
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Endocrinol., 17 August 2017
Sec. Cellular Endocrinology
Volume 8 - 2017 | https://doi.org/10.3389/fendo.2017.00190
This article is part of the Research TopicAstrocytic-neuronal-astrocytic Pathway Selection for Formation and Degradation of Glutamate/GABAView all 18 articles
This article is a correction to:
Protein Kinase C Phosphorylates the System N Glutamine Transporter SN1 (Slc38a3) and Regulates Its Membrane Trafficking and Degradation
A corrigendum on
In the original article, there was a mistake in Figure 1 as published. The figure has two white arrows (in the left upper corner) which are labeled opposite of what they should be. The corrected Figure 1 appears below. The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way.
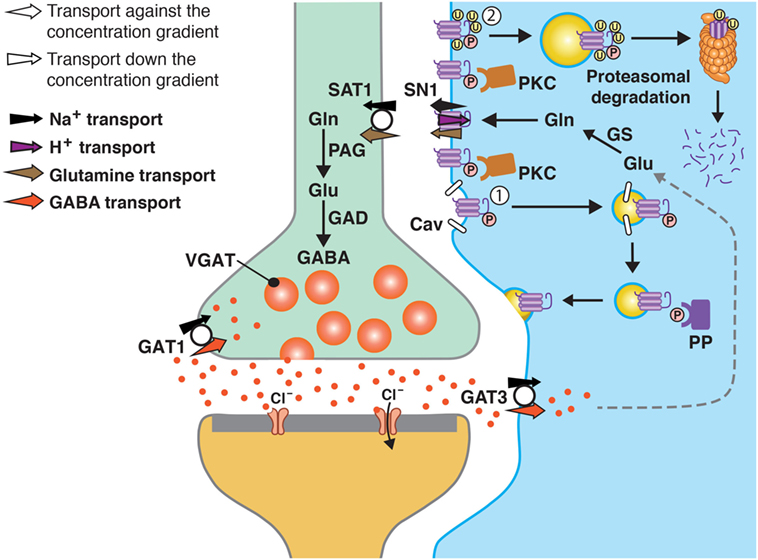
Figure 1. A model of SN1 membrane trafficking based on consolidated data on the regulation of SN1 activity by protein kinase C (PKC). The cartoon depicts a GABAergic synapse in adult rat brain where GABA is released exocytotically and acts upon specific post-synaptic receptors. The signal is terminated by removal of GABA from the synaptic cleft by transport of GABA back into the nerve terminal by the plasma membrane GABA transporter (GAT) 1. A substantial amount of GABA in the synaptic cleft is also transported into perisynaptic astroglial processes by GAT3 (and GAT1) and converted to glutamate, and then to glutamine catalyzed by glutamine synthetase (GS). Glutamine may then be released from the astroglial cells by the electroneutral and bidirectional system N transporter 1, SN1, and may subsequently be accumulated inside GABAergic neurons by the system A transporter SAT1. Here, glutamine is metabolized to yield glutamate and then GABA by the action of phosphate-activated glutaminase (PAG) and glutamic acid decarboxylase (GAD), respectively. Finally, GABA is translocated into synaptic vesicles by the vesicular GABA transporter (VGAT), wherefrom it is ready to be exocytotically released. Astroglial PKC may be activated upon stimulation of specific receptors on the astroglial membranes or e.g., by excessive amounts of Mn2C. PKCa and PKCg (and PKCd) can phosphorylate SN1 on a serine at the 52 position. This results in caveolin (Cav)-dependent internalization of SN1 from the plasma membrane (1). The internalized SN1 may be relocated to the plasma membrane upon dephosphorylation by protein phosphatases (PP). PKC-mediated phosphorylation of SN1 also increases ubiquitination of SN1 (2). This may also internalize the protein into intracellular compartments and target it to the proteasomal degradation pathway. Similar regulation of SN1 activity also takes place at glutamatergic synapses. The same mechanisms are likely to be involved in the regulation of SN1 in hepatocytes, renal tubule cells, and the pancreatic B-cells.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Keywords: SN1, Slc38, glutamine, glutamate, PKC, GABA, neurotransmitter replenishment, transporter
Citation: Nissen-Meyer LSH and Chaudhry FA (2017) Corrigendum: Protein Kinase C Phosphorylates the System N Glutamine Transporter SN1 (Slc38a3) and Regulates Its Membrane Trafficking and Degradation. Front. Endocrinol. 8:190. doi: 10.3389/fendo.2017.00190
Received: 17 July 2017; Accepted: 21 July 2017;
Published: 17 August 2017
Edited and Reviewed by: Leif Hertz, China Medical University, China
Copyright: © 2017 Nissen-Meyer and Chaudhry. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lise Sofie H. Nissen-Meyer, bC5zLmgubmlzc2VuLW1leWVyQG1lZGlzaW4udWlvLm5v;
Farrukh Abbas Chaudhry, Zi5hLmNoYXVkaHJ5QG1lZGlzaW4udWlvLm5v
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.