
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Endocrinol. , 11 June 2018
Sec. Pituitary Endocrinology
Volume 9 - 2018 | https://doi.org/10.3389/fendo.2018.00321
This article is part of the Research Topic Diabetes Secondary to Pituitary Diseases View all 7 articles
Background: The growth hormone (GH)/insulin-like growth factor 1 (IGF-1) axis has a fundamental impact on glucose metabolism. Therefore, both untreated GH deficiency (GHD) and GH treatment (GHT) may be associated with some metabolic alterations, although the abnormalities of glucose metabolism have been investigated by relatively few studies as main outcomes.
Aim: The present review summarizes the available data on glucose metabolism in children with GHD, providing an overview of the current state of the art in order to better clarify the real metabolic impact of GHD and GHT.
Methods: Among all the existing studies, we evaluated all original studies that fulfilled our criteria for analysis reporting parameters of glucose metabolism as the primary or secondary objective.
Results: The reported impact of GHD per se on glucose metabolism is quite homogeneous, with the majority of studies reporting no significant difference in metabolic parameters between GHD children and controls. Conversely, GHT proves to be more frequently associated with a subtle form of insulin resistance, while both fasting glucose and HbA1c levels remain almost always within the normal range.
Conclusion: The different methods to study glucose metabolism, the heterogeneity of the populations evaluated, the different doses of GH used together with the variable duration of follow-up may be responsible for discrepancy in the results. Long-term longitudinal studies having glucose homeostasis as their primary outcome are still needed in order better to clarify the real metabolic impact of GHD and GHT in children.
The growth hormone (GH)/insulin-like growth factor 1 (IGF-1) axis has a fundamental impact on metabolism (1). GH regulates glucose homeostasis directly, by inducing glycogenolysis, gluconeogenesis, and lipolysis and promoting insulin resistance, and indirectly, via IGF-I production. Primarily, GH inhibits insulin-induced suppression of hepatic gluconeogenesis, thus increasing glucose production. In addition, it mainly act in stimulating lipolysis by providing free fatty acids (FFA) in order to switch metabolism from glucose and protein to lipid utilization (2–5) and these evidences were supported by a study that demonstrated that inhibition of lipolysis with acipimox, a free fatty acid blocker, partially prevented GH-induced insulin resistance (6).
Following the reduction of insulin sensitivity, a compensatory increase in insulin secretion is usually observed. In addition, GH is also a potent growth factor for β-cell proliferation and insulin secretion (7). Other mechanisms play a role in the metabolic effects of GH, namely its interaction with the insulin receptor (8, 9) and the presence of a polymorphism in the GH receptor gene (10–15). On the other hand, IGF-1 physiologically improves glucose homeostasis and enhances insulin sensitivity (16, 17). For these reasons, the interplay between GH/IGF-1 axis and glucose metabolism is complex.
Both untreated GH deficiency (GHD) and GH treatment (GHT) may be associated with metabolic alterations and a common mechanism could be the increased flux of FFA caused by body composition alterations (in untreated GHD) or enhanced lipid oxidation secondary to the anti-insulin effect of GH (during GHT), respectively (5, 18).
However, metabolic abnormalities associated with GHD have so far been evaluated only in a small number of children (19) and most studies focused on the main markers of cardiovascular disease, namely abnormalities in body composition (20–22), lipid profile (23, 24), inflammatory markers (25–27), and cardiac function (28–32), while the abnormalities of glucose metabolism have been investigated by relatively few studies and incompletely. The present review summarizes the available data on glucose metabolism in children with GHD, providing an overview of the current state of the art.
PubMed was searched for studies evaluating the glucose metabolism in children with GHD and/or its changes during GHT. The search terms used were “growth hormone deficiency in children,” “growth hormone deficiency and glucose,” “growth hormone deficiency and diabetes,” and “growth hormone deficiency and metabolism.” We excluded from the analysis the review articles. We considered all original studies in English language published from 1970 to January 2018 which reported as the primary or secondary objective of the study at least one of the following metabolic parameters: fasting glucose, fasting insulin, glycosylated hemoglobin (HbA1c), homeostatic model assessment of insulin resistance (Homa-IR), the quantitative insulin sensitivity check index (QUICKI), the insulin sensitivity index (ISI), or the M-value (derived from euglycemic hyperinsulinemic clamp).
Specifically, we carefully evaluated more than 100 studies concerning children who have been treated with GH. We considered case–control prospective studies performed on consecutive patients (levels of evidence I/II). We excluded from the analysis all original studies which evaluated less than five patients, those with a follow-up of less than 1 month of treatment, studies on children treated with long-acting formulations of GH or treated with GH for indications other than GHD. The final analysis comprised 22 (for naïve GHD children compared to controls) and 24 studies (for children during GHT), that fulfilled our criteria for analysis.
Low GH and IGF-1 levels are known to be predictors of unfavorable metabolic profile in healthy subjects (33–35) and in adults with GHD (36–39). Similarly, adolescents with confirmed GHD who discontinued GHT at completion of linear growth may exhibit metabolic alterations (23, 25, 40–48), as well as untreated GHD in children has also been associated with many markers of cardiovascular risk (9, 49, 50).
Substantially, most of the studies did not show a significant impairment in glucose metabolism in naïve GHD children. The Table 1 summarizes the studies on glucose metabolism in naïve GHD children compared to controls.
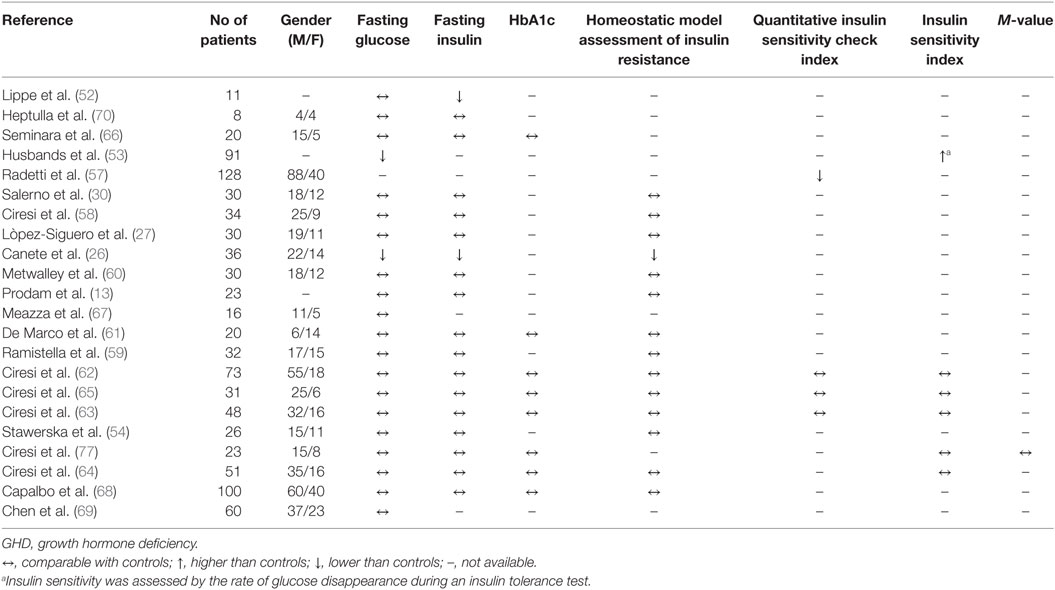
Table 1. Selected studies for analysis of glucose metabolism in naïve GHD children compared to controls.
Fasting hypoglycemia and marked insulin sensitivity have sometimes been observed in GHD children due to diminished hepatic output through decreased gluconeogenesis or abnormal glucose mobilization (51). One of the first metabolic studies performed in 11 GHD children has shown basal hypoinsulinemia with normal fasting glucose compared with controls (52). Partially similar results were documented by Canete et al. in 36 GHD children, who showed lower glucose, insulin, and Homa-IR values than controls (26). The tendency of GHD children to show higher insulin sensitivity has been demonstrated by Husbands et al. (53). In that study, as a direct measure of insulin sensitivity the authors evaluated the rate of glucose disappearance following an insulin tolerance test in 91 GHD children and 142 controls. The authors demonstrated that GHD children had an increased susceptibility to hypoglycemia, thus proving to be more insulin-sensitive than GH-sufficient children.
Partially in accordance with these data, more recently Stawerska et al. demonstrated comparable glucose and insulin levels between GHD children and controls but significantly lower insulin levels and higher insulin sensitivity in GHD children with lower IGF-1 bioavailability than children with higher IGF-1 levels (54).
The insulin sensitivity related to GHD seems to decrease with age, probably due to the physiological effect of steroids production or the modifications of body composition during the time. Indeed, in normal children insulin action is physiologically reduced during puberty and insulin secretion is normally increased. The insulin resistance of puberty, which was found to be directly correlated with IGF-1 levels, leads to compensatory hyperinsulinemia which serves to amplify the anabolic effect of insulin during this period of rapid growth (55, 56). For these reasons, the higher insulin sensitivity observed in young untreated GHD children is widely different from the different degree of insulin resistance shown by adolescents (19) or adults (39) with GHD, in which the change of body composition plays an additional fundamental role (43, 44).
These data contrast with those of Radetti et al., who through evaluation of QUICKI demonstrated a slight degree of insulin resistance in GHD children at baseline compared with controls (57), while other studies did not show any impairment in Homa-IR (12, 27, 30, 58–61) or in ISI (38, 62–65) in naïve children. No significant difference in fasting glucose, insulin, and HbA1c levels between GHD children and controls were found in many other studies (38, 61–70).
Growth hormone treatment has been suggested to impair glucose metabolism because of the anti-insulin effect of GH and its direct effect on β-cell, although the real metabolic impact of GHT in children has not been established. The limitation of most studies is represented by evaluation of glucose metabolism through basal parameters and surrogate indices of insulin sensitivity, i.e., fasting glucose, fasting insulin, or Homa-IR, and not by the more reliable indexes derived from oral glucose tolerance test (OGTT), i.e., ISI Matsuda, or the gold standard technique to measure insulin sensitivity, the euglycemic hyperinsulinemic clamp (71), which is expensive and not routinely applicable.
The Table 2 summarizes the studies on glucose metabolism in GHD children during GHT.
Fasting glucose is undoubtedly the parameter mostly used to evaluate glucose metabolism in almost all studies (21/24; 87.5%). In a recent study, Xue et al. reported a significant increase in fasting glucose in 60 children during 6 months of GHT, although in half of the patients a high GH dose (0.1 U/kg) was used (72). Similar results have been shown by Chen et al. in 60 children after 12 months of GHT, regardless of the GH dose used (68) and these data have been confirmed by four other studies (60, 62–64).
In 2000, a large retrospective analysis of data more than 23,000 children reported that the incidence of type 1 diabetes did not differ from expected values, while the incidence of type 2 diabetes was higher (85 out of 23,333 children, 0.36%) than reported in children not GH-treated, probably as a consequence of an acceleration of the disorder in predisposed individuals. However, it has to be highlighted that not only children with GHD were included in this analysis, but also children with other clinical conditions treated with supraphysiological doses of GH (73).
In 2011 an analysis of data of 11,686 patients showed a slightly higher incidence of type 2 diabetes in GH-treated children than in the general population. Interestingly, most patients who developed diabetes had preexisting risk factors, like preexisting insulin resistance. Thus, monitoring of glucose before and periodically during GHT is recommended by the authors, especially for children with preexisting risk factors (74).
Radetti et al. reported normalization of glucose tolerance in 5 out of 128 children who already presented impaired glucose tolerance before starting GHT (57), while unchanged glucose levels have been found in the majority of studies (14/21; 66.6%) (12, 15, 26, 52, 58, 59, 61, 65, 66, 70, 75–77).
Recently, a large safety-monitoring study enrolling more than 54,000 children who were given GHT and were followed until GHT discontinuation showed no significant increase in incidence of diabetes compared to the general population (78), as well as a recent analysis failed to show an increased incidence of diabetes in adults who were previously treated during childhood (79). Notably, a recent study by our group reported a significant increase in fasting glucose only in children who were given GHT every day compared to those who were given the same dose through three injections per week (80). The limit of the use of fasting glucose as a parameter to detect changes in glucose metabolism during GHT has been documented by Lippe et al., who demonstrated higher plasma glucose concentrations during OGTT than controls after 6 months of GHT, despite mean fasting glucose remaining normal (52).
HbA1c was only evaluated in 9 out of 24 studies and most of these (8 studies) showed no significant change in levels during GHT.
Another metabolic parameter very frequently assessed during GHT is fasting insulin, evaluated in 18 out of 24 studies (75%). An increase in fasting insulin has been documented in 83.3% of studies and unchanged levels in 11.1% of studies. Lippe et al. showed comparable fasting insulin levels between GHD children and controls but with a concomitant increase in insulin secretion following OGTT in a group of 11 children after 6 months of GHT (52).
Insulin sensitivity has been more frequently studied by surrogate indexes derived from fasting glucose and insulin levels, as Homa-IR or, less frequently, QUICKI.
Homeostatic model assessment of insulin resistance was calculated in 15 out of 24 studies. Specifically, it proved to be unchanged in two studies (13%) (61, 66) and increased in the majority of them (87%). An increase in fasting insulin levels and, consequently, in Homa-IR after 2 years of GHT was documented by Salerno et al. (30). Similarly, López-Siguero et al. showed a significant increase in Homa-IR, without significant change in fasting glucose and insulin levels, in 30 GHD children after 12 months of GHT (27). Conversely, Metwalley et al. documented a deterioration in Homa-IR with a concomitant increase in fasting glucose during GHT (60), while Meazza et al. documented a rise in fasting insulin but without changes in Homa-IR (67). Ramistella et al. confirmed an increase in both fasting insulin and Homa-IR in 32 children, although these values were within the normal range during the entire follow-up (59) and a similar deterioration in insulin sensitivity assessed by Homa-IR was reported by other authors (26, 58, 62–64, 68).
Quantitative insulin sensitivity check index was evaluated in very few studies (4 out of 24 studies; 16.6%) and it is proved to decrease during GHT in all of them (57, 62, 63, 65).
Fewer studies evaluated the degree of insulin sensitivity through evaluation of the more reliable ISI (6 out of 24; 25%) and the majority of them (5 out of 6; 83%) are concordant in detecting a reduction in ISI during GHT (63–65, 80).
The clamp was used in very few studies. Heptulla et al. employed the hyperglycemic clamp to evaluate the effects of 6 months of GHT in six children affected by GHD and two children with non-deficient short stature. The authors showed a decrease in insulin sensitivity compensated by a marked increase in insulin responses after GHT, although it remained within the normal range (70). The few studies that directly assessed insulin sensitivity by euglycemic hyperinsulinemic clamp showed a significant reduction in M-value, hence confirming a real deterioration in insulin sensitivity after GHT, though without evident changes in glucose tolerance (62, 77).
Recently, 99 GHD children were annually tested with OGTT to longitudinally study insulin sensitivity and the capacity of β-cells to adapt to changes in insulin sensitivity (through the oral disposition index, DIo) during 6 years of GHT. The results of this study suggested a positive influence of GHT on the β-cell secretory capacity, without a significant impact on insulin sensitivity (81). The DIo was also evaluated in another study enrolling 73 GHD children, who showed a significant reduction in DIo in concomitance with an increase in insulin levels during GHT. The conclusion of the study was that while a direct trophic effect of GH on β-cells cannot be ruled out to explain the increase in fasting insulin secretion, the decrease in DIo can be considered an early marker of inadequate β-cell compensation of decreased insulin sensitivity (62).
It is important to highlight that, overall, the anti-insulin effect of GH seems to be prevalent during the first months of treatment and may be caused by a decline of peripheral glucose utilization and increase in insulin resistance (82) whereas, after initial deterioration, glucose tolerance can improve (83). Capalbo et al. demonstrated that the first year of GHT was associated with an increase in insulin and HOMA-IR, but these parameters did not change further during the following years of treatment. Indeed, at the fifth year of the study a significant increase in insulin and HOMA-IR was documented also in control subjects, making these parameters comparable between GHD and controls (68). This finding is consistent with the results of Radetti et al., who showed a slight deterioration in insulin sensitivity after the first year of GHT, but without a further progressive deterioration in the following 6 years (57).
In synthesis, glycometabolic effects of GHT seem to be biphasic, leading initially to deterioration of glucose metabolism, but in the long-term an improvement of glucose handling is reported. These data can be explained by the long-term beneficial effects of GH on body composition, which probably may overcome the insulin-antagonist effect of GH in the long-term follow-up (84).
Few studies have tried to identify any predictive factors of glucose alterations in children during GHT. Probably, the strongest predictors of the degree of insulin resistance and the development of diabetes during GHT are represented by the individual predisposition of patients or by the presence of preexisting diabetes risk factors (73, 74). Interestingly, 1 h-glucose levels higher than 132.5 mg/dl after OGTT at diagnosis were found to be a predictor of alterations in glucose metabolism during GHT (64), in agreement with other studies performed in non-GHD children (85). Recently, Staverska et al. demonstrated that GHD children are a heterogeneous group as regards the differences in the metabolic profile, probably due to the different IGF-1 bioavailability, and concluded that naïve GHD children have a worse metabolic profile when the IGF-1 bioavailability and the body mass were greater, although the authors do not fully explain this phenomenon (54). In addition, the role of microRNAs expression in skeletal muscles has been hypothesized as a mechanism that contributes to increased insulin resistance during GHT (86).
Glucose metabolism in GHD children is not exhaustively and uniformly evaluated by the available studies. Fasting glucose and insulin are the parameters most frequently evaluated, followed by Homa-IR and HbA1c levels. Very few studies used other metabolic parameters, i.e., QUICKI, ISI, and M-value.
In children, the reported impact of GHD per se on glucose metabolism is quite homogeneous, with the majority of studies reporting no significant difference in metabolic parameters between GHD children and controls. Conversely, GHT proves to be more frequently associated with a subtle form of insulin resistance that can be assumed to be the mechanism by which basal glucose does not significantly change after GHT despite the increase in insulin levels.
The increase in insulin levels and, consequently, in Homa-IR during GHT probably does not indicate with any certainty a condition of insulin resistance, probably due to the inability of basal indexes to reliably assess insulin sensitivity. Indeed, the increase in Homa-IR, as well as the reduction in QUICKI, may just represent an expected consequence of GH-induced basal hyperinsulinemia and very few studies have compared data about the degree of insulin sensitivity in GHD children assessed by different indices (77). However, even in the few studies that used more reliable indices, like ISI or M-value during clamp, a reduction in insulin sensitivity during GHT was almost always confirmed.
Overall, despite the decrease in insulin sensitivity, both fasting glucose and HbA1c levels remain almost always within the normal range. Therefore, the hyperinsulinemia documented during GHT can probably be compared to that observed during puberty (87), although the long-term implications of this prolonged state of hyperinsulinemia are not widely known. Probably, the long-term beneficial effect of GHT on body composition will counterbalance the initial insulin-antagonist effects of GH leading to a general improvement in insulin sensitivity.
For these reasons, to date there are no clear indications on routinely monitoring GHD patients receiving GHT with regards to the risk of diabetes. The only statements from the Growth Hormone Research Society are that “fasting insulin levels are not routinely measured” and “caution should be exercised when considering the decision of continuing GHT in conditions where there is a known risk of diabetes in the transition age” (88). Also a recent GH safety workshop position paper did not indicate any specific recommendations regarding diabetes risk monitoring in GHD children (89). In our opinion, in agreement with Carel et al. (90), particular attention to glucose metabolism is warranted in such patients and especially in children with already known risk factors for diabetes, both before and at least annually, including an OGTT, which is useful for evaluating any changes in both glucose and insulin levels.
The different methods to study glucose metabolism, the heterogeneity of the populations evaluated, the different doses of GH used together with the variable duration of follow-up may be responsible for discrepancy in the results. Long-term longitudinal studies having glucose homeostasis as their primary outcome are still needed in order to better clarify the real metabolic impact of GHD and GHT in children.
AC and CG contributed equally to this work.
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the manuscript.
This research did not receive any specific grant from any funding agency in the public, commercial, or non-profit sector.
1. Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev (2009) 30(2):152–77. doi:10.1210/er.2008-0027
2. Møller N, Jørgensen JO, Abildgard N, Orskov L, Schmitz O, Christiansen JS. Effects of growth hormone on glucose metabolism. Horm Res (1991) 36(Suppl 1):32–5. doi:10.1159/000182185
3. Rizza RA, Mandarino LJ, Gerich JE. Effects of growth hormone on insulin action in man. Mechanisms of insulin resistance, impaired suppression of glucose production, and impaired stimulation of glucose utilization. Diabetes (1982) 31:663–9. doi:10.2337/diabetes.31.8.663
4. Jørgensen JO, Møller L, Krag M, Billestrup N, Christiansen JS. Effects of growth hormone on glucose and fat metabolism in human subjects. Endocrinol Metab Clin North Am (2007) 36(1):75–87. doi:10.1016/j.ecl.2006.11.005
5. Bramnert M, Segerlantz M, Laurila E, Daugaard JR, Manhem P, Groop L. Growth hormone replacement therapy induces insulin resistance by activating the glucose-fatty acid cycle. J Clin Endocrinol Metab (2003) 88(4):1455–63. doi:10.1210/jc.2002-020542
6. Segerlantz M, Bramnert M, Manhem P, Laurila E, Groop LC. Inhibition of the rise in FFA by acipimox partially prevents GH-induced insulin resistance in GH deficient adults. J Clin Endocrinol Metab (2001) 86:5813–8. doi:10.1210/jcem.86.12.8096
7. Nielsen JH, Linde S, Welinder BS, Billestrup N, Madsen OD. Growth hormone is a growth factor for the differentiated pancreatic beta-cell. Mol Endocrinol (1989) 3(1):165–73. doi:10.1210/mend-3-1-165
8. Smith TR, Elmendorf JS, David TS, Turinsky J. Growth hormone-induced insulin resistance: role of the insulin receptor, IRS-1, GLUT-1, and GLUT-4. Am J Physiol (1997) 272(6 Pt 1):E1071–9.
9. Dominici FP, Turyn D. Growth hormone-induced alterations in the insulin-signaling system. Exp Biol Med (Maywood) (2002) 227(3):149–57. doi:10.1177/153537020222700301
10. Strawbridge RJ, Kärvestedt L, Li C, Efendic S, Ostenson CG, Gu HF, et al. GHR exon 3 polymorphism: association with type 2 diabetes mellitus and metabolic disorder. Growth Horm IGF Res (2007) 17(5):392–8. doi:10.1016/j.ghir.2007.04.005
11. Giavoli C, Ferrante E, Profka E, Olgiati L, Bergamaschi S, Ronchi CL, et al. Influence of the d3GH receptor polymorphism on the metabolic and biochemical phenotype of GH-deficient adults at baseline and during short- and long-term recombinant human GH replacement therapy. Eur J Endocrinol (2010) 163(3):361–8. doi:10.1530/EJE-10-0317
12. Sørensen K, Aksglaede L, Munch-Andersen T, Aachmann-Andersen NJ, Leffers H, Helge JW, et al. Impact of the growth hormone receptor exon 3 deletion gene polymorphism on glucose metabolism, lipids, and insulin-like growth factor-I levels during puberty. J Clin Endocrinol Metab (2009) 94(8):2966–9. doi:10.1210/jc.2009-0313
13. Prodam F, Savastio S, Genoni G, Babu D, Giordano M, Ricotti R, et al. Effects of growth hormone (GH) therapy withdrawal on glucose metabolism in not confirmed GH deficient adolescents at final height. PLoS One (2014) 9(1):e87157. doi:10.1371/journal.pone.0087157
14. Mericq V, Román R, Iñiguez G, Angel B, Salazar T, Avila A, et al. Relationship between nocturnal growth hormone concentrations, serum IGF-I/IGFBP-3 levels, insulin sensitivity and GH receptor allelic variant in small for gestational age children. Horm Res (2007) 68(3):132–8. doi:10.1159/000100546
15. Audí L, Carrascosa A, Esteban C, Fernández-Cancio M, Andaluz P, Yeste D, et al. The exon 3-deleted/full-length growth hormone receptor polymorphism does not influence the effect of puberty or growth hormone therapy on glucose homeostasis in short non-growth hormone-deficient small-for-gestational-age children: results from a two-year controlled prospective study. J Clin Endocrinol Metab (2008) 93(7):2709–15. doi:10.1210/jc.2008-0150
16. Pratipanawatr T, Pratipanawatr W, Rosen C, Berria R, Bajaj M, Cusi K, et al. Effect of IGF-I on FFA and glucose metabolism in control and type 2 diabetic subjects. Am J Physiol Endocrinol Metab (2002) 282(6):E1360–8. doi:10.1152/ajpendo.00335.2001
17. Drogan D, Schulze MB, Boeing H, Pischon T. Insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 in relation to the risk of type 2 diabetes mellitus: results from the epic-potsdam study. Am J Epidemiol (2016) 183(6):553–60. doi:10.1093/aje/kwv188
18. Jørgensen JO, Vestergaard E, Gormsen L, Jessen N, Nørrelund H, Christiansen JS, et al. Metabolic consequences of GH deficiency. J Endocrinol Invest (2005) 28(5 Suppl):47–51.
19. Lanes R. Cardiovascular risk in growth hormone deficiency: beneficial effects of growth hormone replacement therapy. Endocrinol Metab Clin North Am (2016) 45(2):405–18. doi:10.1016/j.ecl.2016.01.005
20. Saggese G, Baroncelli GI, Bertelloni S, Barsanti S. The effect of long-term growth hormone (GH) treatment on bone mineral density in children with GH deficiency. Role of GH in the attainment of peak bone mass. J Clin Endocrinol Metab (1996) 81(8):3077–83. doi:10.1210/jc.81.8.3077
21. Boot AM, Engels MA, Boerma GJ, Krenning EP, De Muinck Keizer-Schrama SM. Changes in bone mineral density, body composition, and lipid metabolism during growth hormone (GH) treatment in children with GH deficiency. J Clin Endocrinol Metab (1997) 82(8):2423–8. doi:10.1210/jc.82.8.2423
22. Matusik P, Klesiewicz M, Klos K, Stasiulewicz M, Barylak A, Nazarkiewicz P, et al. Baseline body composition in prepubertal short stature children with severe and moderate growth hormone deficiency. Int J Endocrinol (2016) 2016:4563721. doi:10.1155/2016/4563721
23. Lanes R, Soros A, Gunczler P, Paoli M, Carrillo E, Villaroel O, et al. Growth hormone deficiency, low levels of adiponectin, and unfavorable plasma lipid and lipoproteins. J Pediatr (2006) 149(3):324–9. doi:10.1016/j.jpeds.2006.05.010
24. Capalbo D, Mattace Raso G, Esposito A, Di Mase R, Barbieri F, Meli R, et al. Cluster of cardiometabolic risk factors in children with GH deficiency: a prospective, case-control study. Clin Endocrinol (Oxf) (2014) 80(6):856–62. doi:10.1111/cen.12393
25. Lanes R, Marcano H, Villaroel O, Gunczler P, Morillo E, Paoli M, et al. Circulating levels of high-sensitivity C-reactive protein and soluble markers of vascular endothelial cell activation in growth hormone-deficient adolescents. Horm Res (2008) 70(4):230–5. doi:10.1159/000151595
26. Cañete R, Valle M, Martos R, Sánchez-Carrión A, Cañete MD, van Donkelaar EL. Short-term effects of GH treatment on coagulation, fibrinolysis, inflammation biomarkers, and insulin resistance status in prepubertal children with GH deficiency. Eur J Endocrinol (2012) 167(2):255–60. doi:10.1530/EJE-12-0214
27. López-Siguero JP, López-Canti LF, Espino R, Caro E, Fernández-García JM, Gutiérrez-Macías A, et al. Effect of recombinant growth hormone on leptin, adiponectin, resistin, interleukin-6, tumor necrosis factor-α and ghrelin levels in growth hormone-deficient children. J Endocrinol Invest (2011) 34(4):300–6. doi:10.1007/BF03347090
28. Shulman DI, Root AW, Diamond FB, Bercu BB, Martinez R, Boucek RJ Jr. Effects of one year of recombinant human growth hormone (GH) therapy on cardiac mass and function in children with classical GH deficiency. J Clin Endocrinol Metab (2003) 88(9):4095–9. doi:10.1210/jc.2003-030030
29. Salerno M, Esposito V, Spinelli L, Di Somma C, Farina V, Muzzica S, et al. Left ventricular mass and function in children with GH deficiency before and during 12 months GH replacement therapy. Clin Endocrinol (Oxf) (2004) 60(5):630–6. doi:10.1111/j.1365-2265.2004.02026.x
30. Salerno M, Esposito V, Farina V, Radetti G, Umbaldo A, Capalbo D, et al. Improvement of cardiac performance and cardiovascular risk factors in children with GH deficiency after two years of GH replacement therapy: an observational, open, prospective, case-control study. J Clin Endocrinol Metab (2006) 91(4):1288–95. doi:10.1210/jc.2005-0981
31. Capalbo D, Lo Vecchio A, Farina V, Spinelli L, Palladino A, Tiano C, et al. Subtle alterations of cardiac performance in children with growth hormone deficiency: results of a two-year prospective, case-control study. J Clin Endocrinol Metab (2009) 94(9):3347–55. doi:10.1210/jc.2008-2639
32. Nygren A, Sunnegårdh J, Teien D, Jonzon A, Björkhem G, Lindell S, et al. Rapid cardiovascular effects of growth hormone treatment in short prepubertal children: impact of treatment duration. Clin Endocrinol (Oxf) (2012) 77(6):877–84. doi:10.1111/j.1365-2265.2012.04456.x
33. Sandhu MS, Heald AH, Gibson JM, Cruickshank JK, Dunger DB, Wareham NJ. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. Lancet (2002) 359(9319):1740–5. doi:10.1016/S0140-6736(02)08655-5
34. Carmichael JD, Danoff A, Milani D, Roubenoff R, Lesser ML, Livote E, et al. GH peak response to GHRH-arginine: relationship to insulin resistance and other cardiovascular risk factors in a population of adults aged 50-90. Clin Endocrinol (Oxf) (2006) 65(2):169–77. doi:10.1111/j.1365-2265.2006.02569.x
35. Di Somma C, Ciresi A, Amato MC, Savastano S, Savanelli MC, Scarano E, et al. Alteration of the growth hormone axis, visceral fat dysfunction, and early cardiometabolic risk in adults: the role of the visceral adiposity index. Endocrine (2015) 49(2):492–502. doi:10.1007/s12020-014-0471-z
36. Carroll PV, Christ ER, Bengtsson BA, Carlsson L, Christiansen JS, Clemmons D, et al. Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. Growth Hormone Research Society Scientific Committee. J Clin Endocrinol Metab (1998) 83(2):382–95. doi:10.1210/jcem.83.2.4594
37. Gazzaruso C, Gola M, Karamouzis I, Giubbini R, Giustina A. Cardiovascular risk in adult patients with growth hormone (GH) deficiency and following substitution with GH – an update. J Clin Endocrinol Metab (2014) 99(1):18–29. doi:10.1210/jc.2013-2394
38. Ciresi A, Radellini S, Guarnotta V, Giordano C. The visceral adiposity index is associated with insulin sensitivity and IGF-I levels in adults with growth hormone deficiency. Endocrine (2017) 56(3):579–88. doi:10.1007/s12020-016-1076-5
39. Di Somma C, Scarano E, Savastano S, Savanelli MC, Pivonello R, Colao A. Cardiovascular alterations in adult GH deficiency. Best Pract Res Clin Endocrinol Metab (2017) 31(1):25–34. doi:10.1016/j.beem.2017.03.005
40. Johannsson G, Albertsson-Wikland K, Bengtsson BA. Discontinuation of growth hormone (GH) treatment: metabolic effects in GH-deficient and GH-sufficient adolescent patients compared with control subjects. Swedish Study Group for Growth Hormone Treatment in Children. J Clin Endocrinol Metab (1999) 84(12):4516–24. doi:10.1210/jcem.84.12.6176
41. Nørrelund H, Vahl N, Juul A, Møller N, Alberti KG, Skakkebaek NE, et al. Continuation of growth hormone (GH) therapy in GH-deficient patients during transition from childhood to adulthood: impact on insulin sensitivity and substrate metabolism. J Clin Endocrinol Metab (2002) 85(5):1912–7. doi:10.1210/jcem.85.5.6613
42. Jørgensen JO, Nørrelund H, Vahl N, Juul A, Skakkebaek NE, Christiansen JS. Continuation of growth hormone therapy versus placebo in transition-phase patients with growth hormone deficiency: impact on body composition, insulin sensitivity, and thyroid function. J Pediatr Endocrinol Metab (2002) 15(Suppl 5):1355–60.
43. Carroll PV, Drake WM, Maher KT, Metcalfe K, Shaw NJ, Dunger DB, et al. Comparison of continuation or cessation of growth hormone (GH) therapy on body composition and metabolic status in adolescents with severe GH deficiency at completion of linear growth. J Clin Endocrinol Metab (2004) 89:3890–5. doi:10.1210/jc.2003-031588
44. Tauber M, Jouret B, Cartault A, Lounis N, Gayrard M, Marcouyeux C, et al. Adolescents with partial growth hormone (GH) deficiency develop alterations of body composition after GH discontinuation and require follow-up. J Clin Endocrinol Metab (2003) 88:5101–6. doi:10.1210/jc.2003-030392
45. Lanes R, Gunczler P, Lopez E, Esaa S, Villaroel O, Revel-Chion R. Cardiac mass and function, carotid artery intima-media thickness, and lipoprotein levels in growth hormone-deficient adolescents. J Clin Endocrinol Metab (2001) 86(3):1061–5. doi:10.1210/jcem.86.3.7268
46. Lanes R, Paoli M, Carrillo E, Villaroel O, Palacios A. Cardiovascular risk of young growth-hormone-deficient adolescents. Differences in growth-hormone-treated and untreated patients. Horm Res (2003) 60(6):291–6. doi:10.1159/000074247
47. Lanes R, Paoli M, Carrillo E, Villaroel O, Palacios A. Peripheral inflammatory and fibrinolytic markers in adolescents with growth hormone deficiency: relation to postprandial dyslipidemia. J Pediatr (2004) 145(5):657–61. doi:10.1016/j.jpeds.2004.07.037
48. Lanes R, Soros A, Flores K, Gunczler P, Carrillo E, Bandel J. Endothelial function, carotid artery intima-media thickness, epicardial adipose tissue, and left ventricular mass and function in growth hormone-deficient adolescents: apparent effects of growth hormone treatment on these parameters. J Clin Endocrinol Metab (2005) 90(7):3978–82. doi:10.1210/jc.2005-0091
49. Rothermel J, Reinehr T. Metabolic alterations in paediatric GH deficiency. Best Pract Res Clin Endocrinol Metab (2016) 30(6):757–70. doi:10.1016/j.beem.2016.11.004
50. De Leonibus C, De Marco S, Stevens A, Clayton P, Chiarelli F, Mohn A. Growth hormone deficiency in prepubertal children: predictive markers of cardiovascular disease. Horm Res Paediatr (2016) 85(6):363–71. doi:10.1159/000444143
51. Haymond MW, Karl I, Weldon VV, Pagliara AS. The role of growth hormone and cortisone on glucose and gluconeogenic substrate regulation in fasted hypopituitary children. J Clin Endocrinol Metab (1976) 42(5):846–56. doi:10.1210/jcem-42-5-846
52. Lippe BM, Kaplan SA, Golden MP, Hendricks SA, Scott ML. Carbohydrate tolerance and insulin receptor binding in children with hypopituitarism: response after acute and chronic human growth hormone administration. J Clin Endocrinol Metab (1981) 53(3):507–13. doi:10.1210/jcem-53-3-507
53. Husbands S, Ong KK, Gilbert J, Wass JA, Dunger DB. Increased insulin sensitivity in young, growth hormone deficient children. Clin Endocrinol (Oxf) (2001) 55(1):87–92. doi:10.1046/j.1365-2265.2001.01298.x
54. Stawerska R, Smyczyńska J, Hilczer M, Lewiński A. Relationship between IGF-I concentration and metabolic profile in children with growth hormone deficiency: the influence of children’s nutritional state as well as the ghrelin, leptin, adiponectin, and resistin serum concentrations. Int J Endocrinol (2017) 2017:5713249. doi:10.1155/2017/5713249
55. Caprio S, Plewe G, Diamond MP, Simonson DC, Boulware SD, Sherwin RS, et al. Increased insulin secretion in puberty: a compensatory response to reductions in insulin sensitivity. J Pediatr (1989) 114(6):963–7. doi:10.1016/S0022-3476(89)80438-X
56. Amiel SA, Caprio S, Sherwin RS, Plewe G, Haymond MW, Tamborlane WV. Insulin resistance of puberty: a defect restricted to peripheral glucose metabolism. J Clin Endocrinol Metab (1991) 72(2):277–82. doi:10.1210/jcem-72-2-277
57. Radetti G, Pasquino B, Gottardi E, Contadin IB, Rigon F, Aimaretti G. Insulin sensitivity in growth hormone-deficient children: influence of replacement treatment. Clin Endocrinol (Oxf) (2004) 61(4):473–7. doi:10.1111/j.1365-2265.2004.02113.x
58. Ciresi A, Amato MC, Criscimanna A, Mattina A, Vetro C, Galluzzo A, et al. Metabolic parameters and adipokine profile during GH replacement therapy in children with GH deficiency. Eur J Endocrinol (2007) 156(3):353–60. doi:10.1530/eje.1.02343
59. Ramistella V, Wasniewska M, Arasi S, Catena M, Velletri MR, Corica D, et al. Cross-sectional and prospective study of the effects of GH therapy on metabolic panel in children with GH deficiency. Pediatr Med Chir (2014) 36(5–6):104. doi:10.4081/pmc.2014.104
60. Metwalley KA, Farghaly HS, Abd El-Hafeez HA. Evaluation of left ventricular mass and function, lipid profile, and insulin resistance in Egyptian children with growth hormone deficiency: a single-center prospective case-control study. Indian J Endocrinol Metab (2013) 17(5):876–82. doi:10.4103/2230-8210.117234
61. De Marco S, Marcovecchio ML, Caniglia D, De Leonibus C, Chiarelli F, Mohn A. Circulating asymmetric dimethylarginine and lipid profile in pre-pubertal children with growth hormone deficiency: effect of 12-month growth hormone replacement therapy. Growth Horm IGF Res (2014) 24(5):216–20. doi:10.1016/j.ghir.2014.08.001
62. Ciresi A, Amato MC, Giordano C. Reduction in insulin sensitivity and inadequate β-cell capacity to counteract the increase in insulin resistance in children with idiopathic growth hormone deficiency during 12 months of growth hormone treatment. J Endocrinol Invest (2015) 38(3):351–9. doi:10.1007/s40618-014-0184-4
63. Ciresi A, Cicciò F, Radellini S, Giordano C. Utility of C-peptide for a reliable estimate of insulin secretion in children with growth hormone deficiency. Growth Horm IGF Res (2016) 29:71–7. doi:10.1016/j.ghir.2016.05.001
64. Ciresi A, Giordano C. One-hour post-load plasma glucose level is associated with a worse metabolic profile in children with GH deficiency. J Endocrinol Invest (2017). doi:10.1007/s40618-017-0805-9
65. Ciresi A, Pizzolanti G, Leotta M, Guarnotta V, Teresi G, Giordano C. Resistin, visfatin, leptin and omentin are differently related to hormonal and metabolic parameters in growth hormone-deficient children. J Endocrinol Invest (2016) 39(9):1023–30. doi:10.1007/s40618-016-0475-z
66. Seminara S, Merello G, Masi S, Filpo A, La Cauza F, D’Onghia G, et al. Effect of long-term growth hormone treatment on carbohydrate metabolism in children with growth hormone deficiency. Clin Endocrinol (Oxf) (1998) 49(1):125–30. doi:10.1046/j.1365-2265.1998.00502.x
67. Meazza C, Elsedfy HH, Pagani S, Bozzola E, El Kholy M, Bozzola M. Metabolic parameters and adipokine profile in growth hormone deficient (GHD) children before and after 12-month GH treatment. Horm Metab Res (2014) 46(3):219–23. doi:10.1055/s-0033-1358730
68. Capalbo D, Esposito A, Improda N, Wasniewska MG, Di Mase R, De Luca F, et al. Glucose homeostasis in GHD children during long-term replacement therapy: a case-control study. Endocrine (2018) 59(3):643–50. doi:10.1007/s12020-017-1408-0
69. Chen M, Gan D, Luo Y, Rampersad S, Xu L, Yang S, et al. Effect of recombinant human growth hormone therapy on blood lipid and carotid intima-media thickness in children with growth hormone deficiency. Pediatr Res (2018) 83(5):954–60. doi:10.1038/pr.2017.271
70. Heptulla RA, Boulware SD, Caprio S, Silver D, Sherwin RS, Tamborlane WV. Decreased insulin sensitivity and compensatory hyperinsulinemia after hormone treatment in children with short stature. J Clin Endocrinol Metab (1997) 82(10):3234–8. doi:10.1210/jcem.82.10.4302
71. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care (1999) 22(9):1462–70. doi:10.2337/diacare.22.9.1462
72. Xue Y, Gao Y, Wang S, Wang P. An examination of the effects of different doses of recombinant human growth hormone on children with growth hormone deficiency. Exp Ther Med (2016) 11(5):1647–52. doi:10.3892/etm.2016.3091
73. Cutfield WS, Wilton P, Bennmarker H, Albertsson-Wikland K, Chatelain P, Ranke MB, et al. Incidence of diabetes mellitus and impaired glucose tolerance in children and adolescents receiving growth-hormone treatment. Lancet (2000) 355(9204):610–3. doi:10.1016/S0140-6736(99)04055-6
74. Child CJ, Zimmermann AG, Scott RS, Cutler GB Jr., Battelino T, Blum WF, et al. Prevalence and incidence of diabetes mellitus in GH-treated children and adolescents: analysis from the GeNeSIS observational research program. J Clin Endocrinol Metab (2011) 96(6):E1025–34. doi:10.1210/jc.2010-3023
75. Nozue H, Kamoda T, Matsui A. Serum resistin concentrations in growth hormone-deficient children during growth hormone replacement therapy. Metabolism (2007) 56(11):1514–7. doi:10.1016/j.metabol.2007.06.018
76. Wei Y, Zheng R, Zhou Y, Wang J, Bao P. Correlation between exon 3 polymorphism of growth hormone receptor gene and the responses to rhGH therapy. Int J Clin Exp Pathol (2015) 8(6):7371–7.
77. Ciresi A, Guarnotta V, Pizzolanti G, Giordano C. Comparison between euglycemic hyperinsulinemic clamp and surrogate indices of insulin sensitivity in children with growth hormone deficiency. Growth Horm IGF Res (2017) 39:40–4. doi:10.1016/j.ghir.2017.12.007
78. Bell J, Parker KL, Swinford RD, Hoffman AR, Maneatis T, Lippe B. Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metab (2010) 95(1):167–77. doi:10.1210/jc.2009-0178
79. Poidvin A, Weill A, Ecosse E, Coste J, Carel JC. Risk of diabetes treated in early adulthood after growth hormone treatment of short stature in childhood. J Clin Endocrinol Metab (2017) 102(4):1291–8. doi:10.1210/jc.2016-3145
80. Ciresi A, Cicciò F, Radellini S, Guarnotta V, Calcaterra AM, Giordano C. More favorable metabolic impact of three-times-weekly versus daily growth hormone treatment in Naïve GH-deficient children. Int J Endocrinol (2017) 2017:8469680. doi:10.1155/2017/8469680
81. Baronio F, Mazzanti L, Girtler Y, Tamburrino F, Fazzi A, Lupi F, et al. The influence of growth hormone treatment on glucose homeostasis in growthhormone-deficient children: a six-year follow-up study. Horm Res Paediatr (2016) 86(3):196–200. doi:10.1159/000448841
82. Sesmilo G, Biller BM, Llevadot J, Hayden D, Hanson G, Rifai N, et al. Effects of growth hormone administration on inflammatory and other cardiovascular risk markers in men with growth hormone deficiency. A randomized, controlled clinical trial. Ann Intern Med (2000) 133(2):111–22. doi:10.7326/0003-4819-133-2-200007180-00010
83. Svensson J, Fowelin J, Landin K, Bengtsson BA, Johansson JO. Effects of seven years of GH-replacement therapy on insulin sensitivity in GH-deficient adults. J Clin Endocrinol Metab (2002) 87(5):2121–7. doi:10.1210/jcem.87.5.8482
84. Giavoli C, Porretti S, Ronchi CL, Cappiello V, Ferrante E, Orsi E, et al. Long-term monitoring of insulin sensitivity in growth hormone-deficient adults on substitutive recombinant human growth hormone therapy. Metabolism (2004) 53(6):740–3. doi:10.1016/j.metabol.2003.11.025
85. Marcovecchio ML, Bagordo M, Marisi E, de Giorgis T, Chiavaroli V, Chiarelli F, et al. One-hour post-load plasma glucose levels associated with decreased insulin sensitivity and secretion and early makers of cardiometabolic risk. J Endocrinol Invest (2017) 40(7):771–8. doi:10.1007/s40618-017-0638-6
86. Galimov A, Hartung A, Trepp R, Mader A, Flück M, Linke A, et al. Growth hormone replacement therapy regulates microRNA-29a and targets involved in insulin resistance. J Mol Med (Berl) (2015) 93(12):1369–79. doi:10.1007/s00109-015-1322-y
87. Saenger P. Metabolic consequences of growth hormone treatment in paediatric practice. Horm Res (2000) 53(Suppl 1):60–9. doi:10.1159/000053207
88. Growth Hormone Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. GH Research Society. J Clin Endocrinol Metab (2000) 85(11):3990–3. doi:10.1210/jc.85.11.3990
89. Allen DB, Backeljauw P, Bidlingmaier M, Biller BM, Boguszewski M, Burman P, et al. GH safety workshop position paper: a critical appraisal of recombinant human GH therapy in children and adults. Eur J Endocrinol (2016) 174(2):1–9. doi:10.1530/EJE-15-0873
Keywords: growth hormone, metabolism, glucose, children, insulin sensitivity
Citation: Ciresi A and Giordano C (2018) Glucose Metabolism in Children With Growth Hormone Deficiency. Front. Endocrinol. 9:321. doi: 10.3389/fendo.2018.00321
Received: 25 February 2018; Accepted: 28 May 2018;
Published: 11 June 2018
Edited by:
Margaret Cristina Da Silva Boguszewski, Universidade Federal do Paraná, BrazilReviewed by:
Sonir Roberto Rauber Antonini, Universidade de São Paulo, BrazilCopyright: © 2018 Ciresi and Giordano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandro Ciresi, YS5jaXJlc2lAdmlyZ2lsaW8uaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.