
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 27 November 2018
Sec. Reproduction
Volume 9 - 2018 | https://doi.org/10.3389/fendo.2018.00686
 Laura Melado1*
Laura Melado1* Barbara Lawrenz1,2
Barbara Lawrenz1,2 Junard Sibal1
Junard Sibal1 Emmanuel Abu1
Emmanuel Abu1 Carol Coughlan1
Carol Coughlan1 Alfredo T. Navarro3
Alfredo T. Navarro3 Human Mousavi Fatemi1
Human Mousavi Fatemi1Anti-Müllerian hormone (AMH) is an important ovarian reserve marker for baseline assessment and therapeutic strategy in fertility treatments, which is considered reliable when measured on any day of the cycle. Recent data have pointed toward significant fluctuations of AMH and questioned whether a single measurement is reliable for clinical decision-making. The aim of this study was to evaluate whether the AMH does have significant variations during a natural cycle when a fully automated assay is used for the sample analysis. We performed a prospective study including healthy volunteers with regular cycles, from April to December 2017. Blood samples for AMH, FSH, LH, estradiol, and progesterone were obtained on day 2/3, day 10, day of LH surge, luteal phase and day 2/3 of subsequent menses. AMH analysis was performed with Elecsys® AMH automated assay. Trial was registered with clinical.trials.gov: NCT03106272. One hundred samples from 22 women with a mean age of 30.74 ± 0.11 years and a BMI of 23.23 ± 0.63 kg/m2 were analyzed. There was a substantial longitudinal fluctuation in AMH levels, indicated by the coefficient of variation (CV) intra-cycle of 0.2070 ± 0.143. A positive correlation between LH and AMH concentrations was found at the moment of LH rise (p < 0.0001). Absolute intra-individual inter-cyclic variability was 0.75 ng/mL (range: 0.03–2.81 ng/mL) and inter-cycle CV was 0.28 (Confidence interval: 0.16–0.39; p < 0.0001). According to our results, with the use of a fully automated assay in natural cycle, AMH shows significant intra- and inter-cycle variations, which are not caused by analytical variability. Future investigations, evaluating AMH dynamics and the best time for AMH assessment should be conducted.
Anti-Müllerian hormone (AMH) is a dimeric glycoprotein produced by granulosa cells of pre-antral and small antral follicles in the ovary and its production is independent of follicle stimulating hormone (FSH) (1). The release of AMH from the granulosa cells results in measurable serum levels and these concentrations have been shown to be proportional to the number of developing follicles in the ovaries (2). Therefore, AMH has emerged as one of the most important clinical indirect markers for ovarian reserve (3) and is used by clinicians for predicting ovarian response to hyperstimulation for IVF, facilitating counseling of patients and individualization of stimulation regimens. Furthermore, a recent publication demonstrated a strong positive, age-independent relationship between AMH level and number of euploid blastocysts obtained following IVF/ICSI cycles (4), also with miscarriage rates (5).
It is well recognized that serum AMH displays a high inter-individual variability mainly attributed to the different number in antral follicles within groups of women of similar age (2). It also appears to vary with factors such as hormonal contraceptive use, pregnancy, body mass index, smoking (6), and the use of gonadotropins for ovarian stimulation (7, 8). Serum AMH is generally believed to be a reliable test that may be measured on any day of a natural cycle, with minimal intra-individual variability (6, 9–13). However, recent publications have raised doubts as to the reliability of a single AMH test taken ad-hoc in a natural cycle. Gorkem et al. demonstrated that serum AMH levels seem to be higher during the follicular phase as compared to the luteal phase in infertile women with normal, high, and low ovarian reserve (14). Other authors have described important variability in AMH concentrations during the menstrual cycle, which was deemed higher than the fluctuations expected due to the analysis alone (15, 16). Moreover, it has been proposed that young women may have a pattern of intra-cycle fluctuation that differs from that of older women (17). Circadian fluctuations for AMH serum levels have also been identified (18).
These fluctuations in AMH during the natural cycle were previously attributed to analytical variations caused by different conditions used for sample storage and/or the assay method (19). But now, AMH can be analyzed with fully automated AMH assays which are highly sensitive and precise, with a broad linear range (20) allowing for more efficient sample processing and reducing possible procedural errors (20–22).
Nowadays, it is widespread accepted that a single AMH measurement as an accurate reflection of a patients' ovarian reserve and clinicians take this result into serious consideration when counseling women about their reproductive health. In light of recent publications demonstrating AMH variability both throughout the menstrual cycle and between consecutive cycles, questions rise as to whether a single AMH measurement would be reliable. The aim of this study was to investigate AMH serum levels in healthy, cycling women using fully automated assay ElecsysⓇ AMH (Roche, for Cobas 601 platformⓇ), at defined time-points of the natural cycle and short-term inter-cycle variations.
Twenty-two healthy volunteers were included in this study, which was undertaken from April to December 2017, in IVIRMA Abu Dhabi clinic. Women between the ages of ≥18 to ≤ 38 years old, with regular menstrual cycles between 28 and 32 days and BMI between ≥18 and ≤ 28 kg/m2 were included. Exclusion criteria included the following factors: the intake of hormonal contraceptives for a minimum of 2 months immediately prior to study commencement, pregnancy, breastfeeding, and previous conditions which may adversely affect ovarian reserve (ovarian surgery, chemotherapy, pelvic radiation). No volunteers with infertility background were included. The participants fulfilled a written questionnaire including menstrual cycle pattern, previous pregnancies, deliveries and miscarriages, ethnicity, smoking and possible exclusion criteria. They were informed about the methods, sample processing and objectives of the study and all of them signed informed consent.
Blood samples were obtained by venepuncture in the clinic between 8 am and 2 pm, transferring the samples immediately to the laboratory located in the same center for immediate processing and further analysis. During the natural cycle, samples were collected on day 2/3 (AMH_01), day 10 (mid follicular phase, AMH_MFP), day of LH rise (AMH_LHR), mid luteal phase (AMH_MLP), and day 2/3 of the subsequent menstruation (AMH_02). The LH surge was defined for the purpose of our study to have begun when the concentration of LH rose by 180% above the latest serum value available in that patient and continued to rise thereafter (23). For this purpose, LH, estradiol and progesterone were monitored starting on day 10 of a natural cycle and then every 3 days until LH surge was diagnosed (24, 25). The luteal phase was confirmed by an elevated serum progesterone level (>3 ng/ml) 8 days after LH surge.
One sample of 5 mL of blood was collected each time. Blood was centrifuged and serum separated in two parts: one for AMH analysis and one for gonadotropin and steroid hormone analysis. Analysis of FSH, LH, estradiol and progesterone was performed using a competitive immunoassay manufactured by Roche on the Cobas 601 platform®. Results for these hormones were obtained and evaluated on the same day of blood collection. Serum samples for AMH were aliquoted, frozen at −20°C the same day of collection and stored for batch analysis. All samples from each study participant were analyzed under the same conditions on the same day, using Elecsys® AMH automated assay (for Cobas 601 platform, Roche®) according to manufacturer‘s instructions. Imprecision expected from the assay was <5%, as described by the manufacturer; intra-assay and inter-assay co-efficient of variation for Elecsys® AMH automated assay has been reported as 0.5–1.4 and 0.7–1.9%, respectively (26).
A research database was generated for all the variables that were evaluated. The export data were correctly anonymized and analyzed with SPSS 22.0.
Demographic variables and hormonal values were summarized with mean and standard deviation. In order to describe hormones and their dynamics, we determined the mean value and standard deviations each time and represented it with boxplot figures. Moreover, we performed polynomial interpolation for dynamic values and represented the variations with mean polynomial interpolation and variance band.
AMH values were represented for AMH dynamic description using a 4-degree-polynomial-exponential interpolation of data. This polynomial-exponential interpolation was used as well to impute missing data (10 missing data points). Patients were not categorized according to initial AMH since homogeneous variability in cluster groups could not be previously assumed.
Pearson correlation test was performed between AMH values and other hormones along with temporary marks and clustering analyses were done for correlation between AMH dynamic patterns and demographics parameters. For the evaluation of any AMH dynamic patterns related with demographics parameters, we applied clustering analyses over AMH polynomial coefficients that were previously calculated. A p-value of 0.05 was considered statistically significant.
The study was approved by the internal Ethics Committee under the number REFA011 and registered for clinical.trials.gov: NCT03106272.
A total of 100 samples from the 22 women were analyzed, with a mean age of 30.7 ± 4.11 years and a BMI of 23.2 ± 3.63 Kg/m2 (Table 1). All the samples were analyzed with Elecsys® AMH automated assay for Cobas 601 platform (Roche®).
The values of serum AMH concentrations obtained during the different phases of the menstrual cycle are shown in Table 2 and the fluctuations presented in AMH levels for each participant are represented in Figure 1. A significant longitudinal fluctuation in AMH levels was found per participant, expressed as coefficient of variation (CV) intra-cycle of 20.7%. This was calculated for all individual readings per cycle day (CV = mean 0.207 ± SD 0.143; [CI95: 0.148, 0.267]; Shapiro test = 0.0016). No pattern of fluctuation was observed for these variations.
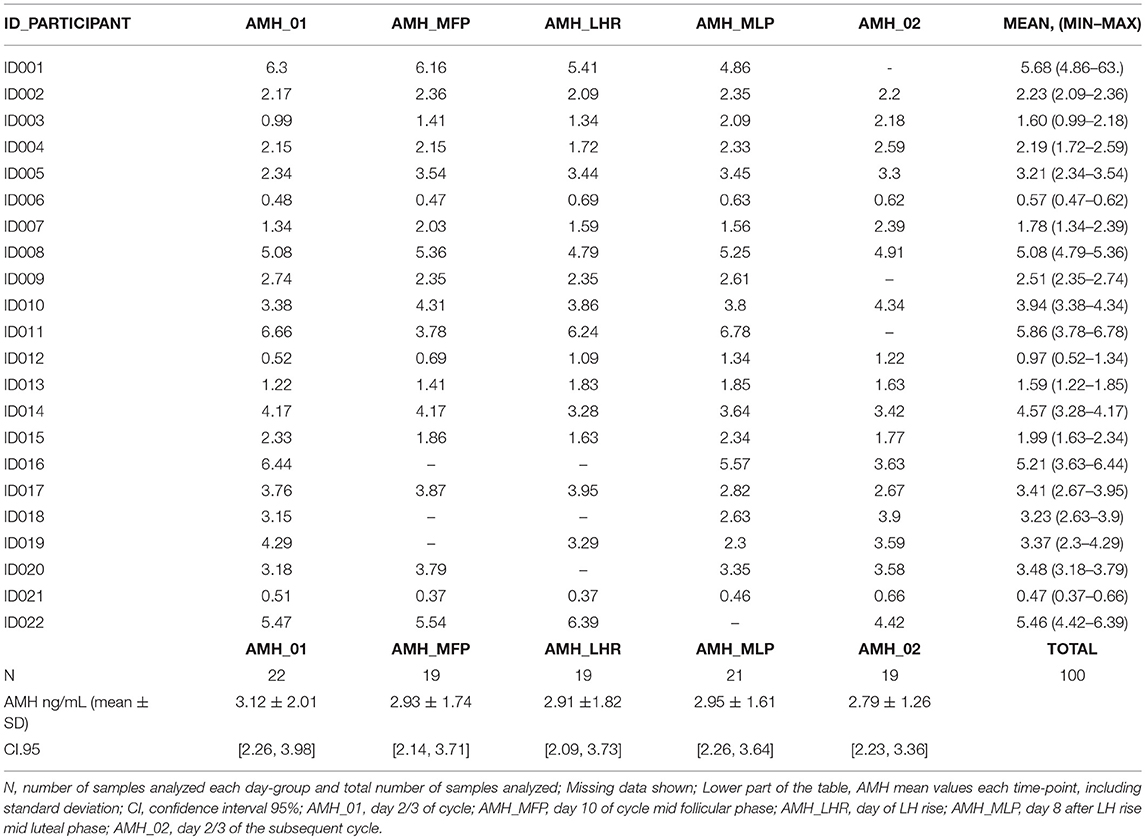
Table 2. AMH values obtained each day of measurement for all the 22 participants, including the mean, minimum value and maximum value.
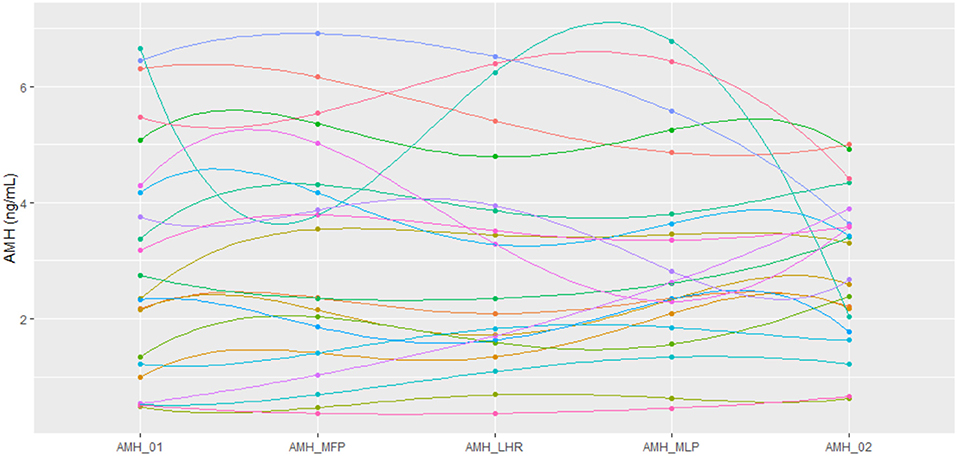
Figure 1. Representation of AMH values using a polynomial-exponential interpolation of data for each patient at every time point. AMH_01: day 2/3 of cycle. AMH_MFP: day 10 of cycle, mid follicular phase. AMH_LHR: day of LH rise. AMH_MLP: day 8 after LH rise, mid luteal phase. AMH_02: day 2/3 of the subsequent cycle.
Comparing the results between day 2/3 of cycle in both consecutive cycles, mean absolute inter-cycle variation of AMH was 0.75 ng/mL, with a range from 0.03 to 2.81 ng/mL. Inter-cycle CV was 0.28 (CI95: 0.16-0.39; p < 0.0001). Hence, in our participants, a 28% of variation between the AMH values measured on day 2/3 of two consecutive menstruations was seen.
The average of the AMH values obtained on each day of measurement for all the 22 participants varies significantly through the cycle as shown by the data in Table 2, as well as represented in boxplot graph (Figure 2), where the intra-cycle variation and inter-cycle variation are shown.
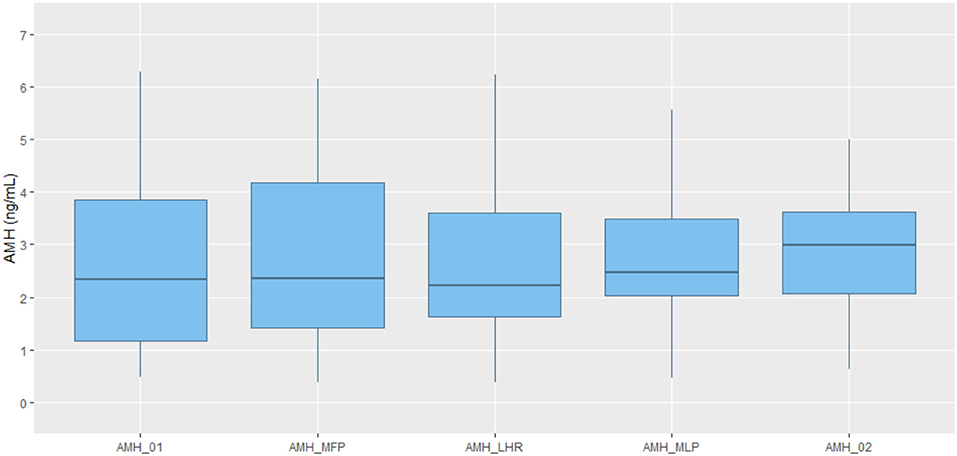
Figure 2. Boxplot representation of AMH values obtained at each time point. Line in the middle of the box representing AMH median value. Box representing 25 and 75% quartile. Upper whisker extends from the hinge to the largest value no further than 1.5*IQR (inter-quartile range). Lower whisker extends from the hinge to the lowest value. AMH_01: day 2/3 of cycle. AMH_MFP: day 10 of cycle, mid follicular phase. AMH_LHR: day of LH rise. AMH_MLP: day 8 after LH rise, mid luteal phase. AMH_02: day 2/3 of the subsequent cycle.
All hormone values for AMH, FSH, LH, estradiol and progesterone are shown in Table 3. No correlation between AMH and FSH, estradiol and progesterone was observed at any time of the measurements. However, a significant positive correlation between LH_LHR and AMH concentrations was found (t-value: 5.962; p < 0.0001).
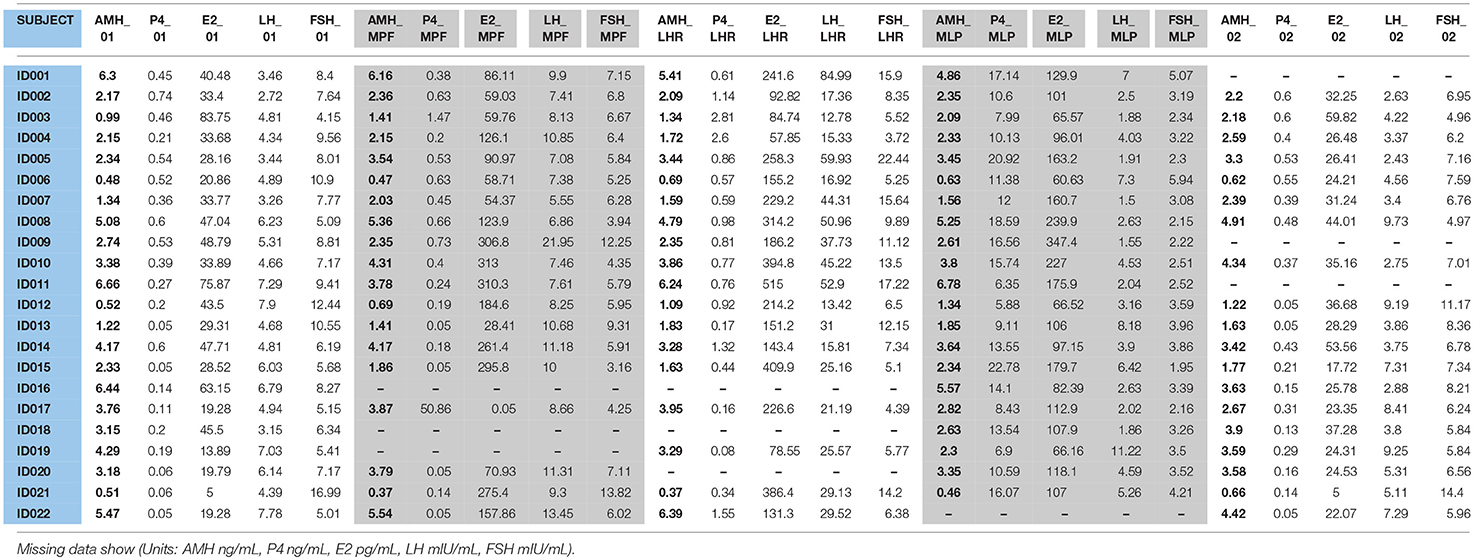
Table 3. AMH, FSH, LH, E2, and P4 values obtained each day of measurement for all the 22 participants.
One of the advantages of AMH as compared to other measures of ovarian reserve such as follicle stimulating hormone (FSH) is that it is thought to have minimal variation within the menstrual cycle and as a result, it can be tested on any day of the cycle. However, studies assessing the stability of AMH levels throughout the menstrual cycle have been conflicting and difficult to interpret due to the differences in assays used. The current study clearly demonstrated a statistically significant variation in serum AMH levels using a fully automated AMH assay during a spontaneous menstrual cycle in a group of healthy reproductive aged women. This finding is in keeping with other previous studies (27–30), some of which identified a particular pattern of change with AMH levels at their highest in the mid-follicular phase, decreasing at the time of ovulation with a rise again in the luteal phase (16, 28, 29, 31). This pattern has been suggested to reflect the role of AMH in follicular growth (32). The current analysis revealed that women, regardless of their ovarian reserve, exhibited a statistically significant intra- and inter-cycle AMH variation, however, a clear fluctuation pattern could not be identified. Notably, a significant positive co-variation between serum levels of AMH and LH was observed during the LH rise. This association between LH and AMH levels was described as well by Bungum et al. (18), when they investigated circadian variations of serum AMH. This correlation between both hormones points to a joint regulatory mechanism between AMH and the secretion of LH, supported by the recent finding of AMH receptors in significant subset of GnRH central neurons in mice and humans (33), which should encourage further research.
For the women included, AMH values varied 20.7% throughout the cycle from the first serum analysis obtained at the beginning of the cycle to day 2/3 of the consecutive menses. These AMH fluctuations are more than three times higher than the expected values attributed to assay imprecision (<5% assay imprecision expected for Elecsys® AMH automated assay) and may, therefore, be reflecting biological fluctuations. Intra-individual variations have been also demonstrated using different assays for the serum analysis (34).
The evidence to date regarding variability of AMH across the menstrual cycle is conflicting (19). Some of these discrepancies may be explained by inclusion of anovulatory cycles, infrequent blood sampling, sample handling or assay interference. Related to that, the present study has several strengths. All blood samples per study subject were assayed in one run on the same day in the same Cobas Elecsys platform, thereby reducing analytical variability and confounding factors. Future studies may consider as well repeating the analysis of the samples on a separate Cobas Elecsys platform for a quality assurance validation. Another important strength is that an average of 4.5 samples per participant were analyzed throughout the natural cycle and ovulation was confirmed by measurement of serum LH and luteal phase progesterone concentrations in all cycles, avoiding the low accuracy of the urine tests to detect ovulation. In addition, a fully automated AMH assay was used for sample analysis in this study, which has previously shown to have low analytical variability across the assay range (22, 35). All the samples were obtained in the morning or early afternoon, to avoid possible circadian variations (18). However, our number of study participants is limited.
The intra-cycle variations in healthy women identified are similar to the data published by Hadlow et al., which revealed an average intra-individual variation of AMH concentration of 20% (combined analytical and biological) in infertile women with ovulatory cycles during a study period of 3 cycles (16). In contrast, the participants of the present study are a representative sample of reproductive aged women with ovulatory cycles. Furthermore, a significant intra-individual variation was identified in a shorter period of time (one ovulatory cycle). The extent of AMH-variation in the current analysis is higher than previously described by Lambert-Messerlian, who concluded that despite a variation of 16% throughout the cycle, sample collection could be performed on any day for assessment of ovarian reserve (15). The variability of more than 20% with a fully automated AMH assay in the present study ought to be a reminder to clinicians that sampling AMH on any day of the menstrual cycle may not adequately reflect the true ovarian reserve.
It is apparent that AMH levels fluctuate not only within cycles, but also between cycles. An inter-cycle variability of 28% (p < 0.0001) between 2 consecutive cycles was identified in the present study. Furthermore, 4 participants with AMH levels <1.2 ng/mL on day 2/3 of the first cycle (AMH_01), would have been categorized as having a low AMH based on the Bologna Criteria (36). Two of these four volunteers showed normal AMH levels after reassessing on day 2/3 of the consecutive cycle (AMH_02). These differences in consecutive cycles may be a reflection of variances in antral follicle counts (AFC). It may also reflect the different ovarian response that can be seen in clinical practice despite prescribing the same treatment protocol to the same patient but in a different cycle (37, 38). This is a very important finding, which could change current clinical practice in terms of AMH testing. Inter-cycle assessment of serum AMH on day 2/3 certainly merits further study.
While real ovarian reserve may not vary during a natural cycle or between consecutive cycles, serum AMH levels do, due to the biological variation and, in addition, unusual AMH isoforms that have been described as well (39). It is clear that AMH is not an easy molecule to quantify. However, important clinical decisions are often based on one random AMH recording, even the dose of gonadotropins to be prescribed in IVF/ICSI cycles (34).
Previous publications have shown that AMH measurement when performed during the first few days of the cycle correlates well with ovarian response to stimulation (basal AMH) (7, 8). Additionally, a combined evaluation of serum AMH and ultrasound evaluation of antral follicle count (AFC) during the first days of the menstrual cycle is recommended prior to counseling of women regarding their reproductive health and the ovarian stimulation outcomes (8, 40). By combining both ovarian markers, AMH and AFC on day 1-3 of cycle, clinicians will be able to determine with greater accuracy the developing follicles expected to grow further following hormone stimulation. This will enable the clinicians to prescribe the most appropriate dosage of gonadotropins for ovarian stimulation at any given cycle, offering even more individualized treatment.
In summary, this study clearly demonstrated significant intra- and inter-cycle variations in serum AMH concentrations throughout natural ovulatory cycles and between two consecutive menstruations using a fully automated AMH assay. Based on these results it is reasonable to question current clinical practice of using a single random AMH measurement in determining ovarian reserve.
The authors listed on this manuscript substantially contributed to the study conception and design, acquisition and interpretation of data, and drafting/revising the manuscript. All have given final approval of the version to be published.
Authors are nowadays employed by IVIRMA. LM, BL, JS, EA, CC, and HF in IVIRMA Middle East—UAE and AN in IVI Foundation—IVIRMA Valencia (Spain).
We thank Mrs. Elissavet Kamperi for data collection.
1. Monniaux D, Clément F, Dalbiés Tran R, Estienne A, Fabre S, Mansanet C, et al. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: What is the link? Biol Reprod. (2014) 90:1–11. doi: 10.1095/biolreprod.113.117077
2. Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Müllerian hormone in women. Human Reprod Update (2014) 20:370–85. doi: 10.1093/humupd/dmt062
3. Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, Groome NP, et al. Serum anti-Müllerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology (2006) 147:3228–34. doi: 10.1210/en.2005-1588
4. La Marca A, Minasi MG, Sighinolfi G, Greco P, Argento C, Grisendi V, et al. Female age, serum Anti-Müllerian hormone level and number of oocytes affect the rate and number of euploid blastocysts in in vitro fertilization / intracytoplasmic sperm injection cycles. Fertil Steril. (2017) 108:777–83. doi: 10.1016/j.fertnstert.2017.08.029
5. Tarasconi B, Tadros T, Ayoudi JM, Belloc S, de Ziegler, Fanchin R. Serum Anti-Müllerian hormone levels are independently related to miscarriage rates after in vitro fertilization – embryo transfer. Fertil Steril. (2017) 108:518–24. doi: 10.1016/j.fertnstert.2017.07.001
6. La Marca A, Grisendi V, Griesinger G. How much does AMH really vary in normal women? Int J Endocrinol. (2013) 2013:959487. doi: 10.1155/2013/959487
7. Lee JR, Kim SH, Kim SM, Jee BC, Ku SY, Suh CS, et al. Anti-Müllerian hormone dynamics during controlled ovarian hyper stimulations and optimal timing of measurement for outcome prediction. Hum Reprod. (2010) 25:2597–604. doi: 10.1093/humrep/deq204
8. Melado L, Fernández-Nistal A, Martínez V, Verdú V, Bruna I, Bajo JM. Anti-Müllerian hormone dynamics during GNRH-antagonist short protocol for IVF/ ICSI in women with varying ovarian reserve levels. Minerva Ginecol. (2017) 69:128–34. doi: 10.23736/S0026-4784.16.03951-4
9. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ, et al. Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. (2006) 91:4057–63. doi: 10.1210/jc.2006-0331
10. Iliodromiti S, Kelsey TW, Wu O, Anderson RA, Nelson SM. The predictive accuracy of anti-Müllerian hormone for live birth after assisted conception: a systematic review and meta-analysis of the literature. Hum. Reprod Update (2014) 20:560–70. doi: 10.1093/humupd/dmu003
11. Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy C, Englert Y. Stable serum levels of anti-Mullerian hormone during the menstrual cycle: a prospective study in normo- ovulatory women. Hum Reprod. (2007) 22:1837–40. doi: 10.1093/humrep/dem101
12. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Müllerian hormone throughout the human menstrual cycle. Hum Reprod. (2006) 21:3103–7. doi: 10.1093/humrep/del291
13. van Disseldorp J, Lambalk CB, Kwee J, Looman CW, Eijkemans MJ, Fauser BC, et al. Comparison of inter- and intra-cycle variability of anti-Müllerian hormone and antral follicle counts. Hum Reprod. (2010) 25:221–7. doi: 10.1093/humrep/dep366
14. Gorkem U, Kucukler FK, Togrul C, Gungor T. Anti-Müllerian hormone exhibits a great variation in infertile women with different ovarian reserve patterns. Aust N Z J Obstet Gynaecol. (2017) 57:464–8. doi: 10.1111/ajo.12625
15. Lambert-Messerlian G, Plante B, Eklund EE, Raker C, Moore RG. Levels of anti-Müllerian hormone in serum during the normal menstrual cycle. Fertil Steril. (2016) 105:208–13. doi: 10.1016/j.fertnstert.2015.09.033
16. Hadlow N, Brown SJ, Habib A, Wardrop R, Joseph J, Gillett M, et al. Quantifying the intraindividual variation of anti-müllerian hormone in the ovarian cycle. Fertil Steril. (2016) 106:1230–7. doi: 10.1016/j.fertnstert.2016.06.009
17. Overbeek A, Broekmans FJ, Hehenkamp WJ. Intra-cycle fluctuations of anti-Müllerian hormone in normal women with regular cycle: a re-analysis. Reprod Biomed Online (2012) 24:664–9. doi: 10.1016/j.rbmo.2012.02.023
18. Bungum L, Jacobsson AK, Rosén F, Becker C, Becker C, Yding Andersen C, Güner N, et al. Circadian variation in concentration of anti-Müllerian hormone in regularly menstruating females: relation to age, gonadotrophin and sex steroid levels. Hum Reprod. (2011) 26:678–84. doi: 10.1093/humrep/deq380
19. Rustamov O, Smith A, Roberts SA, Yates AP, Fitzgerald C, Krishnan M, et al. The measurement of anti-Müllerian hormone: a critical appraisal. J Clin Endocrinol Metab. (2014) 99:723–32. doi: 10.1210/jc.2013-3476
20. Deeks E. Elecsys® AMH Assay: a review in anti-müllerian hormone quantification and assessment of ovarian reserve. Mol Diagn Ther. (2015) 19:245–9. doi: 10.1007/s40291-015-0156-1
21. Gassner D, Jung R. First fully automated immunoassay for anti-Müllerian hormone. Clin Chem Lab Med. (2014) 52:1143–52. doi: 10.1515/cclm-2014-0022
22. Anckaert E, Oktem M, Thies A, Cohen-Bacrie M, Daan N, Schiettecatte J, et al. Multicenter analytical performance evaluation of a fully automated anti-Müllerian hormone assay and reference interval determination. Clin Biochem. (2016) 49:260–7. doi: 10.1016/j.clinbiochem.2015.10.008
23. Fatemi HM, Kyrou D, Bourgain C, Van den Abbeel E, Griesinger G, Devroey P. Cryopreserved-thawed human embryo transfer: spontaneous natural cycle is superior to human chorionic gonadotropin-induced natural cycle. Fertil Steril. (2010) 94:2054–8. doi: 10.1016/j.fertnstert.2009.11.036
24. Park SJ, Goldsmith L, Skurnick J, Wojtczuk A, Weiss G. Characteristics of the urinary luteinizing hormone surge in young ovulatory women. Fertil Steril. (2007) 88:684–90. doi: 10.1016/j.fertnstert.2007.01.045
25. Direito A1, Bailly S, Mariani A, Ecochard R. Relationships between the luteinizing hormone surge and other characteristics of the menstrual cycle in normally ovulating women. Fertil Steril. (2013) 99:279–85. doi: 10.1016/j.fertnstert.2012.08.047
26. Li HWR, Wong BPC, Ip WK, Yeung WSB, Ho PC, Ng EHY. Comparative evaluation of three new commercial immunoassays for anti-Müllerian hormone measurement. Human Reprod. (2016) 31:2796–802. doi: 10.1093/humrep/dew248
27. Sowers M, McConnell D, Gast K, Zheng H, Nan B, McCarthy JD, et al. Anti-Müllerian hormone and inhibin B variability during normal menstrual cycles. Fertil Steril. (2010) 94:1482–6. doi: 10.1016/j.fertnstert.2009.07.1674
28. Cook CL, Siow Y, Taylor S, Fallat M. Serum Müllerian-inhibiting substance levels during normal menstrual cycles. Fertil Steril. (2000) 73:859–61. doi: 10.1016/S0015-0282(99)00639-1
29. Wunder DM, Bersinger NA, Yared M, Kretschmer R, Birkhäuser MH. Statistically significant changes of anti-Müllerian hormone and inhibin levels during the physiologic menstrual cycle in reproductive age women. Fertil Steril. (2008) 89:927–33. doi: 10.1016/j.fertnstert.2007.04.054
30. Kissell KA, Danaher MR, Schisterman EF, Wactawski-Wende J, Ahrens KA, Schliep K, et al. Biological variability in serum anti-Müllerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum Reprod. (2014) 29:1764–72. doi: 10.1093/humrep/deu142
31. Randolph JF, Harlow SD, Helmuth ME, Zheng H, McConnell DS. Updated assays for inhibin B and AMH provide evidence for regular episodic secretion of inhibin B but not AMH in the follicular phase of the normal menstrual cycle. Hum Reprod. (2014) 29:592–600. doi: 10.1093/humrep/det447
32. Gracia C, Shin S, Prewitt M, Chamberlin J, Lofaro L, Jones K, et al. Multi-center clinical evaluation of the Access AMH assay to determine AMH levels in reproductive age women during normal menstrual cycles. J Assist Reprod Genet. (2018) 35:777–83. doi: 10.1007/s10815-018-1141-5
33. Cimino I, Casoni F, Liu X, Messina A, Parkash J, Jamin SP, et al. Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun. (2016) 12:10055. doi: 10.1038/ncomms10055
34. Bungum L, Tagevi J, Jokubkiene L, Bungum M, Giwercman A, Macklon N, et al. The impact of the biological variability or assay performance on AMH measurements: a prospective cohort study with AMH tested on three analytical assay-platforms. Front Endocrinol. (2018) 9:603. doi: 10.3389/fendo.2018.00603
35. Anderson RA, Anckaert E, Bosch E, Dewailly D, Dunlop CE, Fehr D, et al. Prospective study into the value of the automated Elecsys anti-Müllerian hormone assay for the assessment of the ovarian growing follicle pool. Fertil Steril. (2015) 103:1074–80. doi: 10.1016/j.fertnstert.2015.01.004
36. Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. (2011) 26:1616–24. doi: 10.1093/humrep/der092
37. Rombauts L, Onwude JL, Chew HW, Vollenhoven BJ. The predictive value of antral follicle count remains unchanged across the menstrual cycle. Fertil Steril. (2011) 96:1514–8. doi: 10.1016/j.fertnstert.2011.09.005
38. Rombauts L, Lambalk CB, Schultze-Mosgau A, van Kuijk J, Verweij P, Gates D, et al. Intercycle variability of the ovarian response in patients undergoing repeated stimulation with corifollitropin alfa in a gonadotropin-releasing hormone antagonist protocol. Fertil Steril. (2015) 104:884–90. doi: 10.1016/j.fertnstert.2015.06.027
39. Robertson DM, Kumar A, Kalra B, Shah S, Pruysers E, Vanden Brink H, et al. Detection of serum anti-Müllerian hormone in women approaching menopause using sensitive anti-Müllerian hormone enzyme-linked immunosorbent assays. Menopause (2014) 12:1277–86. doi: 10.1097/GME.0000000000000244
Keywords: Anti-Müllerian hormone (AMH), natural cycle, intra-cycle variations, inter-cycle variations, fully automated assay
Citation: Melado L, Lawrenz B, Sibal J, Abu E, Coughlan C, Navarro AT and Fatemi HM (2018) Anti-müllerian Hormone During Natural Cycle Presents Significant Intra and Intercycle Variations When Measured With Fully Automated Assay. Front. Endocrinol. 9:686. doi: 10.3389/fendo.2018.00686
Received: 14 September 2018; Accepted: 02 November 2018;
Published: 27 November 2018.
Edited by:
Katja Teerds, Wageningen University, NetherlandsReviewed by:
Jing Xu, Oregon Health and Science University, United StatesCopyright © 2018 Melado, Lawrenz, Sibal, Abu, Coughlan, Navarro and Fatemi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Melado, bGF1cmEubWVsYWRvQGl2aXJtYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.