
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol., 26 June 2019
Sec. Reproduction
Volume 10 - 2019 | https://doi.org/10.3389/fendo.2019.00398
This article is part of the Research TopicFollicle-Stimulating Hormone: Fertility and BeyondView all 22 articles
 Alessandro Conforti1*
Alessandro Conforti1* Alberto Vaiarelli2
Alberto Vaiarelli2 Danilo Cimadomo2
Danilo Cimadomo2 Francesca Bagnulo1
Francesca Bagnulo1 Stefania Peluso1
Stefania Peluso1 Luigi Carbone1
Luigi Carbone1 Francesca Di Rella3
Francesca Di Rella3 Giuseppe De Placido1
Giuseppe De Placido1 Filippo Maria Ubaldi2
Filippo Maria Ubaldi2 Ilpo Huhtaniemi4
Ilpo Huhtaniemi4 Carlo Alviggi1,5
Carlo Alviggi1,5The purpose of a pharmacogenomic approach is to tailor treatment on the basis of an individual human genotype. This strategy is becoming increasingly common in medicine, and important results have been obtained in oncologic and antimicrobial therapies. The rapid technological developments and availability of innovative methodologies have revealed the existence of numerous genotypes that can influence the action of medications and give rise to the idea that a true “individualized” approach could become in the future a reality in clinical practice. Moreover, compared to the past, genotype analyses are now more easily available at accessible cost. Concerning human reproduction, there is ample evidence that several variants of gonadotropins and their receptors influence female reproductive health and ovarian response to exogenous gonadotropins. In more detail, variants in genes of follicle-stimulating hormone β-chain (FSH-B) and its receptor (FSH-R) seem to be the most promising candidates for a pharmacogenomic approach to controlled ovarian stimulation in assisted reproductive technologies. In the present review, we summarize the evidence regarding FSH-B and FSH-R variants, with special reference to their impact on reproductive health and assisted reproductive technology treatments.
Follicle-stimulating hormone (FSH) is a pituitary gonadotropic hormone, which is fundamental for follicle growth in females and spermatogenesis in males. FSH is an heterodimeric molecule belonging to the glycoprotein hormone family. It consists of the common α-subunit shares as with other glycoprotein hormones (LH, hCG, TSH) and the hormone specific β subunit. FSH, when in the ovary and testis, binds to its cognate receptor (FSH-R), which belongs to the superfamily of the G-protein coupled receptors. It is characterized by a long ligand-binding extracellular domain, seven transmembrane domains, mediating the hormonal stimulus, and an intracellular C-terminal domain participating in receptor internalization and desensitization of the signal. Through the interaction with its receptor, FSH activated several intracellular signaling pathways, the most important of them being adenylyl cyclase and β-arrestins (1, 2).
It has recently been demonstrated that FSH exerts its action outside the reproductive tract, including in the placenta, hepatocytes and tumor blood vessels (3, 4). In addition, FSH was demonstrated to be involved in the pathogenesis of endometriotic lesions (5). Focusing on the female reproductive tract, it was also recently demonstrated that FSH could exert its effect on the endometrial glands. In detail, FSH-R was able to increase in these cells intracellular levels of cAMP, leading to induction of steroidogenesis (6). During the menstrual cycle, FSH has several important actions. Firstly, it promotes folliculogenesis by stimulating estradiol production by the aromatase enzyme system, stimulating granulosa cell growth and inducing the expression of luteinizing hormone receptors (1). Together with LH, FSH levels peak in the mid-cycle which induces important actions in the ovulation process, such as the stimulation of proteolytic enzymes essential for follicular wall rupture (7). Finally, in the early follicular phase FSH is involved in the recruitment of new antral follicles for the next cycle of folliculogenesis (8). On the basis of differences in the terminal sialic acid residues in the carbohydrate moieties that are attached to the FSH protein, numerous isoforms of FSH have been identified (9). Acidic isoforms seems to be involved in follicular recruitment at the end of menstrual cycles, while follicle selection and rupture seem to be promoted by basic FSH isoforms (9). Given its biological importance in folliculogenesis, pharmaceutical FSH products are currently adapted for multiple follicular growth in assisted reproductive technology (ART) (10). The ovarian activity, as well as the ovarian response to exogenous gonadotropin appear to be influenced by specific genetic traits involving gonadotropins and their receptors (11–14) (Table 1). In the present review, we will summarize the most important evidence concerning variants of FSH and FSH-R and their implication in female reproductive functions.
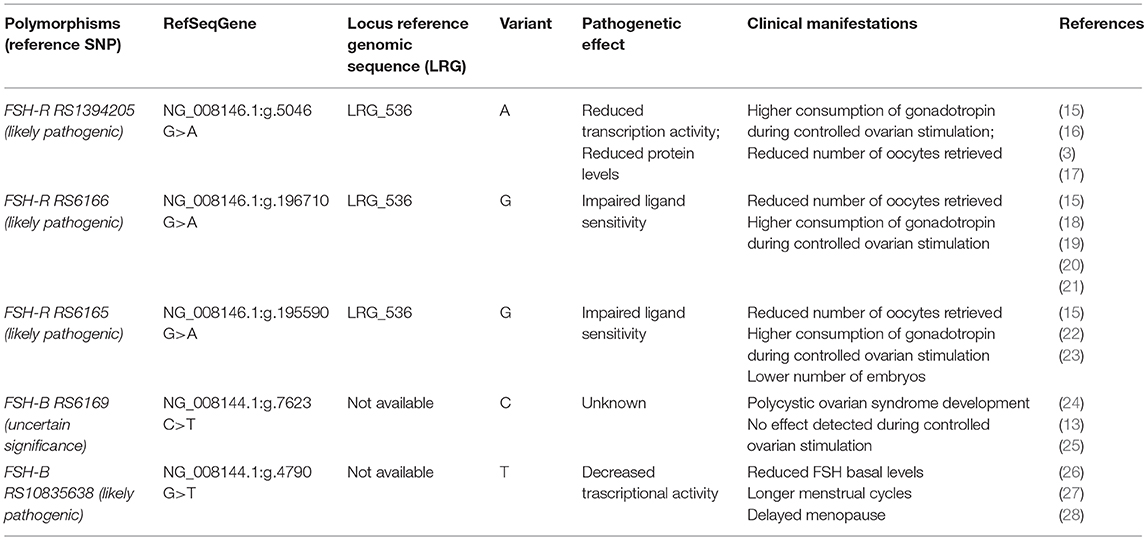
Table 1. Clinical manifestations and pathogenetic effects of FSH-R and FSH-B subunit most common variants (https://www.ncbi.nlm.nih.gov/snp).
A systematic search was carried out using MEDLINE (Pubmed) AND Scopus databases with no restriction of language or time period. The search strategy consisted in the use of the combinations of the following keywords: “controlled ovarian stimulation,” “ART,” “IVF,” “ICSI,” “FIVET,” “IUI,” “intrauterine insemination,” “ovulation induction,” “ovarian stimulation,” “polymorphism,” “SNV,” “Single nucleotide variant,” “FSH Receptor,” “FSHR,” “FSH,” “follicle-stimulating hormone,” “follicle-stimulating hormone,” and “beta subunit.”
In view of the recent meta-analysis published by our group (15) we updated our research adding also more recent papers in the present review (29–32).
As recommended by Human Genome Variation Society (33) every single nucleotide variants (SNV) illustrated in the present paper was reported indicating Locus Reference Genomic sequence (LRG) and RefSeqGene or transcript (Table 1).
In contrast to LH beta subunit, FSH-B appears to be highly conserved (34). Indeed, few variants of the gene encoding for FSH-B subunit have been identified so far. The first variant was identified in 1993 in a women with primary amenorrhea, sexual infantilism and infertility (35, 36). This variant consisted in a two-nucleotide deletion in codon 61 that gave rise to premature stop codon. Thereafter, several other inactivating variants have been identified. Most of them induce an alteration of the cysteine knot structure of FSH which is crucial for its biological activity (34). Thus, the majority of variants inactivating FSH-B are characterized by the absence of puberty, infertility and the absence of breast maturation and few of them show partial puberty development (34, 37).
Considering the conserved structure of FSH, very few clinically significant variants have been identified. Among the 24 SNVs identified (18) only the one located in FSH-B chain promoter (C.-221G>T, RS10835638) seems to have significant clinical impact on male and female reproduction (38, 39). In the first report by Grigorova et al. T homozygous men showed lower FSH levels and reduced testicular volume than other haplotypes (38). Conversely, in 365 women with normal menses the T homozygotes showed elevated FSH and LH levels with reduced progesterone production (39). In another study, the T allele resulted in longer menstrual cycles [0.16 Standard differences; 95% confidence interval (CI) 0.12–0.20; P < 0.05], in delayed age at menopause (0.13 years; 95% CI 0.04–0.22; P < 0.05), and greater female nulliparity [odds ratio (OR) = 1.06; 95% CI 1.02–1.11; P < 0.05] (26). Interestingly, the same study showed lower risk of endometriosis among T carriers compared with other haplotypes [OR = 0.79; 95% CI 0.69–0.90; P < 0.05]. In another study, involving 193 infertile eumenorrheic women, a statistically significant reduction of FSH on cycle day 3 was observed in carriers with the combination of FSH-B (C-211 G>T, RS10835638) GT + TT/FSH-R (C.2039 G>A, RS6166) AA genotype, compared with the FSH-B GG/FSH-R GG genotype (27). More recently, it was confirmed that the T allele carriers were associated with higher FSH and LH levels and idiopathic infertility (40). The T allele of FSH-B C-211 G>T, RS10835638 appears to decrease transcriptional activity of the gene (28). Very recently, Trevisan et al. observed in a cross-sectional study involving 140 infertile women (median age 33 years), that women carrying GT genotype (n = 38) had a lower response to ovarian stimulation compared to GG (wild type) genotype (n = 102) with of number of oocytes retrieved (3.0 vs. 5.0, p = 0.03) and a lower number of embryos at the end of stimulation (2 vs. 3, p = 0.02) (29).
There is also evidence which suggests that another variant of FSH-B subunit (C.228 C>T, RS6169) might be implicated in the development polycystic ovarian syndrome (24, 25). A retrospective analysis of 135 Chinese women between 19 and 38 years of age affected by PCOS with 105 as a normal control, a higher prevalence of homozygous carrier was observed in PCOS than in the control group (12.6 vs. 3.8%) (25). Furthermore, the frequency of this variant was more pronounced in a specific subgroup of PCOS women, namely those with obesity (0.50 and 31.0%, respectively) and hyperandrogenism. These findings support the concept that hyperandrogenic PCOS women could show peculiar characteristics and probably display specific pathogenetic mechanisms (41). In a recent prospective trials involving 30 normogonadotropic women, we did not observe differences in terms of ovarian response or pregnancy rate when comparing different haplotypes of this variant (13). Despite the low number of patients recruited, our study did not support any implications in terms of ovarian response to exogenous gonadotropin and ART success.
Several inactivating and activating variants of FSH-R have been identified (42). The majority of the inactivating variants are located on exons 7 and 10 (42). The most typical clinical manifestations are primary amenorrhea, elevated FSH levels, and infertility. Specific inactivating variants were also associated with polycystic ovarian syndrome (43). Also, the majority of activating variants are located in exon 10. The most common clinical manifestation is a spontaneous occurrence of ovarian hyperstimulation syndrome. In general, while activating variants in the FSH-R gene can manifest in heterozygotes, the inactivating variants alter the phenotype only when present in the homozygous or compound heterozygous form Desai et al. (42).
The FSH-R gene carries more than 2000 single nucleotide variants (SNVs) (18). Among them the most widely studied common variants which apparently impact on female reproduction are: -29 G>A. (RS1394205); C.919G>A (RS6165); C.2039G>A (RS6166). Two FSH-R variants with SNVs in the coding region have been identified and well-characterized (44). The SNV known as the Serine680 variant causes the replacement of asparagine (Asn) for serine (Ser) at the 680 position, which is located in the intracellular domain of the FSH-R protein. The RS6165 SNV replaces threonine (Thr) by alanine (Ala). Except in some African populations, the two SNVs are in linkage disequilibrium (19); this means that carriers who possess Thr307 nearly always have Asn680 present on the same allele and carriers who have Ala307 have Ser680 on the same allele (45). The former (RS6166) introduces a potential phosphorylation site and the latter (RS6165) results in a change from a polar to a non-polar hydrophobic amino acid, thereby removing a potential O-linked glycosylation site (19). In vitro studies conducted using human granulosa cells showed that GG carriers of the FSH-R (RS6166) genotype have greater resistance to FSH than do AA carriers (18, 46) and are characterized by slower kinetics of cAMP production, ERK1/2, and CREB phosphorylation (47). Despite the linkage disequilibrium between these two SNVs, several studies suggest that these two variants could influence ovarian stimulation (OS) outcome in different ways. In detail, Achrekar et al. observed that only the FSH-R RS6165 variant could significantly impact the total FSH consumption during OS (3). Discrepancies between FSH-R (RS6166) and FSH-R (RS6166) were also reported by Trevisan et al. in a cross-sectional study involving 149 infertile women, in which a difference in terms of the number of embryos produced was observed only among different FSH-R (RS6165) haplotype (23).
Our findings in a recent systematic review corroborate these previous observations. Indeed, we found that GG FSH-R (RS6166) carriers had higher ovarian resistance to exogenous gonadotropin and, consequently, had fewer oocytes compared with AA carriers (15). These findings were also confirmed in a more recent study (31). In addition, higher FSH basal levels and resistance to clomiphene citrate were observed in G allele carriers, supporting an higher receptorial resistance even to endogenous level of FSH (15, 27, 48, 49). Conversely, A allele carriers show an higher FSH sensitivity as confirmed in a recent retrospective study of 586 infertile women undergoing their first IVF cycle, where an increased risk for developing OHSS syndrome (OR 1.7 95% CI 1.025–2.839, p = 0.04) was observed in carriers of this allele (30).
The finding of Borgbo et al. that the FSH-R RS6166 and FSH-R RS6165 GG carriers had higher LHCGR gene expression but lower Anti-Müllerian hormone receptor-2 expression vs. carriers of the other haplotypes, suggested that these variants could affect the protein expression of human antral follicles (50). Nonetheless, it remains to be established whether FSH-R RS6166 and RS6165 influence expression of the FSH-R protein.
The FSH-R−29 G>A (RS1394205) variant is located in the 5′-untranslated region of the gene and is able to influence ovarian response. In vitro studies showed that A allele presence is characterized by reduced mRNA transcriptional activity and reduced FSH-R protein level (46, 47).
In ART context, Achrekar et al. reported with homozygous variant genotype AA lower number of oocytes and lower pregnancy rate compared with GG genotype in women who underwent OS (22). This observation was confirmed in a further larger study by Desai et al. involving 100 women (16) where those with the AA genotype at position−29 were at higher risk for poor ovarian response in comparison to the other haplotypes (OR 8.63, 95% CI 1.84–45.79; P = 0.001). In contrast, other authors did not confirm significant effects of this variant concerning the ovarian response (31, 32, 51). The evidence regarding the clinical effect of the FSH-R−29 G>A (RS1394205) variant on OS was summarized in a recent meta-analysis, which showed that higher exogenous FSH consumption is required in homozygotes for the A allele than carriers of the G allele (15).
Demographic and anthropometric characteristics of women and the ovarian reserve tests do not fully explain the ovarian response to exogenous gonadotropin (52, 53). Indeed, there is a subgroup of women that, despite showing normal ovarian reserve in terms of functional and biochemical markers, have an “unexpected” impaired prognosis to ART and poor or suboptimal number of oocytes at the end of stimulation (54). This ovarian resistance to exogenous gonadotropin is also called “hypo-response,” and it was recently included in the new POSEIDON classification of low prognosis patients in ART (55–58).
The fact that several variants could in some way affect the ovarian response to OS opens up the way to a pharmacogenomic approach to ART and might partially explain the hypo-response phenomenon (13, 59). The pharmacogenomic approach is spreading more and more to several fields, and it could provide the explanations for adverse or poor drug effects (60). In the ART scenario, a pharmacogenomic approach to OS could optimize ART treatments, thereby reducing both poor response rates and potentially life-threatening excessive ovarian responses (53). However, although more than 30 studies have already been published, no large randomized clinical trials on this topic have been conducted, indicating that the pharmacogenomic approach to OS is still a largely neglected topic. In addition, it should be underlined that variants, especially those involving FSH receptors are very common in general population (19, 61–63) (Figures 1, 2). Furthermore, the genotype analysis can now be provided at low cost (15). So far, few studies have adopted a pharmacogenomic approach to ART with the FSH-R variant rs6166 being the one most often investigated (2, 20). The first one was conducted by Behre et al. (21). In detail, women undergoing controlled ovarian hyperstimulation for ART, homozygous for the wild-type or for the FSH-R SNV(C.2039G>A [RS6166]), were randomly assigned to group I (GG carriers, n = 24), receiving an FSH daily dose of 150 U/day, or group II (GG carries, n = 25), receiving an FSH dose of 225 U/day. Age- and body mass index-matched AA carriers, receiving a daily dose of 150 IU, served as control group. The wild-type group (AA carriers) had higher estradiol production after treatment with 150 IU/day of FSH compared with the GG carriers who received the same dose. The increment of dosage for 150–225 IU/day was able to compensate for this discrepancy. This finding was confirmed by those reported by Genro et al. (64). The authors showed that the follicle development during OS was not significantly influenced by the presence of FSH-R RS6166/RS6165 when a high FSH dose (300 IU per day) was administrated during OS (64). These results suggested that increasing FSH dosage during OS could mitigate the negative effect exerted by the FSH-R variant on the ovarian response. Finally, a recent meta-analysis including 4,425 observation concluded that GG allele carriers produced a significantly lower number of oocytes compared with AA (Random Weight Mean Difference: 0.84, 95% CI: 0.19–1.49, P = 0.01, P = 0.03) and AG carriers (Random Weight Mean Difference: 0.88, 95% CI: 0.12–1.63, P = 0.02) (15). Furthermore, gonadotropin type seems to influence the number of oocytes collected in relation to the FSH-R (RS6166) genotype distribution. As a matter of fact, the number of oocytes retrieved was significantly higher in AA carriers than in GG carriers when recombinant FSH was used, but not when urinary FSH formulations with LH activity were used (15). Although this finding suggests that the addition of LH in OS (65–67) could also mitigate the effects of FSH-R RS6166 variants more data are needed on this issue.
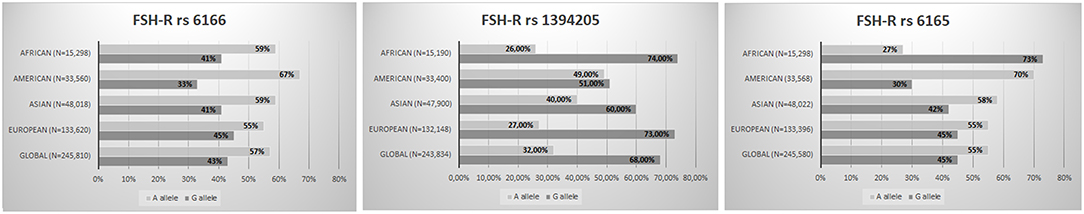
Figure 1. FSH-R variants worldwide distribution (RS6166; RS1394205; RS6165) (The Genome Aggregation Database—https://www.ncbi.nlm.nih.gov/snp/).
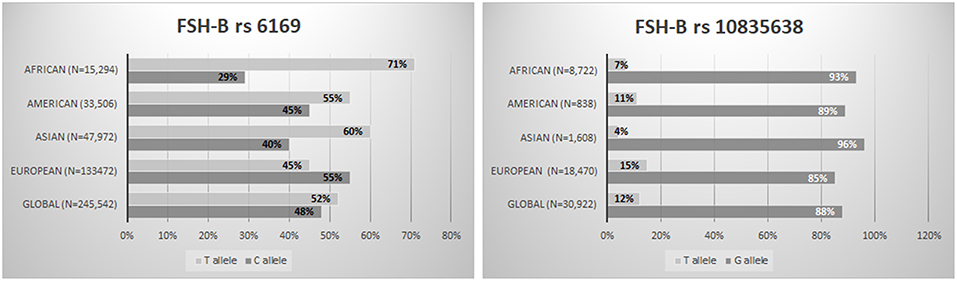
Figure 2. FSH-B subunit variants worldwide distribution (RS6169; RS10835638) (The Genome Aggregation Database—https://www.ncbi.nlm.nih.gov/snp/).
In conclusion, increasing evidence indicates that a pharmacogenomic approach to ovarian stimulation could become a clinical reality in the future. So far, specific variants of FSH-B and FSH-R represent promising genetic markers to better standardize controlled ovarian stimulation in women undergoing ART.
AC and CA idealized the paper and wrote the first draft. All authors participated in literature research and paper editing. The senior authors IH and GD supervised the paper and participate in the elaboration of the final version. All authors listed have made intellectual contribution to the work and approved the final version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Yong EL, Baird DT, Hillier SG. Mediation of gonadotrophin-stimulated growth and differentiation of human granulosa cells by adenosine-3′,5′-monophosphate: one molecule, two messages. Clin Endocrinol. (1992) 37:51–8. doi: 10.1111/j.1365-2265.1992.tb02283.x
2. Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. (1997) 18:739–73. doi: 10.1210/er.18.6.739
3. Achrekar SK, Modi DN, Desai SK, Mangoli VS, Mangoli RV, Mahale SD. Follicle-stimulating hormone receptor polymorphism (Thr307Ala) is associated with variable ovarian response and ovarian hyperstimulation syndrome in Indian women. Fertil Steril. (2009) 91:432–9. doi: 10.1016/j.fertnstert.2007.11.093
4. Radu A, Pichon C, Camparo P, Antoine M, Allory Y, Couvelard A, et al. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med. (2010) 363:1621–30. doi: 10.1056/NEJMoa1001283
5. Ponikwicka-Tyszko D, Chrusciel M, Stelmaszewska J, Bernaczyk P, Sztachelska M, Sidorkiewicz I, et al. Functional expression of FSH receptor in endometriotic lesions. J Clin Endocrinol Metab. (2016) 101:2905–14. doi: 10.1210/jc.2016-1014
6. Sacchi S, Sena P, Degli Esposti C, Lui J, La Marca A. Evidence for expression and functionality of FSH and LH/hCG receptors in human endometrium. J Assist Reprod Genet. (2018) 35:1703–12. doi: 10.1007/s10815-018-1248-8
7. Yoshimura Y, Santulli R, Atlas SJ, Fujii S, Wallach EE. The effects of proteolytic enzymes on in vitro ovulation in the rabbit. Am J Obstet Gynecol. (1987) 157:468–75. doi: 10.1016/S0002-9378(87)80197-7
8. Roseff SJ, Bangah ML, Kettel LM, Vale W, Rivier J, Burger HG, et al. Dynamic changes in circulating inhibin levels during the luteal-follicular transition of the human menstrual cycle. J Clin Endocrinol Metab. (1989) 69:1033–9. doi: 10.1210/jcem-69-5-1033
9. Palermo R. Differential actions of FSH and LH during folliculogenesis. Reprod Biomed Online. (2007) 15:326–37. doi: 10.1016/S1472-6483(10)60347-1
10. Leao RB, Esteves SC. Gonadotropin therapy in assisted reproduction: an evolutionary perspective from biologics to biotech. Clinics. (2014) 69:279–93. doi: 10.6061/clinics/2014(04)10
11. Alviggi C, Conforti A, Caprio F, Gizzo S, Noventa M, Strina I, et al. In estimated good prognosis patients could unexpected “hyporesponse” to controlled ovarian stimulation be related to genetic polymorphisms of FSH receptor? Reprod Sci. (2016) 23:1103–8. doi: 10.1177/1933719116630419
12. Altmae S, Hovatta O, Stavreus-Evers A, Salumets A. Genetic predictors of controlled ovarian hyperstimulation: where do we stand today? Hum Reprod Update. (2011) 17:813–28. doi: 10.1093/humupd/dmr034
13. Conforti A, Alfano S, De Rosa P, Alviggi C, De Placido G. The role of gonadotropin polymorphisms and their receptors in assisted reproductive technologies and controlled ovarian stimulation: a prospective observational study. Italian J Gynaecol Obstetr. (2017) 29:15–21. doi: 10.14660/2385-0868-67
14. Alviggi C, Clarizia R, Pettersson K, Mollo A, Humaidan P, Strina I, et al. Suboptimal response to GnRHa long protocol is associated with a common LH polymorphism. Reprod Biomed Online. (2011) 22(Suppl. 1):S67–72. doi: 10.1016/S1472-6483(11)60011-4
15. Alviggi C, Conforti A, Santi D, Esteves SC, Andersen CY, Humaidan P, et al. Clinical relevance of genetic variants of gonadotrophins and their receptors in controlled ovarian stimulation: a systematic review and meta-analysis. Hum Reprod Update. (2018) 24:599–614. doi: 10.1093/humupd/dmy019
16. Desai SS, Achrekar SK, Pathak BR, Desai SK, Mangoli VS, Mangoli RV, et al. Follicle-stimulating hormone receptor polymorphism (G-29A) is associated with altered level of receptor expression in Granulosa cells. J Clin Endocrinol Metab. (2011) 96:2805–12. doi: 10.1210/jc.2011-1064
17. Nakayama T, Kuroi N, Sano M, Tabara Y, Katsuya T, Ogihara T, et al. Mutation of the follicle-stimulating hormone receptor gene 5'-untranslated region associated with female hypertension. Hypertension. (2006) 48:512–8. doi: 10.1161/01.HYP.0000233877.84343.d7
18. Casarini L, Santi D, Marino M. Impact of gene polymorphisms of gonadotropins and their receptors on human reproductive success. Reproduction. (2015) 150:R175–84. doi: 10.1530/REP-15-0251
19. Simoni M, Casarini L. Mechanisms in endocrinology: genetics of FSH action: a 2014-and-beyond view. Eur J Endocrinol. (2014) 170:R91–107. doi: 10.1530/EJE-13-0624
20. Perez Mayorga M, Gromoll J, Behre HM, Gassner C, Nieschlag E, Simoni M. Ovarian response to follicle-stimulating hormone (FSH) stimulation depends on the FSH receptor genotype. J Clin Endocrinol Metab. (2000) 85:3365–9. doi: 10.1210/jc.85.9.3365
21. Behre HM, Greb RR, Mempel A, Sonntag B, Kiesel L, Kaltwaßer P, et al. Significance of a common single nucleotide polymorphism in exon 10 of the follicle-stimulating hormone (FSH) receptor gene for the ovarian response to FSH: A pharmacogenetic approach to controlled ovarian hyperstimulation. Pharmacogenet Genomics. (2005) 15:451–6. doi: 10.1097/01.fpc.0000167330.92786.5e
22. Achrekar SK, Modi DN, Desai SK, Mangoli VS, Mangoli RV, Mahale SD. Poor ovarian response to gonadotrophin stimulation is associated with FSH receptor polymorphism. Reprod Biomed Online. (2009) 18:509–15. doi: 10.1016/S1472-6483(10)60127-7
23. Trevisan CM, Peluso C, Cordts EB, de Oliveira R, Christofolini DM, Barbosa CP, et al. Ala307Thr and Asn680Ser polymorphisms of FSHR gene in human reproduction outcomes. Cell Physiol Biochem. (2014) 34:1527–35. doi: 10.1159/000366356
24. Simoni M, Tempfer CB, Destenaves B, Fauser BC. Functional genetic polymorphisms and female reproductive disorders: part I: polycystic ovary syndrome and ovarian response. Hum Reprod Update. (2008) 14:459–84. doi: 10.1093/humupd/dmn024
25. Tong Y, Liao WX, Roy AC, Ng SC. Association of AccI polymorphism in the follicle-stimulating hormone beta gene with polycystic ovary syndrome. Fertil Steril. (2000) 74:1233–6. doi: 10.1016/S0015-0282(00)01616-2
26. Ruth KS, Beaumont RN, Tyrrell J, Jones SE, Tuke MA, Yaghootkar H, et al. Genetic evidence that lower circulating FSH levels lengthen menstrual cycle, increase age at menopause and impact female reproductive health. Hum Reprod. (2016) 31:473–81. doi: 10.1093/humrep/dev318
27. La Marca A, Papaleo E, Alviggi C, Ruvolo G, De Placido G, Candiani M, et al. The combination of genetic variants of the FSHB and FSHR genes affects serum FSH in women of reproductive age. Hum Reprod. (2013) 28:1369–74. doi: 10.1093/humrep/det061
28. Hoogendoorn B, Coleman SL, Guy CA, Smith K, Bowen T, Buckland PR, et al. Functional analysis of human promoter polymorphisms. Hum Mol Genet. (2003) 12:2249–54. doi: 10.1093/hmg/ddg246
29. Trevisan CM, de Oliveira R, Christofolini DM, Barbosa CP, Bianco B. Effects of a polymorphism in the promoter region of the follicle-stimulating hormone subunit beta (FSHB) gene on female reproductive outcomes. Genet Test Mol Biomarkers. (2019) 23:39–44. doi: 10.1089/gtmb.2018.0182
30. Nenonen HA, Lindgren IA, Prahl AS, Trzybulska D, Kharraziha I, Hulten M, et al. The N680S variant in the follicle-stimulating hormone receptor gene identifies hyperresponders to controlled ovarian stimulation. Pharmacogenet Genomics. (2019) 29:114–120. doi: 10.1097/FPC.0000000000000374
31. Garcia-Jimenez G, Zarinan T, Rodriguez-Valentin R, Mejia-Dominguez NR, Gutierrez-Sagal R, Hernandez-Montes G, et al. Frequency of the T307A, N680S, and −29G>A single-nucleotide polymorphisms in the follicle-stimulating hormone receptor in Mexican subjects of Hispanic ancestry. Reprod Biol Endocrinol. (2018) 16:100. doi: 10.1186/s12958-018-0420-4
32. Zamaniara T, Taheripanah R, Ghaderian SMH, Zamaniara E, Aghabozorgi SSA. Polymorphism FSHR (-29G/A) as a genetic agent together with ESRI (XbaIG/A) in women with poor response to controlled ovarian hyperstimulation. Hum Antibodies. (2017) 26:143–7. doi: 10.3233/HAB-180332
33. den Dunnen JT, Dalgleish R, Maglott DR, Hart RK, Greenblatt MS, McGowan-Jordan J, et al. HGVS recommendations for the description of sequence variants: 2016 update. Hum Mutat. (2016) 37:564–9. doi: 10.1002/humu.22981
34. Huhtaniemi IT, Themmen AP. Mutations in human gonadotropin and gonadotropin-receptor genes. Endocrine. (2005) 26:207–17. doi: 10.1385/ENDO:26:3:207
35. Matthews CH, Borgato S, Beck-Peccoz P, Adams M, Tone Y, Gambino G, et al. Primary amenorrhoea and infertility due to a mutation in the beta-subunit of follicle-stimulating hormone. Nat Genet. (1993) 5:83–6. doi: 10.1038/ng0993-83
36. Phillip M, Arbelle JE, Segev Y, Parvari R. Male hypogonadism due to a mutation in the gene for the beta-subunit of follicle-stimulating hormone. N Engl J Med. (1998) 338:1729–32. doi: 10.1056/NEJM199806113382404
37. Layman LC, Porto AL, Xie J, da Motta LA, da Motta LD, Weiser W, et al. FSH beta gene mutations in a female with partial breast development and a male sibling with normal puberty and azoospermia. J Clin Endocrinol Metab. (2002) 87:3702–7. doi: 10.1210/jcem.87.8.8724
38. Grigorova M, Punab M, Ausmees K, Laan M. FSHB promoter polymorphism within evolutionary conserved element is associated with serum FSH level in men. Hum Reprod. (2008) 23:2160–6. doi: 10.1093/humrep/den216
39. Schuring AN, Busch AS, Bogdanova N, Gromoll J, Tuttelmann F. Effects of the FSH-beta-subunit promoter polymorphism−211G->T on the hypothalamic-pituitary-ovarian axis in normally cycling women indicate a gender-specific regulation of gonadotropin secretion. J Clin Endocrinol Metab. (2013) 98:E82–6. doi: 10.1210/jc.2012-2780
40. Rull K, Grigorova M, Ehrenberg A, Vaas P, Sekavin A, Nommemees D, et al. FSHB−211 G>T is a major genetic modulator of reproductive physiology and health in childbearing age women. Hum Reprod. (2018) 33:954–66. doi: 10.1093/humrep/dey057
41. Alviggi C, Conforti A, De Rosa P, Strina I, Palomba S, Vallone R, et al. The distribution of stroma and antral follicles differs between insulin-resistance and hyperandrogenism-related polycystic ovarian syndrome. Front Endocrinol. (2017) 8:117. doi: 10.3389/fendo.2017.00117
42. Desai SS, Roy BS, Mahale SD. Mutations and polymorphisms in FSH receptor: functional implications in human reproduction. Reproduction. (2013) 146:R235–48. doi: 10.1530/REP-13-0351
43. Orio F Jr., Ferrarini E, Cascella T, Dimida A, Palomba S, Gianetti E, et al. Genetic analysis of the follicle stimulating hormone receptor gene in women with polycystic ovary syndrome. J Endocrinol Invest. (2006) 29:975–82. doi: 10.1007/BF03349210
44. Lalioti MD. Impact of follicle stimulating hormone receptor variants in fertility. Curr Opin Obstet Gynecol. (2011) 23:158–67. doi: 10.1097/GCO.0b013e3283455288
45. Alviggi C, Humaidan P, Ezcurra D. Hormonal, functional and genetic biomarkers in controlled ovarian stimulation: tools for matching patients and protocols. Reprod Biol Endocrinol. (2012) 10:9. doi: 10.1186/1477-7827-10-9
46. Casarini L, Moriondo V, Marino M, Adversi F, Capodanno F, Grisolia C, et al. FSHR polymorphism p.N680S mediates different responses to FSH in vitro. Mol Cell Endocrinol. (2014) 393:83–91. doi: 10.1016/j.mce.2014.06.013
47. Riccetti L, De Pascali F, Gilioli L, Santi D, Brigante G, Simoni M, et al. Genetics of gonadotropins and their receptors as markers of ovarian reserve and response in controlled ovarian stimulation. Best Pract Res Clin Obstet Gynaecol. (2017) 44:15–25. doi: 10.1016/j.bpobgyn.2017.04.002
48. Laisk-Podar T, Kaart T, Peters M, Salumets A. Genetic variants associated with female reproductive ageing–potential markers for assessing ovarian function and ovarian stimulation outcome. Reprod Biomed Online. (2015) 31:199–209. doi: 10.1016/j.rbmo.2015.05.001
49. Overbeek A, Kuijper EA, Hendriks ML, Blankenstein MA, Ketel IJ, Twisk JW, et al. Clomiphene citrate resistance in relation to follicle-stimulating hormone receptor Ser680Ser-polymorphism in polycystic ovary syndrome. Hum Reprod. (2009) 24:2007–13. doi: 10.1093/humrep/dep114
50. Borgbo T, Jeppesen JV, Lindgren I, Lundberg Giwercman Y, Hansen LL, Yding Andersen C. Effect of the FSH receptor single nucleotide polymorphisms (FSHR 307/680) on the follicular fluid hormone profile and the granulosa cell gene expression in human small antral follicles. Mol Hum Reprod. (2015) 21:255–61. doi: 10.1093/molehr/gau106
51. Tohlob D, Abo Hashem E, Ghareeb N, Ghanem M, Elfarahaty R, Byers H, et al. Association of a promoter polymorphism in FSHR with ovarian reserve and response to ovarian stimulation in women undergoing assisted reproductive treatment. Reprod Biomed Online. (2016) 33:391–7. doi: 10.1016/j.rbmo.2016.06.001
52. Alviggi C, Pettersson K, Longobardi S, Andersen CY, Conforti A, De Rosa P, et al. A common polymorphic allele of the LH beta-subunit gene is associated with higher exogenous FSH consumption during controlled ovarian stimulation for assisted reproductive technology. Reprod Biol Endocrinol. (2013) 11:51. doi: 10.1186/1477-7827-11-51
53. Conforti A, Cariati F, Vallone R, Alviggi C, de Placido G. Individualization of treatment in controlled ovarian stimulation: myth or reality? Biochim Clin. (2017) 41:294–305. doi: 10.19186/BC_2017.051
54. Alviggi C, Conforti A, Esteves SC, Vallone R, Venturella R, Staiano S, et al. Understanding ovarian hypo-response to exogenous gonadotropin in ovarian stimulation and its new proposed marker-the follicle-to-oocyte (FOI) index. Front Endocrinol. (2018) 9:589. doi: 10.3389/fendo.2018.00589
55. Esteves SC, Roque M, Bedoschi GM, Conforti A, Humaidan P, Alviggi C. Defining low prognosis patients undergoing assisted reproductive technology: POSEIDON criteria-the why. Front Endocrinol. (2018) 9:461. doi: 10.3389/fendo.2018.00461
56. Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, Esteves SC, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril. (2016) 105:1452–3. doi: 10.1016/j.fertnstert.2016.02.005
57. Esteves SC, Humaidan P, Alviggi C, Fischer R. The novel POSEIDON stratification of 'Low prognosis patients in Assisted Reproductive Technology' and its proposed marker of successful outcome. F1000Research. (2016) 5:2911. doi: 10.12688/f1000research.10382.1
58. Conforti A, Esteves SC, Picarelli S, Iorio G, Rania E, Zullo F, et al. Novel approaches for diagnosis and management of low prognosis patients in assisted reproductive technology: the POSEIDON concept. Panminerva Med. (2019) 61:24–9. doi: 10.23736/S0031-0808.18.03511-5
59. Alviggi C, Guadagni R, Conforti A, Coppola G, Picarelli S, De Rosa P, et al. Association between intrafollicular concentration of benzene and outcome of controlled ovarian stimulation in IVF/ICSI cycles: a pilot study. J Ovarian Res. (2014) 7:67. doi: 10.1186/1757-2215-7-67
60. Sychev DA, Malova EU. Evidence-based pharmacogenetics: Is it possible? Int J Risk Saf Med. (2015) 27(Suppl. 1):S97–8. doi: 10.3233/JRS-150706
61. Alviggi C, Conforti A, Fabozzi F, De Placido G. Ovarian stimulation for IVF/ICSI cycles: A pharmacogenomic approach. Med Ther Med Reproduct Gynecol Endocrinol. (2009) 11:271–7. doi: 10.1684/mte.2009.0255
62. Alviggi C, Conforti A, Esteves SC. Impact of mutations and polymorphisms of gonadotrophins and their receptors on the outcome of controlled ovarian stimulation. In: Ghumman S, editor. Principles and Practice of Controlled Ovarian Stimulation in ART. New Delhi: Springer (2015). p. 147–56. doi: 10.1007/978-81-322-1686-5_14
63. Nilsson C, Pettersson K, Millar RP, Coerver KA, Matzuk MM, Huhtaniemi IT. Worldwide frequency of a common genetic variant of luteinizing hormone: an international collaborative research. International Collaborative Research Group. Fertil Steril. (1997) 67:998–1004. doi: 10.1016/S0015-0282(97)81430-6
64. Genro VK, Matte U, De Conto E, Cunha-Filho JS, Fanchin R. Frequent polymorphisms of FSH receptor do not influence antral follicle responsiveness to follicle-stimulating hormone administration as assessed by the Follicular Output RaTe (FORT). J Assist Reprod Genet. (2012) 29:657–63. doi: 10.1007/s10815-012-9761-7
65. Alviggi C, Clarizia R, Mollo A, Ranieri A, De Placido G. Who needs LH in ovarian stimulation? Reproductive BioMedicine Online. (2006) 12:599–607. doi: 10.1016/S1472-6483(10)61186-8
66. Alviggi C, Mollo A, Clarizia R, De Placido G. Exploiting LH in ovarian stimulation. Reproduct Bio Med Online. (2006) 12:221–33. doi: 10.1016/S1472-6483(10)60865-6
Keywords: FSH, FSH receptor, polymorphisms, mutations, ovarian stimulation, assisted reproductive technology, IVF, genetic variants
Citation: Conforti A, Vaiarelli A, Cimadomo D, Bagnulo F, Peluso S, Carbone L, Di Rella F, De Placido G, Ubaldi FM, Huhtaniemi I and Alviggi C (2019) Pharmacogenetics of FSH Action in the Female. Front. Endocrinol. 10:398. doi: 10.3389/fendo.2019.00398
Received: 16 March 2019; Accepted: 05 June 2019;
Published: 26 June 2019.
Edited by:
Sandro C. Esteves, Androfert, Andrology and Human Reproduction Clinic, BrazilReviewed by:
Giulia Brigante, University of Modena and Reggio Emilia, ItalyCopyright © 2019 Conforti, Vaiarelli, Cimadomo, Bagnulo, Peluso, Carbone, Di Rella, De Placido, Ubaldi, Huhtaniemi and Alviggi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandro Conforti, Y29uZmFsZUBob3RtYWlsLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.