- 1Pediatric Clinic, Department of Surgical and Biomedical Sciences, Università Degli Studi di Perugia, Perugia, Italy
- 2Università degli Studi di Milano, Milan, Italy
Hashimoto's thyroiditis (HT) is the most common cause of thyroid disease in children and adolescents. Along with significant modifications of thyroid function, HT in pediatric age can be accompanied by relevant thyroid structural alterations. Over time, benign thyroid nodules, carcinoma and, rarely, primary non-Hodgkin lymphoma can develop. However, the relationships between HT and neoplasms are poorly defined. The main aim of this paper is to discuss what is presently known regarding the coexistence of HT and thyroid tumors. Moreover, we attempt to define the pathogenesis of cancer development in children with HT. Literature analysis showed that despite its rarity and relatively promising prognosis, thyroid cancer is associated with HT. Although not all reasons for the coexistence of these diseases are clearly defined, children with HT should be considered at higher risk for thyroid cancer development. Strict correlations between high levels of serum TSH and anti-thyroid antibodies with cancer must be remembered. The same is true for the presence of nodules, especially if multiple nodules are present and ultrasonography and thyroid fine needle aspiration cytology should be promptly used in uncertain cases.
Introduction
Hashimoto's thyroiditis (HT), also termed chronic lymphocytic thyroiditis, is the most common cause of thyroid disease in children and adolescents. It is an autoimmune disease with an estimated prevalence in pediatrics of 1–2%, with variations according to genetic susceptibility, age and gender, ethnicity, iodine status, the presence of other autoimmune diseases or genetic syndromes and the criteria used for diagnosis (1). HT is more common in children aged 6 to 16 years, in females, in Caucasians, and in countries with iodine deficiency. Moreover, it is more frequently diagnosed in children who suffer from type 1 diabetes, coeliac disease, Addison's disease, autoimmune hypoparathyroidism, Down syndrome, Noonan syndrome, and Turner syndrome, as well as when antibody assays and thyroid fine needle aspiration cytology (TFNAC) are available (1).
At the time of diagnosis, most children with HT show few to no symptoms. A small goiter or the presence of mild clinical symptoms of hypothyroidism are observed in ~70% of the causes of hospital referrals (2). Other reasons include findings upon work-up for an unrelated problem or for one of the diseases mentioned above that pose the child at higher risk of developing HT. Thyroid function, as evidenced by blood thyroid hormone levels, is normal in up to 80% of cases (2). Only a minority has low hormone concentrations, suggesting overt hypothyroidism. In rare cases, hyperthyroidism can be demonstrated. Long-term outcomes of HT can significantly vary and are not predictable in single cases. However, both children who are initially euthyroid and those with subclinical hypothyroidism can develop overt hypothyroidism within a few years from diagnosis. Although this is more common in subclinically hypothyroid patients (3–6), conversion to Graves' disease cannot be excluded (7). The presence of goiter and elevated serum concentrations of anti-thyroglobulin antibody (TG-Ab) and anti-thyroid peroxidase antibody (TPO-Ab) at diagnosis or the progressive increase in serum TSH levels suggests an increased risk of hypothyroidism (5, 8). Finally, patients with hyperthyroidism generally become euthyroid and only occasionally develop hypothyroidism (4).
Along with significant modifications of thyroid function, HT in children can be accompanied by relevant thyroid structural alterations. Over time, benign thyroid nodules, carcinoma and, rarely, primary non-Hodgkin lymphoma can develop (9–11). However, the relationships between HT and neoplasms are poorly defined. In particular, it is not known whether HT is a predisposing factor to the development of thyroid neoplasms and whether other clinical manifestations or laboratory biomarkers can permit the early identification of HT children that are at higher risk of tumor development. The main aim of this paper is to discuss what is presently known regarding the coexistence of HT and thyroid tumors. Moreover, we attempt to define the pathogenesis of cancer development in children with HT.
Epidemiology of Pediatric Thyroid Cancer
Although the incidence rates of pediatric thyroid cancer have progressively increased over the past thirty years (9–11), this disease remains rare in children and adolescents compared to adults. Only 2% of the ~60,000 cases annually diagnosed in the USA regard subjects younger than 19 years of age (12). However, thyroid cancer plays a relevant role in pediatric oncology, as, in the USA, it is the eighth most common cancer diagnosed in patients aged 15–19 years and is the second most common cancer among adolescent girls (13, 14).
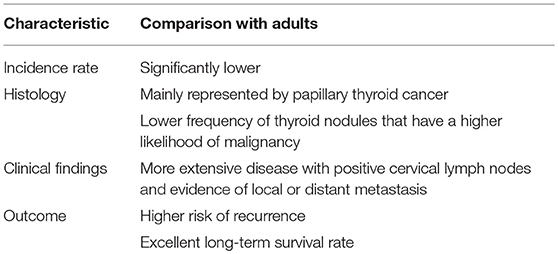
Along with differences in frequency compared to adult cases, pediatric thyroid cancer has several other differences regarding both histology and clinical characteristics. From a histological point of view, differentiated thyroid cancer comprises 90–95% of all childhood thyroid cancers, with papillary thyroid carcinoma (PTC) accounting for the majority of cases. In contrast, medullary thyroid cancer and undifferentiated and anaplastic forms that can be diagnosed in adults are exceptionally rare in children (15). Thyroid nodules are significantly less common in children (16, 17). However, pediatric thyroid nodules have a higher likelihood of malignancy compared to adults (16–20).
Several factors were found to be potential predictors of thyroid nodule malignancy. Microcalcifications, hypoechoic pattern, intranodular vascularization, lymph node alterations, and TSH concentration were identified by Mussa et al. (21). Papendieck et al. (22) added multinodular goiter, solid nodules, irregular margins and TSH values >2.5 mIU/L.
Finally, in contrast to adults, children with cancer at presentation have more extensive disease, with positive cervical lymph nodes and evidence of local or distant metastasis and have a higher risk of recurrence. In contrast, pediatric PTC has an excellent long-term prognosis, with 30-year survival rates of 90–99% (20, 23–26). All these findings, that are summarized in Table 1, have raised the supposition that pediatric thyroid cancer might be distinct from that of adults (27).
Coexistence of Thyroid Cancer and Hashimoto's Thyroiditis (HT) in Children
Most of the studies regarding the potential association between HT and thyroid cancer development have been carried out in adults. With few exceptions (28), most have clearly demonstrated that the coexistence of HT and thyroid tumors, mainly PTC, is common and that the risk of development of thyroid cancer in patients with HT is significantly higher than that in patients without HT. Moreover, HT seemed to have a certain protective effect on the short- and long-term prognosis of cancer. In a meta-analysis of 38 articles published before September 2011, including 10,648 PTC cases (29), the frequency of HT in PTC cases was ~23%, ranging from 5 to 85%. Different diagnostic criteria for HT, various surgical procedures, and heterogeneity of enrolled patient characteristics may explain the differences. HT was more frequently observed in PTC than in benign thyroid diseases and other carcinomas (odds ratio [OR] = 2.8 and 2.4, respectively; P < 0.001). The association was more common in females (OR = 2.7; P < 0.001) and was found to have more favorable clinical and histological characteristics than PTC without HT (29). In patients with HT-associated PTC, carcinoma had no extrathyroidal extension (OR = 1.3; P = 0.002), no lymph node metastasis (OR = 1.3; P = 0.041), and a long recurrence-free survival (hazard ratio [HR] = 0.6; P = 0.001). Similar results were reported in a more recent meta-analysis (30). In this case, 27 studies published until December 2015, enrolling 76,281 patients and including 12,476 cases of thyroid cancer, were analyzed. The mean rate of PTC among patients with HT ranged from 1.1 to 40.1%, with variations strictly related to the methods used to diagnose thyroid cancer, being higher when diagnosis is performed with more effective radiological and laboratory methods (30). The overall pooled OR of the PTC risk for HT (HT vs. non-HT) was 2.12 (95% confidence interval [CI] = 1.78–2.52).
In children, the few available studies had even more severe limitations than those enrolling adults. They were retrospective, frequently included small numbers of children and used different criteria for the diagnosis of both HT and thyroid cancer. This precludes pooling and comparison. However, for children and adolescents, the association between HT and thyroid cancer seems relatively common. The frequency of PTC in children and adolescents with HT was found variable from 0.67 to ~3% (31–33). In patients with PTC, the prevalence of coexisting HT varied from 6.3% to more than 40% of the cases. In a recent study, in which modern histological and laboratory methods, including thyroid fine needle aspiration cytology (TFNAC), were used to diagnose HT and thyroid cancer, HT was detected in 28.7% of the 108 thyroid cancer cases (34).
However, the impact of pediatric HT on the short- and long-term prognosis of cancer was not clearly defined. In the study by Iliadou et al. (34), HT was associated with a more severe clinical manifestation of the neoplasm. Histological examination revealed that infiltration of the thyroid parenchyma, revealing invasive characteristics of the cancer, was more frequent in children with HT (74.2 vs. 48.1%; P = 0.024), but the final prognosis was not influenced by HT. The clinical condition of patients with or without HT was strikingly similar after 5 or 10 years of follow-up. In contrast, in the study by Ren et al. (35), in which a frequent association between HT and cancer was shown (HT in 44.2% of differentiated thyroid cancer and 41.3% of thyroid nodules), no significant differences were observed in the clinical characteristics of the thyroid tumor among children with or without HT. No differences in tumor multifocality (P = 0.7), tumor size (P = 0.09), extrathyroidal infiltration (P = 0.6), or metastasis (P = 0.34) were shown (35). However, as evidenced by Iliadou et al. (34), no effect of HT on short-term disease-free survival was shown.
Supposed Reasons for the Coexistence of Thyroid Cancer and Hashimoto's Thyroiditis
Several hypotheses have been proposed to explain the potential relationship between HT and thyroid cancer (Table 2). One of them regards the role of TSH. It has been shown that the risk of thyroid malignancy is strictly related to the serum level of TSH, even with serum TSH levels within the normal range (36). In a recent prospective study, it was found that the risk of malignancy was ~3-fold higher in patients with TSH levels ≥2.26 μIU/mL than in patients with lower TSH levels (P = 0.001) (37). Thyroid autoimmunity that leads to higher serum TSH concentrations may partially explain the association between HT and PTC. However, it remains unclear whether TSH simply promotes the growth of a pre-existing cancer or truly causes cancer development.
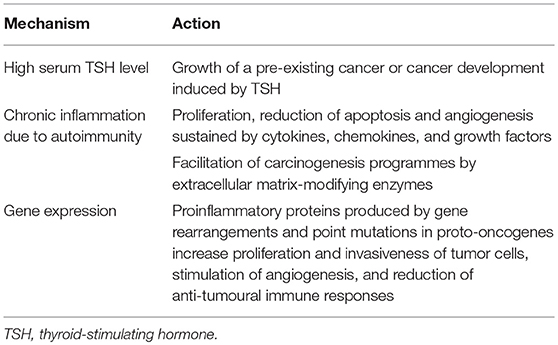
Table 2. Hypotheses proposed to explain the potential relationship between Hashimoto's thyroiditis (HT) and thyroid cancer.
A second hypothesis considers the role of chronic inflammation due to autoimmunity. HT is associated with chronic inflammation of thyroid tissue. Inflammation can increase the risk of cancer by providing bioactive molecules from cells infiltrating the tumor microenvironment (38). Cytokines, chemokines, and growth factors favor sustained proliferation, a significant reduction of apoptosis, and angiogenesis. Moreover, the production of extracellular matrix-modifying enzymes, such as metalloproteinases, is induced. This production promotes epithelial-mesenchymal transition and facilitates other carcinogenesis programmes, such as genome instability, immune evasion, and modifications of energy metabolism (39, 40). On the other hand, a complete concordance of all the markers of thyroid autoimmunity with thyroid cancer development has been reported. Boi et al. (39) carried out a retrospective analysis on 2,053 patients with single/prevalent thyroid nodules submitted to TFNAC and found that a higher prevalence of suspicious/malignant or indeterminate cytological findings was detected in patients with positive TG-Ab and thyroid microsomal antibody (TM-Ab) than in those with benign cytology. Increased independent OR for malignancy was conferred by any anti-thyroid antibody (OR 2.21; 95% CI = 1.49–3.29, P < 0.0001), TPO-Ab (OR 2.15; CI = 1.42–3.25, P < 0.0001) and TG-Ab (OR 1.67; CI = 1.05–2.67, P < 0.05).
A third hypothesis considers genetics. Molecular genetic studies have shown an association of HT with gene rearrangements and point mutations in the proto-oncogenes implicated in PTC, suggesting a potential interrelationship between the two diseases (18). Proinflammatory proteins (several cytokines and chemokines) induced by these mutated genes are relevant for the mobility, proliferation, survival, and invasiveness of tumor cells; stimulation of angiogenesis; and reduction of anti-tumoural immune responses. The best example in this regard is given by the chromosomal rearrangement involving the RET receptor tyrosine kinase gene. The rearrangement, named RET/PTC, fuses the 3′ terminal portion of RET coding for the tyrosine kinase domain with the 5′ terminal sequence of different unrelated genes, leading to constitutive activation of the RET tyrosine kinase. It is frequently identified in patients with PTC, although with significant differences according to several factors, including methodological and ethnic differences. However, one of the most important differences is age: RET/PTC rearrangements are much more frequent in younger patients with PTC, especially in children. These rearrangements constitute 40–70% of sporadic papillary carcinomas diagnosed in children and young adults (18). Regarding association with HT, RET/PTC rearrangement was more frequently observed in PTC associated with HT than in PTC without HT (31 vs. 13%, P = 0.02) (38). It was found that RET/PTC rearrangements were correlated with high TSH levels (P = 0.037) (41). Moreover, thyroid cell lines expressing RET/PTC may induce genes encoding molecules involved in the immune response (42), including CXCL10, which plays an important role in the first steps of HT lymphocytic infiltration (43, 44). Finally, it was reported that RET/PTC could be found both in areas of PTC and in areas with classic histological thyroid modifications typical of HT (45). Other genes have been theoretically implicated in the association between HT and thyroid cancer. Human 8-oxoguanine glycosylase is one of these genes. Mutations of this gene have commonly been found in both PTC (94%) and HT (73%), but not in other thyroid diseases (8%) (46). However, also in this case, children are different from adults because rearrangements appear more common in children, whereas mutations are more frequently detected in adults (46).
Independently of mutations in proto-oncogenes implicated in PTC, it seems likely that other genetic alterations may play a role in favoring the association between HT and thyroid cancer. This is suggested by the description of some clinical reports regarding children with thyroid cancer and HT associated with other autoimmune diseases, such as type 1 diabetes (47) and the autoimmune polyglandular syndrome type II (48).
Conclusion
Despite its rarity and relatively promising prognosis, thyroid cancer remains a significant clinical problem in pediatrics. Its association with HT, despite being based on a significantly lower number of reliable studies than in adults, seems likely. However, although not all reasons for the coexistence of these diseases are clearly defined, children with HT should be considered at highest risk of cancer development. Strict correlations between high levels of serum TSH and anti-thyroid antibodies must be remembered. The same is true for the presence of nodules, especially if multiple nodules are present, Ultrasonography and TFNAC can favor an early identification of patients with malignant changes and should be promptly used in uncertain cases.
Author Contributions
LP conceptualized the work and wrote the first draft of the manuscript. MC, LL, and AL performed the literature analysis. NP gave a significant contribution of the event. SE supervised the work and gave a substantial scientific contribution. All the authors approved the final report.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
2. de Vries L, Bulvik S, Phillip M. Chronic autoimmune thyroiditis in children and adolescents: at presentation and during long-term follow-up. Arch Dis Child. (2009) 94:33–7. doi: 10.1136/adc.2007.134841
3. Aversa T, Corrias A, Salerno M, Tessaris D, Di Mase R, Valenzise M, et al. Five-year prospective evaluation of thyroid function test evolution in children with Hashimoto's thyroiditis presenting with either euthyroidism or subclinical hypothyroidism. Thyroid (2016) 26:1450–6. doi: 10.1089/thy.2016.0080
4. Crisafulli G, Gallizzi R, Aversa T, Salzano G, Valenzise M, Wasniewska M, et al. Thyroid function test evolution in children with Hashimoto's thyroiditis is closely conditioned by the biochemical picture at diagnosis. Ital J Pediatr. (2018) 44:22. doi: 10.1186/s13052-018-0461-5
5. Radetti G, Gottardi E, Bona G, Corrias A, Salardi S, Loche S. The natural history of euthyroid Hashimoto's thyroiditis in children. J Pediatr. (2006) 149:827–32. doi: 10.1016/j.jpeds.2006.08.045
6. Rallison ML, Dobyns BM, Keating FR, Rall JE, Tyler FH. Occurrence and natural history of chronic lymphocytic thyroiditis in childhood. J Pediatr. (1975) 86:675–82. doi: 10.1016/S0022-3476(75)80350-7
7. Ohye H, Nishihara E, Sasaki I, Kubota S, Fukata S, Amino N, et al. Four cases of Graves' disease which developed after painful Hashimoto's thyroiditis. Intern Med. (2006) 45:385–9. doi: 10.2169/internalmedicine.45.1506
8. Champion B, Gopinath B, Ma G, El-Kaissi S, Wall JR. Conversion to Graves' hyperthyroidism in a patient with hypothyroidism due to Hashimoto's thyroiditis documented by real-time thyroid ultrasonography. Thyroid (2008) 18:1135–7. doi: 10.1089/thy.2008.0142
9. Bergamini LB, Frazier AL, Abrantes FL, Ribeiro KB, Rodriguez-Galindo C. Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: a population-based study. J Pediatr. (2014) 164:1481–5. doi: 10.1016/j.jpeds.2014.01.059
10. Holmes L, Hossain J, Opara F. Paediatric thyroid carcinoma incidence and temporal trends in the USA (1973–2007): race or shifting diagnostic paradigm? ISRN Oncol. (2012) 2012:1–10. doi: 10.5402/2012/906197
11. Ieni A, Vita R, Magliolo E, Santarpia M, Di Bari F, Benvenga S, et al. One-third of an archivial series of papillary thyroid cancer (years 2007–2015) has coexistent chronic lymphocytic thyroiditis, which is associated with a more favorable tumor-node-metastasis staging. Front Endocrinol. (2017) 8:337. doi: 10.3389/fendo.2017.00337
12. Hogan AR, Zhuge Y, Perez EA, Koniaris LG, Lew JI, Sola JE. Paediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J Surg Res. (2009) 156:167–72. doi: 10.1016/j.jss.2009.03.098
13. Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer incidence rates and trends among children and adolescents in the United States, 2001–2009. Paediatrics (2014) 134:e945–55. doi: 10.1542/peds.2013-3926
14. Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. (2014) 64:83–103. doi: 10.3322/caac.21219
15. Demidchik YE, Demidchik EP, Reiners C, Biko J, Mine M, Saenko VA, et al. Comprehensive clinical assessment of 740 cases of surgically treated thyroid cancer in children of Belarus. Ann Surg. (2006) 243:525. doi: 10.1097/01.sla.0000205977.74806.0b
16. Niedziela M. Thyroid nodules. Best Pract Res Clin Endocrinol Metab. (2014) 28:245–77. doi: 10.1016/j.beem.2013.08.007
17. Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab. (2008) 22:901–11. doi: 10.1016/j.beem.2008.09.019
18. Yamashita S, Saenko V. Mechanisms of disease: molecular genetics of childhood thyroid cancers. Nat Clin Pract Endocrinol Metab. (2007) 3:22–9. doi: 10.1038/ncpendmet0499
19. Wiersinga WM. Management of thyroid nodules in children and adolescents. Hormones (Athens) (2007) 6:194–9.
20. Jarzab B, Handkiewicz-Junak D. Differentiated thyroid cancer in children and adults: same or distinct disease? Hormones (Athens) (2007) 6:200–9.
21. Mussa A, De Andrea M, Motta M, Mormile A, Palestini N, Corrias A. Predictors of malignancy in children with thyroid nodules. J Pediatr. (2015) 167:886–92. doi: 10.1016/j.jpeds.2015.06.026
22. Papendieck P, Gruñeiro-Papendieck L, Venara M, Acha O, Cozzani H, Mateos F, et al. Differentiated thyroid cancer in children: prevalence and predictors in a large cohort with thyroid nodules followed prospectively. J Pediatr. (2015) 167:199–201. doi: 10.1016/j.jpeds.2015.04.041
23. Gigsby PW, Gal-or A, Michalski JM, Doherty GM. Childhood and adolescent thyroid carcinoma. Cancer (2002) 95:724–9. doi: 10.1002/cncr.10725
24. Hay ID, Gonzalez-Losada T, Reinalda MS, Honetschlager JA, Richards ML, Thompson GB. Long term outcome in 215 children and adolescents with papillary thyroid cancer treated during 1940 through 2008. World J Surg. (2010) 34:1192–202. doi: 10.1007/s00268-009-0364-0
25. La Quaglia MP, Black T, Holcomb TB, Sklar CA, Azizkhan RG, et al. Differentiated thyroid cancer; clinical characteristics, treatment, and outcome in patients under 21 years of age who present with distant metastases. A report from the Surgical Discipline Committee of the Children's Cancer Group. J Pediatr Surg. (2000) 35:955–9. doi: 10.1053/jpsu.2000.6935
26. Rivkees SA, Mazzaferri EL, Verburg FA, Reiners C, Luster M, Breuer CK, et al. The treatment of differentiated thyroid cancer in children; emphasis on surgical approach and radioactive iodine therapy. Endocr Rev. (2011) 32:798–826. doi: 10.1210/er.2011-0011
27. Gupta A, Ly S, Castroneves LA, Frates MC, Benson CB, Feldman HA, et al. A standardized assessment of thyroid nodules in children confirms higher cancer prevalence than in adults. J Clin Endocrinol Metab. (2013) 98:3238–45. doi: 10.1210/jc.2013-1796
28. Jankovic B, Le KT, Hershman JM. Clinical review: Hashimoto's thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab. (2013) 98:474–82. doi: 10.1210/jc.2012-2978
29. Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto's thyroiditis: a meta-analysis. Eur J Endocrinol. (2013) 168:343–9. doi: 10.1530/EJE-12-0903
30. Lai X, Xia Y, Zhang B, Li J, Jiang Y. A meta-analysis of Hashimoto's thyroiditis and papillary thyroid carcinoma risk. Oncotarget (2017) 8:62414–24. doi: 10.18632/oncotarget.18620
31. Keskin M, Savas-Erdeve S, Aycan Z. Co-existence of thyroid nodule and thyroid cancer in children and adolescents with Hashimoto thyroiditis: a single-center study. Horm Res Paediatr. (2016) 85:181–7. doi: 10.1159/000443143
32. Skarpa V, Kousta E, Tertipi A, Anyfandakis K, Vakaki M, Dolianiti M, et al. Epidemiological characteristics of children with autoimmune thyroid disease. Hormones (Athens) (2011) 10:207–14. doi: 10.14310/horm.2002.1310
33. Corrias A, Cassio A, Weber G, Mussa A, Wasniewska M, Rapa A, et al. Thyroid nodules and cancer in children and adolescents affected by autoimmune thyroiditis. Arch Pediatr Adolesc Med. (2008) 162:526–31. doi: 10.1001/archpedi.162.6.526
34. Iliadou PK, Effraimidis G, Konstantinos M, Grigorios P, Mitsakis P, Patakiouta F, et al. Chronic lymphocytic thyroiditis is associated with invasive characteristics of differentiated thyroid carcinoma in children and adolescents. Eur J Endocrinol. (2015) 173:827–33. doi: 10.1530/EJE-14-1046
35. Ren PY, Liu J, Xue S, Chen G. Paediatric differentiated thyroid carcinoma: the clinicopahological features and the coexistence of Hashimoto's thyroiditis. Asian J Surg. (2017). doi: 10.1016/j.asjsur.2017.10.006. [Epub ahead of print].
36. McLeod DS, Watters KF, Carpenter AD, Ladenson PW, Cooper DS, Ding EL. Thyrotropin and thyroid cancer diagnosis: a systematic review and dose-response meta-analysis. J Clin Endocrinol Metab. (2012) 97:2682–92. doi: 10.1210/jc.2012-1083
37. Golbert L, de Cristo AP, Faccin CS, Farenzena M, Folgierini H, Graudenz MS, et al. Serum TSH levels as a predictor of malignancy in thyroid nodules: a prospective study. PLoS ONE (2017) 12:e0188123. doi: 10.1371/journal.pone.0188123
38. Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumour microenvironment. J Immunol Res. (2014) 2014:149185. doi: 10.1155/2014/149185
39. Boi F, Minerba L, Lai ML, Marziani B, Figus B, Spanu F, et al. Both thyroid autoimmunity and increased serum TSH are independent risk factors for malignancy in patients with thyroid nodules. J Endocrinol Invest. (2013) 36:313–20. doi: 10.3275/8579
40. Muzza M, Degl'Innocenti D, Colombo C, Perrino M, Ravasi E, Rossi S, et al. The tight relationship between papillary thyroid cancer, autoimmunity and inflammation: clinical and molecular studies. Clin Endocrinol (Oxf) (2010) 72:702–8. doi: 10.1111/j.1365-2265.2009.03699.x
41. Su X, He C, Ma J, Tang T, Zhang X, Ye Z, et al. RET/PTC Rearrangements are associated with elevated postoperative TSH levels and multifocal lesions in papillary thyroid cancer without concomitant thyroid benign disease. PLoS ONE (2016) 11:e0165596. doi: 10.1371/journal.pone.0165596
42. Borrello MG, Alberti L, Fischer A, Degl'innocenti D, Ferrario C, Gariboldi M, et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci USA. (2005) 102:14825–30. doi: 10.1073/pnas.0503039102
43. Antonelli A, Rotondi M, Fallahi P, Grosso M, Boni G, Ferrari SM, et al. Iodine-131 given for therapeutic purposes modulates differently interferon-gamma-inducible alpha-chemokine CXCL10 serum levels in patients with active Graves' disease or toxic nodular goiter. J Clin Endocrinol Metab. (2007) 92:1485–90. doi: 10.1210/jc.2006-1571
44. Rotondi M, Chiovato L, Romagnani S, Serio M, Romagnani P. Role of chemokines in endocrine autoimmune diseases. Endocr Rev. (2007) 28:492–520. doi: 10.1210/er.2006-0044
45. Mechler C, Bounacer A, Suarez H, Saint Frison M, Magois C, Aillet G, et al. Papillary thyroid carcinoma: 6 cases from 2 families with associated lymphocytic thyroiditis harbouring RET/PTC rearrangements. Br J Cancer (2001) 85:1831–7. doi: 10.1054/bjoc.2001.2187
46. Royer MC, Zhang H, Fan CY, Kokoska MS. Genetic alterations in papillary thyroid carcinoma and Hashimoto thyroiditis: an analysis of hOGG1 loss of heterozygosity. Arch Otolaryngol Head Neck Surg. (2010) 136:240–2. doi: 10.1001/archoto.2010.20
47. Karavanaki K, Karayianni C, Vassiliou I, Tzanela M, Sdogou T, Kakleas K, et al. Multiple autoimmunity, type 1 diabetes (T1DM), autoimmune thyroiditis and thyroid cancer: is there an association? A case report and literature review. J Pediatr Endocrinol Metab. (2014) 27:1011–6. doi: 10.1515/jpem-2013-0370
Keywords: anti-thyroid antibodies, Hashinoto's disease, thyroid cancer, thyroid nodules, TSH
Citation: Penta L, Cofini M, Lanciotti L, Leonardi A, Principi N and Esposito S (2018) Hashimoto's Disease and Thyroid Cancer in Children: Are They Associated?. Front. Endocrinol. 9:565. doi: 10.3389/fendo.2018.00565
Received: 27 July 2018; Accepted: 05 September 2018;
Published: 09 October 2018.
Edited by:
Giampaolo Papi, Azienda Unità Sanitaria Locale di Modena, ItalyReviewed by:
Roberto Vita, Università degli Studi di Messina, ItalyMisa Imaizumi, Radiation Effects Research Foundation, Japan
Copyright © 2018 Penta, Cofini, Lanciotti, Leonardi, Principi and Esposito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susanna Esposito, c3VzYW5uYS5lc3Bvc2l0b0B1bmltaS5pdA==
 Laura Penta
Laura Penta Marta Cofini1
Marta Cofini1 Lucia Lanciotti
Lucia Lanciotti Nicola Principi
Nicola Principi Susanna Esposito
Susanna Esposito