blood levels and splitting of the doses of this hormone. The extent to which such levels are faithfully reflected by diverse tissue requirements further confounds optimal dosing of L-T4/L-T3. Authors from the European Thyroid Association have also suggested a relatively physiologic combination therapy dose ratio L-T4/L-T3 ranging from 13:1 to 20:1 by weight (administering L-T4 once daily and dividing the total daily L-T3 dose in two doses) (32). The ETA guidelines regarding combination therapy were developed to enhance safety (potentially due to harms from treatment with supraphysiologic doses of thyroid hormones) and to counter its indiscriminate use (32). The ETA guidelines have indicated that “the goal of L-T4/L-T3 combination therapy is to resolve persistent complaints despite a normal TSH in L-T4-treated hypothyroid patients” (32). Furthermore, in the interest of safety, the ETA guidelines have recommended close specialist follow-up, with dose adjustments intended to meet the goals of treatment (32). Authors from the Italian Society of Endocrinology and the Italian Thyroid Association have suggested a dose ratio of L-T4:L-T3 of 10 to 20:1, administered in divided daily doses (3). However, the suggested relatively physiologic L-T3 dosages are below that used in many of the trials included in this review. One of the potential risks of using higher dose L-T3 may be TSH suppression, particularly if the L-T4 dosage is not sufficiently reduced, and TSH suppression with combination therapy was reported in some of the included trials. The Italian guidelines also highlight the importance of close monitoring for potential adverse effects, including cardiovascular complications and osteoporosis (3). Relative contraindications to L-T3 combination therapy are important considerations. Clinical practice guideline authors sponsored various organizations have recommended avoiding combination L-T3/L-T4 therapy in the following groups: pregnant women (3, 6, 32, 34) the elderly (3) patients with known cardiac arrhythmias (6, 32), individuals with cardiac risk factors (3), patients with differentiated thyroid cancer with a high risk of disease progression or intermediate to high risk of adverse effects (3).
There are multiple strengths and several limitations of this systematic review and meta-analysis. An important strength is the systematic search for relevant citations conducted in multiple electronic databases by an experienced library information specialist (which was supplemented by a hand search). Furthermore, two reviewers independently review of citations and full-text papers in duplicate, with resolution of any discrepancies in inclusion of papers resolved in discussion with a third content expert reviewer. Two investigators also independently critically appraised included studies and abstracted the data, with the final consensus of reported results. We also contacted some authors of primary studies to obtain critically information, relating to study inclusion and results. Some limitations of this research include: exclusion of non-English studies (due to lack of resources for translation), lack of a comprehensive search of the gray literature, inclusion of a relatively small number of trials (such that publication bias could not be reliably assessed), some methodologic limitations of included trials, and short duration of included trials (precluding analysis of durability of patient preference over time). Additional potential limitations relating to treatment of hypothyroidism that were not addressed by this review nor the included studies include the potential for a symptom-optimized TSH goal that may be narrower than the traditional 95% reference range (35), drug interference with TSH secretion (36), management of treatment-refractory hypothyroidism (i.e., individuals requiring unusually high doses of thyroid hormones) (37), and consideration of interference with gastrointestinal absorption of thyroid hormones (38).
In conclusion, although L-T4 monotherapy is the standard of care in management of hypothyroidism in adults, dissatisfaction among some patients treated with L-T4 as well as significant uncertainties relating to thyroid hormone alternatives, highlights the critical need for more research on effective treatments to optimize the well-being and treatment satisfaction in this population.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
All of the authors provided input on the design of the study and reviewed the manuscript. RF conducted the electronic database search. AA and AS screened citations, reviewed the full-text papers, critically appraised included studies, abstracted the data, and drafted the manuscript. SE provided input, in the event consensus was not achieved by AA and AS on inclusion of studies. AS conducted the statistical analyses and the statistical methods and data were reviewed by LT.
AS was a co-author of a clinical practice guideline on hypothyroidism (American Thyroid Association).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Dr. John Walsh for providing information about his study, including unpublished subgroup analysis data. The authors would also like to thank Dr. Ponnusamy Saravanan for clarifying patient-reported outcomes evaluated in his study. The authors would also like to thank an individual who is on combination therapy for treatment of hypothyroidism, who provided input on our study and the interpretation of our findings from a patient perspective. Finally, the authors would like to thank Mrs. Coreen Marino, administrative assistant, for retrieving the full-text papers for review.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2019.00477/full#supplementary-material
1. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid. (2014). 24:1670–751. doi: 10.1089/thy.2014.0028
2. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: the American association of clinical endocrinologists and the american thyroid association. Thyroid. (2012) 22:1200-1235. doi: 10.1089/thy.2012.0205
3. Guglielmi R, Frasoldati A, Zini M, Grimaldi F, Gharib H, Garber JR, et al. Italian association of clinical endocrinologists statement—replacement therapy for primary hypothyroidism: a brief guide for clinical practice. Endocr Pract. (2016) 22:1319–26. doi: 10.4158/ep161308.or
4. Brenta G, Vaisman M, Sgarbi JA, Bergoglio LM, Andrada NC, Bravo PP, et al. Task force on hypothyroidism of the latin American Thyroid Society (LATS). Clinical practice guidelines for the management of hypothyroidism. Arq Bras Endocrinol Metabol. (2013) 57:265–91. doi: 10.1590/S0004-27302013000400003
5. Dave JA, Klisiewicz A, Bayat Z, Mohamed NA, Stevens Z, Mollentze WF, et al. SEMDSA/ACE-SA guideline for the management of hypothyroidism in adults. JEMDSA. (2015) 20:18–26. doi: 10.1080/16089677.2015.1056468
6. Okosieme O, Gilbert J, Abraham P, Boelaert K, Dayan C, Gurnell M, et al. Management of primary hypothyroidism: statement by the British thyroid association executive committee. Clin Endocrinol. (2016) 84:799–808. doi: 10.1111/cen.12824
7. Biondi B, Bartalena L, Chiovato L, Lenzi A, Mariotti S, Pacini F, et al. (2016). Recommendations for treatment of hypothyroidism with levothyroxine and levotriiodothyronine: A 2016 position statement of the Italian society of endocrinology and the Italian thyroid association. J Endocrinol Invest. (2016) 39: 1465–74. doi: 10.1007/s40618-016-0511-z
8. Peterson SJ, Cappola AR, Castro MR, Dayan CM, Farwell AP, Hennessey JV, et al. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid. (2018) 28:707–21. doi: 10.1089/thy.2017.0681
9. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
10. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.1371/journal.pmed.1000097.
11. Fletcher J. What is heterogeneity and is it important? BMJ. (2007) 334:94–6. doi: 10.1136/bmj.39057.406644.68.
12. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 3277414:557–60. doi: 10.1136/bmj.327.7414.557
13. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34.
14. Appelhof BC, Fliers E, Wekking EM, Schene AH, Huyser J, Tijssen JG, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab. (2005) 90:2666–74. doi: 10.1210/jc.2004-2111.
15. Bunevicius R, Kazanavicius G, Zalinkevicius R, Prange AJ Jr. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Engl J Med. (1999) 340:424–9. doi: 10.1056/NEJM199902113400603
16. Bunevicius R, Jakuboniene N, Jurkevicius R, Cernicat J, Lasas L, Prange AJ Jr. Thyroxine vs thyroxine plus triiodothyronine in treatment of hypothyroidism after thyroidectomy for Graves' disease. Endocrine. (2002) 18:129–33. doi: 10.1385/ENDO:18:2:129.
17. Escobar-Morreale HF, Botella-Carretero JI, Gómez-Bueno M, Galán JM, Barrios V, Sancho J. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med. (2005) 142:412–24. doi: 10.7326/0003-4819-142-6-200503150-00007
18. Nygaard B, Jensen EW, Kvetny J, Jarløv A, Faber J. Effect of combination therapy with thyroxine (T4) and 3,5,3'-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. Eur J Endocrinol. (2009) 161:895–902. doi: 10.1530/EJE-09-0542
19. Rodriguez T, Lavis VR, Meininger JC, Kapadia AS, Stafford LF. Substitution of liothyronine at a 1:5 ratio for a portion of levothyroxine: effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. Endocr Pract. (2005) 11:223–33. doi: 10.4158/EP.11.4.223
20. Walsh JP, Shiels L, Lim EM, Bhagat CI, Ward LC, Stuckey BG, et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab. (2003) 88:4543–50. doi: 10.1210/jc.2003-030249.
21. Appelhof BC, Peeters RP, Wiersinga WM, Visser TJ, Wekking EM, Huyser J, et al. Polymorphisms in type 2 deiodinase are not associated with well-being, neurocognitive functioning, and preference for combined thyroxine/3,5,3'-triiodothyronine therapy. J Clin Endocrinol Metab. (2005) 90:6296–9. doi: 10.1210/jc.2005-0451.
22. Van Der Deure WM, Appelhof BC, Peeters RP, Wiersinga WM, Wekking EM, Huyser J, et al. Polymorphisms in the brain-specific thyroid hormone transporter OATP1C1 are associated with fatigue and depression in hypothyroid patients. Clin Endocrinol. (2008) 69:804–11. doi: 10.1111/j.1365-2265.2008.03267.x
23. Carlé A, Faber J, Steffensen R, Laurberg P, Nygaard B. Hypothyroid patients encoding combined MCT10 and DIO2 gene polymorphisms may prefer L-T3 + L-T4 combination treatment - Data Using a blind, randomized, clinical study. Eur Thyroid J. (2017) 6:143–51. doi: 10.1159/000469709.
24. Clyde PW, Harari AE, Getka EJ, Shakir KMM. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism - A randomized controlled trial. JAMA. (2003) 290:2952–58. doi: 10.1001/jama.290.22.2952.
25. Kaminski J, Miasaki FY, Paz-Filho G, Graf H, Carvalho GA. Treatment of hypothyroidism with levothyroxine plus liothyronine: a randomized, double-blind, crossover study. Arch Endocrinol Metab. (2016) 60:562–572. doi: 10.1590/2359-3997000000192.
26. Saravanan P, Simmons DJ, Greenwood R, Peters TJ, Dayan CM. Partial substitution of thyroxine with tri-iodothyronine in patients on T4 replacement therapy: Results of a large community-based randomized controlled trial. J Clin Endocrinol Metab. (2005) 90:805–12. doi: 10.1210/jc.2004-1672
27. Sawka AM, Gerstein HC, Marriott MJ, MacQueen GM, Joffe RT. Does a Combination regimen of thyroxine and 3,5,3′-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab. (2003) 88:4551–55. doi: 10.1210/jc.2003-030139
28. Siegmund W, Spieker K, Weike AI, Giessmann T, Modess C, Daber T, et al. Replacement therapy with levothyroxine plus triiodothyronine (bioavailable molar ratio 14 : 1) is not superior to thyroxine alone to improve well-being and cognitive performance in hypothyroidism. Clin Endocrinol. (2004) 60:750–7.
29. Valizadeh M, Seyyedmajidi MR, Momtazi S, Musavi N. The efficacy of combined levothyroxine plus liothyronine with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. Iran J Endocrinol Metab. (2009) 10:465.
30. Fadeyev VV, Morgunova TB, Melnichenko GA, Dedov II. Combined therapy with L-Thyroxine and L-Tiiodothyronine compared to L-Thyroxine alone in the treatment of primary hypothyroidism. Hormones. (2010) 9:245–52. doi: 10.14310/horm.2002.1274
31. Smith KM. Controlled clinical trial of combined triiodothyronine and thyroxine in the treatment of hypothyroidism. Br Med J. (1970) 4:145–148.
32. Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012 ETA guidelines: The use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur Thyroid J. (2012) 1:55–71. doi: 10.1159/000339444
33. Saravanan P, Siddique H, Simmons DJ, Greenwood R, Dayan CM. Twenty-four hour hormone profiles of TSH, Free T3 and free T4 in hypothyroid patients on combined T3/T4 therapy. Exp Clin Endocrinol Diabetes. (2007) 115:261–7. doi: 10.1055/s-2007-973071.
34. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. (2017) 27:315–89. doi: 10.1089/thy.2016.0457
35. Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab. (2005) 90:5483–8. doi: 10.1210/jc.2005-0455.
36. Haugen BR. Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab. (2009) 23:793–800. doi: 10.1016/j.beem.2009.08.003.
37. Centanni M, Benvenga S, Sachmechi I. Diagnosis and management of treatment-refractory hypothyroidism: an expert consensus report. J Endocrinol Invest. (2017) 40:1289–301. doi: 10.1007/s40618-017-0706-y
Keywords: hypothyroidism, thyroid hormone, levothyroxine, triiodothyronine, systematic review, meta-analysis, randomized controlled trials
Citation: Akirov A, Fazelzad R, Ezzat S, Thabane L and Sawka AM (2019) A Systematic Review and Meta-Analysis of Patient Preferences for Combination Thyroid Hormone Treatment for Hypothyroidism. Front. Endocrinol. 10:477. doi: 10.3389/fendo.2019.00477
Received: 17 April 2019; Accepted: 01 July 2019;
Published: 24 July 2019.
Edited by:
Francesco S. Celi, Virginia Commonwealth University, United StatesReviewed by:
Riccardo Zucchi, University of Pisa, ItalyCopyright © 2019 Akirov, Fazelzad, Ezzat, Thabane and Sawka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna M. Sawka, c2F3a2FhbUB5YWhvby5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.