
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Endocrinol. , 02 February 2018
Sec. Neuroendocrine Science
Volume 9 - 2018 | https://doi.org/10.3389/fendo.2018.00008
This article is part of the Research Topic Neuroendocrine Disorders after Traumatic Brain Injury: Past, Present and Future View all 7 articles
Traumatic brain injury (TBI) remains a major, global public health concern. Over the last 15 years, a significant body of evidence has emerged demonstrating that post-traumatic hypopituitarism (PTHP) is a common and clinically significant consequence of TBI. Non-specific symptomology and the lack of an agreed approach to screening for PTHP has led to significant under-diagnosis of this debilitating disease. In this review, we will discuss the frequency and clinical significance of acute and chronic PTHP as described in the current literature highlighting the evidence base for screening and hormone replacement in these patients. We will also offer a pragmatic approach to identifying relevant anterior pituitary dysfunction after TBI and a follow-up strategy for those patients. Specific controversies and remaining unanswered questions will be addressed.
The true global incidence of traumatic brain injury (TBI) is difficult to fully ascertain; a recent meta-analysis suggested that the incidence is increasing and affects 134–618 persons per 100,000 per year with the highest incidence appearing in adult men (1). This makes TBI the most common cause of death and disability in young adults living in industrialized countries (2) and as such it presents a major public health concern.
However, the reported incidence of TBI varies wildly depending on numerous geographical and demographic factors. Most TBIs are mild and are not admitted to hospital (3). As a result, the vast majority of the literature on the subject relates to patients who required hospital admission, typically with moderate or severe TBI. The resultant underestimation of the true incidence of TBI has led to it being described as a “silent epidemic.”
Over the recent 15 years, numerous research studies have documented a high rate of pituitary gland dysfunction following moderate or severe TBI with evidence that post-traumatic hypopituitarism (PTHP) contributes significantly to the morbidity and possibly mortality in those patients (4). PTHP can masquerade as a post-concussional syndrome and can be overlooked leading to delayed recovery and impaired rehabilitation (5). Therefore, screening patients for PTHP should be an important part of the standard care provided following severe or moderate TBI.
This review will discuss the evidence supporting the need for screening of anterior pituitary function after TBI and our approach to the endocrine assessment and management of these patients.
Pituitary damage was first recognized as a potential outcome of head injury in 1918 when it was reported in an individual with a fractured base of skull (6). In the decades that followed, it was generally considered to be a rare complication of TBI. A review in 2000 reported only 367 known cases in the literature (7). However, over the last 15 years, there has been numerous retrospective and prospective studies showing a high rate of PTHP among long-term survivors of TBI (Table 1). In one meta-analysis, the pooled data from 19 studies showed a prevalence of 27% (8). The variations in the reported frequency of PTHP reflect patient selection, timing of testing, and methodological heterogeneity in the ascertainment of hypopituitarism. Among the pituitary hormone deficiencies, growth hormone (GH) deficiency, as with other forms of hypopituitarism, appears to be the commonest deficiency in those who were tested 6 months or more following the event (Table 1).
Prospective longitudinal studies have shown that pituitary dysfunction occurs early after acute TBI. The most clinically significant abnormalities in the acute phase of TBI are ACTH-cortisol deficiency and salt and water imbalance (11, 17). Some of these early abnormalities are transient and recover fully within days or weeks after TBI while new pituitary hormone deficiency may become apparent in the post-acute phase (13, 14). The symptoms of hypopituitarism can be subtle (18) and are summarized in Table 2.
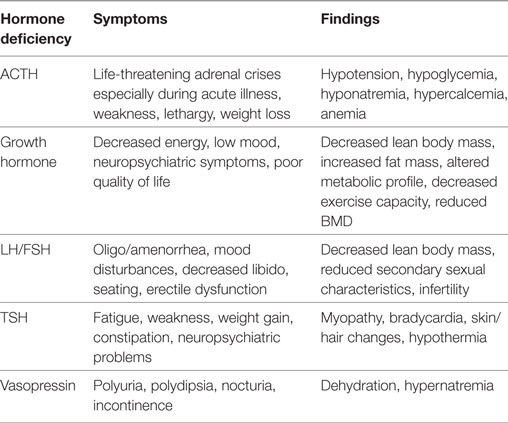
Table 2. A summary of common symptoms and findings associated with specific pituitary hormone deficiencies.
Mechanisms of injury including vascular injury to the hypothalamus, stalk, or the pituitary gland resulting in infarction, direct traumatic injury to the pituitary from base of skull fractures or secondary insults from, hypoxia, hypotension, or raised intracranial pressure (19) have all been hypothesized. It is likely that a vascular injury is the commonest mechanism as autopsy studies have demonstrated pituitary infarction in up to 43% of cases of fatal TBI (20).
The relationship between the severity of the head injury and the risk of hypopituitarism remains controversial. Traditionally, the clinical severity of TBI is measured by the post-resuscitation and pre-intubation Glasgow Coma Scale (GCS) Score. A score of 3–8 indicates severe, 9–12 moderate, and 13–15 mild injury. In patients with moderate or severe TBI, the scores do not seem to predict the risk of PTHP (8) but it is important to emphasize that this scale is limited by significant inter-observer variability and that the GCS scores can fluctuate over time. Some authors have suggested that findings on computed tomography should be factored in to the initial assessment when determining severity of TBI. They have hypothesized that significant pathological findings like cerebral edema or hemorrhage on cranial imaging may infer a more severe TBI. The data on this is limited with one study demonstrating a correlation between diffuse brain swelling and PTHP (19) and another finding no such association (14).
The data for mild TBI and hypopituitarism are limited (3). While some studies showed a significant association (21), one excellent large study showed a very low incidence of PTHP in mild TBI cases (22).
In patients with TBI, acute-phase cortisol deficiency is potentially life threatening and a number of studies have shown an association between increased morbidity and mortality in acute post-traumatic hypoadrenalism (11, 23).
Long-term adult GH deficiency is associated with decreased quality of life (24), reduced lean body mass (25), reduced bone mineral density (26), and impaired cardiac function (27). The sequelae of sex steroids, ACTH, and TSH deficiencies are well known (Table 2). Recent data seem to confirm that anterior pituitary hormone deficiency has a negative impact on functional outcome at 6 months post TBI as assessed by mini-mental state exam and functional independence measure scores (4). Other studies have found a correlation between PTHP and unfavorable metabolic and body composition profiles with associated lower quality of life scores (28, 29) as well as decreased exercise capacity (30) and neuropsychiatric complications (31).
Screening every patient with a TBI for PTHP is clearly not feasible. This approach does not meet the criteria for an appropriate screening tool due to the high cost and the complexity of testing which often requires dynamic and sometimes repeat assessments. The most robust data with regard to incidence of PTHP are in those patients with moderate or severe TBI (14). We therefore do not routinely screen patients with mild TBI for pituitary dysfunction unless there are specific symptoms suggestive of hypopituitarism (21, 22). Features that seem to confer an increased risk of PTHP include diffuse axonal injury on brain imaging, prolonged admission to ICU, basal skull fracture, and increasing age (32).
Screening for mild cases of TBI remains controversial. Schneider et al. demonstrated a high rate (16.8%) of PTHP following a mild TBI (8) although most of the endocrine abnormalities on mild TBI cases were not confirmed by second dynamic tests. In one well-executed study, the frequency of PTHP after simple mild TBI was found to be very low (22). However, it has been suggested that “complicated” cases labeled as mild TBI should also be included in the screening process (33). Features of a complicated mild TBI as described in this review included those patients admitted to hospital for >24 h, patients admitted to the ICU or those requiring neurosurgical intervention, patients suffering pituitary dysfunction within 2 weeks of their TBI, or patients with new anatomical changes on their brain scans. Therefore, selected cases of complicated mild TBI could also be offered screening but this will clearly have considerable resource implications.
There is currently no evidence to support the treatment of acute GH, thyroid hormones or gonadotropins deficiencies, or hyperprolactinemia in the acute phase following TBI. In addition, interpretation of the results in the acute phase is difficult. Therefore, early assessment of these axes is unnecessary.
Assessment of acute anterior pituitary dysfunction following TBI should focus on ACTH deficiency because of its potential contribution to the acute morbidity and mortality (11) in this patient cohort. Under normal circumstances, a dynamic test of the adrenal axis (such as the insulin stress test or the corticotrophin test) would provide the most accurate assessment. However, these dynamic tests are either inappropriate (the insulin stress test) or unreliable (the corticotrophin test) in the acute setting of TBI. For this reason, serial morning cortisol levels can be checked as a guide to possible ACTH deficiency. An acute-phase cortisol level below 300 nmol/l is suggestive of adrenal insufficiency (34) and treatment with glucocorticoids should be instituted (Figure 1). Levels of 300–500 nmol/l should be interpreted in the clinical context. Features suggestive of adrenal insufficiency as listed in Table 2 should alert the clinician and treatment with GC replacement may be warranted.
All patients with moderate and severe TBI should have an assessment of their adrenal, thyroid, and gonadal axes in the post-acute phase, usually at 3–6 months post TBI. The choice of the dynamic test for adrenal reserves will depend on the preference of the endocrinologist but we prefer the corticotrophin test (Synacthen test) as a simple and reliable test (35). Also, thyroid function tests (free T4 and TSH) and sex steroids with corresponding gonadotrophins levels (and menstrual history in premenopausal women) should be assessed. If a deficiency is detected, we recommend replacement as appropriate and repeating the assessment at 1 year as early deficiencies may recover (36).
Early GH deficiency post TBI can be transient (36). Assessment of the GH axis is also more complex due to the need to perform dynamic testing which is often only available in specialist centers. For these reasons, we recommend deferring GH assessment until 1 year post TBI. Assessment of GH reserves should ideally be done with the gold standard insulin tolerance test provided no contraindications (especially seizures and heart disease) are present. Other dynamic tests for GH can also be used (37) (Figure 1).
Traumatic brain injury is one of the world’s leading causes of morbidity and mortality among young men. PTHP is now recognized as a common, but often underdiagnosed, complication which may contribute significantly to the morbidity and possibly the mortality following moderate and severe TBI. Endocrine evaluation and management should be part of standard multidisciplinary care for these patients. Among patients with severe and moderate TBI, acute cortisol deficiency should be diagnosed and managed promptly while a more comprehensive assessment of the pituitary function should be undertaken in the post-acute phase. Randomized clinical trials examining the effect of GH replacement in patients with post-traumatic GH deficiency are needed to assess the potential impact on recovery, rehabilitation, and quality of life.
MQ carried out a detailed literature review on the topic of post-traumatic hypopituitarism and created various drafts under the title “Post-traumatic hypopituitarism—who should be screened, when, and how?” AA corrected each draft and provided additional literature which added to the scope of the review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Nguyen R, Fiest KM, McChesney J, Kwon CS, Jette N, Frolkis AD, et al. The international incidence of traumatic brain injury: a systematic review and meta-analysis. Can J Neurol Sci (2016) 43(6):774–85. doi:10.1017/cjn.2016.290
2. Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD. Surveillance for traumatic brain injury-related deaths – United States, 1997–2007. MMWR Surveill Summ (2011) 60(5):1–32.
3. McKinlay A, Grace RC, Horwood LJ, Fergusson DM, Ridder EM, MacFarlane MR. Prevalence of traumatic brain injury among children, adolescents and young adults: prospective evidence from a birth cohort. Brain Inj (2008) 22(2):175–81. doi:10.1080/02699050801888824
4. Park KD, Kim DY, Lee JK, Nam HS, Park YG. Anterior pituitary dysfunction in moderate-to-severe chronic traumatic brain injury patients and the influence on functional outcome. Brain Inj (2010) 24(11):1330–5. doi:10.3109/02699052.2010.506863
7. Benvenga S, Campenni A, Ruggeri RM, Trimarchi F. Clinical review 113: hypopituitarism secondary to head trauma. J Clin Endocrinol Metab (2000) 85(4):1353–61. doi:10.1210/jcem.85.4.6506
8. Schneider HJ, Kreitschmann-Andermahr I, Ghigo E, Günter K, Agha A. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage. JAMA (2007) 298(12):1429–38. doi:10.1001/jama.298.12.1429
9. Personnier C, Crosnier H, Meyer P, Chevignard M, Flechtner I, Boddaert N, et al. Prevalence of pituitary dysfunction after severe traumatic brain injury in children and adolescents: a large prospective study. J Clin Endocrinol Metab (2014) 99(6):2052–60. doi:10.1210/jc.2013-4129
10. Baxter D, Sharp DJ, Feeney C, Papadopoulou D, Ham TE, Jilka S, et al. Pituitary dysfunction after blast traumatic brain injury: the UK BIOSAP study. Ann Neurol (2013) 74(4):527–36. doi:10.1002/ana.23958
11. Hannon MJ, Crowley RK, Behan LA, O’Sullivan EP, O’Brien MM, Sherlock M, et al. Acute glucocorticoid deficiency and diabetes insipidus are common after acute traumatic brain injury and predict mortality. J Clin Endocrinol Metab (2013) 98(8):3229–37. doi:10.1210/jc.2013-1555
12. Kozlowski Moreau O, Yollin E, Merlen E, Daveluy W, Rousseaux M. Lasting pituitary hormone deficiency after traumatic brain injury. J Neurotrauma (2012) 29:81–9. doi:10.1089/neu.2011.2048
13. Schneider HJ, Schneider M, Saller B, Petersenn S, Uhr M, Husemann B, et al. Prevalence of anterior pituitary insufficiency 3 and 12 months after traumatic brain injury. Eur J Endocrinol (2006) 154(2):259–65. doi:10.1530/eje.1.02071
14. Agha A, Rogers B, Sherlock M, O’Kelly P, Tormey W, Phillips J, et al. Anterior pituitary dysfunction in survivors of traumatic brain injury. J Clin Endocrinol Metab (2004) 89(10):4929–36. doi:10.1210/jc.2004-0511
15. Tanriverdi F, Senyurek H, Unluhizarci K, Selcuklu A, Casanueva FF, Kelestimur F. High risk of hypopituitarism after traumatic brain injury: a prospective investigation of anterior pituitary function in the acute phase and 12 months after trauma. J Clin Endocrinol Metab (2006) 91(6):2105–11. doi:10.1210/jc.2005-2476
16. Aimaretti G, Ambrosio MR, Di Somma C, Gasperi M, Cannavò S, Scaroni C, et al. Residual pituitary function after brain injury-induced hypopituitarism: a prospective 12-month study. J Clin Endocrinol Metab (2005) 90(11):6085–92. doi:10.1210/jc.2005-0504
17. Agha A, Thornton E, O’Kelly P, Tormey W, Phillips J, Thompson CJ. Posterior pituitary dysfunction after traumatic brain injury. J Clin Endocrinol Metab (2004) 89(12):5987–92. doi:10.1210/jc.2004-1058
18. Schneider HJ, Aimaretti G, Kreitschmann-Andermahr I, Stalla G-K, Ghigo E. Hypopituitarism. Lancet (2007) 369(9571):1461–70. doi:10.1016/S0140-6736(07)60673-4
19. Kelly DF, Gonzalo IT, Cohan P, Berman N, Swerdloff R, Wang C. Hypopituitarism following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a preliminary report. J Neurosurg (2000) 93:743–52. doi:10.3171/jns.2000.93.5.0743
20. Salehi F, Kovacs K, Scheithauer BW, Pfeifer EA, Cusimano M. Histologic study of the human pituitary gland in acute traumatic brain injury. Brain Inj (2007) 21(6):651–6. doi:10.1080/02699050701426956
21. Tanriverdi F, Unluhizarci K, Kelestimur F. Pituitary function in subjects with mild traumatic brain injury: a review of literature and proposal of a screening strategy. Pituitary (2010) 13(2):146–53. doi:10.1007/s11102-009-0215-x
22. van der Eerden AW, Twickler MT, Sweep FC, Beems T, Hendricks HT, Hermus AR, et al. Should anterior pituitary function be tested during follow-up of all patients presenting at the emergency department because of traumatic brain injury? Eur J Endocrinol (2010) 162(1):19–28. doi:10.1530/EJE-09-0436
23. Cohan P, Wang C, McArthur DL, Cook SW, Dusick JR, Armin B, et al. Acute secondary adrenal insufficiency after traumatic brain injury: a prospective study*. Crit Care Med (2005) 33(10):2358–66. doi:10.1097/01.CCM.0000181735.51183.A7
24. Cuneo RC, Salomon F, McGauley GA, Sönksen PH. The growth hormone deficiency syndrome in adults. Clin Endocrinol (1992) 37:387–97. doi:10.1111/j.1365-2265.1992.tb02347.x
25. Binnerts A, Deurenberg P, Swart GR, Wilson JH, Lamberts SW. Body composition in growth hormone deficient adults. Am J Clin Nutr (1992) 55:918–23. doi:10.1093/ajcn/55.5.918
26. Kuzma M, Kuzmova Z, Zelinkova Z, Killinger Z, Vanuga P, Lazurova I, et al. Impact of the growth hormone replacement on bone status in growth hormone deficient adults. Growth Horm IGF Res (2014) 24(1):22–8. doi:10.1016/j.ghir.2013.12.001
27. Cittadini A, Cuocolo B, Merola S, Fazio D, Sabatini E, Nicolai A, et al. Impaired cardiac performance in GH-deficient adults and its improvement after GH replacement. Am J Physiol (1994) 267(2):E219–25 doi:10.1152/ajpendo.1994.267.2.E219
28. Klose M, Watt T, Brennum J, Feldt-Rasmussen U. Posttraumatic hypopituitarism is associated with an unfavorable body composition and lipid profile, and decreased quality of life 12 months after injury. J Clin Endocrinol Metab (2007) 92(10):3861–8. doi:10.1210/jc.2007-0901
29. Mossberg KA, Durham WJ, Zgaljardic DJ, Gilkison CR, Danesi CP, Sheffield-Moore M, et al. Functional changes after recombinant human growth hormone replacement in patients with chronic traumatic brain injury and abnormal growth hormone secretion journal of neurotrauma. J Neurotrauma (2017) 34(4):845–52. doi:10.1089/neu.2016.4552
30. Mossberg KA, Masel BE, Gilkison CR, Urban RJ. Aerobic capacity and growth hormone deficiency after traumatic brain injury. J Clin Endocrinol Metab (2008) 93(7):2581–7. doi:10.1210/jc.2008-0368
31. Olver JH, Ponsford JL, Curran CA. Outcome following traumatic brain injury: a comparison between 2 and 5 years after injury. Brain Inj (1996) 10(11):841–8. doi:10.1080/026990596123945
32. Schneider M, Schneider HJ, Yassouridis A, Saller B, von Rosen F, Stalla GK. Predictors of anterior pituitary insufficiency after traumatic brain injury. Clin Endocrinol (2008) 68:206–12. doi:10.1111/j.1365-2265.2007.03020.x
33. Tanriverdi F, Kelestimur F. Pituitary dysfunction following traumatic brain injury: clinical perspectives. Neuropsychiatr Dis Treat (2015) 11:1835–43. doi:10.2147/NDT.S65814
34. Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med (2003) 348:727–34. doi:10.1056/NEJMra020529
35. Agha A, Tomlinson JW, Clark PM, Holder G, Stewart PM. The long-term predictive accuracy of the short synacthen (corticotropin) stimulation test for assessment of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab (2006) 91:43–7. doi:10.1210/jc.2005-1131
36. Agha A, Phillips J, O’Kelly P, Tormey W, Thompson CJ. The natural history of post-traumatic hypopituitarism: implications for assessment and treatment. Am J Med (2005) 118(12):1416. doi:10.1016/j.amjmed.2005.02.042
Keywords: traumatic brain injury, post-traumatic hypopituitarism, hypopituitarism, pituitary injury, growth hormone deficiency, ACTH deficiency, incidence of post-traumatic hypopituitarism, screening for post-traumatic hypopituitarism
Citation: Quinn M and Agha A (2018) Post-Traumatic Hypopituitarism—Who Should Be Screened, When, and How? Front. Endocrinol. 9:8. doi: 10.3389/fendo.2018.00008
Received: 06 November 2017; Accepted: 08 January 2018;
Published: 02 February 2018
Edited by:
Jacques Epelbaum, Institut National de la Santé et de la Recherche Médicale, FranceReviewed by:
Maria Vrontakis, University of Manitoba, CanadaCopyright: © 2018 Quinn and Agha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark Quinn, TWFyay5xdWlubjg2QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.