
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 04 April 2018
Sec. Neuroendocrine Science
Volume 9 - 2018 | https://doi.org/10.3389/fendo.2018.00147
This article is part of the Research Topic The Multiple Facets of Kisspeptin Activity in Biological Systems View all 13 articles
Kisspeptin, a novel neuropeptide product of the Kiss1 gene, activates the G protein-coupled membrane receptor G protein-coupled receptor 54 (now termed Kiss1r). Over the last 15 years, the importance of the kisspeptin system has been the subject of much debate in the mammalian research field. At the heart of the debate is whether kisspeptin is an absolute upstream regulator of gonadotropin-releasing hormone secretion, as it has been proposed to be the master molecule in reproductive events and plays a special role not only during puberty but also in adulthood. The teleostean kisspeptin system was first documented in 2004. Although there have been a number of kisspeptin studies in various fish species, the role of kisspeptin in reproduction remains a subject of controversy and has not been widely recognized. There is an extensive literature on the physiological and endocrinological bases of gametogenesis in fish, largely derived from studying small, model fish species, and reports on non-model species are limited. The reason for this discrepancy is the technical difficulty inherent in developing rigorous experimental systems in many farmed fish species. We have already established methods for the full life-cycle breeding of a commercially important marine fish, the chub mackerel (cm), and are interested in understanding the reproductive function of kisspeptins from various perspectives. Based on a series of experiments clarifying the role of the brain–pituitary–gonad axis in modulating reproduction in cm, we theorize that the kisspeptin system plays an important role in the reproduction of this scombroid species. In this review article, we provide an overview of kisspeptin studies in cm, which substantially aids in elucidating the role of kisspeptins in fish reproduction.
Kisspeptin is an RFamide peptide product of the Kiss1 gene and the natural ligand of the G protein-coupled receptor 54 (GPR54), now named Kiss1r (1, 2). Mature kisspeptins in mammals are cleaved into endogenous fragments: Kp54, Kp16, Kp14, Kp13, and Kp10 (3). The C-terminus decapeptide Kp10 (Kiss-10) region is the minimum active site and is highly conserved across vertebrates. Mutations in the Kiss1R gene are correlated with an absence of puberty onset and hypogonadotropic hypogonadism in humans (4, 5). These abnormalities are due to the disruption of the hierarchical reproductive network, especially the kisspeptin–gonadotropin-releasing hormone (Gnrh)–luteinizing hormone (Lh) pathway. Kisspeptin fibers have been observed in the vicinity of Gnrh neuron cell bodies, and a large population of Gnrh neurons expresses Kiss1r mRNA, clearly indicating that kisspeptin neurons directly signal Gnrh neurons (6–8). Indeed, administration of Kp10 was found to elicit a robust increase in the circulating levels of Gnrh (9). Several studies in mammals have strongly demonstrated the absolute necessity of kisspeptin signaling for puberty onset and ovulation through the regulation of Gnrh secretion.
In 2004, the isolation of the complementary DNA (cDNA) of a piscine ortholog of the kisspeptin receptor (kissr2) in Nile tilapia (Oreochromis niloticus) was the first evidence for the existence of a kisspeptin system in fish (10). In mammals, only one gene (Kiss1) coding for the ligand and one for the receptor (Kiss1r) are present. Teleost fishes, known to have undergone a third genome duplication event, have two paralogous kisspeptin genes (kiss1 and kiss2), and four genes encoding kisspeptin receptors have been reported, although most fish have only two receptors, kissr2 and kissr3 (also known as gpr54-2b and gpr54-1b) (11, 12).
At least two molecular forms of Gnrh are present in the brains of all vertebrate species, with some teleosts expressing three different forms (i.e., Gnrh1, Gnrh2, and Gnrh3) (13). Gnrh1 is considered the major hypophysiotropic hormone controlling the synthesis and release of Lh in all vertebrates. By contrast, Gnrh3 is a teleost-specific form that is expressed in neuronal populations in the olfactory bulb, the terminal nerve ganglion region, and the pre-optic area (POA). Gnrh3 axonal fibers project into different brain regions, suggesting a role in neuromodulation. In fish expressing two Gnrh forms, such as the Salmonidae and Cyprinidae, Gnrh3 not only functions as a neuromodulator but also regulates the secretion of pituitary gonadotropins (14, 15).
The major underlying question is whether fish kisspeptin is an important regulator of the reproductive brain–pituitary–gonad (BPG) axis. Several studies strongly suggest that, similar to its mammalian counterpart, the fish kisspeptin ortholog is a potent activator of the reproductive axis. Gnrh1 neurons express kissr2 mRNA in cichlid fish (e.g., O. niloticus and Astatotilapia burtoni) (10, 16), and striped bass (Morone saxatilis) (17) is a notable example. In addition, several in vivo studies have shown that the injection of Kiss2 peptide promotes the secretion of Gnrh and gonadotropins (18, 19). Furthermore, kisspeptin antagonists were found to inhibit sperm production in striped bass (20). However, in the zebrafish (Danio rerio), kiss- or kissr-knockout mutants exhibited normal gonadal maturation, indicating that kisspeptin signaling is not indispensable for reproduction in this species (21). A few studies have indicated that Gnrh neurons do not express kisspeptin receptors in medaka (Oryzias latipes) (22) or European sea bass (Dicentrarchus labrax) (23). Hence, the true role of kisspeptin in fish reproduction remains open to debate.
The chub mackerel (cm) (Scomber japonicus) is a small marine pelagic fish, which belongs to the order Perciformes and the family Scombridae. This species is one of the top 10 principal food fish contributing to global capture fisheries production. In addition, cm is a suitable experimental model fish for reproductive endocrinological research in Perciformes, which is the most evolved and largest teleost fish group and includes many target aquaculture species. Our team recently developed standardized methods to support the full life cycle of this species in land-based small-scale aquaculture. This system aids in obtaining a series of captive cm samples throughout the entire reproductive cycle for the analysis of key hormones acting in the BPG axis and enables breeding experiments. Using these fish sampling facilities, our group has isolated the key molecular elements of the cm BPG axis in reproduction, namely Gnrhs (Gnrh1, 2, and 3) (24, 25), the Gnrh receptor (GnrhR1) (26), gonadotropic hormones (Gths) (27–30), and Gth receptors (31). Furthermore, steroid hormones involved in vitellogenesis and oocyte maturation have been demonstrated in this species (32). There is an extensive literature on the physiological and endocrinological bases of gametogenesis in fish; this literature is largely derived from small, model fish species, such as zebrafish and medaka. However, in many cases, data on the mechanisms of reproductive regulation in teleosts vary greatly among species. This may be due to the considerable length of their evolutionary process and the diversity of species, reproductive patterns, and habitats. To clarify the general mechanisms underlying the reproductive physiology of fish, studies in non-model species may be informative.
In recent years, we have focused on inducing the potency of kisspeptin peptides in gonadal development. Of particular interest is the period of pubertal transition and gonadal recrudescence, which is important for the establishment of an efficient aquaculture of any fish species. The aim of this article is to review our previous 11 papers on the kisspeptin system in cm reproduction. At the same time, we strongly adhere to a comparative approach involving other model and non-model teleost fish species to provide a comprehensive summary of the knowledge on fish kisspeptins to date.
The cm possesses two kisspeptin genes, kiss1 and kiss2 (33). These sequences were submitted to GenBank as follows: kiss1, GU731672 and kiss2, GU731673. cmkiss1 and cmkiss2 cDNAs encode 105 and 123 amino acids, respectively, and display very low sequence similarity (18%).
Kisspeptin coding sequences have been isolated in many fish species. All reported teleost species possess the kiss2 gene, whereas the genomes of puffer fish and sticklebacks (Gasterosteus aculeatus) lack the kiss1 gene and contain only the kiss2 gene (18).
The C-terminus decapeptide Kp10 region is highly conserved within mammalian and non-mammalian vertebrates. In an initial study of fish kisspeptin, the deduced sequences for Kiss1-10 and Kiss2-10 were assumed to be the minimum functional core peptides. The modified fish KP44 peptide, which lacks the C-terminus KP10 region, has no bioactivity, suggesting that the KP10 region is essential for receptor binding, as is the case with mammalian kisspeptin (34). However, in teleost fish, the Kiss1 precursor contains a conserved dibasic 5-amino acid site upstream of the KP10 region, indicating that it produces a mature pentadecapeptide (Kiss1-15), which should have pyroglutamate at the N-terminus because the residue at the N-terminal end of Kiss1-15 is glutamine in all reported fish species (35). Similarly, a conserved arginine residue at position 13 is present in all available Kiss2 sequences, indicating the presence of a putative cleavage site that produces a mature dodecapeptide (Kiss2-12) (35). Both the Kiss1-15 and Kiss2-12 regions are highly conserved across teleost fishes.
The cmKiss1-15 (QDMSSYNFNSFGLRY-NH2) and cmKiss2-12 (SNFNFNPFGLRF-NH2) peptides showed the highest potency for the activation of cognate receptors, stronger than their corresponding KP10 peptides in cm (36). The same results were reported in zebrafish (35) and European sea bass (37). These results suggest that amino acid sequences other than the KP10 region are also functionally important, perhaps due to factors such as the structure, hydrophobicity, or electric charge of fish kisspeptins. Choosing the right peptide form to test is therefore important for clarifying the bioactivity of fish kisspeptins.
The cmKiss1 precursor contains a putative cleavage site located seven amino acids upstream of the core sequence (33), indicating that it produces a mature hexadecapeptide (cmKiss1-16: HQDMSSYNFNSFGLRY-NH2). This Kiss1-16 showed higher sensitivity for receptor activation than Kiss1-15 (38). Another example, a mature Kiss2 tridecapeptide (Kiss2-13) was isolated in masu salmon (Oncorhynchus masou masou and Oncorhynchus nerka) (39). This may also reflect differences in the basic site of the precursor protein.
Chub mackerel possess two receptor subtypes (kissr2 and kissr3), which have already been submitted to GenBank as kissr2 (gpr54-2): JX982323 and kissr3 (gpr54-1): JX982322 (36). The kissr2 and kissr3 cDNAs have open reading frames of 1,137 bp (378 amino acids) and 1,110 bp (369 amino acids), respectively. The amino acid sequences of the receptors share only 52% identity; however, high sequence identity was found between the transmembrane domains (73%). cmKissR2 exhibited >80% identity with all other teleosts and >93% identity with other Perciform fish KissR2 sequences. cmKissR3 showed high similarity to European sea bass (88%), followed by striped bass (87%); however, slightly lower homology with goldfish (65%) and zebrafish (62%) KissR3 sequences was observed.
Since its first discover in 2004, kisspeptin receptors have been cloned and sequenced in more than 50 different teleost fish species. Almost all these fish possess the piscine ortholog kissr2, whereas few species possess kissr2 and kissr3 sequences in their genomes. There are a few exceptions; for example, the European eel (Anguilla anguilla), coelacanth (Latimeria chalumnae), and spotted gar (Lepisosteus oculatus) possess three types of receptors (kissr1, kissr2, and kissr3), and coelacanth and the spotted gar further possess kissr4 genes, as detailed in the phylogenetic tree (11, 40). It is important to note that the nomenclature of fish kisspeptin receptors has not yet been established, and different research teams use different abbreviations. In this article, the receptor names are adopted from syntenic studies by Pasquier et al. (11, 12). Elsewhere, kissr2 is sometimes referred to as kiss2r or gpr54-2. Furthermore, other designations of kissr3 include kissr1, kiss1r, and gpr54-1, because, in many cases, this receptor showed high sensitivity for the Kiss1 peptide. Our previous kisspeptin papers referred to the kisspeptin receptors as kissr1 and kissr2.
The ligand sensitivities of the two subtypes of cm kisspeptin receptors were examined by reporter gene assays using mammalian cell lines. The results revealed that cm kisspeptin receptor signals are preferentially transduced via the protein kinase C (PKC)/mitogen activated protein kinases rather than by the protein kinase A (PKA) pathway (36). Synthetic cm Kiss1-15 (or Kiss1-16) and Kiss2-12 peptides showed the highest potency for the activation of KissR3 and KissR2, respectively (Figures 1A,B) (36, 38). Thus, we concluded that KissR3 and KissR2 are the intrinsic receptors for the Kiss1 and Kiss2 peptides, respectively, and signals are mainly transduced via the PKC pathway in this species (36).
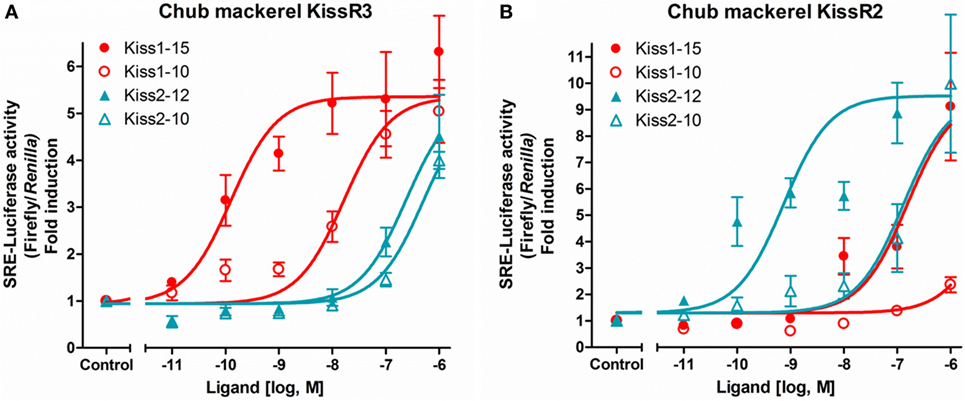
Figure 1. Ligand selectivity of the chub mackerel (cm) kisspeptin receptors. KissR3 (A) and KissR2 (B), each together with SRE-Luc. Transfected cells were treated with graded concentration of each peptides. The data are expressed as the ratio of changes in firefly luciferase activity over the control Renilla luciferase activity. Each point was determined in quadruplicate and is given as a mean ± SEM. Modified from Ref. (36), by permission of Elsevier.
Ligand sensitivity has been analyzed in duplicated kisspeptin systems in zebrafish (35, 41), goldfish (Carassius auratus) (42), Southern bluefin tuna (Thunnus maccoyii) (43), yellowtail kingfish (Seriola lalandi) (43), medaka (22), European sea bass (37), and striped bass (20) and, in one species, the orange-spotted grouper (Epinephelus coioides), with only the Kiss2/KissR2 pair (44). In all studies, intracellular signals were preferentially transduced via the PKC pathway; however, the PKA pathway was also activated by ligand stimulation in goldfish (42), medaka (22), and European sea bass (37). In many cases, KissR2 and KissR3 showed high sensitivity for the Kiss2 and Kiss1 peptides, respectively. Conversely, in goldfish, Kiss1-10 enhanced KissR2 activation, and Kiss2-10 exhibited a higher preference for KissR3 (42). It should also be noted that KissR2 showed equal sensitivity to both Kiss1 and Kiss2 peptides in the zebrafish (35), Southern bluefin tuna, and yellowtail kingfish (43); KissR3 showed the same binding potency for Kiss1 and Kiss2 peptides in striped bass (20). The high species specificity and the complexity of the ligand sensitivity or signaling pathways in duplicated kisspeptin systems in fish is noteworthy.
We evaluated the biological potency of kisspeptin peptides to induce transcriptional changes in gnrh1, follicle-stimulating hormone (fsh)b, and lhb in gonadal recrudescent cm. Synthetic Kiss1-15 and Kiss2-12 were administered at a dose of 100 ng into the intracerebroventricular (i.c.v.) region, and brains were sampled at 6 and 12 h post-administration (Figure 2A). In both sexes, the transcription levels were quantified using quantitative real-time PCR (qRT-PCR). i.c.v. administration of Kiss2-12 but not Kiss1-15 significantly elevated pituitary fshb and lhb transcripts at 12 h post-administration in comparison to saline-injected controls (Figures 2B,C) (45). In addition, in females, the transcription levels of POA gnrh1 were downregulated by both kisspeptin peptides at 12 h post-administration (45). It is clear that central administration of kisspeptin peptides influenced Gnrh and gonadotropin synthesis, suggesting that kisspeptin peptides may play a central role in the regulation of the reproductive BPG axis in cm.
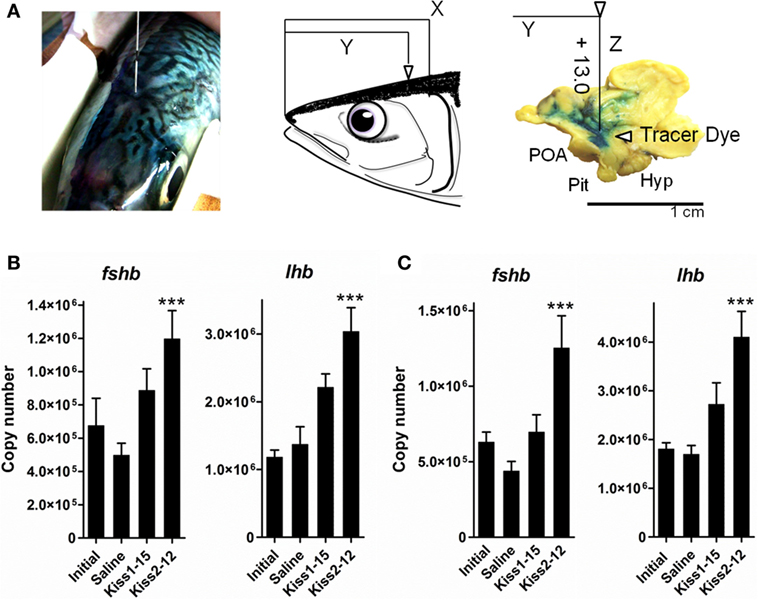
Figure 2. Schematic illustration of coordinates for injection into the intracerebroventricular (i.c.v.) region of chub mackerel (A). We determined that if head length (rostral end to upper end of gill) was assumed to be X and length of the rostral end to immediately above the third ventricle was assumed to be Y, the ratio of X to Y converged from 1.4 to 1.0. Tested peptide or PBS with blue dye was administered into the third ventricle to a depth of 13 mm below the tissue surface. Abbreviations: Hyp, hypothalamus; Pit, pituitary; POA, pre-optic area. (B,C) Analysis of the effect of i.c.v. administration of Kiss1-15 and Kiss2-12 peptides on fshb and lhb mRNA levels in the pituitary at 12 h post-administration, each together in adult male (B) or female (C) immature subjects. Transcription levels are the mean ± SEM of 9–16 independent determinations. ***P < 0.001, one-way ANOVA followed by a Tukey’s multiple comparison test. Modified from Ref. (45), by permission of Elsevier.
The third ventricle was chosen as the site of administration due to its proximity to the brain centers controlling reproductive activity; it is the most accurate way to study the role of centrally acting peptides (45). Thus, delivering exogenous kisspeptins to the central nervous system is important, and our study was the first to demonstrate the effects of kisspeptin peptides on brain gnrh mRNA expression. In the same manner, Espigares et al. evaluated the bioactivity of kisspeptin peptides in immature adult European sea bass. i.c.v. administration of Kiss2-12 stimulated Gnrh1 release into the pituitary and increased serum Fsh, Lh, and sex steroids until 72 h post-treatment (19). These results suggest that kisspeptin potently stimulates reproductive axis activity.
The biological effects of kisspeptin peptides through peripheral [intraperitoneal (i.p.) and intramuscular (i.m.)] administration have been studied in some fish species. In early to mid-pubertal fathead minnows (Pimephales promelas), i.p. administration of mammalian KP10 increased the expression of gnrh3 in the brain after 10 h (46). In sexually mature female zebrafish, i.p. administration of Kiss2-10 upregulated fshb and lhb expression 12 h post-treatment (47). In goldfish, i.p. administration of Kiss1-10 stimulated Lh secretion until 6 h post-treatment (42). In sexually mature female orange-spotted grouper, i.p. administration of Kiss2-10 increased hypothalamic expression of gnrh1 and pituitary fshb expression until 12 h post-treatment (44). Finally, in pubertal hybrid bass (Morone species), i.m. administration of Kiss2-12 upregulated plasma Lh levels until 24 h post-treatment and, during gonadal recrudescence, both Kiss1-15 and Kiss2-12 induced Lh secretion after 24 h (17). Taken together, these data conclusively demonstrate that kisspeptins induce Gnrh and gonadotropin release in many fish species.
We evaluated the potency of Kiss1 and Kiss2 in inducing gonadal development in sexually immature prepubertal and gonadal recrudescent cm. In pubertal fish, synthetic Kiss1-15 or Kiss2-12 peptides were mixed with molten cocoa butter (slow-releasing medium), and peripheral injection was repeated three times at an interval of 2 weeks. In adult fish, the same peptides were administered subcutaneously using Alzet mini-osmotic pumps (Model 2006). In both studies, peptide treatments were continued for a period of 6 weeks.
These results deserve explicit emphasis. In pubertal males, 66.7% of Kiss1-15-treated fish had spermatozoa (SPZ) in their testes, whereas no SPZ were observed in Kiss2-12- and saline-treated fish (48). In addition, the levels of sex steroids 11-ketotestosterone (11-KT) and estradiol-17β (E2) were significantly higher in Kiss1-15-treated fish (48). In pubertal females, Kiss1-15-treated fish displayed yolk vesicles in the growing oocytes, whereas Kiss2-12- and saline-treated fish did not. E2 levels were significantly higher in Kiss1-15-injected fish than in control fish (49). The effects on gonadal recrudescence were more critical. In recrudescent males, Kiss1-15-treated fish exhibited a significantly higher gonadosomatic index (GSI) than did all other treatment groups, and SPZ were present in the testes of Kiss1-15-treated fish at the end of the experiment (Figures 3A–E,K,L) (50). The GSI values of adult females did not differ among treatments; however, the mean oocyte diameters of Kiss1-15-treated fish, representing the early vitellogenic oocytes, were significantly higher than those of the saline- and Kiss2-12-treated fish (Figures 3F–J,M) (50). In both sexes, Kiss1-15-treated fish exhibited higher levels of circulating 11-KT and E2 (Figures 3N,O) (50). These results suggest that synthetic kisspeptin peptides can induce pubertal onset and gonadal recrudescence in cm.
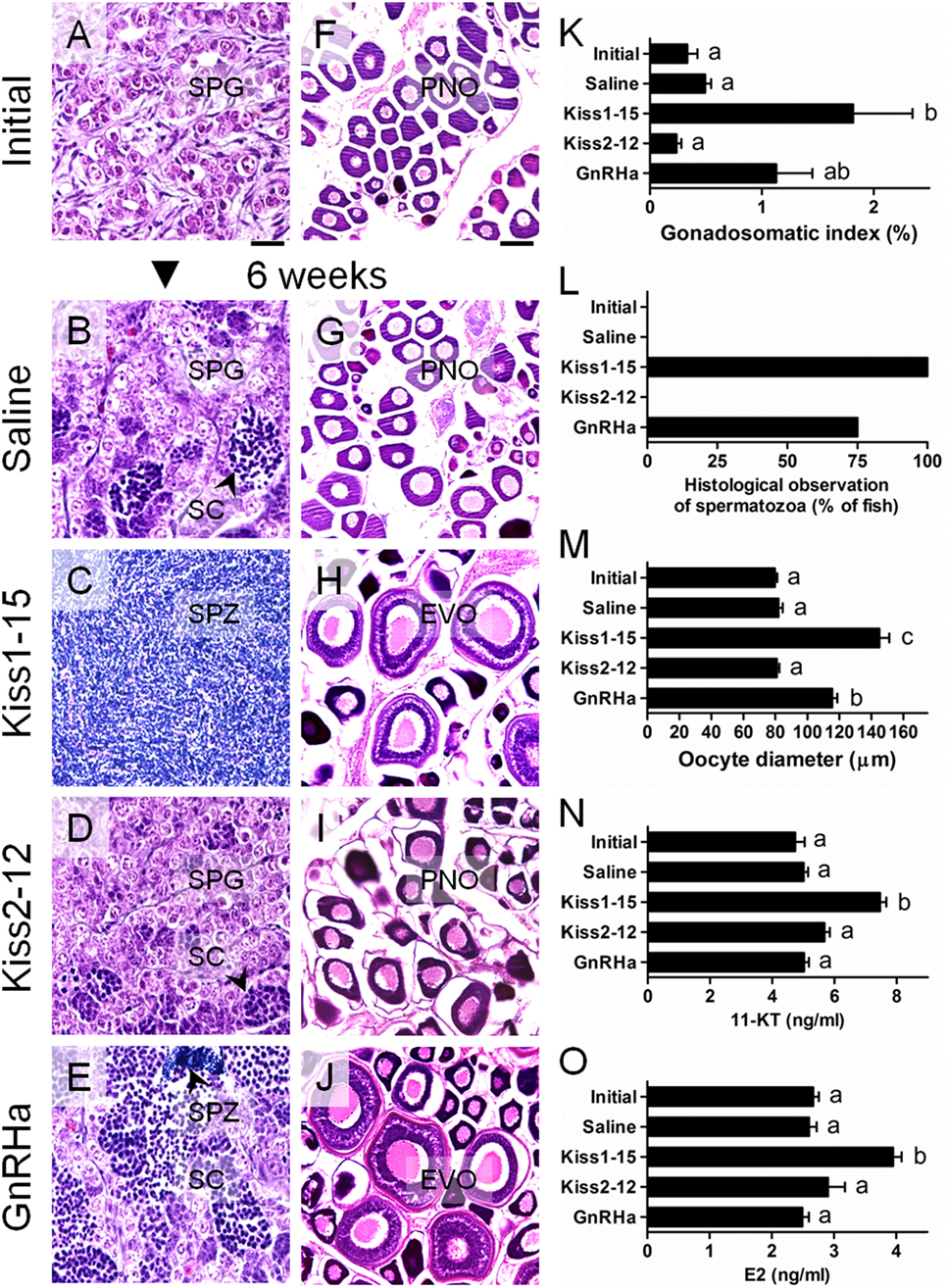
Figure 3. Changes in the gonadal histology of male (A–E) and female (F–J) chub mackerel in different treatments. Abbreviations; SPG, spermatogonia; SC, spermatocytes; SPZ, spermatozoa; PNO, perinucleolar oocyte; EVO, early vitellogenic oocyte. Scale bars = 100 µm. (K) Changes in the gonadosomatic index (GSI) of male fish. (L) Percentage of fish by treatment showing histological presence of SPZ in the testes. (M) Changes the mean oocyte diameter of fish at different treatments, respectively. Changes in serum 11-ketotestosterone (11-KT) in male (N) and estradiol-17b (E2) in female (O), respectively. Values are means ± SEM (n = 4–6 for each treatment). Different letters above the bars represent significant differences between treatments (p < 0.05, one-way ANOVA followed by a Tukey’s multiple comparison test). GnRHa: GnRH analog (D-Ala6, des-Gly10)-LHRH ethylamide. Modified from Ref. (50), by permission of Zoological Society of Japan.
There is ample evidence to indicate the importance of kisspeptin stimulation for gonadotropin secretion and gonadal development in fish. Beck et al. reported that subcutaneous biweekly administration of European sea bass Kiss1-10 but not Kiss2-10 for 7 weeks significantly increased the GSI and SPZ volume in prepubertal male white bass (M. chrysops) (51). Nocillado et al. utilized slow-release implants to chronically deliver synthetic kisspeptin to prepubertal male yellowtail kingfish and reported that fish treated with Kiss1-10 were 100% developed over 4 weeks and exhibited the most advanced stage of development, with testes containing mostly spermatids and SPZ (52). On the other hand, 8-week treatment with Kiss2-10 had a stronger stimulatory effect on testicular development during the non-breeding period (52). In male and female cinnamon clownfish (Amphiprion melanopus), treatment with human KP10 upregulated Gnrhs, Gths, Gth receptors, estrogen, and vitellogenin in the brain, pituitary, gonads, serum, and liver, respectively, and also promoted gonadal development over 6 weeks (53). In male European sea bass, Kiss2-12 treatment induced a significant increase in cumulative milt on days 3 and 7 after i.c.v. administration (19). Finally, in male and female Nile tilapia, cognate Kiss2-10 was administered i.p. twice weekly, and this increased the expression of gnrh1, fshb and lhb mRNA, and plasma levels of E2 and 11-KT (54). This study also showed accelerated testicular development after 4 weeks (with eight total administrations of kisspeptin). Overall, these studies suggest that chronic treatment with kisspeptin peptides modulates gonadotropin secretion and influences gonadal development in many fish species. However, the effects on ovarian development may be slower. For example, oocyte size increased, but there were no histological differences in prepubertal white bass after a 7-week period of Kiss1-10 or Kiss2-10 treatment (51), in prepubertal striped bass after 10 weeks of Kiss1-15 treatment (55), or in immature Nile tilapia after 4 weeks of Kiss2-10 treatment (54). The same trend was confirmed in our study using prepubertal female cm (49). Detailed and long-term research is needed to comprehensively examine both the basic science and commercial application of kisspeptin peptides.
Quantitative real-time PCR analysis revealed that kisspeptin system transcripts are distributed in different tissues of the BPG axis in adult male and female cm. The kiss1 gene was expressed in the brain, pituitary, and gonad (33). The kiss2 gene was expressed only in the brain (33). The kissr2 gene was expressed in the brain, pituitary, and testes but not in the ovary (36). The kissr3 gene was expressed in the brain and testes but not in the ovary (36).
We analyzed expression changes in these genes during early development [0–30 days post-hatching (dph)] and during the period of gonadal sex differentiation (37–60 dph) in cm using qRT-PCR assays. During early development, the expression of kiss1, kiss2, and kissr2 in the whole head did not vary significantly; however, kissr3 expression decreased significantly at 20 dph compared with expression levels just after hatch (56). Interestingly, kiss2, kissr2, and kissr3 were significantly elevated at the start of gonadal sex differentiation in both males and females (56). These results suggest the potential involvement of the kisspeptin system during early development and gonadal sex differentiation in the cm.
There is strong evidence to suggest that the early kisspeptin system modulates the proliferation of the neuronal network. In zebrafish, Zhao et al. showed that early treatment with both Kiss1 and Kiss2 stimulated the proliferation of trigeminal Gnrh3 neurons located in the peripheral nervous system, and Kiss1, but not Kiss2, stimulated the proliferation of the terminal nerve and hypothalamic populations of Gnrh3 neurons in the central nervous system (57). In the same species, qRT-PCR revealed detectable levels of kiss1 and kiss2 mRNA by 1 day post-fertilization (dpf), which increased throughout embryonic and larval development (57). Furthermore, there are several other examples of kisspeptin expression at early developmental stages. In medaka, the expression of kiss1, kiss2, and kissr3 was observed immediately 1 h post-fertilization, and both kiss1 and kiss2 levels peaked at 1 dpf (58). In cobia (Rachycentron canadum), kissr2 expression was detected at 1 dph and peaked at 2 and 3 dph (59). In black rockfish (Sebastes schlegelii), both kiss1 and kissr2 expression displayed an increasing trend during early development (60).
Expression changes during gonadal sex differentiation were also confirmed in some reports. In cobia, kissr2 expression increased in sexually differentiated males but not in females (59). In fathead minnow, kissr2 expression increased in both males and females immediately after gonadal sex differentiation (46). In pejerrey (Odontesthes bonariensis), kiss2 levels increased at 4 weeks after hatching, which is when gonadal differentiation occurs in this species (40). Finally, in tongue sole (Cynoglossus semilaevis), kiss2 and kissr2 transcripts rapidly increased during the early gonadal differentiation period (61). It is likely that an early kisspeptin system is important in brain development and involved in modulating gonadal sex differentiation in teleost fishes.
We examined the temporal patterns of gene expression of the two kisspeptin subtypes and their receptors in the brain during the pubertal process in cm using qRT-PCR. In male fish, kiss2 and gnrh1 expression levels increased significantly just before the onset of meiosis in the testes (62). In female fish, kiss1 and kiss2 levels increased significantly, concomitant with increases in the kissr2, kissr3, and gnrh1 levels, just before the onset of vitellogenesis in the oocytes (Figure 4) (62). Notably, upon pubertal onset, pituitary gnrhr1, fshb, and lhb began to gradually increase in both sexes (26, 63). These results suggest that, as is the case in mammals, the positive involvement of the kisspeptin system in the pubertal process in cm.
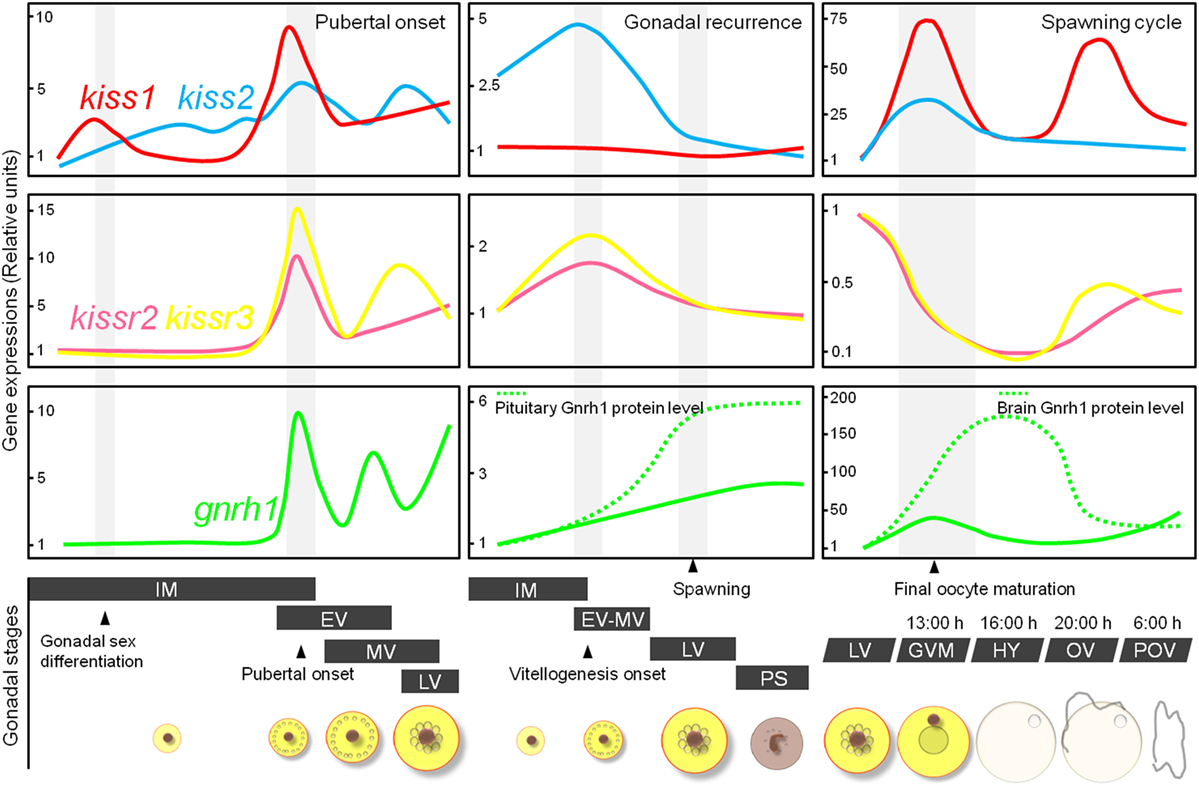
Figure 4. Schematic representation showing relative changes in kiss1, kiss2, kissr2, kissr3, and gnrh1 gene levels in the brain of female chub mackerel during the puberty, gonadal recurrence, and spawning cycle. Abbreviations: IM, immature; EV, early vitellogenesis; MV, mid-vitellogenesis; LV, late vitellogenesis; GVM, germinal vesicle migration; HY, hydration; OV, ovulation; POV, post-ovulation.
For the moment, let us closely examine kisspeptin gene expression at puberty. The levels of kissr2 mRNA in the brain were correlated with pubertal development in the cobia (59), grey mullet (Mugil cephalus) (64), zebrafish (41), fathead minnow (46), Nile tilapia (65), Senegalese sole (Solea senegalensis) (66, 67), Atlantic halibut (Hippoglossus hippoglossus) (68), and Atlantic salmon (Salmo salar) (69). In the brains of Nile tilapia, kissr2 was expressed in a significantly higher percentage of Gnrh neurons in mature males than in immature males (10). Ligand expression during puberty was reported in female zebrafish, and kiss1, kiss2 and gnrh3 all showed increased gene expression at the onset of puberty (47). Similarly, brain kiss2 expression levels increased significantly in flat fish, Senegalese sole (67, 70) and Japanese flounder (71). In Atlantic cod (Gadus morhua), acute kiss2 elevation was observed in maturing individuals (72). In red sea bream (Pagrus major), the number of kiss2-expressing neurons in the hypothalamus was greater during the first spawning season in both males and females compared with fish in the post-spawning periods (73). In female striped bass, kiss1 and kiss2 and their receptors were dramatically elevated in mature females compared with prepubertal levels (17). These results clearly demonstrate that kisspeptin gene expression and pubertal timing are highly correlated in many teleost fish species. These results support the involvement of the kisspeptin system in pubertal onset in fish reproduction.
To elucidate the involvement of multiple kisspeptin systems in annual gonadal recurrence in cm, their relative gene expression profiles in the brain were analyzed at different gonadal stages using qRT-PCR. In males, kiss1 and kiss2 exhibited maximal expression levels between the immature and early spermiation periods and gradually decreased in the post-spawning period (33). In females, kiss2 expression reached a maximal level at the start of the vitellogenic period, and two receptors also showed significantly high expression at this time (Figure 4) (33, 36). Our previous study demonstrated that the gene expression of Gnrh1 was closely related to seasonal ovarian development, and Gnrh1 peptide secretion increased at the start of gonadal recrudescence (25). Collectively, these findings suggest that the activation of kisspeptin systems during gonadal recrudescence may influence Gnrh1 release from the brain to the pituitary.
Similar studies are abundant. A study of the seasonal expression of kisspeptin genes during the seasonal gonadal cycle in the adult grass puffer (Takifugu niphobles) found that the kiss2 and kissr2 genes in the brain were significantly elevated during the prespawning and spawning periods in both sexes (74). The kiss1, kiss2, and kissr2 expression levels in the whole brain in male European sea bass were significantly higher in mid- and late spermatogenesis compared with the post-spawn period (75). Alvarado et al. measured the expression of kisspeptin-related genes in the hypothalamus of adult male and female European sea bass and reported that there genes increased either before or during the advanced stage of oogenesis and decreased during the atretic stage (76). In the rohu (Labeo rohita), the brain kiss1 expression levels were significantly elevated at the prespawning and spawning periods in males and females, respectively (77). Similarly, in golden mahseer (Tor putitora), brain kiss1 and kissr3 expression levels were comparatively higher during the initial stages of gonadal development than during spermiation or ovulation (78). In the sapphire devil (Chrysiptera cyanea), brain kissr2 and kissr3 levels increased during the late vitellogenic and post-spawning periods (79).
Overall, kisspeptin system genes were activated just before or during the advanced stage of gonadal growth in many fish species. These observations suggest that the kisspeptin system is important for reproduction but may also be involved in various functions other than reproduction.
In the same manner, kisspeptin systems were found to be important during the spawning period in cm. We analyzed expression changes of kisspeptin-related genes in the brain during final oocyte maturation (FOM) and ovulation. Both kiss1 and kiss2 expression peaked during the FOM and ovulation stages (Figure 4) (80). Notably, the levels of Gnrh1 peptides also coincided with an increase in kisspeptin expression in the brain, and pituitary Lhb immunoreactivity was consistently high during FOM in the cm (27, 80). In contrast to other reproductive factors, the levels of kisspeptin receptors decreased during the FOM and ovulation phases (Figure 4) (81). In monkeys, continuous administration of human kisspeptin led to the desensitization of its receptor, Kiss1r (82, 83). Thus, desensitization of the kisspeptin receptors may have been involved in the decreased kisspeptin expression observed during the FOM and ovulation phases in cm.
Similarly, during their breeding seasons, medaka and goldfish displayed higher numbers of neurons expressing kiss1 and kiss2, respectively, than they did during their non-breeding seasons (84, 85). In the grass puffer, both kiss2 and kissr2 showed clear diurnal and circadian variations in expression levels during the spawning season (86). In zebrafish, only males and females sampled at the time of spawning displayed strong kiss2 expression in the periventricular hypothalamus (55).
In terms of breeding success, the estrogen feedback mechanism deserves a passing mention. Our previous study demonstrated that serum levels of E2 and its precursor, testosterone, showed characteristic variations during FOM and were significantly elevated when the largest and second largest oocytes were at the germinal vesicle migration stage and middle vitellogenesis stage of development, respectively (32). Interestingly, kiss1 and kiss2 expression levels peaked during FOM in the spawning cycle (80). Interactions between kisspeptins and steroid hormones require further investigation in cm. As in mammals, kisspeptin neurons express estrogen receptors and exhibit steroid sensitivity in medaka (84, 87), zebrafish (88), goldfish (85), and European sea bass (89).
To provide a basic understanding of the involvement of kisspeptins in reproduction, we analyzed their gene expression in the pituitary at different gonadal stages using qRT-PCR. In cm, only kiss1 and kissr2 were expressed in the pituitary and did not show significant fluctuations during the annual reproductive cycle (33, 36). These results revealed that Kiss1 peptides exhibit lower affinity for KissR2 (36). The interactions between Kiss1 and KissR2 in the pituitary of cm remain unclear.
Functional analyses of the pituitary kisspeptin system have been reported in several species. In vitro studies on the actions of kisspeptin peptides have yielded conflicting results about their stimulatory effects on Lh release in goldfish (42, 90, 91). However, kisspeptin receptor expression was detected in immuno-identified gonadotrophs in the same species (90). An inhibitory effect of kisspeptin peptides on Lh secretion directly at the pituitary level was demonstrated in the European eel (92). In this species, kissr1 and kissr2 transcript levels were significantly downregulated in mature eels compared with in eels blocked at the prepubertal stage (11). In contrast, Kiss2-12, but not Kiss1-15, induced Fsh and Lh release from European sea bass pituitary cells, and Kiss2 cells also co-localized with gonadotropin-immunoreactive cells (93). In this species, kiss2 and the two receptors increased either before or during the advanced stages of oogenesis in females (76). In a different study, in double transgenic kiss2:mCherry/gnrh3:EGFP zebrafish, both Kiss2 and Gnrh3 fibers extended to the pituitary via the pituitary stalk and were in direct contact with Gnrh3 fibers in the pars distalis (94). In addition, Zmora et al. reported that the Kiss1 and Kiss2 nucleus lateralis tuberis (NLT) populations probably act directly on pituitary gonadotrophs at the prespawning stage in male and female striped bass (55). The same study also reported that both Kiss1-15 and Kiss2-12 induced Fsh release, and Kiss2-12 induced Lh release from pituitary cells in vitro. The role of the pituitary kisspeptin system is still unknown. However, this system may be important in gonadotropin regulation in several teleost species, as these actions are independent of Gnrh signaling. More detailed studies should be performed in the near future to further clarify the role of the pituitary kisspeptin system in cm.
It should also be noted that the kisspeptin system has an important role at the gonadal level. Our qRT-PCR analysis showed that kiss1 and kissr3 (cognate receptor of Kiss1) were expressed strongly in the testes and dramatically increased at the spermiation stage of the annual reproductive cycle in adult cm (33, 36). In particular, the kissr3 transcript level was 10.8-fold higher at this stage than at the immature and post-spawning periods (36). The possibility that there are autocrine/paracrine effects of Kiss1 peptides on the testes cannot be excluded, but our expression and pharmacological analyses suggest a local role of the Kiss1 system in the testes of cm. Notably, ovarian kiss1 expression significantly increased during vitellogenesis, but the ovaries did not express any kisspeptin receptors (33, 36). One possible explanation is that ovarian kisspeptin may exert effects via other RFamide receptors in this species. As a simple example, kisspeptins mediate physiological effects via the neuropeptide FF1 and FF2 receptors in mammals (95, 96).
At the gonadal level, a positive correlation between gonadal development and kisspeptin system expression has been reported in several species. In the grey mullet, ovarian kissr2 expression showed an increasing trend during early development (64). In European sea bass, testicular kissr2 expression exhibited a significant increase at the beginning of spermiation, and kissr3 levels increased significantly in the full spermiation stage (37). In rohu, gonadal kiss1 expression increased during the prespawning and spawning periods in male and female fish, respectively (77). In male golden mahseer, consistently high transcript levels of kiss1 were observed during testicular development and, in female fish, kiss1 and kissr2 expression peaked in the late vitellogenic ovary (78). In male pejerrey, kiss1 and kissr3 increased during testicular development compared with their levels in immature testes, and female fish showed high levels of expression of receptors in the ovaries at final maturation (97).
The significance of kisspeptin expression in the fish gonad is still unknown but is a promising area of future research not only in cm but also in other fish species.
The lack of anatomical evidence for a neuronal network is the central problem in elucidating the functions of kisspeptin in fish. We analyzed the localization of two kisspeptin neurons in the brains of adult cm using in situ hybridization (ISH). Kiss1 mRNAs were detected in the anterior part of the POA, the ventral hypothalamus, including the NLT, and the dorsal hypothalamus, including the nucleus recessus lateralis (38). Kiss2 mRNAs were detected in the anterior POA and NLT, and a large population was observed in the ventral hypothalamus, including the NRL (38). In our experiment, no sex differences were observed. The localization of neurons expressing the two kisspeptins has been reported in detail in five fish species: medaka (47, 84), zebrafish (88), striped bass (17), European sea bass (23, 89), and goldfish (85, 98). Furthermore, histological kiss2 expression was examined in four species: red sea bream (73), Nile tilapia (99), masu salmon (39), and grass puffer (86). The distribution of cells expressing cm kiss1 and kiss2 roughly matched those of the four species mentioned above.
In fish, highly specific Kiss1 and Kiss2 antibodies were developed in zebrafish (88), and a Kiss2 antibody was developed in European sea bass (23). Immunohistochemistry (IHC) revealed that Kiss2 neurons are mainly located in the hypothalamus and project widely to the subpallium, the POA, the thalamus, the ventral and caudal hypothalamus, and the mesencephalon in zebrafish and European sea bass (23, 88). All these regions strongly expressed the kissr2 mRNAs (see next section), indicating a very strong correlation with the wide distribution of Kiss2-positive fibers. On the other hand, a large population of Kiss2 neurons was localized in the POA region in goldfish and showed a strong sensitivity for estrogen feedback (85). This suggests that POA Kiss2 neurons play an important role in the regulation of reproduction in this species.
Kiss1 localization also varies among species. In zebrafish, medaka, and European sea bass, the main location of Kiss1 neurons and receptors is the habenular nucleus, and they may function by autocrine or paracrine action. Indeed, double-labeled ISH and c-fos expression after Kiss1 administration suggested the existence of autocrine regulation in zebrafish habenular nuclei (100). However, in our study, Kiss1 neurons were not expressed in the habenular nucleus, and the same result was reported in striped bass (17, 38). The physiological function or significance of the fish Kiss1 system remains largely unknown. Studies in goldfish found a dominant role of Kiss1 in the regulation of Lh secretion in vivo and in vitro (42, 90, 91). On the other hand, habenular Kiss1 modulates the serotonergic system and fear response in zebrafish (100, 101). In IHC studies in zebrafish, Kiss1 immunoreactive neurons were found in the ventromedial habenula, with axons contacting the interpeduncular and raphe nuclei, which express serotonergic neurons (88, 100). Clarifying the Kiss1 system in fish is an important and interesting future task; however, this peptide likely contributes to reproduction in cm and will be described later.
We also examined the localization of kissr2- and kissr3-expressing cells in the brains of cm using ISH. The kissr2- (cognate receptor of the Kiss2 peptide) expressing cells displayed a broader distribution in the anterior and posterior parts of the POA and were abundant in the ventral hypothalamus, including the ventral and lateral parts of the NLT; the ventral, lateral, and dorsal parts of the NRL; and the nucleus of the NRP (38). The kissr3- (cognate receptor of the Kiss1 peptide) expressing cells were present in the anterior POA and the dorsal and ventral parts of the habenular nucleus (38). No expression differences between the sexes were observed.
The localization of the two types of kisspeptin receptor-expressing cells has been reported in detail in zebrafish (88), striped bass (17), European sea bass (23), medaka (22), and in one species of African cichlid fish with only the kissr2 transcript (16). The distributions of cells expressing kisspeptin receptors roughly matched and, overall, kissr2 showed widespread, high expression levels, whereas kissr3 was modestly expressed in limited regions of the brain.
Central to the fish kisspeptin issue is the problem of interactions with Gnrh neurons. To investigate this, we conducted dual-labeled ISH and carefully assessed the signals using confocal laser-scanning microscopy. Our study clarified that Gnrh1 neurons co-expressed kissr3 mRNA in the POA region in male and female cm (Figures 5A–G) (38). There is no doubt that the Kiss1 peptide is involved in reproduction, and some of its signals are inputs for Gnrh1 neurons. As noted earlier, Kiss1 treatment strongly induced gonadal development in cm (48, 50). In addition, kissr2 was widely expressed in the POA; in many cases, it was expressed in close proximity to the Gnrh1 neurons, but they did not appear to be co-expressed (38).
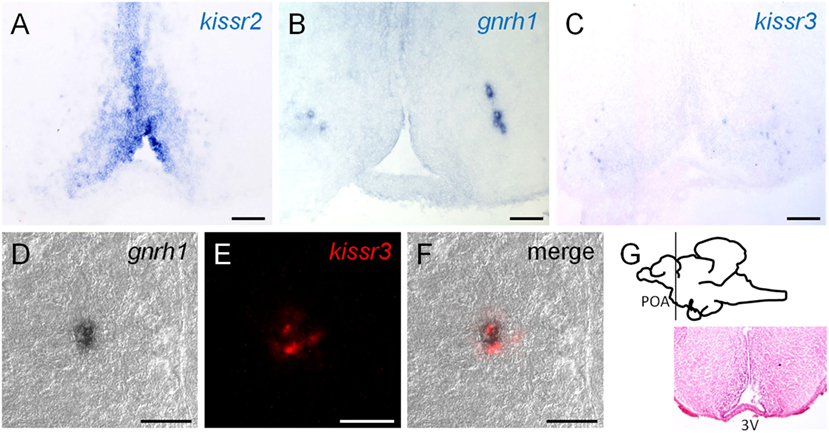
Figure 5. (A–C) Localization of kissr2 expressing cells, Gnrh1 neurons, and kissr3-expressing cells at pre-optic area (POA), respectively. Scale bars, 100 µm. (D–F) Double-label in situ hybridization at the POA region. Scale bars, 30 µm. (D) Differential interference contrast images of Gnrh1 neurons. (E) Red fluorescence indicate the kissr3 mRNA. (C) Merged image of (E,F) and indicate that Gnrh1 neurons co-expressed kissr3 mRNA. (G) Sagittal view of the chub mackerel brain. Planes of the ventral POA region are visualized by hematoxylin–eosin staining. Modified from Ref. (38), by permission of Oxford University Press.
The interactions between kisspeptin receptors and functional Gnrh neurons were first examined in a cichlid fish, Nile tilapia, using laser-captured single-cell PCR (10). The results clearly showed that all three types of Gnrh neuronal cells (Gnrh1, 2, and 3) expressed kissr2 mRNA (10). This observation was supported by double-labeled ISH analysis by Grone et al., which showed that Gnrh1 and Gnrh3 neurons were co-expressed with kissr2 transcripts in cichlid fish (16). Furthermore, in vivo treatment with Kiss2 modulated the reproductive axis and gonadal development in the same species, supporting the histological evidence (54). The same anatomical evidence was reported in a study in striped bass combining ISH and IHC, which showed that Gnrh1 cell bodies co-expressed kissr2 mRNA, and kissr3 was expressed in cells attached to Gnrh1 fibers (17). This may suggest dual modes of Gnrh1 regulation by the two kisspeptin peptides; indeed, both Kiss1 and Kiss2 enhanced Lh secretion in adult fish (17).
Alternatively, Gnrh neurons lack the expression of kisspeptin receptors in some species. An IHC study in zebrafish reported that some Gnrh3 neurons were contacted by Kiss2 fibers (88). Similarly, in double transgenic kiss2:mCherry/gnrh3:EGFP zebrafish, Kiss2 and Gnrh3 fibers were adjacent, and few contacts were observed in the telencephalon and hypothalamus (94). However, both studies similarly failed to find evidence of kisspeptin receptors in Gnrh3 neurons. In addition, morphological evidence from dual-labeled fluorescence ISH has shown that Gnrh1 neurons do not appear to express kissr2 or kissr3 mRNAs in medaka (22). In European sea bass, an antibody against preproGnrh1 was used for coupling with ISH for kisspeptin receptors. In this study, both kissr2- and kissr3-expressing cells in the ventral telencephalon and POA were often found in close proximity to Gnrh1 neurons; however, the authors did not detect a single case of co-expression (23). Nevertheless, administration of Kiss2 peptides upregulated the expression of the gonadotropin subunit genes as well as the secretion of gonadotropins in zebrafish and European sea bass, respectively (18, 19, 47). Based on the numerous studies of fish kisspeptins, it seems reasonable to suggest that kisspeptins affect gonadotropin regulation. However, the pathway of gonadotropin regulation may vary among species. The relationship between kisspeptin and Gnrh alone may not form the core of kisspeptin function in fish reproductive physiology.
Finally, there is one other important factor for kisspeptin function in fish. Despite the lack of direct contact between Kiss2 and Gnrh1 neurons, i.c.v. administration of Kiss2 peptides strongly enhanced the transcription of gonadotropins and influenced gnrh1 expression in cm (45). Our histological observations showed that kissr2 was abundantly expressed in the vicinity of Gnrh1 neurons and hypothalamic regions (38). The transcript levels were quantified by qRT-PCR, and kissr2 transcripts were expressed at far higher levels than were kissr3 transcripts, indicating that the expression of kiss2/kissr2 can be very dynamic, depending on maturational stage, as shown in many previous studies in cm (33, 36). One hypothesis is that Kiss2 indirectly regulates the reproductive axis via interneurons that are expressed in close proximity to Gnrh1 neurons.
In the earliest study, Grone et al. discovered that not only Gnrh1 neurons but also some unknown non-Gnrh cells in the vicinity of Gnrh1 neurons expressed kissr2 mRNA in African cichlid fish (16). Recent studies have clarified that various types of neurons co-express kisspeptin receptors in fish. Escobar et al. reported that neuronal nitric oxide synthase (nNOS), neuropeptide Y (NPY), tyrosine hydroxylase, and somatostatin (SRIF) neurons co-expressed kissr2 transcripts in the brains of European sea bass (23). In mice, Gnrh neurons are surrounded by nNOS neurons, which express Kiss1r, and nNOS may contribute to the direct modulation of Gnrh neuronal activity in a manner codependent with kisspeptin signaling (102, 103). Also, NPY was shown to induce Lh secretion in European sea bass (104). Gnrh1 neurons in European sea bass do not express kissr2 mRNA; it is likely that Kiss2 regulates Gnrh and gonadotropin secretion via various neuronal networks.
Kisspeptin may also play an important role in reproductive behavior. Grone et al. reported that significantly more kissr2 transcripts were found in Gnrh3 neurons than in Gnrh1 neurons in African cichlid fish (16). The same report also clarified that high-status territorial males have higher brain levels of kissr2 mRNA than low-status non-territorial males. Some teleost fishes have three distinct populations of Gnrh neurons, and Gnrh3 neurons are considered to be particularly important for reproductive behaviors (105). On the other hand, Zhao and Wayne reported changes in Gnrh3 electrical activity after the application of Kiss1 through some interneurons in medaka (106). Furthermore, breeding medaka showed changes in hypothalamic kiss1 and telencephalic gnrh3 expression depending on differences in dominance hierarchy (107). In the same species, Kanda et al. showed that arginine vasotocin (Avt) and isotocin (It) neurons co-expressed kissr2 mRNA in the brains (22). In teleost fish, Avt and It are mainly implicated in spawning reflex, courtship and mate-guarding behavior, and hierarchical status (108, 109). These results suggest that kisspeptin neurons directly regulate some sexual behavioral functions via Gnrh3 neurons or Avt and It neurons. The correlation between kisspeptin systems and behavioral factors were similarly reported in striped bass: Avt neurons expressed kissr2 mRNA, and It neurons expressed kissr3 mRNA (20).
In another case, pituitary growth hormone (Gh)-producing cells expressed kiss1 and its receptor transcripts, and pituitary prolactin-producing cells also expressed kissr2 mRNA in goldfish (90). In the same study, Kiss1-10 increased the basal release of Gh and prolactin from pituitary cells in vitro. Furthermore, administration of human KP10 promoted Gh, insulin-like growth factor 1 (IGF-I), and somatolactin secretion and pituitary gene expression in cinnamon clownfish (110). As mentioned earlier, in European sea bass, which is the same marine Perciform species, SRIF neurons co-expressed kisspeptin receptors (23). SRIF is a highly conserved peptide that also acts as an inhibitor of Gh secretion in teleosts (111, 112). Although seemingly contradictory, these results point to the potential function of kisspeptins in regulating somatic growth-related factors.
The kisspeptin/neurokinin B/dynorphin A (KNDy) neurons will play a key role in regulating pulsatile secretion of Gnrh in mammals (113), but very little is known regarding the relationship between neurokinins and kisspeptins in the context of reproduction in fish. In past study, i.p. administration of tachykinins cording peptides, neurokinin B (NKB) and neurokinin F (NKF: unique neurokinin form in fish) elicited significant Lh secretion in sexually mature female zebrafish (114). However, ISH showed no co-expression of tachykinins mRNA with kisspeptins mRNA (115). On the other hand, i.m. administration of NKB and NKF reduced kiss1 and kiss2 gene expression in the brain and pituitary content of Gnrh1 in spermiating striped bass (116). Furthermore, tachykinin (tac3) neurons in the hypothalamus strongly innervated proximal Kiss2 neurons in the dorsal and ventral NRL, which in turn express cognate receptor (tac3r) (116). Finally, we may note in passing that a recent deep-sequencing study suggested that novel neural systems, such as cholecystokinin and neuropeptide B, may also be under the control of kisspeptin signals in medaka (117). The multiple and integral regulation of the reproductive axis or non-reproductive functions by fish kisspeptins are still largely unknown and in need of further consideration.
In this article, we reviewed the current insights on fish kisspeptin physiology based on our cm studies. A take-home message from this article is that the kisspeptin system plays a role in the reproductive success not only of cm but also of many other fish species via various known or unknown neuronal networks. It must be noted that the methods of involvement differ from species to species. The correlation between kisspeptins and Gnrh neurons is a good illustration of the high species specificity of the kisspeptin system. Namely, it may be erroneous to assume that fish kisspeptin is a central and absolute upstream regulator of the Gnrh–Gth pathway as is the case in mammals. Fish are known as the earliest vertebrates, and it may be that their hierarchical reproductive fine network has not yet been completed.
A recent genome editing study clearly showed that kisspeptin-related genes null mutant zebrafish or medaka showed normal gonadal development and maturation (21, 117). However, in fish, various neuropeptidergic, catecholaminergic, and amino-acidergic neurons form direct or indirect contacts with gonadotrophs in the pituitary gland, possibly constituting a multiple back-up system to maintain appropriate gonadotropin release. This does not affect the validity of the reproductive functions of fish kisspeptins.
The strong potency of gonadotropin release is relevant to our research objectives. With capture fishery production relatively static since the late 1980s, aquaculture has been responsible for the impressive growth in the supply of fish for human consumption (118). Hence, the development of efficient aquaculture methods and a model of reproduction are imperative tasks. The “maturation induction” potency of kisspeptin peptides may have the potential to rescue reproductive failure in cultured fish. Additional comparative kisspeptin research will further elucidate the pleiotropic role of kisspeptin in fish and may contribute to the development of not only a basic understanding of fish physiology but also of the applied science used in aquaculture and stock management.
HO wrote a manuscript. HO, SS, and MM contributed substantially to the conception and design of the work, reviewing, final approvals of the version submitted, and agreed to be accountable for accuracy and integrity of content.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Our kisspeptin studies of chub mackerel were supported by JSPS KAKENHI Grant Numbers, JAG0380113, JAG3001406, JAG5003910, and JAG3658163, and by 2015 CASIO Science Promotion Foundation to MM. A part of this work was performed by the “Technological Development for Selection and Secure Stock of Bloodstock for Culture of Bluefin Tuna,” which is supported by the Fisheries Agency of Japan.
1. Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature (2001) 411(6837):613–7. doi:10.1038/35079135
2. Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem (2001) 276(37):34631–6. doi:10.1074/jbc.M104847200
3. Beltramo M, Dardente H, Cayla X, Caraty A. Cellular mechanisms and integrative timing of neuroendocrine control of GnRH secretion by kisspeptin. Mol Cell Endocrinol (2014) 382(1):387–99. doi:10.1016/j.mce.2013.10.015
4. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A (2003) 100(19):10972–6. doi:10.1073/pnas.1834399100
5. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med (2003) 349(17):1614–27. doi:10.1056/NEJMoa035322
6. Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology (2004) 80(4):264–72. doi:10.1159/000083140
7. Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci (2005) 25(49):11349–56. doi:10.1523/JNEUROSCI.3328-05.2005
8. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology (2006) 147(12):5817–25. doi:10.1210/en.2006-0787
9. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A (2005) 102(5):1761–6. doi:10.1073/pnas.0409330102
10. Parhar IS, Ogawa S, Sakuma Y. Laser-captured single digoxigenin-labeled neurons of gonadotropin releasing hormone types reveal a novel G protein-coupled receptor (Gpr54) during maturation in cichlid fish. Endocrinology (2004) 145(8):3613–8. doi:10.1210/en.2004-0395
11. Pasquier J, Lafont AG, Jeng SR, Morini M, Dirks R, van den Thillart G, et al. Multiple kisspeptin receptors in early osteichthyans provide new insights into the evolution of this receptor family. PLoS One (2012) 7(11):e48931. doi:10.1371/journal.pone.0048931
12. Pasquier J, Kamech N, Lafont AG, Vaudry H, Rousseau K, Dufour S. Molecular evolution of GPCRs: kisspeptin/kisspeptin receptors. J Mol Endocrinol (2014) 52(3):T101–17. doi:10.1530/JME-13-0224
13. Fernald RD, White RB. Gonadotropin-releasing hormone genes: phylogeny, structure, and functions. Front Neuroendocrinol (1999) 20(3):224–40. doi:10.1006/frne.1999.0181
14. Kim MH, Oka Y, Amano M, Kobayashi M, Okuzawa K, Hasegawa Y, et al. Immunocytochemical localization of sGnRH and cGnRH-II in the brain of goldfish, Carassius auratus. J Comp Neurol (1995) 356(1):72–82. doi:10.1002/cne.903560105
15. Amano M, Oka Y, Kitamura S, Ikuta K, Aida K. Ontogenic development of salmon GnRH and chicken GnRH-II systems in the brain of masu salmon (Oncorhynchus masou). Cell Tissue Res (1998) 293(3):427–34. doi:10.1007/s004410051134
16. Grone BP, Maruska KP, Korzan WJ, Fernald RD. Social status regulates kisspeptin receptor mRNA in the brain of Astatotilapia burtoni. Gen Comp Endocrinol (2010) 169(1):98–107. doi:10.1016/j.ygcen.2010.07.018
17. Zmora N, Stubblefield J, Zulperi Z, Biran J, Levavi-Sivan B, Muñoz-Cueto JA, et al. Differential and gonad stage-dependent roles of kisspeptin1 and kisspeptin2 in reproduction in the modern teleosts, Morone species. Biol Reprod (2012) 86(6):1–12. doi:10.1095/biolreprod.111.097667
18. Felip A, Zanuy S, Pineda R, Pinilla L, Carrillo M, Tena-Sempere M, et al. Evidence for two distinct KiSS genes in non-placental vertebrates that encode kisspeptins with different gonadotropin-releasing activities in fish and mammals. Mol Cell Endocrinol (2009) 312(1):61–71. doi:10.1016/j.mce.2008.11.017
19. Espigares F, Carrillo M, Gómez A, Zanuy S. The forebrain-midbrain acts as functional endocrine signaling pathway of Kiss2/Gnrh1 system controlling the gonadotroph activity in the teleost fish European sea bass (Dicentrarchus labrax). Biol Reprod (2015) 92(3):1–13. doi:10.1095/biolreprod.114.125138
20. Zmora N, Stubblefield JD, Wong TT, Levavi-Sivan B, Millar RP, Zohar Y. Kisspeptin antagonists reveal kisspeptin 1 and kisspeptin 2 differential regulation of reproduction in the teleost, Morone saxatilis. Biol Reprod (2015) 93(3):1–12. doi:10.1095/biolreprod.115.131870
21. Tang H, Liu Y, Luo D, Ogawa S, Yin Y, Li S, et al. The kiss/kissr systems are dispensable for zebrafish reproduction: evidence from gene knockout studies. Endocrinology (2014) 156(2):589–99. doi:10.1210/en.2014-1204
22. Kanda S, Akazome Y, Mitani Y, Okubo K, Oka Y. Neuroanatomical evidence that kisspeptin directly regulates isotocin and vasotocin neurons. PLoS One (2013) 8(4):e62776. doi:10.1371/journal.pone.0062776
23. Escobar S, Servili A, Espigares F, Gueguen MM, Brocal I, Felip A, et al. Expression of kisspeptins and kiss receptors suggests a large range of functions for kisspeptin systems in the brain of the European sea bass. PLoS One (2013) 8(7):e70177. doi:10.1371/journal.pone.0070177
24. Selvaraj S, Kitano H, Fujinaga Y, Amano M, Takahashi A, Shimizu A, et al. Immunological characterization and distribution of three GnRH forms in the brain and pituitary gland of chub mackerel (Scomber japonicus). Zoolog Sci (2009) 26(12):828–39. doi:10.2108/zsj.26.828
25. Selvaraj S, Kitano H, Amano M, Nyuji M, Kaneko K, Yamaguchi A, et al. Molecular characterization and expression profiles of three GnRH forms in the brain and pituitary of adult chub mackerel (Scomber japonicus) maintained in captivity. Aquaculture (2012) 356:200–10. doi:10.1016/j.aquaculture.2012.05.015
26. Lumayno SDP, Ohga H, Selvaraj S, Nyuji M, Yamaguchi A, Matsuyama M. Molecular characterization and functional analysis of pituitary GnRH receptor in a commercial scombroid fish, chub mackerel (Scomber japonicus). Gen Comp Endocrinol (2017) 247:143–51. doi:10.1016/j.ygcen.2017.01.027
27. Nyuji M, Shiraishi T, Selvaraj S, Van In V, Kitano H, Yamaguchi A, et al. Immunoreactive changes in pituitary FSH and LH cells during seasonal reproductive and spawning cycles of female chub mackerel Scomber japonicus. Fish Sci (2011) 77(5):731. doi:10.1007/s12562-011-0380-5
28. Nyuji M, Selvaraj S, Kitano H, Ohga H, Yoneda M, Shimizu A, et al. Changes in the expression of pituitary gonadotropin subunits during reproductive cycle of multiple spawning female chub mackerel Scomber japonicus. Fish Physiol Biochem (2012) 38(3):883–97. doi:10.1007/s10695-011-9576-y
29. Nyuji M, Selvaraj S, Kitano H, Shiraishi T, Yamaguchi A, Shimizu A, et al. Immunoreactivity of gonadotrophs (FSH and LH cells) and gonadotropin subunit gene expression in the male chub mackerel Scomber japonicus pituitary during the reproductive cycle. Zoolog Sci (2012) 29(9):623–9. doi:10.2108/zsj.29.623
30. Ohga H, Kaneko K, Shimizu A, Kitano H, Selvaraj S, Nyuji M, et al. Steroidogenic and maturation-inducing potency of native gonadotropic hormones in female chub mackerel, Scomber japonicus. Reprod Biol Endocrinol (2012) 10(1):71. doi:10.1186/1477-7827-10-71
31. Nyuji M, Kitano H, Shimizu A, Lee JM, Kusakabe T, Yamaguchi A, et al. Characterization, localization, and stage-dependent gene expression of gonadotropin receptors in chub mackerel (Scomber japonicus) ovarian follicles. Biol Reprod (2013) 88(6):1–14. doi:10.1095/biolreprod.112.107292
32. Matsuyama M, Shiraishi T, Sundaray JK, Rahman M, Ohta K, Yamaguchi A. Steroidogenesis in ovarian follicles of chub mackerel, Scomber japonicus. Zoolog Sci (2005) 22(1):101–10. doi:10.2108/zsj.22.101
33. Selvaraj S, Kitano H, Fujinaga Y, Ohga H, Yoneda M, Yamaguchi A, et al. Molecular characterization, tissue distribution, and mRNA expression profiles of two kiss genes in the adult male and female chub mackerel (Scomber japonicus) during different gonadal stages. Gen Comp Endocrinol (2010) 169(1):28–38. doi:10.1016/j.ygcen.2010.07.011
34. Song H, Wang M, Qi J, Wang Z, Zhang Q. Native recombinant kisspeptin can induce GnRH1 and kissr2 expression in Paralichthys olivaceus in vitro. Comp Biochem Physiol B Biochem Mol Biol (2016) 200:36–43. doi:10.1016/j.cbpb.2016.05.002
35. Lee YR, Tsunekawa K, Moon MJ, Um HN, Hwang JI, Osugi T, et al. Molecular evolution of multiple forms of kisspeptins and GPR54 receptors in vertebrates. Endocrinology (2009) 150(6):2837–46. doi:10.1210/en.2008-1679
36. Ohga H, Fujinaga Y, Selvaraj S, Kitano H, Nyuji M, Yamaguchi A, et al. Identification, characterization, and expression profiles of two subtypes of kisspeptin receptors in a scombroid fish (chub mackerel). Gen Comp Endocrinol (2013) 193:130–40. doi:10.1016/j.ygcen.2013.07.016
37. Felip A, Espigares F, Zanuy S, Gómez A. Differential activation of kiss receptors by kiss1 and kiss2 peptides in the sea bass. Reproduction (2015) 150(3):227–43. doi:10.1530/REP-15-0204
38. Ohga H, Adachi H, Kitano H, Yamaguchi A, Matsuyama M. Kiss1 hexadecapeptide directly regulates gonadotropin-releasing hormone 1 in the scombroid fish, chub mackerel. Biol Reprod (2017) 96(2):376–88. doi:10.1095/biolreprod.116.142083
39. Osugi T, Ohtaki N, Sunakawa Y, Son YL, Ohkubo M, Iigo M, et al. Molecular evolution of kiss2 genes and peptides in vertebrates. Endocrinology (2013) 154(11):4270–80. doi:10.1210/en.2012-2267
40. Bohórquez MOT, Mechaly AS, Hughes LC, Campanella D, Ortí G, Canosa LF, et al. Kisspeptin system in pejerrey fish (Odontesthes bonariensis). Characterization and gene expression pattern during early developmental stages. Comp Biochem Physiol A Mol Integr Physiol (2017) 204:146–56. doi:10.1016/j.cbpa.2016.11.014
41. Biran J, Ben-Dor S, Levavi-Sivan B. Molecular identification and functional characterization of the kisspeptin/kisspeptin receptor system in lower vertebrates. Biol Reprod (2008) 79(4):776–86. doi:10.1095/biolreprod.107.066266
42. Li S, Zhang Y, Liu Y, Huang X, Huang W, Lu D, et al. Structural and functional multiplicity of the kisspeptin/GPR54 system in goldfish (Carassius auratus). J Endocrinol (2009) 201(3):407–18. doi:10.1677/JOE-09-0016
43. Nocillado JN, Biran J, Lee YY, Levavi-Sivan B, Mechaly AS, Zohar Y, et al. The kiss2 receptor (kiss2r) gene in southern bluefin tuna, Thunnus maccoyii and in yellowtail kingfish, Seriola lalandi – functional analysis and isolation of transcript variants. Mol Cell Endocrinol (2012) 362(1):211–20. doi:10.1016/j.mce.2012.06.024
44. Shi Y, Zhang Y, Li S, Liu Q, Lu D, Liu M, et al. Molecular identification of the kiss2/kiss1ra system and its potential function during 17alpha-methyltestosterone-induced sex reversal in the orange-spotted grouper, Epinephelus coioides. Biol Reprod (2010) 83(1):63–74. doi:10.1095/biolreprod.109.080044
45. Ohga H, Selvaraj S, Adachi H, Imanaga Y, Nyuji M, Yamaguchi A, et al. Functional analysis of kisspeptin peptides in adult immature chub mackerel (Scomber japonicus) using an intracerebroventricular administration method. Neurosci Lett (2014) 561:203–7. doi:10.1016/j.neulet.2013.12.072
46. Filby AL, Aerle RV, Duitman J, Tyler CR. The kisspeptin/gonadotropin-releasing hormone pathway and molecular signaling of puberty in fish. Biol Reprod (2008) 78(2):278–89. doi:10.1095/biolreprod.107.063420
47. Kitahashi T, Ogawa S, Parhar IS. Cloning and expression of kiss2 in the zebrafish and medaka. Endocrinology (2009) 150(2):821–31. doi:10.1210/en.2008-0940
48. Selvaraj S, Ohga H, Nyuji M, Kitano H, Nagano N, Yamaguchi A, et al. Subcutaneous administration of Kiss1 pentadecapeptide accelerates spermatogenesis in prepubertal male chub mackerel (Scomber japonicus). Comp Biochem Physiol A Mol Integr Physiol (2013) 166(2):228–36. doi:10.1016/j.cbpa.2013.06.007
49. Selvaraj S, Ohga H, Nyuji M, Kitano H, Nagano N, Yamaguchi A, et al. Effects of synthetic kisspeptin peptides and GnRH analogue on oocyte growth and circulating sex steroids in prepubertal female chub mackerel (Scomber japonicus). Aquac Res (2013) 46(8):1866–77. doi:10.1111/are.12342
50. Selvaraj S, Ohga H, Kitano H, Nyuji M, Yamaguchi A, Matsuyama M. Peripheral administration of kiss1 pentadecapeptide induces gonadal development in sexually immature adult scombroid fish. Zoolog Sci (2013) 30(6):446–54. doi:10.2108/zsj.30.446
51. Beck BH, Fuller SA, Peatman E, McEntire ME, Darwish A, Freeman DW. Chronic exogenous kisspeptin administration accelerates gonadal development in basses of the genus Morone. Comp Biochem Physiol A Mol Integr Physiol (2012) 162(3):265–73. doi:10.1016/j.cbpa.2012.03.019
52. Nocillado JN, Zohar Y, Biran J, Levavi-Sivan B, Elizur A. Chronic kisspeptin administration stimulated gonadal development in pre-pubertal male yellowtail kingfish (Seriola lalandi; Perciformes) during the breeding and non-breeding season. Gen Comp Endocrinol (2013) 191:168–76. doi:10.1016/j.ygcen.2013.06.005
53. Kim NN, Shin HS, Choi YJ, Choi CY. Kisspeptin regulates the hypothalamus–pituitary–gonad axis gene expression during sexual maturation in the cinnamon clownfish, Amphiprion melanopus. Comp Biochem Physiol B Biochem Mol Biol (2014) 168:19–32. doi:10.1016/j.cbpb.2013.11.002
54. Park JW, Jin YH, Oh SY, Kwon JY. Kisspeptin2 stimulates the HPG axis in immature Nile tilapia (Oreochromis niloticus). Comp Biochem Physiol B Biochem Mol Biol (2016) 202:31–8. doi:10.1016/j.cbpb.2016.07.009
55. Zmora N, Stubblefield J, Golan M, Servili A, Levavi-Sivan B, Zohar Y. The medio-basal hypothalamus as a dynamic and plastic reproduction-related kisspeptin-gnrh-pituitary center in fish. Endocrinology (2014) 155(5):1874–86. doi:10.1210/en.2013-1894
56. Selvaraj S, Kitano H, Ohga H, Yamaguchi A, Matsuyama M. Expression changes of mRNAs encoding kisspeptins and their receptors and gonadotropin-releasing hormones during early development and gonadal sex differentiation periods in the brain of chub mackerel (Scomber japonicus). Gen Comp Endocrinol (2015) 222:20–32. doi:10.1016/j.ygcen.2014.09.019
57. Zhao Y, Lin MCA, Mock A, Yang M, Wayne NL. Kisspeptins modulate the biology of multiple populations of gonadotropin-releasing hormone neurons during embryogenesis and adulthood in zebrafish (Danio rerio). PLoS One (2014) 9(8):e104330. doi:10.1371/journal.pone.0104330
58. Hodne K, Weltzien FA, Oka Y, Okubo K. Expression and putative function of kisspeptins and their receptors during early development in medaka. Endocrinology (2013) 154(9):3437–46. doi:10.1210/en.2013-1065
59. Mohamed JS, Benninghoff AD, Holt GJ, Khan IA. Developmental expression of the G protein-coupled receptor 54 and three GnRH mRNAs in the teleost fish cobia. J Mol Endocrinol (2007) 38(2):235–44. doi:10.1677/jme.1.02182
60. Song H, He Y, Ma L, Zhou X, Liu X, Qi J, et al. Characterisation of kisspeptin system genes in an ovoviviparous teleost: Sebastes schlegeli. Gen Comp Endocrinol (2015) 214:114–25. doi:10.1016/j.ygcen.2014.06.010
61. Song H, Wang M, Wang Z, Liu J, Qi J, Zhang Q. Characterization of kiss2 and kissr2 genes and the regulation of kisspeptin on the HPG axis in Cynoglossus semilaevis. Fish Physiol Biochem (2017) 43(3):731–53. doi:10.1007/s10695-016-0328-x
62. Ohga H, Adachi H, Matsumori K, Kodama R, Nyuji M, Selvaraj S, et al. mRNA levels of kisspeptins, kisspeptin receptors, and GnRH1 in the brain of chub mackerel during puberty. Com Biochem Physiol A Mol Integr Physiol (2015) 179:104–12. doi:10.1016/j.cbpa.2014.09.012
63. Nyuji M, Kodama R, Kato K, Yamamoto S, Yamaguchi A, Matsuyama M. Gonadal development and gonadotropin gene expression during puberty in cultured chub mackerel (Scomber japonicus). Zoolog Sci (2014) 31(6):398–406. doi:10.2108/zs130254
64. Nocillado JN, Levavi-Sivan B, Carrick F, Elizur A. Temporal expression of G-protein-coupled receptor 54 (GPR54), gonadotropin-releasing hormones (GnRH), and dopamine receptor D2 (drd2) in pubertal female grey mullet, Mugil cephalus. Gen Comp Endocrinol (2007) 150(2):278–87. doi:10.1016/j.ygcen.2006.09.008
65. Martinez-Chavez CC, Minghetti M, Migaud H. GPR54 and rGnRH I gene expression during the onset of puberty in Nile tilapia. Gen Comp Endocrinol (2008) 156(2):224–33. doi:10.1016/j.ygcen.2008.01.019
66. Mechaly AS, Viñas J, Piferrer F. Identification of two isoforms of the kisspeptin-1 receptor (kiss1r) generated by alternative splicing in a modern teleost, the Senegalese sole (Solea senegalensis). Biol Reprod (2009) 80(1):60–9. doi:10.1095/biolreprod.108.072173
67. Mechaly AS, Viñas J, Piferrer F. Sex-specific changes in the expression of kisspeptin, kisspeptin receptor, gonadotropins and gonadotropin receptors in the Senegalese sole (Solea senegalensis) during a full reproductive cycle. Comp Biochem Physiol A Mol Integr Physiol (2012) 162(4):364–71. doi:10.1016/j.cbpa.2012.04.003
68. Mechaly AS, Viñas J, Murphy C, Reith M, Piferrer F. Gene structure of the kiss1 receptor-2 (kiss1r-2) in the Atlantic halibut: insights into the evolution and regulation of kiss1r genes. Mol Cell Endocrinol (2010) 317(1):78–89. doi:10.1016/j.mce.2009.11.005
69. Chi L, Li X, Liu Q, Liu Y. Photoperiod regulate gonad development via kisspeptin/kissr in hypothalamus and saccus vasculosus of Atlantic salmon (Salmo salar). PLoS One (2017) 12(2):e0169569. doi:10.1371/journal.pone.0169569
70. Mechaly AS, Viñas J, Piferrer F. Gene structure analysis of kisspeptin-2 (kiss2) in the Senegalese sole (Solea senegalensis): characterization of two splice variants of kiss2, and novel evidence for metabolic regulation of kisspeptin signaling in non-mammalian species. Mol Cell Endocrinol (2011) 339(1):14–24. doi:10.1016/j.mce.2011.03.004
71. Song H, Wang M, Wang Z, Yu H, Wang Z, Zhang Q. Identification and characterization of kiss2 and kissr2 homologs in Paralichthys olivaceus. Fish Physiol Biochem (2016) 42(4):1073–92. doi:10.1007/s10695-016-0199-1
72. Cowan M, Davie A, Migaud H. Photoperiod effects on the expression of kisspeptin and gonadotropin genes in Atlantic cod, Gadus morhua, during first maturation. Com Biochem Physiol A Mol Integr Physiol (2012) 163(1):82–94. doi:10.1016/j.cbpa.2012.05.191
73. Shimizu Y, Tomikawa J, Hirano K, Nanikawa Y, Akazome Y, Kanda S, et al. Central distribution of kiss2 neurons and peri-pubertal changes in their expression in the brain of male and female red seabream Pagrus major. Gen Comp Endocrinol (2012) 175(3):432–42. doi:10.1016/j.ygcen.2011.11.038
74. Shahjahan M, Motohashi E, Doi H, Ando H. Elevation of kiss2 and its receptor gene expression in the brain and pituitary of grass puffer during the spawning season. Gen Comp Endocrinol (2010) 169(1):48–57. doi:10.1016/j.ygcen.2010.07.008
75. Migaud H, Ismail R, Cowan M, Davie A. Kisspeptin and seasonal control of reproduction in male European sea bass (Dicentrarchus labrax). Gen Comp Endocrinol (2012) 179(3):384–99. doi:10.1016/j.ygcen.2012.07.033
76. Alvarado MV, Carrillo M, Felip A. Expression of kisspeptins and their receptors, gnrh-1/gnrhr-II-1a and gonadotropin genes in the brain of adult male and female European sea bass during different gonadal stages. Gen Comp Endocrinol (2013) 187:104–16. doi:10.1016/j.ygcen.2013.03.030
77. Saha A, Pradhan A, Sengupta S, Nayak M, Samanta M, Sahoo L, et al. Molecular characterization of two kiss genes and their expression in rohu (Labeo rohita) during annual reproductive cycle. Com Biochem Physiol B Biochem Mol Biol (2016) 191:135–45. doi:10.1016/j.cbpb.2015.10.008
78. Shahi N, Singh AK, Sahoo M, Mallik SK, Thakuria D. Molecular cloning, characterization and expression profile of kisspeptin1 and kisspeptin1 receptor at brain-pituitary-gonad (BPG) axis of golden mahseer, Tor putitora (Hamilton, 1822) during gonadal development. Com Biochem Physiol B Biochem Mol Biol (2017) 205:13–29. doi:10.1016/j.cbpb.2016.11.010
79. Imamura S, Hur SP, Takeuchi Y, Bouchekioua S, Takemura A. Molecular cloning of kisspeptin receptor genes (gpr54-1 and gpr54-2) and their expression profiles in the brain of a tropical damselfish during different gonadal stages. Com Biochem Physiol A Biochem Mol Biol (2017) 203:9–16. doi:10.1016/j.cbpa.2016.07.015
80. Selvaraj S, Kitano H, Amano M, Ohga H, Yoneda M, Yamaguchi A, et al. Increased expression of kisspeptin and GnRH forms in the brain of scombroid fish during final ovarian maturation and ovulation. Reprod Biol Endocrinol (2012) 10(1):64. doi:10.1186/1477-7827-10-64
81. Ohga H, Selvaraj S, Yamaguchi A, Matsuyama M. A unique expression profile of kisspeptin receptor genes during final oocyte maturation in female chub mackerel, Scomber japonicus. J Fac Agr Kyushu Univ (2017) 62(1):87–91.
82. Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley WF Jr, Plant TM. Effect of continuous intravenous administration of human metastin 45–54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta). Endocrinology (2007) 148(7):3364–70. doi:10.1210/en.2007-0207
83. Seminara SB, DiPietro MJ, Ramaswamy S, Crowley WF Jr, Plant TM. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology (2006) 147(5):2122–6. doi:10.1210/en.2005-1550
84. Kanda S, Akazome Y, Matsunaga T, Yamamoto N, Yamada S, Tsukamura H, et al. Identification of KiSS-1 product kisspeptin and steroid-sensitive sexually dimorphic kisspeptin neurons in medaka (Oryzias latipes). Endocrinology (2008) 149(5):2467–76. doi:10.1210/en.2007-1503
85. Kanda S, Karigo T, Oka Y. Steroid sensitive kiss2 neurones in the goldfish: evolutionary insights into the duplicate kisspeptin gene-expressing neurones. J Neuroendocrinol (2012) 24(6):897–906. doi:10.1111/j.1365-2826.2012.02296.x
86. Ando H, Ogawa S, Shahjahan M, Ikegami T, Hattori A, Parhar I. Diurnal and circadian oscillations in expression of kisspeptin, kisspeptin receptor and gonadotrophin-releasing hormone 2 genes in the grass puffer, a semilunar-synchronised spawner. J Neuroendocrinol (2014) 26(7):459–67. doi:10.1111/jne.12165
87. Mitani Y, Kanda S, Akazome Y, Zempo B, Oka Y. Hypothalamic kiss1 but not kiss2 neurons are involved in estrogen feedback in medaka (Oryzias latipes). Endocrinology (2010) 151(4):1751–9. doi:10.1210/en.2009-1174
88. Servili A, Le Page Y, Leprince J, Caraty A, Escobar S, Parhar IS, et al. Organization of two independent kisspeptin systems derived from evolutionary-ancient kiss genes in the brain of zebrafish. Endocrinology (2011) 152(4):1527–40. doi:10.1210/en.2010-0948
89. Escobar S, Felip A, Gueguen MM, Zanuy S, Carrillo M, Kah O, et al. Expression of kisspeptins in the brain and pituitary of the European sea bass (Dicentrarchus labrax). J Comp Neurol (2013) 521(4):933–48. doi:10.1002/cne.23211
90. Yang B, Jiang Q, Chan T, Ko WK, Wong AO. Goldfish kisspeptin: molecular cloning, tissue distribution of transcript expression, and stimulatory effects on prolactin, growth hormone and luteinizing hormone secretion and gene expression via direct actions at the pituitary level. Gen Comp Endocrinol (2010) 165(1):60–71. doi:10.1016/j.ygcen.2009.06.001
91. Chang JP, Mar A, Wlasichuk M, Wong AO. Kisspeptin-1 directly stimulates LH and GH secretion from goldfish pituitary cells in a Ca 2+-dependent manner. Gen Comp Endocrinol (2012) 179(1):38–46. doi:10.1016/j.ygcen.2012.07.028
92. Pasquier J, Lafont AG, Leprince J, Vaudry H, Rousseau K, Dufour S. First evidence for a direct inhibitory effect of kisspeptins on LH expression in the eel, Anguilla anguilla. Gen Comp Endocrinol (2011) 173(1):216–25. doi:10.1016/j.ygcen.2011.05.019
93. Espigares F, Zanuy S, Gómez A. Kiss2 as a regulator of lh and fsh secretion via paracrine/autocrine signaling in the teleost fish european sea bass (Dicentrarchus labrax). Biol Reprod (2015) 93(5):1–12. doi:10.1095/biolreprod.115.131029
94. Song Y, Duan X, Chen J, Huang W, Zhu Z, Hu W. The distribution of kisspeptin (kiss) 1-and kiss2-positive neurones and their connections with gonadotrophin-releasing hormone-3 neurones in the zebrafish brain. J Neuroendocrinol (2015) 27(3):198–211. doi:10.1111/jne.12251
95. Lyubimov Y, Engstrom M, Wurster S, Savola JM, Korpi ER, Panula P. Human kisspeptins activate neuropeptide FF2 receptor. Neuroscience (2010) 170(1):117–22. doi:10.1016/j.neuroscience.2010.06.058
96. Elhabazi K, Humbert JP, Bertin I, Schmitt M, Bihel F, Bourguignon JJ, et al. Endogenous mammalian RF-amide peptides, including PrRP, kisspeptin and 26RFa, modulate nociception and morphine analgesia via NPFF receptors. Neuropharmacology (2013) 75:164–71. doi:10.1016/j.neuropharm.2013.07.012
97. Bohórquez MOT, Mechaly AS, Elisio M, Chalde T, Canosa LF, Miranda LA, et al. Kisspeptins and their receptors in the brain-pituitary-gonadal axis of Odonthestes bonariensis: their relationship with gametogenesis along the reproductive cycle. Gen Comp Endocrinol (2017) 252:209–18. doi:10.1016/j.ygcen.2017.06.028
98. Wang Q, Sham KW, Ogawa S, Li S, Parhar IS, Cheng CH, et al. Regulation of the two kiss promoters in goldfish (Carassius auratus) by estrogen via different ERα pathways. Mol Cell Endocrinol (2013) 375(1–2):130–9. doi:10.1016/j.mce.2013.04.023
99. Ogawa S, Ng KW, Xue X, Ramadasan PN, Sivalingam M, Li S, et al. Thyroid hormone upregulates hypothalamic kiss2 gene in the male Nile tilapia, Oreochromis niloticus. Front Endocrinol (2013) 4:184. doi:10.3389/fendo.2013.00184
100. Ogawa S, Ng KW, Ramadasan PN, Nathan FM, Parhar IS. Habenular kiss1 neurons modulate the serotonergic system in the brain of zebrafish. Endocrinology (2012) 153(5):2398–407. doi:10.1210/en.2012-1062
101. Ogawa S, Nathan FM, Parhar IS. Habenular kisspeptin modulates fear in the zebrafish. Proc Natl Acad Sci U S A (2014) 111(10):3841–6. doi:10.1073/pnas.1314184111
102. Clasadonte J, Poulain P, Beauvillain JC, Prevot V. Activation of neuronal nitric oxide release inhibits spontaneous firing in adult gonadotropin-releasing hormone neurons: a possible local synchronizing signal. Endocrinology (2007) 149(2):587–96. doi:10.1210/en.2007-1260
103. Hanchate NK, Parkash J, Bellefontaine N, Mazur D, Colledge WH, de Tassigny XDA, et al. Kisspeptin-GPR54 signaling in mouse NO-synthesizing neurons participates in the hypothalamic control of ovulation. J Neurosci (2012) 32(3):932–45. doi:10.1523/JNEUROSCI.4765-11.2012
104. Cerdá-Reverter JM, Sorbera LA, Carrillo M, Zanuy S. Energetic dependence of NPY-induced LH secretion in a teleost fish (Dicentrarchus labrax). Am J Physiol (1999) 277(6):R1627–34.
105. Abe H, Oka Y. Mechanisms of neuromodulation by a nonhypophysiotropic GnRH system controlling motivation of reproductive behavior in the teleost brain. J Reprod Dev (2011) 57(6):665–74. doi:10.1262/jrd.11-055E
106. Zhao Y, Wayne NL. Effects of kisspeptin1 on electrical activity of an extrahypothalamic population of gonadotropin-releasing hormone neurons in medaka (Oryzias latipes). PLoS One (2012) 7(5):e37909. doi:10.1371/journal.pone.0037909
107. Kagawa N, Hirose S, Fujimoto K, Nomura C, Fujita Y, Honda A, et al. Social rank-dependent expression of gonadotropin-releasing hormones and kisspeptin in the medaka brain. Gen Comp Endocrinol (2017) 249:48–54. doi:10.1016/j.ygcen.2017.03.001
108. Godwin J, Thompson R. Nonapeptides and social behavior in fishes. Horm Behav (2012) 61(3):230–8. doi:10.1016/j.yhbeh.2011.12.016
109. Yokoi S, Okuyama T, Kamei Y, Naruse K, Taniguchi Y, Ansai S, et al. An essential role of the arginine vasotocin system in mate-guarding behaviors in triadic relationships of medaka fish (Oryzias latipes). PLoS Genet (2015) 11(2):e1005009. doi:10.1371/journal.pgen.1005009
110. Kim NN, Choi YU, Park HS, Choi CY. Kisspeptin regulates the somatic growth-related factors of the cinnamon clownfish Amphiprion melanopus. Comp Biochem Physiol A Mol Integr Physiol (2015) 179:17–24. doi:10.1016/j.cbpa.2014.08.020
111. Sheridan MA, Hagemeister AL. Somatostatin and somatostatin receptors in fish growth. Gen Comp Endocrinol (2010) 167(3):360–5. doi:10.1016/j.ygcen.2009.09.002
112. Canosa LF, Unniappan S, Peter RE. Periprandial changes in growth hormone release in goldfish: role of somatostatin, ghrelin, and gastrin-releasing peptide. Am J Physiol (2005) 289(1):R125–33. doi:10.1152/ajpregu.00759.2004
113. Uenoyama Y, Tsukamura H, Maeda KI. KNDy neuron as a gatekeeper of puberty onset. J Obstet Gynaecol Res (2014) 40(6):1518–26. doi:10.1111/jog.12398
114. Biran J, Palevitch O, Ben-Dor S, Levavi-Sivan B. Neurokinin Bs and neurokinin B receptors in zebrafish-potential role in controlling fish reproduction. Proc Natl Acad Sci U S A (2012) 109(26):10269–74. doi:10.1073/pnas.1119165109
115. Ogawa S, Ramadasan PN, Goschorska M, Anantharajah A, We Ng K, Parhar IS. Cloning and expression of tachykinins and their association with kisspeptins in the brains of zebrafish. J Comp Neurol (2012) 520(13):2991–3012. doi:10.1002/cne.23103
116. Zmora N, Wong TT, Stubblefield J, Levavi-Sivan B, Zohar Y. Neurokinin B regulates reproduction via inhibition of kisspeptin in a teleost, the striped bass. J Endocrinol (2017) 233(2):159–74. doi:10.1530/JOE-16-0575
117. Nakajo M, Kanda S, Karigo T, Takahashi A, Akazome Y, Uenoyama Y, et al. Evolutionally conserved function of kisspeptin neuronal system is non-reproductive regulation as revealed by non-mammalian study. Endocrinology (2017) 159(1):163–83. doi:10.1210/en.2017-00808
Keywords: kisspeptin, puberty, brain–pituitary–gonad axis, marine teleost, perciform, aquaculture
Citation: Ohga H, Selvaraj S and Matsuyama M (2018) The Roles of Kisspeptin System in the Reproductive Physiology of Fish With Special Reference to Chub Mackerel Studies as Main Axis. Front. Endocrinol. 9:147. doi: 10.3389/fendo.2018.00147
Received: 15 December 2017; Accepted: 19 March 2018;
Published: 04 April 2018
Edited by:
Rosanna Chianese, Università degli Studi della Campania Luigi Vanvitelli Caserta, ItalyReviewed by:
Juan F. Asturiano, Universitat Politècnica de València, SpainCopyright: © 2018 Ohga, Selvaraj and Matsuyama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michiya Matsuyama, cmlueWFfbUBhZ3Iua3l1c2h1LXUuYWMuanA=
†Present address: Hirofumi Ohga, Fisheries Research Institute of Karatsu, Kyushu University, Saga, Japan;
Sethu Selvaraj, Fisheries College and Research Institute, Tamil Nadu Fisheries University, Ponneri, Tamil Nadu, India
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.